Abstract
Recent studies demonstrate that several polyphenolic compounds produced from beyond the canonical monolignol biosynthetic pathways can behave as lignin monomers, participating in radical coupling reactions and being incorporated into lignin polymers. Here, we show various classes of flavonoids, the chalconoid naringenin chalcone, the flavanones naringenin and dihydrotricin, and the flavone tricin, incorporated into the lignin polymer of papyrus (Cyperus papyrus L.) rind. These flavonoids were released from the rind lignin by Derivatization Followed by Reductive Cleavage (DFRC), a chemical degradative method that cleaves the β-ether linkages, indicating that at least a fraction of each was integrated into the lignin as β-ether-linked structures. Due to the particular structure of tricin and dihydrotricin, whose C-3ʹ and C-5ʹ positions at their B-rings are occupied by methoxy groups, these compounds can only be incorporated into the lignin through 4ʹ–O–β bonds. However, naringenin chalcone and naringenin have no substituents at these positions and can therefore form additional carbon–carbon linkages, including 3ʹ– or 5ʹ–β linkages that form phenylcoumaran structures not susceptible to cleavage by DFRC. Furthermore, Nuclear Magnetic Resonance analysis indicated that naringenin chalcone can also form additional linkages through its conjugated double bond. The discovery expands the range of flavonoids incorporated into natural lignins, further broadens the traditional definition of lignin, and enhances the premise that any phenolic compound present at the cell wall during lignification could be oxidized and potentially integrated into the lignin structure, depending only on its chemical compatibility. This study indicates that papyrus lignin has a unique structure, as it is the only lignin known to date that integrates such a diversity of phenolic compounds from different classes of flavonoids. This discovery will open up new ways to engineer and design lignins with specific properties and for enhanced value.
A series of flavonoids incorporate into the rind lignin of papyrus, participating as monomers during lignification.
Introduction
Lignin is a complex phenylpropanoid biopolymer characteristic of vascular plants to which it provides structural support and rigidity, as well as a protection against pathogens. Lignin results from the oxidative polymerization of mainly three cinnamyl alcohols (p-coumaryl, coniferyl, and sinapyl alcohols) known as monolignols (Ralph et al., 2004, 2019; Vanholme et al., 2010, 2019). Other phenolic compounds derived from the monolignols biosynthetic pathway, including monolignol esters (with acetic, p-coumaric, p-hydroxybenzoic, or ferulic acids) or intermediate compounds from the monolignols biosynthesis (such as caffeyl alcohol, 5-hydroxyconiferyl alcohol, and the hydroxycinnamaldehydes), have also been found to act as lignin monomers in many plants (del Río et al., 2007, 2008; Ralph, 2010; Chen et al., 2012, 2013; Tobimatsu et al., 2013; Lu et al., 2015; Karlen et al., 2016; Rencoret et al., 2018, 2020; Ralph et al., 2019).
However, recent studies have broadened the range of lignin monomers involved in lignification, and have demonstrated that several polyphenolic compounds produced from other biosynthetic pathways, namely flavonoids, hydroxystilbenes, and hydroxycinnamic amides, can also behave as lignin monomers in several plants participating in radical coupling reactions and being incorporated into the lignin polymer (del Río et al., 2020, 2021). These discoveries have challenged and expanded the traditional, more narrow definition of lignin. The flavone tricin was the first phenolic compound derived from outside the monolignol biosynthetic pathway that was found to participate in lignification (del Río et al., 2012; Lan et al., 2015). Tricin was first described in the lignin from wheat (Triticum durum) straw (del Río et al., 2012) and subsequent investigations revealed that it was present in the lignins of all grasses, as well as in other monocots (Rencoret et al., 2013; del Río et al., 2015; Lan et al., 2016a, 2016b). Tricin was also found in the lignins of the eudicotyledonous plant alfalfa (Medicago sativa) (Lan et al., 2016b) and the related M. truncatula (Lui et al., 2020). Later, another class of polyphenolic compounds, hydroxystilbenes (piceatannol, resveratrol, and isorhapontigenin), was also reported to act as lignin monomers and become embedded into the lignin structure of palm fruit endocarps (del Río et al., 2017; Rencoret et al., 2018). Likewise, the respective hydroxystilbene glucosides (astringin, piceid, and isorhapontin) were found to be incorporated into the lignin of Norway spruce (Picea abies) bark (Rencoret et al., 2019; Neiva et al., 2020). Also several feruloyl amides were found incorporated into the lignins of some plants, such as feruloyltyramine in the lignin of tobacco (Nicotiana tabacum) and potato (Solanum tuberosum) tubers (Negrel and Jeandet, 1987; Negrel et al., 1996; Ralph et al., 1998), and diferuloylputrescine in the lignin of maize (Zea mays) kernels (del Río et al., 2018). Flavonoids, hydroxystilbenes, and feruloyl amides, unlike the monolignols derived from the shikimate biosynthetic pathway, are metabolic hybrids derived from a combination of the shikimate pathway and the acetate/malonate-derived polyketide pathway or the amino acid biosynthetic pathway. In the particular case of flavonoids, they are known to participate in oxidative radical cross-coupling reactions with monolignols to produce “non-conventional” lignans and neolignans (termed flavonolignans) that have two phenylpropanoid units connected through a diversity of linkages (Begum et al., 2010; Chambers et al., 2015). Several flavonoids from different classes (the flavanonol taxifolin, the flavonol quercetin, the flavanones eriodictyol, and dihydrotricin, and the flavones apigenin, luteolin, selgin, and tricin) have been reported to form such flavonolignans (del Río et al., 2020). The wide natural occurrence in plants of these flavonolignans produced from the cross-coupling with monolignols is an indication that at least some members of the flavonoids are also compatible with the radical-coupling reactions implicated in lignification. However, the flavone tricin is the only flavonoid that has been reported so far to be incorporated into the lignins of wild-type plants. The flavanone naringenin and the flavone apigenin were reported to be incorporated into the lignin of rice (Oryza sativa) plants in which the genes of the tricin biosynthetic pathway were mutated (Lam et al., 2017, 2019), but have not yet been reported in wild-type plants.
In this article, we provide evidence of several flavonoids from different classes, the chalconoid naringenin chalcone, the flavanones naringenin, and dihydrotricin, along with the flavone tricin, being present in the rind lignin of papyrus (Cyperus papyrus L.), a nongrass commelinid monocot plant from the Cyperaceae, in which they appear to participate in radical coupling reactions with monolignols and act as true lignin monomers.
