Summary
Background
During the Covid-19 pandemic, children and adolescents faced poverty, potentially dying from preventable causes, or missing out essential vaccines. The aim of this study was to assess potential environmental and individual factors associated to COVID-19 mortality in children and adolescents in Mexico.
Methods
This cross-sectional study analysed the official data of 131,001 children under 10 years of age and adolescents between 10 and 19 years of age with COVID-19 disease, in Mexico. Participants were diagnosticated between March 2020 and June 13, 2021. The environmental variables such as malnutrition, vaccination coverage and social lag index were evaluated at the state level. Multilevel models were fitted to evaluate the association between environmental and individual factors and COVID-19 mortality.
Findings
A total of 773 (0.6%) children and adolescents died due to COVID-19. Younger age (OR = 0.878, 95%CI: 0.869-0.888), diabetes (OR = 3.898, 95%CI: 2.596-5.851), immunosuppression (OR = 5.410, 95%CI: 4.088-7.158), obesity (OR = 1.876, 95%CI: 1.397-2.521), hypertension (OR = 1.906, 95%CI: 1.239-2.932), cardiovascular disease (OR = 2.288, 95%CI: 1.482-3.531), and chronic kidney disease (OR = 13.250, 95%CI: 9.066-19.350) were associated with mortality. COVID-19 mortality was directly associated with social lag index and malnutrition (ORvery high = 2.939, 95%CI: 1.111-7.775, and OR = 1.390, 95%CI: 1.073-1.802, respectively), and inversely associated with population density (OR = 0.374, 95%CI: 0.204-0.688). Finally, children and adolescents living in areas with a higher percentage of people with incomplete education (OR = 1.045, 95%CI: 1.011-1.081), of children of school age of 6–14 years who do not attend school (OR = 1.266, 95%CI: 1.032-1.554), and of illiterate population aged 15 and over (OR = 1.086, 95%CI: 0.999-1.179) were associated with a higher risk of COVID-19 mortality.
Interpretation
Malnutrition, social lag index and population density are key factors to understand COVID-19 mortality in children and adolescents. Also, age and pre-existing comorbidities were also associated with worse COVID-19 prognosis.
Funding
No funding was secured for this study.
Keywords: SARS-CoV 2, COVID-19, Childhood, Risk factors, Inequality
Research in context.
Evidence before this study
Preliminary evidence suggests the relevance of health inequities in COVID-19 outcomes. In Mexico, COVID-19 mortality in children and adolescents was higher compared to high-income countries. Malnutrition and social lag index have been associated with worse health outcomes in other diseases. Previous studies evaluated the impact of environmental factors on COVID-19 mortality in older adults or the general population. Also, it is known that children younger than four years of age with cardiovascular risk or immunosuppressed had an increased risk of mortality from COVID-19. However, no data are reported on the association between environmental and individual factors and COVID-19 mortality in children and adolescents.
Added value of this study
We analyzed COVID-19 mortality and risk factors in 131,001 children and adolescents with COVID-19 in Mexico. To our best knowledge, this is the largest study of COVID-19 in children and adolescents in Mexico aimed to evaluate environmental and individual factors associated with a worse COVID-19 prognosis. This study confirms the impact of factors such as malnutrition, social lag index or density population on COVID-19 mortality.
Implications of all the available evidence
Our results seem to show that COVID-19 is a mild disease in most of the paediatric cases. However, it is recommendable to consider the impact of malnutrition and social lag on COVID-19 outcomes, specially, in a low-middle-income country. Our findings point to the need to consider environmental factors to face future challenges regarding the pandemic. Likewise, this evidence on the impact of environmental characteristics on mortality in children and adolescents should contribute to rethinking public policies that improve the health of children and adolescents, and even help prepare for new pandemics.
Alt-text: Unlabelled box
Introduction
Less than 10% of COVID-19 cases involve children up to the age of 16.1, 2, 3 Although the clinical course of the symptomatic SARS-CoV-2 infection (COVID-19) is usually mild among young patients, children affected by the disease can suffer a moderate-serious infection.4,5 The main COVID-19 complication in children is the multisystem inflammatory syndrome (MIS).6 COVID-19 mortality in young people is low, varing between 0 and 13%,3 however, recent information has revealed that the impact of this disease in pediatric populations is likely to vary between and within countries.7
The World Health Organization (WHO) highlighted the association between a high risk of COVID-19 infection and poverty.8 Factors such as reduced access to healthcare, malnutrition and environmental and living conditions can increase the risk of a worse outcome.9 Differences in health outcomes according to income increased during the pandemic.10 An estimated 47 million children younger than 5 years old were suffering malnutrition previous to the COVID-19 pandemic.11 This condition was associated with prolonged hospitalization due to COVID-19 infection.12 Moreover, patients with COVID‐19 disease are prone to develop significant weight loss, malnutrition, and cachexia13 and, therefore, malnutrition could also be a prognostic factor for COVID-19–related increased mortality.
Comorbidities are associated with worst outcomes in pediatric COVID-19 patients.14 Obesity is associated with a worsened prognosis of the infection in both adults and children.15 Although diabetes, hypertension, malignancies and chronic respiratory disease were previously shown to be associated with COVID-19 prognosis in adults, evidence on the role of these comorbidities in children is still scarce.14,16
This analysis evaluated the impact of environmental and individual factors on COVID-19 mortality in Mexican children and adolescents during the first two years of the pandemic. We hypothesized that children and adolescents living in areas with less advantageous conditions would experience worse COVID-19 outcomes.
