Abstract
We evaluated the genetic diversity of Streptococcus suis isolates of different serotypes by macrorestriction analysis and elucidated possible relationships between the genetic background, expression of potential virulence traits, and source of isolation. Virulence traits included expression of serotype-specific polysaccharides, muramidase-released protein (MRP), extracellular protein factor (EF), hemolysin activity, and adherence to epithelial cells. Macrorestriction analysis of streptococcal DNA digested with restriction enzymes SmaI and ApaI allowed differentiation of single isolates that could be assigned to four major clusters, named A1, A2, B1, and B2. Comparison of the genotypic and phenotypic features of the isolates with their source of isolation showed that (i) the S. suis population examined, which originated mainly from German pigs, exhibited a genetic diversity and phenotypic patterns comparable to those found for isolates from other European countries; (ii) certain phenotypic features, such as the presence of capsular antigens of serotypes 2, 1, and 9, expression of MRP and EF, and hemolysin activity (and in particular, combinations of these features), were strongly associated with the clinical background of meningitis and septicemia; and (iii) isolates from pigs with meningitis and septicemia showed a significantly higher degree of genetic homogeneity compared to that for isolates from pigs with pneumonia and healthy pigs. Since the former isolates are considered highly virulent, this supports the theory of a clonal relationship among highly virulent strains.
Streptococcus suis is a major cause of meningitis, septicemia, arthritis, and bronchopneumonia in young pigs and can cause meningitis in humans (1, 2). Effective control of the disease is hampered by the poor knowledge about its epidemiology and pathogenesis. Since many healthy pigs harbor S. suis as an “early colonizer” (6, 18), identification of virulent S. suis isolates is of particular importance. This is, however, complicated by the pathogen's extreme diversity, in particular with respect to its virulence.
Phenotypic markers used to distinguish highly virulent and avirulent isolates include the presence of serotype-specific capsular polysaccharides (25), expression of muramidase-relased protein (MRP) (27, 35) and extracellular protein factor (EF) (35), and hemolysin activity (10, 11, 16). The role of these factors as virulence markers, however, is still unclear. The capsular polysaccharides are the basis for classification into serotypes (of which 35 are currently known) and have recently been shown to prevent phagocytosis and, thus, have been proposed as an important virulence trait (25). Serotype 2 is considered the most dominant one among highly virulent strains, but disease is also frequently caused by strains of other serotypes (24, 33, 36), suggesting that serotype-independent virulence markers must exist. MRP and EF have previously been found to be strongly associated with highly virulent isolates in Europe (35, 36) but do not appear to confer virulence directly, as recently demonstrated by the use of gene knockout technology (28). Hemolysin activity has been characterized and associated with virulent strains, but its in vivo expression does not seem to correlate with virulence (11, 12, 15). Adherence to epithelial cells has also been suggested as a virulence trait (9), and adhesins that recognize erythrocytes have been found to induce opsonizing antibodies in mice (32). However, studies with adhesins were limited to a very few strains, and information concerning the adherence mechanisms and the possible role of adhesins in the virulence of the bacterium is yet very poor.
Different molecular typing methods such as ribotyping have been evaluated in comparison with conventional phenotyping, indicating that the latter is of limited value for epidemiological analyses (12, 21, 23, 25, 29). Results of these studies also revealed an association between virulence and ribotype, thus demonstrating that a close relationship between highly virulent strains is plausible (23, 26, 29). Nevertheless, most of these studies were restricted to serotype 2 isolates, and the hypothesis about a clonal lineage or relationship of highly virulent strains is still under debate (4, 12, 13, 19).
The purpose of the present study was to elucidate the genetic diversity within an S. suis population belonging to various serotypes, particularly by comparing isolates from pigs with invasive disease, i.e., meningitis and septicemia, versus isolates from pigs with pneumonia and healthy pigs. For this, we studied a collection of 99 isolates, mostly recovered from German pigs, by assessing possible relationships between the genetic background, different phenotypic markers (serotype, expression of MRP and EF, hemolysin activity, adherence to epithelial cells), and clinical backgrounds.
MATERIALS AND METHODS
If not stated otherwise, all chemicals were purchased from Sigma (Munich, Germany).
Bacteria.
S. suis isolates had been randomly collected in the course of routine diagnostic procedures from tissues of healthy and diseased pigs over a period of 3 years (1996 to 1998). Most pigs were 4 to 12 weeks old and from different farms located in different geographic regions in (especially northern) Germany. According to the source of isolation and clinical background, the isolates were assigned to three groups, i.e., (i) those from pigs with meningitis, septicemia, or arthritis (isolates from brains and joints of animals with respective clinical symptoms [also referred to as meningitis-septicemia isolates]), (ii) those from pigs with pneumonia (S. suis was isolated from lungs, either as a pure culture [40% of all pneumonia isolates] or in association with other respiratory pathogens such as Pasteurella spp. and Bordetella bronchiseptica [60% of all pneumonia isolates]), and (iii) those from healthy pigs (isolated from swabs of the upper respiratory or the genital tract). For comparison purposes, a number of reference and control strains were included, such as the suilysin control strain P1/7 described by Jacobs et al. (15), serotype 2 reference strain Henrichsen S735 (DSM 9682; German Culture Collection, Braunschweig, Germany), serotype 1 reference strain Henrichsen S428 (DSM 9683), and MRP-EF control strains D282, T15, and 4005 (34). Bacteria were isolated on blood agar plates, identified as S. suis by standard biochemical testing, and cultured in Todd-Hewitt broth (Oxoid, Wesel, Germany), on blood agar plates, or on tryptic soy agar plates for 18 h at 37°C.
Phenotyping. (i) Serotyping and expression of MRP and EF.
Serotyping and determination of expression of MRP and EF were done as described previously (33). Serotype-specific antisera had been prepared in rabbits (8) against reference strains of serotypes 1 to 28.
(ii) Hemolysin activity.
Determination of hemolysin activity was done as described by Staats et al. (29), with some minor modifications. Briefly, streptococci from an overnight plate culture on tryptic soy agar were washed and suspended in phosphate-buffered saline (PBS) to an optical density (OD) at 560 nm of 1.6. The suspensions were incubated for 1 h at 40°C to optimize hemolysin production and centrifuged, and the supernatants were immediately tested for hemolysin activity by using 1% sheep erythrocytes in PBS. Tests were run in round-bottom 96-well microtiter plates as twofold serial dilutions beginning with 100 μl of supernatant. A total of 100 μl of erythrocytes was added to each dilution. After incubation for 2 h at 37°C in 6% CO2, the plates were centrifuged and the supernatants were transferred to a new plate for spectrophotometric measurements at 410 nm. As controls, streptococcal supernatants were replaced by PBS (negative control) or H2O (positive control). Each experiment was run in triplicate and included a freshly prepared supernatant of hemolysin control strain P1/7 as an internal reference. Results were expressed as mean relative hemolysin activity as a percentage of the activity expressed by strain P1/7. According to the resulting activities, the isolates were grouped as hemolysin negative (<10% of the hemolysin activity of P1/7), intermediate (10 to 80% of the hemolysin activity of P1/7), or positive (>80% of the hemolysin activity of P1/7).
