Abstract
The involvement of Borna disease virus (BDV) in psychiatric diseases in humans remains controversial. T-cell memory response and seroprevalence of BDV in patients with psychiatric disorders and blood donors in Japan were evaluated collectively by Western blot (WB) analysis with inhibition test, electrochemiluminescence immunoassay, immunofluorescence assay, and T-cell proliferative response as well as detection of BDV p24 RNA in peripheral blood mononuclear cells (PBMCs). Positive proliferative responses to both BDV p40 and p24 proteins were detected in 9% of patients with mood disorders (4 of 45), 4% of schizophrenic patients (2 of 45), and 2% of blood donors (1 of 45). By WB analysis, the antibody to BDV p40 was detected only in 2% of patients with mood disorders (1 of 45). The BDV p24 antibody was detected in 2% of patients with mood disorders (1 of 45) and 9% of schizophrenic patients. (4 of 45) No plasma reacted with both BDV proteins. The finding of a lower seroprevalence than previously reported suggests the presence of false-positive cases in the previous report. BDV RNA was detected only in 2% of patients with mood disorders (1 of 45). In these three serological assays, T-cell responses, and PCR analysis, there was no significant difference in the prevalence among the three groups. However, we found three psychiatric patients who were positive for both BDV antibodies and T-cell proliferative responses and one patient who was positive for BDV RNA in PBMCs. These findings suggest the usefulness of the proliferative T-cell response and that certain individuals are infected with BDV or a BDV-related virus.
Borna disease virus (BDV) is the prototype of a new virus family, Bornaviridae, within the Mononegavirales order, which has a nonsegmented, negative-sense, single-stranded RNA genome (12, 42). BDV is a noncytolytic neurotropic virus that infects a wide variety of animal species from birds to primates (7, 17, 26, 36, 51, 52) and causes Borna disease (BD), which is characterized by central nervous system dysfunction with variable manifestations ranging from fatal neuronal damage to almost asymptomatic viral persistence (19, 25, 28, 29). The wide host range of the virus and behavioral disturbances in animals with BD have suggested that BDV infection may be associated with human psychiatric disorders (5, 8, 9, 15, 23, 33, 40, 48, 49, 53). Furthermore, seroepidemiological data and the detection of BDV RNA in peripheral blood mononuclear cells (PBMCs) by nested reverse transcriptase PCR (RT-PCR) have also suggested a possible involvement of BDV in human psychiatric disorders. However, there is controversy over the prevalence of BDV antibodies and BDV RNA in the PBMCs of patients with psychiatric disorders.
In previous serological studies, the prevalence of anti-BDV antibodies in psychiatric patients varied from 0 to 30% in different laboratories. The differences in prevalence could be due to the use of different assay systems (immunofluorescence [IF] assay [4, 5, 37, 38], Western blot [WB] analysis [8, 14, 15, 21, 23, 24, 40, 47, 50], enzyme-linked immunosorbent assay [14, 20, 24], and electrochemiluminescence immunoassay [ECLIA] [53]) with different sensitivities and specificities. Particularly, in most of the previous reports that examined BDV antibodies by WB analysis, the specificity for BDV has not been considered, giving rise to the possibility of false-positive results. One of the aims of the present study was to establish a WB analysis with specificity for the detection of BDV antibodies in human sera and to reevaluate the prevalence of BDV antibodies. Furthermore, we also tried to detect anti-BDV antibodies by ECLIA and IF assay to make elaborate inquires into the prevalence of anti-BDV antibodies. To our knowledge, this is the first report on the evaluation of the prevalence of anti-BDV antibodies examined by three different methods.
Experimental animal models of BD demonstrated that BD is caused by T-cell-mediated immunopathology in the brain (1a, 2, 3, 16, 28, 31, 32, 35, 41, 43–45). The evidence has suggested that BDV-specific CD4+ and CD8+ T cells in the brain play an important role in the development of BD. Studies indicate that BDV has little to no direct cytopathogenity (19, 29), and antiviral antibodies do not play a significant role in the pathogenesis of BD (18, 29). These findings encouraged us to clarify whether T-cell responses to BDV could be detected in human psychiatric patients and whether the responses might have an association with antibody response to BDV. In the present study, we examined T-cell proliferative and antibody responses to BDV as well as BDV RNA of PBMCs in psychiatric patients and blood donors to evaluate more precisely the status of BDV infection. Here, we demonstrate the possibility that some patients with mood disorders and schizophrenia could be infected with BDV, and the T-cell proliferation assay may be an additional tool for the diagnosis of BDV infection in humans.
MATERIALS AND METHODS
Subjects.
Blood specimens from 90 psychiatric patients and 45 blood donors who reside in Fukushima prefecture in the northeast part of Japan were examined for BDV infection. The patients consisted of 45 patients with mood disorders (23 males and 22 females) ranging in age from 17 to 74 years (average, 47 years) and 45 patients with schizophrenia (23 males and 22 females) ranging in age from 21 to 60 years (average, 45 years). All patients met Diagnostic and Statistical Manual of Mental Disorders IV (DSM-IV) (1) criteria for the diagnosis of mood disorders and schizophrenia. Diagnostic codes for these patients are shown in Tables 1 and 2. Forty-five blood donors (22 males and 23 females) ranging in age from 26 to 62 years (average, 48 years) were matched by age and sex to the patients. This study was approved by the Ethical Committee of Fukushima Medical University, and all patients gave their informed consent for this study.
TABLE 1.
Results of proliferation assay, WB analysis. ECLIA, IF assay, and RT-PCR for patients with mood disordersa
| Patient | Sex | Age (yr) | MOB | Diag. | Proliferation assay (SI)b
|
WB analysisc
|
ECLIAd
|
IFc (BDV/MDCK) | RT-PCR (p24) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PHA | p40 | p40 NC | p24 | p24 NC | p40 | p24 | p40 | p24 | |||||||
| M1 | M | 49 | 9 | 296.0 | 211 | 5.6 | 1.3 | 4.2 | 1.2 | −e | − | − | − | − | − |
| M2 | M | 58 | 5 | 296.2 | 198 | 5.4 | 1.4 | 7.5 | 1.4 | − | − | − | − | − | − |
| M3 | F | 74 | 8 | 300.4 | 171 | 3.3 | 1.2 | 2.5 | 0.8 | − | − | − | − | − | − |
| M4 | M | 44 | 12 | 296.3 | 361 | 1.2 | 1.0 | 1.2 | 1.1 | − | − | − | − | − | − |
| M5 | M | 64 | 8 | 296.2 | 150 | 1.0 | 0.7 | 1.9 | 1.4 | − | − | − | − | − | − |
| M6 | M | 30 | 3 | 296.2 | 326 | 1.6 | 1.1 | 1.9 | 0.8 | − | − | − | − | − | − |
| M7 | M | 45 | 11 | 296.3 | 171 | 1.1 | 0.7 | 1.6 | 0.4 | − | − | − | − | − | − |
| M8 | M | 66 | 1 | 296.2 | 180 | 1.5 | 0.5 | 1.9 | 0.7 | − | − | − | − | − | − |
| M9 | F | 57 | 10 | 296.4 | 256 | 2.2 | 1.2 | 2.1 | 1.4 | − | − | − | − | − | − |
| M10 | M | 33 | 8 | 296.5 | 362 | 12.0 | 1.2 | 10.5 | 1.1 | 3,200 | − | 31,172 | − | 160 | − |
| M11 | F | 51 | 10 | 296.2 | 187 | 2.2 | 1.5 | 2.8 | 1.4 | − | − | − | − | − | − |
| M12 | M | 54 | 12 | 311.0 | 147 | 2.8 | 1.5 | 2.8 | 0.8 | − | − | − | − | − | − |
| M13 | M | 67 | 4 | 296.3 | 92 | 2.5 | 0.9 | 7.7 | 0.9 | − | − | − | − | − | − |
