Abstract
Tomato (Solanum lycopersicum) is one of the most important greenhouse vegetables, with a large cultivated area across the world. However, in northern China, tomato plants often suffer from low-temperature stress in solar greenhouse cultivation, which affects plant growth and development and results in economic losses. We previously found that a chloroplast aldolase gene in tomato, SlFBA4, plays an important role in the Calvin-Benson cycle (CBC), and its expression level and activity can be significantly altered when subjected to low-temperature stress. To further study the function of SlFBA4 in the photosynthesis and chilling tolerance of tomato, we obtained transgenic tomato plants by the over-expression and RNA interference (RNAi) of SlFBA4. The over-expression of SlFBA4 led to higher fructose-1,6-bisphosphate aldolase activity, net photosynthetic rate (Pn) and activity of other enzymes in the CBC than wild type. Opposite results were observed in the RNAi lines. Moreover, an increase in thousand-seed weight, plant height, stem diameter and germination rate in optimal and sub-optimal temperatures was observed in the over-expression lines, while opposite effects were observed in the RNAi lines. Furthermore, over-expression of SlFBA4 increased Pn and enzyme activity and decreased malonaldehyde (MDA) content under chilling conditions. On the other hand, Pn and MDA content were more severely influenced by chilling stress in the RNAi lines. These results indicate that SlFBA4 plays an important role in tomato growth and tolerance to chilling stress.
Keywords: tomato; fructose-1,6-bisphosphate aldolase; transgenic; low-temperature stress; photosynthesis; growth
1. Introduction
Fructose-1,6-bisphosphate aldolase (FBA, EC 4.1.2.13) has been extensively found in various organisms, such as bacteria, higher plants and animals [1,2]. It is an essential enzyme that is involved in carbohydrate metabolism, including gluconeogenesis, glycolysis and the Calvin-Benson cycle (CBC) [3,4]. In the CBC, FBA catalyzes the reversible conversion of fructose-1,6-bisphosphate (FBP) to dihydroxyacetone phosphate (DHAP) and glyceraldehyde-3-phosphate (G3P) [5,6,7]. Both DHAP and G3P are substrates or metabolic products in the tricarboxylic acid cycle, phenylpropanoid pathway and oxidative/non-oxidative pentose phosphate pathway [8].
FBAs can be divided into two classes based on their catalytic mechanisms and evolutionary origins [2]. Class I FBAs utilize a lysine residue to generate a Schiff base, intermediate product, and their activity can be inhibited by hydroboron [2]. Class I FBAs often occur in higher plants and animals and have two isoforms that differ in subcellular locations: chloroplast/plastid FBA (CpFBA) and cytosolic FBA (cFBA) [9,10]. CpFBA catalyzes the condensation reaction of FBP and sedoheptulose-1,7-bisphosphate [11], whereas cFBA plays a vital role in glycolysis and gluconeogenesis [9,12]. Class II FBAs are mainly identified in microorganisms [5,11] and only a few class II FBAs occur in wheat and other closely related species [13]. Class I FBAs have been extensively identified and characterized in various plants, such as Arabidopsis [10], rice [14,15], maize [16], spinach [9], soybean [17], potato [18], oat [19], tobacco [20], Sesuvium portulacastrum L. [21], bamboo [22], wheat [13] and tomato [23,24]. Many studies have indicated that FBAs are involved in plant growth and development [7,20]. For example, higher cFBA activity was observed in elongating tissues than in tissues that had finished elongating in moso bamboo (Phyllostachys pubescens Mazel), which indicated an important role of cFBA in the elongation of tissues [22]. In tomato, decreased FBA activity resulted in the slower growth of plants [23,24]. Accumulating evidence indicates that FBAs respond to various biotic [8] and abiotic stresses [10,19,20,21,24,25,26,27,28]. For example, FBA activity was up-regulated in 12-day-old wheat seedlings under salt stress, which helped the seedlings adapt to the stress [26]. In oat (Avena sativa L.), FBA activity can be induced by heat [19], whereas in chickpea (Cicer arietinum L.), FBA activity was repressed by water-deficit stress [28].
We previously identified eight members of the FBA gene family in tomato, which comprised five CpFBAs and three cFBAs. SlFBA7, one of the cFBAs, plays an important role in regulating tomato growth and chilling tolerance [24]. However, little is known about the function of CpFBAs in tomato. In the present study, we focus on SlFBA4, one of the CpFBAs, to investigate the function of this gene and demonstrate that it also plays an important role in regulating tomato growth and chilling tolerance.
2. Results
2.1. Production and Selection of Tomato Transformants
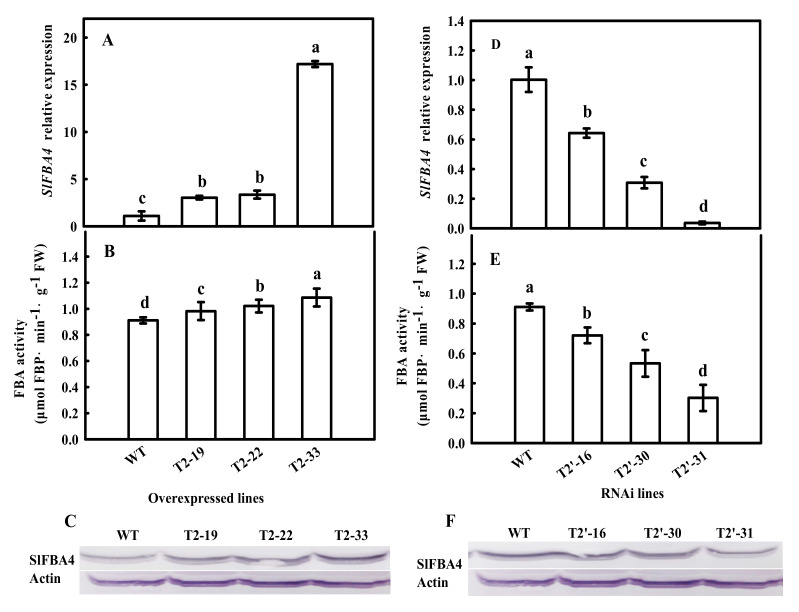
To test whether SlFBA4 regulates plant growth, especially under low temperature, an over-expression cassette that contained the SlFBA4 CDS controlled by the CaMV-35S promoter (35S-SlFBA4) and an RNAi vector that contained a reverse-complementary hairpin structure (RNAi-SlFBA4) were created and introduced into tomato via Agrobacterium tumefaciens-mediated genetic transformation (Figure S1). Transgenic plants that overexpressed and suppressed SlFBA4 were generated. Three independent over-expression lines (T2-19, T2-22 and T2-33) and RNAi lines (T2′-16, T2′-30 and T2′-31) with significantly increased or decreased SlFBA4 expression were used for further investigation (Figure 1). Compared with SlFBA4 expression in wild-type (WT) plants, SlFBA4 transcript abundance in the three over-expression transgenic lines increased by 178.39%, 208.09% and 1480.27% (Figure 1A). The SlFBA4 expression level of the three RNAi lines was decreased by 35.93%, 69.27% and 96.37% (Figure 1D). Meanwhile, the FBA activities of the SlFBA4 over-expression lines were significantly increased compared with WT (Figure 1B). In contrast, the RNAi lines exhibited significantly decreased FBA activities when compared with WT (Figure 1E). Similarly, stronger and weaker protein signals were observed in the over-expression and RNAi transgenic lines, respectively, than that in WT plants using Western blot analysis with an antiserum against SlFBA4.