(iii) Adherence to epithelial cells.
Adherence to epithelial cells was tested as described previously (31) by using human HEp-2 cells (ATCC CCL23) and a porcine testis epithelial cell line (kindly supplied by G. Herrler, Institut fuer Virologie, Tieraerztliche Hochschule Hannover) grown to confluency on glass coverslips. Approximately 100 streptococci per epithelial cell were incubated for 1 h at 37°C. After three washes with PBS, the coverslips were stained with Giemsa and examined microscopically by counting the number of adherent streptococci per 50 epithelial cells. The results were expressed as adherence negative (<25 adherent bacteria), intermediate (25 to 100 adherent bacteria), or positive (>100 adherent bacteria).
PFGE.
Macrorestriction analysis by pulsed-field gel electrophoresis (PFGE) was done by using a modification of the method of Olsen and Skov (22). Streptococcal DNA was prepared from 18-h cultures in Todd-Hewitt broth. After centrifugation of the cultures, the pelleted bacteria were washed and adjusted to an OD at 576 nm of 0.3 with PFGE buffer (1 M NaCl, 10 mM EDTA, 10 mM Tris-HCl [pH 8.0]). Five milliliters of this suspension was pelleted, washed once in PFGE buffer, resuspended in 0.5 ml of PFGE buffer, mixed with 0.5 ml of low-melting-temperature high-purity agarose (1.2%; Bio-Rad, Munich, Germany), and subsequently aliquoted into 100-μl portions and placed into a plug mold. After solidification, five plugs each were placed in 3 ml of fresh lysis buffer (1 M NaCl, 200 mM EDTA, 10 mM Tris-HCl [pH 8.0], 0.5% sarcosyl, 0.2% sodium deoyxycholate, 1 mg of lysozyme per ml, 2 μg of RNase per ml, 13 U of mutanolysin per ml). After incubation for 2 h at 37°C, the lysis solution was replaced by 3 ml of ESP buffer (0.5 M EDTA, 1% sarcosyl, 1 mg of proteinase K per ml) and further incubated overnight at 56°C. Subsequently, the plugs were washed with TE buffer (10 mM Tris-HCl [pH 8.0], 1 mM EDTA) and incubated twice in TE buffer containing 1 mM phenylmethylsulfonic acid fluoride for 30 min at room temperature. Then the plugs were washed three times for 30 min each time with 3 ml of TE buffer. After the final washing, 5 ml of TE buffer was added and the plugs were stored overnight at 4°C. For digestion with the restriction enzymes SmaI and ApaI (Boehringer Mannheim, Mannheim, Germany), approximately 0.5-mm slices of the plugs were cut and equilibrated in 250 μl of restriction enzyme reaction buffer (as supplied by the manufacturer). After 1 h of equilibration at room temperature, the buffer was replaced by 50 μl of freshly prepared restriction enzyme reaction buffer containing 10 U of SmaI or ApaI per ml. After incubation for 4 h at 37°C, the resulting DNA fragments were separated by PFGE for 14 h (SmaI digestion) or 18 h (ApaI digestion) at 6 V/cm with a CHEF DR II apparatus (Bio-Rad). Subsequently, the bands were visualized by staining of the gels with ethidium bromide and documented under UV illumination with (the MulitAnalyst system (Bio-Rad).
Analysis of PFGE patterns.
Fragment sizes were calculated for each lane by comparison with a size standard (ApaI-digested genomic DNA from Staphylococcus aureus strain DSM 1104 [German Culture Collection]) which was run in parallel in each experiment. Bands of less than 50 kb were not included to avoid possible interference of plasmid DNA. On the basis of the calculated sizes of the resulting DNA fragments, the gels were analyzed with GelCompar software (Applied Maths, Kortrijr, Belgium, supplied by HeroLab, Wiesloch, Germany). Dendrograms were generated by using the Dice coefficient, and clustering was done by the unweighted pair group method with arithmetic averages (UPGMA) with a 3% tolerance in band size.
Statistical analysis.
The statistical significance of probabilities of dependences between different phenotypes, assignment to PFGE clusters, and clinical backgrounds was analyzed by linear regression (odds ratio) by the chi-square test. P values of less than 0.01 were considered significant.
RESULTS
Macrorestriction analysis.