| M14 | F | 53 | 11 | 311.2 | 126 | 1.1 | 1.6 | 1.3 | 0.8 | − | − | − | − | − | − |
| M15 | F | 47 | 1 | 300.1 | 67 | 1.5 | 1.2 | 1.1 | 0.8 | − | − | − | − | − | − |
| M16 | M | 52 | 11 | 311.2 | 112 | 1.0 | 1.3 | 0.6 | 0.4 | − | − | − | − | − | − |
| M17 | M | 34 | 4 | 300.4 | 117 | 1.6 | 1.0 | 1.2 | 1.0 | − | − | − | − | − | − |
| M18 | M | 42 | 4 | 300.4 | 109 | 2.0 | 1.4 | 2.9 | 2.4 | − | − | − | − | − | − |
| M19 | F | 47 | 8 | 300.4 | 60 | 2.9 | 2.1 | 2.3 | 1.0 | − | − | − | − | − | − |
| M20 | F | 48 | 1 | 296.2 | 68 | 2.2 | 1.5 | 1.4 | 1.1 | − | − | − | − | − | − |
| M21 | F | 30 | 4 | 311.2 | 89 | 3.1 | 1.2 | 1.9 | 1.0 | − | − | − | − | − | − |
| M22 | F | 57 | 5 | 296.3 | 317 | 4.5 | 0.6 | 2.3 | 1.0 | − | − | − | − | − | − |
| M23 | M | 46 | 11 | 296.2 | 99 | 1.4 | 1.3 | 1.4 | 1.4 | − | − | − | − | − | + |
| M24 | F | 53 | 6 | 311.2 | 107 | 2.7 | 2.1 | 4.7 | 1.8 | − | − | − | − | − | − |
| M25 | M | 29 | 7 | 311.2 | 298 | 2.6 | 0.9 | 3.3 | 0.9 | − | − | − | − | − | − |
| M26 | M | 39 | 7 | 296.3 | 538 | 1.1 | 1.2 | 1.0 | 0.9 | − | − | − | − | − | − |
| M27 | F | 28 | 12 | 300.4 | 140 | 1.7 | 1.0 | 1.1 | 1.0 | − | − | − | − | − | − |
| M28 | F | 24 | 8 | 300.4 | 62 | 2.2 | 1.3 | 2.9 | 2.7 | − | − | − | − | − | − |
| M29 | F | 39 | 2 | 296.2 | 97 | 3.4 | 1.6 | 1.2 | 2.2 | − | − | − | − | − | − |
| M30 | M | 49 | 9 | 296.3 | 127 | 2.2 | 1.3 | 1.8 | 0.8 | − | − | − | − | − | − |
| M31 | M | 52 | 8 | 296.4 | 365 | 1.6 | 1.0 | 1.6 | 1.1 | − | − | − | − | − | − |
| M32 | F | 65 | 10 | 296.2 | 180 | 1.0 | 0.8 | 0.8 | 0.7 | − | − | − | − | − | − |
| M33 | F | 30 | 3 | 296.2 | 210 | 1.9 | 1.1 | 1.7 | 1.2 | − | − | − | − | − | − |
| M34 | F | 17 | 8 | 296.2 | 310 | 1.0 | 0.9 | 1.9 | 1.1 | − | − | − | − | − | − |
| M35 | F | 54 | 11 | 300.4 | 140 | 2.0 | 1.1 | 1.5 | 1.3 | − | − | − | − | − | − |
| M36 | F | 47 | 7 | 296.2 | 110 | 1.1 | 0.9 | 3.5 | 1.2 | − | − | − | − | − | − |
| M37 | F | 65 | 1 | 296.3 | 120 | 2.5 | 1.5 | 8.2 | 1.1 | − | − | − | − | − | − |
| M38 | F | 62 | 11 | 296.2 | 360 | 1.4 | 1.5 | 1.0 | 1.2 | − | − | − | − | − | − |
| M39 | M | 34 | 8 | 311.2 | 380 | 2.9 | 2.1 | 5.9 | 0.8 | − | − | − | − | − | − |
| M40 | M | 59 | 4 | 311.2 | 167 | 2.5 | 1.7 | 1.6 | 1.1 | − | − | − | − | − | − |
| M41 | M | 50 | 5 | 311.2 | 174 | 3.5 | 1.8 | 6.4 | 2.1 | − | − | − | − | − | − |
| M42 | M | 49 | 4 | 300.4 | 321 | 3.3 | 2.1 | 4.1 | 1.1 | − | − | − | − | − | − |
| M43 | M | 47 | 12 | 296.3 | 123 | 4.1 | 1.0 | 5.5 | 1.1 | − | 200 | − | − | − | − |
| M44 | F | 27 | 2 | 300.4 | 115 | 1.3 | 0.7 | 1.0 | 0.9 | − | − | − | − | − | − |
| M45 | F | 28 | 7 | 300.4 | 162 | 1.2 | 1.1 | 1.7 | 1.1 | − | − | − | − | − | − |
| Mean (SD) | 47 (13) | 191 (109) | 2.5 (1.8) | 1.2 (0.4) | 2.8 (2.3) | 1.1 (0.5) | |||||||||
M, male; F, female; MOB, month of birth; Diag., diagnostic code according to DSM-IV.
SIs were calculated as mean counts per minute with BDV antigen divided by mean counts per minute without antigen.
Titers of antibodies are expressed as the highest serum dilution showing a detectable signal.
Results for ECLIA-positive samples are expressed as ECLIA counts.
−, negative.
TABLE 2.
Results of proliferation assay, WB analysis. ECLIA, and RT-PCR for patients with schizophreniaa
| Patient | Sex | Age (yr) | MOB | Diag. | Proliferation assay (SI)b
|
WB analysisc
|
ECLIAd
|
IFc (BDV/MDCK) | RT-PCR (p24) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PHA | p40 | p40 NC | p24 | p24 NC | p40 | p24 | p40 | p24 | |||||||
| S1 | M | 48 | 10 | 295.90 | 150 | 3.3 | 1.3 | 4.2 | 1.3 | −d | − | − | − | − | − |
| S2 | M | 59 | 12 | 295.90 | 360 | 3.0 | 1.1 | 2.2 | 1.0 | −d | − | − | − | − | − |
| S3 | M | 44 | 4 | 295.90 | 236 | 2.6 | 1.2 | 4.9 | 1.3 | − | − | − | − | − | − |
| S4 | F | 48 | 6 | 295.90 | 162 | 1.4 | 0.9 | 1.6 | 0.8 | − | − | − | − | − | − |
| S5 | M | 52 | 4 | 295.90 | 297 | 2.2 | 0.8 | 1.7 | 0.7 | − | − | − | − | − | − |
| S6 | M | 52 | 11 | 295.90 | 279 | 1.4 | 1.3 | 2.4 | 1.0 | − | − | − | − | − | − |
| S7 | M | 29 | 7 | 295.90 | 177 | 4.9 | 1.0 | 10.6 | 2.1 | − | − | − | − | − | − |
| S8 | F | 21 | 8 | 295.90 | 185 | 1.8 | 1.3 | 2.5 | 1.1 | − | − | − | − | − | − |
| S9 | M | 35 | 2 | 295.90 | 370 | 1.3 | 1.2 | 1.4 | 1.0 | − | − | − | − | − | − |
| S10 | M | 50 | 3 | 295.90 | 188 | 1.2 | 1.4 | 1.5 | 0.8 | − | − | − | − | − | − |
| S11 | M | 46 | 3 | 295.90 | 164 | 1.4 | 1.2 | 1.1 | 0.8 | − | − | − | − | − | − |
| S12 | M | 55 | 2 | 295.90 | 447 | 1.2 | 1.1 | 1.3 | 0.9 | − | − | − | − | − | − |
| S13 | F | 27 | 5 | 295.90 | 89 | 1.3 | 0.8 | 1.9 | 1.2 | − | − | − | − | − | − |
| S14 | M | 37 | 12 | 295.90 | 647 | 1.3 | 1.6 | 1.4 | 1.1 | − | 100 | − | − | − | − |
| S15 | F | 39 | 8 | 295.90 | 114 | 1.5 | 1.4 | 1.1 | 0.8 | − | − | − | − | − | − |
| S16 | F | 60 | 4 | 295.90 | 224 | 1.4 | 1.0 | 1.2 | 0.9 | − | − | − | − | − | − |
| S17 | F | 34 | 4 | 295.90 | 243 | 1.9 | 1.1 | 1.5 | 0.8 | − | − | − | − | − | − |
| S18 | M | 48 | 6 | 295.90 | 171 | 3.8 | 1.9 | 3.3 | 1.3 | − | − | − | − | − | − |
| S19 | M | 48 | 10 | 295.90 | 355 | 2.2 | 1.5 | 4.0 | 1.0 | − | − | − | − | − | − |
| S20 | F | 27 | 2 | 295.90 | 322 | 1.4 | 1.0 | 1.5 | 0.9 | − | − | − | − | − | − |
| S21 | F | 49 | 1 | 295.90 | 230 | 2.1 | 1.3 | 2.3 | 0.8 | − | − | − | − | − | − |
| S22 | F | 47 | 3 | 295.91 | 94 | 2.4 | 0.5 | 0.8 | 0.5 | − | − | − | − | − | − |
| S23 | M | 52 | 6 | 295.10 | 160 | 2.7 | 1.4 | 1.3 | 0.8 | − | − | − | − | − | − |
| S24 | M | 45 | 8 | 295.90 | 71 | 6.4 | 1.0 | 1.8 | 0.6 | − | − | − | − | − | − |
| S25 | M | 44 | 5 | 295.90 | 57 | 1.0 | 0.9 | 1.7 | 0.7 | − | − | − | − | − | − |
| S26 | M | 48 | 9 | 295.90 | 380 | 2.9 | 1.4 | 2.8 | 1.1 | − | − | − | − | − | − |
| S27 | M | 44 | 11 | 295.90 | 53 | 2.9 | 0.6 | 3.1 | 0.8 | − | − | − | − | − | − |
| S28 | F | 43 | 1 | 295.90 | 211 | 1.4 | 1.1 | 1.5 | 0.9 | − | − | − | − | − | − |
| S29 | F | 49 | 10 | 295.30 | 259 | 2.7 | 1.8 | 1.5 | 1.2 | − | − | − | − | − | − |
| S30 | F | 44 | 3 | 295.30 | 150 | 2.6 | 1.1 | 1.2 | 0.9 | − | − | − | − | − | − |
| S31 | F | 32 | 5 | 295.30 | 246 | 3.3 | 1.2 | 2.5 | 0.9 | − | − | − | − | − | − |
| S32 | F | 27 | 9 | 295.90 | 289 | 3.3 | 1.1 | 3.3 | 1.1 | − | − | − | − | − | − |
| S33 | F | 53 | 9 | 295.90 | 198 | 3.5 | 1.5 | 2.4 | 1.3 | − | − | − | − | − | − |
| S34 | M | 53 | 7 | 295.90 | 87 | 2.3 | 1.7 | 1.7 | 2.8 | − | − | − | − | − | − |
| S35 | M | 42 | 9 | 295.90 | 247 | 1.7 | 1.3 | 2.2 | 1.2 | − | − | − | − | − | − |
| S36 | F | 46 | 3 | 295.90 | 210 | 1.8 | 1.2 | 1.7 | 1.0 | − | 50 | − | − | − | − |
| S37 | F | 43 | 1 | 295.90 | 243 | 1.1 | 0.9 | 1.3 | 1.0 | − | − | − | − | − | − |
| S38 | F | 50 | 7 | 295.90 | 320 | 1.7 | 1.2 | 1.8 | 1.0 | − | − | − | − | − | − |
| S39 | F | 45 | 6 | 295.90 | 210 | 1.5 | 1.0 | 1.3 | 1.0 | − | − | − | − | − | − |
| S40 | F | 45 | 10 | 295.90 | 169 | 1.6 | 0.9 | 0.9 | 1.4 | − | − | − | − | − | − |
| S41 | F | 47 | 12 | 295.90 | 125 | 1.1 | 0.6 | 0.8 | 1.1 | − | − | − | − | − | − |
| S42 | F | 48 | 2 | 295.90 | 227 | 1.1 | 0.9 | 1.2 | 0.9 | − | − | − | − | − | − |
| S43 | M | 59 | 11 | 295.90 | 461 | 1.8 | 1.2 | 2.7 | 2 | − | − | − | − | − | − |
| S44 | M | 44 | 7 | 295.90 | 272 | 6.8 | 1.5 | 8.6 | 1.4 | − | 100 | − | − | − | − |
| S45 | M | 47 | 9 | 295.90 | 127 | 2.5 | 1.5 | 1.4 | 1.2 | − | 400 | − | − | − | − |
| Mean (SD) | 45 (9) | 228 (117) | 2.3 (1.3) | 1.2 (0.3) | 2.3 (1.8) | 1.1 (0.4) | |||||||||
M, male; F, female; MOB, month of birth; Diag., diagnostic code according to DSM-IV.