Figure 1.
SlFBA4 mRNA abundance, FBA activity and protein levels in WT and transgenic plants. (A,D) SlFBA4 mRNA abundance in overexpression (A) and RNAi (D) plants, total RNA was separately isolated from the third fully expanded leaves of the WT and transgenic seedlings and subjected to real-time PCR analysis. (B,E) FBA activity, the same tissues used for SlFBA4 mRNA analyses were sampled for FBA activity assay. (C,F) SlFBA4 protein levels, 25 μg of protein samples from leaves of WT and transgenic seedlings were separated by SDS-PAGE and polyclonal antibodies were used to detect FBA protein level. All values are presented as the mean ± SD (n = 3). Lowercase letters indicate that the mean values are significantly different among samples (p < 0.05).
2.2. Effects of SlFBA4 on Transgenic Tomato Plant Growth
To investigate the effects of SlFBA4 on tomato seed and seedling development, the thousand-seed weight, germination rate and plant growth parameters, including plant height and stem diameter, were measured. As shown in Table 1, over-expression of SlFBA4 significantly increased the thousand-seed weight compared with that in WT. Conversely, the down-regulation of SlFBA4 decreased the thousand-seed weight compared with that in WT. Under 28 °C, which is the normal temperature for tomato seed germination, the difference in germination rate between WT and over-expressed transgenic seeds was not obvious; however, the germination rate and germination potential of RNAi transgenic seeds was significantly lower than that of WT (Table 1; Figure 2). For plant growth, over-expression of SlFBA4 resulted in plants with a significantly larger plant height and stem diameter than those of WT (Table 1; Figure 2). However, plant height and stem diameter were comparable between the RNAi plants and WT (Table 1; Figure 2).
Table 1.
Comparison of morphological parameters of WT and transgenic plants.
| Lines | Thousand-Seed-Weight | Germination Rate (%) | Plant Height (cm) |
Stem Diameter (mm) |
|
|---|---|---|---|---|---|
| 18 °C | 28 °C | ||||
| WT | 2.2 ± 0.3 d | 37.3 ± 5 b | 97.0 ± 4 a | 25.4 ± 2 d | 14.2 ± 0.7 d |
| T2-19 | 2.7 ± 0.2 c | 41.0 ± 3 ab | 96.6 ± 4 a | 29.0 ± 1 c | 16.5 ± 0.5 c |
| T2-22 | 3.0 ± 0.2 b | 46.0 ± 5 a | 97.0 ± 3 a | 32.2 ± 1 b | 17.2 ± 0.3 b |
| T2-33 | 3.2 ± 0.3 a | 48.6 ± 2 a | 99.3 ± 1 a | 35.4 ± 1 a | 21.0 ± 0.6 a |
| T2′-16 | 2.1 ± 0.1 e | 34.3 ± 5 bc | 60.0 ± 2 b | 25.8 ± 1 d | 14.1 ± 0.7 d |
| T2′-30 | 1.9 ± 0.2 f | 29.0 ± 1 cd | 53.7 ± 5 c | 26 ± 1 d | 13.7 ± 1.1 d |
Note: Values represent means ± SD; lowercase superscript letters indicate that the mean values are significantly different among samples (p < 0.05).
Figure 2.
Seedlings and seed germination under different temperature conditions of the wild-type and transgenic lines of tomato. (A) Seedlings of WT and SlFBA4 over-expression lines; (B) Seedlings of WT and RNAi lines; (C) Seed germination of WT and SlFBA4 over-expression lines under 18 °C, and 28 °C; (D) Seed germination of WT and RNAi lines under 18 °C and 28 °C.
2.3. Effects of SlFBA4 on Net Photosynthetic Rate as well as Gene Expression and Activity of Select Enzymes in the Calvin-Benson Cycle
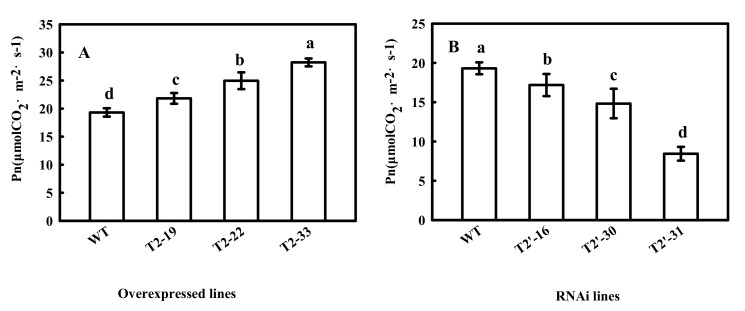
Since CpFBA is a key enzyme in the CBC, which is an important stage of photosynthesis, we also measured Pn in transgenic tomato plants. As expected, Pn significantly increased and decreased in the SlFBA4 over-expression lines and the RNAi lines, respectively, compared with that in WT (Figure 3), which indicated an important role of SlFBA4 in regulating photosynthesis in tomato.
Figure 3.
Net photosynthetic rate (Pn) in WT and transgenic tomato seedlings. Pn were measured at 25 °C under ambient carbon dioxide (CO2) (360 μmol mol−1). All values are presented as the means ± SD (n = 3). Lowercase letters indicate that the mean values are significantly different among the samples (p < 0.05).
