Abstract
Myeloid-derived suppressor cells (MDSCs) are one of the main suppressive cell population of the immune system. They play a pivotal role in the establishment of the tumor microenvironment (TME). In the context of cancers or other pathological conditions, MDSCs can differentiate, expand, and migrate in large quantities during circulation, inhibiting the cytotoxic functions of T cells and NK cells. This process is regulated by ROS, iNOS/NO, arginase-1, and multiple soluble cytokines. The definition of MDSCs and their phenotypes in humans are not as well represented as in other organisms such as mice, owing to the absence of the cognate molecule. However, a comprehensive understanding of the differences between different species and subsets will be beneficial for clarifying the immunosuppressive properties and potential clinical values of these cells during tumor progression. Recently, experimental evidence and clinical investigations have demonstrated that MDSCs have a close relationship with poor prognosis and drug resistance, which is considered to be a leading marker for practical applications and therapeutic methods. In this review, we summarize the remarkable position of MDSCs in solid tumors, explain their classifications in different models, and introduce new treatment approaches to target MDSCs to better understand the advancement of new approaches to cancer treatment.
Keywords: myeloid-derived suppressor cells (MDSCs), solid tumor, immunotherapy, tumor microenvironment (TME)
1. Introduction
In recent decades, myeloid-derived suppressor cells (MDSCs) have been recognized as an indispensable cell population of the innate immune system, highlighted by their strong immunosuppressive activity in cancers and other pathological conditions, altering our adaptive immune response.
MDSCs are a heterogeneous group of immature myeloid cells derived from the bone marrow hematopoietic precursor cells [1]. In healthy individuals, immature myeloid cells (IMCs) differentiate into granulocytes, macrophages, and dendritic cells [2], and enter the corresponding organs and tissues, exerting normal immune functions. However, in pathological and chronic inflammatory conditions, such as cancers, infectious diseases, autoimmune disorders, or sepsis, a block in the normal myeloid differentiation occurs, and the persistent stimulation of myelopoiesis results in the expansion of MDSCs [3,4]. This is regulated by several factors, including, but not limited to, granulocyte/macrophage colony-stimulating factor (GM-CSF), interleukin (IL)-6, IL-10, IL-12, cycloxygenase-2 (COX-2), prostaglandin E2 (PGE2), and vascular endothelial growth factor (VEGF) [5,6]. When MDSCs accumulated and migrated to the periphery, their number and proportion increased by 10-fold, accounting for approximately 10% of peripheral blood mononuclear cells (PBMCs) [7] throughout the entire process of disease. These cells inhibit the normal immune function of T cells in the tumor microenvironment (TME) [8].
MDSCs show a wide range of phenotypes and are involved in the setting of tumor progression. In mice, MDSCs are marked by CD11b and Gr1, and can be divided into two subgroups, including polymorphonuclear MDSCs (PMN-MDSCs) with CD11b+ Ly6Ghi Ly6C−/low and monocytic MDSCs (M-MDSCs) with CD11b+ Ly6G−/low Ly6Chi [9]. However, the cognition of MDSCs in humans is complicated and remains controversial. To imitate the same findings in mice, human MDSCs were specified by surface markers [10]. Human M-MDSCs are defined as CD14+ CD33+ CD11b+ HLA-DR−, and PMN-MDSCs as CD15+ CD33+ CD11b+ HLA-DR− [11]. Both M-MDSCs and PMN-MDSCs can restrain T cells and NK cells by secreting reactive oxygen species (ROS), nitrogen oxide (NO), arginase-1, etc., and inducing regulatory T cells (Tregs) in some circumstances, therefore, suppressing the immune response of the host.
The special feature of MDSCs is their potent inhibitory function, which establishes an immunosuppressive TME that is conducive to tumor immune escape, and further promotes the occurrence and development of tumors. Hence, these cells are worth noting in future clinical immunotherapy applications.
2. MDSCs as the Main Component of Cellular Immune Suppressors
Tregs and MDSCs are considered two crucial immunosuppressive cell populations, participating in accelerating the progression and metastasis of tumors. Tregs contribute mainly to cellular immunity through direct contact between cells, secretion of inhibitory factors, and competition with antigen-presenting cells (APC) for costimulatory molecules [12]. Similarly, a broad range of suppressive indicators in MDSCs prevent immune cells’ (mostly T cells and NK cells) anti-tumor reactivity and support disease progression.
Mechanisms of MDSCs Immunosuppressive Function
There are several mechanisms that consist of MDSC-mediated immunosuppression (Table 1). Firstly, MDSCs exhibit immunosuppressive effects under oxygen pressure, producing ROS and peroxynitrite (ONOO−). In the biological context, ROS take part in maintaining homeostasis [13], while in oxidative stress conditions, increased ROS concentration will hamper T-cell signaling activation, proliferation, and viability, thereby damaging the adaptive immune response [14,15]. ONOO− is a strong nitrifying agent, ascribed to the reaction with superoxide and nitrogen oxides (NO) [16], and enriched in MDSCs and tumor cell aggregation sites [17,18]. It can nitrate T cell receptor (TCR) and CD8 molecules during direct contact between MDSCs and T cells and inhibit the proliferation of CD8+ T cells, causing the down-regulation of the immune activity [19,20]. MDSCs express nitrogen-oxygen synthase 2 (iNOS), which releases NO. The up-regulated expression of iNOS leading to NO production was proven to be relevant for obstructing IFN-γ signaling. This effect is due to a signal transducer and activator of transcription 1 (STAT1) in the anti-tumor immune response [21]. NO also directly impairs T cell function by inhibiting JAK/STAT5 pathways, MHC class II expression, and inducing T cell and NK cell apoptosis in tumor cells [22].
Table 1.
Main mechanisms of the MDSCs immunosuppressive function.
| Mechanisms | Main Factors | Immune Response | References | |
|---|---|---|---|---|
| Oxidative stress | ROS | Inhibit T cell activation, proliferation, and viability | [14,15] | |
| iNOS/NO | Impair T cell function and induce T cell and NK cell apoptosis | [19,20] | ||
| ONOO- | Nitrate TCR/CD8 molecules and inhibit the proliferation of CD8+ T cells | [21,22] | ||
| Amino acid consumption | Arginine depletion | Arginase-1 | Block G0-G1 phase during T cell proliferation | [23,24] |
| Cystine deprivation | Cystine | Prevent T cell activation | [25,26,27] | |
| IDO overexpression | IDO | Influence the function of effector T cells | [28,29] | |
| Typical Cytokines and other mediators support | IL-1β, IL-6, IL-10 | Impose immunosuppressive effects on T cells | [30,31,32] | |
| GM-CSF | Suppress T cells | [33,34,35] | ||
| TGF-β | Block cytotoxic T cell-mediated tumor immunosurveillance | [36,37] | ||
| CCR2 | Affect the transport of T cells to the tumor site | [38] | ||
| VEGF | Prolong the innate immunity suppression | [39,40] | ||
| IFN-γ | Negatively manipulating anti-tumor T cell response | [41] | ||
| TLR2 | Negatively manipulating anti-tumor T cell response | [41] | ||
| PGE2 (COX2) | Inhibit the activation of CD4+ and CD8+ T cells | [43,44,45] | ||
| ADAM17 | Inhibit T cell migration to peripheral lymph nodes and tumor sites. | [5] | ||
| miRNA-155, miRNA-21 | Inhibit helper T cell and cytotoxic T cell proliferation | [46,47] | ||
| Cell–cell transfer | Other immune cells | Reduce T cell anti-tumor immunity | [48,49,50,51,52] | |
Secondly, MDSCs facilitate the consumption of some amino acids needed in our body. Both IL-4 and IL-13 can bind to IL-4Rα, inducing STAT6 activation and arginase-1 expression in myeloid cells (e.g., MDSCs and activated macrophages) [23]. The growing number of arginase-1 generated by MDSCs depletes L-arginine, leading to the G0–G1 phase being blocked during T cell proliferation and the low expression of the CD3ζ chain of the TCR, connected with tumor escape in vivo [24]. Human CD4+ T cells depend on the uptake of exogenous cystine/cysteine for glutathione and DNA synthesis [25,26]. MDSCs, however, prevent T cell activation via depriving cystine and restricting the availability of cysteine [27]. Indoleamine 2,3-dioxygenase (IDO) metabolizing tryptophan is a part of the malignant transformation process. The overexpression of IDO has been referred to support MDSCs in immunosuppressive TME influencing the inflammatory environment [28]. In clinical trials, IDO inhibitors have been used as a regimen for reducing MDSCs and abolishing their suppressive function on effector T cells [29].
Thirdly, MDSCs apply their immunosuppressive capability in tumor progression by multiple molecules. IL-10 produced by cancer and stroma cells hinders the maturation of myeloid cells by activating STAT3. These immature myeloid cells become MDSCs and impose immunosuppressive effects on CD8+ T cells [30]. In a renal cell carcinoma (RCC) a low level of IL-1β may influence adaptive and innate immune resistance on account of increasing MDSC infiltration [31]. IL-1β was also shown to provoke IL-10 production by MDSCs. In murine models, IL-6 was proven to facilitate MDSC expansion and activation, preventing the immune response directly [32]. GM-CSF allows the in vitro generation of MDSCs retaining their suppressive activity in vivo [33]. Tumor-derived G-CSF induced the overexpression of MDSCs, which are responsible for T cell suppression, tumor growth, and metastasis in gynaecological solid tumors [34,35]. It has been shown that transforming growth factor β (TGF-β) was contained in the regulation of hematopoiesis, which can interrupt the maturation of myeloid cells, prompting MDSC generation and expansion [36]. The production of TGF-β in CD11b+ Gr-1+ myeloid cells is one of the mechanisms of blocking cytotoxic T cell-mediated tumor immunosurveillance [37]. MDSC accumulation depends on CC chemokine receptor (CCR) 2-mediated signals. Bone marrow-derived CCR2+ MDSCs may affect the transport of T cells to the tumor site, supporting tumor growth [38]. MDSCs are also known to produce vascular endothelial growth factors (VEGF) that promote neo-angiogenesis and create a pre-metastatic environment, prolonging innate immunity suppression [39,40]. Interferon-γ (IFN-γ) and Toll-like receptor (TLR) 2 were reported to enhance the mediated immunosuppression of M-MDSCs, (not G-MDSCs), negatively manipulating the anti-tumor T cell response [41]. Prostaglandin E2 (PGE2) and cycloxygenase-2 (COX-2) are inflammatory mediators produced by different tumors [42]. PGE2 was confirmed to induce the accumulation of MDSCs, and inhibit the activation of CD4+ and CD8+ T cells [43]. Tumor-derived COX-2 conduces arginase-1 induction of MDSCs, resulting in tumor immune tolerance [44,45]. ADAM17 (a disintegrin and metalloproteinase domain 17), expressed on MDSCs, is likely to down-regulate L-selection on CD4+ and CD8+ T cells and prevent them to migrate to peripheral lymph nodes and tumor sites [5]. Furthermore, some small single-stranded non-coding RNA, such as microRNA-21 and microRNA-155, were also shown to adjust the immune suppression system mediated by MDSCs in tumors [46,47].
Lastly, cell-to-cell contact is another way for MDSCs to suppress the immune response. In the peripheral blood (PB) of pregnant women, M-MDSCs have been observed to significantly suppress T cell responses in a reactive oxygen species-dependent manner and require a mechanism that relies on cell contact [48]. Recently, one report demonstrated that MDSCs could paralyze T cells through the cell–cell transfer of the metabolite methylglyoxal, which may reduce T cell anti-tumor immunity [49]. The interplay between MDSCs and Tregs contribute to the establishment of an immunosuppressive environment. MDSCs promote the production of Tregs via a cell-contact dependent mechanism, inducing immune tolerance under conditions of cancers or other abnormal immune responses [50,51]. In addition, MDSCs also directly incite tumor-associated macrophages (TAMs) to facilitate immunosuppression [52]. Thus, the involvement of MDSCs is regarded as an obstacle to the immune response against solid tumors as well as hematological malignancies. Furthermore, the reciprocal connection between MDSCs and other immune cells is also of great value in tumor promotion.
3. Crosstalk between MDSCs and Other Immune Cells
3.1. Conventional T Cells
Generally, MDSCs immunosuppressor function lies in their ability to restrain T cell proliferation and activation, as well as to impede T cell recruitment to lymph nodes or tumor tissues [19]. This process has been already introduced in the previous section. Conversely, inflammatory molecules, such as IFN-γ, which is released by activated T cells, could also trigger MDSC expansion, migration, and activation at the tumor site, resulting in the growth of solid tumors [53].
The production of IFN-γ and IL-13 by CD8+ T cells could be integrated into MDSCs, having a negative impact on the adaptive immune system [54]. Blocking IFN-γ and IL-13 in combination therapy of tumor–host could obtain the additive effect. Nagaraj et al. [55] reported that CD4+ T cells cause the conversion of MDSCs from antigen-specific to non-specific suppressors via retrograde MHC class II signaling. This characterization is important for understanding the nature of immune defects in cancer. Pinton et al. [56] identified that MDSCs only exhibit their immunosuppression ability on activated but not on resting T cells. This cell–cell interaction induces the release of IL-10 in T cells and in turn, activates STAT3 phosphorylation on MDSCs, enhancing the immunosuppressive activity of these cells.
As critical members of the immune system, T cells are required for guiding the immune response process and controlling cancer development. The complex, in-depth mechanism of interaction between MDSCs and T cells presents a substantial challenge for therapeutic targeting.
3.2. Tregs and Th17
MDSCs and Tregs are both implicated in tumor escape from immunosurveillance. Immunosuppression can be weakened by decreasing the MDSCs and Treg cell populations in solid tumors after removing the tumor burden [57].
