Abstract
This meta-analysis aims to evaluate the effects of exercise in improving cardiometabolic risk factors in overweight children and adolescents until the adolescent age, which is 18 years. A systemic search was conducted using the electronic databases PubMed/Medline, Cochrane Library, and Google Scholar, from inception to 29 June 2021. All statistical analyses were conducted in Review Manager 5.4.1. All studies meeting the inclusion criteria were selected. A random-effect model was used to pool the studies, and the results are reported in the odds ratio (OR) and corresponding 95% Confidence interval (CI). Twelve randomized control trials were selected for meta-analysis. Significant results were obtained for BMI in children after the interventions (0.38 95% CI 0.14, 0.62; p = 0.002; I2 = 65%). LDL level was also found significantly reduced (0.41 95% CI 0.01, 0.82; p = 0.05; I2 = 83%). Other factors such as HDL level, blood pressure, blood glucose level, body weight, and waist circumference were also analyzed. We found that exercise interventions significantly improved several cardiometabolic risk factors such as BMI, LDL level, BP, and blood glucose level. However, no significant effect on HDL concentration, waist circumference, and body weight were found. Long-term interventions are needed to attain improvement in all cardiometabolic risk factors.
Keywords: physical stress, cardiometabolic, risk factors, long term intervention
1. Introduction
In the past 50 years, pediatric obesity (PO) has reached prevalence and severity levels worldwide, resulting in adverse effects on physical and mental health [1,2,3]. Furthermore, there is a significantly increased risk of developing cardiometabolic diseases, such as diabetes mellitus type 2, hypertension, coronary artery disease, and stroke as a consequence of pediatric obesity [4,5]. A high percentage of visceral abdominal fat is demonstrated to exacerbate hyperlipidemia and hypertension and contribute significantly to insulin resistance [6,7,8]. An extensive capillary network surrounds the adipose tissue, resulting in increased blood volume and cardiac output, affecting the cardiac workload [9,10]. The adipose tissue is also a significant source of interleukin-6 (IL-6), lipoprotein lipase, estrogen, angiotensinogen, adiponectin, leptin, insulin binding protein-3, and tumor necrosis factor- α [11,12]. IL-6 leads to a chronic inflammatory state that can trigger acute coronary syndrome. These properties of the adipose tissue increase the risk for cardiometabolic syndromes [13,14,15].
Fang et al., in their two-sample Mendelian randomization study, found a significant relationship between raised BMI and diabetes type 2 by analyzing the characteristics of nine single-nucleotide polymorphisms associated with increased risk of childhood obesity and increased risk of type 2 diabetes, glucose intolerance, insulin resistance, high blood pressure, and atherosclerosis [16]. In addition, obese children are often socially stigmatized and have psychological problems such as anxiety, depression, stress, low self-esteem, distorted body image, difficulties linked to being bullied, social withdrawal, and low quality of life, which results in poor school performance and lack of concentration [17,18,19,20].
Both genetic and environmental factors are found to influence PO. Pathological causes include hypothyroidism, Cushing syndrome, growth hormone deficiency, and central nervous system tumors such as craniopharyngioma [21,22,23,24,25]. Environmental factors such as consumption of fat-rich foods, lack of physical activity, and unhealthy lifestyle, biobehavioral and sociodemographic factors and mental health issues additionally contribute to cardiometabolic risk factors (CMRFs) [26]. Several gestational factors such as small or large gestational age, maternal smoking, maternal obesity, and Cesarean section are also found to increase the risk of childhood obesity [27,28]. Rare genetic defects in the leptin–melanocortin regulatory pathway result in obesity [16]. Body mass index (BMI) and the levels of high-density lipoprotein (HDL) and low-density lipoprotein (LDL) are widely used as strong and independent predictors of cardiovascular events [29,30,31].
Multidisciplinary interventions are considered effective in reducing CMRFs in overweight children and adolescents (overweight being defined as BMI between 25 and 30). These approaches primarily focus on weight reduction and lifestyle modification [32,33,34,35]. In a systematic review published by Rajjo et al., the cardiometabolic outcomes were significantly reduced with weight and BMI reduction. It was found that a reduction in BMI by 1.6 kg/m2 was associated with a reduction by 10 mmHg of systolic blood pressure and by 16 mg/dL of triglycerides and with an increase by 1.7 mg/dL of HDL [36]. The core elements of these programs include exercise and diet; other interventions such as pharmacological medications, psychotherapy, and surgery are less preferred [33,34]. A meta-analysis in 2019 concluded that exercise interventions in overweight children improve body composition by lowering body fat, blood glucose, BMI, and waist circumference [37]. High-intensity interval training (HIIT) is a popular multidisciplinary approach. HIIT was found to significantly improve aerobic capacity levels in overweight youth [38,39,40].
However, despite several previous studies, the results were not consistent or were inconclusive regarding the role of multidisciplinary interventions on HDL, LDL, and blood glucose levels in children. Our aim and objective for conducting this meta-analysis were to evaluate the effectiveness of exercise in reducing body weight, BMI, diastolic blood pressure (DBP), systolic blood pressure (SBP), HDL, LDL, and blood glucose, and waist circumference in overweight children and adolescents.
2. Materials and Methods
2.1. Data Sources and Search Strategy
This systematic literature search was conducted according to the Preferred Reporting items for Systematic Review and Meta-analyses (PRISMA) guidelines [41]. An electronic investigation from PubMed/Medline, Cochrane Library, and Google Scholar was conducted from their inception to 29 June 2021 (detailed strategy provided in Table 1), using the search string: ((physical exercise OR workout OR physical exertion) AND (cardiometabolic)) AND (risk factors OR predisposing Factor OR predictive factors) AND (children OR young OR adolescent OR youth OR teenagers). In addition, we manually screened the articles cited in previous meta-analyses, randomized controlled trials, cohort trials, and review articles to select all relevant studies.
Table 1.
Detailed search strategy.
| Search Engine | Search Strategy |
|---|---|
| Pubmed/Medline | (“exercise”[MeSH Terms] OR “exercise”[All Fields] OR (“physical”[All Fields] AND “exercise”[All Fields]) OR “physical exercise”[All Fields] OR (“workout”[All Fields] OR “workouts”[All Fields]) OR (“physical exertion”[MeSH Terms] OR (“physical”[All Fields] AND “exertion”[All Fields]) OR “physical exertion”[All Fields])) AND (“cardiometabolic”[All Fields] OR “cardiometabolically”[All Fields]) AND (“risk factors”[MeSH Terms] OR (“risk”[All Fields] AND “factors”[All Fields]) OR “risk factors”[All Fields] OR (“causality”[MeSH Terms] OR “causality”[All Fields] OR (“predisposing”[All Fields] AND “factor”[All Fields]) OR “predisposing factor”[All Fields]) OR ((“predict”[All Fields] OR “predictabilities”[All Fields] OR “predictability”[All Fields] OR “predictable”[All Fields] OR “predictably”[All Fields] OR “predicted”[All Fields] OR “predicting”[All Fields] OR “prediction”[All Fields] OR “predictions”[All Fields] OR “predictive”[All Fields] OR “predictively”[All Fields] OR “predictiveness”[All Fields] OR “predictives”[All Fields] OR “predictivities”[All Fields] OR “predictivity”[All Fields] OR “predicts”[All Fields]) AND (“factor”[All Fields] OR “factor s”[All Fields] OR “factors”[All Fields]))) AND (“child”[MeSH Terms] OR “child”[All Fields] OR “children”[All Fields] OR “child s”[All Fields] OR “children s”[All Fields] OR “childrens”[All Fields] OR “childs”[All Fields] OR (“young”[All Fields] OR “youngs”[All Fields]) OR (“adolescences”[All Fields] OR “adolescency”[All Fields] OR “adolescent”[MeSH Terms] OR “adolescent”[All Fields] OR “adolescence”[All Fields] OR “adolescents”[All Fields] OR “adolescent s”[All Fields]) OR (“adolescent”[MeSH Terms] OR “adolescent”[All Fields] OR “youth”[All Fields] OR “youths”[All Fields] OR “youth s”[All Fields]) OR (“adolescent”[MeSH Terms] OR “adolescent”[All Fields] OR “teenage”[All Fields] OR “teenager”[All Fields] OR “teenagers”[All Fields] OR “teenaged”[All Fields] OR “teenager s”[All Fields] OR “teenages”[All Fields])) |
| Cochrane | ((physical exercise OR workout OR physical exertion) AND (cardiometabolic)) AND (risk factors OR predisposing Factor OR predictive factors) AND (children OR young OR adolescent OR youth OR teenagers) |
| Google Scholar | ((physical exercise OR workout OR physical exertion) AND (cardiometabolic)) AND (risk factors OR predisposing Factor OR predictive factors) AND (children OR young OR adolescent OR youth OR teenagers) |
2.2. Study Selection
All studies were included if they met the following eligibility criteria described as PICOS: (a) P (Participants) children and adolescents aged less than 18 years who were overweight; (b) I (Intervention) exercise or any other intervention leading to physical stress; C (Control) baseline values of the participants were considered as controls; (c) O (Outcome) effect of physical factors on cardiometabolic factors (body weight, BMI, diastolic blood pressure, systolic blood pressure, high-density lipoprotein, low-density lipoprotein, and blood glucose levels, and waist circumference); (d) S (Studies) articles with more than 5 participants who were overweight, with full follow-ups, excluding editorials, animal studies, observational studies, review articles, case reports, and case series.
