Abstract
Stroke is a primary debilitating disease in adults, occurring in 15 million individuals each year and causing high mortality and disability rates. The latest estimate revealed that stroke is currently the second leading cause of death worldwide. Post-stroke cognitive impairment (PSCI), one of the major complications after stroke, is frequently underdiagnosed. However, stroke has been reported to increase the risk of cognitive impairment by at least five to eight times. In recent decades, peripheral blood molecular biomarkers for stroke have emerged as diagnostic, prognostic, and therapeutic targets. In this study, we aimed to evaluate some blood-derived proteins for stroke, especially related to brain damage and cognitive impairments, by conducting a systematic review and meta-analysis and discussing the possibility of these proteins as biomarkers for PSCI. Articles published before 26 July 2021 were searched in PubMed, Embase, the Web of Science, and the Cochrane Library to identify all relevant studies reporting blood biomarkers in patients with stroke. Among 1820 articles, 40 were finally identified for this study. We meta-analyzed eight peripheral biomarker candidates: homocysteine (Hcy), high-density lipoprotein cholesterol (HDL-C), C-reactive protein (CRP), low-density lipoprotein cholesterol (LDL-C), total cholesterol (TC), triglyceride (TG), uric acid, and glycated hemoglobin (HbA1c). The Hcy, CRP, TC, and LDL-C levels were significantly higher in patients with PSCI than in the non-PSCI group; however, the HDL-C, TG, uric acid, and HbA1c levels were not different between the two groups. Based on our findings, we suggest the Hcy, CRP, TC, and LDL-C as possible biomarkers in patients with post-stroke cognitive impairment. Thus, certain blood proteins could be suggested as effective biomarkers for PSCI.
Keywords: stroke, dementia, cognitive impairment, post-stroke cognitive impairment, blood biomarker
1. Introduction
Stroke occurs in 15 million individuals each year, causing high mortality and disability rates. The latest estimate revealed that stroke is the second leading cause of death worldwide [1,2]. Most strokes are ischemic, owing to the presence of a reduced blood flow, generally resulting from arterial occlusion. The remaining 10–40% of stroke presentations are hemorrhagic, depending on regional epidemiology and resulting from the rupture of the cerebral arteries [3,4]. Structural damage to the brain in patients with stroke occurs because of both ischemia and hemorrhage [5,6,7]. As a result, even minor stroke affects daily functions, executive functions, and cognition, consequently affecting patients’ activity performance, quality of life, and ability to return to work [8,9].
In particular, the cognitive domains involved in the development of dementia after stroke may vary depending on the stroke characteristics, such as stroke type, volume, number, location, and severity [10,11]. (1) Important critical locations include the dominant hemisphere and lesions affecting the prefrontal–subcortical circuit that mediates executive dysfunction [12,13]. (2) Frontal lobe functions comprising processing speed, reaction time, working memory, and executive task measures are most commonly affected [14]. (3) A single large cortico-subcortical brain ischemic lesion may present with acute cognitive deterioration if located in an area that is functionally critical for cognition [15]. (4) Strategic infarction dementia may be caused by damage to the components of the Papez (hippocampal memory loop) [16] or Yokovlev circuits [11].
Generally, stroke diagnosis depends crucially on neuroimaging; computed tomography remains an essential component of stroke management, although it is not always available [17]. Over the last decades, molecular biomarkers for stroke have gained the attention of clinicians around the world owing to their broad application in facilitating diagnosis, characterizing clinical size and severity, estimating long-term prognoses, and selecting an appropriate treatment option [18,19]. The National Institutes of Health Biomarkers Definitions Working Group proposed a new definition of biomarkers in 2001: “a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention” [20,21]. Thus, biomarkers are beneficial for patients, caregivers, and clinicians for: (a) planning subsequent clinical pathways and goal setting; and (b) identifying whom and when to target and, in some instances, at which level to use, along with interventions for promoting stroke recovery [22]. The application of these biomarkers can improve risk stratification and therefore guide the implementation of tailored treatment modalities [23].
Unlike general stroke, post-stroke cognitive impairment (PSCI), one of the major complications after stroke, is frequently underdiagnosed, as it may be overlooked in the presence of other distressing signs (e.g., motor or visual symptoms). Consequently, the cognitive impact of acute stroke is often underestimated [24,25]. However, stroke has been reported to increase the risk of cognitive impairment by at least five to eight times [26]. To diagnose PSCI, in fact, neuroimaging features of computed tomography (CT) or magnetic resonance imaging (MRI) such as functional MRI (fMRI) and diffusion tensor imaging (DTI) have been used [27,28] and cognitive assessments such as the Information Questionnaire on Cognitive Decline in the Elderly (IQCODE), the Mini-Mental State Examination (MMSE), and the National Institute of Neurological Disease and Stroke (NINDS) battery have been applied [29].
Therefore, we aimed to evaluate some blood-derived proteins, especially those related to brain damage and cognitive impairments, by conducting a systematic review and meta-analysis and discussing the possibility of these proteins as biomarkers for PSCI.
2. Results
2.1. Characteristics of the Included Studies Reporting Potential Biomarkers for PSCI
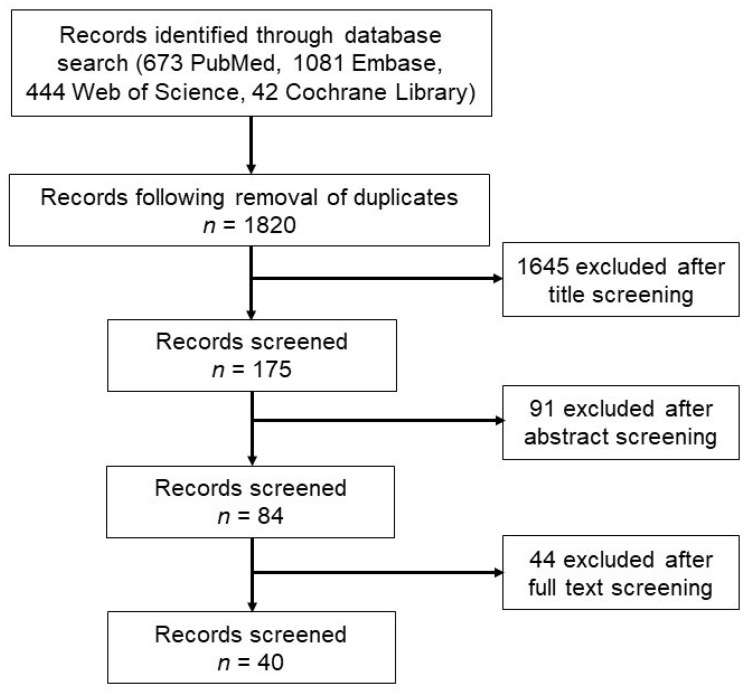
We identified 2240 studies including 673 from PubMed, 1081 from Embase, 444 from the Web of Science, and 42 from the Cochrane Library (Figure 1). Duplicates were then excluded, yielding a total of 1820 studies. Thereafter, 84 studies were assessed for eligibility based on their title and abstract after excluding the following studies: (1) studies that did not investigate potential blood biomarkers for evaluating cognitive function after stroke, (2) studies that used cell or animal models, and (3) commentaries, letters, editorials, conference abstracts, or reviews. After a full-text review, 40 articles were finally included in this study.
Figure 1.
Flow diagram of the study selection.
Table 1 shows the selected studies that reported potential blood biomarkers for PSCI. The included studies were published between 2004 and 2021. The countries of patients were Lithuania, Argentina, Japan, Taiwan, the USA, France, China, Canada, Israel, Poland, the Russian Federation, Sweden, and Turkey. The types of groups used included post-stroke without dementia and post-stroke dementia groups, drug or related molecular concentration groups, stroke progression groups, and Mini-Mental State Examination (MMSE) level groups. The sample size of the case and control groups ranged from 8 to 1029, dividing male and female patients and presenting the mean age in each group. The outcome measurement tools used for evaluating cognitive impairment included the MMSE, Montreal Cognitive Assessment, and clinical dementia rating. Furthermore, the sample specimens used were the plasma or serum. Potential blood biomarkers were identified in the selected studies.
Table 1.
Summary of the 40 selected studies reporting potential biomarkers for PSCI.