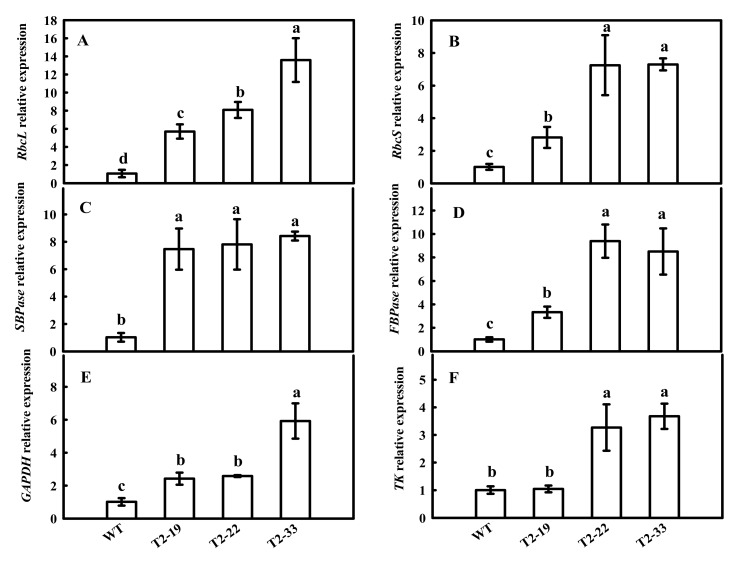
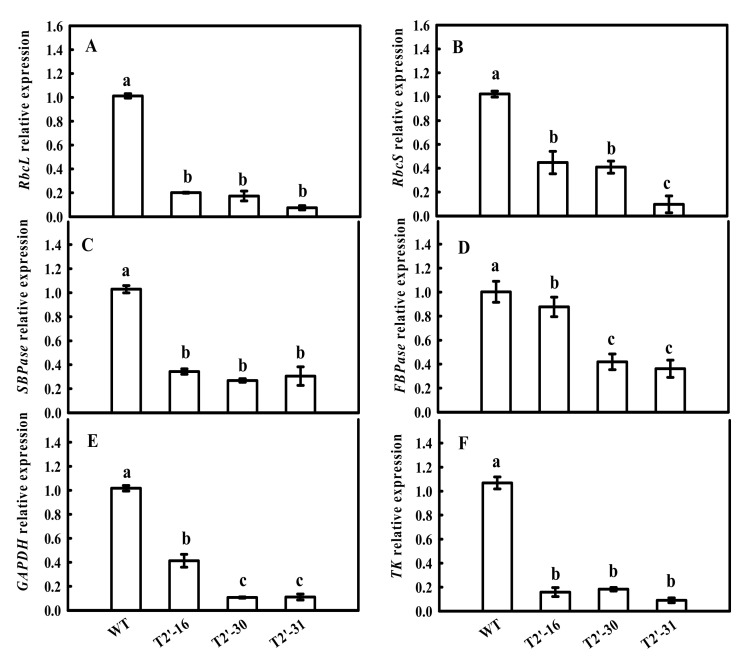
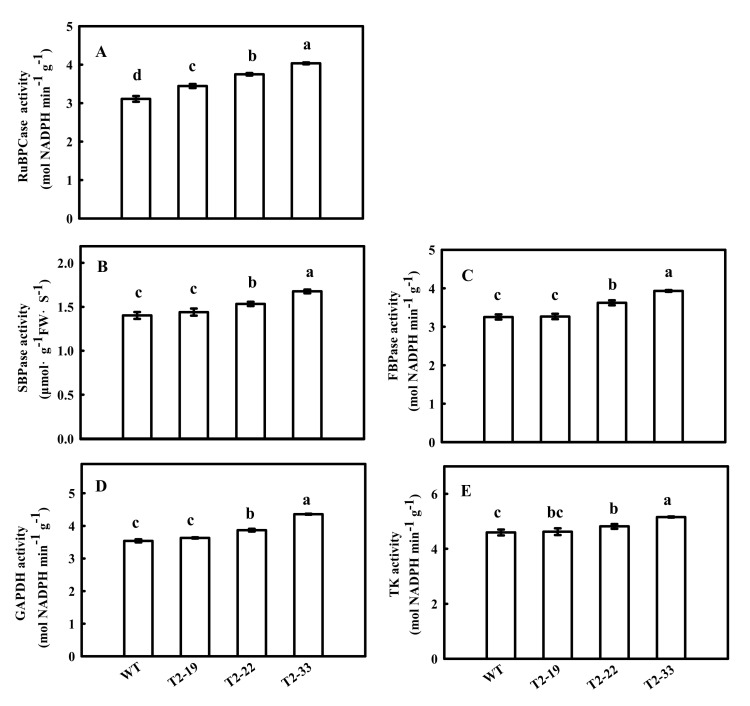
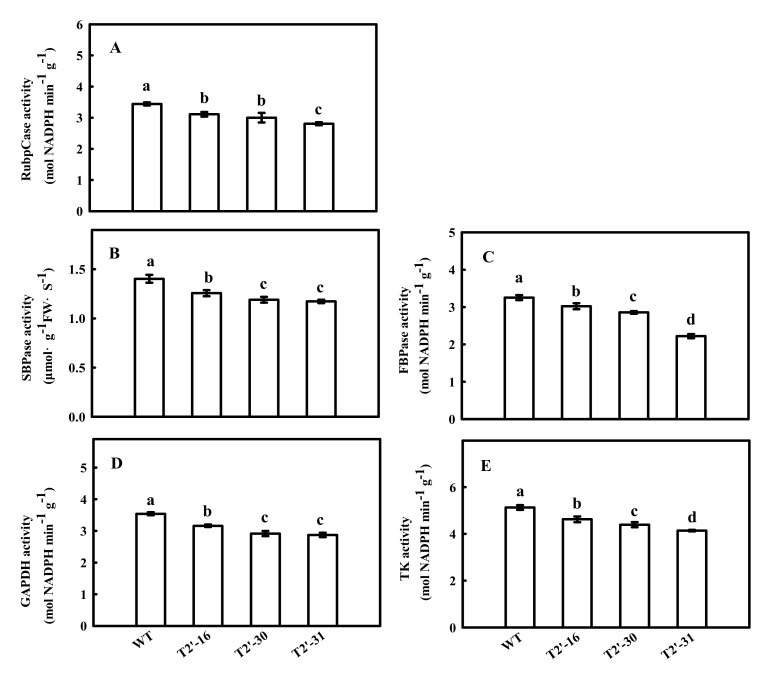
Previously, we found that the down-regulation of tomato FBA7 led to significant changes in the mRNA expression and activity of some enzymes in the CBC, including ibulose 1,5-bisphosphate carboxylase/oxygenase (Rubisco), sedoheptulose-1,7-bisphosphatase (SBPase), fructose 1,6-bisphosphatsse (FBPase), glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and transketolase (TK). Therefore, we investigated the effects of SlFBA4 on the mRNA expression and activity of these enzymes. As shown in Figure 4, the relative expressions of rbcL, rbcS, SBPase, FBPase, GAPDH and TK were significantly increased in the over-expression lines compared with that in WT, except TK in lines T2-19 (Figure 4). However, a significant decrease in the mRNA abundances of the six genes in the RNAi lines was found when compared with WT (Figure 5). As expected, the activities of RuBPCase, SBPase, FBPase, GAPDH and TK were significantly increased in at least two over-expression lines compared with that in WT (Figure 6). Conversely, significantly lower activities of the five enzymes were observed in the RNAi lines than in WT (Figure 5 and Figure 7). Taken together, these results suggest an important role of SlFBA4 in regulating photosynthesis in tomato.
Figure 4.
mRNA abundances of the six genes encoding important enzymes in the Calvin-Benson cycle in overexpressed SlFBA4 transgenic tomato seedlings. Total RNA was separately isolated from the third fully expanded leaves of WT and transgenic plants and subjected to qRT-PCR analyses. (A) rbcL, (B) rbcS, (C) SBPase, (D) FBPase, (E) GAPDH, (F) TK. All values are presented as the means ± SD (n = 3). Lowercase letters indicate that the mean values are significantly different among the samples (p < 0.05).
Figure 5.
mRNA abundances of the six genes encoding important enzymes of the Calvin-Benson cycle in the RNAi seedlings using qRT-PCR. (A) rbcL, (B) rbcS, (C) SBPase, (D) FBPase, (E) GAPDH, (F) TK. All values are presented as the means ± SD (n = 3). Lowercase letters indicate that the mean values are significantly different among the samples (p < 0.05).
Figure 6.