Results and discussion
Release of flavonoids by chemical cleavage of the alkyl-aryl ether linkages in lignin
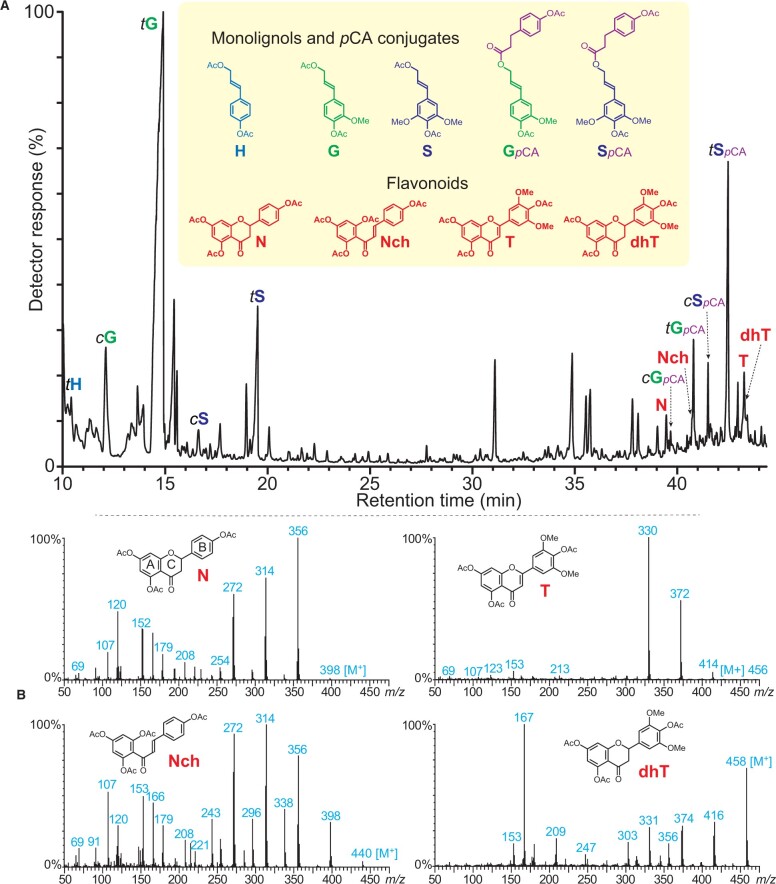
The “milled-wood lignin” (MWL) isolated from the rind of papyrus culms was analyzed by Derivatization Followed by Reductive Cleavage (DFRC), a chemical degradative method that selectively cleaves β-ether linkages (the most abundant linkages in the lignin polymer), and the released compounds were identified by GC/MS (Figure 1). Similar to other commelinid monocots, the DFRC degradation products of the papyrus rind lignin included the cis- and trans-isomers of guaiacyl and syringyl lignin monomers (cG, tG, cS, and tS), as well as their corresponding monolignol p-coumarate conjugates (cGpCA, tGpCA, cSpCA, and tSpCA). But more importantly, the chromatogram also revealed the presence of a series of flavonoid compounds that were identified as the chalconoid naringenin chalcone (Nch), the flavanones naringenin (N) and dihydrotricin (dhT), and the flavone tricin (T). The identities of all of these flavonoids were confirmed by comparison with the retention times and mass spectra of authentic standards (Figure 1). These flavonoids are not contaminants as the plant cell walls were successively and exhaustively extracted with several solvents (acetone, methanol, water), and the prepared MWL was similarly purified as well. The different flavonoid standards were subjected to DFRC degradation to confirm that their structures were not modified during the chemical treatment, and they were released intact; the results also indicated that naringenin did not produce naringenin chalcone upon DFRC degradation (Figure 2). Therefore, the presence of these flavonoids among the DFRC degradation products indicated that at least some fraction of each was originally integrated into the lignin of papyrus rind as β-ether-linked structures.
Figure 1.
Monolignols, monolignol-p-coumarates, and flavonoids released from the rind lignin of papyrus by DFRC. A, Total-ion chromatogram of the DFRC degradation products released from the MWL lignin isolated from papyrus rind, showing the presence of naringenin (N), naringenin chalcone (Nch), dihydrotricin (dhT), and tricin (T), as their acetate derivatives. cGpCA, tGpCA, cSpCA, and tSpCA are the cis- and trans-coniferyl- and sinapyl-dihydro-p-coumarates, as their acetate derivatives. B, Electron-impact mass spectra and structures of naringenin (N), naringenin chalcone (Nch), dihydrotricin (dhT), and tricin (T), as their acetate derivatives. Note that the p-coumarates are converted to dihydro-p-coumarates during DFRC degradation.
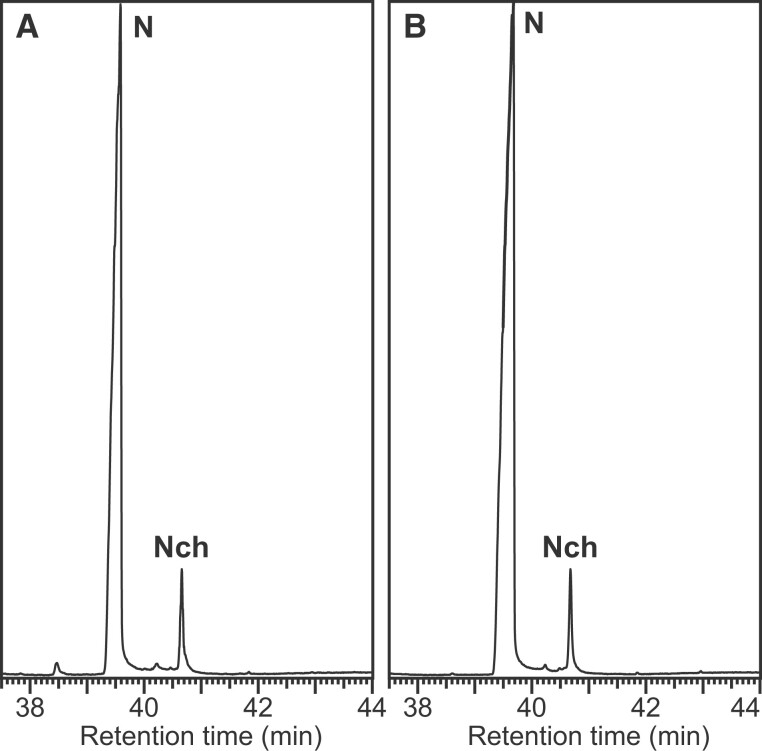
Figure 2.
Demonstration that naringenin (N) does not produce naringenin chalcone (Nch) during the DFRC degradation process. A, Chromatogram of naringenin standard. B, Chromatogram of the DFRC degradation products released from naringenin. Note that small amounts of naringenin chalcone are present in the naringenin standard, but these amounts do not increase after DFRC.
Tricin was the first lignin monomer from outside the classical monolignol biosynthetic pathway to be discovered, and was found to be widely incorporated into the lignin of grasses and other monocotyledonous plants (del Río et al., 2012, 2015, 2020, 2021; Rencoret et al., 2013; Lan et al., 2015, 2016a, 2016b). In this work, the presence of tricin among the products released by DFRC from papyrus rind lignin was not entirely surprising as it has been widely found in the lignins of other commelinid monocots, but the release of the chalconoid naringenin chalcone as well as the flavanones naringenin and dihydrotricin was completely unexpected as they have never been previously reported as being part of the lignin in wild-type plants. Naringenin was, however, previously found integrated into the lignin structure in rice mutants deficient in flavone synthase II (FNSII), the enzyme that converts the flavanone naringenin into the flavone apigenin, which is subsequently hydroxylated and methylated by the corresponding enzymes (flavonoid 3′-hydroxylase, F3′H; flavonoid O-methyltransferase, FOMT; crysoeriol 5′-hydroxylase, C5′H) to ultimately produce tricin (Lam et al., 2017). Disruption of FNSII blocked the conversion of naringenin into apigenin, and ultimately tricin, producing a lignin that incorporated naringenin, instead of tricin. Also, dihydrotricin is known to participate in cross-coupling reactions with monolignols forming several flavonolignans (Chang et al., 2010), indicating its compatibility with lignification.