| Author and Year | Country | Study Groups | Sample Size (M/F) | Age (y) | Outcome Measurement Tool | Specimen | Potential Biomarkers |
|---|---|---|---|---|---|---|---|
| Bunevicius et al., 2015 | Lithuania | Acute ischemic stroke | 53/25 | 72 | MMSE | Serum | NT-proBNP, IL-6, hs-CRP |
| Hemorrhagic stroke | |||||||
| Casas et al., 2017 | Argentina | Control | 20/20 | 70 ± 3/77 ± 1 | MoCA | Plasma | BDNF, NO−2 |
| Acute ischemic stroke | 20/20 | 72 ± 4/83 ± 2 | |||||
| Chei et al., 2014 | Japan | Control | 88/104 | 62.2 ± 4.4 | The dementia status was classified into six ranks. | Serum | hs-CRP |
| Dementia with a history of stroke | 44/52 | 62.4 ± 4.3 | |||||
| Control | 98/260 | 62.8 ± 5.6 | |||||
| Dementia without a history of stroke | 49/130 | 63.1 ± 5.6 | |||||
| Chen et al., 2019a | Taiwan | Post-stroke without dementia | 56/31 | 62.98 ± 9.23 | CDR | Plasma | BChE |
| Post-stroke dementia | 18/12 | 73.20 ± 8.68 | |||||
| Chen et al., 2019b | Taiwan | Post-stroke without dementia | 41/12 | 61.7 ± 8.95 | MMSE | Plasma | D-amino acid oxidase |
| Post-stroke dementia | 11/9 | 69.35 ± 7.24 | |||||
| Choi et al., 2020 | USA | Acute ischemic stroke alone | 27/8 | 64.5 ± 14.1 | Serum | IL-6, CRP, complement component 3, S100B | |
| Acute ischemic stroke and underlying dementia | 5/3 | 85.8 ± 9.6 | |||||
| Cogo et al., 2021 | France | Post-stroke cognitive decline | 6/4 | 64.7 ± 13.3 | MMSE | Serum | Quinolinic acid, quinolinic acid/kynurenic acid ratio, tryptophan, kynurenine, kynurenic acid, kynurenine/tryptophan ratio, indoleamine 2,3-dioxygenase |
| Post-stroke cognitive decline | 8/5 | 69.4 ± 17.8 | |||||
| El Hussini et al., 2020 | USA | Small-vessel-type stroke | 9/13 | 56.5 (49.5–62.0) | A standardized battery of neuropsychological tests | Plasma | VCAM-1, IFN-γ, IL-1 RA, IL-6, IL-8, IL-10, thrombin-antithrombin |
| Feng et al., 2020 | China | Stroke rhGH group | 18/8 | 61.3 ± 10 | MoCA | Plasma | TC, LDL-C, HDL-C, TG, FBG, HbA1c, IGF-1, VEGF |
| Stroke placebo group | 17/9 | 60.8 ± 11.3 | |||||
| Ge et al., 2020 | China | Acute ischemic stroke | 414/184 | 59.9 ± 10.5 | MMSE/MoCA | Serum | TIMP-1, MMP-9 |
| Gold et al., 2011 | Canada | Ischemic stroke | 22/19 | 72.3 ± 12.2 | MMSE | Plasma | Tryptophan, L-kynurenine, L-kynurenine/tryptophan |
| Hou et al., 2019 | China | Total stroke | 140/121 | 66.4 ± 9.3 | MoCA | Serum | TC, TG, LDL-C, HDL-C, hs-CRP, Hcy, retinoic acid |
| Stroke without PSCI | 65/55 | 67.7 ± 9.3 | |||||
| Stroke with PSCI | 75/66 | 67.7 ± 9.3 | |||||
| Kliper et al., 2013 | Israel | First-ever mild to moderate stroke | MoCA | Serum | CRP | ||
| Krzystanek et al., 2007 | Poland | Stroke | 15/17 | 74.13 ± 7.43 | MMSE | Platelet | Phospholipase A2 |
| Vascular dementia | 13/19 | 75.25 ± 9.22 | |||||
| Alzheimer’s disease | 10/27 | 73 ± 6.45 | |||||
| Kulesh et al., 2018 | Russian Federation | Normal cognition | 8/7 | 59.5 ± 10.0 | MMSE/MoCA | Serum | IL-1β, IL-6, IL-10, TNFα |
| Dysexecutive cognitive impairment | 13/8 | 66.4 ± 8.8 | |||||
| Mixed cognitive impairment | 16/5 | 67.8 ± 8.2 | |||||
| Liu et al., 2018 | China | Acute ischemic stroke | 71/37 | MMSE | Plasma | Uric acid, creatinine, urea N, glucose | |
| Better outcome (mRS score of ≤ 2) | 32/19 | 63.9 ± 14.9 | |||||
| Poor outcome (mRS score of >2) | 39/18 | 66.1 ± 16.2 | |||||
| Liu et al., 2017 | China | Non-PSCI | 65/27 | 60 (52.3–65.8) | MMSE | Serum | Malondialdehyde, 8-OHdG |
| PSCI | 56/45 | 66 (56–72) | |||||
| Lu et al., 2016 | China | Acute ischemic stroke | 192/61 | MMSE/MoCA | Non-HDL-C, TC, HDL-C, LDL-C, FBG, TG, Hcy, hs-CRP, HbA1c | ||
| Normal non-HDL-C | 63.1 ± 11.9 | ||||||
| High non-HDL-C | 62.2 ± 10.8 | ||||||
| Mao et al., 2020 | China | Non-PSCI | 79/37 | 65 (60–74) | MoCA | Serum | Aβ42, T3, T4, FT3, FT4, TSH, TC, TG, HDL-C, LDL-C, hs-CRP, Hcy |
| PSCI | 38/34 | 73 (66–80) | |||||
| Marklund et al., 2004 | Sweden | Acute ischemic stroke | 56/32 | 71 ± 11 | MMSE | Serum | Cortisol, DS, cortisol/DS ratio |
| Pedersen et al., 2018 | Sweden | Acute ischemic stroke | 169/99 | 18–69 | BNIS | Plasma/serum | Von Willebrand factor, tissue plasminogen activator, fibrinogen, hs-CRP |
| Stroke for <50 years | 32/35 | ||||||
| Qian et al., 2012 | China | Stroke | 44/20 | 62.1 ± 1.6 | MMSE/MoCA | Serum | sRAGE, BACE, neprilysin |
| Vascular cognitive impairment with no dementia | 19/18 | 65.5 ± 1.7 | |||||
| Vascular dementia | 18/18 | 73.8 ± 2.1 | |||||
| Mixed dementia | 6/9 | 74.6 ± 2.2 | |||||
| Qian et al., 2020 | China | Endostatin concentration group | 431/182 | 60.0 ± 10.5 | MoCA | Plasma | Endostatin |
| Ran et al., 2020 | China | Stroke | 41/74 | 57.72 ± 6.11 | MoCA | Serum | Uric acid, hs-CRP, fibrinogen, TG, cholesterol |
| PSCI | 43/39 | 59.99 ± 7.46 | |||||
| Stokowska et al., 2021 | Sweden | Intervention group | 64/51 | Letter number sequence test | Plasma | NfL | |
| Sun et al., 2020 | China | Non-PSCI | 60/26 | 64.66 ± 11.57 | MoCA | Serum | Uric acid, folic acid, VB12, Hcy, TG, cholesterol, HDL-C, LDL-C |
| PSCI | 110/78 | 71.3 ± 10.88 | |||||
| Tang et al., 2017 | Taiwan | Stroke without vascular dementia | 90/46 | 71.2 ± 6.9 | CDR/MMSE/MoCA | Plasma | sRAGE, esRAGE |
| Stroke with vascular dementia | 21/15 | 75.4 ± 8.8 | |||||
| Tong et al., 2017 | China | Stroke | 21/21 | 75.55 ± 2.39 | MMSE | Plasma | Semicarbazide-sensitive amino oxidase, formaldehyde |
| Post-stroke dementia | 21/21 | 76.14 ± 3.73 | |||||
| Wang et al., 2021 | China | Stable | 148/107 | 64.86 ± 9.37 | MMSE/MoCA | Serum | NfL |
| Progression | 26/23 | 65.18 ± 8.61 | |||||
| Wang et al., 2020 | China | Control | 14/16 | 66.1 ± 5.9 | MMSE/MoCA | Plasma/serum | Aβ40, Aβ42, Aβ42/Aβ40, CRP, TNF-α, IL-6 |
| Observation | 17/13 | 67.2 ± 7.1 | |||||
| Wang et al., 2021 | China | Non-PSCI | 355/200 | 62 ± 13 | MoCA | Plasma | pNfL, HbA1c, hs-CRP, Hcy |
| PSCI | 538/491 | 66 ± 18.5 | |||||
| Weng et al., 2020 | China | Non-PSCI | 130/67 | 64 | MoCA | Blood | CRP, TB, DBIL, IBIL, TC, Ca, uric acid, HbA1c, D-dimer |
| PSCI | 102/74 | 72 | |||||
| Yalbuzdag et al., 2015 | Turkey | Ischemic | 53/43 | 63.78 ± 12.3 | MMSE | Plasma | 25(OH)D |
| Hemorrhagic | 11/13 | 61.8 ± 10.0 | |||||
| Yan et al., 2015 | China | Non-vascular dementia | 56/48 | MMSE/MoCA | Serum | Hcy, hs-CRP, LDL-C | |
| Vascular dementia | |||||||
| Zeng et al., 2019 | China | Cognitive impairment no dementia | 61/20 | 71.40 ± 11.32 | MoCA | Serum | Cystatin C, HbA1c, creatinine, uric acid, TC, TG, HDL-C, LDL-C |
| Vascular cognitive impairment | 45/26 | 76.28 ± 15.16 | |||||
| Zhong et al., 2018 | China | MMP concentration group | 558 | MMSE/MoCA | Serum | MMP-9 | |
| Zhong et al., 2021 | China | Choline/betaine/TMAO | 433/184 | 60 ± 10.5 | MMSE/MoCA | Plasma | Choline, betaine, TMAO |
| Zhu et al., 2020 | China | Non-PSCI | 89/81 | 65 ± 10.8 | MMSE | Plasma | TMAO, TC, TG, LDL-C, HDL-C, hs-CRP, FBG, Hcy |
| PSCI | 50/36 | 71.1 ± 10.4 | |||||
| Zhu et al., 2019 | China | RF concentration group | 582 | MMSE/MoCA | Serum | RF | |
| Zhu et al., 2019 | China | MMSE/MoCA group | 448/190 | 60.7 ± 10.3 | MMSE/MoCA | Serum | aPS, GPS, aCL, GPL, β2-GPI, RF, NT-proBNP, Lp-PLA2 mass, MMP-9, tHcy, eGFR, uric acid, HGF |
25(OH)D: 25-hydroxyvitamin D3, 8-OHdG: 8-hydroxydeoxyquanosine, aCL GPL: anticardiolipin antibodies, IgG anticar-diolipin antibodies units, aPS GPS: anti-phosphatidylserine antibodies, IgG antiphosphatidylserine antibodies units, Aβ40: amyloid β 40, Aβ42: amyloid β 42, BACE: β-secretase enzyme, BChE: butyrylcholinesterase, BDNF: brain-derived neurotrophic factor, CDR: clinical dementia rating, CRP: C-reactive protein, DBIL: direct bilirubin, DS: dehydroepiandrosterone sulphate, eGFR: estimated glomerular filtration rate, esRAGE: endogenous secretory RAGE, FBG: fasting blood glucose, FT3: free triiodothyroinine, FT4: free thyroxin, HbA1c: glycated hemoglobin, Hcy: homocysteine, HDL-C: high-density lipoprotein cholesterol, HGF: hepatocyte growth factor, hs-CRP: high-sensitivity C-reactive protein, IBIL: indirect bilirubin, IFN-γ: interferon-gamma, IGF-1: insulin-like growth factor-1, IL-1 RA: interleukin-1 receptor antagonist, IL-10: interleukin-10, IL-1β: interleukin-1 beta, IL-6: interleukin-6, IL-8: interleukin-8, LDL-C: low-density lipoprotein cholesterol, Lp-PLA2: lipoprotein-associated phospholipase A2, MMP-9: matrix metalloproteinase-9, MMSE: Mini-Mental State Examination, MoCA: Montreal Cognitive Assessment, NfL: neurofilament light, NT-proBNP: N-terminal pro b-type natriuretic peptide, PSCI: post-stroke cognitive impairment, RF: rheumatoid factor, sRAGE: soluble receptor for advanced glycation end products, T3: triiodothyronine, T4: thyroxin, TB: total bilirubin, TC: total cholesterol, TG: triglyceride, tHcy: total homocysteine, TIMP-1: tissue inhibitor metalloproteinase-1, TMAO: trimethylamine N-oxide, TNFα: tumor necrosis factor-alpha, TSH: thyrotropin, VB12: vitamin B12, VCAM-1: vascular cell adhesion molecule 1, VEGF: vascular endothelial growth factor, β2-GPI: beta(2)-glycoprotein 1-dependent anticardiolipin antibodies.