Prisma checklist is added at the end of the study.
2.3. Statistical Analysis
Review Manager (RevMan) [Computer program]. Version 5.4. The Cochrane Collaboration, 2020, was used for all statistical analyses. The data from studies were pooled using a random-effects model to calculate the odds ratio (OR) with respective 95% confidence intervals (CI). Odds Ratio was used to describe the statistical probability of how physical stress affect cardiometabolic factors. The chi square test was performed to assess any differences between the subgroups. Sensitivity analysis was done to see if any individual study drove the results and explore reasons for high heterogeneity. As per the Cochrane handbook, the scale for heterogeneity was as follows: I2 = 25–60%—moderate; 50–90%—substantial; 75–100%—considerable heterogeneity; p < 0.1 indicated significant heterogeneity [42]. A p < 0.05 was considered significant for all analyses.
2.4. Data Extraction and Quality Assessment of the Studies
An independent search of the electronic databases was done. Studies searched were exported to the EndNoteTM 20.0.1 (Clarivate Analytics, Philadelphia, PA, USA), and duplicates were screened and removed.
Data extraction and quality assessment of the included studies were done simultaneously. The Physiotherapy Evidence Database (PEDro) scale was used, which is known as a valid and reliable instrument to assess allocation to groups, blinding of allocation, and comparison between groups at baseline and its outcomes. This scale includes 11 questions with yes or no answers (yes = 1; no = 0), providing a total score which ranges between 0 (poor methodological quality) and 10 (excellent methodological quality; the first item is not included in the rating)). It suggests that the “level of evidence” be inserted. Based on the physiotherapy evidence database scale and in order to assess the evidence of the interventions, the Van Tulder criteria should be applied, according to which the selected studies were grouped by levels of evidence, on the basis of their methodological quality. A study with a physiotherapy evidence database score of 6 or more is considered level 1 (high methodological quality) (6–8: good, 9–10: excellent), while a study with a score of 5 or less is considered level 2 (low methodological quality)) (4–5: moderate; <4: poor). Details of quality assessment are provided in Table 2.
Table 2.
Quality assessment of the selected articles (PEDro Scale).
| Authors | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | Total Score | Methodological Quality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Seo et al. [43] | Y | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 8 | High |
| Dias et al. [44] | Y | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 | High |
| Hobkrik et al. [45] | Y | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 | High |
| Paahoo et al. [46] | Y | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 | High |
| Kamal et al. [47] | Y | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 4 | Low |
| Branco et al. [48] | Y | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 | High |
| Smith et al. [49] | Y | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 | High |
| Seabra et al. [50] | Y | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 4 | Low |
| Shalitin et al. [51] | Y | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 | High |
Abbreviations: 1, Eligibility; 2, Random allocation; 3, Concealed allocation; 4, Baseline comparability; 5, Blind subjects; 6, Blind therapists; 7, Blind assessors; 8, Adequate follow-up; 9, Intention-to-treat analysis; 10, Between-group comparisons; 11, Point estimates and variability; Y, yes; N, No. Note: Eligibility criteria item does not contribute to the total score.
3. Results
3.1. Literature Search Results
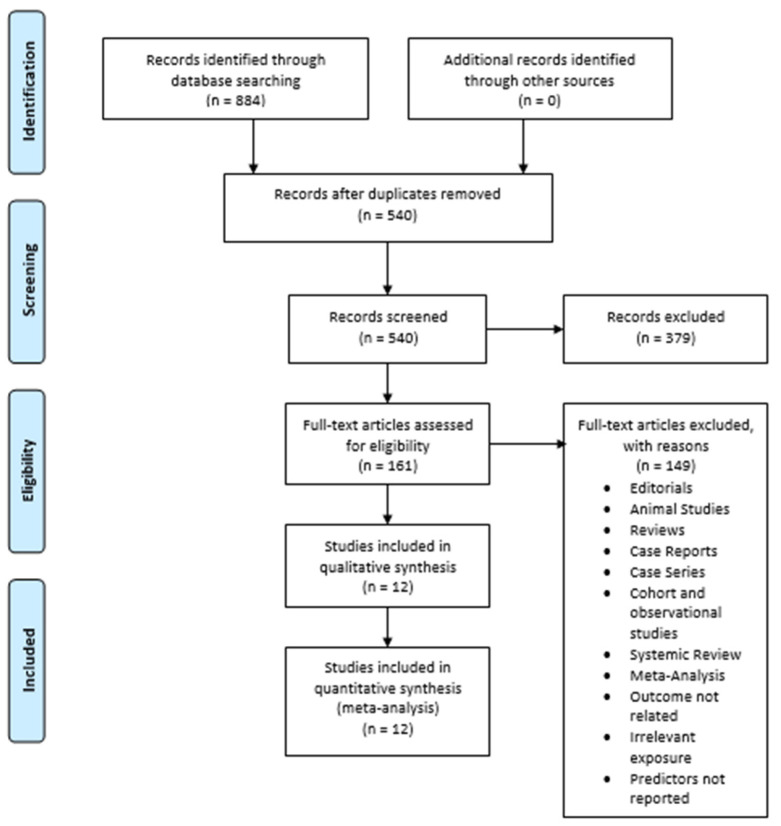
The initial search of the three electronic databases yielded 884 potential studies. After exclusions based on titles and abstracts, the full text of 161 studies was read for possible inclusion. A total of 12 studies were selected for quantitative analysis [43,44,45,46,47,48,49,50,51,52,53,54]. Figure 1 summarizes the results of our literature search.
Figure 1.
Prisma flow chart.
3.2. Study Characteristics
Table 3 and Table 4 provides the basic characteristics of the included studies and a description of the interventions used. Twelve studies had a total of 479 participants. The baseline values of the participants were considered as control values, and the values of factors after intervention were considered as experimental values. We analyzed eight factors: Body weight, BMI, Diastolic blood pressure, Systolic blood pressure, High-density lipoprotein, Low-density lipoprotein, Blood glucose, and Waist circumference (Table 5). In addition, a comparative analysis between the factors’ baseline and post-intervention values was carried out.
Table 3.
Characteristics of the included studies.