SIs were calculated from mean counts per minute with BDV antigen divided by mean counts per minute without antigen.
Titers of antibodies are expressed as the highest serum dilution showing a detectable signal.
−, negative.
Preparation of human PBMCs.
PBMCs were isolated on the day following drawing of 20 ml of blood with EDTA by centrifugation on Ficoll-Conray solution (density, 1.077 g/ml) and used for proliferative responses and RT-PCR.
Preparation of BDV proteins.
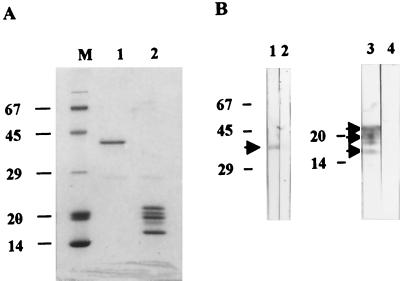
Recombinant BDV proteins were prepared as described previously (20, 53) with modifications. In brief, the full-length cDNA encoding BDV p40 or p24 derived from the He80-1 strain was inserted into the prokaryotic expression vector pGEX-5X-3 (Pharmacia, Uppsala, Sweden). Recombinant proteins were produced in Escherichia coli (JM 109) as the fusion protein with glutathione S-transferase (GST) and were bound on glutathione–Sepharose 4B (Pharmacia). The fusion proteins p40 and p24 were cleaved by factor Xa according to the manufacturer's protocol. GST was also purified by binding on glutathione–Sepharose 4B and elution with a reduced form of glutathione. These proteins were further purified by Mono Q column chromatography (Pharmacia) with a gradient elution buffer (0 to 0.5 M NaCl). The mobility and purity of the proteins were assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and densitometry (see Fig. 1A). Protein concentrations were determined with the Micro bicinchoninic acid protein assay reagent (Pierce, Rockford, Ill.).
FIG. 1.
Purification of BDV p40 and p24 proteins. BDV proteins expressed as GST fusion proteins were purified by cleavage from GST by factor Xa and Mono Q column chromatography. (A) SDS-PAGE of purified BDV proteins. BDV p40 protein was electrophoresed with the expected mobility, 40 kDa (lane 1). Purified BDV p24 protein exhibited three major bands (22, 20, and 17.5 kDa), and two minor bands (21 and 19 kDa) (lane 2). (B) Specificity of BDV proteins. Lanes 1 and 2, immunoreactivities of monospecific rabbit anti-BDV p40 serum and normal rabbit serum (1:16,000 and 1:50, respectively) to BDV p40 protein. Lanes 3 and 4, immunoreactivities of monospecific rabbit anti-BDV p24 serum and normal rabbit serum (1:4,000 and 1:50, respectively) to BDV p24 protein. The electrophoretic mobilities and size (in kilodaltons) of molecular markers are indicated on the left. The positions of p40 and p24 are indicated by arrowheads.
Negative control (NC) antigens for p40 or p24 in proliferation assays were prepared from the culture of E. coli transformed by pGEX-5X-3 by the same procedures for p40 and p24. The same fraction corresponding to the fraction of p40 or p24 in Mono Q chromatography was used as an NC at the same dilution for p40 or p24, respectively. The protein concentrations of the NC fractions were under the limit of detection (<1 μg/ml).
Proliferative response of PBMCs.
PBMCs (2 × 105 cells) were cultured with 2 μg of purified BDV p40 or p24 per ml in 0.2-ml pentaplicates of RPMI 1640 containing 10% human AB serum and 5 × 10−5 M 2-mercaptoethanol in 96-well round-bottom plates at 37°C for 6 days. Control antigens consisted of GST, egg lysozyme (Nacalai Tesque, Kyoto, Japan), and horse muscle myoglobin (Nacalai Tesque) and were used at 2 μg/ml. The NC negative antigens described above were also used. Phytohemagglutinin (PHA) was used as positive control at 1 μg/ml. The PBMCs were pulsed with 0.5 μCi of tritiated thymidine ([3H]TdR) for 16 h before harvest. The cells were harvested using a cell harvester (PHD cell harvester; Cambridge Technology, Watertown, Mass.), and tritiated thymidine incorporation was counted in a liquid scintillation counter (LSC-3500; Aloka, Tokyo, Japan) as counts per minute. The counts per minute of samples without antigens ranged from 103 to 2,612. The stimulation index (SI) was calculated as mean counts per minute with BDV antigen divided by mean counts per minute without antigen. An SI of over 4 was considered positive, compared to the SI of NC antigen in any of three subject groups by statistical analysis (Student's t test; P < 0.001) (an SI of 4 was over the mean plus 3 standard deviations of the SI of the NC antigen).
WB analysis.
WB analysis was performed to detect anti-BDV p40 and p24 antibodies. Purified BDV proteins (0.3 μg/lane) were subjected to SDS-PAGE and electroblotted to nitrocellulose membranes [PROTEIN BA 85 CELLULOSENIT AT(E): Schleider & Schuell, Keene, N.H.]. The membrane was soaked in a blocking solution (phosphate-buffered saline [PBS] containing 3% [wt/vol] nonfat dry milk and 0.2% [wt/vol] bovine serum albumin) overnight at 4°C. After blocking, the strips of the membrane were incubated with human plasma diluted with blocking solution containing 0.5% (vol/vol) Tween 20 at 1:50 for 1 h at room temperature. Rabbit anti-BDV p40 serum (1:4,000) or rabbit anti-BDV p24 serum (1:2,000) (kind gifts from W. I. Lipkin and J. C. de la Torre, respectively) was used as a positive control. After five washes with PBS containing 0.5% Tween 20, the membranes were incubated for 30 min at room temperature with horseradish peroxidase-labeled goat anti-human immunoglobulin G (IgG) antibody (1:600) (DAKO, Kyoto, Japan) or goat anti-rabbit antibody (1:800) (ICN Pharmaceuticals, Inc., Costa Mesa, Calif.). After three washes, the antibodies were visualized with 0.1% 3,3′-diaminobenzidine tetrahydrochloride and 0.03%, H2O2 for 15 min at room temperature. The antibody titer was expressed as the highest plasma dilution showing a detectable signal. To evaluate the specificity of the antibody, inhibition tests were performed (40). The positive human plasma was diluted at the antibody titer and incubated with 20 μg of BDV p40 or p24 protein per ml for 1 h at room temperature prior to the reaction on a nitrocellulose membrane. The membrane was then further processed as described above.
ECLIA.
ECLIA was performed to detect anti-BDV p40 and p24 antibodies as described previously (53). In brief, the plasma sample diluted 1:11 with normal rabbit serum was incubated with highly purified recombinant p40- or p24-coated beads. After washes, a second antibody, anti-human IgG (Fc) mouse monoclonal antibody coupled with ruthenium(II) Tris (bipyridyl)-N-hydroxysuccinimide ester [Ru(bpy)32+], was added to the beads. The beads were conducted into the electrode, and the photon emitted from the Ru(bpy)32+ was counted with a photomultiplier tube as the ECLIA count. The sample was considered positive if the ECLIA count was higher than cutoff counts. The cutoff count was 1,101 for BDV p40- and p24-coated beads, 868 for p40-coated beads, and 1,341 for p24-coated beads. The specificities of the positive samples were confirmed with the inhibition test (53). The plasma samples were finally regarded as positive if the ECLIA counts were inhibited by more than 50% of the original counts. The positive samples were reexamined for ECLIA and reproducibly confirmed to be positive.
IF assay.
Acetone-fixed Mardin-Darby canine kidney (MDCK) cells persistently infected with BDV (BDV/MDCK) (kindly provided by R. Rott, Justus-Liebig-Universitat Giessen, Giessen, Germany) were incubated with serially diluted human plasma for 30 min at room temperature. After three washes with PBS containing 0.1% Tween 20, the cells were incubated with fluorescein isothiocyanate-conjugated goat anti-human IgG antibody for 30 min at room temperature. The antibody titer was expressed as the highest dilution showing detectable signals. Rabbit anti-BDV p40 was used as a positive control. Normal rabbit serum and uninfected MDCK cells were used as NCs.
Preparation of RNA.
Total cellular RNA was prepared from 107 PBMCs with an RNA extraction kit (Isogen; Nippon Gene, Tokyo, Japan). The approximate concentration of extracted RNA was determined by spectrophotometry as described previously (22).