Methods
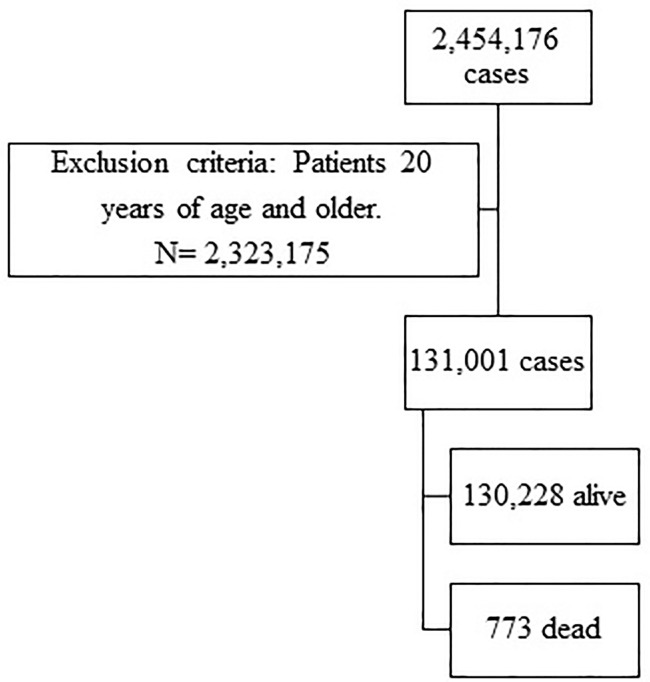
This is a retrospective analysis of the open COVID-19 registry dataset collected by the Mexican government in its official coronavirus web page and updated daily (available at https://www.gob.mx/salud/documentos/datos-abiertos-152127). The study sample included children (0–9 years old) and adolescents (10–19 years old) with COVID-19 diagnosis from February 27, 2020 to June 13, 2021 (Figure 1). The WHO defines adolescence as the phase of life between childhood and adulthood, from ages 10–19; early adolescents are those aged 10–14 years, and late adolescents are those aged 15–19 years. COVID-19 diagnosis was made according to the Diagnosis and Treatment Guideline for COVID-19 published by the Mexican Secretary of Health (available at https://coronavirus.gob.mx/). A positive COVID-19 case was considered if the child or adolescent presented a positive RT-PCR test, a positive rapid Ag-T test, or a diagnosis defined by patients with a clinical-epidemiological relation compatible with SARS-CoV-2 infection, as well as deceased persons that were assigned the diagnosis by a specialized committee.17 The average time between onset of symptoms and death from COVID-19 was 13 days.
Figure 1.
Flow diagram for study participants.
We used a harmonized dataset of individual and state-level data for 32 states (Mexico is a federal republic composed of 32 states). Based on the patient's place of residence, we linked individual level with state-level data using unique state codes.
Outcomes
The dependent variable was death in patients with COVID-19, which was assessed using date that the person died (alive=0; dead=1).
Clinical covariates
For this study, socio-demographic information on the patient was used, such as age, sex, state of residence, and ethnicity (indigenous/not indigenous). Clinical information included the presence of pneumonia, type of patient care (outpatient vs. inpatient), admission at intensive care unit (ICU), and treatment with intubation. Pre-existing comorbidities were determined based on the patient's self-report on admission, and included obesity, diabetes, hypertension, chronic obstructive pulmonary disease (COPD), asthma, immunosuppression, cardiovascular disease, chronic kidney disease (CKD), and other comorbidities.
Exposure variables of the environment
The influence of environmental factors on mortality in children and adolescents with COVID-19 was assessed according to different data sources. Exposure variables were all measured at the entity level.
Social lag index
The 2020 social lag index is an indicator that measures social development in Mexico, which is calculated by the National Council for the Evaluation of Social Development Policy (CONEVAL) for each federal entity.18 The index contains 11 indicators: the percentage of the population (1) aged 15 and over that is illiterate, (2) with children of school age of 6–14 years who do not attend school, (3) aged 15 and over with incomplete basic education, and (4) not affiliated to any health service, and (5–11) percentage of inhabited private housing units without the following basic infrastructure: drainage (5), floor covering (6), toilets or sanitary services (7), piped water from the public network (8), electricity services (9), washing machine (10), or refrigerator (11). The social lag index was categorized as very low, low, medium, high and very high.
Malnutrition
The number of people with malnutrition by area in 2020 was obtained from the Mexican Secretary of Health.19 These persons were identified using the International Classification of Diseases, Tenth Revision (ICD-10) diagnosis codes E40-E46.20,21
Vaccination
Information on vaccination coverage was captured from the National Survey on Health and Nutrition (ENSANUT) 2018. Two categories of vaccination schedule were analyzed as “complete schedule” and “schedule with 4 vaccines” for children of one year of age and up to two years old. A vaccination scheme was considered complete if there was a record that the child received a dose of BCG, three doses of hepatitis B, three doses of pentavalent, two doses of pneumococcal vaccine, two doses of anti-rotavirus, and one dose of MMR (triple viral). The 4-vaccine schedule completely excludes rotavirus and pneumococcal vaccines.22
Population density
This variable was extracted from the National Institute of Statistics and Geography (INEGI, for its Spanish acronym) (inhabitants per km2).23
Statistical analysis
Individual and environmental characteristics according to whether the patient was alive or dead were described, and means for continuous variables and percentages for categorical variables were used. Bivariate analyses were conducted to determine the association between individual and environmental variables and COVID-19 mortality. Finally, multilevel multivariable logistic regression models were performed with individuals nested within states, to test the association between each variable and COVID-19 mortality.
Models were adjusted for sex, age, diabetes, COPD, asthma, immunosuppression, hypertension, cardiovascular disease and obesity. Furthermore, the model with the malnutrition variable was also adjusted for population density, since this variable is in absolute numbers. Therefore, a set of adjusted models were fitted separately for each environmental variable, adjusting for individual covariates. Also, a model was fitted for each indicator related to social lag index. Models where the environmental variables were associated significantly with mortality at the 0.05 level are shown.
The overall model is showed bellow
∼ Binomial(1, )
Where πij is the probability of dying for individual i in state j, Xi is the set of explanatory variables at the individual level (sex, age, diabetes, obesity, immunosuppression, hypertension, cardiovascular disease and CKD), Zj is the set of explanatory variables defined for the states (social lag index or its indicators, vaccination coverage in children, people with malnutrition or population density), and uj are the residuals of level 2, for which it is assumed that they are independent and follow a normal distribution with mean 0 and variance .
To quantify the random effect, the Median Odds Ratio (MOR) was used, which can be interpreted as the increase in the median risk of worse control of a patient if he/she changes from one state to another with a higher risk.24 All statistical analyses were carried out using STATA version 16.0 (StataCorp, Stata Statistical Software, 2019). P-value < 0.05 was considered as statistically significant.