| Author | Country | Total Sample by Gender (n) | Age (Years) (M ± SD) | Groups (n) | Body Fat (%) | Body Mass (kg) | BMI (kg/m2) | Registered Protocol |
|---|---|---|---|---|---|---|---|---|
| Seo et al., 2019 [43] | Korea | 25F | CG = 12.09 ± 2.20 | CG = 44 | CG = 41.26 ± 4.25 | CG = 72.1 ± 19.88 | CG = 29.43 ± 4.81 | Yes |
| EG = 12.8 ± 1.72 | EG = 26 | EG = 41.77 ± 4.23 | EG = 77.4 ± 11.34 | EG = 30.06 ± 3.40 | ||||
| Dias et al. 2017 [44] | Australia | 103F | CG = 11.5 ± 2.4 | CG = 100 | CG = 44.1 ± 6.2 | CG = 31.3 ± 10.6 | CG = 29.5 ± 4.4 | Yes |
| EG = 12.0 ± 2.3 | EG = 99 | EG = 19.5 ± 7.5 | EG = 7.7 ± 3.6 | EG = 17.6 ± 2.1 | ||||
| Hobkrik et al., 2016 [45] | United Kingdom | N/R | N/R | 75 | N/R | 94.2 ± 22.1 | 34. 2 ± 6.4 | N/R |
| Pahoo et al., 2020 [46] | Iran | N/R | CG = 11.20 ± 0.94 | CG = 15 | CG = 27.88 ± 1.06 | CG = 54.20 ± 4.45 | CG = 25.02 ± 1.89 | N/R |
| HIITG = 11.13 ± 0.99 | HIITG = 15 | HIITG = 28.04 ± 1.46 | HIITG = 56.00 ± 4.51 | HIITG = 25.33 ± 1.01 | ||||
| AG = 10.86 ± 1.06 | AG = 15 | AG = 27.87 ± 1.06 | AG = 55.06 ± 3.75 | AG = 25.01 ± 0.69 | ||||
| Kamal et al., 2012 [47] | Egypt | 40F | CG = 10.1 ± 1.21 | CG = 49 | N/R | CG = 62.6 ± 4.1 | CG = 67.1 ± 6.3 | N/R |
| EG without MS = 10.2 ± 1.2 | EG without MS = 32 | EG without MS = 50.2 ± 3.7 | EG without MS = 17.2 ± 2.5 | |||||
| EG with MS = 11.04 ± 1.15 | EG with MS = 12 | EG with MS = 67.1 ± 6.3 | EG with MS = 29.4 ± 4.8 | |||||
| Branco et al., 2016 [48] | Brazil | N/R | FG = 16 ± 1 | FG = 9 | FG = 43.9 ± 4 | FG = 99 ± 20.5 | FG = 34.7 ± 3.8 | Yes |
| WG = 16 ± 1 | WG = 9 | WG = 38.7 ± 9.2 | WG = 97.8 ± 24.2 | WG = 33.2 ± 8.3 | ||||
| Smith et al., 2017 [49] | United Kingdom | 20F | CG = 16.8 ± 0.5 | CG = 30 | N/R | CG = 66.2 ± 13.8 | CG = 21.8 ± 2.1 | N/R |
| EG = 17 ± 0.3 | EG = 22 | EG = 67.1 ± 14.4 | EG = 22.5 ± 2.5 | |||||
| Seabra et al., 2020 [50] | Germany | 40M | CG = 10.1 ± 1.5 | CG = 20 | CG = 35.1 ± 8.3 | CG = 57.6 ± 15.7 | N/R | N/R |
| EG = 10.5 ± 1.5 | EG = 20 | EG = 34.4 ± 6 | EG = 54.5 ± 13.4 | |||||
| Shalitin et al., 2009 [51] | Israel | 81F | EG = 8.21 ± 1.78 | EG = 52 | EG = 41.2 ± 1.36 | EG = 46 ± 1.6 | EG = 25.5 ± 0.52 | N/R |
| D + EG = 8.2 ± 1.56 | D + EG = 55 | D + EG = 41.3 ± 1.3 | D + EG = 46.4 ± 1.51 | D + EG = 25.9 ± 0.51 | ||||
| DG = 8.51 ± 1.52 | DG = 55 | DG = 43.1 ± 1.24 | DG = 47.5 ± 1.52 | DG = 25.5 ± 0.52 | ||||
| Sigal et al., 2014 [52] |
Canada | 213F | AG = 15.5 ± 1.4 | AG = 75 | AG = 47.1 ± 1.3 | AG = 97.1 ± 1.8 | AG = 34.7 ± 0.5 | N/R |
| RG = 15.9 ± 1.5 | RG = 78 | RG = 48 ± 1.3 | RG = 100.1 ± 1.7 | RG = 35.1 ± 0.5 | ||||
| CTG = 15.5 ± 1.3 | CTG = 75 | CTG = 48.4 ± 1.3 | CTG = 97.8 ± 1.8 | CTG = 34.7 ± 0.5 | ||||
| CG = 15.6 ± 1.3 | CG = 76 | CG = 46.6 ± 1.3 | CG = 97.9 ± 1.8 | CG = 34.2 ± 0.5 | ||||
| Biljon et al., 2018 [53] | South Africa | 67F | 11.1 ± 0.8 | MICTG = 29 | N/R | MICTG = 41.0 ± 10.6 | MICTG = 19.2 ± 3.5 | N/R |
| HIITG = 29 | HIITG = 41.0 ± 10.6 | HIITG = 18.5 ± 3.2 | ||||||
| MICTG + HIITG = 27 | MICTG + HIITG = 38.7 ± 9.3 | MICTG + HIITG = 18.0 ± 3.8 | ||||||
| CG = 24 | CG = 42.6 ± 9.6 | CG = 20.3 ± 3.7 | ||||||
| Brand et al., 2020 [54] | Brazil | 22F | CG = 8.27 | CG = 18 | CG = 14.13 | CG = 39.29 | CG = 21.88 | Yes |
| EG = 8.17 | EG = 17 | EG = 13.6 | EG = 40.9 | EG = 21.97 |
Abbreviations: N, number; M, mean; SD, standard deviation; CG, control group; EG, experimental group; F, female; M, male; USA, united states; BMI, body mass index; kg, kilograms; NR, not reported; HIITG, high-intensity interval training group; AG, aerobic group; MS, metabolic syndrome; FG, functional group; WG, weight training group; D, diet; DG, diet group; CTG, combined training group MIITG, moderate intensity interval training group.
Table 4.
Description of the interventions performed in the included studies.
| Author | Exercise Modality | Exercise (Names) | Frequency (Days/Week) | Intensity | Sets/Exercise (n) | Reps per Set (n) | Rest | Intervention Duration (Weeks) | Session Duration (min) | Eccentric Velocity (s) | Supervised? |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Seo et al., 2019 [43] | Cardio training | Aerobic exercise and ICAAN exercise | 3 | moderate-intensity intervention | N/R | N/R | 30–40 s | 16 | 60 | N/R | Yes |
| Dias et al. [44] | Cardio training | treadmill | 3 | High- and Moderate-Intensity Interval Training | N/R | N/R | 3 min | 12 weeks | 36 (High intensity) 44 (Moderate intensity) |
N/R | Yes |
| Hobkrik et al. [45] | Cardio training | N/R | N/R | N/R | N/R | N/R | N/A | 4 | N/R | N/R | Yes |
| Pahoo et al. [46] | Cardio training and Flexibility | warm-up and other exercise | 3 | High-Intensity Interval Training and Aerobic Exercise | 3 | N/R | 5 min | 12 | 45 | N/R | Yes |
| Kamal et al. [47] | Cardio training | warm-up, walking–jogging, and relaxation exercises | 3 | N/R | N/R | N/R | N/R | 12 weeks | 30–65 | N/R | Yes |
| Branco et al. [48] | Cardio training and Strength training | warm-up, CP, HS, SP, Leg extension, Triceps pulley, ACM, OAM, Scott curl machine, Aerobics, CRM, Incline row, Leg curl, LCGP, Push-ups, SWS, MBV, GUD, Triceps bench dips, SSU, HLKF, RTRX, TPNR, BTRX, OSSB | 3 | High- and Moderate- Intensity Interval Training | 3 | N/R | 3 min | 12 weeks | 46 | N/R | |
| Smith et al. [49] | Cardio training | “all out” running sprints | 3 | Sprint Interval Training | 5–6 | N/R | 30 s | 4 weeks | 4.5–5.5 | N/R | Yes |
| Seabra et al. [50] | Cardio training | soccer | 2 | N/R | N/R | N/R | 10 min | 6 months | 60–90 | N/R | Yes |
| Shalitin et al. [51] | Cardio training and Strength training | Sports, running games, sit-ups, hand-lifting of small weights, and ball exercise | 3 | Aerobic exercise and resistance training exercises | N/R | N/R | N/R | 12 weeks | 45 (each group) | N/R | Yes |
| Sigal et al. [52] |
Cardio training and Strength training | Gymnasiums, weight machines or free weights | 2–4 | aerobic and resistance training | 2–3 | 8–15 | N/R | 6 months | 20–45 | N/R | Yes |
| Biljon et al. [53] | Cardio training | Warm-up and cool-down periods consisting of jogging at a low intensity, followed by static stretching |
3 | High- and Moderate- Intensity Interval Training | N/R | N/R | 5 min | 5 weeks | 23 (High intensity) 33 (Moderate intensity) |
N/R | Yes |
| Brand et al. [54] | Multicomponent | Exercise | 2 | N/R | N/R | N/R | N/R | 12 weeks | 60 | N/R | Yes |
Abbreviations: N, number; M, mean; SD, standard deviation; CG, control group; EG, experimental group; F, female; M, male; BMI, body mass index; kg, kilograms; NR, not reported. CP, Chest press; SP, Shoulder press; HS. Hack squat; ACM, Abdominal crunch machine; OAM, Oblique abdominal machine; CRM, Calf raise machine; LCGP, Lever close grip pulldown; SWS, Squat with Swiss-ball on the back; MBV, Medicine ball vertical throw; GUD, Go up and down a plinth; SSU, Straight sit-ups on ball; HLKF, Hip lift with knee flexion on Swiss-ball; RTRX, Row on TRX with supinated grip; BTRX, Biceps curl on TRX; OSSB, Oblique sit-ups on Swiss-ball; TPNR, Tire pulling with naval rope.
Table 5.