Enzyme activities of RuBPCase (A), SBPase (B), FBPase (C), GAPDH (D) and TK (E) in Calvin-Benson cycle in SlFBA4 overexpressed transgenic tomato seedlings. The same tissues for mRNA analyses were sampled for the enzyme activities assay. All values are presented as the means ± SD (n = 3). Lowercase letters indicate that the mean values are significantly different among the samples (p < 0.05).
Figure 7.
Enzyme activities of RuBPCase (A), SBPase (B), FBPase (C), GAPDH (D) and TK (E) in Calvin-Benson cycle in RNAi transgenic seedlings. All values are presented as the means ± SD (n = 3). Lowercase letters indicate that the mean values are significantly different among the samples (p < 0.05).
2.4. Effects of SlFBA4 on Germination Rate, Net Photosynthetic Rate and Malonaldehyde Content under Chilling Stress
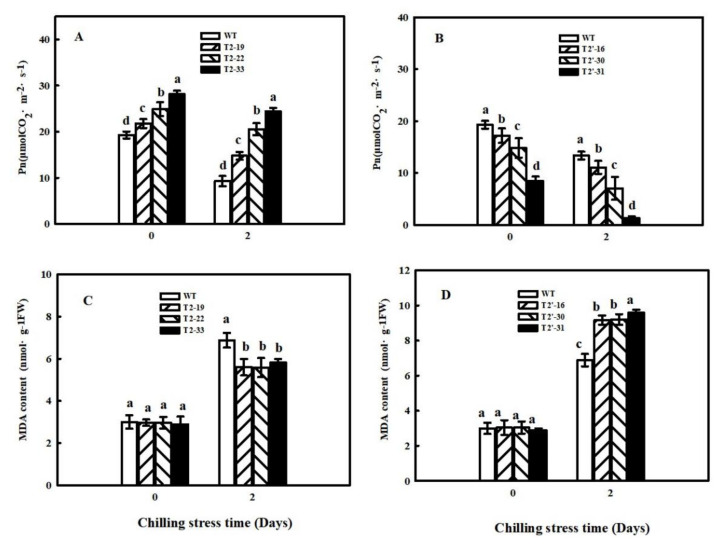
To explore the effects of SlFBA4 on chilling tolerance in tomato, germination rate, Pn and MDA content were investigated in WT and transgenic seedlings under chilling conditions. Under 18 °C, which is a sub-low temperature for tomato seed germination, germination rate increased and decreased in SlFBA4-over-expressing and RNAi lines, respectively, compared with that in WT (Table 1). Similar changes in Pn were also observed in transgenic lines compared with that in WT under 5 °C treatment for 48 h (Figure 8A,B). MDA is the main product of lipid peroxidation and its content can indicate damage to plant membranes. As shown in Figure 8C,D, MDA content in WT and over-expression seedlings T2-19, T2-22 and T2-33 under chilling for two days significantly increased by 129.33%, 89.19%, 88.51% and 102.08%, respectively, compared with that under normal conditions. MDA content in RNAi seedlings T2′-16, T2′-30 and T2′-31 increased by 201.32%, 202.63% and 230.34%, respectively, compared with that under normal conditions. These results indicate that the over-expression of SlFBA4 increased chilling tolerance and interference of SlFBA4 expression increased damage to the cell membranes of tomato seedlings by chilling stress.
Figure 8.
Effects of chilling stress on the net photosynthetic rate (A,B) and MDA content (C,D) in WT and transgenic lines. Seedlings were treated at 5 °C for 0 day and 2 days under 100 μmol m−2 s−1 PFD. After treatment, the third fully expanded leaves were used for the Pn and MDA content measurement. All values are presented as the means ± SD (n = 3). Lowercase letters indicate that the mean values are significantly different among the samples (p < 0.05).
3. Discussion
Tomato is a vegetable crop cultivated worldwide. In northern China, tomato is widely cultivated in greenhouses during winter and spring. Thus, tomato plants often suffer from low-temperature stress, leading to reduced productivity and economic losses. Chilling stress is a common factor that leads to a dramatic reduction in photosynthesis in chilling-sensitive plants, with the consequence of damaging the photosynthetic apparatus [29,30].
It has long been known that the limitations of carbon assimilation in C3 plants are largely due to the catalytic properties of ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) [31]. However, since the 1990s, studies have found that reductions in the activity of Rubisco have little impact on carbon assimilation under normal environmental conditions [32,33,34]. Rubisco may not be the primary limiting factor that leads to poor performance under chilling conditions but instead helps plants recover faster from these conditions [35]. Recent studies have shown that there is a non-regulated enzyme, aldolase, that is sensitive to abiotic stresses [36,37,38]. It is well known that FBAs are key positive regulators of photosynthesis [39,40]; however, the knowledge of SlFBAs in regulating photosynthesis and chilling stress in tomato is still limited.
We previously identified eight FBA genes in the tomato genome, which can be divided into two subfamilies [23]. A previous study showed that SlFBA4 is highly similar to CpFBA in Arabidopsis (AtFBA1/2/3), indicating that SlFBA4 is likely located in the chloroplast where the CBC occurs [23]. Notably, we found that the expression of all eight FBAs was affected by low-temperature stress, especially SlFBA4, which were gradually upregulated by low-temperature stress from 0 day to three days [23]. This result indicates the possible roles of SlFBA4 in response to chilling stress. In addition, given that FBAs are key positive regulators of photosynthesis [39,40] and the fact that increasing the expression levels of SlFBA7 resulted in enhanced chilling tolerance in tomato [24], we studied the functions of SlFBA4 in tomato by creating over-expressed or silenced SlFBA4 transgenic lines in tomato.
In this study, we observed that an increase in FBA activity led to increases in seed and plant growth (Table 1) and Pn (Figure 3). Furthermore, we showed that a decrease in FBA activity led to an inhibitory effect on seed germination in optimal and sub-optimal temperature conditions (Table 1), indicating that SlFBA4 is important for tomato growth and development, especially under low-temperature conditions. Similar results were also observed in other plants [41,42].
The root system is the most important structure to absorb water and nutrients, provide structural support and ensure tolerance against abiotic stresses [43,44]. Importantly, vigorous root system architecture leads to better growth and greater stress tolerance [40,44]. To our best knowledge, little is known about the role of FBAs in regulating root growth. In the present study, the promotion of root growth was clearly seen in over-expression transgenic lines and the roots look comparable between WT and RNAi lines (Figure 2A,B). It might be one of the reasons for the increased chilling stress tolerance of over-expression transgenic lines. It is worthy of further study to ascertain the role of SlFBA4 in root growth.