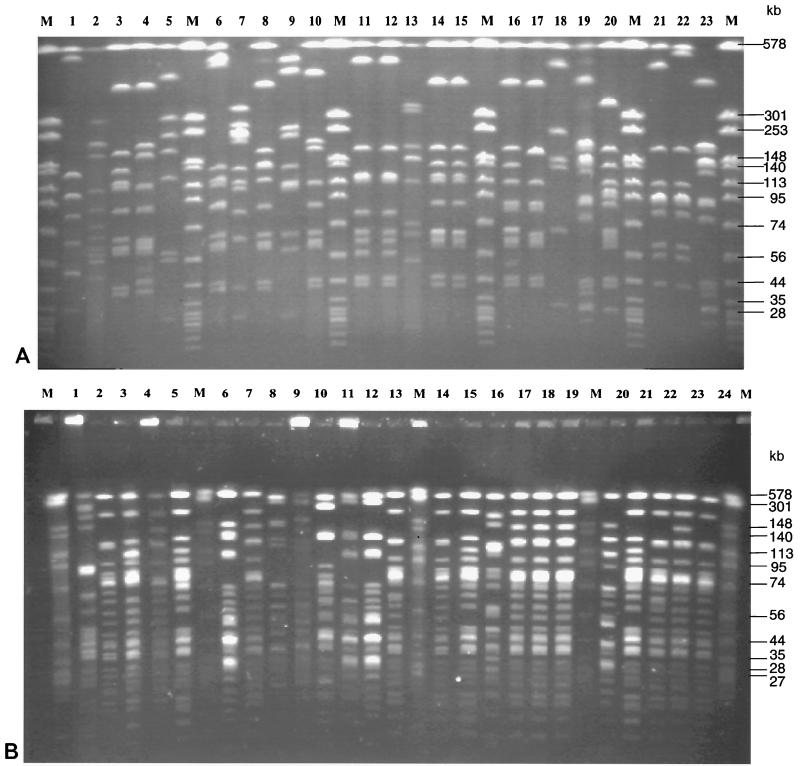
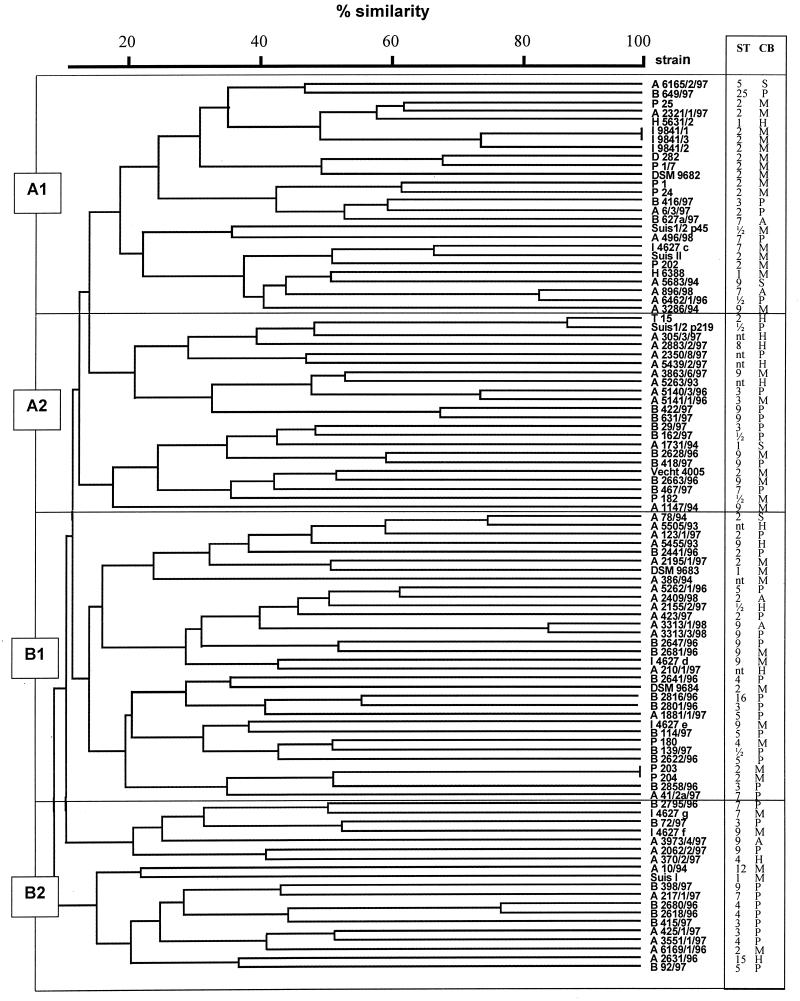
Macrorestriction analysis was done by PFGE of streptococcal DNA digested with restriction enzymes SmaI and ApaI. The number of bands obtained with SmaI-digested DNA ranged from 6 to 13, and the number of bands obtained with ApaI-digested DNA ranged from 15 to 23. Genome sizes calculated by addition of DNA fragments of various sizes varied; for most isolates a genome size of 1.4 × 106 to 2.0 × 106 bp was calculated. This corresponded to the genome size of streptococci as indicated in the literature (30) (additional details can be found at the genome website University of Oklahoma's Advanced Center for Genome Technology (http://www.genome.ou.de/strep.html). Representative PFGE gels of SmaI-digested DNAs of isolates mainly belonging to serotype 9 are shown in Fig. 1A, and PFGE gels of ApaI-digested DNAs of isolates mainly belonging to serotype 2 are shown in Fig. 1B. The data show that PFGE allowed differentiation of isolates between and within serotypes. Moreover, PFGE enabled us to monitor isolates from various locations of a single animal (Fig. 1A, lanes 11 and 12; Fig. 1B, lanes 17 to 19) as well as from different animals within the same herd (Fig. 1A, lanes 10, 16, and 20). Strongly related but not identical isolates could also easily be discriminated (Fig. 1A, lanes 21 and 22; Fig. 1B, lanes 20 to 24). Cluster analysis of PFGE patterns of SmaI-digested DNA by UPGMA revealed a dendrogram with four major groups, designated groups A1, A2, B1, and B2, each comprising isolates with a similarity of at least 20% (Fig. 2). Isolates which appeared to be identical by visual inspection of the gels showed similarities of approximately 80 to 100% by cluster analysis. Examples are isolates I9841/1-3 (Fig. 2, cluster A1, corresponding to Fig. 1B, lanes 17 to 19) as well as isolates A3313/1/98 and A3313/3/98 (Fig. 2, cluster B1, corresponding to Fig. 1A, lanes 11 and 12). Closely related isolates showed similarities of approximately 70% by cluster analysis, e.g., strains P1/7 and D282 (Fig. 2, cluster A1, corresponding to Fig. 1A, lanes 21 and 22), as well as isolates B422/97 and 631/97 (Fig. 2, cluster A2, corresponding to Fig. 1B, lanes 14 and 15). All isolates determined to be closely related by PFGE with SmaI-digested DNA could be more clearly differentiated visually in PFGE gels of ApaI-digested DNA (data not shown). A dendrogram constructed from the PFGE patterns of ApaI-digested DNA was almost similar to the dendrogram constructed from the PFGE patterns of SmaI-digested DNA, except that the degree of differentiation was higher (data not shown).
FIG. 1.
Macrorestriction analysis of S. suis isolates by PFGE. (A) PFGE of SmaI-digested DNA of S. suis serotype 9 isolates (lanes 1 to 20 and 23) and, in addition, serotype 2 hemolysin activity-positive control strain P1/7 (lane 21) and serotype 2 MRP- and EF-positive control strain D282 (lane 22). Lane M, size standard (ApaI-digested genomic DNA from S. aureus strain DSM 1104) indicating molecular sizes (in kilobases). (B) PFGE of ApaI-digested DNA of S. suis serotype 2 isolates (lanes 1 to 24) including the type 2 reference strain (lane 5), MRP- and EF-positive control strains 4005 (lane 7), T15 (lane 8), and D282 (lane 23), as well as hemolysin activity-positive control strain P1/7 (lane 20). Lane M, size standard (ApaI-digested genomic DNA from S. aureus, strain DSM 1104) indicating molecular sizes (in kilobases).
FIG. 2.