A few reports indicated that MDSCs are related to the induction of Treg [57,58] by direct contact. The high expression of IDO and arginase-1 in MDSCs fostered Treg expansion and differentiation [59,60]. In contrast, Tregs have also been shown to influence the survival and/or proliferation of MDSCs due to the production of certain cytokines. Lee et al. [61] demonstrated that Tregs control the regulation of MDSCs via TGF-β during murine colitis. Fujimura et al. [62] also signified that in melanoma that the depletion of Tregs lessened the production of IL-10 by MDSCs, readily decreasing the suppressive function of MDSCs. The interconnection between Tregs and MDSCs was dedicated to the acquisition of immunosuppressive effects, which may be utilized to achieve clinical benefit.
Th17 cells tend to promote immune reactions in many pathological conditions. A high level of arginase-1, expressed by MDSCs, enhances Th17 polarization [63]. Some studies suggested that the recruitment of MDSCs at tumor sites are often accompanied by aggregation of Th17 cells [64,65,66]. However, the potential connection between these two cell types demands further systematic exploration.
3.3. B Cells
Nowadays, many studies have pay attention to the interaction between MDSCs and B cells in the progression of tumor diseases. Unfortunately, the mechanisms on how MDSCs affect B cells and whether they could be applied to all circumstances remain unclear. Some studies supply new points of view. Wang et al. [67] implied that the upregulation of IL-7 and STAT5 influenced by MDSCs in lung cancer hindered the proliferation and differentiation of B cells. Moreover, M-MDSCs could weaken the B cell immune response by several suppressive mediators such as arginase-1, ROS, NO, and IDO [68,69]. MDSCs also directly act on B cells to decline IgM and IgG production (thereby reducing their levels in serum) through the V-domain Ig suppressor of T-cell activation (VISTA) from mice infected with an immunodeficiency-causing retrovirus [70].
B-regulatory cells (B-regs) are a newly identified immunosuppressive cell population [71], which are not yet well investigated. However, these cells are deemed significant contributors to neoplastic disorders [72]. Bodogai et al. [73] indicated that cancer-induced Bregs fully mobilized the immunomodulatory and tumor-promoting function of MDSCs, which are manifested by the increase of ROS and NO, inhibiting the CD4+ and CD8+ T cell proliferation. Lee-Chang et al. [74] uncovered that MDSCs represented a major immunosuppressive cell compartment in glioblastoma (GBM) patients and mice. They could deliver membrane-bound programmed death-ligand 1 (PD-L1) to B cells by microvesicles, resulting in the transformation of naïve B cells to Bregs and inhibiting regular immune responses.
3.4. Natural Killer (NK) Cells
MDSCs could advance tumor progression by affecting the anti-tumor activities of NK cells. The reduction of NK cells is relevant to the marked increase of MDSCs, not Tregs [75,76].
Liu et al. [77] verified that the cell–cell contact between MDSCs and NK cells in tumor-bearing mice could inhibit the IL-2–mediated activation of NK cells and perforin production, decreasing the ability of NK cells to attack tumor cells. MDSCs could also block IFN-γ secretion by NK cells, and NO produced by MDSCs further interferes with NK Fc Receptor to inhibit the cytotoxicity of NK cells [78].
Greene et al. [79] stated that the prevention of MDSC trafficking with a CXCR1/2 small molecule inhibitor can improve the therapeutic efficacy of adoptively transfer of NK cells, which clarified the important ability of MDSC to suppress NK cell function in TME. The PD-1/PD-L1 pathway has been validated to be the main index in cancer immunotherapy. A recent study reported a novel NK cell line, PD-L1, targeting high-affinity natural killer (t-haNK) cells, which retained its in vitro cytotoxic potency against various human cancer cells. These cells could effectively lyse tumor cells and MDSCs without affecting T and NK cells, relieving the immunosuppression of MDSCs in the TME [80]. Clinical trials should be implemented to ensure safety and efficacy in future applications.
3.5. Neutrophils
Neutrophils and G-MDSCs (or PMN-MDSCs) share the same origin and many morphological and phenotypic features [81]. Pillay et al. [82] proposed a hypothesis that G-MDSCs could be a unique phenotype of neutrophils. A few studies demonstrated that the tumor-promoting activity of neutrophils could be attributed to PMN-MDSCs. Semerad et al. [83] suggested that G-CSF was a principal regulator of neutrophil transport from bone marrow to blood, which was also confirmed to be involved in MDSCs suppression function. In addition, the production of ROS in both neutrophils and MDSCs is considered a major mechanism of immune suppression in the TME [84]. It appeared that G-MDSCs (or PMN-MDSCs) could represent neutrophils at distinct maturation stages, and the activated neutrophils are able to obtain the inhibitory activity of MDSCs.
3.6. Tumor-Associated Macrophages (TAMs)
In the case of chronic inflammation and tumors, the bidirectional nature of MDSC–macrophage interactions significantly amplified the level of IL-10 and declined the level of IL-6, IL-12 and MHC II, prompting the differentiation of CD4+ T cells (Th2), the absence of NK cells, and the development of Tregs [85].
Immature myeloid cells could rapidly differentiate into TAMs mediated by ROS elevation and STAT1 signaling activation [86,87]. Corzo et al. [88] made use of a tumor mouse model to explore the nature of tumor-associated MDSCs under hypoxia. Their data indicated that the existence of hypoxia-inducible factor (HIF) 1α directly controls the differentiation of MDSCs to TAMs at tumor sites. Kumar et al. [89] observed that the fate of M-MDSCs was modulated by the activation of STAT3. When this transcriptional factor was selectively inhibited by hypoxia-induced CD45 tyrosine phosphatase upregulation, M-MDSCs would differentiate into TAMs, which introduced a new mechanism for the design of targeted therapy. Loeuillard et al. [90] characterized that the blockade of TAMs did not reduce the tumor growth in cholangiocarcinoma (CCA) but supplied a compensatory accumulation of G-MDSCs. This MDSC subset could facilitate tumor invasion and metastasis. After the combined removal of TAM and G-MDSC populations, the effect of the immune checkpoint blockade (ICB) with anti–PD-1 was improved, supporting a prospective therapeutic approach on targeting both macrophages and MDSCs.
3.7. Dendritic Cells (DCs)
The cancer cell-induced immunosuppressive microenvironment does not only limit the activity of mature and functional DCs but also expand the tumor-promoting immature DC populations [91]. The recruitment of MDSCs seems to be linked to this impaired differentiation of DCs; however, the basic mechanisms are still not fully understood.
An early study evidenced that the overexpression of Ca2+ binding myeloid-related proteins (S100A8 and S100A9) induced by STAT3 could be one of the reasons for DC differentiation disorder and MDSC hyperproduction, enhancing the immunosuppression of a tumor-bearing host [92]. This underlying connection between immune cells and pro-inflammatory factors might interpret a pathway to negatively regulate the immune response in cancer. Another finding in melanoma patients showed that MDSCs did not affect the viability of DCs, but impaired DC maturity by restricting antigen uptake due to the blockage of DC capacity to stimulate IFN-γ-producing T cells, as well skewing DC cytokine production towards an anti-inflammatory phenotype [93]. Moreover, the highly suppressive M-MDSCs (CD14+ HLA-DR− cells) were found to impair the quality of the overall DC vaccine; therefore, their removal could be beneficial [93]. The mutual transformation and interaction between MDSCs and DCs could be a focus of future immunotherapy research.
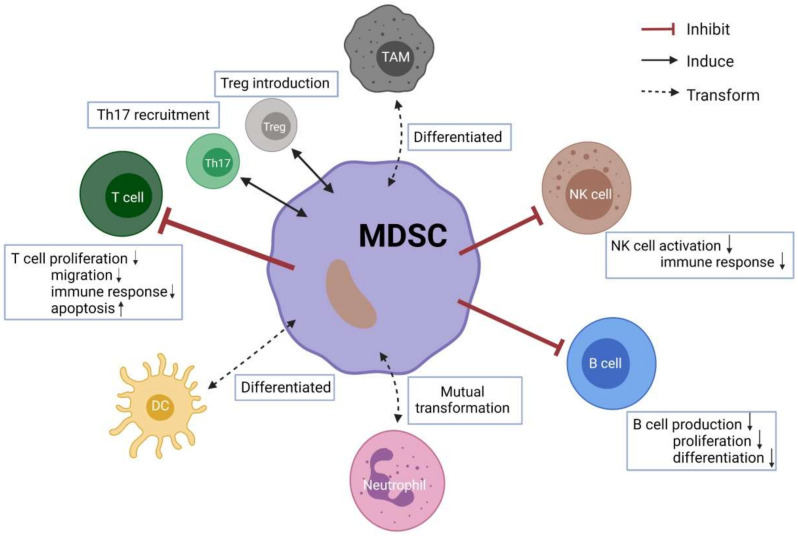
To summarize, given the ability of MDSCs to modulate various immune cells in facilitating tumor progression, this immune cell population may be an important therapeutic target. (Figure 1, created with BioRender.com (accessed on 29 November 2021)) Next, we will focus on their potential clinical application in both mouse models and human solid tumors.
Figure 1.
Crosstalk between MDSCs and other immune cells. Up arrows mean increased, and the down arrows mean decreased.
4. Defining MDSCs in Mouse and Human
As mentioned previously, MDSCs are a very heterogenic group not only on the basis of the cellular origin but also in the context of their surface markers. In mice, MDSCs present high levels of CD11b (a subunit of the β2 integrin Mac-1) and Gr1 (granulocytic marker) [94]. Gr1 antigen detected by the RB6-8C5 antibody was discovered to be made up of two phosphatidylinositol-anchored cell surface glycoproteins, Ly6C and Ly6G [95]. Based on their expression, murine MDSCs can be classified into two subtypes, monocytic MDSCs (M-MDSCs) and polymorphonuclear MDSCs (PMN-MDSCs), also known as granulocytic MDSCs (G-MDSCs). M-MDSCs display a high levels of Ly6C expression while low or no expression of Ly6G, resembling monocytes in phenotypical and morphological features, while PMN-MDSCs express low levels of Ly6C and high levels of Ly6G, similar to neutrophils [96]. Although these two subpopulations are both involved in immune suppression, current views assume that their proportions and inhibitory pathways in different tumors are not completely the same. M-MDSCs mainly up-regulate NO and arginase, secreting soluble cytokines [81], whereas G-MDSCs need to interact with T cells directly [97] and usually exert their suppressive function utilizing ROS. The nature of chemokines produced by tumor cells might support the infiltration of M-MDSCs in early tumor sites and transmission from the primary site [81], while G-MDSCs could facilitate tumor cell expansion and metastatic growth via reverting epithelial-mesenchymal transition (EMT)/cancer stem cell (CSC) phenotype [98]. A recent study published in 2021 provided evidence that endoplasmic reticulum (ER) stress response appears to be a dominant factor in surveillance the immune-suppressive activity of PMN-MDSCs by the IRE1α and ATF6 pathways in tumor-bearing mice. In contrast, the induction of M-MDSC depends on IFN-γR signaling instead of the ER stress response [99].
Unlike mouse MDSCs, human MDSCs are less well characterized due to the lack of Gr1; therefore, they are usually express high levels of CD33, CD11b surface markers and low levels of HlA-DR [100]. Similar to murine MDSCs, human MDSCs can also be subdivided into CD14+ M-MDSC and CD15+ PMN-MDSC populations [101,102]. Notably, another type of MDSCs with CD11b+ CD14− CD15− was defined as early-MDSCs (E-MDSCs) [9], comprising more immature progenitors.
At the moment, a debate on the subsets, origin, and functions of human MDSCs still exists; thus, other methods need to be discussed to clarify the characterization of human MDSCs. CCAAT/enhancer-binding protein (c/EBPβ) plays a key role in the generation of in vivo tumor-induced MDSCs [103]. Moreover, STAT3 signaling was revealed to promote MDSC differentiation and expansion [104]. Although these proteins are not surface markers and are therefore difficult for analysis, they helped to define two separate MDSC groups, CD33+ HLA-DR−/low STAT3+ and CD11b+ HLA-DR−/low c/EBPβ+, which provided novel diagnostic and therapeutic tools for cancer immunotherapy [105]. Other myeloid markers, such as PD-L1, CD40, CD49d, CD80, CD115, and CD124, were also discovered to describe specific patterns of MDSCs [106,107,108].
Since MDSCs are a group of continuums in different phases of differentiation, characterizing MDSCs subtypes in various model systems and species, especially distinguishing between G-MDSCs and neutrophil, M-MDSCs and TAMs or monocyte-derived dendritic cells (mDCs), would help us to comprehend their functions for distinct organisms and activation procedures under pathological conditions.
5. MDSCs in Murine Models of Solid Tumors
MDSCs are abundant in the TME and could be employed in disease prediction, clinical outcome evaluation, prognosis value analysis, and immunotherapies testing. Compared with human models, most early mechanistic and functional studies on MDSCs were carried out in mice. It has been reported that MDSCs can be detected from peripheral blood [109], lymph nodes [110], spleen [111], and tumor sites [112] in murine tumor models.