It is important to note that this is the first time that such a diversity of phenolic compounds from different flavonoid classes (chalcones, flavanones, and flavones) has been found incorporated into the same lignin, which makes the papyrus lignin unique (at least to date). Due to their particular structure, with an S-type B-ring, tricin and dihydrotricin can only form 4′–O–β linkages upon cross-coupling with monolignols, which implies that they can only be present at the starting end of the lignin chain. This is the reason why tricin was proposed to act as a nucleation site for lignification in grasses and other monocots (Lan et al., 2015), and it is likely that dihydrotricin could play an analogous role in papyrus lignin. However, unlike tricin and dihydrotricin, naringenin chalcone and naringenin have no substituents at the C-3′ and C-5′ positions and therefore can form additional carbon–carbon linkages, which are not prone to DFRC degradation. In fact, the related flavanone eriodictyol is known to form the flavonolignans silyhermin and neosilyhermins A and B that present phenylcoumaran structures formed through 5′–β and or 2′–β cross-coupling, respectively, with coniferyl alcohol (Csupor et al., 2016). Therefore, the actual contents of naringenin chalcone and naringenin incorporated into the lignin of papyrus rind could be higher than indicated by their DFRC release.
Identification and incorporation of flavonoids in papyrus rind lignin as observed by 2D-NMR analyses
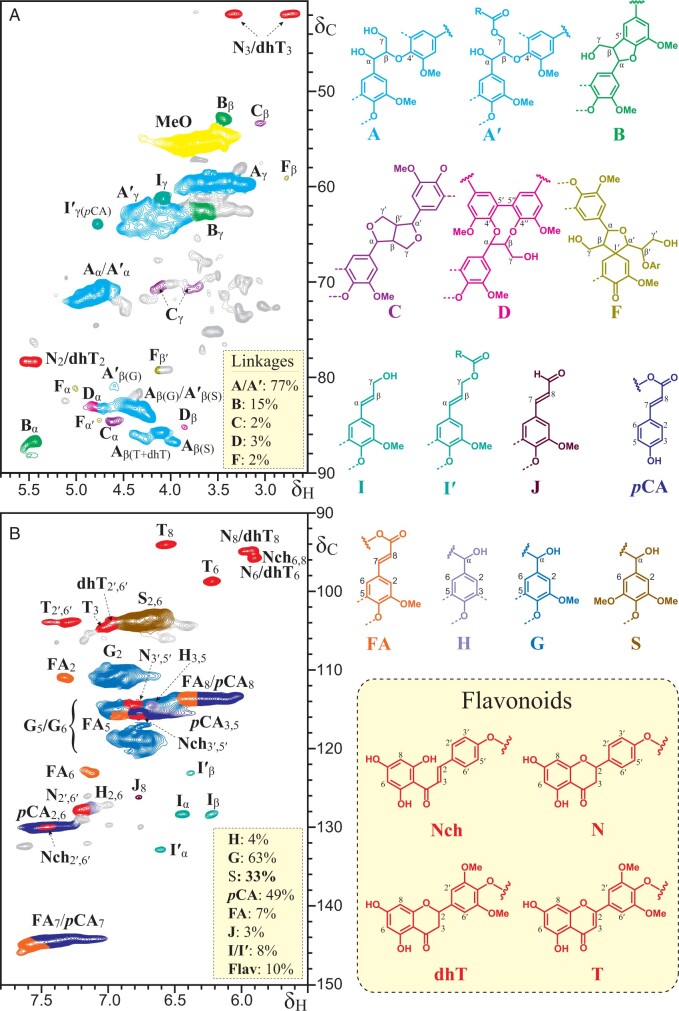
2D-Nuclear magnetic resonance (NMR) spectroscopic analyses provided further information on the identities of the flavonoids present in the lignin of papyrus rind and their mode of incorporation into the lignin structure. The 2D-heteronuclear single quantum coherence (HSQC) spectrum of papyrus rind lignin is shown in Figure 3. The most relevant structural characteristics of the papyrus rind lignin are shown in appropriate boxes in Figure 3, including the abundances of the different lignin units (H, G, and S), inter-unit linkages, p-hydroxycinnamyl end-groups, and the contents of p-hydroxycinnamates and flavonoids, estimated from the integration of the signals in the HSQC spectrum.
Figure 3.
2D HSQC NMR spectrum (in DMSO-d6) of the MWL preparation isolated from papyrus rind. A, Aliphatic-oxygenated (δC/δH 40 − 90/2.5 − 5.8) and B, aromatic (δC/δH 90 − 150/5.5 − 7.8) regions. The main structures found are as follows: A: β–O–4′ alkyl-aryl ethers; A′: β–O–4′ alkyl-aryl ethers acylated at the γ-OH; B: β–5′ phenylcoumarans; C: β–β′ resinols; D: 5–5′ dibenzodioxocins; F: β–1′ spirodienones; I: p-hydroxycinnamyl alcohol end-groups; I′: p-hydroxycinnamyl alcohol end-groups acylated at the γ-OH; J: p-hydroxycinnamaldehyde end-groups; pCA: p-coumarates; FA: ferulates; H: p-hydroxyphenyl units; G: guaiacyl units; S: syringyl units; Nch: naringenin chalcone; N: naringenin; dhT: dihydritricin; T: tricin. The relative abundances of the different H-, G-, and S-lignin units, inter-unit linkages and p-hydroxycinnamyl end-groups, p-hydroxycinnamates, and flavonoids (Flav = Nch + N + dhT + T), estimated from the integration of the signals in the HSQC spectrum are shown in appropriate boxes. Lignin inter-unit linkages (A/A′, B, C, D, and F) are expressed as fractions of the total linkages types, whereas pCA, FA, J, I/I′ and Flav contents are reported as percentages of the sum of the lignin units (H + G + S = 100).
Besides the signals from typical lignin structures, the HSQC spectrum also showed prominent signals from the different flavonoids. To assist the assignments, the HSQC spectrum was compared with analogous spectra from authentic standards of naringenin chalcone, naringenin, dihydrotricin, and tricin (Figure 4). The HSQC spectrum of papyrus rind lignin displayed the typical signals of tricin incorporated into the lignin, such as the correlations for C6/H6 at δC/δH 98.8/6.22 (T6), and C8/H8 at δC/δH 94.1/6.57 (T8), as well as the correlations for C3/H3 at δC/δH 104.6/7.03 (T3) and C2′,6′/H2′,6′ at δC/δH 104.0/7.31 (T2′,6′) (del Río et al., 2012). The HSQC spectrum also showed a series of signals that are characteristic of the A and C-rings of flavanones, including the correlations of C2/H2 at δC/δH 78.5/5.45 (N2/dhT2), and C3/H3 at δC/δH 42.1/3.33 and 42.1/2.72 (N3/dhT3), as well as other signals for the correlations of C6/H6 at δC/δH 95.7/5.91 (N6/dhT6), and C8/H8 at δC/δH 94.6/5.93 (N8/dhT8), and that exactly matched those observed in the HSQC spectra of the authentic standards of naringenin and dihydrotricin (Figure 4), confirming their presence in this lignin. Interestingly, the characteristic signals of flavanones (N8/dhT8 and N6/dhT6) could also be seen in the HSQC spectrum of lignified cell walls of papyrus stems collected from a different location (Karlen et al., 2016), although the correlations were not assigned at that time. HMBC and HSQC-TOCSY experiments (Supplemental Figure S1) confirmed all the A- and C-ring bond connectivities and, together with the HSQC spectra of the standards, helped to assign the C2′,6′/H2′,6′ and C3′,5′/H3′,5′ correlation signals of naringenin at δC/δH 127.8/7.21 (N2′,6′) and δC/δH 114.5/6.77 (N3′,5′), as well as the C2′,6′/H2′,6′ correlations of dihydrotricin at δC/δH 103.6/6.98 (dhT2′,6′). Finally, the C6,8/H6,8 correlation signal of naringenin chalcone that appears at δC/δH 94.6/5.84 (Nch6,8) in the HSQC spectrum of the authentic standard seems to overlap with the signals of N6/dhT6 and N8/dhT8 of the flavanones naringenin and dihydrotricin in the HSQC spectrum of papyrus rind lignin. More importantly, however, the C2/H2 and C3/H3 correlation signals for the double bond, which are distinctively present in the HSQC spectrum of the authentic naringenin standard at δC/δH 141.8/7.64 (Nch2) and δC/δH 123.5/7.95 (Nch3), were not detected in the HSQC spectrum of the lignin from papyrus rind, indicating that the double bond must participate prominently in radical coupling reactions with monolignols or oligolignols (via a 3–O–4′ ether linkage, as will be shown below). If naringenin chalcone couples via a 3–O-linkage within the lignin, then the C3/H3 correlation would disappear whereas the C2/H2 correlation would shift to a different (and yet unknown) region in the NMR spectrum. This would also be in line with the release of naringenin chalcone (maintaining the double bond) upon cleavage of the 3–O–4′ ether bond by DFRC.