2.2. Classification of Potential Blood Biomarkers for PSCI
The potential blood biomarkers identified in the selected studies were categorized into blood and vascular functions, inflammatory and immune functions, metabolic function, neuronal function, kidney function, oxidative stress, hormones, and others (Table 2).
Table 2.
Changes in the potential blood biomarkers for PSCI.
| Category | Level | Potential Biomarkers |
|---|---|---|
| Blood and vascular functions | Increase | D-dimer, Hcy, endostatin, fibrinogen, VCAM-1 |
| No change | Direct bilirubin, fibrinogen, Hcy, indirect bilirubin, total bilirubin, tissue plasminogen activator, vitamin B12, VEGF, von Willebrand factor, thrombin-antithrombin | |
| Inflammatory and immune functions | Increase | esRAGE, hs-CRP (CRP), indoleamine 2,3-dioxygenase, IL-10, IL-1β, IL-6, kynurenine, MMP-9, phospholipase A2, quinolinic acid, RF, sRAGE, semicarbazide-sensitive amino oxidase, TIMP-1, TMAO, TNF-α, kynurenine/tryptophan ratio, quinolinic acid/kynurenic acid ratio |
| Decrease | BChE, hs-CRP (CRP), sRAGE | |
| No change | aCL GPL, aPS GPS, β2-GPI, complement component 3, hs-CPR (CRP), kynurenic acid, Lp-PLA2 mass, tryptophan, IFN-γ, IL-1 RA, IL-6, IL-8, IL-10 | |
| Metabolic function | Increase | FBG, HbA1c, HDL-C, LDL-C, non-HDL-C, TC, TG |
| Decrease | Betaine, TC levels | |
| No change | FBG, glucose, HbA1c, HDL-C, HGF, LDL-C, TC, TG, IGF-1 | |
| Neuronal function | Increase | BACE1, neprilysin, NfL |
| Decrease | BDNF, Aβ42, Aβ42/Aβ40, NfL | |
| No change | S100B, Aβ42, Aβ40, AChE, neprilysin | |
| Kidney function | Increase | Cystatin C, uric acid |
| Decrease | eGFR, uric acid | |
| No change | Creatinine, uric acid, urea N | |
| Oxidative stress | Increase | 8-OHdG, D-amino acid oxidase, malondialdehyde |
| Hormone | Increase | NT-proBNP, cortisol |
| Decrease | 25(OH)D, FT4, T3 | |
| No change | Cortisol/DS ratio, DS, FT3, NT-proBNP, T4, TSH | |
| Others | Decrease | Choline, formaldehyde, NO−2 |
| No change | Ca, folic acid, TMAO, retinoic acid |
25(OH)D: 25-hydroxyvitamin D3, 8-OHdG: 8-hydroxydeoxyquanosine, aCL GPL: anticardiolipin antibodies, IgG anticar-diolipin antibodies units, aPS GPS: anti-phosphatidylserine antibodies, IgG antiphosphatidylserine antibodies units, Aβ40: amyloid β 40, Aβ42: amyloid β 42, β2-GPI: beta(2)-glycoprotein 1-dependent anticardiolipin antibodies, BACE: β-secretase enzyme, BChE: butyrylcholinesterase, BDNF: brain-derived neurotrophic factor, CDR: clinical dementia rating, CRP: C-reactive protein, DS: dehydroepiandrosterone sulphate, eGFR: estimated glomerular filtration rate, esRAGE: endogenous secretory RAGE, FBG: fasting blood glucose, FT3: free triiodothyroinine, FT4: free thyroxin, HbA1c: glycated hemoglobin, Hcy: homocysteine, HDL-C: high-density lipoprotein cholesterol, HGF: hepatocyte growth factor, hs-CRP: high-sensitivity C-reactive protein, IFN-γ: interferon-gamma, IGF-1: insulin-like growth factor-1, IL-1 RA: interleukin-1 receptor antagonist, IL-10: interleukin-10, IL-1β: interleukin-1 beta, IL-6: interleukin-6, IL-8: interleukin-8, LDL-C: low-density lipoprotein cholesterol, Lp-PLA2: lipoprotein-associated phospholipase A2, MMP-9: matrix metalloproteinase-9, MMSE: Mini-Mental State Examination, MoCA: Montreal Cognitive Assessment, NfL: neurofilament light, NT-proBNP: N-terminal pro b-type natriuretic peptide, PSCI: post-stroke cognitive impairment, RF: rheumatoid factor, sRAGE: soluble receptor for advanced glycation end products, T3: triiodothyronine, T4: thyroxin, TC: total cholesterol, TG: triglyceride, tHcy: total homocysteine, TIMP-1: tissue inhibitor metalloproteinase-1, TMAO: trimethylamine N-oxide, TNFα: tumor necrosis factor-alpha, TSH: thyrotropin, VB12: vitamin B12, VCAM-1: vascular cell adhesion molecule 1, VEGF: vascular endothelial growth factor.
Of the identified potential biomarkers, the D-dimer, homocysteine (Hcy), endostatin, fibrinogen, vascular cell adhesion molecule 1 (VCAM-1), endogenous secretory receptor for advanced glycation end products (esRAGE), hs-CRP (high-sensitivity C-reactive protein) or CRP, indoleamine 2,3-dioxygenase, interleukin-10 (IL-10), interleukin-1 beta (IL-1β), interleukin-6 (IL-6), kynurenine, matrix metalloproteinase-9 (MMP-9), phospholipase A2, quinolinic acid, rheumatoid factor (RF), soluble receptor for advanced glycation end products (sRAGE), semicarbazide-sensitive amino oxidase, tissue inhibitor metalloproteinase-1 (TIMP-1), trimethylamine N-oxide (TMAO), tumor necrosis factor-alpha (TNF-α), kynurenine/tryptophan ratio, quinolinic acid/kynurenic acid ratio, fasting blood glucose (FBG), glycated hemoglobin (HbA1c), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), non-HDL-C, total cholesterol (TC), triglyceride (TG), β-secretase enzyme (BACE1), neprilysin, neurofilament light (NfL), cystatin C, uric acid, 8-hydroxydeoxyquanosine (8-OHdG), D-amino acid oxidase, malondialdehyde, N-terminal pro b-type natriuretic peptide (NT-proBNP), and cortisol levels increased among patients with PSCI.
Meanwhile, the butyrylcholinesterase (BChE), hs-CRP or CRP, sRAGE, betaine, TC, brain-derived neurotrophic factor (BDNF), amyloid beta 42 (Aβ42), Aβ42/amyloid beta (Aβ40), NfL, estimated glomerular filtration rat (eGFR), uric acid, 25-hydroxyvitamin D3 (25(OH)D), free thyroxin (FT4), triiodothyronine (T3), choline, formaldehyde, and NO−2 levels decreased.
The following extracted potential blood biomarkers did not change: direct bilirubin, fibrinogen, Hcy, indirect bilirubin, total bilirubin, tissue plasminogen activator, vitamin B12, vascular endothelial growth factor, von Willebrand factor, thrombin-antithrombin, anticardiolipin antibodies, IgG anticardiolipin antibodies units (aCL GPL), anti-phosphatidylserine antibodies, IgG antiphosphatidylserine antibodies units (aPS GPS), beta(2)-glycoprotein 1-dependent anticardiolipin antibodies (β2-GPI), complement component 3, CRP, kynurenic acid, lipoprotein-associated phospholipase A2 mass (Lp-PLA2 mass), tryptophan, interferon-gamma (IFN- γ), interleukin-1 receptor antagonist (IL-1 RA), IL-6, IL-8, IL-10, FBG, glucose, HbA1c, HDL-C, hepatocyte growth factor (HGF), LDL-C, TC, TG, insulin-like growth factor 1 (IGF-1), S100B protein, Aβ42, Aβ40, acetylcholinesterase (AChE), neprilysin, creatinine, uric acid, urea N, dehydroepiandrosterone sulphate (DS), free triiodothyroinine (FT3), NT-proBNP, thyroxin (T4), thyrotropin (TSH), Ca, folic acid, TMAO, retinoic acid, and cortisol/DS ratio.
