Abstract
Background: the establishment of periodontitis is regulated by the primary etiological factor and several individual conditions including the immune response mechanism of the host and individual genetic factors. It results when the oral homeostasis is interrupted, and biological reactions favor the development and progression of periodontal tissues damage. Different strategies have been explored for reinforcing the therapeutic effect of non-surgical periodontal treatment of periodontal tissue damage. Gaseous ozone therapy has been recognized as a promising antiseptic adjuvant, because of its immunostimulating, antimicrobial, antihypoxic, and biosynthetic effects. Then, we hypothesized that the adjunct of gaseous ozone therapy to standard periodontal treatment may be leveraged to promote the tissue healing response. Methods: to test this hypothesis, we conducted a prospective randomized study comparing non-surgical periodontal treatment plus gaseous ozone therapy to standard therapy. A total of 90 healthy individuals with moderate or severe generalized periodontitis were involved in the study. The trial was conducted from September 2019 to October 2020. Forty-five patients were randomized to receive scaling and root-planning (SRP) used as conventional non-surgical periodontal therapy plus gaseous ozone therapy (GROUP A); forty-five were allocated to standard treatment (GROUP B). The endpoint was defined as the periodontal response rate after the application of the ozone therapy at 3 months and 6 months, defined as no longer meeting the criteria for active periodontitis. Statistical analysis was performed employing SPSS v.18 Chicago: SPSS Inc. Results: periodontal parameters differed significantly between patients treated with the two distinct procedures at 3 months (p ≤ 0.005); a statistically significant difference between groups was observed from baseline in the CAL (p ≤ 0.0001), PPD (p ≤ 0.0001) and BOP (p ≤ 0.0001) scores. Conclusions: The present study suggests that SRP combined with ozone therapy in the treatment of periodontitis revealed an improved outcome than SRP alone.
Keywords: gaseous ozone therapy, ozone, non-surgical periodontal treatment, moderate periodontitis, severe periodontitis, periodontal disease
1. Introduction
Periodontal disease (PD) is one of the most common inflammatory illnesses affecting the individuals, and the global burden of periodontal disorders, as measured in prevalence, is between 20 and 50%, with severe periodontitis affecting 11.2% worldwide [1,2,3]. The term encompasses a wide spectrum of pathological conditions, ranging from reversible gingival inflammation to severe form, characterized by progressive destruction of alveolar bone [3]. All clinical manifestations have the same pathogenic pathway, with a dramatic increase in bacterial pathogens aggregation (bacterial plaque) as a mainly etiologic factor and important genetic and immunoregulatory individual determinants of the severity of the disease [4,5,6,7,8,9]. In general, the conventional treatment for periodontal lesions is a mechanical and manual non-surgical procedure, named scaling and root planning (SRP), aimed at eliminating supra and sub-gingival bacterial plaque and calculus [10,11]. Several studies have examined the application of add-on therapy in the treatment of periodontitis (e.g., laser or photodynamic therapies) to improve immunogenic responses [12,13,14,15,16,17], and inter-individual variability of response to various adjuvant treatments and therapeutic procedures has been widely reported [18,19,20,21]. Recently, the treatment with gaseous ozone has been studied as a support for SRP for its important effects of immune modulation and healing [22,23,24]. Ozone therapy has been extensively studied in medicine because of its physicochemical properties and its unbelievable versatility for many biomedical applications, specifically degenerative, neurological, orthopaedic and genitourinary disorders [25,26,27,28,29,30]. Also in dentistry, it has an extensive application which fluctuates from endodontia to conservative as well as the treatment of tooth sensitivity [31,32,33,34]. In the context of periodontal infection, oxygen/ozone gas can act as a powerful device for the targeted antiseptic action, potentially reducing the impact of microbial burden, and contemporary increasing the immune system capability [18,27,28,29,30,31,32,33,34,35]. The efficacy of ozone (O3) is greatly related to the beneficial chemical and physical properties, that makes it eligible for employment in periodontal area [12,19,30]. As extensively documented, the ozone has an immunomodulatory, anti-inflammatory and biocide action [36,37,38]. Its antiseptic activity is mediated by disruption of bacterial cell membrane integrity, resulting in their lysis and death [39,40]. In addition, the ozone exerts a double damage: on sulfhydryl groups of specific enzymes, disrupting the normal cellular enzymatic activity and diminishing their function; on the base components of nucleic acids, the purines and pyrimidine, resulting in damage to DNA [41,42]. The anti-inflammatory property is due to the disruption of the self-perpetuating inflammatory cycle altering the breakdown of Arachidonic acid-derived prostaglandins that contribute to the development of inflammation [43,44,45]. Furthermore, O3 contributes to activate the immune cells and it is involved in the production of cytokines [19,25,40]. According to these pharmacological properties, the purpose of this trial was to determine the effectiveness of gaseous ozone therapy in patients with periodontitis, by assuming the superiority of treatment respect to the SRP only. To test this hypothesis, we conducted a prospective randomized controlled study investigating the effectiveness of gaseous ozone therapy in patients with moderate and severe periodontitis.
2. Materials and Methods
2.1. Ethical Considerations
The protocol was conducted in compliance with the Ethics Committee Approval INTL_ALITMKCOOP/HealthMicroPath/HMM2019_IPM and according to Good Clinical Practice and the Declaration of Helsinki Declaration of 1975, as revised in 2013 [46]. Written informed consent was obtained from all patients before the study.
2.2. Study Design and Participants
The trial was conducted from September 2020 to October 2021 at School of Technical Medical Sciences, University A. Xhuvani, Elbasan, Albania. This randomized double-masked clinical trial was carried out to test the hypothesis that the gaseous ozone therapy application as adjunct to SRP leads to significant improvements of periodontal parameters compared with SRP alone. Periodontal examinations were performed by one blinded examiner (BR), and three operators (dentist, AS; dentist, FI, dental hygienist, EF) carried out the treatment at each time point. One blinded statistician (AG) performed the data analysis. Based on limited data available at the time, a sample size of 80 participants (40 for each group) were required to achieve 90% study power using a two-group t-test assuming an α-level of 0.05. Considering a drop-out rate of 10% total sample size, was planned a recruitment of 90 participants.
2.3. Inclusion and Exclusion Criteria
Adult patients were considered eligible for inclusion in the trial if they had a diagnosis for moderate-to-severe periodontitis. Periodontitis diagnosis was determined according to the new criteria presented in the World Workshop on the Classification of Periodontal and Peri-implant Diseases and Conditions [2]. Qualifying patients met all the following inclusion criteria: having a periodontitis diagnosis; having at least 16 teeth with a minimum of four teeth in each quadrant; men and women, aged ≥18 years; could provide informed consent. Reasons for non-enrolment were the following: unable to meet the inclusion criteria (1) underwent administration of any systemic antibiotic regimen within the previous 6 months before enrolment (2); having undergone periodontal therapy within the 12 months prior to the randomization (3); history of systemic diseases (4); medical conditions that contraindicated ozone therapy (e.g., respiratory diseases) (5); current daily smokers with a number of >10 cigarettes/day (6); cognitive or serious mental illness; and pregnancy (7). The source population for this study consisted of subjects with a mean age of 51.56 ± 10.35. The follow-up started on the date of the baseline and ended at 6 months; an interim analysis report addressing the impact of ozone therapy on periodontal outcomes was conducted at 14 days.