Figure 4.
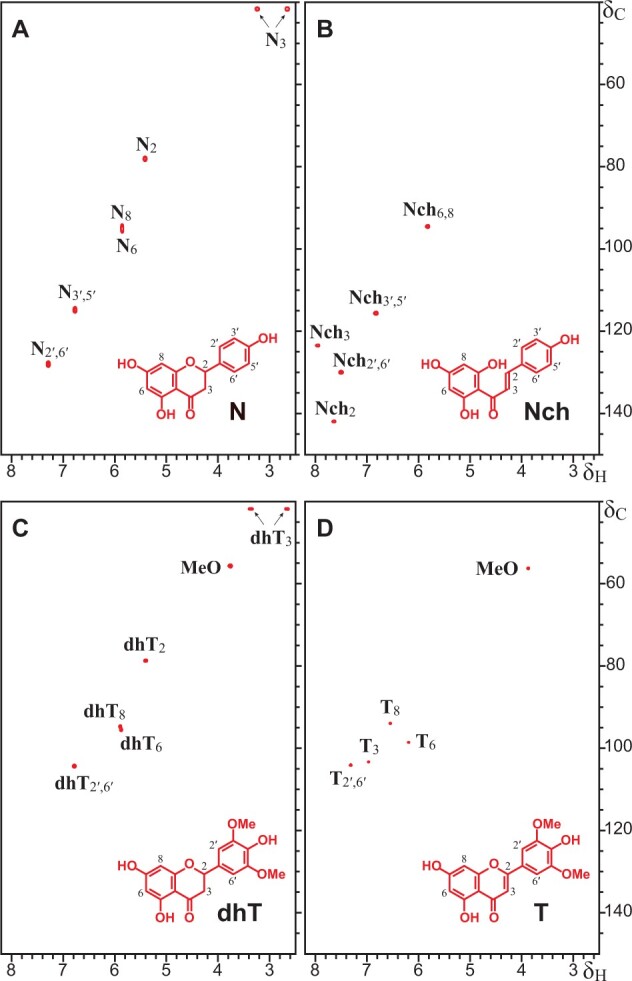
2D HSQC NMR spectra in DMSO-d6 (δC/δH 48 − 150/2.5 − 8.2) of authentic standards. (A) Naringenin, N; (B) naringenin chalcone, Nch; (C) dihydrotricin, dhT; (D) tricin, T.
The HMBC spectrum of the lignin isolated from papyrus rind also provided information on how these flavonoids are incorporated into the lignin polymer. The HMBC showed two signals for the correlations of C-2 of flavanones (at δC 78.5) with the H-2′ and H-6′ of the B-ring of the flavanones naringenin and dihydrotricin at around δH 6.98 and 6.83. These two signals appear to be a superposition of several signals that include the correlations for H-2′ and H-6′ of naringenin forming phenylcoumaran structures, as well as the correlations for H-2′,6′ of naringenin and also dihydrotricin in 4′–O–β ethers structures. The HMBC spectrum also clearly showed the cross-signals of tricin and dihydrotricin linked to the lignin through 4′–O–β ether linkages, as shown by the correlations for β-protons in β-ether units (Figure 5). However, signals for 4′–O–β ether linkages involving naringenin and naringenin chalcone could not be detected in the HMBC spectrum although their incorporation into the papyrus rind lignin through 4′–O–β linkages was supported by the release of these compounds upon DFRC. However, detailed analysis of biomimetic radical coupling products is required to fully assign the signals here.
Figure 5.
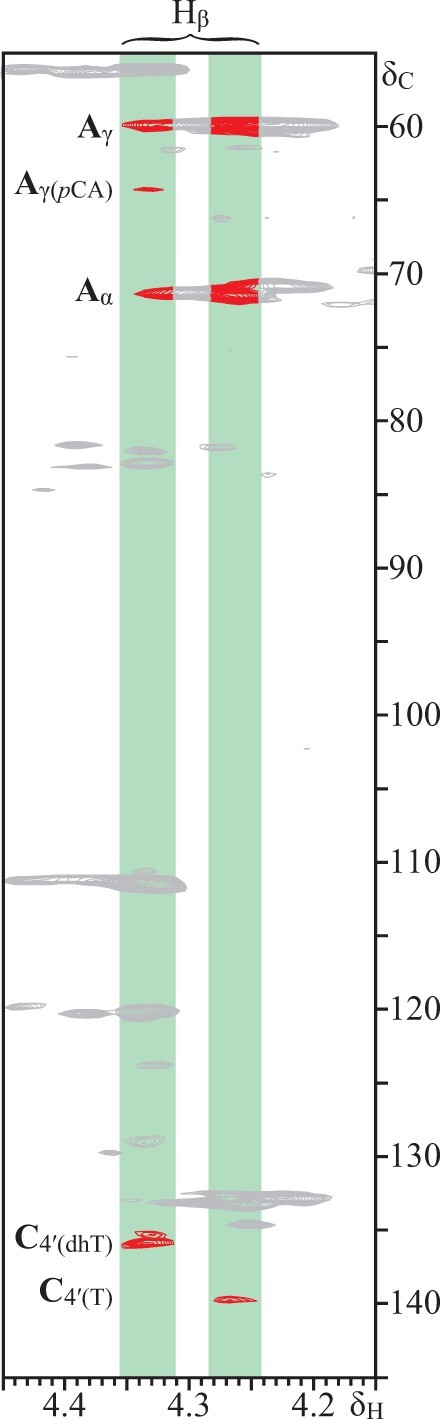
Selected region of the HMBC spectrum of the lignin from papyrus rind that shows the correlations Hβ–C4ʹ in 4′–O–β linkages, A. The correlations for dihydrotricin (dhT) and tricin (T) are indicated. pCA: p-coumarates.
Cross-coupling of naringenin, naringenin chalcone, dihydrotricin, and tricin with monolignols
Flavonoids are known to participate in oxidative radical cross-coupling reactions with monolignols to produce the so-called flavonolignans that have two phenylpropanoid units connected through a diversity of linkages, including simple 4′–O–β ethers, and phenylcoumaran structures, among others (Begum et al., 2010; Chambers et al., 2015). The occurrence of these flavonolignans is an indication that these phenolic compounds are compatible with lignification, and it is likely that the same types of linkages that occur in flavonolignans can also occur upon their incorporation into the lignin polymer during lignification.