5.1. Functional Studies in Mice
In various tumor-bearing mouse models, MDSCs increased with tumor progression, also in some premalignant states, but were less infiltrated in normal tissues [50,113,114]. Karakhanova et al. [115] demonstrated a pronounced accumulation of MDSCs in tumor and spleen of the tumor-bearing mice in an orthotopic mouse model of pancreatic ductal adenocarcinoma (PDAC). These cells were highly immunosuppressive and were associated with VEGF enrichment during PDAC progression. Meyer et al, using a melanoma mouse model, have shown that accumulated MDSCs inhibited tumor-reactive T cells and down-regulated their TCR ζ-chain [116]. Siret et al. [50] analyzed MDSC populations in PDAC mice by immunohistochemistry (IHC) and multiparametric flow cytometry. It was observed that the expression of B7-H1 (PD-L1) and CD40 (markers involved in MDSC-mediated immunosuppression) were up-regulated on the two MDSC subgroups both in tumor and in the spleen. However, F4/80 and CD124 (MDSCs associated surface markers) displayed increased level in M-MDSCs than G-MDSCs. Additionally, the quantity of ROS and arginase-1 were more ascendant in tumor mice than the naïve controls, showing a strong suppressive capacity against CD8+ T cells. This indicated that it is necessary to identify MDSCs and their detailed classification in cancer, and the recruitment and activation of these cells is a hallmark of abnormal immune function.
Metastasis is one of the leading causes of death in patients with solid cancer. PMN-MDSCs preferentially accumulated in melanoma mice, and then disseminated cancer cells to lymph nodes and lung by inducing EMT of tumor cells [117], establishing a favorable microenvironment for tumor migration. Hamilton et al. [118] found in mice bearing metastatic 4T1 or 4TO7 murine mammary tumors, that the pulmonary accumulation of MDSCs could promote metastatic tumor growth. Bosiljcic et al. [119] also examined the high level of MDSCs in the lungs, metastases from primary 4T1 tumors, and confirmed that the surgical removal of primary tumor diminished the quantity of G-MDSCs and M-MDSCs, as well as the metastatic growth in the lungs.
Stress response after surgical trauma has a link to a poor clinical outcome. The amplification of iNOS/NO released by MDSCs was sensitive in injury-induced immune dysfunction, and using a potent NO scavenger could protect against lymphocyte changes following mouse abdominal surgery trauma [120]. Highly suppressive MDSCs expanded after operation in the melanoma mouse model, and impaired the proliferation and functions of T cells, resulting in postoperative cancer recurrence [121].
Taken together, MDSC expansion represents immunological deficits and negative effects in mouse tumor models, and has shown potential use as a metastatic marker. However, more in-depth investigations should be conducted to explore the value of the targeting MDSCs in clinical application.
5.2. Therapy Testing in Mice
Several studies have mentioned that limiting the numbers of MDSCs and/or their immunosuppressive ability could restore the function of T cells and delay tumor progression. To target MDSCs, we can take measures from the following aspects: (i) promote the differentiation of MDSCs; (ii) block the expansion and accumulation of MDSCs; (iii) inhibit the immunosuppressive function of MDSCs; and (iv) reduce the numbers of MDSCs (Table 2).
Table 2.
MDSCs-mediated immunotherapy strategies in solid tumors.
| Treatment Strategies | Representative Treatment | Cancer | Factors | References |
|---|---|---|---|---|
| Promote the differentiation of MDSCs | Very Small Size Proteoliposomes (VSSP) | Sarcoma | Arginase-1, Nos2 | [122] |
| All-trans retinoic acid (ATRA) | Sarcomas/Breast cancer/Pancreatic cancer | ERK pathway, ROS | [123,124,125,126] | |
| Ibrutinib | Breast cancer | VEGF, MMP9, CXCL1 | [127,128] | |
| Chloroquine | Melanoma/Hepatocarcinoma | Arginase-1, iNOS, IDO-1, IL-10, TGF-β | [129] | |
| Docetaxel | Breast cancer | ROS, IL-10, IL-12 | [130] | |
| Block the expansion and accumulation of MDSCs | Amino-biphosphonates | Breast cancer | VEGF, MMP9 | [131] |
| SX-682 | Oral and Lewis lung carcinoma | CXCR1/2 | [132] | |
| Sunitinib | Renal cell carcinoma | VEGF, G-CSF | [136] | |
| PD-1 ablation | Fibrosarcoma/Colon carcinoma/Melanoma | PI3K/Akt pathway, G-CSF | [137,138] | |
| Inhibit the immunosuppressive function of MDSCs | Celecoxib | Glioma/Mesothelioma/Endometrial cancer | COX-2, PGE2, IL-6, G-CSF, ROS | [139,140,141] |
| Sildenafil or Tadalafil | Breast cancer/Melanoma/Hepatocellular carcinoma | PDE5, arginase-1 and iNOS | [142,143,144] | |
| Nitro-aspirin | Colon cancer/Breast cancer | Arginase and iNOS | [145] | |
| Reduce the numbers of MDSCs | Gemcitabine and 5-Fu | Melanoma/Lewis lung carcinoma/Colon cancer/Pancreatic cancer | TGF-β, IL-6 and IL-10, IL-1β | [146,147,148,150] |
| Naltrexone | Solid Ehrlich carcinoma | IFN-γ | [151] | |
| Synthetic Nanoparticle Antibodies (SNAbs) | Breast cancer | S100A8/A9 | [152] |
The differentiation of MDSCs into DCs or macrophages is achievable in many experiments. In vivo, Very Small Size Proteoliposomes (VSSP) were verified to differentiate MDSCs into phenotypically mature DCs, diminishing MDSCs immunosuppression effects in tumor-bearing mice [122]. All-trans retinoic acid (ATRA), a derivative of vitamin A, has a strong ability in inducing the differentiation of MDSCs into DCs and/or macrophages in vitro, primarily via the neutralization of high ROS production and up-regulating the protein level of glutathione synthase (GSS) in these cells [123]. This has also helped to improve the efficacy of other therapies, such as chimeric antigen receptor (CAR) therapies [124], antiangiogenic therapies (ATT) [125], DC vaccinations [126], and so on. Varikuti et al. [127] found that ibrutinib can reprogram MDSCs to mature DCs using an orthotopic mouse breast cancer model, which at least in part improved their anti-tumor immunity. A systematic review concluded some preclinical studies and publications to assess ibrutinib’s efficacy in cell lines and animal models of gynecological malignancies [128]. Their results also prove that this drug could regulate the cytokine production, PD-L1 expression, as well as MDSC and other myeloid cell movement, which could be a promising therapy on solid tumors. Additionally, chloroquine (CQ) [129] and docetaxel [130] both strengthen the anti-tumor immune response by resetting MDSCs toward a M1 macrophages-like phenotype, exerting pro-inflammatory and phagocytic functions.
The expansion and accumulation of MDSCs rely on tumor-derived factors; thus accordingly, the depletion of these elements would be beneficial for increasing the effectiveness of clinical therapies on MDSCs. Melani et al. [131] showed in breast cancer mice that amino-biphosphonates could stop MDSCs expansion by inhibiting MMP-9 activity and overcoming tumor-induced immune suppression. Sun and her colleagues [132] proposed that SX-682, a small-molecule inhibitor of CXCR1 and CXCR2, obstructs MDSCs trafficking while enhancing T cell immunotherapy in syngeneic models of oral and Lewis lung carcinoma. VEGF receptor tyrosine kinase inhibitors (TKI) have been implicated in dealing with solid tumors, but drug resistance is still a clinical management problem [133]. Increased MDSCs in TME was believed to be correlated with resistance to immune therapies [134,135]. Diaz-Montero et al. [136] investigated treatment with sunitinib (a member of VEGF TKI class) and a MEK inhibitor in renal cell carcinoma (RCC) patient-derived xenograft (PDX) mouse models, concluding that decreasing the level of G-CSF and the accumulation of G-MDSCs, significantly delaying drug resistance. PD-1 is highly expressed in myeloid cells induced by lipopolysaccharide (LPS) via the NF-κB signaling pathway [137]. Strauss et al. [138] revealed that the myeloid- but not T cell-specific deletion of PD-1 in different mouse models prevented the accumulation of granulocyte/macrophage progenitors (GMPs) and MDSCs, whereas the systemic output of myeloid effector cells and T effector memory cells (Tem) with the improved anti-tumor function was increased. This study pointed out that myeloid-specific PD-1 blockade mediates myeloid cell-intrinsic effects, promoting a systemic anti-tumor response.
Another strategy aims to block MDSCs immunosuppressive functions and revoke the immune activity of T cells. For example, COX-2 inhibitors, such as celecoxib, could minimize the production of PGE2 and ROS in solid tumor development, diminishing the suppression ability of MDSC subpopulations and reinforcing the infiltration of CD8+ T cells [139,140]. Yokoi et al. [141] also used a mouse model of endometrial cancer to demonstrate that celecoxib could inhibit the MDSC activity by cancelling the increase of IL-6 production and restraining tumor progression. Phosphodiesterase-5 (PDE-5) inhibitors, such as sildenafil or tadalafil, have been reported to reduce the expression of arginase-1 and iNOS and thus inhibit the immune escape mechanism mediated by MDSCs while restoring T cell proliferation and inducing tumor cell apoptosis [142,143,144]. Likewise, nitro-aspirin blunted both iNOS and arginase activity in tumor-associated MDSCs and corrected immune dysfunction in colon cancer mouse models [145].
In addition, some clinically approved drugs have been applied to deplete MDSCs. Suzuki et al. [146] explored the potential use of gemcitabine in anti-tumor immune activity, indicating that this chemotherapeutic agent could eliminate splenic MDSCs in tumor-bearing mice. Recently, gemcitabine has been identified as an MDSC depleting chemotherapeutic agent in various cancers. The drug can reduce the expression of immunosuppressive mediators, including TGF-β, IL-6, and IL-10, promoting the consumption of MDSCs [147]. At the same time, several studies have reported that the combination of gemcitabine and capecitabine (a 5-FU pro-drug) could more effectively lower the MDSC levels and exert higher anti-tumor effects, which may also enhance the efficacy of other treatments [148,149,150]. Aboalsoud et al. [151] used a low dose of naltrexone (LDN) in solid Ehrlich carcinoma in mice to investigate its impact on tumor growth. The results implied that LDN could reduce MDSC count and tumor weight, suggesting that LDN could be potentially beneficial in clinical practice. Synthetic Nanoparticle Antibodies (SNAbs) were developed to efficiently deplete MDSCs. The systemic injection of MDSC-targeting SNAbs in a mouse triple-negative breast cancer model was affirmed to selectively lower the circulating MDSCs and promote T cell and NK cell infiltration into the tumor [152].
Currently, therapeutic plans based on reversing immunosuppression of MDSCs, directly or indirectly, have increasingly achieved gratifying results. However, men are not similar to mice, and more studies are required to prove clinical safety and efficacy in future applications.
6. MDSCs in Patients with Solid Tumors
6.1. Clinical Importance of MDSCs in Humans
Human MDSCs and their phenotypes are not as well defined as in mice, but the clinical importance of these cells has been widely studied.
The accumulation of MDSCs is most pronounced in patients with stage IV, but can also be detected as early as in stage I patients [153]. Sieminska [113] reviewed that MDSCs can be detected throughout the course of colorectal cancer, especially related to tumor metastasis and poor prognosis. The same is true for melanoma [154,155], hepatocellular carcinoma (HCC) [156,157], and non-small cell lung carcinoma (NSCLC) [158,159] patients. Therefore, the idea occurs to use MDSCs as prognostic biomarkers. Concerning the prognostic value of MDSCs, some meta-analyses have already been published, which illustrated that patients with a large number of MDSCs tend to have poorer clinical outcomes [160,161,162,163]. One study [164] conveyed that elevated MDSCs in gastrointestinal cancers could serve as an independent prognostic factor for decreased survival, normally linked with an elevation of IL-13, arginase-1, and Tregs. Similar findings are also described in gynecological cancers [165,166]. Shimura et al. [167] indicated that immune suppression mediated by G-CSF-induced MDSCs could be the reason leading to the poor prognosis in gynecological cancer patients. Moreover, MDSC-induced immune suppression could facilitate metastasis in the circulation. The rising percentage and absolute number of MDSCs have an association with an increased metastatic tumor burden in patients with breast cancer [168] and colorectal cancer [169].
It is also essential to assess the different roles of two MDSC subtypes (M-MDSCs and G-MDSCs) in solid tumors. Circulating M-MDSCs increased in various cancers, associated with promoting tumor growth, and had a detrimental impact on the survival of patients with malignancies [154,156,170]. Yuan et al. [171] found that M-MDSCs can attenuate T cell proliferation and IFN-γ production and showed a certain correlation with clinical cancer staging and pathological grades. Wu et al. [172] characterized that M-MDSCs are the typical MDSCs in the PB and ascites from ovarian cancer (OC) patients. This phenomenon may be attributed to the overexpression of IL-6 and IL-10 and their downstream STAT3 signal. The higher level of M-MDSCs in OC patients indicated shorter recurrence-free survival. G-MDSCs are usually elevated in terminal cancer patients [173,174,175], accompanied by poor physical status and prognosis. In light of the differences between these two subgroups of MDSCs, it is necessary to consider their respective characteristics at different stages of the disease, which would be meaningful for predicting the development of cancer and evaluating the response of cancer patients to immunotherapy. Of particular note, the survival kinetics of M-MDSCs and G-MDSCs from human PBMCs after blood draw are not equal in terms of time point analysis. There is no obvious difference in G-MDSC levels over time, but M-MDSCs should be studied within 4 h after blood sampling [176]. Therefore, for analyzing the subsets of MDSCs in PBMCs, caution in the selection of appropriate time points is required.
Furthermore, MDSCs are incorporated in estimating therapeutic response in cancer patients, and the blockade of MDSCs would intensify the response to antiangiogenic therapy (AAT), chemotherapy, radiotherapy, or perioperative therapy [177,178].
6.2. Targeting of MDSCs for Clinical Applications
The clinical significance of MDSCs in cancer is established; therefore, approaches for selectively targeting MDSCs and their inhibitory activities to treat patients with solid tumors are under development. Many clinical trials on MDSCs are ongoing (Supplementary Tables S1 and S2).