2.4. Randomization and Blinding
Eligible patients were randomly assigned, in a 1:1 ratio, to following groups: SRP + OZONE (Test Group A, n = 45); SRP (Control Group B, n = 45). Examiners and statistician were blinded to group assignment. Randomization was performed with computer generated random number list. At baseline, at 3 and 6 months after SRP each patient received periodontal examination by two calibrated examiners, blinded to the treatment group. The examiners calibration was conducted before the study. The alignment exercise resulted in 80% inter-examiner reliability and 90% intra-examiner reproducibility [41].
2.5. Periodontal Clinical Parameters Measurement
To assess the periodontal status before and subsequent the intervention and infer the difference inter-groups, the following clinical outcome parameters were revealed: Bleeding on probing (BOP) was recorded to assess gingival inflammation and it was registered as the percentage at four sites per tooth showing bleeding 30 s after probing [47]; Probing pocket depth (PPD), which is established by calculating the distance from the gingival margin (GM) to the base of the sulcus/pocket with a calibrated periodontal probe; and clinical attachment level (CAL), which is determined by measuring the distance from the cemento-enamel-junction (CEJ) to the base of the sulcus/pocket [41]. Probing pocket depth and CAL were recorded at six sites per tooth. The assessment of clinical status was carried out employing the standard probing measurements using a marked periodontal probe (UNC15 probe, Hu Friedy, Chicago, IL, USA).
2.6. Outcomes
The outcomes were the probing pocket depth reduction, clinical attachment level improvement at 3 and 6 months.
2.7. Treatment
After enrolment, for each patient of both groups, scaling and root planning (SRP) treatment was performed. The objective of scaling is to remove supra- and sub-gingival calculus deposits and root planning to smooth root surfaces.
Each participant received hygiene education session and appropriate motivation and underwent supra- and subgingival prophylaxis with ultrasonic instruments. All sites with probing pocket depth (PPD) ≥4 mm were root planed by using manual instruments (Gracey curets, Hu Friedy, Chicago, IL, USA), under local anaesthesia. No rinsing with chlorhexidine digluconate solution was recommended so as not to affect the results. Follow up was planned in three- and six-months’ time. In the group assigned to SRP + OZONE, gaseous ozone treatment was performed in three steps after instrumentation by ultrasonic instruments employing an ozone generator (Ozone DTA, Sweden & Martina Company; Carrara San Giorgio, Veneto, Italy), according to manufacturer instructions, as follows: Step 1. 2-min rinse with ozonated water at a ratio of 1:3; Full-mouth decontamination; Topical irrigation with ozonated water; 1–2 cycles of ozone gas at 8–10 power in correspondence of pathological pockets, under local anaesthesia. Step 2. Quadrant root planning; 2-min rinse with ozonated water at a ratio of 1:3; Deplaquing; 1–2 cycles of ozone gas at 8–10 power in correspondence of pathological pockets for each quadrant, under local anaesthesia. Step 3. Maintenance: 2-min rinse with ozonated water at a ratio of 1:3; Deplaquing; 1–2 cycles of ozone gas at 4–5 power in correspondence of pathological pockets for all quadrants, two weeks after completion of treatment.
To maintain a state of optimal periodontal health for a correct view of the periodontal ligament, each patient was motivated to a correct home management using a roto oscillating or sonic toothbrush and toothpastes to keep the periodontium intact and avoid the progression of the disease with the destruction of the tissue itself [48,49].
2.8. Statistical Analysis
The Shapiro-Wilk test was used to confirm normal distribution of the data related to each numerical variable for each follow-up time point. Continuous variables were presented as mean ± standard deviation (SD) and all categorical data are expressed as a frequency or a percentage. The comparison of data from the two groups at each time point was performed by using the unpaired 2-sample t test. A mixed model multivariate analysis of covariance (MANCOVA) with two within-subjects factors and one between-subjects factor was conducted to determine whether significant differences exist among the time points for PPD and CAL between the levels of treatment (SRP+ OZONE or SRP) after controlling for stage of disease (moderate or severe), age, and sex as covariates. A p value of <0.05 was considered statistically significant. Statistical analysis was performed employing SPSS Statistics for Windows, version 18 (SPSS Inc., Chicago, IL, USA).
3. Results
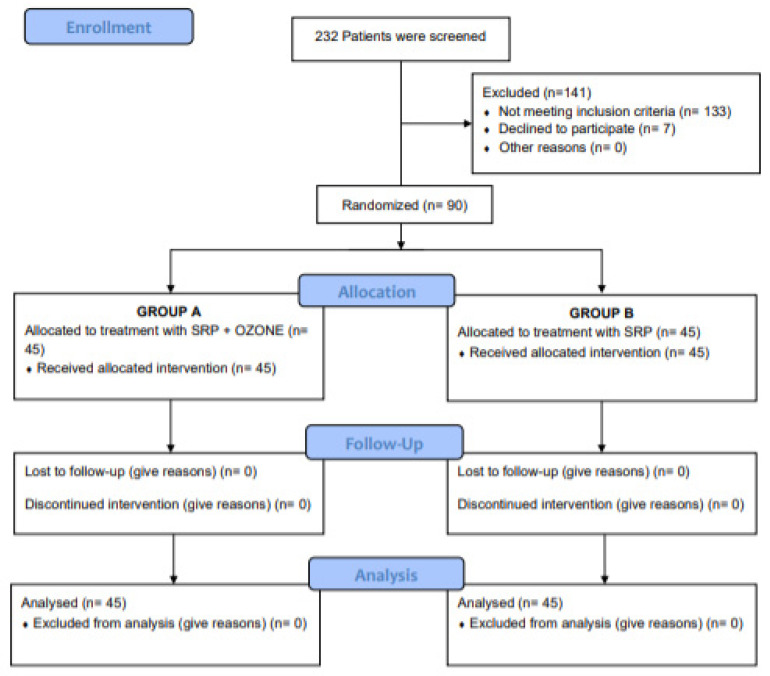
The enrollment was started in September 2019 and ended in December 2019. During this phase, 232 patients were screened. Figure 1 shows the trial profile.
Figure 1.
Consort diagram showing the screening, enrolment and randomization of study patients.
A total of 90 patients were included in the study. Baseline demographic characteristics and clinical periodontal parameters of the 90 patients included in our analysis are illustrated in Table 1 and Table 2, respectively.
Table 1.
Baseline demographic characteristics of participants.
| Group A * | Group B ** | |
|---|---|---|
| Age (mean ± SD) | 51.62 ± 9.56 | 49.88 ± 10.54 |
| Sex | M 87% F 13% |
M 78% F 22% |
| Prevalence of Moderate Periodontitis (%) | 78 | 83 |
| Prevalence of Severe Periodontitis (%) | 22 | 17 |
Group A *: Test Group (SRP + OZONE); Group B **: Control Group (SRP).
Table 2.
Baseline clinical periodontal parameters of both groups.
| PPD (mm) Group A * |
PPD (mm) Group B ** |
p Value | CAL (mm) Group A |
CAL (mm) Group B |
p Value | BOP (%) Group A |
BOP (%) Group B |
p Value | |
|---|---|---|---|---|---|---|---|---|---|
| Mean | 5.39 | 5.37 | 0.81 | 5.53 | 5.78 | <0.05 | 49 | 50.83 | 0.62 |
| Std. Deviation | 0.31 | 0.2 | - | 0.27 | 0.3 | - | 14.74 | 18.11 | - |
Group A *: Test Group (SRP + OZONE); Group B **: Control Group (SRP); PPD: Probing pocket depth; CAL: Clinical attachment level; BOP: Bleeding on probing. p Value: statistically significant at <0.05.