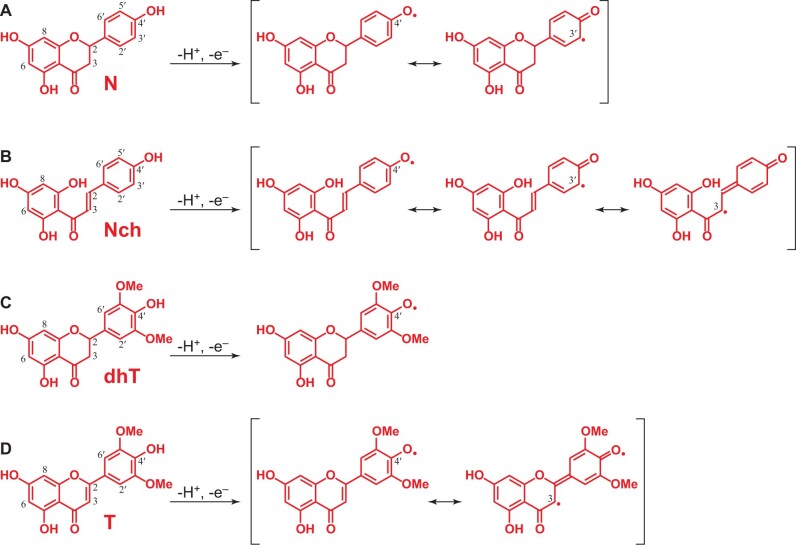
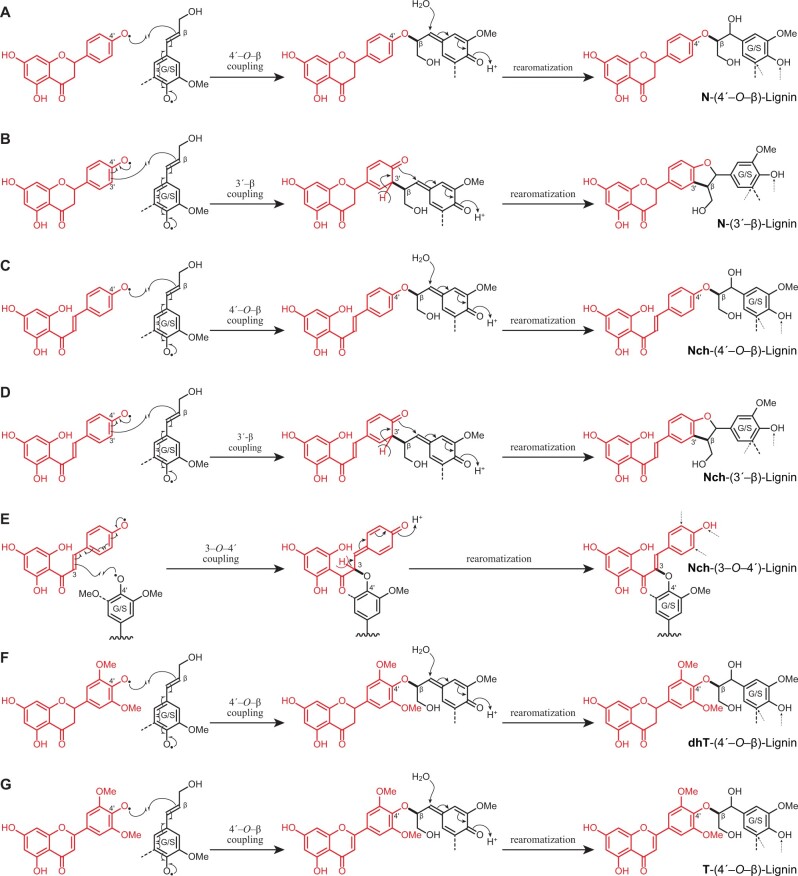
Flavonoids can be oxidized to form radicals that are stabilized by resonance, as seen in Figure 6. During lignification, these radicals will cross-couple with monolignol and oligolignol radicals producing different linkages once incorporated into the lignin. The resonance forms of the different flavonoids present in the lignin of papyrus rind show how coupling can occur primarily at the 4′–O-position of all of them. In addition, in the case of naringenin chalcone and naringenin, because their position C-3′ (and C-5′) is free, cross-coupling can additionally occur at these positions. In the case of naringenin chalcone, the radical delocalized to the double bond in the side-chain, and cross-coupling can also occur at the C-3 position; a similar resonance form may be drawn for tricin but coupling at the 3-position has not been observed (Lan et al., 2015). Hence, the different flavonoids found in the lignin of papyrus rind (naringenin chalcone, naringenin, dihydrotricin, tricin) can be incorporated into the lignin polymer through different linkages, as indicated by the mechanisms in Figure 7. As noted above, all the flavonoids identified can cross-couple with monolignols via 4′–O–β coupling, forming a para-quinone methide intermediate that can subsequently rearomatize to produce the 4′–O–β ether linkage (Figure 7, A, C, F, and G). In addition, naringenin chalcone and naringenin, which have nonsubstituted H-type B-rings, can also cross-couple with monolignols via radical coupling of their C-3′ position with the β-position of a monolignol, followed by a subsequent trapping and α–O–4′ bond formation upon rearomatization of the para-quinone methide intermediate producing a phenylcoumaran ring (pathways 7B and 7D). Moreover, in the case of naringenin chalcone, cross-coupling with generic phenolic end-units in lignin can also take place at its C-3 position, producing 3–O–4′ ether linkages and, because of the acidic β-proton elimination to rearomatize the quinone methide, maintaining the double bond (Figure 7E).
Figure 6.
One-electron oxidation of the different flavonoids and the major resonance forms of their phenolic radicals. (A) Naringenin, N; (B) naringenin chalcone, Nch; (C) dihydrotricin, dhT; (D) tricin, T.
Figure 7.
Dehydrodimerization products arising from oxidative cross-coupling of the different flavonoids (naringenin, naringenin chalcone, dihydrotricin, and tricin) with monolignols. A, 4′–O–β Coupling of naringenin and a monolignol producing aryl alkyl ethers; B, 3′–β Coupling of naringenin and a monolignol producing phenylcoumaran structures; C, 4′–O–β Coupling of naringenin chalcone and a monolignol producing aryl-alkyl ethers; D, 3′–β Coupling of naringenin chalcone and a monolignol producing phenylcoumaran structures; E, 3–O–4′ Coupling of naringenin chalcone and the growing lignin chain producing alkyl-aryl ethers and conserving the double bond; F, 4′O–β Coupling of dihydrotricin and a monolignol producing aryl-alkyl ethers; G, 4′–O–β Coupling of tricin and a monolignol producing aryl-alkyl ethers. Sites of possible further radical coupling on the products are shown via small dashed arrows; obviously coupling can only occur if the site is unoccupied, when the dashed bond is an H and not, for example, an OMe in a syringyl unit.