Monoclonal antibodies (mAbs) against checkpoint inhibitors aiming to boost anti-tumor immunity have shown a great challenge in the combat against cancer and could improve outcomes even more in combination with other therapies. Pembrolizumab, a PD-1 blocking mAb, was approved to treat unresectable or metastatic solid tumors [179], such as head and neck squamous cell carcinoma (HNSCC) [180], NSCLC [181], and melanoma [182]. A phase 2 trial on the combination of pembrolizumab and BL-8040 (a CXCR4 antagonist) in metastatic PDAC showed that BL-8040 reduced MDSC numbers and increased effector T cell tumor infiltration, suggesting that BL-8040 could expand the benefit of pembrolizumab for PDAC patients [183]. Ipilimumab is a fully humanized mAb that potentiates the anti-tumor T-cell response by blocking cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) [184]. Sade-Feldman et al. [185] discovered that treating melanoma patients with anti-CTLA4 (ipilimumab) therapy could lower the frequency of MDSCs, which may change the suppressive environment and obtain better disease outcomes. Tobin et al. [186] combined ipilimumab with ATRA to target MDSCs in melanoma patients. Compared to ipilimumab treatment alone, adding ATRA to a standard of care ipilimumab appears to be safe, and ATRA may upgrade ipilimumab efficacy by reducing the frequency of MDSCs, increasing the activation of CD8+ T cells, as well as ameliorating the incidence of grade 3 or 4 adverse events. At the same time, a follow-up clinical trial is being recruited to appraise their potential safety and efficacy.
Moreover, other mAbs, such as bevacizumab (NCT02669173), nivolumab (NCT03486119, NCT02917772), and atezolizumab (NCT03201458, NCT02992912), have also been extensively investigated to be added to clinical trials aiming at MDSC function, looking forward to introducing better treatments for patients with solid tumors.
At present, altering the production and biological functions of Tregs and MDSCs to decrease immunosuppression and improve solid tumor treatment has been adopted in preclinical and clinical trials. Gemcitabine can decrease G-MDSCs, but not M-MDSCs, and increase the ratio of effector T cells/Tregs, boosting the immune status of PDAC patients [187]. Tivozanib inhibits Tregs and MDSCs function by mediating c-Kit/SCF signaling, which reversed the tumor immune suppression and correlated with survival of hepatocellular carcinoma (HCC) patients [188]. Weed et al. [189] carried out a randomized phase 2 trial to evaluate the presence of MDSCs and Tregs in HNSCC patients before and after being treated with tadalafil. It seems that daily tadalafil treatment could reduce the infiltration of MDSCs and Tregs at the tumor site, promoting CD8+ T cell proliferation and activation and changing the tumor macro- and microenvironment. Another phase 2 trial in advanced urothelial carcinoma certified that cabozantinib, a multikinase inhibitor, could also be a new therapeutic option for these cancer patients by lowering the percentage of Tregs among CD4+ T cells and the G-MDSC populations [190].
Tumor-induced PD-L1 expression was restricted to the myeloid cells and, specifically, to the TAMs and MDSCs. One study suggested that CCA patients with a high level of TAMs or MDSCs seemed to be beneficial from immune checkpoint inhibitors (ICIs) [90]. Prima et al. [191] showed that in murine bladder carcinoma, PGE2-forming enzymes microsomal PGE 2 synthase 1 (mPGES1) and COX2 were involved in the regulation of PD-L1 expression on TAMs and MDSCs. Utilizing celecoxib targeting PGE 2 metabolism will help to diminish PD-L1 mediated immune suppression. Okla [192] demonstrated that PD-L1 expression on TAMs and M-MDSCs were increased in the blood of OC patients. However, no results can prove that PD-L1 is related to the prognosis and clinicopathologic parameters. These data should be concerned in future clinical studies/trials.
The co-dependency of MDSCs and DCs in tumor growth appears to be a potential member in immunotherapy. Kong et al. [193] showed that GM-CSF accelerated DCs and MDSCs response speed to pro-inflammatory stimuli differentially regulated via TLR4. These data unveil the substantial role of GM-CSF in a substantial initiation of immunity, with promising implications for future immunotherapy development based on DCs. A few clinical trials on DC vaccination are in progress (NCT01622933, NCT01808820, NCT02479230).
7. Conclusions
MDSCs accumulate, expand, and activate in cancer growth, playing a critical role in tumor maintenance and progression because of their powerful immunosuppressive functions. According to the preceding observations, it is reasonable to consider MDSCs as principal predictors of tumor prognosis and metastasis and to include them in future clinical therapeutic strategies. Not only can they provide more effective individualized treatment for patients with solid tumors, but they also amplify the efficiency of other therapies. Importantly, human MDSCs are more complex than mouse MDSCs; thus more attention should be paid to the possible effectiveness and safety of new drugs targeting MDSCs in clinical use. To address the limitations of current research, further studies should be conducted to fully understand the existence, heterogeneity, phenotypes, and adaptative conditions of MDSCs in practical application, bringing a more favorable prognosis for patients with malignant tumors.
Acknowledgments
The authors would like to thank the anonymous reviewers of our manuscript for providing us with helpful comments and suggestions to improve our work.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cells11020310/s1, Table S1: List of clinical trials with published results targeting on MDSCs in solid tumors; Table S2: List of completed or recruiting clinical trials without results submitted targeting on MDSCs in solid tumors.
Author Contributions
Conceptualization, A.V.B.; writing—initial draft and review, T.M. and A.V.B.; writing and editing, B.W.R., M.I., D.K., Y.Y. and J.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Veglia F., Perego M., Gabrilovich D. Myeloid-derived suppressor cells coming of age. Nat. Immunol. 2018;19:108–119. doi: 10.1038/s41590-017-0022-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Groth C., Hu X., Weber R., Fleming V., Altevogt P., Utikal J., Umansky V. Immunosuppression mediated by myeloid-derived suppressor cells (MDSCs) during tumour progression. Br. J. Cancer. 2019;120:16–25. doi: 10.1038/s41416-018-0333-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Consonni F.M., Porta C., Marino A., Pandolfo C., Mola S., Bleve A., Sica A. Myeloid-Derived Suppressor Cells: Ductile Targets in Disease. Front. Immunol. 2019;10:949. doi: 10.3389/fimmu.2019.00949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lim H.X., Kim T.S., Poh C.L. Understanding the Differentiation, Expansion, Recruitment and Suppressive Activities of Myeloid-Derived Suppressor Cells in Cancers. Int. J. Mol. Sci. 2020;21:3599. doi: 10.3390/ijms21103599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li K., Shi H., Zhang B., Ou X., Ma Q., Chen Y., Shu P., Li D., Wang Y.S. Myeloid-derived suppressor cells as immunosuppressive regulators and therapeutic targets in cancer. Signal Transduct. Target. Ther. 2021;6:362. doi: 10.1038/s41392-021-00670-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar V., Patel S., Tcyganov E., Gabrilovich D.I. The Nature of Myeloid-Derived Suppressor Cells in the Tumor Microenvironment. Trends Immunol. 2016;37:208–220. doi: 10.1016/j.it.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Safarzadeh E., Hashemzadeh S., Duijf P.H.G., Mansoori B., Khaze V., Mohammadi A., Kazemi T., Yousefi M., Asadi M., Mohammadi H., et al. Circulating myeloid-derived suppressor cells: An independent prognostic factor in patients with breast cancer. J. Cell. Physiol. 2019;234:3515–3525. doi: 10.1002/jcp.26896. [DOI] [PubMed] [Google Scholar]
- 8.Nakamura K., Smyth M.J. Myeloid immunosuppression and immune checkpoints in the tumor microenvironment. Cell. Mol. Immunol. 2020;17:1–12. doi: 10.1038/s41423-019-0306-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bronte V., Brandau S., Chen S.H., Colombo M.P., Frey A.B., Greten T.F., Mandruzzato S., Murray P.J., Ochoa A., Ostrand-Rosenberg S., et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat. Commun. 2016;7:12150. doi: 10.1038/ncomms12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lv M., Wang K., Huang X.J. Myeloid-derived suppressor cells in hematological malignancies: Friends or foes. J. Hematol. Oncol. 2019;12:105. doi: 10.1186/s13045-019-0797-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hegde S., Leader A.M., Merad M. MDSC: Markers, development, states, and unaddressed complexity. Immunity. 2021;54:875–884. doi: 10.1016/j.immuni.2021.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Workman C.J., Szymczak-Workman A.L., Collison L.W., Pillai M.R., Vignali D.A. The development and function of regulatory T cells. Cell. Mol. Life Sci. CMLS. 2009;66:2603–2622. doi: 10.1007/s00018-009-0026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li R., Jia Z., Trush M.A. Defining ROS in Biology and Medicine. React. Oxyg. Species. 2016;1:9–21. doi: 10.20455/ros.2016.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belikov A.V., Schraven B., Simeoni L. T cells and reactive oxygen species. J. Biomed. Sci. 2015;22:85. doi: 10.1186/s12929-015-0194-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohl K., Tenbrock K. Reactive Oxygen Species as Regulators of MDSC-Mediated Immune Suppression. Front. Immunol. 2018;9:2499. doi: 10.3389/fimmu.2018.02499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Szabo C., Ischiropoulos H., Radi R. Peroxynitrite: Biochemistry, pathophysiology and development of therapeutics. Nat. Rev. Drug Discov. 2007;6:662–680. doi: 10.1038/nrd2222. [DOI] [PubMed] [Google Scholar]
- 17.Bentz B.G., Haines G.K., 3rd, Radosevich J.A. Increased protein nitrosylation in head and neck squamous cell carcinogenesis. Head Neck. 2000;22:64–70. doi: 10.1002/(SICI)1097-0347(200001)22:1<64::AID-HED10>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 18.Cobbs C.S., Whisenhunt T.R., Wesemann D.R., Harkins L.E., Van Meir E.G., Samanta M. Inactivation of wild-type p53 protein function by reactive oxygen and nitrogen species in malignant glioma cells. Cancer Res. 2003;63:8670–8673. [PubMed] [Google Scholar]
- 19.Gabrilovich D.I., Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schouppe E., Van Overmeire E., Laoui D., Keirsse J., Van Ginderachter J.A. Modulation of CD8(+) T-cell activation events by monocytic and granulocytic myeloid-derived suppressor cells. Immunobiology. 2013;218:1385–1391. doi: 10.1016/j.imbio.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 21.Burrack K.S., Morrison T.E. The role of myeloid cell activation and arginine metabolism in the pathogenesis of virus-induced diseases. Front. Immunol. 2014;5:428. doi: 10.3389/fimmu.2014.00428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Umansky V., Schirrmacher V. Nitric oxide-induced apoptosis in tumor cells. Adv. Cancer Res. 2001;82:107–131. doi: 10.1016/s0065-230x(01)82004-2. [DOI] [PubMed] [Google Scholar]