Dendrogram of similarity among the observed PFGE macrorestriction patterns of SmaI-digested DNAs from 99 S. suis isolates. Serotypes (ST) and clinical backgrounds (CB) are indicated on the right. Isolates originated either from pigs with typical invasive disease (meningitis [M], septicemia [S], arthritis [A]), from pigs with pneumonia (P), or from healthy pigs (H). Major clusters are indicated by A1, A2, B1, and B2. nt, nontypeable.
Comparison of the clinical backgrounds of isolates with their assignment to the different clusters revealed that 20 of 48 isolates from pigs with meningitis-septicemia belonged to cluster A1 (Table 1). In contrast, isolates from pigs with pneumonia or from healthy pigs were almost equally distributed among the different clusters except for cluster A1 (Table 1). The phenotypic features of the cluster A1 isolates are described below.
TABLE 1.
Assignment to PFGE clusters among S. suis isolates from pigs with various clinical backgrounds
| PFGE clustera | No. of isolates | No. (%) of isolates from:
|
||
|---|---|---|---|---|
| Pigs with meningitis-septicemia | Pigs with pneumonia | Healthy pigs | ||
| A1 | 26 | 20 (42) | 5 (12.5) | 1 (9) |
| A2 | 22 | 9 (19) | 9 (22.5) | 4 (36) |
| B1 | 32 | 13 (27) | 15 (37.5) | 4 (36) |
| B2 | 19 | 6 (12) | 11 (27.5) | 2 (18) |
| Total | 99 | 48 (100) | 40 (100) | 11 (100) |
Isolates were assigned to four different clusters as revealed from cluster analysis of macrorestriction patterns of SmaI-digested streptococcal DNA.
Serotyping and analysis of potential virulence traits.
Results from serotyping and phenotyping of isolates are summarized in Tables 2 to 4. The distribution of serotypes within the different isolates shows that the majority belonged to serotypes 2 (25%) and 9 (20%), followed by serotypes 3, 7, ½, 5, 4, and 1 (Table 2). The remaining isolates (indicated as “others” in Table 2) either belonged to serotype 8, 15, 16, or 25 or were nontypeable with the antisera used (i.e., antisera to serotypes 1 to 28). The nontypeable isolates represented 7% of the total number tested. The most dominant serotypes among all isolates tested, serotypes 2 and 9, also represented the majority of isolates from pigs with meningitis-septicemia (44% were serotype 2, 25% were serotype 9), followed by serotype 1 (8% of all meningitis-septicemia isolates) (Table 2). On the other hand, the serotypes of isolates from pigs with pneumonia showed a broader distribution, with most of these isolates belonging to serotype 3, 5, 7, or 9 (Table 2). Interestingly, most isolates from healthy pigs were nontypeable or belonged to one of the rarely represented serotypes (both indicated as “others” in Table 2).
TABLE 2.
Distribution of serotypes among S. suis isolates from pigs with various clinical backgrounds
| Serotype | No. of isolates | No. (%) of isolates from:
|
||
|---|---|---|---|---|
| Pigs with meningitis-septicemia | Pigs with pneumonia | Healthy pigs | ||
| 1 | 5 | 4 (8) | 0 | 1 (9) |
| ½ | 8 | 2 (4) | 5 (10) | 1 (9) |
| 2 | 25 | 21 (44) | 3 (6) | 1 (9) |
| 3 | 9 | 1 (2) | 8 (20) | 0 |
| 4 | 6 | 1 (2) | 4 (10) | 1 (9) |
| 5 | 7 | 2 (4) | 5 (12.5) | 0 |
| 7 | 8 | 3 (6) | 5 (12.5) | 0 |
| 9 | 20 | 12 (25) | 7 (17.5) | 1 (9) |
| Othersa | 11 | 2 (4) | 3 (6) | 6 (55) |
| Total | 99 | 48 (100) | 40 (100) | 11 (100) |
Isolates belonged to serotype 8, 15, 16, or 25 or were nontypeable.
TABLE 4.
Hemolysin activities among S. suis isolates from pigs with various clinical backgrounds
| Hemolysin activitya | No. of isolates | No. (%) of isolates from:
|
||
|---|---|---|---|---|
| Pigs with meningitis-septicemia | Pigs with pneumonia | Healthy pigs | ||
| + | 27 | 21 (43.7) | 2 (5) | 4 (36) |
| ± | 22 | 8 (16.6) | 13 (32.5) | 1 (9) |
| − | 50 | 19 (39.5) | 25 (62.5) | 6 (55) |
| Total | 99 | 48 (100) | 40 (100) | 11 (100) |
Isolates were grouped as hemolysin positive (+; >80% activity), intermediate (±; 10 to 80% activity), or negative (−; <10% activity) in comparison to the activity of control strain P1/7.
Analysis of expression of virulence-associated proteins MRP and EF (summarized in Table 3) demonstrated that the majority (67%) of all isolates expressed MRP, either alone or in combination with expression of EF. None of the isolates expressed solely EF. Isolates from pigs with meningitis-septicemia mostly either expressed MRP alone (52%) or expressed MRP in combination with EF (29%). Among isolates from pigs with pneumonia, only one expressed MRP and EF; the remaining isolates expressed either MRP only (52.5%) or neither of these proteins (45%). All isolates were also tested for hemolysin activity, another potential virulence marker. The results are shown in Table 4. Isolates were classified as hemolysin negative, intermediate, or positive with respect to their relative activity in comparison with the activity of control strain P1/7 (see Materials and Methods). A high proportion of all isolates were hemolysin negative (50%); only 27% were positive, and 22% were intermediate. This distribution was comparable among isolates from pigs with meningitis-septicemia and healthy pigs, whereas a significantly higher proportion (95%) of isolates from pigs with pneumonia were hemolysin negative or intermediate. Adherence of streptococci to epithelial cells was tested since adherence capacity and/or presence of adhesins has been related to the virulence of S. suis (and other bacteria) in earlier studies (10, 32). Surprisingly, the adherence of all isolates to two commonly used cell lines, porcine testis cells and human HEp-2 epithelial cells, was generally very low. Substantial adherence was observed for only 25% of all isolates by using porcine cells and only 18% of all isolates by using HEp-2 cells. The isolates that adhered well to HEp-2 cells were the same ones that adhered well to porcine cells. There was no association of adherence capacities with source of isolation (data not shown).
TABLE 3.