Details about the selected factors.
| Factors | No. of Studies | Odds Ratio (OR 95% CI) | p-Value | Heterogeneity (I2) |
|---|---|---|---|---|
| (n) | (%) | |||
| Body weight | 7 | 0.11 95% CI −0.05, 0.28 | 0.18 | 27 |
| BMI | 8 | 0.38 95% CI 0.14, 0.62 | 0.002 | 65 |
| Diastolic blood pressure | 8 | 0.71 95% CI 0.11, 1.31 | 0.02 | 93 |
| Systolic blood pressure | 8 | 0.89 95% CI −0.02, 1.80 | 0.05 | 97 |
| High-density lipoprotein | 10 | −0.48 95% CI −1.06, 0.11 | 0.11 | 92 |
| Low-density lipoprotein | 9 | 0.41 95% CI 0.01, 0.82 | 0.05 | 83 |
| Blood glucose | 7 | 0.58 95% CI 0.13, 1.03 | 0.01 | 85 |
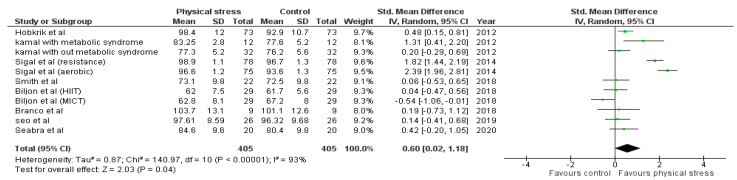
| Waist circumference | 8 | 0.60 95% CI 0.02, 1.18 | 0.04 | 93 |
3.3. Publication Bias Assessment
As no factor was analyzed in more than 10 studies, no funnel plot is presented to show publication bias.
3.4. Results of the Meta-Analysis
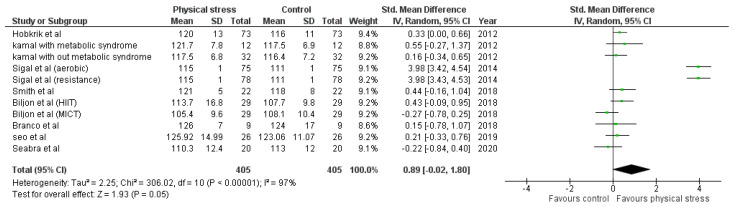
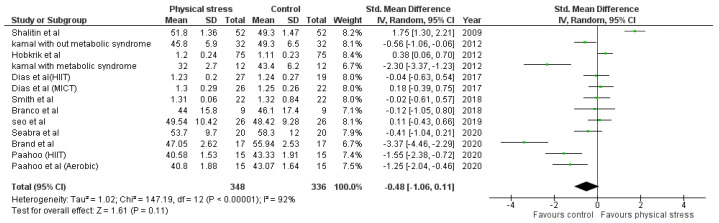
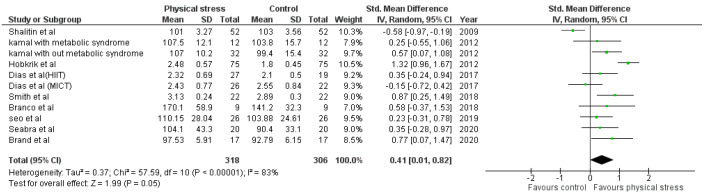
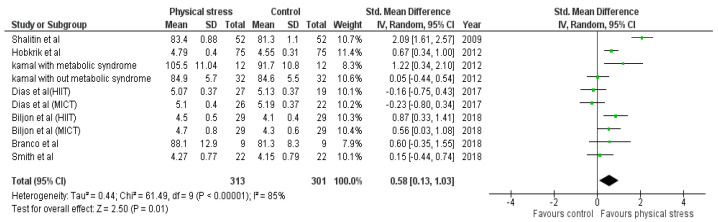
Detailed forest plots outlining the effect size of Body weight (Figure 2), Body mass index (Figure 3), Diastolic blood pressure (Figure 4), Systolic blood pressure (Figure 5), levels of High-density lipoprotein (Figure 6), Low-density lipoprotein (Figure 7), and Serum glucose (Figure 8), and Waist circumference (Figure 9) are provided in the manuscript.
Figure 2.
Forest plot showing the results for body weight.
Figure 3.
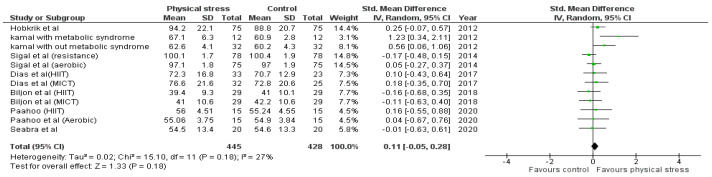
Forest plot showing the results for BMI.
Figure 4.
Forest plot showing the results for DBP.
Figure 5.
Forest plot showing the results for SBP.
Figure 6.
Forest plot showing the results for HDL.
Figure 7.
Forest plot showing result of LDL.
Figure 8.
Forest plot showing the results for blood glucose.
Figure 9.
Forest plot showing the results for waist circumference.
3.4.1. Body Weight
Seven out of the 12 studies reported data for Body weight. Pooled results (Figure 2) showed that Body weight was not statistically significant in the comparison with the control group (0.11 95% CI −0.05, 0.28; p = 0.18; I2 = 27%).
3.4.2. Body Mass Index (BMI)
Out of 12 studies, 8 reported data for BMI. Pooled results (Figure 3) showed that BMI was statistically significant in the comparison with the control group (0.38 95% CI 0.14, 0.62; p = 0.002; I2 = 65%).
3.4.3. Diastolic Blood Pressure
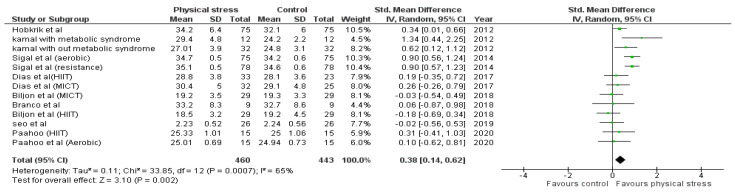
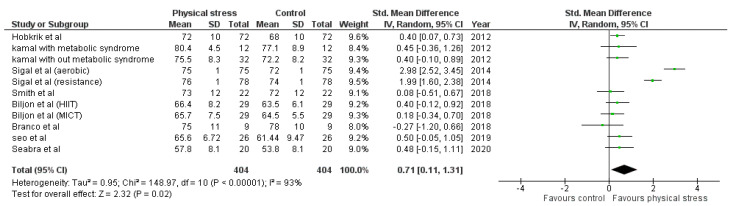
Out of 12 studies, 8 reported data for Diastolic blood pressure. Pooled results (Figure 4) showed that Diastolic blood pressure was statistically significant in the comparison with the control group (0.71 95% CI 0.11, 1.31; p = 0.02; I2 = 93%).
3.4.4. Systolic Blood Pressure
Out of 12 studies, 8 reported data for Systolic blood pressure. Pooled results (Figure 5) showed that Systolic blood pressure was not statistically significant in the comparison with the control group (0.89 95% CI −0.02, 1.80; p = 0.05; I2 = 97%).
3.4.5. High-Density Lipoprotein (HDL)
Out of 12 studies, 10 reported data for HDL. Pooled results (Figure 6) showed that HDL level was not statistically significant in the comparison with the control group (−0.48 95% CI −1.06, 0.11; p = 0.11; I2 = 92%).
3.4.6. Low-Density Lipoprotein (LDL)
Out of 12 studies, 9 reported data for LDL. Pooled results (Figure 7) showed that LDL level was statistically significant in the comparison with the control group (0.41 95% CI 0.01, 0.82; p = 0.05; I2 = 83%).
3.4.7. Blood Glucose
Out of 12 studies, 7 reported data for Blood glucose. Pooled results that (Figure 8) showed Blood glucose was statistically significant in the comparison with the control group (0.58 95% CI 0.13, 1.03; p = 0.01; I2 = 85%).
3.4.8. Waist Circumference
Out of 12 studies, 8 reported data for Waist circumference. Pooled results (Figure 9) showed that Waist circumference was not statistically significant in the comparison with the control group (0.60 95% CI 0.02, 1.18; p = 0.04; I2 = 93%).
3.5. Sensitivity Analysis
A sensitivity analysis was conducted to assess the influence of each study on the overall effect by excluding one study at a time, followed by the generation of pooled Odds Ratio (OR) for the rest of the studies. No significant change was observed after the exclusion of any individual study, suggesting the results were robust.
4. Discussion
In this study, we present the assessment of the evidence from 12 randomized control trials (n = 479), evaluating the effectiveness of exercise in reducing cardiometabolic risk factors in overweight children and adolescents from 6 to 18 years of age. During the intervention period from 5 weeks to 6 months, a reduction in BMI, blood glucose level, DBP, and LDL level was found. In contrast, there was no significant effect of exercise on body weight, SBP, HDL level, and waist circumference. HIIT intervention was used in four studies [44,46,49,53], moderate-intensity continuous training (MICT) was used as an intervention in four studies [44,48,52,53], and two studies used aerobic exercise [46,52]; the data regarding the type of exercise was not available in five studies [43,45,47,51,54]. Seabra et al. used school-based soccer practice as an intervention [50]. Children with metabolic syndrome were included by Kamal et al. [47].