Detection of BDV RNA in PBMCs by nested RT-PCR.
PBMC RNA was screened for the presence of BDV p24 sequences by nested RT-PCR as described previously (22). Specificity of the amplification products was demonstrated by Southern blot hybridization using an alkaline phosphatase-labeled oligonucleotide specific for BDV p24 sequences. To confirm the presence of BDV RNA, RT-PCR was repeated for all of the positive samples, and in some cases RNA obtained at the second time of blood collection was also tested. We regarded the samples as positive if they showed positive at least twice and showed negative twice in nested RT-PCR without reverse transcriptase.
RESULTS
Purification and specificity of BDV proteins.
Recombinant BDV p40 and p24 proteins for the proliferation assay and WB analysis were purified by Mono Q column chromatography and analyzed by SDS-PAGE (Fig. 1A). One band of expected molecular mass was observed for the p40 protein, but in the case of p24, three major bands of 22, 20, and 17.5 kDa and two minor bands of 21 and 19 kDa were observed. The specificity of the bands was tested by WB analysis with rabbit monospecific anti-BDV sera and normal rabbit sera (1:50 diluted). Rabbit anti-BDV p40 antibody reacted with the purified BDV p40 protein. Monospecific rabbit anti-BDV p24 antibody reacted with all of five bands of the purified BDV p24 protein, indicating that these bands represented degradation products of p24 (Fig. 1B). Normal rabbit sera did not react with either BDV p40 or p24 protein, indicating that the purified BDV antigens are BDV specific. The purity of p40 and p24 was >98%.
Establishment of a WB analysis to detect antibodies to BDV proteins.
To detect antibodies to BDV p40 and p24 in sera from psychiatric patients and blood donors, we established a WB analysis with purified recombinant BDV proteins. To test the sensitivity of a WB analysis, rabbit anti-BDV p40 antibody or anti-BDV p24 antibody was diluted and subjected to the reaction. The maximum dilutions that showed a detectable signal were 1:32,000 for rabbit anti-BDV p40 antibody and 1:4,000 for rabbit anti-BDV p24 antibody (Fig. 2A). To evaluate the specificity of the WB analysis, the inhibition test was performed. Rabbit anti-BDV p40 antibody diluted at 1:16,000 or rabbit anti-BDV p24 antibody diluted at 1:2,000 was preincubated with 20 μg ml of BDV p40 or p24 protein per ml, respectively, and subjected to the WB analysis. The reactions of both rabbit anti-BDV antibodies were inhibited by purified BDV proteins, suggesting specific binding of the anti-BDV antibodies to BDV proteins (Fig. 2B).
FIG. 2.
Sensitivity and specificity of Western blot analysis. (A) Titration of rabbit anti-BDV p40 (a) and p24 (b) antibodies. (a) Rabbit anti-BDV p40 antibody was serially diluted twofold from 1:2,000 to 1:64,000 (lanes 1 to 6). (b) Rabbit anti-BDV p24 antibody was serially diluted twofold from 1:500 to 1:16,000 (lanes 1 to 6). Lanes 7 illustrate immunoreactivity of normal rabbit serum (1:50). (B) Specificity of rabbit anti-BDV p40 (a) and p24 (b) antibodies. (a) Lane 1, immunoreactivity of monospecific rabbit anti-BDV p40 antibody to BDV p40 protein. Specificity of the immunoreactivity of rabbit anti-BDV p40 antibodies was demonstrated by inhibiting the reaction with 20 μg of BDV p40 (lane 2) and GST (lane 3) protein per ml. (b) Lane 1, immunoreactivity of monospecific rabbit anti-BDV p24 antibody to BDV p24 protein. Specificity of the immunoreactivity of rabbit anti-BDV p24 antibodies was demonstrated by inhibiting the reaction with 20 μg of BDV p24 (lane 2) and GST (lane 3) protein per ml. The electrophoretic mobilities and sizes (in Kilodaltons) of molecular markers are indicated on the left. Arrowheads indicate the positions of BDV p40 and p24.
Detection of anti-BDV antibodies in human plasma by WB analysis.
We investigated the prevalence of antibodies to BDV p40 and p24 in plasma specimens from 90 psychiatric patients and 45 blood donors by WB analysis (Fig. 3). Plasma samples were regarded as positive only when their corresponding immunoreactivities in WB analysis were inhibited by preincubation with purified BDV proteins. In the primary screening without an inhibition test, positive bands to BDV p40 were detected in 8 of 45 patients with mood disorders (18%), 13 of 45 in schizophrenic patients (29%), and 4 of 45 blood donors (9%). Positive bands to BDV p24 were detected in 4 of 45 patients with mood disorders (9%), 5 of 45 schizophrenic patients (11%), and 1 of 45 blood donors (2%). To evaluate the specificity of the detected signals, inhibition tests were performed. In patients with mood disorders, only one out of five patients who had a positive band to p40 (2%; 1 of 45) was found to have specific antibody to p40. There were no specific antibodies in patients with schizophrenia or in blood donors. In the case of anti-p24 antibody, one out of five patients with mood disorders (2%; 1 of 45) and four out of five patients with schizophrenia (9%; 4 of 45) had the specific antibody. There was no plasma that reacted with both BDV p40 and p24. There was no significant difference among the three groups in the prevalences of the antibodies to BDV p40 and p24.
FIG. 3.
Detection of antibodies to BDV proteins in human plasma. Recombinant BDV p40 and p24 proteins were separated by SDS-PAGE and analyzed by Western blot analysis. (A) Screening test for antibodies to BDV p40 (a) and p24 (b) in human sera. Lanes 1 to 20, immunoreactivities of 18 representative human plasma samples (lanes 1 to 9 and 11 to 19), rabbit anti-p40 antibody (lane 10), and rabbit anti-p24 antibody (lane 20). (B) Specificity of human plasma to BDV p40 (left panel) and p24 (right panel) proteins was demonstrated by inhibition tests. M10 and M43 show the reactions of two positive plasma samples, and M2 and C10 show the reactions of two negative plasma samples. Lanes a, immunoreactivity of human plasma without preincubation with p40 or p24 protein. Lanes b and c, immunoreactivity of human plasma preincubated with 20 μg of p40 or p24 (lanes b) and GST (lanes c) proteins, per ml. The electrophoretic mobilities and sizes (in kilodaltons) of molecular markers are indicated on the left. Arrowheads indicate the positions of p40 and p24.
Detection of anti-BDV antibodies by ECLIA.
All of the blood samples were also tested for anti-BDV antibodies by ECLIA. Of the samples, the patient with mood disorders M10 and the blood donor C23 were positive for anti-BDV p40 antibody (count, 31,172, inhibition, 62.9%) and anti-BDV p24 antibody (count, 3,089, inhibition, 72.1%), respectively (Tables 1 and 3). The prevalences of anti-p40 antibody were 2% (1 of 45), 0%, and 0% in patients with mood disorders, patients with schizophrenia, and blood donors, respectively. The prevalences of anti-p24 antibody were 0, 0, and 2% (1 of 45) in patients with mood disorders, patients with schizophrenia, and blood donors, respectively. None of the plasma samples reacted with both p40 and p24.
TABLE 3.