Ethics approval and consent to participate
The conduct of this study did not require the approval of an institutional ethics committee, because it is a secondary analysis of public data available on the platform of the Mexican Secretary of Health under the Mexico's open government data (OGD) policy, in order to facilitate the access, use, reuse and redistribution of information.
Role of the funding source
This research received no external funding. All authors had full accesses to all data in the study and had final responsibility for the decision to submit for publication.
Results
Table 1 summarizes characteristics of 131,001 children and adolescents with COVID-19 by outcome. A total of 773 (0.60%) patients died due to COVID-19: 421 were male (54.46%) and 352 female (45.54%), 419 (54.20%) patients were less than 10 years old. The mean age at the moment of COVID-19 diagnosis was 13.5 years (13.6 years for alive cases versus 8.2 years for deceased cases). Among indigenous children with COVID-19, observed mortality was 2.25% (17/757).] Also, 905 (0.69%) patients required ICU admission and 210 (27.17%) of those patients died. Comorbidities were more frequent in patients who died: 10.31% immunosuppression, 7.76% hypertension, 7.28% CKD, 6.64% diabetes, 5.07% cardiovascular disease, 3.99% obesity, 1.56% asthma, and 0.52% COPD. A total of 127 (16.43%) deceased cases presented more than one comorbidity, and the percentage of deceased patients with pneumonia was 66.36%.
Table 1.
Characteristics of children and adolescents according to COVID-19 outcome.
| Alive (n = 130,228) | Dead (n = 773) | Total (n = 131,001) | p-value | ||
|---|---|---|---|---|---|
| Sex, n (%) | Female | 65551 (50.34%) | 352 (45.54%) | 65903 (50.31%) | 0.007 |
| Male | 64677 (49.66%) | 421 (54.46%) | 65098 (49.69%) | ||
| Edad, mean (SD) | 13.56 (5.27) | 8.23 (7.46) | 13.53 (5.3) | <0.001 | |
| Age group (years) | < 10 | 27203 (20.89%) | 419 (54.20%) | 27622 (21.09%) | <0.001 |
| 10 - 14 | 31143 (23.91%) | 104 (13.45%) | 31247 (23.85%) | ||
| ≥ 15 | 71882 (55.20%) | 250 (32.34%) | 72132 (55.06%) | ||
| Intubated, n (%) | No | 129813 (99.71%) | 465 (60.63%) | 130278 (99.48%) | <0.001 |
| Yes | 378 (0.29%) | 302 (39.37%) | 680 (0.52%) | ||
| Pneumonia, n (%) | No | 127137 (97.63%) | 260 (33.64%) | 127397 (97.25%) | <0.001 |
| Yes | 3091 (2.37%) | 513 (66.36%) | 3604 (2.75%) | ||
| Indigenous, n (%) | No | 121488 (99.32%) | 740 (97.75%) | 122228 (99.31%) | <0.001 |
| Yes | 835 (0.68%) | 17 (2.25%) | 852 (0.69%) | ||
| Diabetes, n (%) | No | 129294 (99.40%) | 717 (93.36%) | 130011 (99.36%) | <0.001 |
| Yes | 780 (0.60%) | 51 (6.64%) | 831 (0.64%) | ||
| Obesity, n (%) | No | 124933 (96.04%) | 698 (90.53%) | 125631 (96.01%) | <0001 |
| Yes | 5154 (3.96%) | 73 (9.47%) | 5227 (3.99%) | ||
| COPD, n (%) | No | 129970 (99.91%) | 765 (99.48%) | 130735 (99.90%) | <0.001 |
| Yes | 121 (0.09%) | 4 (0.52%) | 125 (0.10%) | ||
| Asthma, n (%) | No | 126275 (97.07%) | 757 (98.44%) | 127032 (97.08%) | 0.025 |
| Yes | 3806 (2.93%) | 12 (1.56%) | 3818 (2.92%) | ||
| Immunosuppression | No | 129258 (99.37%) | 687 (89.69%) | 129945 (99.31%) | <0.001 |
| Yes | 820 (0.63%) | 79 (10.31%) | 899 (0.69%) | ||
| Hypertension, n (%) | No | 129409 (99.48%) | 717 (93.24%) | 130126 (99.45%) | <0.001 |
| Yes | 674 (0.52%) | 52 (6.76%) | 726 (0.55%) | ||
| Cardiovascular disease, n (%) | No | 129471 (99.53%) | 730 (94.93%) | 130201 (99.50%) | <0.001 |
| Yes | 615 (0.47%) | 39 (5.07%) | 654 (0.50%) | ||
| CKD, n (%) | No | 129753 (99.75%) | 713 (92.72%) | 130466 (99.70%) | <0.001 |
| Yes | 331 (0.25%) | 56 (7.28%) | 387 (0.30%) | ||
| Patient type, n (%) | Outpatients | 124094 (95.29%) | 50 (6.47%) | 124144 (94.77%) | <0.001 |
| Inpatients | 5439 (4.18%) | 513 (66.36%) | 5952 (4.54%) | ||
| ICU | 695 (0.53%) | 210 (27.17%) | 905 (0.69%) | ||
| Comorbidities, n (%) | 0 | 117708 (90.39%) | 400 (51.75%) | 118108 (90.16%) | <0.001 |
| 1 | 10860 (8.34%) | 246 (31.82%) | 11106 (8.48%) | ||
| Mayor de 1 | 1660 (1.27%) | 127 (16.43%) | 1787 (1.36%) |
COPD, chronic obstructive pulmonary disease; CKD, chronic kidney disease; ICU, intensive care unit.
Table 2 presents the environmental characteristics where children and adolescents resided at the time of diagnosis of SARS-CoV-2 infection. A total of 198 (25.61%) deaths occurred in children and adolescents living in areas with high and very high social lag index. COVID-19 mortality was significantly different among the indicators related to social lag index, except for population not affiliated to any health service. Deceased children and adolescents lived in areas with a greater number of people with malnutrition compared to children and adolescents who survived (an average of 2022.68 vs 1872.03 inhabitants). Finally, children and adolescents living in areas with higher rates of complete vaccination coverage who were one year old and up to two years old showed lower mortality percentage than those with “schedule with 4 vaccines”.