In our literature review, we found that the first meta-analysis evaluating the effects of exercise and behavioral therapy on overweight children was published in 2012 and reported significant improvement after exercise in cardiometabolic outcomes compared with overweight children without treatment [55]. Several systematic reviews and meta-analyses have been published that evaluate the positive effects of HIIT on CMRFs and cardiorespiratory fitness [56,57]. School-based exercise programs had also a significant effect in decreasing CMRFs in a meta-analysis [58]. Several RCTs conducted on children with metabolic syndrome to evaluate the effectiveness of metformin showed significant results [59,60]. Various studies assessed the association of dietary patterns with obesity [61,62,63,64,65]. The association of sleeping patterns with obesity appeared evident [66,67,68]. A meta-analysis was published in 2016, which showed a significant decrease in BMI and improvement in body composition in overweight children after exercise [69]. However, the data regarding the effectiveness of exercise on HDL, LDL, and blood glucose levels were inconclusive in this study.
BMI is the most reliable and accessible screening tool in clinical settings to assess obesity and overweight in children and adolescents [70,71,72,73]. We found a significant effect of exercise in reducing BMI in children and adolescents [73]. BMI was reported in 8 studies, and the studies were further divided based on their intervention; therefore, 13 interventions were analyzed. Both aerobic and MICT interventions, examined in Sigal et al. [52], in two patients’ populations, as reported by Kamal et al., had a significant effect in reducing BMI [47]. On the other hand, nine interventions did not significantly affect BMI [43,44,45,46,48,53], as shown in Figure 3. The most prolonged intervention period was used by Sigal et al. and corresponded to 6 months. It resulted in a significant decrease in BMI, i.e., −0.6 (95% CI, −1.1 to 0) after aerobic training and −0.5 (95% CI, −1.1 to 0) after MICT [52]. Seabra et al. also used an intervention period of 6 months in their school-based soccer program and also found a significant decrease in BMI z-score in overweight children and adolescents (BMI z-score = 2.2 ± 0.5, %BFM = 32.3 ± 6.1) [50]. The intervention period of Kamal et al. was 12 weeks, and significant results were attained in patients with and without metabolic syndrome. However, better effects were obtained for children with metabolic syndrome. Metabolic syndrome decreased from 12.9% to 7.5%, and BMI was reduced from 47.3% to 32.6%, suggesting an improvement in metabolic syndrome with exercise [47]. An intervention period from 4 weeks to 12 weeks was used in the rest of the studies and showed non-significant results [43,44,45,46,48,53].
No significant change was found regarding body weight and waist circumference. Seven studies were analyzed reporting 12 interventions for body weight reduction; only one intervention had a significant effect [47]. Eight studies reported 11 waist circumference interventions; 4 had significant effects [45,47,52]. Figure 9 shows the data regarding body weight and waist circumference. For body weight, significant changes were only seen in children with metabolic syndrome after 12 weeks of intervention, as reported by Kamal et al. (standard mean difference (SMD) 1.23; 95% CI 0.34 to 2.11) [47]. Waist circumference was improved in the metabolic syndrome patients examined by Kamal et al. (SMD 1.31; 95% CI 0.41 to 2.20), Hobkrik et al. (SMD 0.48; 95% CI 0.15 to 0.8) and Sigal et al. with two interventions had; (SMD 1.82; 95% CI 1.44 to 2.19) and SMD 2.39; 95% CI 1.96 to 2.81) [45,47,52]. The results suggest that waist circumference, body weight, and BMI were improved in children and adolescents with metabolic syndrome. Long-term interventions also significantly affected these parameters.
The interactions between different genetic and environmental factors play a significant role in the pathogeny of cardiometabolic syndrome [74]. Increased LDL and blood glucose levels play an essential role in the manifestation of atherosclerosis, leading to coronary artery diseases (CAD) [75,76,77,78]. Overweight children and adolescents are more prone to develop adulthood CAD [79,80]. We found the exercise has a vital role in decreasing LDL and blood glucose levels in children. Nine studies using 11 interventions were analyzed to examine the effects on LDL. Four interventions indicated a positive effect of physical exercise [45,47,49,54], one intervention showed non-significant results [51]. Seven studies reported the blood glucose levels after 10 interventions; 4 interventions had significant effects [45,47,51,53] (Figure 8). Shalitin et al. showed a significant increase in LDL level after an intervention of 12 weeks (SMD −0.58; 95% CI −0.97 to −0.19); however, in a follow-up after 52 weeks, it was found that LDL level was significantly reduced (p = 0.004) [51]. The 12-week intervention of Shalitin et al. was significantly effective in reducing blood glucose level (SMD 2.09; 95% CI 1.61 to 2.57). Significant results were also obtained for children and adolescents affected by metabolic syndrome [47]. No significant effect on HDL was found after the analysis (Figure 6). For HDL, 13 interventions reported in 10 studies were analyzed; 5 interventions had no effect [46,47,54], and 2 interventions improved physical stress [45,51]. Children and adolescents with metabolic syndrome showed a significant increase in HDL concentration (SMD −2.30; 95% CI −3.37 to −1.23). Shalitin et al. showed a significant reduction in HDL levels after a 12-week intervention (SMD 1.75; 95% CI 1.30 to 2.21).
According to the World Health Organization (WHO), hypertension is the leading cause of death worldwide [81]. Weight loss shows a significant effect in reducing blood pressure [82,83]. Therefore, we emphasize the significance of exercise for blood pressure reduction. We found a significant decrease in diastolic blood pressure but not in systolic blood pressure (Figure 4 and Figure 5). Eight studies reported 11 interventions for blood pressure. Two interventions significantly reduced SBP [52], while 3 interventions significantly reduced DBP [45,52]. Sigal et al.’s 6-month intervention showed significant results in decreasing both SBP and DBP [52]. Hobkrik et al. showed a significant change in DBP but not in SBP (SMD 0.40; 95% CI 0.07 to 0.73).
Therefore, we conclude that long-term interventions can obtain significant results, regardless of the type of intervention. Furthermore, early effects were seen only in children and adolescents affected by metabolic syndrome.
5. Limitations
Our study is limited by the following factors: (a) all studies were randomized controlled trials in nature, (b) fewer studies were available with significant publication bias (the reason is described in the above section), (c) the number of study subjects was low, (d) studies reporting on different types of physical activity were pooled together, which might be the reason for the high heterogeneity observed. Nevertheless, these studies were pivotal for our analysis. More studies providing greater numbers of subjects and random controls should be conducted.
6. Conclusions
The current evidence suggests that exercise interventions in overweight children and adolescents improve BMI, DBP, and LDL and blood glucose levels; however, no significant effects were obtained for HDL level, body weight, SBP, and waist circumference, suggesting that exercise interventions are substantial in improving some cardiometabolic risk factors. However, long-term interventions might significantly improve other cardiometabolic risk factors. Therefore, the risks of cardiometabolic diseases in overweight adolescents can be reduced with a long-term exercise program and lifestyle modification.