The adaptation of photosynthetic apparatus in cucumber seedlings to suboptimal conditions was related to the activation mechanisms of photosynthetic enzymes [45]. Both stomatal and nonstomatal factors influence photosynthesis in higher plants. Previous studies have shown that one of the most important nonstomatal factors is the activity of photosynthetic enzymes [45,46,47]. An increase in CBC enzyme activity may be the main factor that promotes the cucumber Pn increases during chilling conditions [48,49]. An increase in FBA activity promotes the reaction from dihydroxyacetone phosphate (DHAP) to fructose-1,6-diphosphate (FBP), and, as a consequence, the downstream reactions, including the reaction from FBP to fructose-6-phosphate (F6P) and a series of reactions to form RuBP, are also promoted. Increased RuBP regeneration could be the reason that Pn, growth and development increased. Low expression of SlFBA4 in tomato leaves [23] was significantly enhanced in our over-expression transgenic plants (Figure 1A). Our results show that over-expression of SlFBA4 led to higher CBC enzyme activity and Pn, whereas down-regulation of SlFBA4 led to lower enzyme activity and Pn (Figure 3, Figure 6 and Figure 7). Many genetic engineering studies have confirmed that changes in CBC enzyme activity lead to changes in Pn and differences in chilling tolerance [23,50,51,52]. MDA, which is one of the best indexes of lipid peroxidation, may accumulate quickly in plant tissues and lead to DNA and biological membrane damage when exposed to chilling conditions [53]. However, our results show that SlFBA4 over-expressing lines had a lower accumulation of MDA, whereas RNAi transgenic plants had a higher accumulation of MDA (Figure 8). These results indicate an important role of SlFBA4 in protecting the cell membrane under chilling stress.
In conclusion, SlFBA4 plays a crucial role in tomato growth and tolerance to chilling stress. Over-expression of SlFBA4 enhanced photosynthetic capability in tomato and protected the cell membrane from MDA. Our findings shed new light on how CpFBA influences plant growth and development and regulates chilling tolerance. This provides a foundation to enhance plant adaptations to chilling conditions in solar greenhouses.
4. Materials and Methods
4.1. Plant Materials, Growth Conditions and Measurements
Seeds of the tomato inbred line ‘FF’ were used for germination tests and genetic transformation. The thousand-seed weight was measured using an analytical balance with 0.1 mg resolution. Germination tests were carried out in Petri dishes with moist filter paper at 28 °C and 18 °C in darkness. Seeds were grown in a solar greenhouse at Shandong Agriculture University in Tai’an under 25 °C/16 h days and 20 °C/8 h nights at a light intensity of 400 µmol m−2 s−1 and relative humidity of 75%. Seedlings at the five-true-leaf expansion stage were used for the following treatments. Plant height and stem diameter were measured using a ruler and a Vernier caliper, respectively. For the physiological parameters and analysis of enzyme activity, seedlings cultured in the greenhouse were used. For the low-temperature treatment, seedlings were transferred to a growth chamber at low temperature (8 °C days, 5 °C nights) and low light intensity (100 μmol m−2 s−1) for 0 h and 48 h. Fully expanded leaves were then harvested and frozen in liquid nitrogen. Samples were stored at −80 °C for subsequent experiments. Each experiment was repeated three times.
4.2. Gene Cloning, Vector Construction and Generation of Transgenic Lines
To isolate the full-length coding sequence (CDS) for SlFBA4, young leaves were harvested from three-week-old seedlings and frozen immediately in liquid nitrogen. Total RNA was extracted using Trizol (Invitrogen, Solarbio Life Sciences, A208, Zicheng Pioneer Park, Xianghuangqi East Road, Haidian District, Beijing, China), and synthesis of cDNA was completed using the SuperScript® First-Strand Synthesis System (Invitrogen) following the manufacturer’s instructions. The full-length CDS of SlFBA4 was introduced into the plant expression vector pROKII driven by the CaMV-35S promoter. For RNA interference (RNAi) vector construction, two RNAi fragments with the same sequence (180 bp) and different restriction sites were cloned into pUCCRNAi to form a reverse-complementary hairpin structure as described previously [24]. Then, the hairpin structure was introduced into the plant expression vector pBI121 under control of the CaMV-35S promoter.
For genetic transformation, the tomato inbred line ‘FF’ (obtained from Qinghua Shi, Shandong Agricultural University) was used. Positive plasmids were used for Agrobacterium-mediated tomato transformation as described by Fillatti et al. [54]. Regenerated shoots were screened on MS medium containing kanamycin (50 μg mL−1) and carbenicillin (300 μg mL−1). Resistant transformants that contained the over-expression plasmid were confirmed by polymerase chain reaction (PCR) using a 35S promoter forward primer and SlFBA4 reverse primer (Table S1). Resistant shoots containing the RNAi vector were also confirmed by PCR (Table S1). The primary transformants (T0 generation) were self-fertilized in the solar greenhouse. The progeny obtained from T0 were named T1, and the progeny obtained from T1 were named T2. The T2 plants were used in the experiment.
4.3. Quantitative Reverse Transcription Polymerase Chain Reaction Analysis
For analysis of gene expression, total RNA was isolated using Trizol according to the manufacturer’s instructions. First-strand cDNA was synthesized from 1 μg of total RNA with the PrimeScript 1st Strand cDNA Synthesis Kit (TaKaRa Bio Engineering Co., Ltd., Dalian, China). The relative mRNA expression from the transgenic plants was analyzed by quantitative reverse transcription PCR (qRT-PCR) using the TransStart TipTop Green qPCR SuperMix (Transgen, Yongtaizhuang North Road, Haidian District, Beijing, China), according to the manufacturer’s instructions. β-actin was used as an internal control. Amplification of targeted genes was performed using the LightCycler® 480 II system (Roche, Penzberg, Germany). The analysis of relative mRNA expression data was performed using the 2−ΔΔCt method [55]. qRT-PCR primers were designed to avoid conserved regions and to amplify 150 to 300 bp products (Table S2). qRT-PCR was performed with three independent biological replicates.
4.4. Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis and Western Blot Analysis
Total protein from transgenic plants was extracted as previously described [56]. Approximately 1.0 g of clean leaf tissue was homogenized in 2 mL of extraction buffer (100 mM Tris-HCl (pH 8.0), containing 1 mM EDTA-Na2, 1% PVP, 10 mM mercaptoethanol and 0.2 M sucrose) followed by two centrifugations for 15 min at 4 °C and 12,000 rpm each. The liquid supernatant was stored at −80 °C and used for protein analysis. The polypeptide of the SlFBA4 protein was used for the polyclonal antibody preparation. The primary antibody was prepared by GenScript Biotechnology Co., Ltd. in Nanjing. Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting were carried out according to methods previously described [57]. Total protein was solubilized in 2 × SDS loading buffer and separated by SDS-PAGE using 12% separating gels and 5% concentrating gels containing 10% SDS. Proteins were then transferred to polyvinylidene fluoride membranes (Millipore, Burlington, MA, USA), which was followed by combination with the primary and secondary antibodies.
4.5. Measurement of Net Photosythetic Rate
The net photosynthetic rate (Pn) of expanded leaves was measured using a portable photosynthetic system (Ciras-3, PP Systems International, Hitchin, Hertfordshire, UK). Photon flux density (600 μmol m−2 s−1), CO2 concentration (380 mg L−1) and leaf temperature (25 °C) were maintained at a constant for all of the measurements.
4.6. Enzyme Activity Assay
Ribulose-1,5-bisphosphate (RuBP) carboxylase (RuBPCase), fructose-1,6-bisphosphatase (FBPase), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), sedoheptulose-1,7-bisphosphatase (SBPase), transketolase (TK) and FBA activities were determined as previously described [24,58].