Table 2.
Neighborhood characteristics for the entities where children and adolescents resided according to COVID-19 outcome.
| Alive (n = 130,228) | Dead (n = 773) | Total (n = 131,001) | p-value* | ||
|---|---|---|---|---|---|
| Social lag index, n (%) | Very low | 65564 (50.35%) | 148 (19.15%) | 65712 (50.16%) | <0.001 |
| Low | 39436 (30.28%) | 375 (48.51%) | 39811 (30.39%) | ||
| Medium | 10842 (8.33%) | 52 (6.73%) | 10894 (8.32%) | ||
| High | 8943 (6.87%) | 95 (12.29%) | 9038 (6.90%) | ||
| Very high | 5443 (4.18%) | 103 (13.32%) | 5546 (4.23%) | ||
| Indicators related to social lag, mean (SD) | Percentage of population aged 15 and over that is illiterate | 3.05 (2.41) | 4.42 (3.21) | 3.05 (2.42) | <0.001 |
| Percentage of population with children of school age of 6–14 years who do not attend school | 5.48 (0.77) | 5.84 (0.97) | 5.48 (0.78) | <0.001 | |
| Percentage of population with incomplete basic education | 23.76 (7.34) | 28.74 (7.78) | 23.79 (7.35) | <0.001 | |
| Percentage of population not affiliated to any health service | 25.78 (4.88) | 25.69 (5.88) | 25.78 (4.89) | 0.626 | |
| Percentage of inhabited private housing units without floor covering | 2.04 (2.47) | 3.4 (3.56) | 2.05 (2.48) | <0.001 | |
| Percentage of inhabited private housing units without toilets or sanitary services | 1.29 (1.45) | 1.96 (1.88) | 1.29 (1.46) | <0.001 | |
| Percentage of inhabited private housing units without piped water from the public network | 2.31 (2.22) | 3.29 (2.94) | 2.32 (2.23) | <0.001 | |
| Percentage of inhabited private housing units without drainage | 2.35 (3.31) | 4.18 (4.26) | 2.36 (3.32) | <0.001 | |
| Percentage of inhabited private housing units without electricity services | 0.44 (0.49) | 0.72 (0.56) | 0.44 (0.49) | <0.001 | |
| Percentage of inhabited private housing units without washing machine | 21.83 (8.47) | 26.56 (12.16) | 21.86 (8.5) | <0.001 | |
| Percentage of inhabited private housing units without refrigerator | 9.06 (5.51) | 12.12 (7.74) | 9.08 (5.53) | <0.001 | |
| Indicators related to malnutrition, mean (SD) | Number of people with malnutrition | 1872.03 (1105.02) | 2022.68 (1431.78) | 1872.92 (1107.29) | <0.001 |
| Vaccination coverage for children of one year of age, mean (SD) | Complete schedule | 21.22 (7.04) | 17.69 (8.46) | 21.2 (7.06) | <0.001 |
| Schedule with 4 vaccines | 22.01 (6.93) | 18.9 (8.65) | 21.99 (6.94) | <0.001 | |
| Vaccination coverage for children up to age two, mean (SD) | Complete schedule | 34.75 (6.46) | 32.84 (9.23) | 34.74 (6.48) | <0.001 |
| Schedule with 4 vaccines | 37.61 (6.51) | 35.08 (9.44) | 37.6 (6.54) | <0.001 | |
| Population density, mean (SD) | 2782.28 (2960.73) | 674.12 (1585.66) | 2769.84 (2958.9) | <0.001 |
Significant value (p < 0.05).
Table 3 shows results of the multilevel model to estimate association between COVID-19 mortality and individual characteristics, adjusted for population density. From this model, it was estimated that the median odds of mortality due to COVID-19 in an area with a high mortality would be approximately twice that of one with a low mortality (MOR = 1.797, 95% CI: 1.482–2.113). This analysis demonstrated a negative association between COVID-19 mortality and age (OR = 0.878, 95% CI: 0.869–0.888). Children and adolescents with diabetes (OR = 3.898, 95% CI: 2.596–5.851), immunosuppression (OR = 5.410, 95% CI: 4.088–7.158), obesity (OR = 1.876, 95% CI: 1.397–2.521), hypertension (OR = 1.906, 95% CI: 1.239–2.932), cardiovascular disease (OR = 2.288, 95% CI: 1.482–3.531), and chronic kidney disease (OR = 13.250, 95% CI: 9.066–19.350) showed a higher risk of mortality.
Table 3.
Factors associated with COVID-19 mortality in children and adolescents in Mexico. Results from the multilevel logistic regression models adjusted for sex, age and comorbidities.
| OR | IC 95% | P-value* | |
|---|---|---|---|
| Sex (Ref. Female) | 1.115 | 0.962 - 1.292 | 0.147 |
| Age | 0.878 | 0.869 - 0.888 | 0.000 |
| Diabetes | 3.898 | 2.596 - 5.851 | 0.000 |
| Obesity | 1.876 | 1.397 - 2.521 | 0.000 |
| Immunosuppression | 5.410 | 4.088 - 7.158 | 0.000 |
| Hypertension | 1.906 | 1.239 - 2.932 | 0.003 |
| Cardiovascular disease | 2.288 | 1.482 - 3.531 | 0.000 |
| CKD | 13.250 | 9.066 - 19.350 | 0.000 |
| Population density | 0.374 | 0.204 - 0.688 | 0.002 |
| MOR | 1.797 | 1.482 - 2.113 | 0.000 |
Significant value (p < 0.05).
CKD, chronic kidney disease.