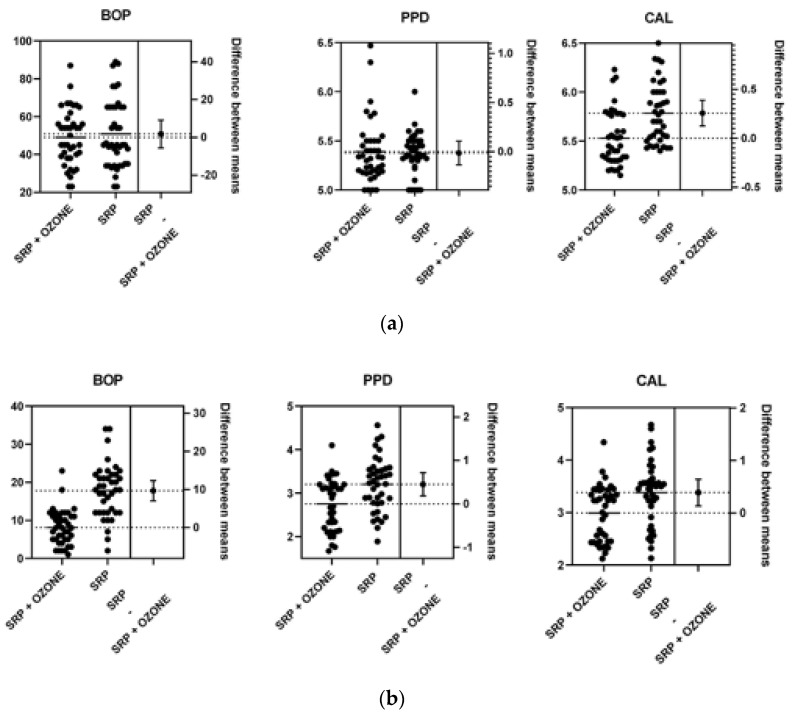
Mean age was 51.62 ± 14.42 for Group A and 49.88 ± 10.54 for the Control Group. Twenty-two percentage of the patients had moderate periodontitis, 78% had diagnosis of severe periodontitis. As shown in Table 2, no significant difference was detected between the two groups in mean score of two periodontal parameters at baseline (PPD 5.39 vs. 5.37, p = 0.81; BOP 49 vs. 50.83, p = 0.62). However, the Control group showed a higher mean CAL score than the Test group (5.78 vs. 5.53, p ≤ 0.0002). At 3 months a statistically significant difference in the PPD (p ≤ 0.0001), CAL (p ≤ 0.003) and BOP (p ≤ 0.0001) was observed between the groups, as shown in Table 3.
Table 3.
Change of periodontal clinical parameters of both groups at 3 months.
| PPD (mm) Group A * |
PPD (mm) Group B ** | p Value | CAL (mm) Group A | CAL (mm) Group B | p Value | BOP (%) Group A | BOP (%) Group B | p Value | |
|---|---|---|---|---|---|---|---|---|---|
| Mean | 2.75 | 3.2 | < 0.001 | 2.99 | 3.38 | < 0.003 | 8.12 | 17.78 | < 0.0001 |
| Median | 2.93 | 3.25 | - | 3.18 | 3.47 | - | 8 | 18 | - |
| Std. Deviation | 0.59 | 0.6 | - | 0.53 | 0.6 | - | 4.6 | 7.05 | - |
Group A *: Test Group (SRP + OZONE); Group B **: Control Group (SRP); PPD: Probing pocket depth; CAL: Clinical attachment level; BOP: Bleeding on probing. p Value: statistically significant at <0.05.
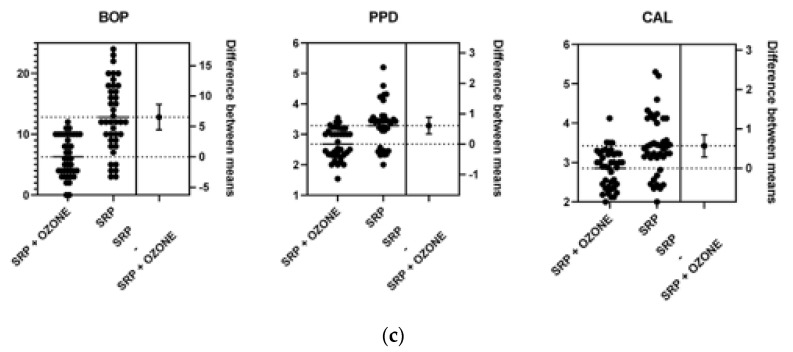
At 6 months a significant decrease was observed in the PPD, CAL and BOP (p ≤ 0.0001, p ≤ 0.0001, and p ≤ 0.0001 respectively) in the test group compared to control group (Table 4).
Table 4.
Change of periodontal clinical parameters of both groups at 6 months.
| PPD (mm) Group A * | PPD (mm) Group B ** | p Value | CAL (mm) Group A | CAL (mm) Group B | p Value | BOP (%) Group A | BOP (%) Group B | p Value | |
|---|---|---|---|---|---|---|---|---|---|
| Mean | 2.67 | 3.28 | <0.0001 | 2.85 | 3.42 | <0.0001 | 6.27 | 12.83 | <0.0001 |
| Median | 2.52 | 3.41 | - | 2.94 | 3.37 | - | 6 | 12 | - |
| Std. Deviation | 0.48 | 0.71 | - | 0.48 | 0.75 | - | 3.32 | 5.7 | - |
Group A *: Test Group (SRP + OZONE); Group B **: Control Group (SRP); PPD: Probing pocket depth; CAL: Clinical attachment level; BOP: Bleeding on probing. p Value: statistically significant at <0.05.
Data resulting from the Unpaired T test are shown in Table 5 and Figure 2.
Table 5.
Unpaired T test results at 3 and 6 months.
| PPD (mm) 3 Months |
PPD (mm) 6 Months |
CAL (mm) 3 Months |
CAL (mm) 6 Months |
BOP (%) 3 Months |
BOP (%) 6 Months |
|
|---|---|---|---|---|---|---|
| p value | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 |
| T value | 3.35 | 4.43 | 3.06 | 4.02 | 7.23 | 6.27 |
| Df | 78 | 78 | 78 | 78 | 78 | 78 |
| Differences between the means ± SEM | 0.45 ± 0.13 | 0.6 ± 0.13 | 0.39 ± 0.12 | 0.57 ± 0.14 | 9.65 ± 1.33 | 6.55 ± 1.04 |
| CI 95% | 0.18 to 0.71 | 0.33 to 0.88 | 0.13 to 0.64 | 0.28 to 0.85 | 6.99 to 12.3 | 4.47 to 8.62 |
| R | 0.12 | 0.2 | 0,1 | 0.17 | 0.4 | 0.33 |
| F | 1.058 | 2.14 | 1.3 | 2.36 | 2.34 | 2.95 |
| p value | 0.86 | 0.01 | 0.4 | 0.008 | 0.009 | 0.001 |
p Value: statistically significant at <0.05.
Figure 2.
The difference between means at baseline (a), 3 (b) and 6 (c) months.