- 23.Cane S., Bronte V. Detection and functional evaluation of arginase-1 isolated from human PMNs and murine MDSC. Methods Enzymol. 2020;632:193–213. doi: 10.1016/bs.mie.2019.07.022. [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez P.C., Quiceno D.G., Ochoa A.C. L-arginine availability regulates T-lymphocyte cell-cycle progression. Blood. 2007;109:1568–1573. doi: 10.1182/blood-2006-06-031856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levring T.B., Hansen A.K., Nielsen B.L., Kongsbak M., von Essen M.R., Woetmann A., Odum N., Bonefeld C.M., Geisler C. Activated human CD4+ T cells express transporters for both cysteine and cystine. Sci. Rep. 2012;2:266. doi: 10.1038/srep00266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levring T.B., Kongsbak M., Rode A.K., Woetmann A., Odum N., Bonefeld C.M., Geisler C. Human CD4+ T cells require exogenous cystine for glutathione and DNA synthesis. Oncotarget. 2015;6:21853–21864. doi: 10.18632/oncotarget.5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Srivastava M.K., Sinha P., Clements V.K., Rodriguez P., Ostrand-Rosenberg S. Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer Res. 2010;70:68–77. doi: 10.1158/0008-5472.CAN-09-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prendergast G.C., Malachowski W.J., Mondal A., Scherle P., Muller A.J. Indoleamine 2,3-Dioxygenase and Its Therapeutic Inhibition in Cancer. Int. Rev. Cell Mol. Biol. 2018;336:175–203. doi: 10.1016/bs.ircmb.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prendergast G.C., Smith C., Thomas S., Mandik-Nayak L., Laury-Kleintop L., Metz R., Muller A.J. Indoleamine 2,3-dioxygenase pathways of pathogenic inflammation and immune escape in cancer. Cancer Immunol. Immunother. CII. 2014;63:721–735. doi: 10.1007/s00262-014-1549-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sevko A., Umansky V. Myeloid-derived suppressor cells interact with tumors in terms of myelopoiesis, tumorigenesis and immunosuppression: Thick as thieves. J. Cancer. 2013;4:3–11. doi: 10.7150/jca.5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aggen D.H., Ager C.R., Obradovic A.Z., Chowdhury N., Ghasemzadeh A., Mao W., Chaimowitz M.G., Lopez-Bujanda Z.A., Spina C.S., Hawley J.E., et al. Blocking IL1 Beta Promotes Tumor Regression and Remodeling of the Myeloid Compartment in a Renal Cell Carcinoma Model: Multidimensional Analyses. Clin. Cancer Res. 2021;27:608–621. doi: 10.1158/1078-0432.CCR-20-1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oh K., Lee O.Y., Shon S.Y., Nam O., Ryu P.M., Seo M.W., Lee D.S. A mutual activation loop between breast cancer cells and myeloid-derived suppressor cells facilitates spontaneous metastasis through IL-6 trans-signaling in a murine model. Breast Cancer Res. BCR. 2013;15:R79. doi: 10.1186/bcr3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morales J.K., Kmieciak M., Knutson K.L., Bear H.D., Manjili M.H. GM-CSF is one of the main breast tumor-derived soluble factors involved in the differentiation of CD11b-Gr1- bone marrow progenitor cells into myeloid-derived suppressor cells. Breast Cancer Res. Treat. 2010;123:39–49. doi: 10.1007/s10549-009-0622-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kawano M., Mabuchi S., Matsumoto Y., Sasano T., Takahashi R., Kuroda H. Kozasa, K.; Hashimoto, K.; Isobe, A.; Sawada, K.; et al. The significance of G-CSF expression and myeloid-derived suppressor cells in the chemoresistance of uterine cervical cancer. Sci. Rep. 2015;5:18217. doi: 10.1038/srep18217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sasano T., Mabuchi S., Kozasa K., Kuroda H., Kawano M., Takahashi R., Komura N., Yokoi E., Matsumoto Y., Hashimoto K., et al. The Highly Metastatic Nature of Uterine Cervical/Endometrial Cancer Displaying Tumor-Related Leukocytosis: Clinical and Preclinical Investigations. Clin. Cancer Res. 2018;24:4018–4029. doi: 10.1158/1078-0432.CCR-17-2472. [DOI] [PubMed] [Google Scholar]
- 36.Ostrand-Rosenberg S., Sinha P. Myeloid-derived suppressor cells: Linking inflammation and cancer. J. Immunol. 2009;182:4499–4506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Terabe M., Matsui S., Park J.M., Mamura M., Noben-Trauth N., Donaldson D.D., Chen W., Wahl S.M., Ledbetter S., Pratt B., et al. Transforming growth factor-beta production and myeloid cells are an effector mechanism through which CD1d-restricted T cells block cytotoxic T lymphocyte-mediated tumor immunosurveillance: Abrogation prevents tumor recurrence. J. Exp. Med. 2003;198:1741–1752. doi: 10.1084/jem.20022227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lesokhin A.M., Hohl T.M., Kitano S., Cortez C., Hirschhorn-Cymerman D., Avogadri F. Monocytic CCR2(+) myeloid-derived suppressor cells promote immune escape by limiting activated CD8 T-cell infiltration into the tumor microenvironment. Cancer Res. 2012;72:876–886. doi: 10.1158/0008-5472.CAN-11-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shojaei F., Wu X., Malik A.K., Zhong C., Baldwin M.E., Schanz S., Fuh G., Gerber H.P., Ferrara N. Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+Gr1+ myeloid cells. Nat. Biotechnol. 2007;25:911–920. doi: 10.1038/nbt1323. [DOI] [PubMed] [Google Scholar]
- 40.Ye X.Z., Yu S.C., Bian X.W. Contribution of myeloid-derived suppressor cells to tumor-induced immune suppression, angiogenesis, invasion and metastasis. J. Genet. Genom. = Yi Chuan Xue Bao. 2010;37:423–430. doi: 10.1016/S1673-8527(09)60061-8. [DOI] [PubMed] [Google Scholar]
- 41.Shime H., Maruyama A., Yoshida S., Takeda Y., Matsumoto M., Seya T. Toll-like receptor 2 ligand and interferon-gamma suppress anti-tumor T cell responses by enhancing the immunosuppressive activity of monocytic myeloid-derived suppressor cells. Oncoimmunology. 2017;7:e1373231. doi: 10.1080/2162402X.2017.1373231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taketo M.M. Cyclooxygenase-2 inhibitors in tumorigenesis (part I) J. Natl. Cancer Inst. 1998;90:1529–1536. doi: 10.1093/jnci/90.20.1529. [DOI] [PubMed] [Google Scholar]
- 43.Sinha P., Clements V.K., Fulton A.M., Ostrand-Rosenberg S. Prostaglandin E2 promotes tumor progression by inducing myeloid-derived suppressor cells. Cancer Res. 2007;67:4507–4513. doi: 10.1158/0008-5472.CAN-06-4174. [DOI] [PubMed] [Google Scholar]
- 44.Rodriguez P.C., Hernandez C.P., Quiceno D., Dubinett S.M., Zabaleta J., Ochoa J.B., Gilbert J., Ochoa A.C. Arginase I in myeloid suppressor cells is induced by COX-2 in lung carcinoma. J. Exp. Med. 2005;202:931–939. doi: 10.1084/jem.20050715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lechner M.G., Liebertz D.J., Epstein A.L. Characterization of cytokine-induced myeloid-derived suppressor cells from normal human peripheral blood mononuclear cells. J. Immunol. 2010;185:2273–2284. doi: 10.4049/jimmunol.1000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meng G., Wei J., Wang Y., Qu D., Zhang J. miR-21 regulates immunosuppression mediated by myeloid-derived suppressor cells by impairing RUNX1-YAP interaction in lung cancer. Cancer Cell Int. 2020;20:495. doi: 10.1186/s12935-020-01555-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang J., Yu F., Jia X., Iwanowycz S., Wang Y., Huang S., Ai W., Fan D. MicroRNA-155 deficiency enhances the recruitment and functions of myeloid-derived suppressor cells in tumor microenvironment and promotes solid tumor growth. Int. J. Cancer. 2015;136:E602–E613. doi: 10.1002/ijc.29151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pan T., Zhong L., Wu S., Cao Y., Yang Q., Cai Z., Cai X., Zhao W., Ma N., Zhang W., et al. 17 beta-Oestradiol enhances the expansion and activation of myeloid-derived suppressor cells via signal transducer and activator of transcription (STAT)-3 signalling in human pregnancy. Clin. Exp. Immunol. 2016;185:86–97. doi: 10.1111/cei.12790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baumann T., Dunkel A., Schmid C., Schmitt S., Hiltensperger M., Lohr K., Laketa V., Donakonda S., Ahting U., Depiereux B.L., et al. Regulatory myeloid cells paralyze T cells through cell-cell transfer of the metabolite methylglyoxal. Nat. Immunol. 2020;21:555–566. doi: 10.1038/s41590-020-0666-9. [DOI] [PubMed] [Google Scholar]
- 50.Siret C., Collignon A., Silvy F., Robert S., Cheyrol T., Andre P., Rigot V., Iovanna J., Pavert S., Lombardo D., et al. Deciphering the Crosstalk Between Myeloid-Derived Suppressor Cells and Regulatory T Cells in Pancreatic Ductal Adenocarcinoma. Front. Immunol. 2019;10:3070. doi: 10.3389/fimmu.2019.03070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang T., Li J., Li R., Yang C., Zhang W., Qiu Y., Yang C., Rong R. Correlation between MDSC and Immune Tolerance in Transplantation: Cytokines, Pathways and Cell-cell Interaction. Curr. Gene Ther. 2019;19:81–92. doi: 10.2174/1566523219666190618093707. [DOI] [PubMed] [Google Scholar]
- 52.Gomez S., Tabernacki T., Kobyra J., Roberts P., Chiappinelli K.B. Combining epigenetic and immune therapy to overcome cancer resistance. Semin. Cancer Biol. 2020;65:99–113. doi: 10.1016/j.semcancer.2019.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Melero I., Rouzaut A., Motz G.T., Coukos G. T-cell and NK-cell infiltration into solid tumors: A key limiting factor for efficacious cancer immunotherapy. Cancer Discov. 2014;4:522–526. doi: 10.1158/2159-8290.CD-13-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gallina G., Dolcetti L., Serafini P., De Santo C., Marigo I., Colombo M.P., Basso G., Brombacher F., Borrello I., Zanovello P., et al. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J. Clin. Investig. 2006;116:2777–2790. doi: 10.1172/JCI28828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nagaraj S., Nelson A., Youn J.I., Cheng P., Quiceno D., Gabrilovich D.I. Antigen-specific CD4(+) T cells regulate function of myeloid-derived suppressor cells in cancer via retrograde MHC class II signaling. Cancer Res. 2012;72:928–938. doi: 10.1158/0008-5472.CAN-11-2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pinton L., Solito S., Damuzzo V., Francescato S., Pozzuoli A., Berizzi A., Mocellin S., Rossi C.R., Bronte V., Mandruzzato S. Activated T cells sustain myeloid-derived suppressor cell-mediated immune suppression. Oncotarget. 2016;7:1168–1184. doi: 10.18632/oncotarget.6662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.OuYang L.Y., Wu X.J., Ye S.B., Zhang R.X., Li Z.L., Liao W., Pan Z.Z., Zheng L.M., Zhang X.S., Wang Z., et al. Tumor-induced myeloid-derived suppressor cells promote tumor progression through oxidative metabolism in human colorectal cancer. J. Transl. Med. 2015;13:47. doi: 10.1186/s12967-015-0410-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li F., Zhao Y., Wei L., Li S., Liu J. Tumor-infiltrating Treg, MDSC, and IDO expression associated with outcomes of neoadjuvant chemotherapy of breast cancer. Cancer Biol. Ther. 2018;19:695–705. doi: 10.1080/15384047.2018.1450116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zoso A., Mazza E.M., Bicciato S., Mandruzzato S., Bronte V., Serafini P., Inverardi L. Human fibrocytic myeloid-derived suppressor cells express IDO and promote tolerance via Treg-cell expansion. Eur. J. Immunol. 2014;44:3307–3319. doi: 10.1002/eji.201444522. [DOI] [PubMed] [Google Scholar]
- 60.Pang B., Zhen Y., Hu C., Ma Z., Lin S., Yi H. Myeloid-derived suppressor cells shift Th17/Treg ratio and promote systemic lupus erythematosus progression through arginase-1/miR-322-5p/TGF-beta pathway. Clin. Sci. 2020;134:2209–2222. doi: 10.1042/CS20200799. [DOI] [PubMed] [Google Scholar]
- 61.Lee C.R., Kwak Y., Yang T., Han J.H., Park S.H., Ye M.B., Lee W., Sim K.Y., Kang J.A., Kim Y.C., et al. Myeloid-Derived Suppressor Cells Are Controlled by Regulatory T Cells via TGF-beta during Murine Colitis. Cell Rep. 2016;17:3219–3232. doi: 10.1016/j.celrep.2016.11.062. [DOI] [PubMed] [Google Scholar]
- 62.Fujimura T., Kambayashi Y., Aiba S. Crosstalk between regulatory T cells (Tregs) and myeloid derived suppressor cells (MDSCs) during melanoma growth. Oncoimmunology. 2012;1:1433–1434. doi: 10.4161/onci.21176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ma Z., Zhen Y., Hu C., Yi H. Myeloid-Derived Suppressor Cell-Derived Arginase-1 Oppositely Modulates IL-17A and IL-17F Through the ESR/STAT3 Pathway During Colitis in Mice. Front. Immunol. 2020;11:687. doi: 10.3389/fimmu.2020.00687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Novitskiy S.V., Pickup M.W., Gorska A.E., Owens P., Chytil A., Aakre M., Wu H., Shyr Y., Moses H.L. TGF-beta receptor II loss promotes mammary carcinoma progression by Th17 dependent mechanisms. Cancer Discov. 2011;1:430–441. doi: 10.1158/2159-8290.CD-11-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wen L., Gong P., Liang C., Shou D., Liu B., Chen Y., Bao C., Chen L., Liu X., Liang T., et al. Interplay between myeloid-derived suppressor cells (MDSCs) and Th17 cells: Foe or friend? Oncotarget. 2016;7:35490–35496. doi: 10.18632/oncotarget.8204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ghiringhelli F., Bruchard M., Apetoh L. Immune effects of 5-fluorouracil: Ambivalence matters. Oncoimmunology. 2013;2:e23139. doi: 10.4161/onci.23139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang Y., Schafer C.C., Hough K.P., Tousif S., Duncan S.R., Kearney J.F., Ponnazhagan S., Hsu H.C., Deshane J.S. Myeloid-Derived Suppressor Cells Impair B Cell Responses in Lung Cancer through IL-7 and STAT5. J. Immunol. 2018;201:278–295. doi: 10.4049/jimmunol.1701069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jaufmann J., Lelis F.J.N., Teschner A.C., Fromm K., Rieber N., Hartl D., Hammer S.B. Human monocytic myeloid-derived suppressor cells impair B-cell phenotype and function in vitro. Eur. J. Immunol. 2020;50:33–47. doi: 10.1002/eji.201948240. [DOI] [PubMed] [Google Scholar]