Results of proliferation assay. WB analysis, ECLIA, and RT-PCR for blood donorsa
| Donor | Sex | Age (yr) | Proliferation assay (SI)b
|
WB analysisc
|
ECLIAd
|
IFc (BDV/MDCK) | RT-PCR (p24) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PHA | p40 | p40 NC | p24 | p24 NC | p40 | p24 | p40 | p24 | |||||
| C1 | F | 53 | 231 | 4.7 | 1 | 1.6 | 0.7 | −e | − | − | − | − | − |
| C2 | F | 54 | 394 | 0.9 | 0.9 | 3.6 | 1.1 | − | − | − | − | − | − |
| C3 | F | 59 | 281 | 2.3 | 0.8 | 1.8 | 0.7 | − | − | − | − | − | − |
| C4 | F | 51 | 265 | 4.5 | 1.1 | 2.5 | 1 | − | − | − | − | − | − |
| C5 | F | 56 | 463 | 2.7 | 1 | 1.9 | 0.8 | − | − | − | − | − | − |
| C6 | F | 59 | 115 | 2.8 | 1.2 | 7.8 | 1.2 | − | − | − | − | − | − |
| C7 | F | 55 | 181 | 2.2 | 0.8 | 3.8 | 0.8 | − | − | − | − | − | − |
| C8 | F | 57 | 217 | 1.1 | 1.2 | 2.4 | 1.3 | − | − | − | − | − | − |
| C9 | F | 50 | 270 | 0.8 | 0.9 | 1.9 | 1.1 | − | − | − | − | − | − |
| C10 | M | 45 | 158 | 0.9 | 1.4 | 2.1 | 1.0 | − | − | − | − | − | − |
| C11 | M | 44 | 81 | 1.7 | 1.1 | 1.3 | 1.1 | − | − | − | − | − | − |
| C12 | M | 37 | 60 | 1.2 | 1.5 | 1.9 | 1.0 | − | − | − | − | − | − |
| C13 | F | 43 | 82 | 1.2 | 1.1 | 1.8 | 0.9 | − | − | − | − | − | − |
| C14 | F | 26 | 84 | 1.2 | 1.7 | 2.3 | 1.3 | − | − | − | − | − | − |
| C15 | F | 30 | 98 | 1.0 | 1.8 | 1.2 | 0.9 | − | − | − | − | − | − |
| C16 | M | 52 | 129 | 1.2 | 0.8 | 1.5 | 0.8 | − | − | − | − | − | − |
| C17 | M | 53 | 101 | 2.5 | 1.6 | 1.8 | 1.1 | − | − | − | − | − | − |
| C18 | M | 53 | 60 | 4.2 | 1.0 | 2.9 | 0.7 | − | − | − | − | − | − |
| C19 | M | 53 | 52 | 2.2 | 0.9 | 4.7 | 0.9 | − | − | − | − | − | − |
| C20 | M | 48 | 522 | 0.9 | 1.0 | 1.3 | 1.1 | − | − | − | − | − | − |
| C21 | M | 44 | 203 | 2.6 | 1.1 | 1.3 | 0.9 | − | − | − | − | − | − |
| C22 | M | 62 | 88 | 2.2 | 0.7 | 4.2 | 0.7 | − | − | − | − | − | − |
| C23 | F | 43 | 211 | 1.2 | 1.3 | 1.2 | 0.8 | − | − | − | 3,089 | − | − |
| C24 | M | 58 | 60 | 2.4 | 1.8 | 1.4 | 1.2 | − | − | − | − | − | − |
| C25 | M | 51 | 57 | 1.3 | 1.1 | 2.4 | 1.3 | − | − | − | − | − | − |
| C26 | F | 42 | 248 | 1.5 | 0.7 | 2.1 | 1.1 | − | − | − | − | − | − |
| C27 | M | 42 | 258 | 3.5 | 1.2 | 3.7 | 1.0 | − | − | − | − | − | − |
| C28 | M | 42 | 248 | 1.6 | 1.1 | 1.5 | 1.1 | − | − | − | − | − | − |
| C29 | M | 50 | 88 | 1.3 | 1.3 | 1.0 | 1.3 | − | − | − | − | − | − |
| C30 | M | 50 | 158 | 1.5 | 1.0 | 1.2 | 0.8 | − | − | − | − | − | − |
| C31 | F | 47 | 71 | 1.2 | 1.4 | 0.9 | 0.8 | − | − | − | − | − | − |
| C32 | F | 40 | 244 | 1.9 | 1.0 | 1.6 | 1.1 | − | − | − | − | − | − |
| C33 | F | 45 | 163 | 1.2 | 0.8 | 1.3 | 0.8 | − | − | − | − | − | − |
| C34 | F | 43 | 61 | 0.7 | 0.6 | 0.9 | 1.0 | − | − | − | − | − | − |
| C35 | F | 52 | 221 | 6.6 | 1.8 | 5.5 | 1.5 | − | − | − | − | − | − |
| C36 | F | 57 | 222 | 2.9 | 1.9 | 1.5 | 0.8 | − | − | − | − | − | − |
| C37 | M | 60 | 320 | 1.5 | 1.0 | 2.5 | 1.0 | − | − | − | − | − | − |
| C38 | F | 28 | 69 | 1.3 | 1.1 | 2.6 | 0.9 | − | − | − | − | − | − |
| C39 | F | 29 | 300 | 1.8 | 1.2 | 1.4 | 1.2 | − | − | − | − | − | − |
| C40 | M | 48 | 235 | 4.1 | 1.2 | 1.8 | 1.2 | − | − | − | − | − | − |
| C41 | M | 55 | 219 | 1.3 | 0.8 | 1.3 | 1.0 | − | − | − | − | − | − |
| C42 | M | 49 | 238 | 1.2 | 0.8 | 1.7 | 1.0 | − | − | − | − | − | − |
| C43 | M | 40 | 239 | 1.5 | 0.9 | 1.8 | 1.1 | − | − | − | − | − | − |
| C44 | M | 58 | 190 | 1.5 | 0.9 | 1.7 | 1.0 | − | − | − | − | − | − |
| C45 | F | 48 | 210 | 1.2 | 0.9 | 1.7 | 1.1 | − | − | − | − | − | − |
| Mean (SD) | 48 (9) | 188 (109) | 2.0 (1.2) | 1.1 (0.3) | 2.2 (1.3) | 1.0 (0.2) | |||||||
M, male; F, female; Diag., diagnostic code according to DSM-IV.
SIs were calculated from mean counts per minute with BDV antigen divided by mean counts per minute without antigen.
Titers of antibodies are expressed as the highest serum dilution showing a detectable signal.
Results for ECLIA-positive samples are expressed as ECLIA counts.
−, negative
Detection of anti-BDV antibodies by IF assay.
IF assay was also performed to detect anti-BDV antibodies in all samples. Of the samples, only those from patient M10 with mood disorders was positive for BDV antibodies, with characteristic dot-like staining in the nuclei of infected cells (Fig. 4A). The antibody titer was 1:160, and this plasma did not react with uninfected MDCK cells (Fig. 4B). In the three antibody assays, patient M10 was positive for p40 antibody by both WB analysis and ECLIA and positive for BDV antibody by IF. Plasma samples from five patients (M43, S14, S36, S44, and S45) were positive for p24 antibody by WB analysis but negative by ECLIA and IF. Conversely, blood donor C23 was only positive for p24 antibody by ECLIA.
FIG. 4.
Detection of antibodies to BDV in patient M10 with mood disorders by IF. The patient plasma at a dilution of 1:160 was applied on acetone-fixed BVD-infected MDCK cells (A) and MDCK cells (B). Note the dot-like staining in the nuclei-of BDV-infected MDCK cells but not in MDCK cells.
Proliferative responses of PBMCs to BDV proteins.
To examine whether cell-mediated immune responses to BDV can be detected in psychiatric patients and blood donors, T-cell proliferative responses to BDV p40 and p24 proteins were analyzed in vitro (Tables 1 to 4). Among patients with mood disorders, 11% of the patients (5 of 45) responded to BDV p40 with an SI of over 4.0, 22% (10 of 45) responded to BDV p24, and 9% (4 of 45) responded to both BDV p40 and p24. Among patients with schizophrenia, 7% of the patients (3 of 45) responded to BDV p40, 11% (5 of 45) responded to BDV p24, and 4% (2 of 45) responded to both BDV p40 and p24. Among blood donors, 11% (5 of 45) responded to BDV p40, 9% (4 of 45) responded to BDV p24, and 2% (1 of 45) responded to both BDV p40 and p24. There was no significant difference among the three groups in the prevalences of the positive proliferative responses to either p40 or p24 or to both p40 and p24 antigens. The mean SIs for NC antigens of p40 and p24 were 1.1 to 1.2 and 1.0 to 1.1, respectively in the three groups. The mean SIs for GST, myoglobin, and lysozyme were 0.8 to 1.7, 1.1 to 1.3, and 0.8 to 1.0, respectively. To confirm the positive proliferative responses, PBMCs from one patient with mood disorders (M10) and one patient with schizophrenia (S44) were recollected and reexamined by the proliferation assay. These two samples showed positive proliferative responses reproducibly, with SIs of over 4 (Table 5).
TABLE 4.
Prevalence of immunological responses to BDV and BDV RNA
| Group | Prevalence % (no. positive/total no.)
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Proliferation assay
|
WB analysis
|
ECLIA
|
IF (BDV/MDCK) | RT-PCR (p24) | |||||||
| p40 | p24 | p40 and p24 | p40 | p24 | p40 and p24 | p40 | p24 | p40 and p24 | |||
| Mood disorder patients | 11 (5/45) | 22 (10/45) | 9 (4/45) | 2 (1/45) | 2 (1/45) | 0 (1/45) | 2 (1/45) | 0 (0/45) | 0 (0/45) | 2 (1/45) | 2 (1/45) |
| Schizophrenia patients | 7 (3/45) | 11 (5/45) | 4 (2/45) | 0 (0/45) | 9 (4/45) | 0 (4/45) | 0 (0/45) | 0 (0/45) | 0 (0/45) | 0 (0/45) | 0 (0/45) |
| Blood donors | 11 (5/45) | 9 (4/45) | 2 (1/45) | 0 (0/45) | 0 (0/45) | 0 (0/45) | 0 (0/45) | 2 (1/45) | 0 (0/45) | 0 (0/45) | 0 (0/45) |
TABLE 5.
BDV status and clinical features of individuals who are seropositive for BDV or positive for BDV RNAa
| Patient | Sex | Age (yr) | MOB | Proliferation assay (SI)b
|
WB analysisc
|
ECLIAd
|
IFc (BDV/MDCK) | RT-PCR (p24) | Clinical features
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PHA | p40 | p40 NC | p24 | p24 NC | p40 | p24 | p40 | p2424 | Diag | Hospitilization status | Psychiatric state | ||||||
| M10-1c | M | 33 | 8 | 362 | 12.0 | 1.2 | 10.5 | 1.1 | 3,200 | −f | 31,172 | − | 160 | − | 296.55 | Outpatient | Partial remission |
| M10-2e | M | 33 | 8 | 135 | 7.5 | 1.3 | 5.5 | 1.0 | 3,200 | − | NT | NT | 160 | − | 296.55 | Outpatient | Partial remission |
| M23 | M | 46 | 9 | 99 | 1.4 | 1.3 | 1.4 | 1.4 | − | − | − | − | − | + | 296.22 | Impatient | Moderate depression |
| M43 | M | 47 | 12 | 123 | 4.1 | 1.0 | 5.5 | 1.1 | − | 200 | − | − | − | − | 296.32 | Inpatient | Moderate depression |
| S14 | M | 37 | 12 | 647 | 1.3 | 1.6 | 1.4 | 1.1 | − | 100 | − | − | − | − | 295.90 | Inpatient | Chronic schizophrenia |
| S36 | F | 46 | 3 | 210 | 1.8 | 1.2 | 1.7 | 1.0 | − | 50 | − | − | − | − | 295.90 | Inpatient | Chronic schizophrenia |
| S44-1e | M | 44 | 7 | 272 | 6.8 | 1.5 | 8.6 | 1.4 | − | 100 | − | − | − | − | 295.90 | Impatient | Chronic schizophrenia |
| S44-2e | M | 44 | 7 | 228 | 4.8 | 1.2 | 7.6 | 1.3 | − | 100 | − | − | − | − | 295.90 | Impatient | Chronic schizopherenia |
| S45 | M | 47 | 10 | 127 | 2.4 | 2.5 | 1.4 | 2.0 | − | 400 | − | − | − | − | 295.90 | Inpatient | Chronic schizophernia |
| C23 | F | 43 | NA | 211 | 1.2 | 1.3 | 1.2 | 0.8 | − | − | − | 3,089 | − | − | |||
M, male; F, female; MOB, month of birth; Diag., diagnostic code according to DSM-IV; NA, not available; NT = not tested.
