Abstract
Background:
Perennial aeroallergen sensitization is associated with greater asthma morbidity and is required for treatment with omalizumab.
Objective:
To investigate the predictive relationship between the number of aeroallergen sensitizations, total serum IgE, and serum eosinophil count, and response to omalizumab in children and adolescents with asthma treated during the fall season.
Methods:
This analysis includes inner-city patients with persistent asthma and recent exacerbations aged 6-20 years comprising the placebo and omalizumab-treated groups in two completed randomized clinical trials, the Inner-City Anti-IgE Therapy for Asthma (ICATA) study and the Preventative Omalizumab or Step-Up Therapy for Fall Exacerbations (PROSE) study. Logistic regression modeled the relationship between greater degrees of markers of allergic inflammation and the primary outcome of fall season asthma exacerbations.
Results:
The analysis included 761 participants who were 62% male and 59% African American with a median age of 10 years. Fall asthma exacerbations were significantly higher in children with greater numbers of aeroallergen-specific sensitizations in the placebo group (OR 1.33, 95% CI 1.11–1.60, p<0.01), but not in the omalizumab-treated children (OR 1.08, 95% CI 0.91–1.28, p=0.37) indicating a significant differential effect (p<0.01). Likewise, there was a differential effect of omalizumab treatment in children with greater baseline total serum IgE levels (p<0.01) or greater baseline serum eosinophil counts (p<0.01). Multiple aeroallergen sensitization was the best predictor of response to omalizumab; treated participants sensitized to ≥4 different groups of aeroallergens had a 51% reduction in the odds of a fall exacerbation (OR 0.49, 95% CI 0.30–0.81, p<0.01).
Conclusions:
In preventing fall season asthma exacerbations, treatment with omalizumab was most beneficial in children with a greater degree of allergic inflammation.
Keywords: Asthma, Omalizumab, IgE, Aeroallergen Sensitization, Allergic Inflammation
INTRODUCTION
Asthma is the most common chronic disease in childhood with prevalence and morbidity particularly high in urban areas. In recent years, there have been significant advances in targeted therapies for pediatric asthma including the use of biologic medications.(2) One such medication is omalizumab, a monoclonal antibody that binds to circulating IgE and thereby prevents IgE binding to effector cells.(3) Omalizumab is an effective treatment for persistent allergic asthma in both adult and pediatric populations.(4-6) For example, in a year-long study of inner-city children with persistent allergic asthma, the addition of omalizumab decreased exacerbations and daily asthma symptoms.(5) In particular, this study demonstrated treatment with omalizumab markedly reduced the spike of asthma exacerbations during the fall season when rhinovirus infections are highly prevalent and children return to school.(5) These results prompted a follow-up study in which short-term omalizumab treatment was given during the fall season, starting a few weeks prior to the first day of school and continuing for the first 90 days of each participant’s school year. This strategy reduced asthma exacerbations when the children returned to school.(7)
The success of omalizumab treatment during the fall season raises the question of which children should be prioritized for such a treatment. It has been noted that patients with recent or frequent asthma exacerbations have greater efficacy with omalizumab.(7, 8) Assessment of biomarkers to predict success on omalizumab therapy have ended with conflicting results; for example, aeroallergen sensitization and serum IgE have been associated with improved outcomes on omalizumab in some, but not all, studies.(9-13) Although sensitization to at least one perennial aeroallergen is a requirement for treatment with omalizumab in uncontrolled asthma, it is uncertain if children will respond differently if they have a greater total number of sensitizations, total serum IgE, or serum eosinophils. This analysis investigated if omalizumab treatment is more effective in preventing exacerbations in children with greater degrees of these three variables which are all representative of allergic inflammation.
METHODS
Overview
This retrospective analysis with a prespecified study design examines aeroallergen sensitization profiles, serum IgE, and serum eosinophils in children and young adults treated by asthma specialists with either omalizumab or placebo in addition to ongoing guidelines-based asthma care (Expert Panel Report-3 [EPR3]).(14) Participant data were included from two studies from the Inner-City Asthma Consortium: (a) the Inner-City Anti-IgE Therapy for Asthma (ICATA) study(5) and (b) the Preventative Omalizumab or Step-Up Therapy for Fall Exacerbations (PROSE) study.(7) In the ICATA study, treatment with omalizumab or placebo lasted 60 weeks. In the PROSE study, treatment with omalizumab or placebo began 4 to 6 weeks before each participant’s school start date and continued for the first 90 days of school. For this analysis, outcomes during the fall season (September to November) from the ICATA study were combined with outcomes from the fall-based PROSE study. For inclusion in either study, the serum IgE level at baseline must have been in the range of 30 to 1,300 IU/mL. Baseline predictors including aeroallergen sensitization testing, serum IgE levels, and serum eosinophil counts were determined prior to randomization. Both studies enrolled predominantly minority participants with persistent asthma from urban neighborhoods. The protocols for each study were approved by each institution’s institutional review board.