Expression of MRP and EF among S. suis isolates from pigs with various clinical backgrounds
| MRP/EF expression statusa | No. of isolates | No. (%) of isolates from:
|
||
|---|---|---|---|---|
| Pigs with meningitis-septicemia | Pigs with pneumonia | Healthy pigs | ||
| +/+ | 15 | 14 (29) | 1 (2.5) | 0 |
| +/− | 52 | 25 (52) | 21 (52.5) | 6 (54.5) |
| −/+ | 0 | 0 | 0 | 0 |
| −/− | 32 | 9 (19) | 18 (45) | 5 (45.5) |
| Total | 99 | 48 (100) | 40 (100) | 11 (100) |
Isolates expressed MRP and EF (+/+), only MRP (+/−), or none of the two proteins (−/−); none of the isolates expressed only EF (−/+).
Taken together, these results showed that certain phenotypic features were striking among isolates from pigs with meningitis-septicemia, such as the presence of capsular antigens of serotypes 2 and 9, expression of MRP and EF, and hemolysin activity. A more heterogeneous background seemed to exist within isolates from pigs with pneumonia or healthy pigs.
Relation of individual features of the isolates with clinical background.
We next calculated the probabilities that isolates with certain features, alone or in combination with each other, originated from pigs with meningitis-septicemia, which would indicate a high degree of virulence. A meningitis-septicemia background was found among 4 of the 5 serotype 1 isolates, 21 of the 25 serotype 2 isolates, and 12 of the 19 serotype 9 isolates (Table 2), indicating a high probability that isolates of serotypes 2 and 1 and, to a lesser extent, of serotype 9 originated from pigs with meningitis. On the other hand, most isolates belonging to serotypes ½ (five of eight), 3 (eight of nine), 4 (four of six), 5 (five of seven), or 7 (five of eight) were from pigs with pneumonia; most isolates designated “others” originated from healthy animals (Table 2). We also found a very high probability of the clinical background meningitis-septicemia among isolates expressing MRP and EF and hemolysin-positive isolates: 14 of the 15 MRP- and EF-positive isolates and 21 of the 27 hemolysin-positive isolates were from pigs with meningitis-septicemia (Tables 3 and 4). Interestingly, a high proportion of isolates that lacked hemolysin activity (25 of 50) or that expressed intermediate hemolysin activity (13 of 22) had been isolated from pigs with pneumonia (Table 4). Moreover, the majority of isolates of PFGE cluster A1 (20 of 26) had a meningitis-septicemia background, and most isolates of cluster B2 (11 of 19) were from pigs with pneumonia (Table 1). Taken together, these data show that there was a high probability that isolates exhibiting either the feature serotype 2 or serotype 1 capsular antigen expression, expression of MRP and EF, strong hemolysin activity, or assignment to PFGE cluster A1 had a clinical background of meningitis-septicemia. On the other hand, the features serotype 3, 4, or 5 capsular antigen expression and a lack of or intermediate hemolysin activity indicated a high probability of pneumonia as the clinical background.
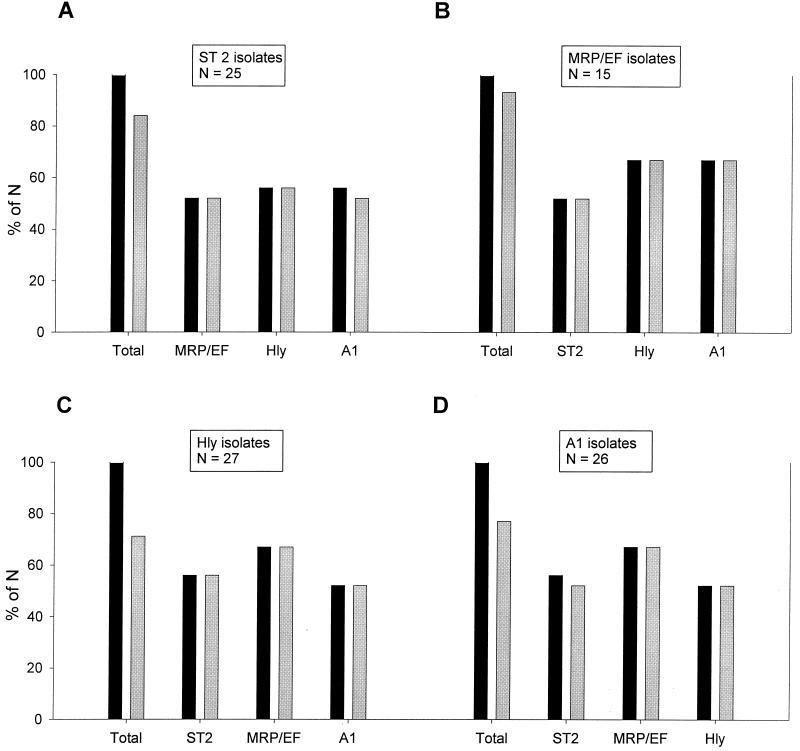
The probability of the clinical background meningitis-septicemia was even higher when some of the features mentioned above were found in combination (Fig. 3). We found that of all serotype 2 isolates, 52% also expressed MRP and EF, 56% were hemolysin positive, and 56% belonged to cluster A1. All serotype 2 isolates that were either MRP and EF positive or hemolysin positive as well as 93% of the serotype 2 isolates assigned to cluster A1 originated from pigs with meningitis (Fig. 3A). Of the MRP- and EF-positive isolates, 52% belonged to serotype 2, 67% were hemolysin positive, and 67% belonged to cluster A1, and all isolates with any of these combinations had been isolated from pigs with meningitis-septicemia (Fig. 3B). Features of the hemolysin-positive and cluster A1 isolates in relation to the clinical background meningitis-septicemia are partially included in Fig. 3A and B but are also shown separately in Fig. 3C and D, respectively.
FIG. 3.
Comparison of combinations of different features of S. suis isolates with their clinical backgrounds. Additional features of serotype 2 isolates (A), isolates expressing MRP and EF (MRP/EF isolates) (B), hemolysin-positive isolates (Hly isolates) (C), and cluster A1 isolates (A1 isolates) (D) are shown. The leftmost pair of bars in each graph (labeled Total) refers to all isolates expressing the indicated feature; labeling of the remaining pairs of bars indicates the additional feature found in isolates of the respective group. Each pair of bars shows the proportion of isolates from pigs with meningitis-septicemia (gray bars) compared to the total proportion of isolates with this combination of features (black bars). Results are expressed as a percentage of the total number for each group (N), which is indicated in boxes above the respective groups.