SIs were calculated from mean counts per minute with BDV antigen divided by mean counts per minute without antigen.
Titers of antibodies are expressed as the highest serum dilution showing a detectable signal.
Results for ECLIA-positive samples are expressed as ECLIA counts.
Specimens were recollected from patients M10 and S44.
−, negative
Detection of BDV p24 RNA in PBMCs of psychiatric patients and blood donors by nested RT-PCR.
To detect BDV RNA in PBMCs of psychiatric patients and blood donors, RNA samples from PBMCs were tested by nested RT-PCR. A BDV p24-specific DNA fragment was detected by Southern blot analysis in 1 of 45 patients with mood disorders (2%) (M23) (Tables 1 and 5), none of 45 patients with schizophrenia (0%) and none of 45 blood donors (0%) (Tables 1 to 4). To confirm the positive result by RT-PCR, we repeated the entire assay, including the sample collection and RNA preparation. This positive sample was confirmed to be positive by repeating RT-PCR and was also confirmed to be reverse transcriptase dependent. Sequence analysis of this PCR product showed a BDV p24 sequence that was highly conserved with that of BDV/MDCK, with mutations at three positions (data not shown). A 529-bp GAPDH (glyceraldehyde-3-phosphate dehydrogenase) fragment as an internal control was amplified in all samples analyzed. Although the rates of BDV RNA positivity in both patients with schizophrenia and blood donors were relatively low compared with our previous results (4 and 2%, respectively), there were no significant differences in the prevalences of BDV RNA among three groups. These results were consistent with those of our recent report (22). To evaluate the sensitivity of nested RT-PCR for BDV p24, a positive control containing 100 molecules of the in vitro-transcribed BDV p24 RNA fragment was included in each RT-PCR assay and was always found to be positive (22).
DISCUSSION
In the present study, we have detected antibodies to BDV p40 and p24 proteins in plasma samples of psychiatric patients by WB analysis with inhibition tests. The prevalences of antibody to BDV p40 or p24 in patients with mood disorders (2 and 2%, respectively) and schizophrenia (0 and 9%, respectively) were not significantly different from those in blood donors (0 and 0%). Previous seroepidemiological studies by WB analysis have suggested a possible association between BDV infection and human psychiatric diseases (8, 15, 40, 49), with a higher prevalence of BDV antibodies in psychiatric patients, ranging from 12 to 38%. However, our results did not support these previous reports. The discrepancy between our results and the previous results may be mostly due to the specificity of the WB analysis, because all but one of the previous studies did not confirm the positive results by inhibition tests with purified BDV proteins. Another probable reason for the false-positive results would be the purity of BDV proteins used as antigens. Contaminations by E. coli- or MDCK cell-derived antigens of the same molecular weight as p40 or p24 which react with antibodies in plasma samples could cause a false-positive reaction. We used highly purified BDV proteins (>98% pure) as antigens to exclude false-positive reactions. The prevalences of BDV antibodies in previous reports (12 to 38%) are similar to the prevalences in the primary screening of our study (18 and 9% in patients with mood disorders, 29 and 11% in patients with schizophrenia, and 9 and 2% in blood donors for anti-BDV p40 and p24 antibodies, respectively). These findings suggest that the previous reports on the prevalence of BDV antibodies are likely to include false-positive cases. The inhibition test is important and is required to avoid a false-positive reaction. In our WB analysis, the false-positive reaction may be due to newly developed epitopes caused by SDS denaturation to which unrelated antibodies may bind. Since the intact BDV proteins do not have these new epitopes, these proteins could not inhibit the reaction.
In contrast to our findings, one study by WB analysis with inhibition tests demonstrated a higher BDV seroprevalence of 9.6 and 1.4% in psychiatric patients and healthy controls respectively, in Homburg, Germany (40). Furthermore, prominent immunoreactivity to p40 rather than p24 was found in the patients, whereas the patients in our study displayed predominant p24 specificity. The difference in seroprevalence between the two studies may be due to different areas and/ or different patient population. Another recent report revealed that all plasma samples derived from 89 psychiatric patients and 210 healthy volunteers in a western area of Japan were negative for BDV p40, p24, and gp 18 antibodies by WB analysis without an inhibition test, while BDV RNA was detected in 1 to 2% of the patients (47). The difference in seroprevalence may be due to different conditions of the WB assay.
We demonstrated cellular immune responses to BDV p40 and p24 proteins in PBMCs of psychiatric patients and blood donors by proliferation assay in vitro. The relatively high prevalence of a positive proliferative response in comparison with a low prevalence of BDV antibody in each group suggests that most of the positive responses may be due to cross-reactive responses of T cells to undefined antigens which share epitopes with BDV proteins. This finding is consistent with that reported in the case of human immunodeficiency virus type 1 p24 (10). However, it may be possible that the positive responses to both p40 and p24 proteins are due to anamnestic T-cell responses to BDV, although cross-reactive responses can not be ruled out. The prevalence of positive responses to both p40 and p24 in patients with mood disorders (9%) and schizophrenia (4%) were not significantly different from that in blood donors (2%) (Table 4). Likewise, the prevalences of positive responses to either p40 or p24 in patients with mood disorders (11 and 22%, respectively) and schizophrenia (7 and 11%, respectively) were not significantly different from those in blood donors (11 and 9%, respectively).
For human immunodeficiency virus HIV and hepatitis C virus (HCV) infection, there has been a hypothesis that individuals who have positive T-cell proliferative responses to the viruses despite the absence of virus-specific antibodies might be exposed to viruses or viral antigens at a level sufficient to prime T-cell immunity but insufficient to induce antibody production (6, 10, 39). In the case of HCV, the T-cell proliferative responses to HCV antigens have been detected in 4 out of 20 seronegative individuals whose spouses are HCV-positive patients, suggesting possible in vivo T-cell priming with HCV antigens which did not induce seroconversion (6).
The BDV status and clinical features of the patients and a blood donor who are seropositive for BDV by at least one of the three antibody assays or positive for BDV RNA are summarized in Table 5. Patient M10 was strongly positive for p40 antibody by WB analysis and ECLIA and was also positive for BDV antibodies by IF. The PBMCs of this patient showed reproducible positive proliferative responses to both p40 and p24 antigens. These findings suggest that this patient should be strongly considered to be infected with BDV and that the T cells might be primed by BDV p24 despite the absence of p24 antibody.
The five patients (M43, S14, S36, S44, and S45) who were seropositive for BDV p24 by WB analysis were seronegative by both ECLIA and IF. This result (seropositive in WB analysis and seronegative in IF assay) may be due to the low sensitivity of IF compared to WB analysis. However, ECLIA is twice as sensitive as WB analysis when compared using rabbit anti-p24 antibody (data not shown). One of the reasons for this discrepancy (seropositive in WB analysis and seronegative in ECLIA) is that anti-p24 antibodies in humans may be oligoclonal, and epitopes of BDV p24 exposed on the nitrocellulose membrane in WB analysis may not be exposed on the beads in ECLIA (namely, the binding of p24 to the beads may be directional). Since rabbit anti-p24 antibody is polyclonal, it could be detected by both assays. The cause of this discrepancy should be clarified because of the evaluation of efficacy of these BDV antibody assays. Two patients (M43 and S44) out of the five who were seropositive for p24 showed positive proliferative responses to both BDV p40 and p24 despite the absence of p40 antibody. In the case of patient S44, the response was reproducible. These findings suggest that these two patients are probably infected with BDV. In the other three patients, who are seropositive only for p24, we cannot conclude whether they are infected with BDV or not. Blood donor C23 was seropositive for p24 only by ECLIA. This discrepancy may be due to higher sensitivity of ECLIA. However, since the ECLIA count was relatively low and the T cells responded to neither p40 nor p24, it should not be concluded that this donor is infected with BDV. Clinical features of these BDV-seropositive or BDV RNA-positive patients are also shown in Table 5. There were no identifiable differences in the clinical course of their disease compared with other seronegative patients, although Waltrip et al. argued that there are neuroanatomical and behavioral features that may differentiate BDV-seropositive from -seronegative schizophrenic patients (50).
We have identified one mood disorder patient (M23) who was positive for BDV RNA in PBMCs by nested RT-PCR. We have carefully performed RT-PCR experiments to avoid the contamination of BDV amplicons. The prevalences of BDV p24 RNA in patients with mood disorders (2%) and schizophrenia (0%) were not significantly different from that in blood donors (0%), consistent with our previous report (22). Patient M23 showed negative T-cell and antibody responses to BDV (Tables 1 and 5). BDV RNA-positive but seronegative cases have been reported previously (23, 40), and HCV RNA-positive cases with negative proliferation responses have also been reported (6). These findings suggest that this patient may be a nonresponder to BDV, or the virus may have latently infected without production of viral antigens in this patient (13, 46).
In the present study, we employed conserved BDV p24 oligonucleotides as primers to detect the reference strain of BDV/MDCK (22) in PBMCs of psychiatric patients and blood donors. Although this primer set may detect some BDV subtypes which these oligonucleotides can anneal, it did not detect a significantly different variant which recently has been found in a horse (30). Therefore, further RT-PCR study will be necessary to detect additional subtypes by using oligonucleotide sequences of newly defined variants.