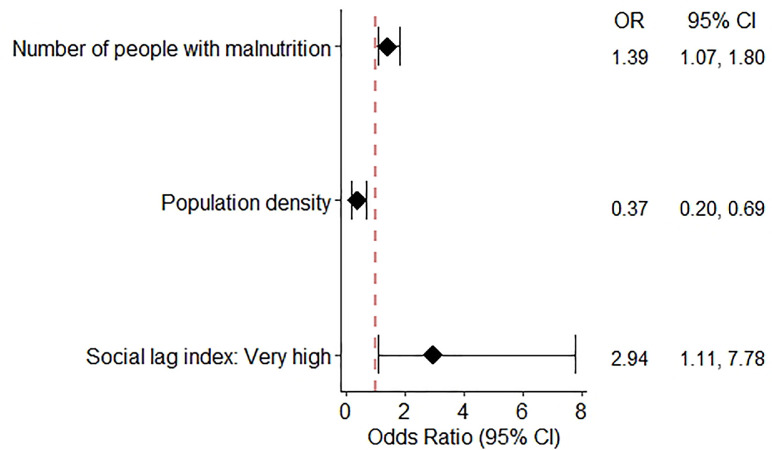
Regarding environmental factors, social lag index and malnutrition were positively associated with COVID-19 mortality (ORvery high = 2.939, 95%CI: 1.111-7.775, and OR = 1.390, 95%CI: 1.073-1.802, respectively). Population density presented a negative association with COVID-19 mortality (OR = 0.374, 95%CI: 0.204–0.688). The models were adjusted by age, sex, diabetes, obesity, immunosuppression, hypertension, and cardiovascular disease. In addition, the malnutrition model was also adjusted for population density (Figure 2).
Figure 2.
Adjusted associations between environmental factors and COVID-19 mortality.
Odds Ratio (OR); 95% Confidence interval (CI). Values were obtained using multivariable multilevel logistic regression models adjusted for sex, age, diabetes, obesity, immunosuppression, hypertension, cardiovascular disease and CKD. The models with the malnutrition variables were also adjusted for population density.
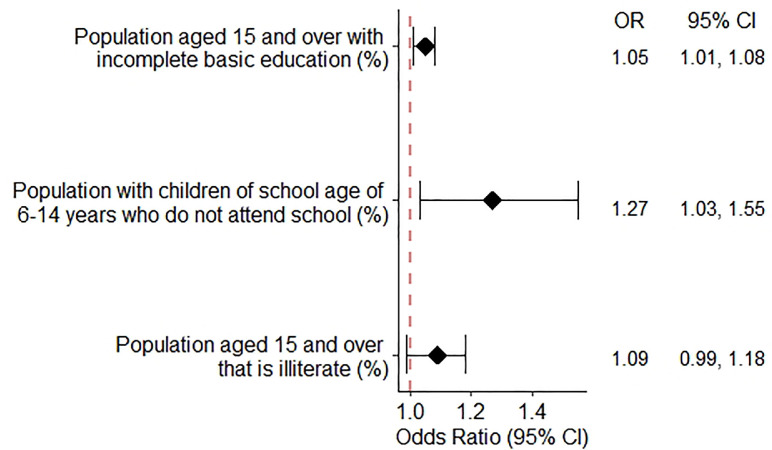
Figure 3 presents the odds of the indicators that make up the social lag index and COVID-19 mortality adjusted by state and individual characteristics. A higher percentage of population aged 15 and over with incomplete education was associated with a higher risk of COVID-19 mortality (OR = 1.045, 95%CI: 1.011–1.081), as well as a higher percentage of population with children of school age of 6-14 years who do not attend school (OR = 1.266, 95%CI 1.032–1.554), and a higher percentage of population aged 15 and over that are illiterate (OR = 1.086, 95%CI 0.999-1.179).
Figure 3.
Adjusted associations between indicators relates to social lag index and COVID-19 mortality.
Odds Ratio (OR); 95% Confidence interval (CI). Values were obtained using multivariable multilevel logistic regression models adjusted for sex, age, diabetes, obesity, immunosuppression, hypertension, cardiovascular disease and CKD.
Discussion
In this study, we assessed the influence of environmental and individual factors on COVID-19 mortality in children and adolescents in Mexico during the first two years of the pandemic. The most relevant findings of this work can be summarized as follows: (i) Children and adolescents living in areas with higher prevalence of malnutrition and very high social lag indexes (especially its indicators on access to education) showed greater odds of dying from COVID-19; and (ii) Regarding individual characteristics, age and pre-existing comorbidities such as diabetes, obesity, immunosuppression, hypertension, cardiovascular disease, and CKD were associated with an increased risk of mortality.
Child malnutrition is a serious public health issue in Mexico.25,26 Malnutrition was associated with socially disadvantaged populations and the pandemic could have worsened this problem.27 Malnutrition is actually a risk factor associated with mortality in some viral infection such as influenza A (H1N1).28 The impact of undernutrition in COVID-19 outcomes became a subject of interest.29 Our findings highlight the relevance of malnutrition in COVID-19 mortality among children and adolescents in Mexico. Mertens and Peñalvo described a potential relationship between COVID-19 mortality and areas with an elevated burden of undernutrition.30 Although this analysis used country-level data, its conclusions were robust enough to consider the importance of malnutrition in the prognosis of the COVID-19 disease. A study conducted in India concluded that malnutrition among under-five children was a risk factor of COVID-19.31 Alec Kurtz et al. showed that malnutrition was correlated with COVID-19 severity in an age-dependent way, and found the worst odds for severe COVID-19 in children between ages 6 and 17 with a history of malnutrition.32
Social inequalities in COVID-19 mortality were previously studied. In the present cohort, a very high category in social lag index was associated with a worse COVID-19 disease prognosis in children. Previous research found similar results in other age groups.33 Other studies from Brazil and Mexico showed that the poorest population groups have higher mortality from COVID-19.34,35 Lower education level showed a strong association with COVID-19 mortality in recent studies in the United States36,37 Ribeiro et al. showed similar findings in Brazil: Lower education level was the strongest association with COVID-19 fatalities.38
Additionally, our findings showed that living in an area with a high population density is associated with lower odds of dying from COVID-19. Some studies conducted by researchers at the Johns Hopkins Bloomberg School of Public Health found that the death rate is inversely related to population density, since dense counties had a significantly lower rate of COVID-19 mortality.39,40 This is in contrast to others assumptions, but density could lead to better access to health care facilities and infrastuctures, as well as achieving a greater impact with other measures like physical distancing and lockdown.41
Our findings also pointed to a connection between obesity and a worse COVID-19 prognosis. Several studies found a strong association between this variable and short-term mortality caused by COVID-19 in the general population.42, 43, 44 Tripathi et al. focused on the impact of previous conditions on COVID-19 outcomes in children. This work, based on a large cohort in the United States, found that obesity was a key factor in understanding the evolution of COVID-19 in this group45.