Taken together, the probability of having a clinical background of meningitis-septicemia was highest (100%) among isolates with a combination of serotype 2 with MRP and EF positivity or hemolysin activity, of MRP and EF positivity with hemolysin activity or assignment to cluster A1, and of hemolysin activity with assignment to cluster A1. In addition, there was a striking correlation of serotypes 7 and 9 with a certain MRP-EF pattern, in that none of these isolates expressed EF. Furthermore, none of the serotype 7 isolates expressed MRP, whereas all but one of the serotype 9 isolates expressed MRP, but most of these expressed a size variant of MRP (37). Only two (10%) of all serotype 9 isolates were hemolysin positive. Most serotype 9 isolates were clustered in clusters A2 and B1 (35% each). Only one of the five serotype 1 isolates expressed MRP and EF (the other four isolates were negative for both), and all were positive for hemolysin activity. There was no specific combination of features that was strongly associated with a background of pneumonia. Furthermore, there was no association of adherence capacity with a certain other feature.
DISCUSSION
S. suis is a major pathogen in swine and can also infect humans. The presence of S. suis in pigs, however, is not a good indicator of disease, since carrier rates of this “early colonizer” can be up to 100%, with prevalences of disease generally being not more than 5% (5, 20). This indicates that the level of virulence varies extremely between strains. Therefore, many recent studies have been aimed at the identification of phenotypic and/or genotypic virulence markers which might be useful to distinguish virulent from less virulent or avirulent isolates (23, 26, 29, 35). As with many other pathogens, a single marker that is sufficient for the identification of all highly virulent strains has not yet been identified (and probably does not exist), but there is strong evidence that highly virulent strains share certain features and might be more related than others (4, 21, 23, 26). Some of these virulence-associated features seem to be production of serotype-specific capsular polysaccharides (25), expression of proteins MRP and EF (35), and expression of hemolysin activity and suilysin (10, 17, 18). However, on the basis of the high prevalence of serotype 2 isolates, most studies have been restricted to serotype 2. Furthermore, some results might have been overinterpreted with respect to the terms virulent and avirulent, as recently discussed by Gottschalk et al. (M. Gottschalk, R. Higgins, and S. Quessy. Letter, J. Clin. Microbiol. 37:4202–4203, 1999).
The ongoing debate about the relatedness of highly virulent strains and the poor knowledge about the features of the S. suis population in Germany prompted us to perform the present study, in which we characterized a collection of 99 S. suis isolates including some of the major reference strains. Isolates were first serotyped to ensure that serotypes other than serotype 2 were also represented. The results of serotyping correlate well with those of others (7), in that serotypes 2 and 9 are the dominant serotypes in most European countries except the United Kingdom, where serotype 1 is most prevalent. Furthermore, we showed that other serotypes such as 1, ½, 3, 4, 5, and 7 seem to be quite common in the German S. suis population, which is in agreement with recently published data (36).
Nearly half (48 of 99) of the isolates analyzed for the present study originated from pigs with typical signs of disease (meningitis, septicemia, arthritis [referred to below as “typical isolates”]); 40 isolates were from pigs with pneumonia. In contrast to the importance of S. suis as a primary pathogen in pigs with meningitis, septicemia, or arthritis, it's causative role in pneumonia is not as clear since in many cases S. suis is isolated together with other lung pathogens such as Pasteurella spp., B. bronchiseptica, or Mycoplasma spp. (18, 24). Similarly, in our study only 16 of 40 S. suis isolates from pigs with pneumonia were isolated as pure cultures. In agreement with the current knowledge of the pathogenicity of S. suis (14, 18, 24), we therefore assumed that the isolates from pigs with typical signs (i.e., meningitis, etc.) represented the most virulent strains, whereas isolates from pigs with pneumonia were less invasive and, thus, less virulent. The remaining 11 of the 99 isolates studied were from healthy pigs and therefore represented strains from carriers. Certainly, it must be considered that the origin of isolation can only give some hints as to an isolate's capacity to cause disease but is never proof of an isolate's virulence.
Taking this into account, we analyzed three S. suis subpopulations (i.e., typical isolates, isolates from pigs with pneumonia, and isolates from potential carriers) by examining all currently known putative virulence traits including adherence to epithelial cells. In addition, all isolates were genotyped by macrorestriction analysis by PFGE, a technique with a high resolution power which hitherto has not been applied to S. suis. In our hands, this technique proved to be most suitable for differentiation of single isolates, even those of the same serotype within an infected herd, and thus should be most valuable in epidemiological studies with S. suis. Due to the high degree of sensitivity and the discriminatory power of this technique, however, isolates that appeared to be identical by visual inspection could also be found in very closely related instead of identical branches after cluster analysis. Therefore, it seemed reasonable to define a cutoff value for identity (i.e., >80%) to exclude possible overinterpretation of differentiation of two isolates.
The most prominent features among the typical isolates were the presence of capsular antigens of serotype 2, 9, or 1, expression of MRP and/or EF, and strong hemolysin activity. However, these features were found in only about half of this population; the remaining isolates had more heterogeneous backgrounds, suggesting that their virulence is attributed to other characteristics. PFGE and subsequent cluster analysis confirmed these assumptions, since most of the isolates exhibiting one more of these most prominent features were found in one cluster (cluster A1), whereas the others were more homogeneously distributed in the resulting dendrogram. A different picture was seen in isolates from pigs with pneumonia: serotype 2 was rarely found, and in addition to serotype 9, serotypes 3, 5, and 7 were most prominent. Moreover, only one of the isolates from pigs with pneumonia expressed both MRP and EF, and only two isolates from pigs with pneumonia expressed strong hemolysin activity. This might suggest a lower capacity of isolates from pigs with pneumonia to express virulence properties, which would correlate well with the assumption that isolates from pigs with pneumonia are less invasive and virulent than the typical isolates. No significant clustering of the isolates from pigs with pneumonia was found by cluster analysis. This indicates that the isolates from pigs with pneumonia had more heterogeneous phenotypic and genotypic backgrounds than typical isolates. Among the isolates from healthy animals that were potential carriers, the high proportion (55%) of rarely seen serotypes and nontypeable isolates was most striking, as was the high proportion (36%) of hemolysin-positive isolates. This suggests that S. suis strains from carriers are more diverse than virulent strains, but this must be confirmed by analysis of a larger population of isolates.
A surprising finding of the present study was that adherence to epithelial cells was not correlated at all to the clinical backgrounds of the isolates, independent of the epithelial cell type used (human or porcine). This raises the question of the significance of adherence as a virulence trait, as suggested by others (9, 32). An explanation for the poor adherence observed in our study might be either that adherence is in fact no virulence trait or that a more specific detection of adhesins is needed to evaluate the virulence potential of a given strain or isolate. Concerning the latter, other target cell types (e.g., porcine tonsillar epithelial or endothelial cells) and/or different conditions for bacterial cultivation might have to be tested. In addition, possible inhibitory effects of encapsulation on adherence might have to be ruled out in future studies, even though recently published data by Charland et al. (3) did not support an inhibitory role of the capsule in adherence.