Author Contributions
All authors whose names are marked with “†” contributed equally and are considered first authors. Conceptualization, S.S.B., L.I.S., A.E.L., C.L.A., C.E.J., M.S., A.S.; Methodology, S.S.B., L.I.S., A.E.L., C.L.A., C.E.J., A.S.; Software, S.S.B., L.I.S.; Validation S.S.B., L.I.S., A.S.; Formal analysis, A.S.; Investigation S.S.B., L.I.S., A.E.L., C.L.A., C.E.J., A.S.; Resources S.S.B., L.I.S., A.E.L., C.L.A., C.E.J., A.S.; Data curation, M.S., A.E.L., C.L.A., C.E.J., A.S.; Writing—original draft preparation S.S.B., L.I.S., A.E.L., C.L.A., C.E.J.; writing—review and editing, S.S.B., L.I.S., A.E.L., C.L.A., C.E.J., A.S.; Visualization S.S.B., A.S.; Supervision, S.S.B., L.I.S., A.S.; Project administration, S.S.B., L.I.S., C.L.A., A.S.; Funding acquisition, S.S.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that the present article has been written independently, without any financial or professional help, and reflects only the opinion of the authors, without any role of the industry.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sabin A.M., Kao K.-T., Juonala M., Baur L., Wake M. Viewpoint article: Childhood obesity—Looking back over 50 years to begin to look forward. J. Paediatr. Child Health. 2015;51:82–86. doi: 10.1111/jpc.12819. [DOI] [PubMed] [Google Scholar]
- 2.Kelsey M.M., Zaepfel A., Bjornstad P., Nadeau K. Age-Related Consequences of Childhood Obesity. Gerontology. 2014;60:222–228. doi: 10.1159/000356023. [DOI] [PubMed] [Google Scholar]
- 3.Nathan B.M., Moran A. Metabolic complications of obesity in childhood and adolescence: More than just diabetes. Curr. Opin. Endocrinol. Diabetes Obes. 2008;15:21–29. doi: 10.1097/MED.0b013e3282f43d19. [DOI] [PubMed] [Google Scholar]
- 4.Weihrauch-Blüher S., Wiegand S. Risk Factors and Implications of Childhood Obesity. Curr. Obes. Rep. 2018;7:254–259. doi: 10.1007/s13679-018-0320-0. [DOI] [PubMed] [Google Scholar]
- 5.Bass R., Eneli I. Severe childhood obesity: An under-recognised and growing health problem. Postgrad. Med. J. 2015;91:639–645. doi: 10.1136/postgradmedj-2014-133033. [DOI] [PubMed] [Google Scholar]
- 6.Ibrahim M.M. Subcutaneous and visceral adipose tissue: Structural and functional differences. Obes. Rev. 2010;11:11–18. doi: 10.1111/j.1467-789X.2009.00623.x. [DOI] [PubMed] [Google Scholar]
- 7.Abootorabi M.S., Ayremlou P., Behroozi-Lak T., Nourisaeidlou S. The effect of vitamin D supplementation on insulin resistance, visceral fat and adiponectin in vitamin D deficient women with polycystic ovary syndrome: A randomized placebo-controlled trial. Gynecol. Endocrinol. 2018;34:489–494. doi: 10.1080/09513590.2017.1418311. [DOI] [PubMed] [Google Scholar]
- 8.Rosqvist F., Iggman D., Kullberg J., Cedernaes J., Johansson H.-E., Larsson A., Johansson L., Ahlström H., Arner P., Dahlman I., et al. Overfeeding Polyunsaturated and Saturated Fat Causes Distinct Effects on Liver and Visceral Fat Accumulation in Humans. Diabetes. 2014;63:2356–2368. doi: 10.2337/db13-1622. [DOI] [PubMed] [Google Scholar]
- 9.O’Rourke R.W. Adipose tissue and the physiologic underpinnings of metabolic disease. Surg. Obes. Relat. Dis. 2018;14:1755–1763. doi: 10.1016/j.soard.2018.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goossens G.H. The Metabolic Phenotype in Obesity: Fat Mass, Body Fat Distribution, and Adipose Tissue Function. Obes. Facts. 2017;10:207–215. doi: 10.1159/000471488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smitka K., Marešová D. Adipose Tissue as an Endocrine Organ: An Update on Pro-inflammatory and Anti-inflammatory Microenvironment. Prague Med Rep. 2015;116:87–111. doi: 10.14712/23362936.2015.49. [DOI] [PubMed] [Google Scholar]
- 12.Haluzík M., Trachta P., Haluzíková D. Hormony tukové tkáne [Adipose tissue hormones] Vnitřní Lékařství. 2010;56:1028–1034. [PubMed] [Google Scholar]
- 13.Booth A., Magnuson A., Fouts J., Foster M.T. Adipose tissue: An endocrine organ playing a role in metabolic regulation. Horm. Mol. Biol. Clin. Investig. 2016;26:25–42. doi: 10.1515/hmbci-2015-0073. [DOI] [PubMed] [Google Scholar]
- 14.Li Z.-Y., Wang P., Miao C.-Y. Adipokines in inflammation, insulin resistance and cardiovascular disease. Clin. Exp. Pharmacol. Physiol. 2011;38:888–896. doi: 10.1111/j.1440-1681.2011.05602.x. [DOI] [PubMed] [Google Scholar]
- 15.Meijer R.I., Serne E.H., Smulders Y.M., Van Hinsbergh V.W.M., Yudkin J.S., Eringa E.C. Perivascular Adipose Tissue and Its Role in Type 2 Diabetes and Cardiovascular Disease. Curr. Diabetes Rep. 2011;11:211–217. doi: 10.1007/s11892-011-0186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fang X., Zuo J., Zhou J., Cai J., Chen C., Xiang E., Li H., Cheng X., Chen P. Childhood obesity leads to adult type 2 diabetes and coronary artery diseases. Medicine. 2019;98:e16825. doi: 10.1097/MD.0000000000016825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Small L., Aplasca A. Child Obesity and Mental Health. Child Adolesc. Psychiatr. Clin. N. Am. 2016;25:269–282. doi: 10.1016/j.chc.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 18.Halfon N., Larson K., Slusser W. Associations Between Obesity and Comorbid Mental Health, Developmental, and Physical Health Conditions in a Nationally Representative Sample of US Children Aged 10 to 17. Acad. Pediatr. 2013;13:6–13. doi: 10.1016/j.acap.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 19.Swallen K.C., Reither E.N., Haas S.A., Meier A.M. Overweight, Obesity, and Health-Related Quality of Life Among Adolescents: The National Longitudinal Study of Adolescent Health. Podiatrics. 2005;115:340–347. doi: 10.1542/peds.2004-0678. [DOI] [PubMed] [Google Scholar]
- 20.Huang I.-C., Frangakis C., Wu A.W. The relationship of excess body weight and health-related quality of life: Evidence from a population study in Taiwan. Int. J. Obes. 2006;30:1250–1259. doi: 10.1038/sj.ijo.0803250. [DOI] [PubMed] [Google Scholar]
- 21.Kostovski M., Tasic V., Laban N., Polenakovic M., Danilovski D., Gucev Z. Obesity in Childhood and Adolescence, Genetic Factors. Prilozi. 2017;38:121–133. doi: 10.2478/prilozi-2018-0013. [DOI] [PubMed] [Google Scholar]
- 22.Nielsen L.A., Nielsen T.R.H., Holm J.-C. The Impact of Familial Predisposition to Obesity and Cardiovascular Disease on Childhood Obesity. Obes. Facts. 2015;8:319–328. doi: 10.1159/000441375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hemmingsson E. Early Childhood Obesity Risk Factors: Socioeconomic Adversity, Family Dysfunction, Offspring Distress, and Junk Food Self-Medication. Curr. Obes. Rep. 2018;7:204–209. doi: 10.1007/s13679-018-0310-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams A.S., Ge B., Petroski G., Kruse R.L., McElroy J.A., Koopman R.J. Socioeconomic Status and Other Factors Associated with Childhood Obesity. J. Am. Board Fam. Med. 2018;31:514–521. doi: 10.3122/jabfm.2018.04.170261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.An R. Diet quality and physical activity in relation to childhood obesity. Int. J. Adolesc. Med. Health. 2017;29:1515. doi: 10.1515/ijamh-2015-0045. [DOI] [PubMed] [Google Scholar]
- 26.Solmi M., Ioannidis J.P., Carvalho A.F. Environmental risk factors and interventions for obesity. Eur. J. Clin. Investig. 2019;49:e13080. doi: 10.1111/eci.13080. [DOI] [PubMed] [Google Scholar]
- 27.Weihrauch-Blüher S., Schwarz P., Klusmann J.-H. Childhood obesity: Increased risk for cardiometabolic disease and cancer in adulthood. Metabolism. 2019;92:147–152. doi: 10.1016/j.metabol.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 28.Catalano P.M., Shankar K. Obesity and pregnancy: Mechanisms of short term and long term adverse consequences for mother and child. BMJ. 2017;356:j1. doi: 10.1136/bmj.j1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Llewellyn A., Simmonds M.C., Owen C., Woolacott N. Childhood obesity as a predictor of morbidity in adulthood: A systematic review and meta-analysis. Obes. Rev. 2016;17:56–67. doi: 10.1111/obr.12316. [DOI] [PubMed] [Google Scholar]