3.1. MANCOVA Analysis
3.1.1. Assumptions
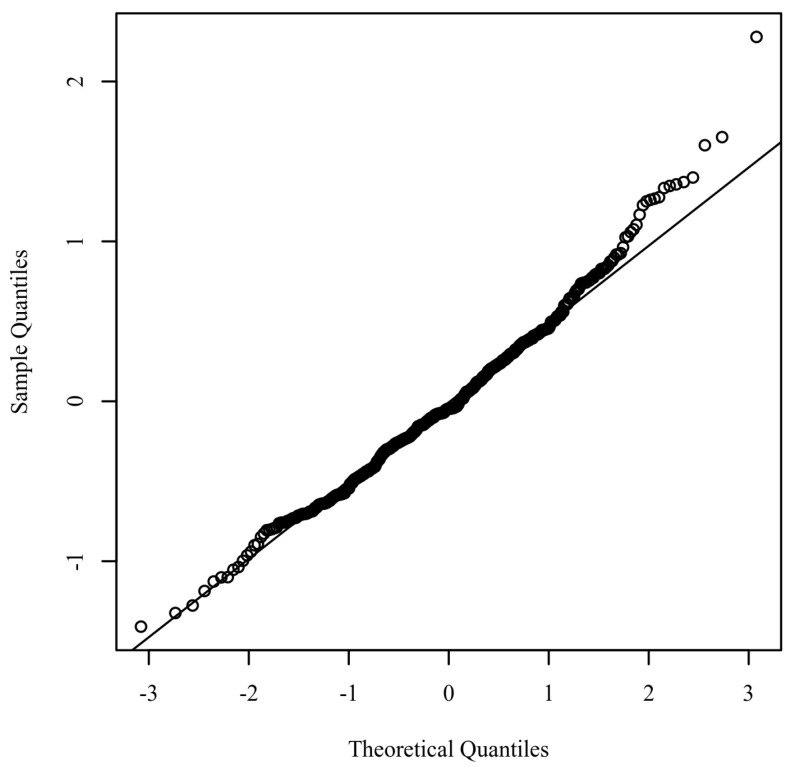
Normality. The assumption of normality was assessed by plotting the quantiles of the model residuals against the quantiles of a Chi-square distribution. Figure 3 presents a Q-Q scatterplot of model residuals.
Figure 3.
Q-Q scatterplot for normality of the residuals for the regression model.
Homoscedasticity. Homoscedasticity was evaluated by plotting the residuals against the predicted values [48,49,50]. Figure 4 presents a scatterplot of predicted values and model residuals.
Figure 4.
Residuals scatterplot testing homoscedasticity.
Sphericity. Mauchly’s test was used to assess the assumption of sphericity [49]. The results showed that the variances of difference scores across the levels of Time Factor were all similar based on an alpha of 0.05, p = 0.483, indicating the sphericity assumption was met for Time Factor. The results showed that the variances of difference scores across the levels of Time Factor:Dipendent variable (Dv) Factor were significantly different from one another based on an alpha of 0.05, p < 0.001, indicating the sphericity assumption was violated for Time Factor:Dv Factor.
Multivariate Outliers. To identify influential points in the residuals, Mahalanobis distances were calculated and compared to a χ2 distribution [51]. An outlier was defined as any Mahalanobis distance that exceeds 22.46, the 0.999 quantile of a χ2 distribution with 6 degrees of freedom [52]. There were no outliers detected in the model.
Homogeneity of regression slopes. The assumption for homogeneity of regression slopes was assessed by rerunning the mixed model MANCOVA, but this time including interaction terms between each independent variable and covariate [49,50]. The model with covariate-independent variable interactions did not explain significantly more variance in the dependent variables than the original model, F(18, 207) = 1.1, p = 0.356. This implies that none of the covariates interacted with the independent variables and the assumption of homogeneity of regression slopes was met.
Covariate-IV independence. An ANOVA was conducted for each pair of numeric covariates and independent variables to assess independence [49]. A multinomial regression model was conducted and compared to the null model for each pair of categorical covariates and independent variables to assess independence. There were no significant models for any combination of covariates and independent variables based on an alpha of 0.05, indicating the assumption of independence between covariates and independent variables was met.
3.1.2. Mixed Model MANCOVA Results
The results were examined based on an alpha of 0.05. Table 6 presents the MANCOVA results.
Table 6.
Mixed Model MANCOVA Results.
| Source | df | SS | MS | F | p | η2p |
|---|---|---|---|---|---|---|
| Between-Subjects | ||||||
| Treatment | 1 | 8.76 | 8.76 | 23.28 | <0.001 | 0.24 |
| Stage_of_disease | 1 | 1.78 | 1.78 | 4.73 | 0.033 | 0.06 |
| Age | 1 | 2.90 | 2.90 | 7.69 | 0.007 | 0.09 |
| sex | 1 | 0.92 | 0.92 | 2.43 | 0.123 | 0.03 |
| Residuals | 75 | 28.24 | 0.38 | |||
| Within-Subjects | ||||||
| Time Factor | 2 | 4.41 | 2.20 | 8.11 | <0.001 | 0.10 |
| Treatment:Time Factor | 2 | 13.82 | 6.91 | 25.45 | <0.001 | 0.25 |
| Stage_of_disease:Time Factor | 2 | 0.99 | 0.49 | 1.82 | 0.166 | 0.02 |
| Age:Time Factor | 2 | 0.40 | 0.20 | 0.73 | 0.482 | 0.01 |
| sex:Time Factor | 2 | 0.71 | 0.36 | 1.32 | 0.271 | 0.02 |
| Time Factor Residuals | 150 | 40.74 | 0.27 | |||
| Dv Factor | 1 | 0.23 | 0.23 | 0.86 | 0.358 | 0.01 |
| Treatment:Dv Factor | 1 | 2.38 | 2.38 | 8.74 | 0.004 | 0.10 |
| Stage_of_disease:Dv Factor | 1 | 0.07 | 0.07 | 0.26 | 0.611 | 0.00 |
| Age:Dv Factor | 1 | 0.04 | 0.04 | 0.15 | 0.698 | 0.00 |
| sex:Dv Factor | 1 | 0.13 | 0.13 | 0.47 | 0.494 | 0.01 |
| Dv Factor Residuals | 75 | 20.43 | 0.27 | |||
| Time Factor:Dv Factor | 2 | 0.11 | 0.06 | 0.20 | 0.778 | 0.00 |
| Treatment:Time Factor:Dv Factor | 2 | 5.43 | 2.71 | 9.57 | <0.001 | 0.11 |
| Stage_of_disease:Time Factor:Dv Factor | 2 | 0.01 | 0.01 | 0.03 | 0.955 | 0.00 |
| Age:Time Factor:Dv Factor | 2 | 0.24 | 0.12 | 0.42 | 0.617 | 0.01 |
| sex:Time Factor:Dv Factor | 2 | 0.73 | 0.37 | 1.29 | 0.276 | 0.02 |
| Time Factor:Dv Factor Residuals | 150 | 42.56 | 0.28 |
Degrees of Freedom (df): Refers to the number of values used to compute a statistic; an F-test has two values for df: the first is determined by the number of groups being compared—1, and the second is approximately the number of observations in the sample; used with the F to determine the p-value; F Ratio (F): The ratio of explained variance to error variance; used with the two df values to determine the p-value; Partial Eta Squared (η2p): Effect size for the ANOVA/MANOVA and determines the strength of the differences among the groups; p-value: The probability of obtaining the observed results if the null hypothesis is true; Residuals: Refers to the difference between the predicted value for the dependent variable and the actual value of the dependent variable.