2.3. Meta-Analysis Results of the Hcy, hs-CRP, Uric Acid, HbA1c, TC, TG, HDL-C, and LDL-C Levels
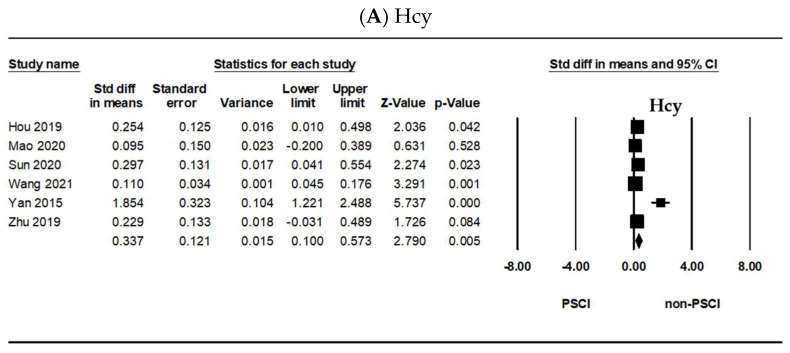
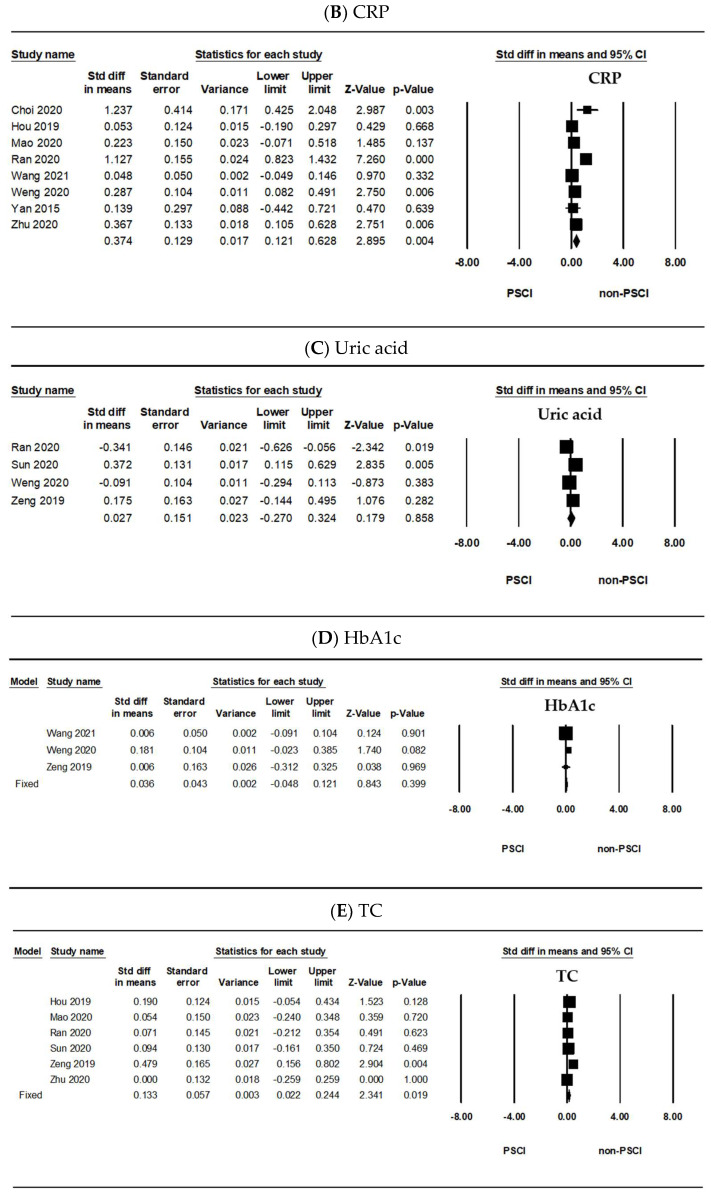
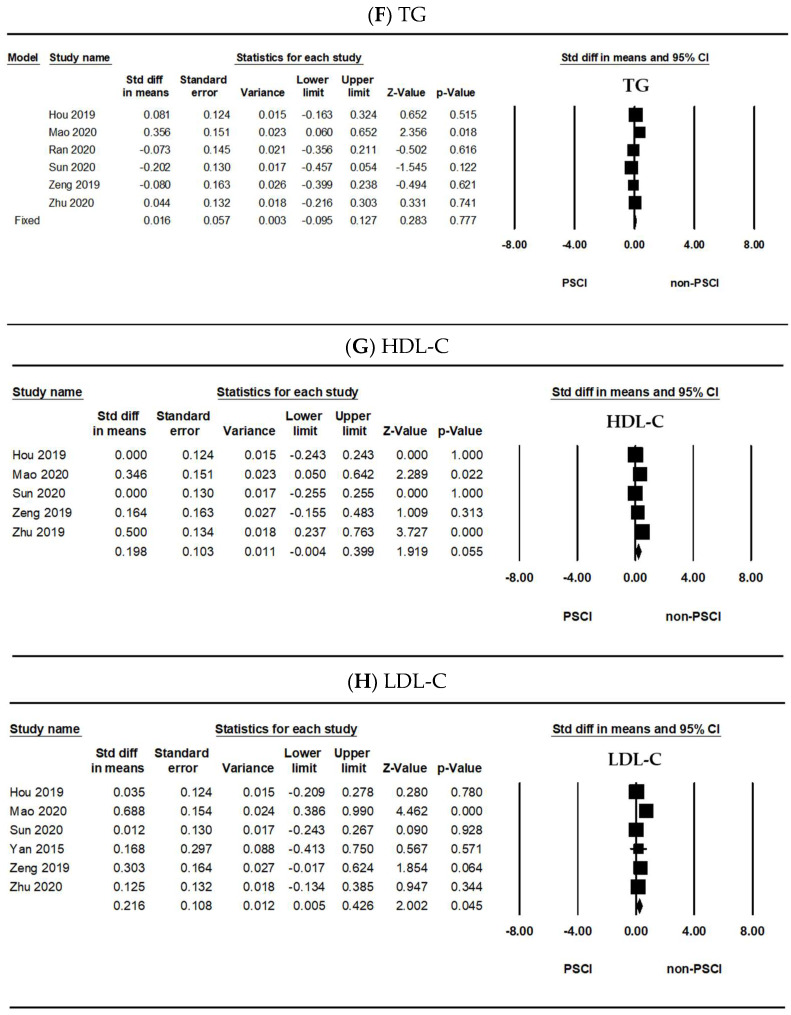
Figure 2 shows the meta-analysis results of the potential blood biomarkers between the PSCI and the non-PSCI group that were identified in five or more articles. As shown in Figure 2A, the Hcy level significantly differed between the PSCI and the non-PSCI groups (SMD = 0.337, 95% confidence interval (CI) = 0.100–0.573, p = 0.005). The CRP level also significantly differed between them (SMD = 0.374, 95% CI = 0.121–0.628, p = 0.004) (Figure 2B). Meanwhile, the uric acid (SMD = 0.027, 95% CI = −0.270–0.324, p = 0.858) (Figure 2C) and HbA1c levels (SMD = 0.036, 95% CI = −0.048–0.121, p = 0.399) (Figure 2D) did not significantly differ between the two groups. Furthermore, the TC level significantly differed between the PSCI and the non-PSCI groups (SMD = 0.133, 95% CI = 0.022–0.244, p = 0.019) (Figure 2E). The TG (SMD = 0.016, 95% CI = −0.095–0.127, p = 0.777) (Figure 2F) and HDL-C levels (SMD = 0.198, 95% CI = −0.004–0.399, p = 0.055) (Figure 2G) did not significantly differ between them. As shown in Figure 2H, the LDL-C level significantly differed between the two groups (SMD = 0.216, 95% CI = 0.005–0.426, p = 0.045).
Figure 2.
Forest plots of the (A) Hcy, (B) CRP, (C) uric acid, (D) HbA1c, (E) TC, (F) TG, (G) HDL-C, and (H) LDL-C levels. Hcy: homocysteine, CRP: C-reactive protein, HbA1c: glycated hemoglobin, TC: total cholesterol, TG: triglyceride, HDL-C: high-density lipoprotein cholesterol, LDL-C: low-density lipoprotein cholesterol, Std diff: standard difference, CI: confidence interval, PSCI: post-stroke cognitive impairment.
As shown in Figure 2A (I2 = 84%, p < 0.001), 2B (I2 = 87%, p < 0.001), 2C (I2 = 81%, p = 0.002), 2G (I2 = 63%, p = 0.028), and 2H (I2 = 66%, p = 0.012), the heterogeneity was significant; thus, we used the random-effects model. The fixed-effects model was applied in Figure 2D (I2 = 14%, p = 0.311), 2E (I2 = 19%, p = 0.291), and 2F (I2 = 44%, p = 0.114). Publication bias was evaluated using Egger’s regression test. All data did not show an obvious risk of publication bias (Figure 2A: p = 0.11, 2B: p = 0.10, 2C: p = 0.84, 2D: p = 0.66, 2E: p = 0.30, 2F: p = 0.76, 2G: p = 0.56, 2H: p = 0.57). Data that were included in this meta-analysis are shown in Supplementary Table S1.
3. Discussion
Recent studies have highlighted the potential of blood-derived parameters as biomarkers for timely patient triage, therapeutics, and stroke mechanisms [30,31,32]. Easily accessible fluid biomarkers can provide an objective evaluation of the real-time panorama, supporting stroke diagnosis or predicting the patients’ outcome and ultimately guiding clinical decisions [20,32,33,34,35]. Cognitive impairment tends to progressively worsen following stroke, with 20–30% of patients developing dementia [9,36]. Hence, international guidelines recommend cognitive assessment as a routine neurological examination for all stroke survivors [37]. Cognitive function refers to mental processes that are crucial for conducting activities of daily living. Such mental processes include attention, short-term and long-term memory, reasoning, coordination of movement, and planning of tasks [38]. The prevalence of brain disorders affecting cognition, such as stroke and dementia, increases steadily in a linear fashion with age [39]. Therefore, we evaluated the levels of eight proteins in the peripheral blood as biomarkers for stroke, especially related to brain damage and cognitive impairments.