- 69.Lelis F.J.N., Jaufmann J., Singh A., Fromm K., Teschner A.C., Poschel S., Schafer I., Hammer S.B., Rieber N., Hartl D. Myeloid-derived suppressor cells modulate B-cell responses. Immunol. Lett. 2017;188:108–115. doi: 10.1016/j.imlet.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 70.Green K.A., Wang L., Noelle R.J., Green W.R. Selective Involvement of the Checkpoint Regulator VISTA in Suppression of B-Cell, but Not T-Cell, Responsiveness by Monocytic Myeloid-Derived Suppressor Cells from Mice Infected with an Immunodeficiency-Causing Retrovirus. J. Virol. 2015;89:9693–9698. doi: 10.1128/JVI.00888-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rosser E.C., Mauri C. Regulatory B cells: Origin, phenotype, and function. Immunity. 2015;42:607–612. doi: 10.1016/j.immuni.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 72.Shang J., Zha H., Sun Y. Phenotypes, Functions, and Clinical Relevance of Regulatory B Cells in Cancer. Front. Immunol. 2020;11:582657. doi: 10.3389/fimmu.2020.582657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bodogai M., Moritoh K., Lee-Chang C., Hollander C.M., Sherman-Baust C.A., Wersto R.P. Immunosuppressive and Prometastatic Functions of Myeloid-Derived Suppressive Cells Rely upon Education from Tumor-Associated B Cells. Cancer Res. 2015;75:3456–3465. doi: 10.1158/0008-5472.CAN-14-3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee-Chang C., Rashidi A., Miska J., Zhang P., Pituch K.C., Hou D., Xiao T., Fischietti M., Kang S.J., Appin C.L., et al. Myeloid-Derived Suppressive Cells Promote B cell-Mediated Immunosuppression via Transfer of PD-L1 in Glioblastoma. Cancer Immunol. Res. 2019;7:1928–1943. doi: 10.1158/2326-6066.CIR-19-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang C., Wang S., Yang C., Rong R. The Crosstalk between Myeloid Derived Suppressor Cells and Immune Cells: To Establish Immune Tolerance in Transplantation. J. Immunol. Res. 2016;2016:4986797. doi: 10.1155/2016/4986797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li H., Han Y., Guo Q., Zhang M., Cao X. Cancer-expanded myeloid-derived suppressor cells induce anergy of NK cells through membrane-bound TGF-beta 1. J. Immunol. 2009;182:240–249. doi: 10.4049/jimmunol.182.1.240. [DOI] [PubMed] [Google Scholar]
- 77.Liu C., Yu S., Kappes J., Wang J., Grizzle W.E., Zinn K.R., Zhang H.G. Expansion of spleen myeloid suppressor cells represses NK cell cytotoxicity in tumor-bearing host. Blood. 2007;109:4336–4342. doi: 10.1182/blood-2006-09-046201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stiff A., Trikha P., Mundy-Bosse B., McMichael E., Mace T.A., Benner B., Kendra K., Campbell A., Gauta S., Abood D., et al. Nitric Oxide Production by Myeloid-Derived Suppressor Cells Plays a Role in Impairing Fc Receptor-Mediated Natural Killer Cell Function. Clin. Cancer Res. 2018;24:1891–1904. doi: 10.1158/1078-0432.CCR-17-0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Greene S., Robbins Y., Mydlarz W.K., Huynh A.P., Schmitt N.C., Friedman J., Horn L.A., Palena C., Schlom J., Maeda D.Y., et al. Inhibition of MDSC Trafficking with SX-682, a CXCR1/2 Inhibitor, Enhances NK-Cell Immunotherapy in Head and Neck Cancer Models. Clin. Cancer Res. 2020;26:1420–1431. doi: 10.1158/1078-0432.CCR-19-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fabian K.P., Padget M.R., Donahue R.N., Solocinski K., Robbins Y., Allen C.T., Lee J.H., Rabizadeh S., Shiong P.S., Schlom J., et al. PD-L1 targeting high-affinity NK (t-haNK) cells induce direct antitumor effects and target suppressive MDSC populations. J. Immunother. Cancer. 2020;8:e000450. doi: 10.1136/jitc-2019-000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Youn J.I., Gabrilovich D.I. The biology of myeloid-derived suppressor cells: The blessing and the curse of morphological and functional heterogeneity. Eur. J. Immunol. 2010;40:2969–2975. doi: 10.1002/eji.201040895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pillay J., Tak T., Kamp V.M., Koenderman L. Immune suppression by neutrophils and granulocytic myeloid-derived suppressor cells: Similarities and differences. Cell. Mol. Life Sci. CMLS. 2013;70:3813–3827. doi: 10.1007/s00018-013-1286-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Semerad C.L., Liu F., Gregory A.D., Stumpf K., Link D.C. G-CSF is an essential regulator of neutrophil trafficking from the bone marrow to the blood. Immunity. 2002;17:413–423. doi: 10.1016/S1074-7613(02)00424-7. [DOI] [PubMed] [Google Scholar]
- 84.Liu Y., Wei J., Guo G., Zhou J. Norepinephrine-induced myeloid-derived suppressor cells block T-cell responses via generation of reactive oxygen species. Immunopharmacol. Immunotoxicol. 2015;37:359–365. doi: 10.3109/08923973.2015.1059442. [DOI] [PubMed] [Google Scholar]
- 85.Ostrand-Rosenberg S., Sinha P., Beury D.W., Clements V.K. Cross-talk between myeloid-derived suppressor cells (MDSC), macrophages, and dendritic cells enhances tumor-induced immune suppression. Semin. Cancer Biol. 2012;22:275–281. doi: 10.1016/j.semcancer.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kusmartsev S., Gabrilovich D.I. Inhibition of myeloid cell differentiation in cancer: The role of reactive oxygen species. J. Leukoc. Biol. 2003;74:186–196. doi: 10.1189/jlb.0103010. [DOI] [PubMed] [Google Scholar]
- 87.Kusmartsev S., Gabrilovich D.I. STAT1 signaling regulates tumor-associated macrophage-mediated T cell deletion. J. Immunol. 2005;174:4880–4891. doi: 10.4049/jimmunol.174.8.4880. [DOI] [PubMed] [Google Scholar]
- 88.Corzo C.A., Condamine T., Lu L., Cotter M.J., Youn J.I., Cheng P., Cho H.I., Celis E., Quiceno D.G., Padhya T., et al. HIF-1alpha regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J. Exp. Med. 2010;207:2439–2453. doi: 10.1084/jem.20100587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kumar V., Cheng P., Condamine T., Mony S., Languino L.R., McCaffrey J.C., Hockstein N., Guarino M., Masters G., Penman E., et al. CD45 Phosphatase Inhibits STAT3 Transcription Factor Activity in Myeloid Cells and Promotes Tumor-Associated Macrophage Differentiation. Immunity. 2016;44:303–315. doi: 10.1016/j.immuni.2016.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Loeuillard E., Yang J., Buckarma E., Wang J., Liu Y., Conboy C., Pavelko K.D., Li Y., O’Brien D., Wang C., et al. Targeting tumor-associated macrophages and granulocytic myeloid-derived suppressor cells augments PD-1 blockade in cholangiocarcinoma. J. Clin. Investig. 2020;130:5380–5396. doi: 10.1172/JCI137110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liao Y.P., Schaue D., McBride W.H. Modification of the tumor microenvironment to enhance immunity. Front. Biosci. A J. Virtual Libr. 2007;12:3576–3600. doi: 10.2741/2336. [DOI] [PubMed] [Google Scholar]
- 92.Cheng P., Corzo C.A., Luetteke N., Yu B., Nagaraj S., Bui M.M., Ortiz M., Nacken W., Sorg C., Vogl T., et al. Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J. Exp. Med. 2008;205:2235–2249. doi: 10.1084/jem.20080132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Poschke I., Mao Y., Adamson L., Salazar-Onfray F., Masucci G., Kiessling R. Myeloid-derived suppressor cells impair the quality of dendritic cell vaccines. Cancer Immunol. Immunother. CII. 2012;61:827–838. doi: 10.1007/s00262-011-1143-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bronte V., Apolloni E., Cabrelle A., Ronca R., Serafini P., Zamboni P., Restifo N.P., Zanovello P. Identification of a CD11b(+)/Gr-1(+)/CD31(+) myeloid progenitor capable of activating or suppressing CD8(+) T cells. Blood. 2000;96:3838–3846. doi: 10.1182/blood.V96.12.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fleming T.J., Fleming M.L., Malek T.R. Selective expression of Ly-6G on myeloid lineage cells in mouse bone marrow. RB6-8C5 mAb to granulocyte-differentiation antigen (Gr-1) detects members of the Ly-6 family. J. Immunol. 1993;151:2399–2408. [PubMed] [Google Scholar]
- 96.Youn J.I., Nagaraj S., Collazo M., Gabrilovich D.I. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J. Immunol. 2008;181:5791–5802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nagaraj S., Gupta K., Pisarev V., Kinarsky L., Sherman S., Kang L., Herber D.L., Schneck J., Gabrilovich D.I. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat. Med. 2007;13:828–835. doi: 10.1038/nm1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ouzounova M., Lee E., Piranlioglu R., El Andaloussi A., Kolhe R., Demirci M.F., Marasco D., Asm I., Chadli A., Hassan K.A., et al. Monocytic and granulocytic myeloid derived suppressor cells differentially regulate spatiotemporal tumour plasticity during metastatic cascade. Nat. Commun. 2017;8:14979. doi: 10.1038/ncomms14979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tcyganov E.N., Hanabuchi S., Hashimoto A., Campbell D., Kar G., Slidel T.W., Cayatte C., Landry A., Pilataxi F., Hayes S., et al. Distinct mechanisms govern populations of myeloid-derived suppressor cells in chronic viral infection and cancer. J. Clin. Investig. 2021;131:1–14. doi: 10.1172/JCI145971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lindau D., Gielen P., Kroesen M., Wesseling P., Adema G.J. The immunosuppressive tumour network: Myeloid-derived suppressor cells, regulatory T cells and natural killer T cells. Immunology. 2013;138:105–115. doi: 10.1111/imm.12036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hoechst B., Ormandy L.A., Ballmaier M., Lehner F., Kruger C., Manns M.P., Greten T.F., Korangy F. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology. 2008;135:234–243. doi: 10.1053/j.gastro.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 102.Peranzoni E., Zilio S., Marigo I., Dolcetti L., Zanovello P., Mandruzzato S., Bronte V. Myeloid-derived suppressor cell heterogeneity and subset definition. Curr. Opin. Immunol. 2010;22:238–244. doi: 10.1016/j.coi.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 103.Wang W., Xia X., Mao L., Wang S. The CCAAT/Enhancer-Binding Protein Family: Its Roles in MDSC Expansion and Function. Front. Immunol. 2019;10:1804. doi: 10.3389/fimmu.2019.01804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wu L., Du H., Li Y., Qu P., Yan C. Signal transducer and activator of transcription 3 (Stat3C) promotes myeloid-derived suppressor cell expansion and immune suppression during lung tumorigenesis. Am. J. Pathol. 2011;179:2131–2141. doi: 10.1016/j.ajpath.2011.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lechner M.G., Megiel C., Russell S.M., Bingham B., Arger N., Woo T., Epstein A.L. Functional characterization of human Cd33+ and Cd11b+ myeloid-derived suppressor cell subsets induced from peripheral blood mononuclear cells co-cultured with a diverse set of human tumor cell lines. J. Transl. Med. 2011;9:90. doi: 10.1186/1479-5876-9-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mandruzzato S., Brandau S., Britten C.M., Bronte V., Damuzzo V., Gouttefangeas C. Toward harmonized phenotyping of human myeloid-derived suppressor cells by flow cytometry: Results from an interim study. Cancer Immunol. Immunother. CII. 2016;65:161–169. doi: 10.1007/s00262-015-1782-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yang Y., Zhang R., Xia F., Zou T., Huang A., Xiong S., Zhang J. LPS converts Gr-1(+)CD115(+) myeloid-derived suppressor cells from M2 to M1 via P38 MAPK. Exp. Cell Res. 2013;319:1774–1783. doi: 10.1016/j.yexcr.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 108.Talmadge J.E., Gabrilovich D.I. History of myeloid-derived suppressor cells. Nat. Rev. Cancer. 2013;13:739–752. doi: 10.1038/nrc3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cassetta L., Baekkevold E.S., Brandau S., Bujko A., Cassatella M.A., Dorhoi A., Krieg C., Lin A., Lore K., Marini O., et al. Deciphering myeloid-derived suppressor cells: Isolation and markers in humans, mice and non-human primates. Cancer Immunol. Immunother. CII. 2019;68:687–697. doi: 10.1007/s00262-019-02302-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lechner M.G., Karimi S.S., Barry-Holson K., Angell T.E., Murphy K.A., Church C.H., Ohlfest J.R., Hu P., Epstein A.L. Immunogenicity of murine solid tumor models as a defining feature of in vivo behavior and response to immunotherapy. J. Immunother. 2013;36:477–489. doi: 10.1097/01.cji.0000436722.46675.4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Maenhout S.K., Van Lint S., Emeagi P.U., Thielemans K., Aerts J.L. Enhanced suppressive capacity of tumor-infiltrating myeloid-derived suppressor cells compared with their peripheral counterparts. Int. J. Cancer. 2014;134:1077–1090. doi: 10.1002/ijc.28449. [DOI] [PubMed] [Google Scholar]
- 112.Maenhout S.K., Thielemans K., Aerts J.L. Location, location, location: Functional and phenotypic heterogeneity between tumor-infiltrating and non-infiltrating myeloid-derived suppressor cells. Oncoimmunology. 2014;3:e956579. doi: 10.4161/21624011.2014.956579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sieminska I., Baran J. Myeloid-Derived Suppressor Cells in Colorectal Cancer. Front. Immunol. 2020;11:1526. doi: 10.3389/fimmu.2020.01526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Turbitt W.J., Xu Y., Sosnoski D.M., Collins S.D., Meng H., Mastro A.M., Rogers C.J. Physical Activity Plus Energy Restriction Prevents 4T1.2 Mammary Tumor Progression, MDSC Accumulation, and an Immunosuppressive Tumor Microenvironment. Cancer Prev. Res. 2019;12:493–506. doi: 10.1158/1940-6207.CAPR-17-0233. [DOI] [PubMed] [Google Scholar]