The p-values for and any interaction with these within-subjects factors were calculated using the Greenhouse-Geisser corrections to adjust for the violation of the sphericity assumption.
Between-Subjects. The main effect for Treatment was significant F(1, 75) = 23.28, p < 0.001, indicating that there were significant differences in PPD and CAL between the levels of Treatment after controlling for stage of disease, age, and sex. The covariate, Stage_of_disease, was significantly related to PPD and CAL, F(1, 75) = 4.73, p = 0.033. The covariate, age, was significantly related to PPD and CAL, F(1, 75) = 7.69, p = 0.007. The covariate, sex, was not significantly related to PPD and CAL, F(1, 75) = 2.43, p = 0.123.
Within-Subjects. The main effect for Time Factor was significant F(2, 150) = 8.11, p < 0.001, indicating there were significant differences in PPD and CAL across the levels of Time Factor ignoring Dv Factor after controlling for stage of disease, age, and sex. The main effect for Dv Factor was not significant F(1, 75) = 0.86, p = 0.358, indicating the values for across the levels of Dv Factor, PPD and CAL, were all similar regardless of Time Factor after controlling for stage of disease, age, and sex. The main effect for Time Factor and Dv Factor was not significant F(2, 150) = 0.20, p = 0.778, indicating that the relationships between the levels of Dv Factor were similar across the levels of Time Factor after controlling for Stage of disease, age, and sex.
Within-Between Interactions. The interaction effect between Time Factor and treatment was significant F(2, 150) = 25.45, p < 0.001, indicating that the relationships between the levels of Time Factor differed significantly between the levels of treatment ignoring Dv Factor after controlling for stage of disease, age, and sex.
The interaction effect between Dv Factor and Treatment was significant F(1, 75) = 8.74, p = 0.004, indicating that the relationships between the levels of Dv Factor differed significantly between the levels of treatment regardless of Time Factor after controlling for stage of disease, Age, and sex.
The interaction effect between Time Factor, Dv Factor, and treatment was significant F(2, 150) = 9.57, p < 0.001, indicating that the relationships between the combinations of Time Factor and Dv Factor differed significantly between the levels of treatment after controlling for stage of disease, age, and sex.
Within-Covariate Interactions. The interaction effect between Time Factor and stage of disease was not significant, F(2, 150) = 1.82, p = 0.166, indicating that the relationships between the levels of Time Factor were similar for all values of stage of disease. The interaction effect between Time Factor and age was not significant, F(2, 150) = 0.73, p = 0.482, indicating that the relationships between the levels of Time Factor were similar for all values of age. The interaction effect between Time Factor and sex was not significant, F(2, 150) = 1.32, p = 0.271, indicating that the relationships between the levels of Time Factor were similar between the levels of sex.
The interaction effect between Dv Factor and stage of disease was not significant, F(1, 75) = 0.26, p = 0.611, indicating that the relationships between the levels of Dv Factor were similar for all values of stage of disease. The interaction effect between Dv Factor and age was not significant, F(1, 75) = 0.15, p = 0.698, indicating that the relationships between the levels of Dv Factor were similar for all values of age. The interaction effect between Dv Factor and sex was not significant, F(1, 75) = 0.47, p = 0.494, indicating that the relationships between the levels of Dv Factor were similar between the levels of sex.
The interaction effect between Time Factor, Dv Factor, and Stage of disease was not significant, F(2, 150) = 0.03, p = 0.955, indicating that the relationships between the combinations of Time Factor and Dv Factor were similar for all values of stage of disease. The interaction effect between Time Factor, Dv Factor, and age was not significant, F(2, 150) = 0.42, p = 0.617, indicating that the relationships between the combinations of Time Factor and Dv Factor were similar for all values of age. The interaction effect between Time Factor, Dv Factor, and sex was not significant, F(2, 150) = 1.29, p = 0.276, indicating that the relationships between the combinations of Time Factor and Dv Factor were similar between the levels of sex.
4. Discussion
The key to onset and progression of periodontitis consists of two canonical pathways: the oral microbial subversion, the central stimulus, resulting in the expression of proinflammatory cytokines to eradicate pathogens and repair the damage tissues; in parallel, the genetic, environmental and systemic health status which contribute cumulatively to the disease etiology and development. The goal of periodontal therapy is based on the eradication of pathogenic bacteria responsible for the onset of the disease to control the inflammatory. The aim of this clinical trial was to determine the impact of gaseous ozone therapy in conjunction to conventional periodontal treatment on conditions and severity of periodontal disease in healthy subjects diagnosed with moderate or severe periodontitis, in comparison with standard treatment. To provide a compelling comparison be-tween the two therapies, a randomized controlled trial was designed. Ozone therapy is a practice of complementary medicine and its effects have been widely confirmed [14,48,49,50]. Beginning in the 1960 [51], multiple trials assessed the safety and efficacy of ozone in medicine for several therapeutic indications. Humans’ studies have exposed the biological plausibility of ozone-induced beneficial impact on several pathological conditions [43,44,45,46,47,48,49] and described the mechanism of action of ozone, which encompasses the capacity to inactivate bacteria, viruses, fungi, yeast and protozoa by disrupting the integrity of the bacterial cell; the ability to stimulate the increase in the red blood cell glycolysis rate; the capacity to activate the immune response by causing the increase in the production of interleukin-2 which determines a cascade of subsequent immunological reactions [17,52,53,54]. Application methods include in-direct and direct procedures, such as the intramuscular injection, ozone bag and others. In dentistry, the indirect technical methods including the ozonated water, ozonated oil and gaseous ozone generator are employed. The aqueous (1.25–20 µmgL−1) and gaseous ozone (1–53 g m−3) are predominantly employed against periodontopathogenic and endodontic bacteria, including the Enterococcus faecalis, the mainly endodontic pathogen [55,56]. Boch et al., reported 85.38% reduction of bacterial count after gaseous ozone application on Enterococcus faecalis biofilm in root canals and 99.5% eradication of bacteria when the ozone was combined with NaOCl [56]. Case et al., demonstrated the efficacy of ozone combined with ultrasonic agitation and ozone alone on E. fecalis [57]. Further, the antimicrobial activity of the ozone has been documented against the Staphylococcus aureus and Staphylococcus epidermidis [13], registering a significantly de-crease in absolute counts of microorganisms. Emerging studies have examined gaseous ozone therapy in addition to non-surgical periodontal therapy. The rationale behind the use of ozone therapy is based on the concept of the specifically inflammatory target pathway, as well as the antimicrobial activity. The microbial pathogenesis of periodontitis and the immune response are the two determinants of this choice. Our results support previous recent studies showing that patients who have been treated with ozone exhibited statistically and clinically significant improvement in periodontal inflammation after gaseous ozone treatment [22,28]. In our previous study of diabetic patients with periodontitis, we observed a sensitive improvement of periodontal status after the application of gaseous ozone [28]. The significant difference between the two groups in a decrease in periodontal outcomes at 3 months in the gaseous ozone-treated group rationalize the improvement of the periodontal stability condition at 6 months in test groups. We hypothesized that the significant reduction displayed in the test group may reflect the biological activity on periodontal tissues and hence an improvement on disease [15,16,58,59]. We theorized that the antimicrobial activity is a key step. Periodontitis is a disease whose course essentially feeds on the presence of pathogenic bacteria that alter homeostasis and induce the establishment of the disease. Then, we assumed that the ozone therapy might promote the healing consequent improvement of the state of the disease by stimulating the immune response and a more rapid lowering of the microbial load. Our results are in contrast with findings reported by Tasdemir et al. [60], which are also based on topical gaseous ozone application into periodontal pockets. They reported no significant differences between the two groups during the follow up in periodontal parameters. Although significant differences in CAL between the groups, some factors in our study could be considered, plausible interindividual differences and the time between ozone treatment and the subsequent 3 months could reveal a different healing pattern in these individuals. The addition of ozone treatment showed a marked improvement in periodontal conditions compared with the test group, while both groups manifested a significant reduction in pocket probing at 3 months. Each patient received the same oral hygiene instruction and motivation, and this may influence long-term outcomes. The current study has some limitations, first, the use of ozone therapy was only granted during the SRP phase, without any recall, and the follow-up was limited to explore the potential benefit of recall. The limitation of this study could be related to the use of criteria to define success as changes in PD and CAL [61,62,63,64,65,66,67], because of the potential limited representativeness of the effectiveness of ozone therapy, which includes additional benefits. Further studies investigating biochemical parameters of oxidative stress might be useful for a more in-depth evaluation on periodontal tissue healing restoration.