4.7. Measurement of Malonaldehyde Content
Malonaldehyde (MDA) content was measured as described by Cho and Park in 2000 [59] and Heath and Packer in 1968 [60]. Briefly, approximately 0.5 g of fresh leaf tissue was prepared and homogenized in 4 mL of pre-cooling phosphate buffer. The liquid supernatant was stored at 4 °C for later use. Thiobarbituric acid colorimetry was used for measurement of MDA content.
4.8. Statistical Analysis
Values are presented as means ± standard deviation of the three biological replicates. The data were statistically analyzed through the DPS software using one-way analysis of variance and Duncan’s multiple range test. Different lowercase letters indicate significant differences at p < 0.05.
Acknowledgments
The authors would like to thank the plantform and all the other people for assistance during the preparation of this manuscript.
Abbreviations
| CBC | Calvin-Benson cycle |
| CDS | Coding sequence |
| cFBA | Cytosolic fructose-1,6-bisphosphate aldolase |
| CpFBA | Chloroplast/plastid fructose-1,6-bisphosphate aldolase |
| DHAP | Dihydroxyacetone phosphate |
| FBA | Fructose-1,6-bisphosphate aldolase |
| FBP | Fructose-1,6-bisphosphate |
| FBPase | Fructose-1,6-bisphosphatase |
| G3P | Glyceraldehyde-3-phosphate |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| MDA | Malonaldehyde |
| PCR | Polymerase chain reaction |
| Pn | Net photosynthetic rate |
| qRT-PCR | Quantitative reverse transcription polymerase chain reaction |
| RNAi | RNA interference |
| Rubisco | Ribulose-1,5-bisphosphate carboxylase/oxygenase |
| RuBP | Ribulose-1,5-bisphosphate |
| RuBPCase | RuBP carboxylase |
| SBPase | Sedoheptulose-1,7-bisphosphatase |
| SDS-PAGE | Sodium dodecyl sulfate polyacrylamide gel electrophoresis |
| TK | Transketolase |
| WT | Wild-type |
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms23020728/s1.
Author Contributions
X.A. conceived the idea and designed the project. B.C. performed the experiment, and wrote and edited the manuscript. Y.N. helped to interpret the data. Q.L. (Qiang Li) and Q.L. (Qingyun Li) helped to modify the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Science Foundation of China (31572170), the Introduce Talents Start-Up fund (NCCIK2020RC-14) of State Key Laboratory of North China Crop Improvement and Regulation in Hebei Agricultural University, 2021 Project for the Introduction of Oversea Students in Hebei (C20210510), and The Modern Agricultural Industrial Technology System in Hebei Province (HBCT2018030211).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
All authors consent to the publication of this article.
Data Availability Statement
Data is contained within the article or Supplementary Materials.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tolan D., Niclas J., Bruce B.D., Lebo R.V. Evolutionary implications of the human aldolase-A, -B, -C, and -pseudogene chromosome locations. Am. J. Hum. Genet. 1987;41:907–924. [PMC free article] [PubMed] [Google Scholar]
- 2.Marsh J.J., Lebherz H.G. Fructose-bisphosphate aldolases: An evolutionary history. Trends Biochem. Sci. 1992;17:110–113. doi: 10.1016/0968-0004(92)90247-7. [DOI] [PubMed] [Google Scholar]
- 3.Berg I., Kockelkorn D., Ramos-Vera W.H., Say R.F., Zarzycki J., Hügler M., Alber B.E., Fuchs G. Autotrophic carbon fixation in archaea. Nat. Rev. Genet. 2010;8:447–460. doi: 10.1038/nrmicro2365. [DOI] [PubMed] [Google Scholar]
- 4.Anderson L.E., Ringenberg M.R., Brown V.K., Carol A.A. Both chloroplastic and cytosolic phosphofructoaldolase isozymes are present in the pea leaf nucleus. Protoplasma. 2005;225:235–242. doi: 10.1007/s00709-005-0099-1. [DOI] [PubMed] [Google Scholar]
- 5.Rutter W.J. Evolution of aldolase. Fed. Proc. 1964;23:1248–1257. [PubMed] [Google Scholar]
- 6.Murad A.M., Molinari H.B.C., Magalhães B.S., Franco A.C., Takahashi F.S.C., De Oliveira N.G., Franco O.L., Quirino B.F. Physiological and Proteomic Analyses of Saccharum spp. Grown under Salt Stress. PLoS ONE. 2014;9:e98463. doi: 10.1371/journal.pone.0098463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zeng Y., Tan X., Zhang L., Jiang N., Cao H. Identifification and expression of fructose-1,6-bisphosphate aldolase genes and their relations to oil content in developing seeds of tea oil tree (Camellia oleifera) PLoS ONE. 2014;9:e107422. doi: 10.1371/journal.pone.0107422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mutuku J.M., Nose A. Changes in the Contents of Metabolites and Enzyme Activities in Rice Plants Responding to Rhizoctonia solani Kuhn Infection: Activation of Glycolysis and Connection to Phenylpropanoid Pathway. Plant Cell Physiol. 2012;53:1017–1032. doi: 10.1093/pcp/pcs047. [DOI] [PubMed] [Google Scholar]
- 9.Lebherz H.G., Leadbetter M.M., Bradshaw R.A. Isolation and characterization of the cytosolic and chloroplast forms of spinach leaf fructose diphosphate aldolase. J. Biol. Chem. 1984;259:1011–1017. doi: 10.1016/S0021-9258(17)43558-7. [DOI] [PubMed] [Google Scholar]
- 10.Lu W., Tang X., Huo Y., Xu R., Qi S., Huang J., Zheng C., Wu C. Identification and characterization of fructose 1,6-bisphosphate aldolase genes in Arabidopsis reveal a gene family with diverse responses to abiotic stresses. Gene. 2012;503:65–74. doi: 10.1016/j.gene.2012.04.042. [DOI] [PubMed] [Google Scholar]
- 11.Flechner A., Gross W., Martin W.F., Schnarrenberger C. Chloroplast class I and class II aldolases are bifunctional for fructose-1,6-biphosphate and sedoheptulose-1,7-biphosphate cleavage in the Calvin cycle. FEBS Lett. 1999;447:200–202. doi: 10.1016/S0014-5793(99)00285-9. [DOI] [PubMed] [Google Scholar]
- 12.Gross W., Lenze D., Nowitzki U., Weiske J., Schnarrenberger C. Characterization, cloning, and evolutionary history of the chloroplast and cytosolic class I aldolases of the red alga Galdieria sulphuraria. Gene. 1999;230:7–14. doi: 10.1016/S0378-1119(99)00059-1. [DOI] [PubMed] [Google Scholar]