One recent report indicated that 20% of psychiatric patients (3 of 15) were positive for BDV RNA in their granulocytes and that granulocytes rather than PBMCs are the primary reservoir for BDV in peripheral blood (34). We tried to detect BDV RNA in granulocytes from 16 schizophrenic patients by the same method used in that study. No BDV RNA was detected in these granulocytes by our RT-PCR system, which can detect 100 copies of BDV RNA (22; Y. Iwata, unpublished data). Therefore, we could not confirm the higher prevalence of BDV RNA in granulocytes than in PBMCs. The reason for this discrepancy is unknown.
Another recent report suggested an etiological association of BDV with schizophrenia by isolating BDV and detecting BDV antigens and genomes in postmortem brain tissues from a young seropositive schizophrenia patient (27). In this patient, BDV RNA was not detected in the PBMCs. These findings suggest the possibility that human brain tissues harbor the BDV genome even in the absence of BDV RNA in patients' PBMCs. Isolation of BDV from human brain tissues is a very critical issue for defining the role of BDV in human neuropathologies.
In conclusion, we have found three psychiatric patients who were positive for both BDV antibodies and T-cell proliferative responses and one patient who was positive for BDV RNA in PBMCs. These four patients are strongly considered to be infected with BDV, although a significant association between BDV infection and human psychiatric disorders was not confirmed, unlike in previous studies (5, 8, 9, 15, 23, 33, 40, 48, 49, 53). Further studies including a larger number of subjects may be necessary. It is possible that some psychiatric diseases may be associated with BDV or BDV-related virus infection (11). To evaluate this hypothesis, precise molecular histopathological and virological studies of BDV infection in the brain may be required.
ACKNOWLEDGMENTS
We thank Juan Carlos de la Torre and Ian W. Lipkin, for plasmid clones, anti-BDV antibodies, and valuable discussions; R. Rott for BDV/MDCK; Sibylle Herzog for IF assay of some specimens; Kiyoshi Ariga, Minako Osonoe, and Noboru Yokoyama for obtaining samples from patients; and Kent Christensen for reviewing the manuscript.
REFERENCES
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders IV. Washington, D.C.: American Psychiatric Association; 1994. [Google Scholar]
- 1a.Berg A L, Johannisson A, Johansson M, Hein A, Berg M, Dorries R L. Peripheral and intracerebral T cell immune response in cats naturally infected with Borna disease virus. Vet Immunol Immunopathol. 1999;68:241–253. doi: 10.1016/s0165-2427(99)00030-6. [DOI] [PubMed] [Google Scholar]
- 2.Bilzer T, Planz O, Lipkin W I, Stitz L. Presence of CD4+ and CD8+ T cells and expression of MHC class I and MHC class II antigen in horses with Borna disease virus-induced encephalitis. Brain Pathol. 1995;5:223–230. doi: 10.1111/j.1750-3639.1995.tb00598.x. [DOI] [PubMed] [Google Scholar]
- 3.Bilzer T, Stitz L. Immunopathogenesis of virus diseases affecting the central nerve system. Crit Rev Immunol. 1996;16:145–222. doi: 10.1615/critrevimmunol.v16.i2.20. [DOI] [PubMed] [Google Scholar]
- 4.Bode L. Human infections with Borna disease virus (BDV) and potential pathogenic implications. Curr Top Microbiol Immunol. 1995;190:103–130. doi: 10.1007/978-3-642-78618-1_7. [DOI] [PubMed] [Google Scholar]
- 5.Bode L, Ferszt R, Czech G. Borna disease virus infection and affective disorders in man. Arch Virol Suppl. 1993;7:159–167. doi: 10.1007/978-3-7091-9300-6_13. [DOI] [PubMed] [Google Scholar]
- 6.Bronowicki J P, Vetter D, Uhl G, Hudziak H, Uhrlacher A, Vetter J M, Doffel M. Lymphocyte reactivity to hepatitis C virus (HCV) antigens shows evidence for exposure to HCV in HCV-seronegative spouses of HCV-infected patients. J Infect Dis. 1997;176:518–522. doi: 10.1086/517279. [DOI] [PubMed] [Google Scholar]
- 7.Carbone K M, Rubin S A, Sierra-Honigmann A M, Lederman H M. Characterization of a glial cell line persistently infected with Borna disease virus (BDV): influence of neurotrophic factors on BDV protein and RNA expression. J Virol. 1993;67:1453–1460. doi: 10.1128/jvi.67.3.1453-1460.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen C H, Chiu Y L, Wei F C, Koong F J, Liu H C, Shaw C K, Hwu H G, Hsiao K J. High seroprevalence of Borna virus infection in schizophrenic patients, family members and mental health workers in Taiwan. Mol Psychiatry. 1999;4:33–38. doi: 10.1038/sj.mp.4000484. [DOI] [PubMed] [Google Scholar]
- 9.Chen C H, Chiu Y L, Shaw C K, Tsai M T, Hwang A L, Hsiao K J. Detection of Borna disease virus RNA from peripheral blood cells in schizophrenic patients and mental health workers. Mol Psychiatry. 1999;4:566–571. doi: 10.1038/sj.mp.4000568. [DOI] [PubMed] [Google Scholar]
- 10.Clerici M, Giorge J V, Chou C-C, Gudeman V K, Zack J A, Gupta P, Ho H-N, Nishianian P G, Berzofsky J A, Shearer M. Cell-mediated immune response to human immunodeficiency virus (HIV) type 1 in seronegative homosexual men with recent sexual exposure to HIV-1. J Infect Dis. 1992;165:1012–1019. doi: 10.1093/infdis/165.6.1012. [DOI] [PubMed] [Google Scholar]
- 11.Czygan M, Hallensleben W, Hofer M, Pollak S, Sauder C, Bilzer T, Blumcke I, Riederer P, Bogerts B, Falkai P, Schwarz M J, Masliah E, Staeheli P, Hufert F T, Lieb K. Borna disease virus in human brains with a rare form of hippocampal degeneration but not in brains of patients with common neuropsychiatric disorders. J Infect Dis. 1999;180:1695–1699. doi: 10.1086/315068. [DOI] [PubMed] [Google Scholar]
- 12.de la Torre J C. Molecular biology of Borna disease virus: prototype of a new group of animal viruses. J Virol. 1994;68:7669–7675. doi: 10.1128/jvi.68.12.7669-7675.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de la Torre J C, Oldstone M B A. Anatomy of viral persistence: mechanism of persistence and associated disease. Adv Virus Res. 1996;46:311–343. doi: 10.1016/s0065-3527(08)60075-5. [DOI] [PubMed] [Google Scholar]
- 14.Evengard B, Briese T, Lindh G, Lee S, Lipkin W I. Absence of evidence of Borna disease virus infection in Swedish patients with chronic fatigue syndrome. J Neurovirol. 1999;5:495–499. doi: 10.3109/13550289909045378. [DOI] [PubMed] [Google Scholar]
- 15.Fu Z F, Amsterdam J D, Kao M, Shankar V, Koprowski H, Dietzschold B. Detection of Borna disease virus-reactive antibodies from patients with affective disorders by Western immunoblot technique. J Affect Disord. 1993;27:61–68. doi: 10.1016/0165-0327(93)90098-5. [DOI] [PubMed] [Google Scholar]
- 16.Hallensleben W, Schwemmle M, Hausmann J, Stitz L, Volk B, Pagenstecher A, Staeheli P. Borna disease virus-induced neurological disorder in mice: infection of neonates results in immunopathology. J Virol. 1998;72:4379–4386. doi: 10.1128/jvi.72.5.4379-4386.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hatalski C G, Lewis A J, Lipkin W I. Borna disease. Emerg Infect Dis. 1997;3:129–135. doi: 10.3201/eid0302.970205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herzog S, Wonigeit K, Frese K, Hedrich H J, Rott R. Effect of Borna disease virus infection in athymic rats. J Gen Virol. 1985;66:503–508. doi: 10.1099/0022-1317-66-3-503. [DOI] [PubMed] [Google Scholar]
- 19.Hirano H, Kao M, Ludwig H. Persistent, tolerant or subacute infection in Borna disease virus infected rats. J Gen Virol. 1983;64:1521–1530. doi: 10.1099/0022-1317-64-7-1521. [DOI] [PubMed] [Google Scholar]
- 20.Horimoto T, Takahashi H, Sakaguchi M, Horikoshi K, Iritani S, Kazamatsuri H, Ikeda K, Tashiro M. A reverse-type sandwich enzyme-linked immunosorbent assay for detecting antibodies to Borna disease virus. J Clin Microbiol. 1997;35:1661–1666. doi: 10.1128/jcm.35.7.1661-1666.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iwahashi K, Watanabe M, Nakamura K, Suwaki H, Nakaya T, Nakamura Y, Takahashi H, Ikuta K. Positive and negative syndromes, and Borna disease virus infection in schizophrenia. Neuropsychobiology. 1998;37:59–64. doi: 10.1159/000026477. [DOI] [PubMed] [Google Scholar]