First, we investigated whether the levels of four plasma lipids could be potential biomarkers for PSCI individually. As shown in Figure 2, the plasma levels of both TC and LDL-C were higher in the PSCI group than in the non-PSCI group. However, there was no difference in the HDL-C and TG levels between them. In fact, disorders of lipid homeostasis are common risk factors for cardiovascular diseases, which are linked to Alzheimer’s disease (AD) (Dement, 2016). Abnormal levels of lipids or lipoproteins in the blood, which include high levels of LDL-C and low levels of HDL-C, TC, and TG, were related to the cause of the disease outbreak [40,41]. Cholesterol, an important constituent of mammalian cell membranes, modulates membrane fluidity and permeability. It is also the precursor of all steroid hormones and bile acids and plays a key role in membrane trafficking, transmembrane signaling processes, and cell proliferation [42,43,44,45,46]. Cholesterol is typically transported as lipoproteins, which consist of TG and cholesterol in the center surrounded by a phospholipid shell with apolipoprotein embedded in them [47,48]. The relationship between lipids and cognition is controversial. Peripheral cholesterol levels have been evaluated in some studies [13,49,50,51,52]; Kumral et al., (2015). Most studies did not find any association between cholesterol levels and PSCI [13,50,51,52]; however, two studies found a significant association. One study showed that higher levels of LDL-C and lower levels of HDL-C were independently associated with PSCI [53]. The TG levels were studied in only four studies [49,51,52,53]. One study found an association between the baseline TG levels and PSCI [49,54]. Nevertheless, in the central nervous system (CNS), altered levels of cholesterol appear to be involved in several neurodegenerative diseases, such as AD, Niemann–Pick C disease, and major depressive disorder [55,56,57]. In addition, cholesterol-driven inflammation seems to affect neuroplasticity, altering membrane fluidity and permeability, vesicular trafficking and monoamine release, and neuroendocrine function [57,58,59,60,61]. The possibility that the differentiation and function of the CNS were partly influenced by the level of LDL-C or HDL-C circulating in the plasma received further support from recent developments in our understanding of the molecular events involved in transmembrane cholesterol movement [62]. Therefore, we insist that the TC and LDL-C levels could be potential biomarkers for PSCI, and understanding the prognostic impact of these levels in relation to PSCI may be clinically relevant. Although our results on the HDL-C and TG levels were not significant, it is necessary to consider them in future research.
Second, the levels of Hcy were higher in the PSCI group than in the non-PSCI group, as shown in Figure 2. Hcy is produced in all cells and is involved in the metabolism of cysteine and methionine [63]. In previous cross-sectional and prospective studies, elevated plasma Hcy levels have been associated with more than 100 diseases, syndromes, or outcomes [64]. Hcy metabolism is largely dependent on B vitamins, including folate, vitamin B12, and vitamin B6. Substantial lowering of the Hcy level can be achieved through B vitamin supplementation, suggesting a safe and inexpensive intervention strategy for reducing age-related cognitive decline and the risk of AD and overall dementia [65]. Whether Hcy contributes to cerebrovascular changes, cognitive function, or both remains controversial; nevertheless, it appears that elevated Hcy levels are a risk factor for dementia in older adults, regardless of the mechanism. The relationship between the Hcy levels and cognition is controversial. Although there were negative results that suggest that the Hcy levels were not associated with cognitive decline after stroke [49,66], it is a known fact that a high Hcy level is an independent risk factor for cerebrovascular events [67,68] and cognitive impairment [69,70,71,72]. In addition, a recent study showed that patients with higher Hcy levels had greater cortical and hippocampal atrophy than those with lower levels [73]. Therefore, the Hcy level could be a potential biomarker for PSCI, and understanding its prognostic impact in relation to PSCI may be clinically relevant.
Third, the levels of CRP were higher in the PSCI group than in the non-PSCI group (Figure 2). CRP is a plasma protein synthesized by the liver and is often used as a nonspecific biomarker of inflammation. Elevated CRP levels are associated with an increased risk of cerebrovascular diseases and dementia [24]. Upregulation of CRP is considered a marker of systemic inflammation in autoimmune conditions, infection, and obesity [74]. Thus, CRP plays dual roles as a marker of inflammation and a driver of the induction of inflammation [75]. There is evidence that it infiltrates the brain following BBB disruption by hemorrhagic or ischemic stroke [76,77,78]. CRP in ischemic tissues drives the progression of AD following ischemic stroke [75,79]. The relationship between the CRP level and cognition is controversial. Inconsistent results on the link between specific cognitive functions and proinflammatory markers have been reported. For example, the CRP levels have been demonstrated to be negatively related to performance in tests of episodic memory and to be predictive of poorer memory performance in some studies [80,81], but not in all [82,83]. Nevertheless, increased CRP levels may cause cognitive impairment. A previous study has found an independent association between the CRP levels and PSCI [84]. In addition, the CRP levels were negatively associated with a composite score of executive function and processing speed [85]. Therefore, the CRP level could be a potential biomarker for PSCI, and understanding its prognostic impact in relation to PSCI may be clinically relevant.
Fourth, there was no difference in the HbA1c and uric acid levels between the PSCI and non-PSCI groups. The relationship between the HbA1c levels and cognition is controversial. The HbA1c levels were evaluated in some studies. One study showed no significant association with PSCI [13]; another study showed an independent association between higher HbA1c levels and PSCI [86]. Further, there has been an accumulation of studies investigating the link between the uric acid levels and neurodegenerative diseases, mainly including dementia, Parkinson’s disease, amyotrophic lateral sclerosis, and multiple system atrophy [87]. A study of cognitively healthy adults found that elevated baseline serum uric acid levels were associated with decreased attention and visuospatial abilities in men [88]. Although our results on the HbA1c and uric acid levels were not significant, it is necessary that these levels be considered in future research.
In addition to the eight proteins selected in our study, we demonstrated that many other proteins could be considered as possible biomarkers; however, we could not meta-analyze these proteins because of insufficient data, as shown in Table 2. Many potential biomarkers for PSCI have been suggested. (1) The levels of the blood and vascular-function-related proteins, such as D-dimer, endostatin, fibrinogen, and VCAM-1, were higher in patients with PSCI than in those without; (2) the levels of the inflammatory and immune-function-related proteins, such as esRAGE, indoleamine 2,3-dioxygenase, IL-10, IL-1β, IL-6, kynurenine, kynurenine/tryptophan ratio, MMP-9, phospholipase A2, quinolinic acid, quinolinic acid/kynurenic acid ratio, RF, sRAGE, SSAO, TIMP-1, TMAO, TNF-α, and BChE, significantly differed between patients with PSCI and those without; (3) the levels of the metabolic-function-related proteins, such as FBG, non-HDL-C, and betaine, significantly differed between them; (4) the levels of the neuronal-function-related proteins, such as BACE1, neprilysin, NfL, BDNF, Aβ42, and Aβ42/Aβ40, also significantly differed between them; (5) the levels of the kidney-function-related proteins, such as cystatin C and epidermal growth factor receptor, also significantly differed between them; (6) the levels of the oxidative-stress-related proteins, such as 8-OHdG, D-amino acid oxidase, and malondialdehyde, were higher in patients with PSCI than in patients with stroke only; (7) the levels of the hormones, such as NT-proBNP, cortisol, 25(OH)D, FT4, and T3, significantly differed between patients with PSCI and those without; and (8) the levels of the other compounds, such as choline, formaldehyde, and NO−2, were lower in patients with PSCI than in those without. Nevertheless, further research on these candidate biomarkers is needed to validate, identify, and introduce useful biomarkers for stroke recurrence or diagnosis in a scalable manner in medical practice [17].
This study had certain limitations. Although potential blood biomarkers for PSCI were collected in various study groups, the meta-analysis was performed using available data between the PSCI and the non-PSCI group. Furthermore, this study did not consider the characteristics of the patient groups, such as the stage and duration of stroke. It has been addressed that the meta-analysis itself has poor quality of included studies, heterogeneity, and publication bias [89]. However, our study is obviously meaningful in selecting new potential biomarkers for PSCI because meta-analysis can be conceived as a systematic study of all studies that have been conducted to answer a specific question or hypothesis [90]. Our study also included patients after stroke onset, regardless of comparison with other neurodegenerative diseases. Therefore, further research is needed to compare patients with stroke with patients with mild cognitive impairment in the early stage of cognitive decline or with patients with other neurodegenerative diseases.
Nevertheless, our study demonstrated that the levels of some proteins, including TC, LDL-C, Hcy, and CRP, remarkably changed in patients with post-stroke cognitive impairment. Therefore, some blood-derived proteins, such as TC, LDL-C, Hcy, and CRP, could be potential biomarkers for the diagnosis, prognosis prediction, and progression evaluation of stroke, especially related to brain damage and cognitive impairments.
4. Materials and Methods
4.1. Literature Search and Selection Criteria
This systematic review was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. All publications that described the association between PSCI and human blood biomarkers were searched in PubMed, Embase, the Web of Science, and the Cochrane Library from inception to 26 July 2021. In PubMed, we used the following search terms: (stroke OR stroke [Mesh] OR “cerebral infarct” OR “brain infarct” OR “cerebral hemorrhage” OR “cerebral hemorrhage” OR “cerebral ischemia” OR “cerebral ischemia” OR “cerebral hematoma” OR “cerebral hematoma” OR “brain hemorrhage” OR “brain hemorrhage”) AND (dementia OR dementia [Mesh] OR “cognitive decline” OR “cognitive impairment” OR cognition disorder OR cognition disorders [Mesh]) AND (biomarker OR biomarker [Mesh]) AND (blood OR serum OR plasma). In Embase, the Web of Science, and the Cochrane Library, the following terms were used: (stroke OR “cerebral infarct” OR “brain infarct” OR “cerebral hemorrhage” OR “cerebral hemorrhage” OR “cerebral ischemia” OR “cerebral ischemia” OR “cerebral hematoma” OR “cerebral hematoma” OR “brain hemorrhage” OR “brain hemorrhage”) AND (dementia OR “cognitive decline” OR “cognitive impairment” OR “cognition disorder”) AND (biomarker) AND (blood OR serum OR plasma). The inclusion criteria were as follows: (1) articles that included patients with cognitive impairment or dementia after stroke; and (2) articles that identified blood biomarkers for cognitive function after stroke.