Biosynthesis, role of flavonoids in the lignin of papyrus rind, and prospects for metabolic engineering
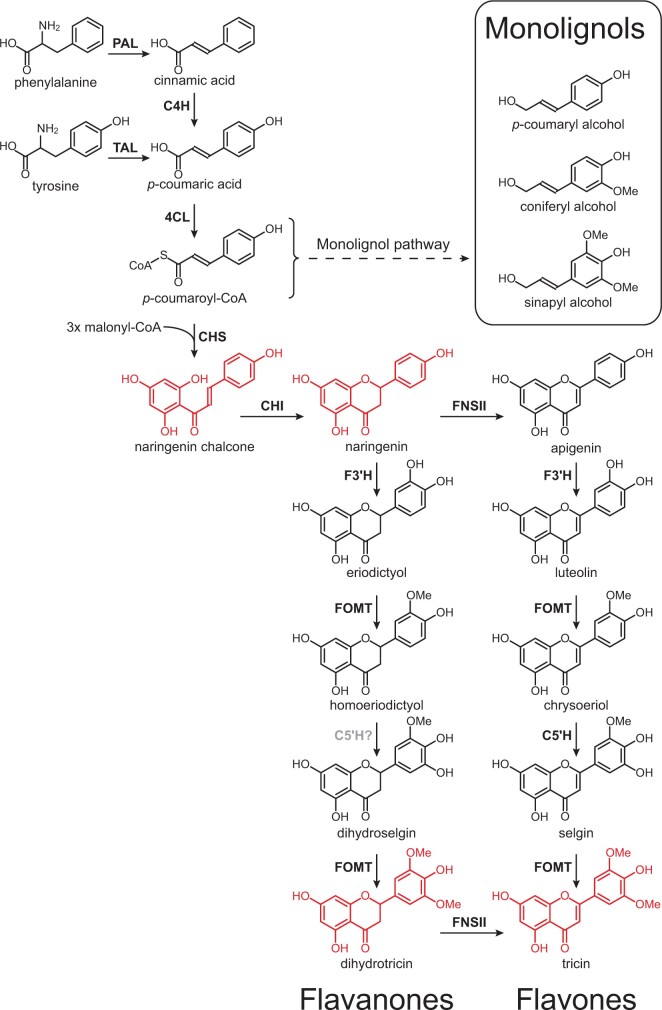
The biosynthesis of flavonoids is modulated by the enzyme chalcone synthase (CHS), responsible for converting malonyl-CoA and 4-coumaryl-CoA into naringenin chalcone, which is then transformed into naringenin by the chalcone isomerase (CHI). The flavanone naringenin is the precursor for the biosynthesis of most types of flavonoids, including the flavones and flavanones, among others (Andersen and Markham, 2006; Quideau et al., 2011; Ferreyra et al., 2012). The possible biosynthetic pathway leading to the flavonoids identified in the lignin of papyrus rind, such as to the naringenin chalcone, the flavanones naringenin and dihydrotricin, and the flavone tricin, is shown in Figure 8. The flavanone naringenin is transformed, in a reaction catalyzed by the FNSII, into the flavone apigenin, which can then undergo sequential hydroxylations and O-methylations reactions at its B-ring by different enzymes (F3′H, FOMT, C5′H) to produce luteolin, chrysoeriol, selgin, and finally tricin. Likewise, naringenin itself can also undergo sequential hydroxylations and O-methylations at the B-ring in the same manner by the same or similar enzymes producing eriodictyol, homoeriodictyol, dihydroselgin, and finally dihydrotricin. Although it is not clear if FNSII in papyrus can act on the other flavanones in the pathway to produce the respective flavones, it was already demonstrated that FNSII in other plants was able to convert different flavanones (i.e., liquiritigenin, naringenin, eriodictyol) into the corresponding flavones (Martens and Forkmann, 1999; Fliegmann et al., 2010). The occurrence of naringenin, dihydrotricin, and tricin in the papyrus lignin suggests that tricin is likely produced from naringenin through sequential hydroxylation and methylation reactions to produce dihydrotricin, which may be then converted into tricin via desaturation possibly by an FNSII enzyme.
Figure 8.
Proposed biosynthetic pathway for flavanones and flavones in papyrus. PAL, phenylalanine ammonia-lyase; TAL, tyrosine ammonia-lyase; C4H, cinnamate 4-hydroxylase; 4CL, 4-coumarate:CoA ligase; CHS, chalcone synthase; CHI: chalcone isomerase; FNSII: flavone synthase II; F3′H: flavonoid 3′-hydroxylase; FOMT: flavonoid O-methyltransferase; C5′H: crysoeriol 5′-hydroxylase. The structures of flavonoids found incorporated into the papyrus lignin are colored in red.
The flavonoids incorporated into the lignin of papyrus rind may have several roles, including UV protection agents or defense against pathogens and animal ingestion, among others (Ferreyra et al., 2012). In addition, tricin was previously suggested to have a role as an initiation site for the biosynthesis of lignin (Lan et al., 2015), and it is likely that the other flavonoids may also have a similar role as initiation sites for the synthesis of lignin in papyrus; ferulate present in grasses has long been proposed to have this role (Ralph, 2010). Without other initiation sites, the polymerization of lignin begins through the dimerization of monolignols by β–β′-, β–O–4′-, or β–5′-coupling to produce resinol, β-ether, or phenylcoumaran dimers (Ralph et al., 2004). In the case of β–β′-coupling, acylated monolignols can also dimerize but, with the monolignol acylated at the γ-OH, rearomatization of the dimeric quinone methide by attack of the γ-OH to the α-carbon of the second unit cannot occur, and β–β′ tetrahydrofurans will be the dimerization products formed instead to start the lignin chain (Lu and Ralph, 2008). However, the lignin of papyrus rind has remarkably low amounts of resinols (2% of all linkages), and γ-acylated tetrahydrofurans (which cannot be seen at this low intensity in the HSQC spectrum of the rind lignin) are present in only trace amounts (<1%). This is probably related to the fact that the lignin chains in papyrus are more commonly initiated by the ferulate and flavonoids present in the cell wall.
The discovery of this series of flavonoids in the lignin of papyrus may have other important implications. Modulating the expression of the genes involved in flavonoid biosynthesis in the papyrus plant may produce lignins with altered content of valuable flavonoids. In this sense, overexpression of the gene that encodes the CHS enzyme could result in higher flavonoid contents. Specific alterations of the biosynthetic pathways could produce lignins that accumulate desired flavonoids. For example, a recent study demonstrated that disruption of FNSII in rice plants, resulted in a lignin that incorporated the intermediate naringenin, instead of tricin (Lam et al., 2017). Moreover, apigenin was also found integrated into the lignin in mutant rice plants lacking F3′H, the enzyme that converts apigenin into luteolin, as detailed in Figure 8 (Lam et al., 2019). Furthermore, incorporation of nonconventional polyphenolic compounds, as the flavonoids described above, can suggest new alternatives to genetically modify the lignin composition and structure to obtain polymers with new or improved properties. Metabolic engineering to accumulate or introduce specific flavonoids into the plant lignins would be of great interest to obtain polyphenolic polymers with unique or enhanced properties, such as a better UV protection, antioxidant, antifungal, and antimicrobial properties. To the extent that some of these valuable components may be released from the lignin, there is the possibility of adding value to biorefinery operations.
Conclusion
Flavonoids of various classes, namely naringenin chalcone, naringenin, dihydrotricin, and tricin, were found to be monomers incorporated into the lignin polymer of papyrus rind. Identification of these flavonoids was performed by analyzing the compounds released from the lignin by the DFRC degradation method, and by 2D-NMR spectroscopic analysis, both using comparison with authentic standards. The data indicated that, whereas dihydrotricin and tricin were incorporated into the lignin exclusively through 4′–O–β ether bonds, it is reasonable to expect that naringenin chalcone and naringenin are incorporated into the lignin through other linkage types, including 3′–β linkages forming phenylcoumaran structures. In conclusion, the lignin of papyrus rind presents a unique structure with the incorporation of several flavonoids from different classes paving the way for even more wide-ranging lignin modifications in the future. However, despite the importance of the findings presented here, further research is still needed to learn more about the mechanism of flavonoid biosynthesis in this plant and its relationship to lignin production.
Materials and methods
Samples
The rind of the papyrus (Cyperus papyrus L.) used for this study was obtained from the stems of papyrus plants obtained from farmers in Qaramos, Egypt. The papyrus rind is the outer part of the stem, a highly lignified tissue made up of sclerenchyma cells and vascular bundles. The air-dried sample was milled using a knife-mill (1-mm screen) and then Soxhlet-extracted with acetone (12 h), methanol (16 h), and hot water (12 h). Klason lignin content, corrected for ash and protein content, was estimated according to the TAPPI method T222 om-88 (Tappi, 2004), having a value of 24.2 ± 0.5 (three replicates were used).