- 22.Iwata Y, Takahashi K, Xie P, Fukuda K, Ohno H, Ogawa T, Gonda K, Mori N, Niwa S, Shigeta S. Detection and sequence analysis of Borna disease virus p24 RNA from peripheral blood mononuclear cells of patients with mood disorders or schizophrenia and of blood donors. J Virol. 1998;72:10044–10049. doi: 10.1128/jvi.72.12.10044-10049.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kishi M, Nakaya T, Nakamura Y, Zhong Q, Ikeda K, Senjo M, Kakinuma M, Kato S, Ikuta K. Demonstration of human Borna disease virus RNA in human peripheral blood mononuclear cells. FEBS Lett. 1995;364:293–297. doi: 10.1016/0014-5793(95)00406-y. [DOI] [PubMed] [Google Scholar]
- 24.Kishi M, Nakaya T, Nakamura Y, Kakinuma M, Takahashi T, Sekiguchi S, Uchikawa M, Tadokoro K, Ikeda K, Ikuta K. Prevalence of Borna disease virus RNA in peripheral blood mononuclear cells from blood donors. Med Microbiol Immunol. 1995;184:135–138. doi: 10.1007/BF00224350. [DOI] [PubMed] [Google Scholar]
- 25.Lipkin W I, Schneemann A, Solbrig M V. Borna disease virus: implications for human neuropsychiatric illness. Trends Microbiol. 1995;3:64–69. doi: 10.1016/s0966-842x(00)88877-0. [DOI] [PubMed] [Google Scholar]
- 26.Lundgren A L, Zimmermann W, Bode L, Czech G, Gosztonyi G, Lindberg R, Ludwig H. Staggering disease in cats: isolation and characterization of the feline Borna disease virus. J Gen Virol. 1995;76:2215–2222. doi: 10.1099/0022-1317-76-9-2215. [DOI] [PubMed] [Google Scholar]
- 27.Nakamura Y, Takahashi H, Shoya Y, Nakaya T, Watanabe M, Tomonaga K, Iwahashi K, Ameno K, Momiyama N, Taniyama H, Sata T, Kurata T, de la Torre J C, Ikuta K. Isolation of Borna disease virus from human brain tissue. J Virol. 2000;74:4601–4611. doi: 10.1128/jvi.74.10.4601-4611.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Narayan O, Herzog S, Frese K, Scheefers K, Rott R. Behavioral disease in rats caused by immunopathological response to persistent Borna disease virus in the brain. Science. 1983;220:1401–1403. doi: 10.1126/science.6602380. [DOI] [PubMed] [Google Scholar]
- 29.Narayan O, Herzog S, Frese K, Scheefers K, Rott R. Pathogenesis of Borna disease in rats: immune-mediated viral ophthalmoencephalopathy causing blindness and behavioral abnormalities. J Infect Dis. 1983;148:305–315. doi: 10.1093/infdis/148.2.305. [DOI] [PubMed] [Google Scholar]
- 30.Nowotny N, Kolodziejek J, Jehle C O, Suchy A, Staeheli P, Schwemmle M. Isolation and characterization of a new subtype of Borna disease virus. J Virol. 2000;74:5655–5658. doi: 10.1128/jvi.74.12.5655-5658.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Planz O, Bilzer T, Sobbe M, Stitz L. Lysis of MHC class I-bearing cells in Borna disease virus-induced degenerative encephalopathy. J Exp Med. 1993;178:163–174. doi: 10.1084/jem.178.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Planz O, Bilzer T, Stitz L. Immunopathogenic role of T-cell subsets in Borna disease virus-induced progressive encephalitis. J Virol. 1995;69:896–903. doi: 10.1128/jvi.69.2.896-903.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Planz O, Rentzsch C, Batra A, Rziha H J, Stitz L. Persistence of Borna disease virus-specific nucleic acid in blood of psychiatric patient. Lancet. 1998;352:623. doi: 10.1016/S0140-6736(05)79577-5. [DOI] [PubMed] [Google Scholar]
- 34.Planz O, Rentzsch C, Batra A, Winkler T, Buttner M, Rziha H J, Stitz L. Pathogenesis of Borna disease virus: granulocyte fractions of psychiatric patients harbor infectious virus in the absence of antiviral antibodies. J Virol. 1999;73:6251–6556. doi: 10.1128/jvi.73.8.6251-6256.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Richt J A, Stitz L, Wekerle H, Rott R. Borna disease, a progressive meningioencephalomyelitis, as a model for CD4+ T cell-mediated immunopathology in the brain. J Exp Med. 1989;170:1045–1050. doi: 10.1084/jem.170.3.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Richt J A, Pfeuffer I, Christ M, Frese K, Bechter K, Herzog S. Borna disease virus infection in animals and humans. Emerg Infect Dis. 1997;3:343–352. doi: 10.3201/eid0303.970311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rott R, Herzog S, Bechter K, Frese K. Borna disease, a possible hazard for man? Arch Virol. 1991;118:143–149. doi: 10.1007/BF01314025. [DOI] [PubMed] [Google Scholar]
- 38.Rott R, Herzog S, Fleischer B, Winokur A, Amsterdam J, Dyson W, Koprowski H. Detection of serum antibodies to Borna disease virus in patients with psychiatric disorders. Science. 1985;228:755–756. doi: 10.1126/science.3922055. [DOI] [PubMed] [Google Scholar]
- 39.Rowland-Jones S, Sutton J, Ariyoshi K. HIV-specific cytotoxic T-cells in HIV-exposed but uninfected Gambian women. Nat Med. 1995;1:59–64. doi: 10.1038/nm0195-59. [DOI] [PubMed] [Google Scholar]
- 40.Sauder C, Muller A, Cubitt B, Mayer J, Steinmetz J, Trabert W, Ziegler B, Wanke K, Mueller-Lantzsch N, de la Torre J C, Grasser F A. Detection of Borna disease virus (BDV) antibodies and BDV RNA in psychiatric patients: evidence for high sequence conservation of human blood-derived BDV RNA. J Virol. 1996;70:7713–7724. doi: 10.1128/jvi.70.11.7713-7724.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sauder C, Hallensleben W, Pagenstecher A, Schneckenburger S, Biro L, Pertlik D, Hausmann J, Suter M, Staeheli P. Chemokine gene expression in astrocytes of Borna disease virus-infected rats and mice in the absence of inflammation. J Virol. 2000;74:9267–9280. doi: 10.1128/jvi.74.19.9267-9280.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schneemann A, Schneider P A, Lamb R A, Lipkin W I. The remarkable cording strategy of Borna disease virus: a new member of the nonsegmented negative strand RNA viruses. Virology. 1995;210:1–8. doi: 10.1006/viro.1995.1311. [DOI] [PubMed] [Google Scholar]
- 43.Sobbe M, Bilzer T, Gommel S, Nöske K, Planz O, Stitz L. Induction of degenerative brain lesions after adoptive transfer of brain lymphcytes from Borna disease virus-infected rats: presence of CD8+ T cells and mRNA. J Virol. 1997;71:2400–2407. doi: 10.1128/jvi.71.3.2400-2407.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stitz L, Dietzschold B, Carbone K M. Immunopathogenesis of Borna disease. In: Koprowski H, Lipkin W I, editors. Borna disease. Springer-Verlag, Berlin, Germany. 1995. pp. 75–92. [DOI] [PubMed] [Google Scholar]
- 45.Stitz L, Soeder D, Desxhl U, Frese K, Rott R. Inhibition of immune-mediated meningioencephalitis in persistently Borna disease virus infected rats by cyclosporin A. J immunol. 1989;143:4250–4256. [PubMed] [Google Scholar]
- 46.Tishon A, Borrow P, Evance C, Oldstone M B A. Virus-induced immunosuppression. Virology. 1993;195:397–405. doi: 10.1006/viro.1993.1389. [DOI] [PubMed] [Google Scholar]
- 47.Tsuji K, Toyomasu K, Imamura Y, Maeda H, Toyoda T. No association of Borna disease virus with psychiatric disorders among patients in northern Kyushu, Japan. J Med Virol. 2000;61:336–340. [PubMed] [Google Scholar]
- 48.VandeWoude S, Richt J A, Zink M C, Rott R, Narayan O, Clements J E. A Borna virus cDNA encoding a protein recognized by antibodies in humans with behavioral diseases. Science. 1990;250:1278–1281. doi: 10.1126/science.2244211. [DOI] [PubMed] [Google Scholar]
- 49.Waltrip R W, II, Buchanan R W, Carpenter W T, Jr, Kirkpatrick B, Summerfelt A, Breier A, Rubin S A, Carbone K M. Borna disease virus antibodies and the deficit syndrome of schizophrenia. Schizophr Res. 1997;23:253–257. doi: 10.1016/s0920-9964(96)00114-4. [DOI] [PubMed] [Google Scholar]
- 50.Waltrip R W, II, Buchanan R W, Summerfelt A, Breier A, Carpenter W T, Jr, Bryant N, Rubin S A, Carbone K M. Borna disease virus and schizophrenia. Psychiatry Res. 1995;56:33–44. doi: 10.1016/0165-1781(94)02600-n. [DOI] [PubMed] [Google Scholar]
- 51.Weisman Y, Huminer D, Malkinson M, Meir R, Kliche S, Lipkin W I, Pitlik S I. Borna disease virus antibodies among workers exposed to infected ostriches. Lancet. 1994;344:1232–1233. doi: 10.1016/s0140-6736(94)90550-9. [DOI] [PubMed] [Google Scholar]
- 52.Weissenbock H, Nowotny N, Caplazi P, Kolodziejek J, Ehrensperger F. Borna disease in a dog with lethal meningoencephalitis. J Clin Microbiol. 1998;36:2127–2130. doi: 10.1128/jcm.36.7.2127-2130.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamaguchi K, Sawada T, Naraki T, Igata-Yi R, Shiraki H, Horii Y, Ishii T, Ikeda K, Asou N, Okabe H, Mochizuki M, Takahashi K, Yamada S, Kubo K, Yashiki S, Waltrip II W R, Carbone K M. Detection of Borna disease virus-reactive antibodies from patients with psychiatric disorders and from horses by electrochemiluminescence immunoassay. Clin Diagn Lab Immunol. 1999;6:696–700. doi: 10.1128/cdli.6.5.696-700.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]