4.2. Data Extraction and Analysis
Two authors (K.Y. Kim and K.-A. Chang) independently screened and selected relevant studies according to the inclusion criteria. Any disagreements on every step were resolved via constant discussion with all authors. The following relevant data were extracted from the 40 selected studies: article information, including the title, name of first author, year of publication, and country of patients; group information, including the types of group, sample size, sex, age, and measurement tool used for evaluating cognitive function; and biomarker information, including the type of specimen and type and level of potential biomarkers. For the meta-analysis, the standardized mean difference (SMD) in the potential biomarkers for evaluating PSCI was analyzed between patients with and without PSCI using the Comprehensive Meta-Analysis software version 3 (Biostats Inc., Englewood, NJ, USA). A fixed- or random-effects model was used after analyzing the Q statistic, while the I2 method was applied to assess heterogeneity. Funnel plots and Egger’s intercept tests were used to evaluate publication bias. The statistical significance level was set at p-values of < 0.05.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ijms23020602/s1.
Author Contributions
Conceptualization, K.-A.C. and K.Y.K.; formal analysis, K.-A.C. and K.Y.K.; investigation, K.Y.S.; writing—original draft, K.Y.S. and K.Y.K.; writing—review and editing, K.Y.S. and K.-A.C.; supervision, K.-A.C.; project administration, K.-A.C.; funding acquisition, K.-A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean government (MSIT) (2020M3A9E4104384) and by the Basic Science Research Program through the NRF of Korea funded by the Ministry of Education (2020R1I1A1A01070793). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Krishnamurthi R.V., Ikeda T., Feigin V.L. Global, regional and country-specific burden of ischaemic stroke, intracerebral haemorrhage and subarachnoid haemorrhage: A systematic analysis of the global burden of disease study 2017. Neuroepidemiology. 2020;54:171–179. doi: 10.1159/000506396. [DOI] [PubMed] [Google Scholar]
- 2.Jia J., Zhang H., Liang X., Dai Y., Liu L., Tan K., Ma R., Luo J., Ding Y., Ke C. Application of metabolomics to the discovery of biomarkers for ischemic stroke in the murine model: A comparison with the clinical results. Mol. Neurobiol. 2021 doi: 10.1007/s12035-021-02535-2. [DOI] [PubMed] [Google Scholar]
- 3.Campbell B.C.V., Khatri P. Stroke. Lancet. 2020;396:129–142. doi: 10.1016/S0140-6736(20)31179-X. [DOI] [PubMed] [Google Scholar]
- 4.Zhang L.F., Yang J., Hong Z., Yuan G.G., Zhou B.F., Zhao L.C., Huang Y.N., Chen J., Wu Y.F., Collaborative Group of China Multicenter Study of Cardiovascular, E Proportion of different subtypes of stroke in China. Stroke. 2003;34:2091–2096. doi: 10.1161/01.STR.0000087149.42294.8C. [DOI] [PubMed] [Google Scholar]
- 5.Woodruff T.M., Thundyil J., Tang S.C., Sobey C.G., Taylor S.M., Arumugam T.V. Pathophysiology, treatment, and animal and cellular models of human ischemic stroke. Mol. Neurodegener. 2011;6:11. doi: 10.1186/1750-1326-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang P., Wang C., Wu J., Zhang S. A systematic review of the predictive value of plasma D-dimer levels for Predicting stroke outcome. Front. Neurol. 2021;12:693524. doi: 10.3389/fneur.2021.693524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grotta J.C., Albers G.W., Broderick J.P., Day A.L., Kasner S.E., Lo E.H., Sacco R.L., Wong L.K.S. Stroke: Pathophysiology, Diagnosis, and Management. 7th. ed. Elsevier, Inc.; Philadelphia, PA, USA: 2021. [Google Scholar]
- 8.Fride Y., Adamit T., Maeir A., Ben Assayag E., Bornstein N.M., Korczyn A.D., Katz N. What are the correlates of cognition and participation to return to work after first ever mild stroke? Top Stroke Rehabil. 2015;22:317–325. doi: 10.1179/1074935714Z.0000000013. [DOI] [PubMed] [Google Scholar]
- 9.Mijajlovic M.D., Pavlovic A., Brainin M., Heiss W.D., Quinn T.J., Ihle-Hansen H.B., Hermann D.M., Assayag E.B., Richard E., Thiel A., et al. Post-stroke dementia—A comprehensive review. BMC Med. 2017;15:11. doi: 10.1186/s12916-017-0779-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Onyike C.U. Cerebrovascular disease and dementia. Int. Rev. Psychiatry. 2006;18:423–431. doi: 10.1080/09540260600935421. [DOI] [PubMed] [Google Scholar]
- 11.Kalaria R.N., Akinyemi R., Ihara M. Stroke injury, cognitive impairment and vascular dementia. Biochim. Biophys. Acta. 2016;1862:915–925. doi: 10.1016/j.bbadis.2016.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vataja R., Pohjasvaara T., Mantyla R., Ylikoski R., Leppavuori A., Leskela M., Kalska H., Hietanen M., Aronen H.J., Salonen O., et al. MRI correlates of executive dysfunction in patients with ischaemic stroke. Eur. J. Neurol. 2003;10:625–631. doi: 10.1046/j.1468-1331.2003.00676.x. [DOI] [PubMed] [Google Scholar]
- 13.Kandiah N., Wiryasaputra L., Narasimhalu K., Karandikar A., Marmin M., Chua E.V., Sitoh Y.Y. Frontal subcortical ischemia is crucial for post stroke cognitive impairment. J. Neurol. Sci. 2011;309:92–95. doi: 10.1016/j.jns.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 14.Stephens S., Kenny R.A., Rowan E., Allan L., Kalaria R.N., Bradbury M., Ballard C.G. Neuropsychological characteristics of mild vascular cognitive impairment and dementia after stroke. Int. J. Geriatr. Psychiatry. 2004;19:1053–1057. doi: 10.1002/gps.1209. [DOI] [PubMed] [Google Scholar]
- 15.Roman G.C., Tatemichi T.K., Erkinjuntti T., Cummings J.L., Masdeu J.C., Garcia J.H., Amaducci L., Orgogozo J.M., Brun A., Hofman A., et al. Vascular dementia—Diagnostic-criteria for research studies—Report of the Ninds-Airen international workshop. Neurology. 1993;43:250–260. doi: 10.1212/WNL.43.2.250. [DOI] [PubMed] [Google Scholar]
- 16.Schnider A., Regard M., Landis T. Anterograde and retrograde amnesia following bitemporal infarction. Behav. Neurol. 1994;7:87–92. doi: 10.1155/1994/653736. [DOI] [PubMed] [Google Scholar]
- 17.Baez S.D., del Barco D.G., Hardy-Sosa A., Nieto G.G., Bringas-Vega M.L., Llibre-Guerra J.J., Valdes-Sosa P. Scalable Bio marker combinations for early stroke diagnosis: A systematic review. Front. Neurol. 2021;12 doi: 10.3389/fneur.2021.638693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maas M.B., Furie K.L. Molecular biomarkers in stroke diagnosis and prognosis. Biomark. Med. 2009;3:363–383. doi: 10.2217/bmm.09.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alex Matos Ribeiro J., Fernanda Garcia-Salazar L., Regina Saade-Pacheco C., Shirley Moreira Silva E., Garcia Oliveira S., Flavia Silveira A., Sanches Garcia-Araujo A., Luiz Russo T. Prognostic molecular markers for motor recovery in acute hemorrhagic stroke: A systematic review. Clin. Chim. Acta. 2021;522:45–60. doi: 10.1016/j.cca.2021.08.006. [DOI] [PubMed] [Google Scholar]
- 20.Biomarkers Definitions Working, Group Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 2001;69:89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- 21.Andone S., Bajko Z., Motataianu A., Mosora O., Balasa R. The role of biomarkers in atherothrombotic stroke—A systematic review. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22169032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boyd L.A., Hayward K.S., Ward N.S., Stinear C.M., Rosso C., Fisher R.J., Carter A.R., Leff A.P., Copland D.A., Carey L.M., et al. Biomarkers of stroke Recovery: Consensus-based core recommendations from the stroke recovery and rehabilitation roundtable. Neurorehabil. Neural Repair. 2017;31:864–876. doi: 10.1177/1545968317732680. [DOI] [PubMed] [Google Scholar]
- 23.Troiani Z., Ascanio L., Rossitto C.P., Ali M., Mohammadi N., Majidi S., Mocco J., Kellner C.P. Prognostic utility of serum biomarkers in intracerebral hemorrhage: A systematic review. Neurorehabil. Neural Repair. 2021:15459683211041314. doi: 10.1177/15459683211041314. [DOI] [PubMed] [Google Scholar]
- 24.Zhang X., Bi X. Post-stroke cognitive impairment: A review focusing on molecular biomarkers. J. Mol. Neurosci. 2020;70:1244–1254. doi: 10.1007/s12031-020-01533-8. [DOI] [PubMed] [Google Scholar]