- 115.Karakhanova S., Link J., Heinrich M., Shevchenko I., Yang Y., Hassenpflug M., Bunge H., Anh K., Brecht R., Mathes A., et al. Characterization of myeloid leukocytes and soluble mediators in pancreatic cancer: Importance of myeloid-derived suppressor cells. Oncoimmunology. 2015;4:e998519. doi: 10.1080/2162402X.2014.998519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Meyer C., Sevko A., Ramacher M., Bazhin A.V., Falk C.S., Osen W., Borrello I., Kato M., Schadendorf D., Baniyash M., et al. Chronic inflammation promotes myeloid-derived suppressor cell activation blocking antitumor immunity in transgenic mouse melanoma model. Proc. Natl. Acad. Sci. USA. 2011;108:17111–17116. doi: 10.1073/pnas.1108121108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Filipazzi P., Valenti R., Huber V., Pilla L., Canese P., Iero M., Castelli C., Marinai L., Parmiani G., Rivoltini L. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J. Clin. Oncol. 2007;25:2546–2553. doi: 10.1200/JCO.2006.08.5829. [DOI] [PubMed] [Google Scholar]
- 118.Hamilton M.J., Bosiljcic M., Lepard N.E., Halvorsen E.C., Ho V.W., Banath J.P., Krystal G., Bennewith K.L. Macrophages are more potent immune suppressors ex vivo than immature myeloid-derived suppressor cells induced by metastatic murine mammary carcinomas. J. Immunol. 2014;192:512–522. doi: 10.4049/jimmunol.1300096. [DOI] [PubMed] [Google Scholar]
- 119.Bosiljcic M., Cederberg R.A., Hamilton M.J., LePard N.E., Harbourne B.T., Collier J.L., Halvorsen E.C., Shi R., Franks S.E., Kim A.Y., et al. Targeting myeloid-derived suppressor cells in combination with primary mammary tumor resection reduces metastatic growth in the lungs. Breast Cancer Res. BCR. 2019;21:103. doi: 10.1186/s13058-019-1189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Diaz-Montero C.M., Finke J., Montero A.J. Myeloid-derived suppressor cells in cancer: Therapeutic, predictive, and prognostic implications. Semin. Oncol. 2014;41:174–184. doi: 10.1053/j.seminoncol.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ananth A.A., Tai L.H., Lansdell C., Alkayyal A.A., Baxter K.E., Angka L., Zhang J., Souza C.T., Stephenson K.B., Parato K., et al. Surgical Stress Abrogates Pre-Existing Protective T Cell Mediated Anti-Tumor Immunity Leading to Postoperative Cancer Recurrence. PLoS ONE. 2016;11:e0155947. doi: 10.1371/journal.pone.0155947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fernandez A., Oliver L., Alvarez R., Hernandez A., Raymond J., Fernandez L.E., Mesa C. Very small size proteoliposomes abrogate cross-presentation of tumor antigens by myeloid-derived suppressor cells and induce their differentiation to dendritic cells. J. Immunother. Cancer. 2014;2:5. doi: 10.1186/2051-1426-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Draghiciu O., Lubbers J., Nijman H.W., Daemen T. Myeloid derived suppressor cells-An overview of combat strategies to increase immunotherapy efficacy. Oncoimmunology. 2015;4:e954829. doi: 10.4161/21624011.2014.954829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Long A.H., Highfill S.L., Cui Y., Smith J.P., Walker A.J., Ramakrishna S., Etriby R.E., Galli S., Tsokos M.G., Orentas R.J., et al. Reduction of MDSCs with All-trans Retinoic Acid Improves CAR Therapy Efficacy for Sarcomas. Cancer Immunol. Res. 2016;4:869–880. doi: 10.1158/2326-6066.CIR-15-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bauer R., Udonta F., Wroblewski M., Ben-Batalla I., Santos I.M., Taverna F., Kuhlencord M., Gensch V., Pasler S., Vinckier S., et al. Blockade of Myeloid-Derived Suppressor Cell Expansion with All-Trans Retinoic Acid Increases the Efficacy of Antiangiogenic Therapy. Cancer Res. 2018;78:3220–3232. doi: 10.1158/0008-5472.CAN-17-3415. [DOI] [PubMed] [Google Scholar]
- 126.Xiao L., Erb U., Zhao K., Hackert T., Zoller M. Efficacy of vaccination with tumor-exosome loaded dendritic cells combined with cytotoxic drug treatment in pancreatic cancer. Oncoimmunology. 2017;6:e1319044. doi: 10.1080/2162402X.2017.1319044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Varikuti S., Singh B., Volpedo G., Ahirwar D.K., Jha B.K., Saljoughian N., Viana A.G., Verma C., Hamza O., Halsey G., et al. Ibrutinib treatment inhibits breast cancer progression and metastasis by inducing conversion of myeloid-derived suppressor cells to dendritic cells. Br. J. Cancer. 2020;122:1005–1013. doi: 10.1038/s41416-020-0743-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Metzler J.M., Burla L., Fink D., Imesch P. Ibrutinib in Gynecological Malignancies and Breast Cancer: A Systematic Review. Int. J. Mol. Sci. 2020;21:4154. doi: 10.3390/ijms21114154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chen D., Xie J., Fiskesund R., Dong W., Liang X., Lv J., Jin X., Liu J., Mo Q., Zhang T., et al. Chloroquine modulates antitumor immune response by resetting tumor-associated macrophages toward M1 phenotype. Nat. Commun. 2018;9:873. doi: 10.1038/s41467-018-03225-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chen L., Zhou L., Wang C., Han Y., Lu Y., Liu J., Hu X., Yao T., Lin Y., Liang S., et al. Tumor-Targeted Drug and CpG Delivery System for Phototherapy and Docetaxel-Enhanced Immunotherapy with Polarization toward M1-Type Macrophages on Triple Negative Breast Cancers. Adv. Mater. 2019;31:e1904997. doi: 10.1002/adma.201904997. [DOI] [PubMed] [Google Scholar]
- 131.Melani C., Sangaletti S., Barazzetta F.M., Werb Z., Colombo M.P. Amino-biphosphonate-mediated MMP-9 inhibition breaks the tumor-bone marrow axis responsible for myeloid-derived suppressor cell expansion and macrophage infiltration in tumor stroma. Cancer Res. 2007;67:11438–11446. doi: 10.1158/0008-5472.CAN-07-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sun L., Clavijo P.E., Robbins Y., Patel P., Friedman J., Greene S., Das R., Silvin C., Waes C.V., Horn L.A., et al. Inhibiting myeloid-derived suppressor cell trafficking enhances T cell immunotherapy. JCI Insight. 2019;4:e126853. doi: 10.1172/jci.insight.126853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Buczek M., Escudier B., Bartnik E., Szczylik C., Czarnecka A. Resistance to tyrosine kinase inhibitors in clear cell renal cell carcinoma: From the patient’s bed to molecular mechanisms. Biochim. Et Biophys. Acta. 2014;1845:31–41. doi: 10.1016/j.bbcan.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 134.Lu X., Horner J.W., Paul E., Shang X., Troncoso P., Deng P., Jiang S., Chang Q., Spring D.J., Sharma P., et al. Effective combinatorial immunotherapy for castration-resistant prostate cancer. Nature. 2017;543:728–732. doi: 10.1038/nature21676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Erin N., Grahovac J., Brozovic A., Efferth T. Tumor microenvironment and epithelial mesenchymal transition as targets to overcome tumor multidrug resistance. Drug Resist. Updates. 2020;53:100715. doi: 10.1016/j.drup.2020.100715. [DOI] [PubMed] [Google Scholar]
- 136.Diaz-Montero C.M., Mao F.J., Barnard J., Parker Y., Zamanian-Daryoush M., Pink J.J., Finke J.H., Rini B.I., Lindner D.J. MEK inhibition abrogates sunitinib resistance in a renal cell carcinoma patient-derived xenograft model. Br. J. Cancer. 2016;115:920–928. doi: 10.1038/bjc.2016.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nam S., Lee A., Lim J., Lim J.S. Analysis of the Expression and Regulation of PD-1 Protein on the Surface of Myeloid-Derived Suppressor Cells (MDSCs) Biomol. Ther. 2019;27:63–70. doi: 10.4062/biomolther.2018.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Strauss L., Mahmoud M.A.A., Weaver J.D., Tijaro-Ovalle N.M., Christofides A., Wang Q., Pal R., Yuan M., Asara J., Patsoukis N., et al. Targeted deletion of PD-1 in myeloid cells induces antitumor immunity. Sci. Immunol. 2020;5:1–14. doi: 10.1126/sciimmunol.aay1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Fujita M., Kohanbash G., Fellows-Mayle W., Hamilton R.L., Komohara Y., Decker S.A., Ohlfest J.R., Okada H. COX-2 blockade suppresses gliomagenesis by inhibiting myeloid-derived suppressor cells. Cancer Res. 2011;71:2664–2674. doi: 10.1158/0008-5472.CAN-10-3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Veltman J.D., Lambers M.E., van Nimwegen M., Hendriks R.W., Hoogsteden H.C., Aerts J.G., Hegmans J.P.J.J. COX-2 inhibition improves immunotherapy and is associated with decreased numbers of myeloid-derived suppressor cells in mesothelioma. Celecoxib influences MDSC function. BMC Cancer. 2010;10:464. doi: 10.1186/1471-2407-10-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yokoi E., Mabuchi S., Komura N., Shimura K., Kuroda H., Kozasa K., Takahashi R., Sasano T., Kawano M., Matsumoto Y., et al. The role of myeloid-derived suppressor cells in endometrial cancer displaying systemic inflammatory response: Clinical and preclinical investigations. Oncoimmunology. 2019;8:e1662708. doi: 10.1080/2162402X.2019.1662708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yu S.J., Ma C., Heinrich B., Brown Z.J., Sandhu M., Zhang Q., Fu Q., Agdashian D., Rosato U., Korangy F., et al. Targeting the crosstalk between cytokine-induced killer cells and myeloid-derived suppressor cells in hepatocellular carcinoma. J. Hepatol. 2019;70:449–457. doi: 10.1016/j.jhep.2018.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hassel J.C., Jiang H., Bender C., Winkler J., Sevko A., Shevchenko I., Halama N., Strauss A.D., Haefeli W.E., Jager D., et al. Tadalafil has biologic activity in human melanoma. Results of a pilot trial with Tadalafil in patients with metastatic Melanoma (TaMe) Oncoimmunology. 2017;6:e1326440. doi: 10.1080/2162402X.2017.1326440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tai L.H., Alkayyal A.A., Leslie A.L., Sahi S., Bennett S., Tanese de Souza C., Katherine B., Angka L., Xu R., Kennedy M.A., et al. Phosphodiesterase-5 inhibition reduces postoperative metastatic disease by targeting surgery-induced myeloid derived suppressor cell-dependent inhibition of Natural Killer cell cytotoxicity. Oncoimmunology. 2018;7:e1431082. doi: 10.1080/2162402X.2018.1431082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.De Santo C., Serafini P., Marigo I., Dolcetti L., Bolla M., Del Soldato P., Melani C., Guiducci C., Colomno M.P., Iezzi M., et al. Nitroaspirin corrects immune dysfunction in tumor-bearing hosts and promotes tumor eradication by cancer vaccination. Proc. Natl. Acad. Sci. USA. 2005;102:4185–4190. doi: 10.1073/pnas.0409783102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Suzuki E., Kapoor V., Jassar A.S., Kaiser L.R., Albelda S.M. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin. Cancer Res. 2005;11:6713–6721. doi: 10.1158/1078-0432.CCR-05-0883. [DOI] [PubMed] [Google Scholar]
- 147.Zhang Y., Bush X., Yan B., Chen J.A. Gemcitabine nanoparticles promote antitumor immunity against melanoma. Biomaterials. 2019;189:48–59. doi: 10.1016/j.biomaterials.2018.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bruchard M., Mignot G., Derangere V., Chalmin F., Chevriaux A., Vegran F., Boireau W., Simon B., Ryffel B., Connat J.L., et al. Chemotherapy-triggered cathepsin B release in myeloid-derived suppressor cells activates the Nlrp3 inflammasome and promotes tumor growth. Nat. Med. 2013;19:57–64. doi: 10.1038/nm.2999. [DOI] [PubMed] [Google Scholar]
- 149.Huang S., Wang Z., Zhou J., Huang J., Zhou L., Luo J., Wan Y.Y., Long H., Zhu B. EZH2 Inhibitor GSK126 Suppresses Antitumor Immunity by Driving Production of Myeloid-Derived Suppressor Cells. Cancer Res. 2019;79:2009–2020. doi: 10.1158/0008-5472.CAN-18-2395. [DOI] [PubMed] [Google Scholar]
- 150.Annels N.E., Shaw V.E., Gabitass R.F., Billingham L., Corrie P., Eatock M., Valle J., Smith D., Wadsley J., Cunningham D., et al. The effects of gemcitabine and capecitabine combination chemotherapy and of low-dose adjuvant GM-CSF on the levels of myeloid-derived suppressor cells in patients with advanced pancreatic cancer. Cancer Immunol. Immunother. CII. 2014;63:175–183. doi: 10.1007/s00262-013-1502-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Aboalsoud A., El-Ghaiesh S.H., Abd Elmonem F.F., Salem M.L., Abdel Rahman M.N. The effect of low-dose naltrexone on solid Ehrlich carcinoma in mice: The role of OGFr, BCL2, and immune response. Int. Immunopharmacol. 2020;78:106068. doi: 10.1016/j.intimp.2019.106068. [DOI] [PubMed] [Google Scholar]