- 13.Lv G.-Y., Guo X.-G., Xie L.-P., Xie C.-G., Zhang X.-H., Yang Y., Xiao L., Tang Y.-Y., Pan X.-L., Guo A.-G., et al. Molecular characterization, gene evolution, and expression analysis of the fructose-1, 6-bisphosphate aldolase (FBA) gene family in wheat (Triticum aestivum L.) Front. Plant Sci. 2017;8:1030. doi: 10.3389/fpls.2017.01030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Konishi H., Yamane H., Maeshima M., Komatsu S. Characterization of fructose-bisphosphate aldolase regulated by gibberellin in roots of rice seedling. Plant Mol. Biol. 2004;56:839–848. doi: 10.1007/s11103-004-5920-2. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y. Master’s Thesis. Huazhong Agricultural University; Wuhan, China: 2014. Functional Analysis of a Fructose-1,6-Diphosphatase Aldolase Gene ALDY in Rice; pp. 35–37. [Google Scholar]
- 16.Kellay P.M., Freeling M. Anaerobic expression of maize glucose phosphate isomerase I. J. Biol. Chem. 1984;259:673–677. doi: 10.1016/S0021-9258(17)43714-8. [DOI] [PubMed] [Google Scholar]
- 17.Russell D.A., Wong D.M.-L., Sachs M.M. The Anaerobic Response of Soybean. Plant Physiol. 1990;92:401–407. doi: 10.1104/pp.92.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haake V., Zrenner R., Sonnewald U., Stitt M. A moderate decrease of plastid aldolase activity inhibits photosynthesis, alters the levels of sugars and starch, and inhibits growth of potato plants. Plant J. 1998;14:147–157. doi: 10.1046/j.1365-313X.1998.00089.x. [DOI] [PubMed] [Google Scholar]
- 19.Michelis R., Gepstein S. Identification and characterization of a heat-induced isoform of aldolase in oat chloroplast. Plant Mol. Biol. 2000;44:487–498. doi: 10.1023/A:1026528319769. [DOI] [PubMed] [Google Scholar]
- 20.Yamada S., Komori T., Hashimoto A., Kuwata S., Imaseki H., Kubo T. Differential expression of plastidic aldolase genes in Nicotiana plants under salt stress. Plant Sci. 2000;154:61–69. doi: 10.1016/S0168-9452(00)00188-6. [DOI] [PubMed] [Google Scholar]
- 21.Fan W., Zhang Z., Zhang Y. Cloning and molecular characterization of fructose-1,6-bisphosphate aldolase gene regulated by high-salinity and drought in Sesuvium portulacastrum. Plant Cell Rep. 2009;28:975–984. doi: 10.1007/s00299-009-0702-6. [DOI] [PubMed] [Google Scholar]
- 22.Lao X., Azuma J.-I., Sakamoto M. Two cytosolic aldolases show different expression patterns during shoot elongation in Moso bamboo, Phyllostachys pubescens Mazel. Physiol. Plant. 2013;149:422–431. doi: 10.1111/ppl.12052. [DOI] [PubMed] [Google Scholar]
- 23.Cai B., Li Q., Xu Y.-C., Yang L., Bi H., Ai X. Genome-wide analysis of the fructose 1,6-bisphosphate aldolase (FBA) gene family and functional characterization of FBA7 in tomato. Plant Physiol. Biochem. 2016;108:251–265. doi: 10.1016/j.plaphy.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 24.Cai B., Li Q., Liu F., Bi H., Ai X. Decreasing fructose-1,6-bisphosphate aldolase activity reduces plant growth and tolerance to chilling stress in tomato seedlings. Physiol. Plant. 2018;163:247–258. doi: 10.1111/ppl.12682. [DOI] [PubMed] [Google Scholar]
- 25.Purev M., Kim M.K., Samdan N., Yang D.-C. Isolation of a novel fructose-1,6-bisphosphate aldolase gene from Codonopsis lanceolata and analysis of the response of this gene to abiotic stresses. Mol. Biol. 2008;42:179–186. doi: 10.1134/S0026893308020027. [DOI] [PubMed] [Google Scholar]
- 26.Kamal A.H.M., Cho K., Kim D.-E., Uozumi N., Chung K.-Y., Lee S.Y., Choi J.-S., Cho S.-W., Shin C.-S., Woo S.H. Changes in physiology and protein abundance in salt-stressed wheat chloroplasts. Mol. Biol. Rep. 2012;39:9059–9074. doi: 10.1007/s11033-012-1777-7. [DOI] [PubMed] [Google Scholar]
- 27.Michelet L., Zaffagnini M., Morisse S., Sparla F., Pérez-Pérez M.E., Francia F., Danon A., Marchand C.H., Fermani S., Trost P., et al. Redox regulation of the Calvin-Benson cycle: Something old, something new. Front. Plant Sci. 2013;4:470. doi: 10.3389/fpls.2013.00470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khanna S.M., Taxak P.C., Jain P.K., Saini R., Srinivasan R. Glycolytic enzyme activities and gene expression in Cicer arietinum exposed to water-deficit stress. Appl. Biochem. Biotechnol. 2014;173:2241–2253. doi: 10.1007/s12010-014-1028-6. [DOI] [PubMed] [Google Scholar]
- 29.Payton P., Webb R., Kornyeyev D., Allen R., Holaday A.S. Protecting cotton photosynthesis during moderate chilling at high light intensity by increasing chloroplastic antioxidant enzyme activity. J. Exp. Bot. 2001;52:2345–2354. doi: 10.1093/jexbot/52.365.2345. [DOI] [PubMed] [Google Scholar]
- 30.Yang W., Wang F., Liu L.-N., Sui N. Responses of membranes and the photosynthetic apparatus to salt stress in cyanobacteria. Front. Plant Sci. 2020;11:713. doi: 10.3389/fpls.2020.00713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Portis A.R., Parry M.A.J. Discoveries in Rubisco (Ribulose 1,5-bisphosphate carboxylase/oxygenase): A historical perspective. Photosynth. Res. 2007;94:121–143. doi: 10.1007/s11120-007-9225-6. [DOI] [PubMed] [Google Scholar]
- 32.Quick W.P., Schurr U., Scheibe R., Schulze E.-D., Rodermel S.R., Bogorad L., Stitt M. Decreased ribulose-1,5-bisphosphate carboxylase-oxygenase in transgenic tobacco transformed with “antisense” rbcS. Planta. 1991;183:542–554. doi: 10.1007/BF00194276. [DOI] [PubMed] [Google Scholar]
- 33.Hudson G.S., Evans J., Von Caemmerer S., Arvidsson Y.B.C., Andrews T.J. Reduction of ribulose-1,5-bisphosphate carboxylase/oxygenase content by antisense RNA reduces photosynthesis in transgenic tobacco plants. Plant Physiol. 1992;98:294–302. doi: 10.1104/pp.98.1.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stitt M., Lunn J., Usadel B. Arabidopsis and primary photosynthetic metabolism—More than the icing on the cake. Plant J. 2010;61:1067–1091. doi: 10.1111/j.1365-313X.2010.04142.x. [DOI] [PubMed] [Google Scholar]
- 35.Salesse-Smith C.E., Sharwood R.E., Busch F.A., Stern D.B. Increased Rubisco content in maize mitigates chilling stress and speeds recovery. Plant Biotechnol. J. 2020;18:1409–1420. doi: 10.1111/pbi.13306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shu S., Chen L., Lu W., Sun J., Guo S., Yuan Y., Li J. Effects of exogenous spermidine on photosynthetic capacity and expression of Calvin cycle genes in salt-stressed cucumber seedlings. J. Plant Res. 2014;127:763–773. doi: 10.1007/s10265-014-0653-z. [DOI] [PubMed] [Google Scholar]