- 30.Whitlock E.P., Williams S.B., Gold R., Smith P., Shipman S. Screening and Interventions for Childhood Overweight [In-ternet]. Rockville (MD): Agency for Healthcare Research and Quality (US) Pediatrics. 2005;116:e125–e144. doi: 10.1542/peds.2005-0242. [DOI] [PubMed] [Google Scholar]
- 31.Stadler J.T., Marsche G. Obesity-Related Changes in High-Density Lipoprotein Metabolism and Function. Int. J. Mol. Sci. 2020;21:8985. doi: 10.3390/ijms21238985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gurnani M., Birken C., Hamilton J. Childhood Obesity. Pediatr. Clin. N. Am. 2015;62:821–840. doi: 10.1016/j.pcl.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 33.Frank A. A Multidisciplinary Approach to Obesity Management: The Physician’s Role and Team Care Alternatives. J. Am. Diet. Assoc. 1998;98:S44–S48. doi: 10.1016/S0002-8223(98)00710-X. [DOI] [PubMed] [Google Scholar]
- 34.Welbourn R., on behalf of the Guidance Development Group. Dixon J., Barth J.H., Finer N., Hughes C.A., le Roux C.W., Wass J. NICE-Accredited Commissioning Guidance for Weight Assessment and Management Clinics: A Model for a Specialist Multidisciplinary Team Approach for People with Severe Obesity. Obes. Surg. 2016;26:649–659. doi: 10.1007/s11695-015-2041-8. [DOI] [PubMed] [Google Scholar]
- 35.Neeland I.J., Poirier P., Després J.-P. Cardiovascular and Metabolic Heterogeneity of Obesity. Circulation. 2018;137:1391–1406. doi: 10.1161/CIRCULATIONAHA.117.029617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rajjo T., Almasri J., Al Nofal A., Farah W., Alsawas M., Ahmed A.T., Mohammed K., Kanwar A., Asi N., Wang Z., et al. The Association of Weight Loss and Cardiometabolic Outcomes in Obese Children: Systematic Review and Meta-regression. J. Clin. Endocrinol. Metab. 2016;101:4764–4768. doi: 10.1210/jc.2016-2575. [DOI] [PubMed] [Google Scholar]
- 37.Skrede T., Steene-Johannessen J., Anderssen S.A., Resaland G.K., Ekelund U. The prospective association between objectively measured sedentary time, moderate-to-vigorous physical activity and cardiometabolic risk factors in youth: A systematic review and meta-analysis. Obes. Rev. 2018;20:55–74. doi: 10.1111/obr.12758. [DOI] [PubMed] [Google Scholar]
- 38.García-Hermoso A., Urbina A.J.C., Herrera-Valenzuela T., Cristi-Montero C., Saavedra J.M., Martínez-Vizcaíno V. Is high-intensity interval training more effective on improving cardiometabolic risk and aerobic capacity than other forms of exercise in overweight and obese youth? A meta-analysis. Obes. Rev. 2016;17:531–540. doi: 10.1111/obr.12395. [DOI] [PubMed] [Google Scholar]
- 39.Ross L.M., Porter R.R., Durstine J.L. High-intensity interval training (HIIT) for patients with chronic diseases. J. Sport Health Sci. 2016;5:139–144. doi: 10.1016/j.jshs.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Astorino T.A., Schubert M.M. Changes in fat oxidation in response to various regimes of high intensity interval training (HIIT). Graefe’s Arch. Clin. Exp. Ophthalmol. 2018;118:51–63. doi: 10.1007/s00421-017-3756-0. [DOI] [PubMed] [Google Scholar]
- 41.Hutton B., Salanti G., Caldwell D.M., Chaimani A., Schmid C.H., Cameron C., Ioannidis J.P., Straus S., Thorlund K., Jansen J.P., et al. The PRISMA Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-analyses of Health Care Interventions: Checklist and Explanations. Ann. Intern. Med. 2015;162:777–784. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 42.Cumpston M., Li T., Page M., Chandler J., Welch A.V., Higgins J., Thomas J. Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019;10:ED000142. doi: 10.1002/14651858.ED000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seo Y.-G., Lim H., Kim Y., Ju Y.-S., Lee H.-J., Jang H.B., Park S.I., Park K.H. The Effect of a Multidisciplinary Lifestyle Intervention on Obesity Status, Body Composition, Physical Fitness, and Cardiometabolic Risk Markers in Children and Adolescents with Obesity. Nutrition. 2019;11:137. doi: 10.3390/nu11010137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dias K.A., Ingul C.B., Tjønna A.E., Keating S.E., Gomersall S., Follestad T., Hosseini M.S., Hollekim-Strand S.M., Ro T.B., Haram M., et al. Effect of High-Intensity Interval Training on Fitness, Fat Mass and Cardiometabolic Biomarkers in Children with Obesity: A Randomised Controlled Trial. Sports Med. 2017;48:733–746. doi: 10.1007/s40279-017-0777-0. [DOI] [PubMed] [Google Scholar]
- 45.Hobkirk J.P., King R.F., Gately P., Pemberton P., Smith A., Barth J.H., Carroll S. Longitudinal Factor Analysis Reveals a Distinct Clustering of Cardiometabolic Improvements During Intensive, Short-Term Dietary and Exercise Intervention in Obese Children and Adolescents. Metab. Syndr. Relat. Disord. 2012;10:20–25. doi: 10.1089/met.2011.0050. [DOI] [PubMed] [Google Scholar]
- 46.Paahoo A., Tadibi V., Behpoor N. Effect of Two Chronic Exercise Protocols on Pre-Atherosclerotic and Anti-Atherosclerotic Biomarkers Levels in Obese and Overweight Children. Iran. J. Pediatr. 2020;30:e99760. doi: 10.5812/ijp.99760. [DOI] [Google Scholar]
- 47.Kamal N.N., Ragy M.M. The effects of exercise on C-reactive protein, insulin, leptin and some cardiometabolic risk factors in Egyptian children with or without metabolic syndrome. Diabetol. Metab. Syndr. 2012;4:27. doi: 10.1186/1758-5996-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Branco B.H.M., Carvalho I.Z., de Oliveira H.G., Fanhani A.P., dos Santos M.C.M., de Oliveira L.P., Boni S.M., Nardo N., Jr. Effects of 2 Types of Resistance Training Models on Obese Adolescents’ Body Composition, Cardiometabolic Risk, and Physical Fitness. J. Strength Cond. Res. 2020;34:2672–2682. doi: 10.1519/JSC.0000000000002877. [DOI] [PubMed] [Google Scholar]
- 49.Martin-Smith R., Buchan D.S., Baker J.S., Macdonald M.J., Sculthorpe N.F., Easton C., Knox A., Grace F.M. Sprint Interval Training and the School Curriculum: Benefits Upon Cardiorespiratory Fitness, Physical Activity Profiles, and Cardiometabolic Risk Profiles of Healthy Adolescents. Pediatr. Exerc. Sci. 2019;31:296–305. doi: 10.1123/pes.2018-0155. [DOI] [PubMed] [Google Scholar]
- 50.Seabra A., Brito J., Figueiredo P., Beirão L., Seabra A., Carvalho M.J., Abreu S., Vale S., Pedretti A., Nascimento H., et al. School-based soccer practice is an effective strategy to improve cardiovascular and metabolic risk factors in overweight children. Prog. Cardiovasc. Dis. 2020;63:807–812. doi: 10.1016/j.pcad.2020.07.007. [DOI] [PubMed] [Google Scholar]
- 51.Shalitin S., Ashkenazi-Hoffnung L., Yackobovitch-Gavan M., Nagelberg N., Karni Y., Hershkovitz E., Loewenthal N., Shtaif B., Gat-Yablonski G., Phillip M. Effects of a Twelve-Week Randomized Intervention of Exercise and/or Diet on Weight Loss and Weight Maintenance, and Other Metabolic Parameters in Obese Preadolescent Children. Horm. Res. 2009;72:287–301. doi: 10.1159/000245931. [DOI] [PubMed] [Google Scholar]
- 52.Sigal R.J., Alberga A.S., Goldfield G.S., Prud’Homme D., Hadjiyannakis S., Gougeon R., Phillips P., Tulloch H., Malcolm J., Doucette S., et al. Effects of Aerobic Training, Resistance Training, or Both on Percentage Body Fat and Cardiometabolic Risk Markers in Obese Adolescents: The Healthy Eating Aerobic and Resistance Training in Youth Randomized Clinical Trial. JAMA Pediatr. 2014;168:1006–1014. doi: 10.1001/jamapediatrics.2014.1392. [DOI] [PubMed] [Google Scholar]
- 53.Van Biljon A., McKune A.J., DuBose K.D., Kolanisi U., Semple S.J. Do Short-Term Exercise Interventions Improve Cardiometabolic Risk Factors in Children? J. Pediatr. 2018;203:325–329. doi: 10.1016/j.jpeds.2018.07.067. [DOI] [PubMed] [Google Scholar]
- 54.Brand C., Martins C.M.D.L., Lemes V.B., Pessoa M.L.F., Dias A.F., Cadore E.L., Mota J., Gaya A.C.A., Gaya A.R. Effects and prevalence of responders after a multicomponent intervention on cardiometabolic risk factors in children and adolescents with overweight/obesity: Action for health study. J. Sports Sci. 2020;38:682–691. doi: 10.1080/02640414.2020.1725384. [DOI] [PubMed] [Google Scholar]
- 55.Ho M., Garnett S.P., Baur L., Burrows T., Stewart L., Neve M., Collins C. Effectiveness of Lifestyle Interventions in Child Obesity: Systematic Review with Meta-analysis. Pediatrics. 2012;130:e1647–e1671. doi: 10.1542/peds.2012-1176. [DOI] [PubMed] [Google Scholar]