- 152.Liu J., Toy R., Vantucci C., Pradhan P., Zhang Z., Kuo K.M., Kubelick K.P., Huo D., Wen J., Kim J., et al. Bifunctional Janus Particles as Multivalent Synthetic Nanoparticle Antibodies (SNAbs) for Selective Depletion of Target Cells. Nano Lett. 2021;21:875–886. doi: 10.1021/acs.nanolett.0c04833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Diaz-Montero C.M., Salem M.L., Nishimura M.I., Garrett-Mayer E., Cole D.J., Montero A.J. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol. Immunother. CII. 2009;58:49–59. doi: 10.1007/s00262-008-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Weide B., Martens A., Zelba H., Stutz C., Derhovanessian E., Di Giacomo A.M., Maio M., Sucker A., Schilling B., Schadendorf D., et al. Myeloid-derived suppressor cells predict survival of patients with advanced melanoma: Comparison with regulatory T cells and NY-ESO-1- or melan-A-specific T cells. Clin. Cancer Res. 2014;20:1601–1609. doi: 10.1158/1078-0432.CCR-13-2508. [DOI] [PubMed] [Google Scholar]
- 155.Jiang H., Gebhardt C., Umansky L., Beckhove P., Schulze T.J., Utikal J., Umansky V. Elevated chronic inflammatory factors and myeloid-derived suppressor cells indicate poor prognosis in advanced melanoma patients. Int. J. Cancer. 2015;136:2352–2360. doi: 10.1002/ijc.29297. [DOI] [PubMed] [Google Scholar]
- 156.Arihara F., Mizukoshi E., Kitahara M., Takata Y., Arai K., Yamashita T., Nakamoto Y., Kaneko S. Increase in CD14+HLA-DR -/low myeloid-derived suppressor cells in hepatocellular carcinoma patients and its impact on prognosis. Cancer Immunol. Immunother. CII. 2013;62:1421–1430. doi: 10.1007/s00262-013-1447-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gao X.H., Tian L., Wu J., Ma X.L., Zhang C.Y., Zhou Y., Sun Y.F., Hu B., Qiu S.J., Zhou J., et al. Circulating CD14(+) HLA-DR(-/low) myeloid-derived suppressor cells predicted early recurrence of hepatocellular carcinoma after surgery. Hepatol. Res. 2017;47:1061–1071. doi: 10.1111/hepr.12831. [DOI] [PubMed] [Google Scholar]
- 158.Feng P.H., Lee K.Y., Chang Y.L., Chan Y.F., Kuo L.W., Lin T.Y., Chung F.T., Kuo C.S., Yu C.T., Lin S.M., et al. CD14(+)S100A9(+) monocytic myeloid-derived suppressor cells and their clinical relevance in non-small cell lung cancer. Am. J. Respir. Crit. Care Med. 2012;186:1025–1036. doi: 10.1164/rccm.201204-0636OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Vetsika E.K., Koinis F., Gioulbasani M., Aggouraki D., Koutoulaki A., Skalidaki E., Mavroudis D., Georgoulias V., Kotsakis A. A circulating subpopulation of monocytic myeloid-derived suppressor cells as an independent prognostic/predictive factor in untreated non-small lung cancer patients. J. Immunol. Res. 2014;2014:659294. doi: 10.1155/2014/659294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhang S., Ma X., Zhu C., Liu L., Wang G., Yuan X. The Role of Myeloid-Derived Suppressor Cells in Patients with Solid Tumors: A Meta-Analysis. PLoS ONE. 2016;11:e0164514. doi: 10.1371/journal.pone.0164514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ai L., Mu S., Wang Y., Wang H., Cai L., Li W., Hu Y. Prognostic role of myeloid-derived suppressor cells in cancers: A systematic review and meta-analysis. BMC Cancer. 2018;18:1220. doi: 10.1186/s12885-018-5086-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhang X., Fu X., Li T., Yan H. The prognostic value of myeloid derived suppressor cell level in hepatocellular carcinoma: A systematic review and meta-analysis. PLoS ONE. 2019;14:e0225327. doi: 10.1371/journal.pone.0225327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wang P.F., Song S.Y., Wang T.J., Ji W.J., Li S.W., Liu N., Yan C.X. Prognostic role of pretreatment circulating MDSCs in patients with solid malignancies: A meta-analysis of 40 studies. Oncoimmunology. 2018;7:e1494113. doi: 10.1080/2162402X.2018.1494113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gabitass R.F., Annels N.E., Stocken D.D., Pandha H.A., Middleton G.W. Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13. Cancer Immunol. Immunother. CII. 2011;60:1419–1430. doi: 10.1007/s00262-011-1028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Liang Y., Lu B., Zhao P., Lu W. Increased circulating GrMyeloid-derived suppressor cells correlated with tumor burden and survival in locally advanced cervical cancer patient. J. Cancer. 2019;10:1341–1348. doi: 10.7150/jca.29647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Mabuchi S., Sasano T. Myeloid-Derived Suppressor Cells as Therapeutic Targets in Uterine Cervical and Endometrial Cancers. Cells. 2021;10:1073. doi: 10.3390/cells10051073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Shimura K., Mabuchi S., Komura N., Yokoi E., Kozasa K., Sasano T., Kawano M., Matsumoto Y., Watabe T., Kodama M., et al. Prognostic significance of bone marrow FDG uptake in patients with gynecological cancer. Sci. Rep. 2021;11:2257. doi: 10.1038/s41598-021-81298-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Shou D., Wen L., Song Z., Yin J., Sun Q., Gong W. Suppressive role of myeloid-derived suppressor cells (MDSCs) in the microenvironment of breast cancer and targeted immunotherapies. Oncotarget. 2016;7:64505–64511. doi: 10.18632/oncotarget.11352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zhang B., Wang Z., Wu L., Zhang M., Li W., Ding J., Zhu J., Wei H., Zhao K. Circulating and tumor-infiltrating myeloid-derived suppressor cells in patients with colorectal carcinoma. PLoS ONE. 2013;8:e57114. doi: 10.1371/journal.pone.0057114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Stenzel A.E., Abrams S.I., Joseph J.M., Goode E.L., Tario J.D., Jr., Wallace P.K., Kaur D., Adamson A.K., Buas M.F., Lugade A.A., et al. Circulating CD14(+) HLA-DR(lo/-) monocytic cells as a biomarker for epithelial ovarian cancer progression. Am. J. Reprod. Immunol. 2021;85:e13343. doi: 10.1111/aji.13343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Yuan X.K., Zhao X.K., Xia Y.C., Zhu X., Xiao P. Increased circulating immunosuppressive CD14(+)HLA-DR(-/low) cells correlate with clinical cancer stage and pathological grade in patients with bladder carcinoma. J. Int. Med. Res. 2011;39:1381–1391. doi: 10.1177/147323001103900424. [DOI] [PubMed] [Google Scholar]
- 172.Wu L., Deng Z., Peng Y., Han L., Liu J., Wang L., Li B., Zhao J., Jiao S., Wei H. Ascites-derived IL-6 and IL-10 synergistically expand CD14(+)HLA-DR(-/low) myeloid-derived suppressor cells in ovarian cancer patients. Oncotarget. 2017;8:76843–76856. doi: 10.18632/oncotarget.20164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Liu C.Y., Wang Y.M., Wang C.L., Feng P.H., Ko H.W., Liu Y.H., Wu Y.C., Chu Y., Chung F.T., Kuo C.H., et al. Population alterations of L-arginase- and inducible nitric oxide synthase-expressed CD11b+/CD14(-)/CD15+/CD33+ myeloid-derived suppressor cells and CD8+ T lymphocytes in patients with advanced-stage non-small cell lung cancer. J. Cancer Res. Clin. Oncol. 2010;136:35–45. doi: 10.1007/s00432-009-0634-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Choi J., Suh B., Ahn Y.O., Kim T.M., Lee J.O., Lee S.H., Heo D.S. CD15+/CD16low human granulocytes from terminal cancer patients: Granulocytic myeloid-derived suppressor cells that have suppressive function. Tumour Biol. 2012;33:121–129. doi: 10.1007/s13277-011-0254-6. [DOI] [PubMed] [Google Scholar]
- 175.Sasidharan Nair V., Saleh R., Toor S.M., Alajez N.M., Elkord E. Transcriptomic Analyses of Myeloid-Derived Suppressor Cell Subsets in the Circulation of Colorectal Cancer Patients. Front. Oncol. 2020;10:1530. doi: 10.3389/fonc.2020.01530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Grutzner E., Stirner R., Arenz L., Athanasoulia A.P., Schrodl K., Berking C., Bogner J.R., Draenert R. Kinetics of human myeloid-derived suppressor cells after blood draw. J. Transl. Med. 2016;14:2. doi: 10.1186/s12967-015-0755-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Achyut B.R., Arbab A.S. Myeloid cell signatures in tumor microenvironment predicts therapeutic response in cancer. OncoTargets Ther. 2016;9:1047–1055. doi: 10.2147/OTT.S102907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Tang F., Tie Y., Tu C., Wei X. Surgical trauma-induced immunosuppression in cancer: Recent advances and the potential therapies. Clin. Transl. Med. 2020;10:199–223. doi: 10.1002/ctm2.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Syn N.L., Teng M.W.L., Mok T.S.K., Soo R.A. De-novo and acquired resistance to immune checkpoint targeting. Lancet Oncol. 2017;18:e731–e741. doi: 10.1016/S1470-2045(17)30607-1. [DOI] [PubMed] [Google Scholar]
- 180.Fuereder T. Immunotherapy for head and neck squamous cell carcinoma. Memo. 2016;9:66–69. doi: 10.1007/s12254-016-0270-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Vachhani P., Chen H. Spotlight on pembrolizumab in non-small cell lung cancer: The evidence to date. OncoTargets Ther. 2016;9:5855–5866. doi: 10.2147/OTT.S97746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Redman J.M., Gibney G.T., Atkins M.B. Advances in immunotherapy for melanoma. BMC Med. 2016;14:20. doi: 10.1186/s12916-016-0571-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Bockorny B., Semenisty V., Macarulla T., Borazanci E., Wolpin B.M., Stemmer S.M., Golan T., Geva R., Borad M.J., Pedersen K.S., et al. BL-8040, a CXCR4 antagonist, in combination with pembrolizumab and chemotherapy for pancreatic cancer: The COMBAT trial. Nat. Med. 2020;26:878–885. doi: 10.1038/s41591-020-0880-x. [DOI] [PubMed] [Google Scholar]
- 184.Hodi F.S., O’Day S.J., McDermott D.F., Weber R.W., Sosman J.A., Haanen J.B., Gonzalez R., Robert C., Schadendorf D., Hassel J.C., et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Sade-Feldman M., Kanterman J., Klieger Y., Ish-Shalom E., Olga M., Saragovi A., Shtainberg H., Lotem M., Baniyash M. Clinical Significance of Circulating CD33+CD11b+HLA-DR- Myeloid Cells in Patients with Stage IV Melanoma Treated with Ipilimumab. Clin. Cancer Res. 2016;22:5661–5672. doi: 10.1158/1078-0432.CCR-15-3104. [DOI] [PubMed] [Google Scholar]
- 186.Tobin R.P., Jordan K.R., Robinson W.A., Davis D., Borges V.F., Gonzalez R., Lewis K.D., McCarter M.D. Targeting myeloid-derived suppressor cells using all-trans retinoic acid in melanoma patients treated with Ipilimumab. Int. Immunopharmacol. 2018;63:282–291. doi: 10.1016/j.intimp.2018.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Eriksson E., Wenthe J., Irenaeus S., Loskog A., Ullenhag G. Gemcitabine reduces MDSCs, tregs and TGFbeta-1 while restoring the teff/treg ratio in patients with pancreatic cancer. J. Transl. Med. 2016;14:282. doi: 10.1186/s12967-016-1037-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kalathil S.G., Wang K., Hutson A., Iyer R., Thanavala Y. Tivozanib mediated inhibition of c-Kit/SCF signaling on Tregs and MDSCs and reversal of tumor induced immune suppression correlates with survival of HCC patients. Oncoimmunology. 2020;9:1824863. doi: 10.1080/2162402X.2020.1824863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Weed D.T., Vella J.L., Reis I.M., De la Fuente A.C., Gomez C., Sargi Z., Nazarian R., Califano J., Borrello I., Serafini P. Tadalafil reduces myeloid-derived suppressor cells and regulatory T cells and promotes tumor immunity in patients with head and neck squamous cell carcinoma. Clin. Cancer Res. 2015;21:39–48. doi: 10.1158/1078-0432.CCR-14-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Apolo A.B., Nadal R., Tomita Y., Davarpanah N.N., Cordes L.M., Steinberg S.M., Cao L., Parnes H.L., Costello R., Merino M.J., et al. Cabozantinib in patients with platinum-refractory metastatic urothelial carcinoma: An open-label, single-centre, phase 2 trial. Lancet Oncol. 2020;21:1099–1109. doi: 10.1016/S1470-2045(20)30202-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Prima V., Kaliberova L.N., Kaliberov S., Curiel D.T., Kusmartsev S. COX2/mPGES1/PGE2 pathway regulates PD-L1 expression in tumor-associated macrophages and myeloid-derived suppressor cells. Proc. Natl. Acad. Sci. USA. 2017;114:1117–1122. doi: 10.1073/pnas.1612920114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Okla K., Rajtak A., Czerwonka A., Bobinski M., Wawruszak A., Tarkowski R., Bednarek W., Szumilo J., Kotarski J. Accumulation of blood-circulating PD-L1-expressing M-MDSCs and monocytes/macrophages in pretreatment ovarian cancer patients is associated with soluble PD-L1. J. Transl. Med. 2020;18:220. doi: 10.1186/s12967-020-02389-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kong Y.Y., Wilson K., Apostolopoulos V., Plebanski M. Dendritic Cells and Myeloid Derived Suppressor Cells Fully Responsive to Stimulation via Toll-Like Receptor 4 Are Rapidly Induced from Bone-Marrow Cells by Granulocyte-Macrophage Colony-Stimulating Factor. Vaccines. 2020;8:522. doi: 10.3390/vaccines8030522. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.