5. Conclusions
This randomized clinical trial suggests that gaseous ozone therapy in conjunction with the conventional periodontal treatment may reduce the likelihood of periodontitis advancing. Based on previous research we hypothesized that gaseous ozone treatment of periodontitis, as adjuvant of SRP, may have encouraging therapeutic effects.
Author Contributions
Conceptualization, B.R.; methodology, A.G.; software, B.R. and E.F.; validation, B.R., E.F., L.S., S.T., G.D., A.M., M.D.D., G.M.T., A.S., F.I. and A.G.; formal analysis, A.G. and E.F.; investigation, B.R. and E.F.; resources, B.R.; data curation, B.R., F.I. and A.S.; writing—original draft preparation, B.R.; writing—review and editing, B.R., E.F., F.I. and A.S.; visualization, B.R. and G.M.T.; supervision, B.R. and G.M.T.; project administration B.R., F.I. and A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of Albania (INTL_ALITMKCOOP/HealthMicroPath/HMM2019_IPM).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nazir M.A. Prevalence of periodontal disease, its association with systemic diseases and prevention. Int. J. Health Sci. 2017;11:72–80. [PMC free article] [PubMed] [Google Scholar]
- 2.Caton J.G., Armitage G., Berglundh T., Chapple I.L.C., Jepsen S., Kornman K.S., Mealey B.L., Papapanou P.N., Mariano Sanz M., Tonetti M.S. A new classification scheme for periodontal and peri-implant diseases and conditions—Introduction and key changes from the 1999 classification. J. Clin. Periodontol. 2018;45:S1–S8. doi: 10.1111/jcpe.12935. [DOI] [PubMed] [Google Scholar]
- 3.Ng E., Tay J.R.H., Ong M.M.A. Minimally Invasive Periodontology: A Treatment Philosophy and Suggested Approach. Int. J. Dent. 2021;2021:2810264. doi: 10.1155/2021/2810264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaur G., Grover V., Bhaskar N., Kaur R.K., Jain A. Periodontal infectogenomics. Inflamm. Regen. 2018;38:8. doi: 10.1186/s41232-018-0065-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loos B.G., Van Dyke T.E. The role of inflammation and genetics in periodontal disease. Periodontology 2000. 2020;83:26–39. doi: 10.1111/prd.12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dowsett S.A., Archila L., Foroud T., Koller D., Eckert G.J., Kowolik M.J. The effect of shared genetic and environmental factors on periodontal disease parameters in untreated adult siblings in Guatemala. J. Periodontol. 2002;73:1160–1168. doi: 10.1902/jop.2002.73.10.1160. [DOI] [PubMed] [Google Scholar]
- 7.Taba M., Jr., Souza S.L., Mariguela V.C. Periodontal disease: A genetic perspective. Braz. Oral Res. 2012;26:32–38. doi: 10.1590/S1806-83242012000700006. [DOI] [PubMed] [Google Scholar]
- 8.Barros S.P., Offenbacher S. Modifiable risk factors in periodontal disease: Epigenetic regulation of gene expression in the inflammatory response. Periodontology 2000. 2014;64:95–110. doi: 10.1111/prd.12000. [DOI] [PubMed] [Google Scholar]
- 9.Montemurro N., Perrini P., Rapone B. Clinical Risk and Overall Survival in Patients with Diabetes Mellitus, Hyperglycemia and Glioblastoma Multiforme. A Review of the Current Literature. Int. J. Environ. Res. Public Health. 2020;17:8501. doi: 10.3390/ijerph17228501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quaglia E., Moscufo L., Corsalini M., Coscia D., Sportelli P., Cantatore F., De Rinaldis C., Rapone B., Carossa M., Carossa S. Polyamide vs. silk sutures in the healing of postextraction sockets: A split mouth study. Oral Implantol. 2018;11:115–120. [Google Scholar]
- 11.Cobb C.M., Sottosanti J.S. A re-evaluation of scaling and root planing. J. Periodontol. 2021;92:1370–1378. doi: 10.1002/JPER.20-0839. [DOI] [PubMed] [Google Scholar]
- 12.Suvan J., Leira Y., Moreno Sancho F.M., Graziani F., Derks J., Tomasi C. Subgingival instrumentation for treatment of periodontitis. A systematic review. J. Clin. Periodontol. 2020;47:155–175. doi: 10.1111/jcpe.13245. [DOI] [PubMed] [Google Scholar]
- 13.Salvi G.E., Stähli A., Schmidt J.C., Ramseier C.A., Sculean A., Walter C. Adjunctive laser or antimicrobial photodynamic therapy to non-surgical mechanical instrumentation in patients with untreated periodontitis: A systematic review and meta-analysis. J. Clin. Periodontol. 2020;47:176–198. doi: 10.1111/jcpe.13236. [DOI] [PubMed] [Google Scholar]
- 14.Corsalini M., Di Venere D., Carossa M., Ripa M., Sportelli P., Cantatore F., De Rinaldis C., Di Santantonio G., Lenoci G., Barile G., et al. Comparative clinical study between zirconium-ceramic and metal-ceramic fixed rehabilitations. Oral Implantol. 2018;11:150–160. [Google Scholar]
- 15.Gandhi K.K., Pavaskar R., Cappetta E.G., Drew H.J. Effectiveness of Adjunctive Use of Low-Level Laser Therapy and Photodynamic Therapy After Scaling and Root Planing in Patients with Chronic Periodontitis. Int. J. Periodontics Restor. Dent. 2019;39:837–843. doi: 10.11607/prd.4252. [DOI] [PubMed] [Google Scholar]
- 16.Grassi F.R., Grassi R., Rapone B., Gianfranco A., Balena A., Kalemaj Z. Dimensional changes of buccal bone plate in immediate implants inserted through open flap, open flap and bone grafting, and flapless technique. A CBCT randomized controlled clinical trial. Clin. Oral Implant. Res. 2019;30:1155–1164. doi: 10.1111/clr.13528. [DOI] [PubMed] [Google Scholar]
- 17.Ren C., McGrath C., Jin L., Zhang C., Yang Y. The effectiveness of low-level laser therapy as an adjunct to non-surgical periodontal treatment: A meta-analysis. J. Periodontal Res. 2017;52:8–20. doi: 10.1111/jre.12361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grassi F.R., Rapone B., Scarano Catanzaro F., Corsalini M., Kalemaj Z. Effectiveness of computer-assisted anesthetic delivery system (STA™) in dental implant surgery: A prospective study. Oral Implantol. 2017;10:381–389. doi: 10.11138/orl/2017.10.4.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Di Venere D., Corsalini M., Nardi G.M., Laforgia A., Grassi F.R., Rapone B., Pettini F. Obstructive site localization in patients with Obstructive Sleep Apnea Syndrome: A comparison between otolaryngologic data and cephalometric values. Oral Implantol. 2017;10:295–310. doi: 10.11138/orl/2017.10.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gou H., Fan R., Chen X., Li L., Wang X., Xu Y., Svensson P., Wang K. Adjunctive effects of laser therapy on somatosensory function and vasomotor regulation of periodontal tissues in patients with periodontitis: A randomized controlled clinical trial. J. Periodontol. 2020;91:1307–1317. doi: 10.1002/JPER.19-0562. [DOI] [PubMed] [Google Scholar]