Other comorbidities were associated with COVID-19 mortality in children and adolescents in our analysis. According to previous studies, other underlying comorbidities may lead to a worse outcome in children and adolescents.14,46,47 A recent meta-analysis showed that excessive adipose tissue, deficit in lean mass, insulin resistance, dyslipidemia, hypertension, high levels of proinflammatory cytokines, and low intake of essential nutrients are associated with damage to immune, cardiovascular, respiratory, and urinary systems, as well as with modification of the intestinal microbiota (dysbiosis).48 Moreira et al. found that children with pre-existing conditions, such as asthma, autoimmune disease, cardiovascular disease, chronic lung disease, gastrointestinal/liver disease or hypertension, had an increased risk of hospitalization and death.49 Differences between baseline characteristics in these cohorts and access to healthcare systems should be considered when interpreting these findings.
Regarding age, our analysis showed that younger patients showed a higher risk of COVID-19 death. This finding is controversial considering previous studies. A large cohort in the United States found that younger age increases the risk of hospitalization, but not death.49 However, the review by Kitano et al. found that over 90% of pediatric COVID-19 deaths were from Low-Middle-Income Countries and that mortality risk was highest in children under 1-year old.10 Potential explanations to the higher risk of COVID-19 mortality Mexican younger patients could involve individual factors as immune function differences and lack of prior exposure to other coronaviruses.50 Also, the important role of social determinants of health and the quality of health care system could be related to the negative results.51
This study has several strengths. Data from a relevant number of Mexican patients were analyzed. To our knowledge, this is the largest pediatric evaluation assessing factors associated with COVID-19 mortality in children and adolescents in Mexico. Nonetheless, some limitations must be considered. First, the presence of comorbidities was self-reported (or reported by a caregiver), which could lead to misclassification bias. In addition, there is a small group of patients who reported other comorbidities that are nonspecific and unrelated to an increased risk of severe infection and poor prognosis. Second, we used cross-sectional information, limiting our ability to establish a clear causal link. Third, the use of the sentinel surveillance system to detect and report COVID-19 cases in Mexico likely skews detection towards more severe cases. Also, since we rely on data from the population who had symptoms and sought health care, we do not have any information on patients who were asymptomatic or mildly symptomatic and did not seek health care. Another limitation of our study is that we cannot exclude the possibility that the number of deaths in patients who had COVID-19 could be underreported. Finally, the environmental factors were measured at the state level, since our ability to geocode the patients was restricted to that level. States are fairly large and heterogeneous, composed by a variable mix of rich and poor neighborhoods. Nevertheless, despite its limitations, we used a large national registry of COVID-19 cases manteined by the Mexican government and many of the cases were tested using both Rapid Ag-T and RT-PCR.
Our findings show the impact of malnutrition, poverty and pre-existing comorbidities in the prognosis of COVID-19 pandemic. The relevance of malnutrition seems to be associated with population density. This finding could be related to the scarce access to health care services in rural areas. Our study can have relevant implications for reaching a more comprehensive understanding of the factors associated with COVID-19 mortality in children and adolescents.
Contributors
CSP, and FJPG conceptualised the analysis. CSP, and FJPG accessed and verified the data, completed the formal analysis, and drafted the original manuscript. All authors had access to all data and contributed to study design, data collection, and manuscript editing. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Data sharing
The datasets generated and/or analyzed during the current study are available in: https://datos.gob.mx/busca/dataset/informacion-referente-a-casos-covid-19-en-mexico/resource/f9418a4c-10e6-4099-8036-f51234b0e81b
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.lana.2022.100184.
Appendix. Supplementary materials
References
- 1.Alsohime F., Temsah M.H., Al-Nemri A.M., Somily A.M., Al-Subaie S. COVID-19 infection prevalence in pediatric population: Etiology, clinical presentation, and outcome. J Infect Public Health. 2020;13(12):1791–1796. doi: 10.1016/j.jiph.2020.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saatci D., Ranger T.A., Garriga C., Clift A.K., Zaccardi F., San Tan P., et al. Association between race and COVID-19 outcomes among 2.6 million children in England. JAMA Pediatr. 2021;175(9):928–938. doi: 10.1001/jamapediatrics.2021.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siebach M.K., Piedimonte G., Ley S.H. COVID-19 in childhood: transmission, clinical presentation, complications and risk factors. Pediatr Pulmonol. 2021;56(6):1342–1356. doi: 10.1002/ppul.25344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shekerdemian L.S., Mahmood N.R., Wolfe K.K., Riggs B.J., Ross C.E., McKiernan C.A., et al. Characteristics and outcomes of children with coronavirus disease 2019 (COVID-19) infection admitted to US and Canadian pediatric intensive care units. JAMA Pediatr. 2020;174(9):868–873. doi: 10.1001/jamapediatrics.2020.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swann O.V., Holden K.A., Turtle L., Pollock L., Fairfield C.J., Drake T.M., et al. Clinical characteristics of children and young people admitted to hospital with covid-19 in United Kingdom: prospective multicentre observational cohort study. BMJ. 2020;370:m3249 doi: 10.1136/bmj.m3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yasuhara J., Watanabe K., Takagi H., Sumitomo N., Kuno T. COVID-19 and multisystem inflammatory syndrome in children: a systematic review and meta-analysis. Pediatr Pulmonol. 2021;56(5):837–848. doi: 10.1002/ppul.25245. %@ 8755-6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martins-Filho P.R., Quintans-Júnior L.J., de Souza Araújo A.A., Sposato K.B., Tavares C.S.S., Gurgel R.Q., et al. Socio-economic inequalities and COVID-19 incidence and mortality in Brazilian children: a nationwide register-based study. Public Health. 2021;190:4–6. doi: 10.1016/j.puhe.2020.11.005. %@ 0033-3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris M., Reza JN. World Health Organization; 2012. Global report for research on infectious diseases of poverty. [Google Scholar]