- 56.Batacan R.B., Duncan M.J., Dalbo V.J., Tucker P.S., Fenning A.S. Effects of high-intensity interval training on cardiometabolic health: A systematic review and meta-analysis of intervention studies. Br. J. Sports Med. 2017;51:494–503. doi: 10.1136/bjsports-2015-095841. [DOI] [PubMed] [Google Scholar]
- 57.Thivel D., Masurier J., Baquet G., Timmons B.W., Pereira B., Berthoin S., Duclos M., Aucouturier J. High-intensity interval training in overweight and obese children and adolescents: Systematic review and meta-analysis. J. Sports Med. Phys. Fit. 2019;59:310–324. doi: 10.23736/S0022-4707.18.08075-1. [DOI] [PubMed] [Google Scholar]
- 58.Pozuelo-Carrascosa D.P., Cavero-Redondo I., Herraiz-Adillo Á., Fernández A.D., López M.S., Martínez-Vizcaíno V. School-Based Exercise Programs and Cardiometabolic Risk Factors: A Meta-analysis. Pediatrics. 2018;142:e20181033. doi: 10.1542/peds.2018-1033. [DOI] [PubMed] [Google Scholar]
- 59.Díaz M., Bassols J., López-Bermejo A., De Zegher F., Ibáñez L. Metformin treatment to reduce central adiposity after prenatal growth restraint: A placebo-controlled pilot study in prepubertal children. Pediatr. Diabetes. 2014;16:538–545. doi: 10.1111/pedi.12220. [DOI] [PubMed] [Google Scholar]
- 60.Bassols J., Martínez-Calcerrada J.-M., Osiniri I., Díaz-Roldán F., Xargay-Torrent S., Mas-Parés B., Dorado-Ceballos E., Prats-Puig A., Carreras-Badosa G., De Zegher F., et al. Effects of metformin administration on endocrine-metabolic parameters, visceral adiposity and cardiovascular risk factors in children with obesity and risk markers for metabolic syndrome: A pilot study. PLoS ONE. 2019;14:e0226303. doi: 10.1371/journal.pone.0226303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Partsalaki I., Karvela A., Spiliotis B.E. Metabolic impact of a ketogenic diet compared to a hypocaloric diet in obese children and adolescents. J. Pediatr. Endocrinol. Metab. 2012;25:697–704. doi: 10.1515/jpem-2012-0131. [DOI] [PubMed] [Google Scholar]
- 62.Macknin M., Kong T., Weier A., Worley S., Tang A.S., Alkhouri N., Golubic M. Plant-Based, No-Added-Fat or American Heart Association Diets: Impact on Cardiovascular Risk in Obese Children with Hypercholesterolemia and Their Parents. J. Pediatr. 2015;166:953–959.e3. doi: 10.1016/j.jpeds.2014.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rajakumar K., Moore C.G., Khalid A.T., Vallejo A.N., Virji A.M., Holick M.F., Greenspan S.L., Arslanian S., Reis E.S. Effect of vitamin D3 supplementation on vascular and metabolic health of vitamin D–deficient overweight and obese children: A randomized clinical trial. Am. J. Clin. Nutr. 2020;111:757–768. doi: 10.1093/ajcn/nqz340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Specht I.O., Rohde J.F., Olsen N.J., Heitmann B.L. Duration of exclusive breastfeeding may be related to eating behaviour and dietary intake in obesity prone normal weight young children. PLoS ONE. 2018;13:e0200388. doi: 10.1371/journal.pone.0200388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dalrymple K.V., Flynn A.C., Seed P.T., Briley A.L., O’Keeffe M., Godfrey K.M., Poston L. Associations between dietary patterns, eating behaviours, and body composition and adiposity in 3-year-old children of mothers with obesity. Pediatr. Obes. 2020;15:e12608. doi: 10.1111/ijpo.12608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pelletier J.E., Lytle L.A., Laska M.N. Stress, Health Risk Behaviors, and Weight Status among Community College Students. Health Educ. Behav. 2015;43:139–144. doi: 10.1177/1090198115598983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brady E., Bodicoat D., Hall A., Khunti K., Yates T., Edwardson C., Davies M. Sleep duration, obesity and insulin resistance in a multi-ethnic UK population at high risk of diabetes. Diabetes Res. Clin. Prac. 2018;139:195–202. doi: 10.1016/j.diabres.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 68.Taylor B., Gray A., Galland B., Heath A.-L.M., Lawrence J., Sayers R.M., Cameron S., Hanna M., Dale K., Coppell K., et al. Targeting Sleep, Food, and Activity in Infants for Obesity Prevention: An RCT. Pediatrics. 2017;139:e20162037. doi: 10.1542/peds.2016-2037. [DOI] [PubMed] [Google Scholar]
- 69.Stoner L., Rowlands D., Morrison A., Credeur D., Hamlin M., Gaffney K., Lambrick D., Matheson A. Efficacy of Exercise Intervention for Weight Loss in Overweight and Obese Adolescents: Meta-Analysis and Implications. Sports Med. 2016;46:1737–1751. doi: 10.1007/s40279-016-0537-6. [DOI] [PubMed] [Google Scholar]
- 70.Tyson N., Frank M. Childhood and adolescent obesity definitions as related to BMI, evaluation and management options. Best Pr. Res. Clin. Obstet. Gynaecol. 2018;48:158–164. doi: 10.1016/j.bpobgyn.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 71.Gutin I. In BMI we trust: Reframing the body mass index as a measure of health. Soc. Theory Health. 2018;16:256–271. doi: 10.1057/s41285-017-0055-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bosello O., Donataccio M.P., Cuzzolaro M. Obesity or obesities? Controversies on the association between body mass index and premature mortality. Eat. Weight. Disord. Stud. Anorexia Bulim. Obes. 2016;21:165–174. doi: 10.1007/s40519-016-0278-4. [DOI] [PubMed] [Google Scholar]
- 73.Trinh A., Campbell M., Ukoumunne O.C., Gerner B., Wake M. Physical Activity and 3-Year BMI Change in Overweight and Obese Children. Pediatrics. 2013;131:e470–e477. doi: 10.1542/peds.2012-1092. [DOI] [PubMed] [Google Scholar]
- 74.Suceveanu A.-I., Mazilu L., Katsiki N., Parepa I., Voinea F., Pantea-Stoian A., Rizzo M., Botea F., Herlea V., Serban D., et al. NLRP3 Inflammasome Biomarker—Could Be the New Tool for Improved Cardiometabolic Syndrome Outcome. Metabolites. 2020;10:448. doi: 10.3390/metabo10110448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Khosravi M., Hosseini-Fard R., Najafi M. Circulating low density lipoprotein (LDL) Horm. Mol. Biol. Clin. Investig. 2018;35:20180024. doi: 10.1515/hmbci-2018-0024. [DOI] [PubMed] [Google Scholar]
- 76.Naito R., Miyauchi K. Coronary Artery Disease and Type 2 Diabetes Mellitus. Int. Heart J. 2017;58:475–480. doi: 10.1536/ihj.17-191. [DOI] [PubMed] [Google Scholar]
- 77.Hartley A., Haskard D., Khamis R. Oxidized LDL and anti-oxidized LDL antibodies in atherosclerosis—Novel insights and future directions in diagnosis and therapy. Trends Cardiovasc. Med. 2019;29:22–26. doi: 10.1016/j.tcm.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 78.Luo Y., Duan H., Qian Y., Feng L., Wu Z., Wang F., Feng J., Yang D., Qin Z., Yan X. Macrophagic CD146 promotes foam cell formation and retention during atherosclerosis. Cell Res. 2017;27:352–372. doi: 10.1038/cr.2017.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Meng L.X., Que X.M., Gao X., Wang T. Childhood obesity and coronary artery disease: A Mendelian randomization study. Front. Genet. 2019;40:839–843. doi: 10.3760/cma.j.issn.0254-6450.2019.07.019. [DOI] [PubMed] [Google Scholar]
- 80.Gomes F., Telo D.F., Souza H.P., Nicolau J.C., Halpern A., Serrano C.V., Jr. Obesidade e doença arterial coronariana: Papel da inflamação vascular. Arquivos Brasileiros de Cardiologia. 2010;94:273–279. doi: 10.1590/S0066-782X2010000200021. [DOI] [PubMed] [Google Scholar]
- 81.World Health Organization (WHO) Office of World Health Reporting . The World Health Report: 2002: Reducing Risks, Promoting Healthy Life: Overview. World Health Organization; Geneva, Switzerland: 2002. [Google Scholar]
- 82.Berchtold P., Jörgens V., Kemmer F.W., Berger M. Obesity and hypertension: Cardiovascular response of weight reduction. Hypertension. 1982;4:III50–III55. doi: 10.1161/01.HYP.4.5_Pt_2.III50. [DOI] [PubMed] [Google Scholar]
- 83.Dascalu A.M., Stoian A.P., Cherecheanu A.P., Serban D., Costea D.O., Tudosie M.S., Stana D., Tanasescu D., Sabau A.D., Gangura G.A., et al. Outcomes of Diabetic Retinopathy Post-Bariatric Surgery in Patients with Type 2 Diabetes Mellitus. J. Clin. Med. 2021;10:3736. doi: 10.3390/jcm10163736. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.