- 25.Verdelho A., Wardlaw J., Pavlovic A., Pantoni L., Godefroy O., Duering M., Charidimou A., Chabriat H., Biessels G.J. Cognitive impairment in patients with cerebrovascular disease: A white paper from the ESO Dementia Committee. Eur. Stroke J. 2021;6:5–17. doi: 10.1177/23969873211000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kulesh A., Drobakha V., Kuklina E., Nekrasova I., Shestakov V. Cytokine response, tract-specific fractional anisotropy, and brain morphometry in post-stroke cognitive impairment. J. Stroke Cerebrovasc. Dis. 2018;27:1752–1759. doi: 10.1016/j.jstrokecerebrovasdis.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 27.Ball E.L., Sutherland R., Squires C., Mead G.E., Religa D., Lundstrom E., Cheyne J., Wardlaw J.M., Quinn T.J., Shenkin S.D. Predicting post-stroke cognitive impairment using acute CT neuroimaging: A systematic review and meta-analysis. Int. J. Stroke. 2021:17474930211045836. doi: 10.1177/17474930211045836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lim J.S., Lee J.J., Woo C.W. Post-stroke cognitive impairment: Pathophysiological insights into brain disconnectome from advanced neuroimaging analysis techniques. J. Stroke. 2021;23:297–311. doi: 10.5853/jos.2021.02376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Potter T., Lioutas V.A., Tano M., Pan A., Meeks J., Woo D., Seshadri S., Selim M., Vahidy F. Cognitive impairment after intracerebral Hemorrhage: A systematic review of current evidence and knowledge gaps. Front. Neurol. 2021;12:716632. doi: 10.3389/fneur.2021.716632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalra L.P., Khatter H., Ramanathan S., Sapehia S., Devi K., Kaliyaperumal A., Bal D., Sebastian I., Kakarla R., Singhania A., et al. Serum GFAP for stroke diagnosis in regions with limited access to brain imaging (BE FAST India) Eur. Stroke J. 2021;6:176–184. doi: 10.1177/23969873211010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bustamante A., Penalba A., Orset C., Azurmendi L., Llombart V., Simats A., Pecharroman E., Ventura O., Ribo M., Vivien D., et al. Blood biomarkers to differentiate Ischemic and hemorrhagic strokes. Neurology. 2021;96:e1928–e1939. doi: 10.1212/WNL.0000000000011742. [DOI] [PubMed] [Google Scholar]
- 32.Dias A., Silva I., Pinto I.M., Maia L.F. Timely and blood-based multiplex molecular profiling of acute stroke. Life. 2021;11:816. doi: 10.3390/life11080816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park D., Joo S.S., Lee H.J., Choi K.C., Kim S.U., Kim Y.B. Microtubule-associated protein 2, an early blood marker of ischemic brain injury. J. Neurosci. Res. 2012;90:461–467. doi: 10.1002/jnr.22769. [DOI] [PubMed] [Google Scholar]
- 34.Misra S., Kumar A., Kumar P., Yadav A.K., Mohania D., Pandit A.K., Prasad K., Vibha D. Blood-based protein biomarkers for stroke differentiation: A systematic review. Proteomics Clin. Appl. 2017;11 doi: 10.1002/prca.201700007. [DOI] [PubMed] [Google Scholar]
- 35.Wang L., Song Q., Wang C., Wu S., Deng L., Li Y., Zheng L., Liu M. Neutrophil to lymphocyte ratio predicts poor outcomes after acute ischemic stroke: A cohort study and systematic review. J. Neurol. Sci. 2019;406:116445. doi: 10.1016/j.jns.2019.116445. [DOI] [PubMed] [Google Scholar]
- 36.Pendlebury S.T., Rothwell P.M. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: A systematic review and meta-analysis. Lancet Neurol. 2009;8:1006–1018. doi: 10.1016/S1474-4422(09)70236-4. [DOI] [PubMed] [Google Scholar]
- 37.Hachinski V., Iadecola C., Petersen R.C., Breteler M.M., Nyenhuis D.L., Black S.E., Powers W.J., DeCarli C., Merino J.G., Kalaria R.N., et al. National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke. 2006;37:2220–2241. doi: 10.1161/01.STR.0000237236.88823.47. [DOI] [PubMed] [Google Scholar]
- 38.de Champlain J., Wu R., Girouard H., Karas M.A., El Midaoui A., Laplante M.A., Wu L. Oxidative stress in hypertension. Clin. Exp. Hypertens. 2004;26:593–601. doi: 10.1081/CEH-200031904. [DOI] [PubMed] [Google Scholar]
- 39.Verhaeghen P., Salthouse T.A. Meta-analyses of age-cognition relations in adulthood: Estimates of linear and nonlinear age effects and structural models. Psychol. Bull. 1997;122:231–249. doi: 10.1037/0033-2909.122.3.231. [DOI] [PubMed] [Google Scholar]
- 40.Alzheimer’s, Association 2016 Alzheimer’s disease facts and figures. Alzheimers Dement. 2016;12:459–509. doi: 10.1016/j.jalz.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 41.Liu Y., Zhong X., Shen J., Jiao L., Tong J., Zhao W., Du K., Gong S., Liu M., Wei M. Elevated serum TC and LDL-C levels in Alzheimer’s disease and mild cognitive impairment: A meta-analysis study. Brain Res. 2020;1727:146554. doi: 10.1016/j.brainres.2019.146554. [DOI] [PubMed] [Google Scholar]
- 42.Fernandez C., Lobo M.D.V.T., Gomez-Coronado D., Lasuncion M.A. Cholesterol is essential for mitosis progression and its deficiency induces polyploid cell formation. Exp. Cell Res. 2004;300:109–120. doi: 10.1016/j.yexcr.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 43.Fernandez C., Martin M., Gomez-Coronado D., Lasuncion M.A. Effects of distal cholesterol biosynthesis inhibitors on cell proliferation and cell cycle progression. J. Lipid Res. 2005;46:920–929. doi: 10.1194/jlr.M400407-JLR200. [DOI] [PubMed] [Google Scholar]
- 44.Hooper N.M. Detergent-insoluble glycosphingolipid/cholesterol-rich membrane domains, lipid rafts and caveolae. Mol. Membr. Biol. 1999;16:145–156. doi: 10.1080/096876899294607. [DOI] [PubMed] [Google Scholar]
- 45.Nwokoro N.A., Wassif C.A., Porter F.D. Genetic disorders of cholesterol biosynthesis in mice and humans. Mol. Genet. Metab. 2001;74:105–119. doi: 10.1006/mgme.2001.3226. [DOI] [PubMed] [Google Scholar]
- 46.Goedeke L., Fernandez-Hernando C. Regulation of cholesterol homeostasis. Cell Mol. Life Sci. 2012;69:915–930. doi: 10.1007/s00018-011-0857-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ohvo-Rekila H., Ramstedt B., Leppimaki P., Slotte J.P. Cholesterol interactions with phospholipids in membranes. Prog. Lipid Res. 2002;41:66–97. doi: 10.1016/S0163-7827(01)00020-0. [DOI] [PubMed] [Google Scholar]
- 48.Laudanski K. Persistence of lipoproteins and cholesterol alterations after sepsis: Implication for atherosclerosis progression. Int. J. Mol. Sci. 2021;22:517. doi: 10.3390/ijms221910517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barba R., Martinez-Espinosa S., Rodriguez-Garcia E., Pondal M., Vivancos J., Del Ser T. Poststroke dementia: Clinical features and risk factors. Stroke. 2000;31:1494–1501. doi: 10.1161/01.STR.31.7.1494. [DOI] [PubMed] [Google Scholar]
- 50.Rasquin S.M., Verhey F.R., van Oostenbrugge R.J., Lousberg R., Lodder J. Demographic and CT scan features related to cognitive impairment in the first year after stroke. J. Neurol. Neurosurg. Psychiatry. 2004;75:1562–1567. doi: 10.1136/jnnp.2003.024190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baum L., Chen X., Cheung W.S., Cheung C.K., Cheung L.W., Chiu K.F., Wen H.M., Poon P., Woo K.S., Ng H.K., et al. Polymorphisms and vascular cognitive impairment after ischemic stroke. J. Geriatr. Psychiatry Neurol. 2007;20:93–99. doi: 10.1177/0891988706298627. [DOI] [PubMed] [Google Scholar]
- 52.Tamam B., Tasdemir N., Tamam Y. The prevalence of dementia three months after stroke and its risk factors. Turk. Psikiyatri. Derg. 2008;19:46–56. [PubMed] [Google Scholar]
- 53.Kumral E., Gulluoglu H., Alakbarova N., Deveci E.E., Colak A.Y., Caginda A.D., Evyapan D., Orman M. Cognitive decline in patients with leukoaraiosis within 5 years after initial stroke. J. Stroke Cerebrovasc. Dis. 2015;24:2338–2347. doi: 10.1016/j.jstrokecerebrovasdis.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 54.Casolla B., Caparros F., Cordonnier C., Bombois S., Henon H., Bordet R., Orzi F., Leys D. Biological and imaging predictors of cognitive impairment after stroke: A systematic review. J. Neurology. 2019;266:2593–2604. doi: 10.1007/s00415-018-9089-z. [DOI] [PubMed] [Google Scholar]
- 55.Ikonen E. Mechanisms for cellular cholesterol transport: Defects and human disease. Physiol. Rev. 2006;86:1237–1261. doi: 10.1152/physrev.00022.2005. [DOI] [PubMed] [Google Scholar]
- 56.Maxfield F.R., Tabas I. Role of cholesterol and lipid organization in disease. Nature. 2005;438:612–621. doi: 10.1038/nature04399. [DOI] [PubMed] [Google Scholar]
- 57.Gliozzi M., Musolino V., Bosco F., Scicchitano M., Scarano F., Nucera S., Zito M.C., Ruga S., Carresi C., Macri R., et al. Cholesterol homeostasis: Researching a dialogue between the brain and peripheral tissues. Pharmacol. Res. 2021;163 doi: 10.1016/j.phrs.2020.105215. [DOI] [PubMed] [Google Scholar]
- 58.Quan G., Xie C., Dietschy J.M., Turley S.D. Ontogenesis and regulation of cholesterol metabolism in the central nervous system of the mouse. Brain Res. Dev. Brain Res. 2003;146:87–98. doi: 10.1016/j.devbrainres.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 59.Saher G., Brugger B., Lappe-Siefke C., Mobius W., Tozawa R., Wehr M.C., Wieland F., Ishibashi S., Nave K.A. High cholesterol level is essential for myelin membrane growth. Nat. Neurosci. 2005;8:468–475. doi: 10.1038/nn1426. [DOI] [PubMed] [Google Scholar]