- 37.Ozturk Z.N., Talamè V., Deyholos M., Michalowski C.B., Galbraith D.W., Gozukirmizi N., Tuberosa R., Bohnert H.J. Monitoring large-scale changes in transcript abundance in drought- and salt-stressed barley. Plant Mol. Biol. 2002;48:551–573. doi: 10.1023/A:1014875215580. [DOI] [PubMed] [Google Scholar]
- 38.Xue G.-P., McIntyre C.L., Glassop D., Shorter R. Use of expression analysis to dissect alterations in carbohydrate metabolism in wheat leaves during drought stress. Plant Mol. Biol. 2008;67:197–214. doi: 10.1007/s11103-008-9311-y. [DOI] [PubMed] [Google Scholar]
- 39.Moon S.-J., Shin D.-J., Kim B.-G., Byun M.-O. Putative fructose-1,6-bisphosphate aldolase 1 (AtFBA1) affects stress tolerance in yeast and Arabidopsis. J. Plant Biotechnol. 2012;39:106–113. doi: 10.5010/JPB.2012.39.2.106. [DOI] [Google Scholar]
- 40.Uematsu K., Suzuki N., Iwamae T., Inui M., Yukawa H. Increased fructose 1,6-bisphosphate aldolase in plastids enhances growth and photosynthesis of tobacco plants. J. Exp. Bot. 2012;63:3001–3009. doi: 10.1093/jxb/ers004. [DOI] [PubMed] [Google Scholar]
- 41.Bi H., Wang M., Dong X., Ai X. Cloning and expression analysis of transketolase gene in Cucumis sativus L. Plant Physiol. Biochem. 2013;70:512–521. doi: 10.1016/j.plaphy.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 42.Zeng Y.L. Ph.D. Thesis. Central South University of Forestry & Technology; Changsha, China: 2013. Analysis of Glycolysis Way and Study on the Function of Aldolase Family Genes in Camellia oleifera Seed; pp. 50–51. [Google Scholar]
- 43.Hussain H.A., Hussain S., Khaliq A., Ashraf U., Anjum S.A., Men S., Wang L. Chilling and drought stresses in crop plants: Implications, cross talk, and potential management opportunities. Front. Plant Sci. 2018;9:393. doi: 10.3389/fpls.2018.00393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hussain H.A., Men S., Hussain S., Zhang Q., Ashraf U., Anjum S.A., Ali I., Wang L. Maize tolerance against drought and chilling stresses varied with root morphology and antioxidative defense system. Plants. 2020;9:720. doi: 10.3390/plants9060720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bi H.G., Wang M.L., Jiang Z.S., Dong X.B., Ai X.Z. Impacts of suboptimal temperature and low light intensity on the activities and gene expression of photosynthetic enzymes in cucumber seedling leaves. Ying Yong Sheng Tai Xue Bao/J. Appl. Ecol. 2011;22:2894–2900. (In Chinese) [PubMed] [Google Scholar]
- 46.Friesen P.C., Sage R.F. Photosynthetic responses to chilling in a chilling-tolerant and chilling-sensitive Miscanthus hybrid. Plant Cell Environ. 2016;39:1420–1431. doi: 10.1111/pce.12699. [DOI] [PubMed] [Google Scholar]
- 47.Sun S.N., Wang Q., Sun C.C., Liu F.J., Bi H.G., Ai X.Z. Response and adaptation of photosynthesis of cucumber seedlings to high temperature stress. Ying Yong Sheng Tai Xue Bao/J. Appl. Ecol. 2017;28:1603–1610. doi: 10.13287/j.1001-9332.201705.009. [DOI] [PubMed] [Google Scholar]
- 48.Xu X.Y., Yu J.H., Xie J.M., Hu L.L., Li J. Effects of exogenous salicylic acid and brassinolide on photosynthesis of cucumber seedlings under low temperature stress. Ying Yong Sheng Tai Xue Bao/J. Appl. Ecol. 2016;27:3009–3015. doi: 10.13287/j.1001-9332.201609.020. [DOI] [PubMed] [Google Scholar]
- 49.Xu X.Y., Yu J.H., Xie J.M., Hu L.L. Effects of 2,4-epibrassinolide on photosynthesis characteristics and antioxidant system of cucumber seedlings under sub-optimal temperature and low light. J. Nucl. Agric. Sci. 2017;31:979–986. [Google Scholar]
- 50.Ding F., Wang M., Zhang S., Ai X. Changes in SBPase activity influence photosynthetic capacity, growth, and tolerance to chilling stress in transgenic tomato plants. Sci. Rep. 2016;6:32741. doi: 10.1038/srep32741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ding F., Wang M., Zhang S. Overexpression of a Calvin cycle enzyme SBPase improves tolerance to chilling-induced oxidative stress in tomato plants. Sci. Hortic. 2017;214:27–33. doi: 10.1016/j.scienta.2016.11.010. [DOI] [Google Scholar]
- 52.Bi H., Liu P., Jiang Z., Ai X. Overexpression of the rubisco activase gene improves growth and low temperature and weak light tolerance in Cucumis sativus L. Physiol. Plant. 2017;161:224–234. doi: 10.1111/ppl.12587. [DOI] [PubMed] [Google Scholar]
- 53.Tsikas D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017;524:13–30. doi: 10.1016/j.ab.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 54.Fillatti J.J., Kiser J., Rose R., Comai L. Efficient transfer of a glyphosate tolerance gene into tomato using a binary Agrobacterium tumefaciens vector. Nat. Biotechnol. 1987;5:726–730. doi: 10.1038/nbt0787-726. [DOI] [Google Scholar]
- 55.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 56.Wang M., Bi H., Liu P., Ai X. Molecular cloning and expression analysis of the gene encoding sedoheptulose-1, 7-bisphosphatase from Cucumis sativus L. Sci. Hortic. 2011;129:414–420. doi: 10.1016/j.scienta.2011.04.010. [DOI] [Google Scholar]
- 57.Shigeoka S., Takeda T., Hanaoka T. Characterization and immunological properties of selenium-containing glutathione peroxidase induced by selenite in Chlamydomonas reinhardtii. Biochem. J. 1991;275:623–627. doi: 10.1042/bj2750623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bi H., Dong X., Wu G., Wang M., Ai X. Decreased TK activity alters growth, yield and tolerance to low temperature and low light intensity in transgenic cucumber plants. Plant Cell Rep. 2015;34:345–354. doi: 10.1007/s00299-014-1713-5. [DOI] [PubMed] [Google Scholar]
- 59.Cho U.-H., Park J.-O. Mercury-induced oxidative stress in tomato seedlings. Plant Sci. 2000;156:1–9. doi: 10.1016/S0168-9452(00)00227-2. [DOI] [PubMed] [Google Scholar]
- 60.Heath R.L., Packer L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968;125:189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is contained within the article or Supplementary Materials.