- 9.Wise P.H., Kotelchuck M., Wilson M.L., Mills M. Racial and socioeconomic disparities in childhood mortality in Boston. N Engl J Med. 1985;313(6):360–366. doi: 10.1056/NEJM198508083130605. [DOI] [PubMed] [Google Scholar]
- 10.Kitano T., Kitano M., Krueger C., Jamal H., Al Rawahi H., Lee-Krueger R., et al. The differential impact of pediatric COVID-19 between high-income countries and low-and middle-income countries: a systematic review of fatality and ICU admission in children worldwide. PLoS One. 2021;16(1) doi: 10.1371/journal.pone.0246326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Headey D., Heidkamp R., Osendarp S., Ruel M., Scott N., Black R., et al. Impacts of COVID-19 on childhood malnutrition and nutrition-related mortality. Lancet North Am Ed. 2020;396(10250):519–521. doi: 10.1016/S0140-6736(20)31647-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu Y., Ye J., Chen M., Jiang C., Lin W., Lu Y., et al. Malnutrition prolongs the hospitalization of patients with COVID-19 infection: a clinical epidemiological analysis. J Nutr Health Aging. 2021;25(3):369–373. doi: 10.1007/s12603-020-1541-y. %@ 1760-4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anker M.S., Landmesser U., von Haehling S., Butler J., Coats A.J.S., Anker SD. Wiley Online Library; 2021. Weight loss, malnutrition, and cachexia in COVID-19: facts and numbers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsankov B.K., Allaire J.M., Irvine M.A., Lopez A.A., Sauvé L.J., Vallance B.A., et al. Severe COVID-19 infection and pediatric comorbidities: a systematic review and meta-analysis. Int J Infect Dis. 2021;103:246–256. doi: 10.1016/j.ijid.2020.11.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caussy C., Wallet F., Laville M., Disse E. Obesity is associated with severe forms of COVID-19. Obesity (Silver Spring) 2020;28(7):1175. doi: 10.1002/oby.22842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang J., Zheng Y., Gou X., Pu K., Chen Z., Guo Q., et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dirección general de epidemiología. Lineamiento estandarizado para la vigilancia epidemiológica y por laboratorio de la enfermedad respiratoria viral. Secretaría de Salud. Agosto de. 2020 https://coronavirus.gob.mx/wp-content/uploads/2020/09/Lineamiento_VE_y_Lab_Enf_Viral_Ago-2020.pdf. [Google Scholar]
- 18.Vargas-Chanes D., Valdés-Cruz S. A longitudinal study of social lag: regional inequalities of growth in Mexico 2000 to 2015. J Chin Sociol. 2019;6(1):1–18. %@ 2198-635. [Google Scholar]
- 19.Dirección General de Epidemiología. Histórico boletín epidemiológico. Secretaría de Salud. México. 2020 https://www.gob.mx/salud/acciones-y-programas/historico-boletin-epidemiologico [Google Scholar]
- 20.Márquez-González H., García-Sámano V.M., de L., Caltenco-Serrano M., García-Villegas E.A., Márquez-Flores H., Villa-Romero AR. Clasificación y evaluación de la desnutrición en el paciente pediátrico. El Resid. 2012;7(2):59–69. %@ 2007-783. [Google Scholar]
- 21.United Nations Children's Fund (UNICEF), World Health Organization, International Bank for Reconstruction and Development/The World Bank. Levels and trends in child malnutrition: Key Findings of the 2020 Edition of the Joint Child Malnutrition Estimates. Geneva: World Health Organization; 2020. Licence: CC BY-NC-SA 3.0 IGO.
- 22.Romero-Martínez M., Shamah-Levy T., Vielma-Orozco E., Heredia-Hernández O., Mojica-Cuevas J., Cuevas-Nasu L., et al. Encuesta nacional de salud y nutrición 2018-19: metodología y perspectivas. Salud Pública de México. 2019;61(6, nov-dic):917–923. doi: 10.21149/11095. [DOI] [PubMed] [Google Scholar]
- 23.INEGI. INEGI [Internet]. México en Cifras. 2015. Available from: http://cuentame.inegi.org.mx/poblacion/asistencia.aspx?tema=P.
- 24.Merlo J., Chaix B., Ohlsson H., Beckman A., Johnell K., Hjerpe P., et al. A brief conceptual tutorial of multilevel analysis in social epidemiology: using measures of clustering in multilevel logistic regression to investigate contextual phenomena. J Epidemiol Community Health. 2006;60(4):290–297. doi: 10.1136/jech.2004.029454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kroker-Lobos M.F., Pedroza-Tobías A., Pedraza L.S., Rivera JA. The double burden of undernutrition and excess body weight in Mexico. Am J Clin Nutr. 2014;100(6):1652S–1658S. doi: 10.3945/ajcn.114.083832. %@ 0002-9165. [DOI] [PubMed] [Google Scholar]
- 26.Batis C., Denova-Gutiérrez E., Estrada-Velasco B.I., Rivera J. Malnutrition prevalence among children and women of reproductive age in Mexico by wealth, education level, urban/rural area and indigenous ethnicity. Public Health Nutr. 2020;23(S1):s77–s88. doi: 10.1017/S1368980019004725. %@ 1368-9800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Romero-Michel J.C., Mokay-Ramírez K.A., Delgado-Machuca M., Delgado-Enciso J., Aurelien-Cabezas N.S., Tiburcio-Jimenez D., et al. Health and economic measures in response to the COVID-19 pandemic-effect on street vendors. J Infect Dev Ctries. 2021;15(02):198–203. doi: 10.3855/jidc.13465. [DOI] [PubMed] [Google Scholar]