- 21.Mestnik M.J., Feres M., Figueiredo L.C., Soares G., Teles R.P., Fermiano D., Duarte P.M., Faveri M. The effects of adjunctive metronidazole plus amoxicillin in the treatment of generalized aggressive periodontitis: A 1-year double-blinded, placebocontrolled, randomized clinical trial. J. Clin. Periodontol. 2012;39:955–961. doi: 10.1111/j.1600-051X.2012.01932.x. [DOI] [PubMed] [Google Scholar]
- 22.Corsalini M., Di Venere D., Rapone B., Stefanachi G., Laforgia A., Pettini F. Evidence of signs and symptoms of Craniomandibular Disorders in Fibromyalgia patients. Open Dent. J. 2017;11:91–98. doi: 10.2174/1874210601711010091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marconcini S., Giammarinaro E., Cosola S., Oldoini G., Genovesi A., Covani U. Effects of Non-Surgical Periodontal Treatment on Reactive Oxygen Metabolites and Glycemic Control in Diabetic Patients with Chronic Periodontitis. Antioxidants. 2021;10:1056. doi: 10.3390/antiox10071056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Azarpazhooh A., Limeback H. The application of ozone in dentistry: A systematic review of literature. J. Dent. 2008;36:104–116. doi: 10.1016/j.jdent.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 25.Cosola S., Giammarinaro E., Genovesi A.M., Pisante R., Poli G., Covani U., Marconcini S. A short-term study of the effects of ozone irrigation in an orthodontic population with fixed appliances. Eur. J. Paediatr. Dent. 2019;20:15–18. doi: 10.23804/ejpd.2019.20.01.03. [DOI] [PubMed] [Google Scholar]
- 26.Gupta G., Mansi B. Ozone therapy in periodontics. J. Med. life. 2012;5:59. [PMC free article] [PubMed] [Google Scholar]
- 27.Di Venere D., Nardi G.M., Lacarbonara V., Laforgia A., Stefanachi G., Corsalini M., Grassi F.R., Rapone B., Pettini F. Early mandibular canine-lateral incisor transposition: Case Report. Oral Implantol. 2017;10:181–189. doi: 10.11138/orl/2017.10.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rapone B., Ferrara E., Corsalini M., Converti I., Grassi F.R., Santacroce L., Topi S., Gnoni A., Scacco S., Scarano A., et al. The Effect of Gaseous Ozone Therapy in Conjunction with Periodontal Treatment on Glycated Hemoglobin Level in Subjects with Type 2 Diabetes Mellitus: An Unmasked Randomized Controlled Trial. Int. J. Environ. Res. Public Health. 2020;17:5467. doi: 10.3390/ijerph17155467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saini R. Ozone therapy in dentistry: A strategic review. J. Nat. Sci. Biol. Med. 2011;2:151–153. doi: 10.4103/0976-9668.92318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Di Paolo N., Bocci V., Gaggiotti E. Ozone therapy. Int. J. Artif. Organs. 2004;27:168–175. doi: 10.1177/039139880402700303. [DOI] [PubMed] [Google Scholar]
- 31.Hodson N., Dunne S.M. Using ozone to treat dental caries. J. Esthet. Restor. Dent. 2007;19:303–305. doi: 10.1111/j.1708-8240.2007.00127.x. [DOI] [PubMed] [Google Scholar]
- 32.Iliadis D., Millar B. Ozone and its use in periodontal treatment. Open J. Stomatol. 2013;3:197–202. doi: 10.4236/ojst.2013.32034. [DOI] [Google Scholar]
- 33.Scarano A., Inchingolo F., Rapone B., Festa F., Tari S.R., Lorusso F. Protective Face Masks: Effect on the Oxygenation and Heart Rate Status of Oral Surgeons during Surgery. Int. J. Environ. Res. Public Health. 2021;18:2363. doi: 10.3390/ijerph18052363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rapone B., Corsalini M., Converti I., Loverro M.T., Gnoni A., Trerotoli P., Ferrara E. Does Periodontal Inflammation Affect Type 1 Diabetes in Childhood and Adolescence? A Meta-Analysis. Front. Endocrinol. 2020;11:27. doi: 10.3389/fendo.2020.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoffman R.K. Ozone. In: Hugo W.B., editor. Inhibition and Destruction of the Microbial Cell. Academic Press; London, UK: 1971. pp. 251–253. [Google Scholar]
- 36.Korich D.G., Mead J.R., Madore M.S., Sinclair N.A., Sterling C.R. Effects of ozone, chlorine dioxide, chlorine and monochloramine on Cryptosporidium parvuum oocyst viability. Appl. Environ. Microbiol. 1990;56:1423–1428. doi: 10.1128/aem.56.5.1423-1428.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim J., Yousef A., Dave S. Application of ozone for enhancing the microbiological safety and quality of foods: A review. J. Food Prot. 1999;62:1071–1087. doi: 10.4315/0362-028X-62.9.1071. [DOI] [PubMed] [Google Scholar]
- 38.Rapone B., Nardi G.M., Di Venere D., Pettini F., Grassi F.R., Corsalini M. Oral hygiene in patients with oral cancer undergoing chemotherapy and/or radiotherapy after prosthesis rehabilitation: Protocol proposal. Oral Implantol. 2016;9:90–97. doi: 10.11138/orl/2016.9.1S.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Di Venere D., Pettini F., Nardi G.M., Laforgia A., Stefanachi G., Notaro V., Rapone B., Grassi F.R., Corsalini M. Correlation between parodontal indexes and orthodontic retainers: Prospective study in a group of 16 patients. Oral Implantol. 2017;10:78–86. doi: 10.11138/orl/2017.10.1.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kalemaj Z., Scarano A., Valbonetti L., Rapone B., Grassi F.R. Bone response to four dental implants with different surface topography: A histologic and histometric study in minipigs. Int. J. Periodontics Restor. Dent. 2016;36:745–754. doi: 10.11607/prd.2719. [DOI] [PubMed] [Google Scholar]
- 41.Michalowicz B.S., Hodges J.S., Pihlstrom B.L. Is change in probing depth a reliable predictor of change in clinical attachment loss? J. Am. Dent. Assoc. 2013;144:171–178. doi: 10.14219/jada.archive.2013.0096. [DOI] [PubMed] [Google Scholar]
- 42.Bocci V.A. Scientific and medical aspects of ozone therapy. State Art Arch. Med. Res. 2006;37:425–435. doi: 10.1016/j.arcmed.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 43.Corsalini M., Di Venere D., Sportelli P., Magazzino D., Ripa M., Cantatore F., Cagnetta C., De Rinaldis C., Montemurro N., De Giacomo A., et al. Evaluation of prosthetic quality and masticatory efficiency in patients with total removable prosthesis: Study of 12 cases. Oral Implantol. 2018;11:230–240. [Google Scholar]