As described above, the phenotypic and genotypic backgrounds of the isolates from diseased pigs revealed some variety but also confirmed features that were commonly found. Therefore, we analyzed our data with respect to the probabilities that the commonly found features, alone or in combination with others, were associated with the clinical background. The results showed that certain combinations of features such as the presence of the capsular antigen of serotype 2 and expression of MRP and EF or the presence of the capsular antigen of serotype 2 and strong hemolysin activity were highly correlated with the invasive clinical background meningitis-septicemia. Furthermore, the probability of such a clinical background was even higher when isolates expressing one of those features could be assigned to PFGE cluster A1. In agreement with the findings of others on the specific ribotype profiles of virulent strains and isolates (21, 29), our clustering results strongly support the hypothesis of a relatively conserved genetic background of highly virulent strains. Together with our observations on the serotypes and potential virulence traits of the isolates tested, these data could indicate that certain highly virulent clones have established themselves successfully within the European S. suis population.
In conclusion, we have presented evidence that (i) PFGE analysis is a most valuable tool for epidemiological studies of S. suis, (ii) several known and yet unknown virulence traits exist in S. suis which, when combined, can give important hints as to whether a given isolate is highly virulent, and (iii) highly virulent isolates or strains from pigs with clinical backgrounds of invasive disease seem to be more related than isolates from pigs with the less invasive pneumonia or typical isolates from carriers. This raises the perspective that in the near future it should be possible to develop strategies for rapid identification of highly virulent isolates and for monitoring of the epidemiology of S. suis infections.
ACKNOWLEDGMENTS
We are in debt to L. Kreienbrock and R. Meyer (Tieraerztliche Hochschule Hannover) for excellent help with statistical analyses and S. Schwarz (FAL, Celle, Germany) for great support with PFGE. We also thank G. Amtsberg and M. Ganter (both from the Tieraerztliche Hochschule Hannover) and C. Laemmler (FB Veterinaermedizin, JLU Giessen) for kindly providing bacterial isolates.
One of us (A.A.) was supported by a grant from the Impfstoffwerk Dessau-Tornau, Rosslau Germany.
REFERENCES
- 1.Arends J P, Zanen H C. Meningitis caused by Streptococcus suis in humans. Rev Infect Dis. 1988;10:131–137. doi: 10.1093/clinids/10.1.131. [DOI] [PubMed] [Google Scholar]
- 2.Chanter N, Jones P W, Alexander T J L. Meningitis in pigs caused by Streptococcus suis—a speculative review. Vet Microbiol. 1993;36:39–55. doi: 10.1016/0378-1135(93)90127-s. [DOI] [PubMed] [Google Scholar]
- 3.Charland N, Nizet V, Rubens C E, Kim K S, Lacouture S, Gottschalk M. Streptococcus suis serotype 2 interactions with human brain microvascular endothelial cells. Infect Immun. 2000;68:637–643. doi: 10.1128/iai.68.2.637-643.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chatellier S, Gottschalk M, Higgins R, Brousseau R, Harel J. Relatedness of Streptococcus suis serotype 2 isolates from different geographic origins as evaluated by molecular fingerprinting and phenotyping. J Clin Microbiol. 1999;37:362–366. doi: 10.1128/jcm.37.2.362-366.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clifton-Hadley F A. The epidemiology, diagnosis, treatment and control of Streptococcus suis type 2 infection. In: McKean J D, editor. Proceedings of the American Association of Swine Practioners. Minneapolis, Minn: American Association of Swine Practioners; 1986. pp. 471–491. [Google Scholar]
- 6.Clifton-Hadley F A, Alexander T J L. The carrier site and carrier rate of Streptococcus suis type II in pigs. Vet Rec. 1980;107:40–41. doi: 10.1136/vr.107.2.40. [DOI] [PubMed] [Google Scholar]
- 7.Esteopangestie S, Laemmler C. Distribution of capsular types 1 to 28 and further characteristics of Streptococcus suis isolates from various European countries. Zentbl Bakteriol Parasitenkd Infektkrank Hyg Abt 1 Orig. 1993;279:394–403. doi: 10.1016/s0934-8840(11)80372-5. [DOI] [PubMed] [Google Scholar]
- 8.Gottschalk M, Higgins R, Jacques M. Production of capsular material by Streptococcus suis serotype 2 under different growth conditions. Can J Vet Res. 1993;57:49–52. [PMC free article] [PubMed] [Google Scholar]
- 9.Gottschalk M, Petitbois S, Higgins R, Jaques M. Adherence of Streptococcus suis capsular type 2 to porcine lung sections. Can J Vet Res. 1991;55:302–304. [PMC free article] [PubMed] [Google Scholar]
- 10.Gottschalk M, Lacouture S, Dubreull J D. Characterization of Streptococcus suis capsular type 2 haemolysin. Microbiology. 1995;141:189–195. doi: 10.1099/00221287-141-1-189. [DOI] [PubMed] [Google Scholar]
- 11.Gottschalk M, Lebrun A, Wisselink H J, Dubreuil J D, Smith H E, Vecht U. Production of virulence-related proteins by Canadian strains of Streptococcus suis capsular type 2. Can J Vet Res. 1998;62:75–79. [PMC free article] [PubMed] [Google Scholar]
- 12.Hampson D J, Trott D J, Clarke I L, Mwaniki C G, Robertson I D. Population structure of Australian isolates of Streptococcus suis. J Clin Microbiol. 1993;31:2895–2900. doi: 10.1128/jcm.31.11.2895-2900.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harel J, Higgins R, Gottschalk M, Bigras-Poulin M. Genomic relatedness among reference strains of different Streptococcus suis serotypes. Can J Vet Res. 1994;58:259–262. [PMC free article] [PubMed] [Google Scholar]
- 14.Hoefling D C. Tracking the incidence of porcine respiratory diseases. Vet Med. 1998;93:391–398. [Google Scholar]
