Abstract
A rare case of Mycobacterium microti infection in a human immunodeficiency virus-positive patient is described. Because of unusual morphological and cultural features, the pathogen was analyzed by spoligotyping and identified as the Mycobacterium microti llama type. Although culture of M. microti is difficult, drug susceptibility testing could be performed, which correlated with the clinical outcome.
CASE REPORT
A 48-year-old white male was admitted with a 3-month history of night sweats, productive cough, progressive dyspnea, and weight loss of 10 kg, which he attributed to dysphagia. The patient smoked 20 cigarettes a day and denied foreign travel and exposure to household, farm, or wild animals or to persons infected with tuberculosis (TB). Upon physical examination, the patient appeared chronically ill, but in no acute distress. There was an extensive oral thrush. Occasionally crackles were heard over the right upper chest.
A chest X-ray showed a right upper lobe infiltrate without cavitation. Ziehl-Neelsen staining of the initial sputum specimen revealed sharply curved acid-fast bacilli (AFB) (Fig. 1A). The patient was isolated in a low-pressure chamber, and antituberculous treatment with isoniazid, rifampin, and pyrazinamide was initiated. Tests for human immunodeficiency virus type 1 (HIV-1) were positive. His CD4+ count was 104 × 106/liter, the CD4+/CD8+ ratio was 0.14, and the viral load as >750,000 copies/ml, indicating long-standing and advanced HIV infection. Oral thrush and dysphagia resolved after treatment with oral fluconazole. The patient was discharged after 31 days, when sputum samples were AFB smear negative. Mycobacterial cultures remained negative. After 3 months, the pulmonary infiltrate had almost completely resolved, and the treatment was changed to isoniazid and rifampin. Meanwhile, the CD4+ count had declined to 54 × 106/liter. Antiretroviral treatment with zidovudine, lamivudine, nelfinavir, and efavirenz was initiated. After 2 months of antiretroviral therapy, the CD4+ count increased to 256 × 106/liter, while the viral load decreased to 237 copies/ml.
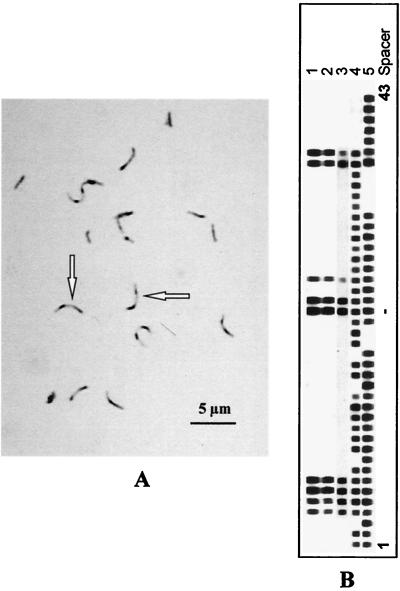
FIG. 1.
(A and B) Ziehl-Neelsen-stained sputum showing sharply curved AFB (arrows) (A) and spoligotype patterns of three sputum cultures (lanes 1 to 3) obtained from the patient, as well as those of a clinical M. bovis isolate (lane 4) and M. tuberculosis H37Rv (lane 5) as controls (B). All three sputum cultures showed a spoligotype pattern observed previously in an M. microti strain obtained from a zoo llama (4).
A total of six mycobacterial isolates could be grown from sputum specimens (Table 1). Mycobacterial growth was first indicated in liquid medium (Bactec 12B; Becton Dickinson, Cockeysville, Md.). Growth of mycobacteria was also detected on Stonebrink medium (1), a solid medium which contains pyruvate instead of glycerol, but not on Loewenstein-Jensen medium (Table 1). The isolates were identified as M. tuberculosis complex with the ACCUProbe culture confirmation test (GenProbe, San Diego, Calif.).
TABLE 1.
Culture results for M. microti from sputum of an HIV-1-infected patient
| Time of sampling | Day after admission | Isolate | Ziehl-Neelsen stain resulta | No. of days until detection on medium:
|
Spoligotyping result | ||
|---|---|---|---|---|---|---|---|
| Bactec 12B | Stonebrink | Loewenstein-Jensen | |||||
| Before therapy | 2 | 1 | 2+ | 14 | 43 | No growth | x |
| 3 | 2 | 0 | 33 | No growth | No growth | x | |
| After start of therapy | 11 | 3 | 0 | 32 | No growth | No growth | x |
| 12 | 4 | 1+ | 18 | No growth | No growth | ||
| 13 | 5 | 3+ | No growth | 38 | No growth | ||
| 17 | 6 | 0 | 40 | No growth | No growth | ||
| Before discharge on day 31 | 20 | 0 | No growth | No growth | No growth | ||
| 26 | 0 | No growth | No growth | No growth | |||
| 27 | 0 | No growth | No growth | No growth | |||
| 30 | 0 | No growth | No growth | No growth | |||
AFB smear reporting (2). 0, no AFB seen; 1+, 1 to 9/100 fields (1,000); 2+, 1 to 9/10 fields (1,000); 3+, 1 to 9/field (1,000).