Lignin isolation and purification
The MWL was obtained from extractive-free papyrus rind according to the classical procedure (Björkman, 1956) and was purified as described elsewhere (del Río et al., 2012). The amount of purified MWL represented around 20% of the Klason lignin content.
Derivatization followed by reductive cleavage
DFRC was carried out using the experimental conditions previously described (Lu and Ralph, 1997; del Río et al., 2012). The DFRC degradation products were acetylated (acetic anhydride/pyridine, 1:1 v/v, 2 h) and analyzed by GC/MS analysis on a Shimadzu GC/MS-QP2010plus instrument using the chromatographic conditions described elsewhere (del Río et al., 2012). Naringenin and naringenin chalcone were purchased from Sigma. Tricin was synthesized as previously described (Lan et al., 2015). Dihydrotricin was not commercially available and it was synthesized as previously published (Chemler et al., 2010).
NMR spectroscopy
2D-NMR spectra (HSQC, HMBC, HSQC-TOCSY) experiments were acquired on an AVANCE III 500 MHz instrument (Bruker, Karlsruhe, Germany) equipped with a 5 mm TCI cryoprobe. Around 60 mg of lignin sample were dissolved in 0.6 mL of DMSO-d6. The central solvent peaks were used as internal references (δC/δH 39.5/2.49). The HSQC experiments used Bruker’s standard “hsqcetgpsisp2.2” pulse program, whereas the HMBC experiments used the “hmbcgplpndqf” pulse program with long-range J-coupling evolution times of 62.5 ms [JLR = 8 Hz, and/or 80 ms (JLR = 6.25 Hz)], and the HSQC-TOCSY experiments used the “hsqcdietgpsisp.2” pulse program with a TOCSY mixing time of 80 ms. The detailed experimental conditions have been described elsewhere (del Río et al., 2012).
Supplemental data
The following material is available in the online version of this article.
Supplemental Figure S1 . Selected regions of the HMBC (δC/δH 70-200/2.5-12.5) and HSQC-TOCSY (δC/δH 40-80/2.5-5.7) spectra of the MWL isolated from papyrus rind displaying the main correlations for the A- and C-rings of the flavanones naringenin (N) and dihydrotricin (dhT).
Supplementary Material
Acknowledgments
We acknowledge Dr Manuel Angulo from SGI-CITIUS-University of Seville for his help in acquiring the NMR spectra.
Funding
This work was funded by the Spanish State Research Agency and the European Regional Development Fund (project AGL2017-83036-R), the Consejería de Transformación Económica, Industria, Conocimiento y Universidades, Junta de Andalucía (project P20-00017), the DOE Great Lakes Bioenergy Research Center (DOE BER Office of Science DE-SC0018409), and the Austrian Biorefinery Center Tulln (ABCT). M.J.R. thanks the Spanish Ministry of Science, Innovation and Universities for a FPI fellowship (PRE2018-083267).
Conflict of interest statement. None declared.
J.C.dR. conceived the study and wrote the article. F.B., T.R., and A.P. obtained the initial samples. J.Re. and M.J.R. carried out the experiments, including the lignin isolation. J.C.dR. and J.Re. discovered the flavonoids incorporated into the lignin. V.I.T. synthesized the authentic dihydrotricin. A.G., H.K., V.I.T., and J.Ra. participated in designing the experimental part and in the discussion of the figures and results. All authors critically revised and accepted the final article.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://academic.oup.com/plphys/pages/general-instructions) is José C. del Río (delrio@irnase.csic.es).
References
- Andersen ØM, Markham KR (2006) Flavonoids – Chemistry, Biochemistry and Applications. CRS Press/Taylor & Francis, Boca Raton, FL [Google Scholar]
- Begum SA, Sahai M, Ray AB (2010) Non-conventional lignans: Coumarinolignans, flavonolignans, and stilbenolignans. Fort Chem Org Nat 93: 1–70 [DOI] [PubMed] [Google Scholar]
- Björkman A (1956) Studies on finely divided wood. Part I. Extraction of lignin with neutral solvents. Sven Papperstidn 59: 477–485. [Google Scholar]
- Chambers SC, Valentová K, Křen V (2015) Non-taxifolin derived flavonolignans: phytochemistry and biology. Curr Pharm Des 21: 5489–5500 [DOI] [PubMed] [Google Scholar]
- Chang CL, Wang GJ, Zhang LJ, Tsai WJ, Chen RY, Wu YC, Kuo YH (2010) Cardiovascular protective flavonolignans and flavonoids from Calamus quiquesetinervius. Phytochemistry 71: 271–279 [DOI] [PubMed] [Google Scholar]
- Chemler JA, Lim CG, Daiss JL, Koffas AG (2010) A versatile microbial system for biosynthesis of novel polyphenols with altered estrogen receptor binding activity. Chem Biol 17: 392–401 [DOI] [PubMed] [Google Scholar]
- Chen F, Tobimatsu Y, Havkin-Frenkel D, Dixon RA, Ralph J (2012) A polymer of caffeyl alcohol in plant seeds. Proc Nat Acad Sci USA 109: 1772–1777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Tobimatsu Y, Jackson L, Nakashima J, Ralph J, Dixon RA (2013) Novel seed coat lignins in the Cactaceae: structure, distribution and implications for the evolution of lignin diversity. Plant J 73: 201–211 [DOI] [PubMed] [Google Scholar]
- Csupor D, Csorba A, Hohmann J (2016) Recent advances in the analysis of flavonolignans of Silybum marianum. J Pharm Biomed Anal 130: 301–317 [DOI] [PubMed] [Google Scholar]
- del Río JC, Lino AG, Colodette JL, Lima CF, Gutiérrez A, Martínez AT, Lu F, Ralph J, Rencoret J (2015) Differences in the chemical structure of the lignins from sugarcane bagasse and straw. Biomass Bioenerg 81: 322–328 [Google Scholar]
- del Río JC, Marques G, Rencoret J, Martínez AT, Gutiérrez A (2007) Occurrence of naturally acetylated lignin units. J Agric Food Chem 55: 5461–5468 [DOI] [PubMed] [Google Scholar]
- del Río JC, Rencoret J, Gutiérrez A, Elder T, Kim H, Ralph J (2020) Lignin monomers from beyond the canonical monolignol biosynthetic pathway: Another brick in the wall. ACS Sustain Chem Eng 8: 4997–5012 [Google Scholar]
- del Río JC, Rencoret J, Gutiérrez A, Kim H, Ralph J (2017) Hydroxystilbenes are monomers in palm fruit endocarp lignins. Plant Physiol 174: 2072–2082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Río JC, Rencoret J, Gutiérrez A, Kim H, Ralph J (2018) Structural characterization of lignin from maize (Zea mays L.) fibers: Evidence for diferuloylputrescine incorporated into the lignin polymer in maize kernels. J Agric Food Chem 66: 4402–4413 [DOI] [PubMed] [Google Scholar]
- del Río JC, Rencoret J, Gutiérrez A, Kim H, Ralph J (2021) Lignin monomers derived from the flavonoid and hydroxystilbene biosynthetic pathways. InReed JD, de Freitas VAP, Quideau S, eds, Recent Advances in Polyphenol Research, Vol 7. Wiley-Blackwell Publishing, Oxford, pp 177–206 [Google Scholar]