- 60.Morell P., Jurevics H. Origin of cholesterol in myelin. Neurochem. Res. 1996;21:463–470. doi: 10.1007/BF02527711. [DOI] [PubMed] [Google Scholar]
- 61.Maiuolo J., Maretta A., Gliozzi M., Musolino V., Carresi C., Bosco F., Mollace R., Scarano F., Palma E., Scicchitano M., et al. Ethanol-induced cardiomyocyte toxicity implicit autophagy and NFkB transcription factor. Pharmacol. Res. 2018;133:141–150. doi: 10.1016/j.phrs.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 62.Dietschy J.M., Turley S.D. Thematic review series: Brain lipids. Cholesterol metabolism in the central nervous system during early development and in the mature animal. J. Lipid Res. 2004;45:1375–1397. doi: 10.1194/jlr.R400004-JLR200. [DOI] [PubMed] [Google Scholar]
- 63.Selhub J. Homocysteine metabolism. Annu. Rev. Nutr. 1999;19:217–246. doi: 10.1146/annurev.nutr.19.1.217. [DOI] [PubMed] [Google Scholar]
- 64.Smith A.D., Refsum H. Homocysteine—From disease biomarker to disease prevention. J. Intern. Med. 2021;290:826–854. doi: 10.1111/joim.13279. [DOI] [PubMed] [Google Scholar]
- 65.McCaddon A., Miller J.W. Assessing the association between homocysteine and cognition: Reflections on Bradford Hill, meta-analyses, and causality. Nutr. Rev. 2015;73:723–735. doi: 10.1093/nutrit/nuv022. [DOI] [PubMed] [Google Scholar]
- 66.Sachdev P.S., Brodaty H., Valenzuela M.J., Lorentz L., Looi J.C., Berman K., Ross A., Wen W., Zagami A.S. Clinical determinants of dementia and mild cognitive impairment following ischaemic stroke: The Sydney stroke study. Dement. Geriatr. Cogn. Disord. 2006;21:275–283. doi: 10.1159/000091434. [DOI] [PubMed] [Google Scholar]
- 67.Hassan A., Hunt B.J., O’Sullivan M., Bell R., D’Souza R., Jeffery S., Bamford J.M., Markus H.S. Homocysteine is a risk factor for cerebral small vessel disease, acting via endothelial dysfunction. Brain. 2004;127:212–219. doi: 10.1093/brain/awh023. [DOI] [PubMed] [Google Scholar]
- 68.Ostrakhovitch E.A., Tabibzadeh S. Homocysteine and age-associated disorders. Ageing Res. Rev. 2019;49:144–164. doi: 10.1016/j.arr.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 69.Garcia A., Zanibbi K. Homocysteine and cognitive function in elderly people. Can. Med. Assoc. J. 2004;171:897–904. doi: 10.1503/cmaj.1031586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Price B.R., Wilcock D.M., Weekman E.M. Hyperhomocysteinemia as a risk factor for vascular contributions to cognitive impairment and dementia. Front. Aging Neurosci. 2018;10 doi: 10.3389/fnagi.2018.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sala I., Sanchez-Saudinos M.B., Molina-Porcel L., Lazaro E., Gich I., Clarimon J., Blanco-Vaca F., Blesa R., Gomez-Isla T., Lleo A. Homocysteine and cognitive impairment relation with diagnosis and neuropsychological performance. Dement. Geriatr. Cogn. Disord. 2008;26:506–512. doi: 10.1159/000173710. [DOI] [PubMed] [Google Scholar]
- 72.Zhou S., Chen J., Cheng L., Fan K., Xu M., Ren W., Chen Y., Geng D., Cheng H., Luan X., et al. Age-dependent association between elevated homocysteine and cognitive impairment in a post-stroke population: A prospective study. Front. Nutr. 2021;8:691837. doi: 10.3389/fnut.2021.691837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Den Heijer T., Vermeer S.E., Clarke R., Oudkerk M., Koudstaal P.J., Hofman A., Breteler M.M. Homocysteine and brain atrophy on MRI of non-demented elderly. Brain. 2003;126:170–175. doi: 10.1093/brain/awg006. [DOI] [PubMed] [Google Scholar]
- 74.Hsuchou H., Kastin A.J., Mishra P.K., Pan W. C-reactive protein increases BBB permeability: Implications for obesity and neuroinflammation. Cell Physiol. Biochem. 2012;30:1109–1119. doi: 10.1159/000343302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jeon M.T., Kim K.S., Kim E.S., Lee S., Kim J., Hoe H.S., Kim D.G. Emerging pathogenic role of peripheral blood factors following BBB disruption in neurodegenerative disease. Ageing Res. Rev. 2021;68:101333. doi: 10.1016/j.arr.2021.101333. [DOI] [PubMed] [Google Scholar]
- 76.Di Napoli M., Godoy D.A., Campi V., Masotti L., Smith C.J., Parry Jones A.R., Hopkins S.J., Slevin M., Papa F., Mogoanta L., et al. C-reactive protein in intracerebral hemorrhage: Time course, tissue localization, and prognosis. Neurology. 2012;79:690–699. doi: 10.1212/WNL.0b013e318264e3be. [DOI] [PubMed] [Google Scholar]
- 77.Di Napoli M., Parry-Jones A.R., Smith C.J., Hopkins S.J., Slevin M., Masotti L., Campi V., Singh P., Papa F., Popa-Wagner A., et al. C-reactive protein predicts hematoma growth in intracerebral hemorrhage. Stroke. 2014;45:59–65. doi: 10.1161/STROKEAHA.113.001721. [DOI] [PubMed] [Google Scholar]
- 78.Di Napoli M., Slevin M., Popa-Wagner A., Singh P., Lattanzi S., Divani A.A. Monomeric C-reactive protein and cerebral Hemorrhage: From bench to bedside. Front. Immunol. 2018;9:1921. doi: 10.3389/fimmu.2018.01921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Slevin M., Matou S., Zeinolabediny Y., Corpas R., Weston R., Liu D., Boras E., Di Napoli M., Petcu E., Sarroca S., et al. Monomeric C-reactive protein—A key molecule driving development of Alzheimer’s disease associated with brain ischaemia? Sci. Rep. 2015;5:13281. doi: 10.1038/srep13281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Komulainen P., Lakka T.A., Kivipelto M., Hassinen M., Penttila I.M., Helkala E.L., Gylling H., Nissinen A., Rauramaa R. Serum high sensitivity C-reactive protein and cognitive function in elderly women. Age Ageing. 2007;36:443–448. doi: 10.1093/ageing/afm051. [DOI] [PubMed] [Google Scholar]
- 81.Bettcher B.M., Wilheim R., Rigby T., Green R., Miller J.W., Racine C.A., Yaffe K., Miller B.L., Kramer J.H. C-reactive protein is related to memory and medial temporal brain volume in older adults. Brain Behav. Immun. 2012;26:103–108. doi: 10.1016/j.bbi.2011.07.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hoth K.F., Haley A.P., Gunstad J., Paul R.H., Poppas A., Jefferson A.L., Tate D.F., Ono M., Jerskey B.A., Cohen R.A. Elevated C-reactive protein is related to cognitive decline in older adults with cardiovascular disease. J. Am. Geriatr. Soc. 2008;56:1898–1903. doi: 10.1111/j.1532-5415.2008.01930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wersching H., Duning T., Lohmann H., Mohammadi S., Stehling C., Fobker M., Conty M., Minnerup J., Ringelstein E.B., Berger K., et al. Serum C-reactive protein is linked to cerebral microstructural integrity and cognitive function. Neurology. 2010;74:1022–1029. doi: 10.1212/WNL.0b013e3181d7b45b. [DOI] [PubMed] [Google Scholar]
- 84.Guo J., Su W., Fang J.H., Chen N., Zhou M.K., Zhang Y., He L. Elevated CRP at admission predicts post-stroke cognitive impairment in Han Chinese patients with intracranial arterial stenosis. Neurol. Res. 2018;40:292–296. doi: 10.1080/01616412.2018.1438224. [DOI] [PubMed] [Google Scholar]
- 85.Tegeler C., O’Sullivan J.L., Bucholtz N., Goldeck D., Pawelec G., Steinhagen-Thiessen E., Demuth I. The inflammatory markers CRP, IL-6, and IL-10 are associated with cognitive function—data from the Berlin aging study II. Neurobiol. Aging. 2016;38:112–117. doi: 10.1016/j.neurobiolaging.2015.10.039. [DOI] [PubMed] [Google Scholar]
- 86.Ben Assayag E., Eldor R., Korczyn A.D., Kliper E., Shenhar-Tsarfaty S., Tene O., Molad J., Shapira I., Berliner S., Volfson V., et al. Type 2 diabetes mellitus and impaired renal function are associated with brain alterations and poststroke cognitive decline. Stroke. 2017;48:2368–2374. doi: 10.1161/STROKEAHA.117.017709. [DOI] [PubMed] [Google Scholar]
- 87.Qiao M., Chen C., Liang Y., Luo Y., Wu W. The influence of serum uric acid level on Alzheimer’s Disease: A narrative review. BioMed. Res. Int. 2021;2021:5525710. doi: 10.1155/2021/5525710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kueider A.M., An Y., Tanaka T., Kitner-Triolo M.H., Studenski S., Ferrucci L., Thambisetty M. Sex-dependent associations of serum uric acid with brain function during aging. J. Alzheimers Dis. 2017;60:699–706. doi: 10.3233/JAD-170287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Noble J.H., Jr. Meta-analysis: Methods, strengths, weaknesses, and political uses. J. Lab. Clin. Med. 2006;147:7–20. doi: 10.1016/j.lab.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 90.Esterhuizen T.M., Thabane L. Con: Meta-analysis: Some key limitations and potential solutions. Nephrol. Dial. Transplant. 2016;31:882–885. doi: 10.1093/ndt/gfw092. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon reasonable request.