Due to the sharply curved appearance of the AFB, which is unusual for the M. tuberculosis complex, and because of the slow growth on solid media, these mycobacteria were analyzed by spoligotyping, a technique based on the mycobacterial strain-dependent presence or absence of short nonrepetitive spacer sequences that intersperse the repetitive direct repeat sequences. The mycobacteria were identified as M. microti type llama (4) by the characteristic spoligotype pattern (Fig. 1B). Drug susceptibility testing in liquid medium (Bactec 460TB; Becton Dickinson) revealed susceptibility to isoniazid, rifampin, pyrazinamide, ethambutol, and streptomycin.
Mycobacterium microti, which belongs to the Mycobacterium tuberculosis complex, has been described to cause TB, mainly in small rodents, but has been considered to be nonpathogenic for humans (4–6). More recently, van Soolingen et al. (4) demonstrated different strains of M. microti isolated from vole, hyrax, llama, pig, and ferret by molecular methods. In retrospective analyses, they additionally found four infections of humans in The Netherlands caused by M. microti strains that showed a high degree of similarity to strains from a ferret and a pig (4; K. Kremer, D. van Soolingen, J. D. A. van Embden, S. Hughes, J. Inwald, and G. Hewinson, Letter, J. Clin. Microbiol. 36:2793–2794, 1998). We have reported the first M. microti llama-type infection presenting as pulmonary TB in an HIV-positive patient in Germany.
To our knowledge, this is the first report of an M. microti infection with the llama type presenting as a pulmonary TB in an HIV-positive patient. The only other case of an M. microti llama-type infection in humans was not described in detail (Kremer et al., letter). The first four M. microti isolates from humans in The Netherlands showed spoligotype patterns identical to those of M. microti strains obtained from a ferret and a pig and various isolates obtained from voles (vole type [4]). However, they differed markedly from those of our isolates, which displayed a spoligotype pattern previously observed in an isolate obtained from a zoo llama (llama type [4]). Moreover, the time spans required for cultivation of the other M. microti isolates from humans (3 and 4 months, respectively) were significantly longer than those for our M. microti llama-type isolate (Table 1).
Three of the four M. microti isolates from humans (2, 4) were found in immunocompromised patients (two had experienced kidney transplantation, one was HIV infected). Only in two patients did empirical triple therapy with antituberculous drugs result in regression of the pulmonary symptoms and disappearance of the AFB. The pulmonary infiltrate of the HIV-infected patient, however, disappeared only when a six-drug therapeutic regimen including clarithromycin and ofloxacin was followed (2). However, in our patient, antituberculous therapy including isoniazid, rifampin, and pyrazinamide was successful, which indicates that regular TB therapy is sufficient for treatment of patients with M. microti infection. These findings also correlated with results obtained by drug susceptibility testing in liquid media.
Contacts with mice by two patients with M. microti infections were found to be suggestive for zoonotic transmission (4). However, a possible source of infection could not be identified in our patient.
In conclusion, the case presented here emphasizes the necessity to consider M. microti as a relevant pathogen in immunocompromised patients. The prevalence and clinical importance of different types of M. microti may have been underestimated so far because of difficulties with primary isolation and differentiation. Hence, further studies applying molecular methods are necessary to analyze the epidemiology of M. microti more thoroughly.
Acknowledgments
We thank Stefanie Lahn for technical assistance.
REFERENCES
- 1.Burkhardt F. Besondere Arbeitsverfahren. Herstellung von Nährböden. In: Burkhardt F, Thieme Georg, editors. Mikrobiologische Diagnostik. Berling Germany: Verlag; 1992. p. 638. [Google Scholar]
- 2.Foudraine N A, van Soolingen D, Noordhoek G T, Reiss P. Pulmonary tuberculosis due to Mycobacterium microti in a human immunodeficiency virus-infected patient. Clin Infect Dis. 1998;27:1543–1544. doi: 10.1086/517747. [DOI] [PubMed] [Google Scholar]
- 3.Kent P T, Kubica G P. Public health mycobacteriology: a guide for the level III laboratory. Atlanta, Ga: Centers for Disease Control, U.S. Department of Health and Human Services; 1985. [Google Scholar]
- 4.Van Soolingen D, van der Zanden A G M, de Haas P E W, Noordhoek G T, Kiers A, Foudraine N A, Portaels F, Kolk A H J, Kremer K, van Embden J D A. Diagnosis of Mycobacterium microti infections among humans by using novel genetic markers. J Clin Microbiol. 1998;36:1840–1845. doi: 10.1128/jcm.36.7.1840-1845.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wayne L G, Kubica G P. The mycobacteria. In: Sneath P H A, Holt J G, editors. Bergey's manual of systematic bacteriology. Vol. 2. Baltimore, Md: The Williams & Wilkins Co.; 1986. pp. 1435–1457. [Google Scholar]
- 6.Wells, A. Q. 1937. Tuberculosis in wild voles. Lancet 1221.