- 15.Jacobs A A C, Loeffen P L W, Van Den Berg A J G, Storm P K. Identification, purification, and characterization of a thiol-activated hemolysin (suilysin) of Streptococcus suis. Infect Immun. 1994;62:1742–1748. doi: 10.1093/benz/9780199773787.article.b00034458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacobs A A C, Van Den Berg A J G, Baars J C, Nielsen B, Johannsen L W. Production of suilysin, the thiol-activated haemolysin of Streptococcus suis, by field isolates from diseased pigs. Vet Rec. 1995;139:295–296. doi: 10.1136/vr.137.12.295. [DOI] [PubMed] [Google Scholar]
- 17.Jacobs A A C, Van Den Berg A J G, Loeffen P L W. Protection of experimentally infected pigs by suilysin, the thiol-activated haemolysin of Streptococcus suis. Vet Rec. 1996;139:225–228. doi: 10.1136/vr.139.10.225. [DOI] [PubMed] [Google Scholar]
- 18.Macinnes J I, Desrosiers R. Agents of the “suis-ide diseases” of swine: Actinobacillus suis, Haemophilus suis, and Streptococcus suis. Can J Vet Res. 1999;63:83–89. [PMC free article] [PubMed] [Google Scholar]
- 19.Mogollon J D, Pijoan C, Murtaugh M P, Collins J E, Cleary P P. Identification of epidemic strains of Streptococcus suis by genomic fingerprinting. J Clin Microbiol. 1991;29:782–787. doi: 10.1128/jcm.29.4.782-787.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mwaniki C G, Robertson I D, Trott D J, Atyeo R F, Lee B J, Hampson D J. Clonal analysis and virulence of Australian isolates of Streptococcus suis type 2. Epidemiol Infect. 1994;113:321–334. doi: 10.1017/s095026880005175x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okwumabua O, Staats J, Chengappa M M. Detection of genomic heterogeneity in Streptococcus suis isolates by DNA restriction fragment length polymorphism of rRNA genes (ribotyping) J Clin Microbiol. 1995;4:968–972. doi: 10.1128/jcm.33.4.968-972.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olsen J E, Skov M. Genomic lineage of Salmonella enterica serovar Dublin. Vet Microbiol. 1984;40:271–282. doi: 10.1016/0378-1135(94)90116-3. [DOI] [PubMed] [Google Scholar]
- 23.Rasmussen S R, Aarestrup F M, Jensen N E, Jorsasl S E. Associations of Streptococcus suis serotype 2 ribotype profiles with clinical disease and antimicrobial resistance. J Clin Microbiol. 1999;37:404–408. doi: 10.1128/jcm.37.2.404-408.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reams R Y, Harrington D D, Glickman L T, Thacker H L, Bowersock T L. Multiple serotypes and strains of Streptococcus suis in naturally infected swine herds. J Vet Diagn Investig. 1996;8:119–121. doi: 10.1177/104063879600800121. [DOI] [PubMed] [Google Scholar]
- 25.Smith H E, Damman M, Van Der Velde J, Wagenaar F, Wisselink H J, Stockhofe-Zurwieden N, Smits M A. Identification and characterization of the cps locus of Streptococcus suis serotype 2: the capsule protects against phagocytosis and is an important virulence factor. Infect Immun. 1999;67:1750–1756. doi: 10.1128/iai.67.4.1750-1756.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith H E, Rijnsburger M, Stockhofe-Zurwieden N, Wisselink H J, Vecht U, Smits M A. Virulent strains of Streptococcus suis serotype 2 and highly virulent strains of Streptococcus suis serotype 1 can be recognized by a unique ribotype profile. J Clin Microbiol. 1997;35:1049–1053. doi: 10.1128/jcm.35.5.1049-1053.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith H E, Vecht U, Gielkens A L J, Smits M A. Cloning and nucleotide sequence of the gene encoding the 136-kilodalton surface protein (muraminidase-released protein) of Streptococcus suis type 2. Infect Immun. 1996;60:2361–2367. doi: 10.1128/iai.60.6.2361-2367.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith H E, Vecht U, Wisselink H J, Stockhofe-Zurwieden N, Biermann Y, Smits M A. Mutants of Streptococcus suis types 1 and 2 impaired in expression of muramidase-released protein and extracellular protein induce disease in newborn germfree pigs. Infect Immun. 1996;64:4409–4412. doi: 10.1128/iai.64.10.4409-4412.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Staats J J, Plattner B L, Nietfeld J, Dritz S, Chengappa M M. Use of ribotyping and hemolysin activity to identify highly virulent Streptococcus suis type 2 isolates. J Clin Microbiol. 1998;36:15–19. doi: 10.1128/jcm.36.1.15-19.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suvrov A N, Ferretti J J. Physical and genetic map of an M type 1 strain of Streptococcus pyogenes. J Bacteriol. 1996;178:5546–5549. doi: 10.1128/jb.178.18.5546-5549.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Talay S R, Valentin-Weigand P, Jerlstroem P G, Timmis K N, Chhatwal G S. Fibronectin binding protein of Streptococcus pyogenes: sequence of the binding domain involved in adherence of streptococci to epithelial cells. Infect Immun. 1992;60:3837–3844. doi: 10.1128/iai.60.9.3837-3844.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tikkanen K, Haataja S, Finne J. The galactosyl-(1-4)-galactose-binding adhesin of Streptococcus suis: occurrence in strains of different hemagglutination activities and induction of opsonic antibodies. Infect Immun. 1996;64:3659–3665. doi: 10.1128/iai.64.9.3659-3665.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vecht U, Arends J P, Van Der Molen E J, Van Leengoed L A M G. Differences in virulence between two strains of Streptococcus suis type II after experimentally induced infection of newborn germ-free pigs. Am J Vet Res. 1989;50:1037–1043. [PubMed] [Google Scholar]
- 34.Vecht U, Stockhofe-Zurwieden N, Tetenburg B J, Wisselink H J, Smith H E. Virulence of Streptococcus suis type 2 for mice and pigs appeared host-specific. Vet Microbiol. 1997;58:53–60. doi: 10.1016/s0378-1135(97)00131-4. [DOI] [PubMed] [Google Scholar]
- 35.Vecht U, Wisselink H J, Jellema M L, Smith H E. Identification of two proteins associated with virulence of Streptococcus suis type 2. Infect Immun. 1991;59:3156–3162. doi: 10.1128/iai.59.9.3156-3162.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wisselink H J, Smith H E, Stockhofe-Zurwieden N, Peperkamp K, Vecht U. Distribution of capsular types and production of murimidase-released protein (MRP) and extracellular factor (EF) of Streptococcus suis isolated from diseased pigs in seven European countries. Vet Microbiol. 2000;74:237–248. doi: 10.1016/s0378-1135(00)00188-7. [DOI] [PubMed] [Google Scholar]