- del Río JC, Rencoret J, Marques G, Gutiérrez A, Ibarra D, Santos JI, Jiménez-Barbero J, Zhang LM, Martínez AT (2008) Highly acylated (acetylated and/or p-coumaroylated) native lignins from diverse herbaceous plants. J Agric Food Chem 56: 9525–9534 [DOI] [PubMed] [Google Scholar]
- del Río JC, Rencoret J, Prinsen P, Martínez AT, Ralph J, Gutiérrez A (2012) Structural characterization of wheat straw lignin as revealed by analytical pyrolysis, 2D-NMR, and reductive cleavage methods. J Agric Food Chem 60: 5922–5935 [DOI] [PubMed] [Google Scholar]
- Ferreyra MLF, Rius SP, Casati P (2012) Flavonoids: biosynthesis, biological functions, and biotechnological applications. Front Plant Sci 3: 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliegmann J, Furtwängler K, Malterer G, Cantarello C, Schüler G, Ebel J, Mithöfer A (2010) Flavone synthase II (CYP93B16) from soybean (Glycine max L.). Phytochemistry 71: 508–514 [DOI] [PubMed] [Google Scholar]
- Karlen SD, Zhang C, Peck ML, Smith RA, Padmakshan D, Helmich KE, Free HCA, Lee S, Smith BG, Lu F, et al. (2016) Monolignol ferulate conjugates are naturally incorporated into plant lignins. Sci Adv 2: e1600393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam PY, Lui ACW, Yamamura M, Wang L, Takeda Y, Suzuki S, Liu H, Zhu FY, Chen MX, Zhang J, et al. (2019) Recruitment of specific flavonoid B-ring hydroxylases for two independent biosynthesis pathways of flavone-derived metabolites in grasses. New Phytol 223: 204–219 [DOI] [PubMed] [Google Scholar]
- Lam PY, Tobimatsu Y, Takeda Y, Suzuki S, Yamamura M, Umezawa T, Lo C (2017) Disrupting Flavone Synthase II alters lignin and improves biomass digestibility. Plant Physiol 174: 972–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan W, Lu F, Regner M, Zhu Y, Rencoret J, Ralph SA, Zakai UI, Morreel K, Boerjan W, Ralph J (2015) Tricin, a flavonoid monomer in monocot lignification. Plant Physiol 167: 1284–1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan W, Morreel K, Lu F, Rencoret J, del Río JC, Vooren W, Vermerris W, Boerjan W, Ralph J (2016a) Maize tricin-oligolignol metabolites and their implications for monocot lignification. Plant Physiol 171: 810–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan W, Rencoret J, Lu F, Karlen S, Smith BG, Harris PJ, del Río JC, Ralph J (2016b) Tricin-lignins: occurrence and quantitation of tricin in relation to phylogeny. Plant J 88: 1046–1057 [DOI] [PubMed] [Google Scholar]
- Lu F, Ralph J (1997) Derivatization followed by reductive cleavage (DFRC method), a new method for lignin analysis: Protocol for analysis of DFRC monomers. J Agric Food Chem 45: 2590–2592 [Google Scholar]
- Lu F, Ralph J (2008) Novel tetrahydrofuran structures derived from β–β-coupling reactions involving sinapyl acetates in kenaf lignins. Org. Biomol. Chem. 6, 3681–3694. [DOI] [PubMed] [Google Scholar]
- Lu F, Karlen S, Regner M, Kim H, Ralph S, Sun RC, Kuroda K, Augustin M, Mawson R, Sabarez H, et al. (2015) Naturally p-hydroxybenzoylated lignins in palms. Bioenergy Res 8: 934–952 [Google Scholar]
- Lui ACW, Lam PY, Chan KH, Wang L, Tobimatsu Y, Lo C (2020) Convergent recruitment of 50-hydroxylase activities by CYP75B flavonoid B-ring hydroxylases for tricin biosynthesis in Medicago legumes. New Phytol 228: 269–284 [DOI] [PubMed] [Google Scholar]
- Martens SG, Forkmann G (1999) Cloning and expression of flavone synthase II from Gerbera hybrids. Plant J 20: 611–618 [DOI] [PubMed] [Google Scholar]
- Negrel J, Jeandet P (1987) Metabolism of tyramine and feruloyltyramine in TMV inoculated leaves of Nicotiana tabacum. Phytochemistry 26: 2185–2190 [Google Scholar]
- Negrel J, Pollet B, Lapierre C (1996) Ether-linked ferulic acid amides in natural and wound periderms of potato tuber. Phytochemistry 43: 1195–1199 [Google Scholar]
- Neiva D, Rencoret J, Marques G, Gutiérrez A, Gominho J, Pereira H, del Río JC (2020) Lignin from tree barks: chemical structure and valorization. ChemSusChem 13: 4537–4547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quideau S, Deffieux D, Douat-Casassus C, Pouysegu L (2011) Plant polyphenols: chemical properties, biological activities, and synthesis. Angew Chem Int Ed 50: 586–621 [DOI] [PubMed] [Google Scholar]
- Ralph J (2010) Hydroxycinnamates in lignification. Phytochem Rev 9: 65–83 [Google Scholar]
- Ralph J, Hatfield RD, Piquemal J, Yahiaoui N, Pean M, Lapierre C, Boudet AM (1998) NMR characterization of altered lignins extracted from tobacco plants down-regulated for lignification enzymes cinnamyl-alcohol dehydrogenase and cinnamoyl-CoA reductase. Proc Natl Acad Sci USA 95: 12803–12808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph J, Lapierre C, Boerjan W (2019) Lignin structure and its engineering. Curr Opin Biotechnol 56: 240–249 [DOI] [PubMed] [Google Scholar]
- Ralph J, Lundquist K, Brunow G, Lu F, Kim H, Schatz PF, Marita JM, Hatfield RD, Ralph SA, Christensen JH, et al. (2004) Lignins: natural polymers from oxidative coupling of 4-hydroxyphenylpropanoids. Phytochem Rev 3: 29–60 [Google Scholar]
- Rencoret J, Kim H, Evaristo AB, Gutiérrez A, Ralph J, del Río JC (2018) Variability in lignin composition and structure in cell walls of different parts of macaúba (Acrocomia aculeata) palm fruit. J Agric Food Chem 66: 138–153 [DOI] [PubMed] [Google Scholar]
- Rencoret J, Neiva D, Marques G, Gutiérrez A, Kim H, Gominho J, Pereira H, Ralph J, del Río JC (2019) Hydroxystilbene glucosides are incorporated into Norway spruce bark lignin. Plant Physiol 180: 1310–1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rencoret J, Marques G, Serrano O, Kaal J, Martínez AT, del Río JC, Gutiérrez A (2020) Deciphering the unique structure and acylation pattern of Posidonia oceanica lignin. ACS Sustain Chem Eng 8: 12521–12533 [Google Scholar]
- Rencoret J, Ralph J, Marques G, Gutiérrez A, Martínez AT, del Río JC (2013) Structural characterization of the lignin from coconut (Cocos nucifera) coir fibers. J Agric Food Chem 61: 2434–2445 [DOI] [PubMed] [Google Scholar]
- Tappi (2004) Tappi Test Methods 2004-2005. Tappi Press, Norcoss, GA. [Google Scholar]
- Tobimatsu Y, Chen F, Nakashima J, Escamilla-Treviño LL, Jackson L, Dixon RA, Ralph J (2013) Coexistence but independent biosynthesis of catechyl and guaiacyl/syringyl lignin polymers in seed coats. Plant Cell 25: 2587–2600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanholme R, De Meester B, Ralph J, Boerjan W (2019) Lignin biosynthesis and its integration into metabolism. Curr Opin Biotechnol 56: 230–239 [DOI] [PubMed] [Google Scholar]
- Vanholme R, Demedts B, Morreel K, Ralph J, Boerjan W (2010) Lignin biosynthesis and structure. Plant Phys 153: 895–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.