- 44.Rapone B., Ferrara E., Santacroce L., Cesarano F., Arazzi M., Di Liberato L., Scacco S., Grassi R., Grassi F.R., Gnoni A., et al. Periodontal Microbiological Status Influences the Occurrence of Cyclosporine-A and Tacrolimus-Induced Gingival Overgrowth. Antibiotics. 2019;8:124. doi: 10.3390/antibiotics8030124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lorusso F., Noumbissi S., Inchingolo F., Rapone B., Khater A.G.A., Scarano A. Scientific Trends in Clinical Research on Zirconia Dental Implants: A Bibliometric Review. Materials. 2020;13:5534. doi: 10.3390/ma13235534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Holt G.R. Declaration of Helsinki—The World’s Document of Conscience and Responsibility. South Med. J. 2014;107:407. doi: 10.14423/SMJ.0000000000000131. [DOI] [PubMed] [Google Scholar]
- 47.Mühlemann H.R., Son S. Gingival sulcus bleeding—A leading symptom in initial gingivitis. Helv. Odontol. Acta. 1971;15:107–113. [PubMed] [Google Scholar]
- 48.Bates D., Mächler M., Bolker B., Walker S. Fitting linear mixed-effects models using lme4. arXiv. 20141406.5823 [Google Scholar]
- 49.Field P.A., Wilcox R.R. Robust statistical methods: A primer for clinical psychology and experimental psychopathology researchers. Behav. Res. Ther. 2017;98:19–38. doi: 10.1016/j.brat.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 50.Osborne W.J., Waters E. Four assumptions of multiple regression that researchers should always test. Pract. Assess. Res. Eval. 2002;8:2. [Google Scholar]
- 51.Newton R.R., Rudestam K.E. Your Statistical Consultant. 2nd ed. Sage Publications, Inc.; Newbury Park, CA, USA: 2013. [Google Scholar]
- 52.Kline R.B. Principles and Practice of Structural Equation Modeling. 4th ed. Guilford Publications; New York City, NY, USA: 2015. [Google Scholar]
- 53.Butera A., Gallo S., Maiorani C., Preda C., Chiesa A., Esposito F., Pascadopoli M., Scribante A. Management of Gingival Bleeding in Periodontal Patients with Domiciliary Use of Toothpastes Containing Hyaluronic Acid, Lactoferrin, or Paraprobiotics: A Randomized Controlled Clinical Trial. Appl. Sci. 2021;11:8586. doi: 10.3390/app11188586. [DOI] [Google Scholar]
- 54.Preda C., Butera A., Pelle S., Pautasso E., Chiesa A., Esposito F., Oldoini G., Scribante A., Genovesi A.M., Cosola S. The Efficacy of Powered Oscillating Heads vs. Powered Sonic Action Heads Toothbrushes to Maintain Periodontal and Peri-Implant Health: A Narrative Review. Int. J. Environ. Res. Public Health. 2021;18:1468. doi: 10.3390/ijerph18041468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fuccio C., Luongo C., Capodanno P., Giordano C., Scafuro M.A., Siniscalco D., Lettieri B., Rossi F., Maglione S., Berrino L. A single subcutaneous injection of ozone prevents allodynia and decreases the over-expression of pro-inflammatory caspases in the orbito-frontal cortex of neuropathic mice. Eur. J. Pharmacol. 2008;603:42–49. doi: 10.1016/j.ejphar.2008.11.060. [DOI] [PubMed] [Google Scholar]
- 56.Johansson E., Claesson R., van Dijken J.W. Antibacterial effect of ozone on cariogenic bacterial species. J. Dent. 2009;37:449–453. doi: 10.1016/j.jdent.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 57.Di Filippo C., Cervone C., Rossi C., Di Ronza C., Marfella R., Capodanno P., Luongo C., Rossi F., D’Amico M. Antiarrhythmic effect of acute oxygen-ozone administration to rats. Eur. J. Pharmacol. 2010;629:89–95. doi: 10.1016/j.ejphar.2009.11.061. [DOI] [PubMed] [Google Scholar]
- 58.Stoker G. Ozone in chronic middle ear deafness. Lancet. 1902;160:1187–1188. doi: 10.1016/S0140-6736(01)45068-9. [DOI] [Google Scholar]
- 59.Oldoini G., Ricci Frabattista G., Saragoni M., Cosola S., Giammarinaro E., Genovesi A.M., Marconcini S. Ozone Therapy for Oral Palatal Ulcer in a Leukaemic Patient. Eur. J. Case Rep. Intern. Med. 2020;7:001406. doi: 10.12890/2020_001406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baysan A., Whiley R., Lynch E. Antimicrobial effects of a novel ozone generating device on microorganisms associated with primary root carious lesion in vitro. Caries Res. 2000;34:498–501. doi: 10.1159/000016630. [DOI] [PubMed] [Google Scholar]
- 61.Elvis A.M., Ekta J.S. Ozone therapy: A clinical review. J. Nat. Sci. Biol. Med. 2011;2:66–70. doi: 10.4103/0976-9668.82319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Balmes J.R., Arjomandi M., Bromberg P.A., Costantini M.G., Dagincourt N., Hazucha M.J., Hollenbeck-Pringle D., Rich D.Q., Stark P., Frampton M.W. Ozone effects on blood biomarkers of systemic inflammation, oxidative stress, endothelial function, and thrombosis: The Multicenter Ozone Study in older Subjects (MOSES) PLoS ONE. 2019;14:e0222601. doi: 10.1371/journal.pone.0222601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Boch T., Tennert C., Vach K., Al-Ahmad A., Hellwig E., Polydorou O. Effect of gaseous ozone on Enterococcus faecalis biofilm-an in vitro study. Clin. Oral Investig. 2016;20:1733–1739. doi: 10.1007/s00784-015-1667-1. [DOI] [PubMed] [Google Scholar]
- 64.Case P.D., Bird P.S., Kahler W.A., George R., Walsh L.J. Treatment of Root Canal Biofilms of Enterococcus faecalis with Ozone Gas and Passive Ultrasound Activation. J. Endod. 2012;38:523–526. doi: 10.1016/j.joen.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 65.Tasdemir Z., Oskaybas M.N., Alkan A.B., Cakmak O. The effects of ozone therapy on periodontal therapy: A randomized placebo-controlled clinical trial. Oral Dis. 2019;25:1195–1202. doi: 10.1111/odi.13060. [DOI] [PubMed] [Google Scholar]
- 66.Seydanur Dengizek E., Serkan D., Abubekir E., Aysun Bay K., Onder O., Arife C. Evaluating clinical and laboratory effects of ozone in non-surgical periodontal treatment: A randomized controlled trial. J. Appl. Oral Sci. 2019;27:e20180108. doi: 10.1590/1678-7757-2018-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Isler S.C., Unsal B., Soysal F., Ozcan G., Peker E., Karaca I.R. The effects of ozone therapy as an adjunct to the surgical treatment of peri-implantitis. J. Periodontal Implant Sci. 2018;48:136–151. doi: 10.5051/jpis.2018.48.3.136. [DOI] [PMC free article] [PubMed] [Google Scholar]