- 28.Reyes L., Arvelo W., Estevez A., Gray J., Moir J.C., Gordillo B., et al. Population-based surveillance for 2009 pandemic influenza A (H1N1) virus in Guatemala, 2009. Influenza Respir Viruses. 2010;4(3):129–140. doi: 10.1111/j.1750-2659.2010.00138.x. %@ 1750-2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu H., Zhou L., Wang H., Wang X., Qu G., Cai J., et al. Malnutrition is associated with hyperinflammation and immunosuppression in COVID-19 patients: a prospective observational study. Nutr Clin Practice. 2021;36(4):863–871. doi: 10.1002/ncp.10679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mertens E., Peñalvo J.L. The burden of malnutrition and fatal COVID-19: a global burden of disease analysis. Front Nutr. 2021;7:351. doi: 10.3389/fnut.2020.619850. %@ 2296-861X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saha J., Chouhan P. Do malnutrition, pre-existing morbidities, and poor household environmental conditions aggravate susceptibility to Coronavirus disease (COVID-19)? A study on under-five children in India. Child Youth Serv Rev. 2021;128 doi: 10.1016/j.childyouth.2021.105962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kurtz A., Grant K., Marano R., Arrieta A., Feaster W., Steele C., et al. Long-term effects of malnutrition on severity of COVID-19. Sci Rep. 2021;11(1):1–8. doi: 10.1038/s41598-021-94138-z. %@ 2045-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bello-Chavolla O.Y., González-Díaz A., Antonio-Villa N.E., Fermín-Martínez C.A., Márquez-Salinas A., Vargas-Vázquez A., et al. Unequal impact of structural health determinants and comorbidity on COVID-19 severity and lethality in older Mexican Adults: considerations beyond chronological aging. J Gerontol Ser A. 2021;76(3):e52–ee9. doi: 10.1093/gerona/glaa163. %@ 1079-5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martins-Filho P.R., de Souza Araújo A.A., Quintans-Júnior L.J., Santos V.S. COVID-19 fatality rates related to social inequality in Northeast Brazil: a neighbourhood-level analysis. J Travel Med. 2020;27(7) doi: 10.1093/jtm/taaa128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Millán-Guerrero R.O., Caballero-Hoyos R., Monárrez-Espino J. Poverty and survival from COVID-19 in Mexico. J Public Health (Oxf) 2021;43(3):437–444. doi: 10.1093/pubmed/fdaa228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seligman B., Ferranna M., Bloom D.E. Social determinants of mortality from COVID-19: a simulation study using NHANES. PLoS Med. 2021;18(1) doi: 10.1371/journal.pmed.1003490. %@ 1549-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hawkins R.B., Charles E.J., Mehaffey JH. Socio-economic status and COVID-19–related cases and fatalities. Public Health. 2020;189:129–134. doi: 10.1016/j.puhe.2020.09.016. %@ 0033-3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ribeiro K.B., Ribeiro A.F., MAdSM V., de Castro M.C. Social inequalities and COVID-19 mortality in the city of São Paulo, Brazil. Int J Epidemiol. 2021;50(3):732–742. doi: 10.1093/ije/dyab022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hamidi S., Ewing R., Sabouri S. Longitudinal analyses of the relationship between development density and the COVID-19 morbidity and mortality rates: early evidence from 1,165 metropolitan counties in the United States. Health Place. 2020;64 doi: 10.1016/j.healthplace.2020.102378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hamidi S., Sabouri S., Ewing R. Does density aggravate the COVID-19 pandemic? J Am Plann Assoc. 2020;86(4):495–509. [Google Scholar]
- 41.Bhadra A., Mukherjee A., Sarkar K. Impact of population density onCovid-19 infected and mortality rate in India. Model Earth Syst Environ. 2020;14:1–7. doi: 10.1007/s40808-020-00984-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vera-Zertuche J.M., Mancilla-Galindo J., Tlalpa-Prisco M., Aguilar-Alonso P., Aguirre-García M.M., Segura-Badilla O., et al. Obesity is a strong risk factor for short-term mortality and adverse outcomesin Mexican patients with COVID-19: a national observational study. Epidemiol Infect. 2021;149:e109. doi: 10.1017/S0950268821001023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gardiner J., Oben J., Sutcliffe A. Obesity as a driver of international differences in COVID-19 death rates. Diabetes Obes Metab. 2021;23(7):1463–1470. doi: 10.1111/dom.14357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hussain A., Mahawar K., Xia Z., Yang W., El-Hasani S. Obesity and mortality of COVID-19. Meta-analysis. Obes Res Clin Pract. 2020;14(4):295. doi: 10.1016/j.orcp.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 45.Tripathi S., Christison A.L., Levy E., McGravery J., Tekin A., Bolliger D., et al. The Impact of Obesity on Disease Severity and Outcomes Among Hospitalized Children With COVID-19. Hosp Pediatr. 2021;11(11):e297–e316. doi: 10.1542/hpeds.2021-006087. [DOI] [PubMed] [Google Scholar]
- 46.Oliveira E.A., Colosimo E.A., e Silva A.C.S., Mak R.H., Martelli D.B., Silva L.R., et al. Clinical characteristics and risk factors for death amonghospitalised children and adolescents with COVID-19 in Brazil: an analysis of a nationwide database. Lancet Child Adolesc Health. 2021;5(8):559–568. doi: 10.1016/S2352-4642(21)00134-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sousa B.L.A., Brentani A., Ribeiro C.C.C., Dolhnikoff M., Grisi S., Ferrer A.P.S., et al. Non-communicable diseases, sociodemographic vulnerability and the risk of mortality in hospitalised children and adolescents with COVID-19 in Brazil: a cross-sectional observational study. BMJ Open. 2021;11(9) doi: 10.1136/bmjopen-2021-050724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nogueira-de-Almeida C.A., Del Ciampo L.A., Ferraz I.S., Del Ciampo I.R.L., Contini A.A., Ued F.D.V. COVID-19 and obesity in childhood and adolescence: a clinical review. J Pediatr (Rio J) 2020;96(5):546–558. doi: 10.1016/j.jped.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moreira A., Chorath K., Rajasekaran K., Burmeister F., Ahmed M., Moreira A. Demographic predictors of hospitalization and mortality in US children with COVID-19. Eur J Pediatr. 2021;180(5):1659–1663. doi: 10.1007/s00431-021-03955-x. %@ 432-076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zimmermann P., Curtis N. Why is COVID-19 less severe in children? A review of the proposed mechanisms underlying the age-related difference in severity of SARS-CoV-2 infections. Arch Dis Child. 2020 doi: 10.1136/archdischild-2020-320338. [DOI] [PubMed] [Google Scholar]
- 51.Richardus J.H., Graafmans W.C., Verloove-Vanhorick S.P., Mackenbach J.P. The perinatal mortality rate as an indicator of quality of care in international comparisons. Med Care. 1998;36(1):54–66. doi: 10.1097/00005650-199801000-00007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.