ICATA population and study design
The ICATA study was a randomized, double-blind, placebo-controlled, parallel-group, multicenter trial that compared omalizumab with placebo added to guideline-based therapy in inner-city children and young adults (aged 6-20 years) with persistent asthma and at least one positive skin test for a perennial allergen.(5) At initial screening visits, each participant was assessed for asthma symptoms, previous asthma treatment, pulmonary function, allergen sensitivity, and blood testing including IgE levels and absolute eosinophil counts. Asthma medications covered by the participants’ insurance plans were prescribed but were not supplied, with the exception of omalizumab or placebo study injections and oral prednisone for exacerbations.
PROSE population and study design
The PROSE study was a 3-arm, randomized, double-blind, double placebo-controlled, multicenter clinical trial conducted among participants receiving ongoing guidelines-based asthma care in inner-city children (aged 6-17 years) with persistent uncontrolled asthma, at least one asthma exacerbation since the beginning of the previous school year, and at least one positive skin test for a perennial allergen.(7) Similar to the ICATA study, at screening visits, each participant was assessed for asthma symptoms, previous asthma treatment, pulmonary function, allergen sensitivities, and serum IgE levels. Similar to the ICATA study, asthma medications covered by the participants’ insurance plans were prescribed but were not supplied, with the exception of omalizumab or placebo study injections and oral prednisone for exacerbations. The PROSE study design differed from that of ICATA in including a third arm in which inhaled corticosteroids were increased during the fall season. Participants from that third arm were not included in this current analysis which focused on the comparison between omalizumab and placebo when added to standard guidelines-based therapy.
Omalizumab therapy
In both studies, after a run-in period, each participant was randomized to receive subcutaneous injections of omalizumab or placebo every 2 or 4 weeks for the duration of the study period. The injection dose of omalizumab (75 to 375 mg) was calculated based on each individual’s weight and total serum IgE level to ensure a minimum monthly dose of 0.016 mg per kilogram of body weight per international unit of IgE per milliliter. Placebo injections were the same composition as the active study drug injection, but without the omalizumab, and were administered in the same volume and with the same frequency as the omalizumab injections. Injections were administered by unblinded nurses who had no other role in the trials. All other study procedures and assessments were performed by study staff blinded to the randomization assignments.
Definition of aeroallergen sensitization
Aeroallergen sensitization testing before randomization in both studies included skin prick testing of up to 14 aeroallergen extracts (Greer Laboratories, Lenoir, NC), total serum IgE level, and serum allergen-specific IgE measurements. A positive skin test was defined as a wheal that was larger than the negative control by 3 mm. A positive serum allergen-specific IgE was defined as a value ≥ 0.35 kU/L. A positive sensitization to an aeroallergen was defined by either a positive skin test or a positive serum test for that specific aeroallergen.
Sensitization to aeroallergen testing was then classified by the following seven groupings, which combined results for tests that were biologically similar: (1) cat: positive skin or serum testing for cat hair, (2) dog: positive skin or serum testing for dog epithelium, (3) cockroach: positive skin or serum testing for either American cockroach or German cockroach, (4) dust mite: positive skin or serum testing for either Dermatophagoides farina or D. pteronyssiunus, (5) rodent: positive skin or serum testing for either mouse or rat epithelia, (6) mold: positive skin or serum testing for either Alternaria tenuis or aspergillus species, and (7) ragweed: positive skin or serum testing for ragweed mix. For the purpose of this fall-based analysis, pollens from other seasons were not investigated. The total number of sensitizations was calculated for each individual ranging from a minimum of 1 to a maximum of 7. By the main studies’ inclusion criteria, a participant must have been sensitized to at least one perennial allergen.
Outcome measure
For this retrospective analysis, the prespecified primary outcome was an asthma exacerbation during the fall season. An exacerbation was defined by a worsening of asthma control requiring systemic corticosteroids or hospitalization.(15) The fall-based PROSE trial only had outcomes during this season defined by the 90-day period beginning at the start of each participant’s school year. For the yearlong ICATA trial, outcome data from the fall season (September to November) was extracted and analyzed to ensure the time period for both studies matched.
Statistical analysis
To compare baseline characteristics between treatment groups, a chi-square test was used for categorical variables, and t-tests or Wilcoxon tests were used for normally distributed and non-normally distributed continuous variables, respectively. Total IgE and eosinophil counts were skewed, therefore log base 10 and square root transformations, respectively, were applied for all statistical models. All models were adjusted for study site. Logistic models for the odds of fall exacerbations were fitted to number of sensitizations, total serum IgE, and total serum eosinophil count ( all continuous variables). For logistic models, we report the adjusted probabilities of an exacerbation and/or the odds ratio and 95% confidence interval (CIs). In our analysis using participants from two ICAC Asthma studies (ICATA and PROSE), we sought to detect subgroups of participants in which the subgroups differ in the effect of treatment with omalizumab.
Subgroup analyses were explored by means of model-based recursive partitioning.(16) This approach incorporates recursive partitioning into the logistic model with adjustments. As a first step, a logistic model is fitted to the whole dataset. Then, parameter instability is tested over all potential splitting variables, and the parent node is split by a variable at a specific cutoff point which results in the highest parameter instability. The highest parameter instability is searched to ensure the daughter nodes have the largest difference in model parameters. In our situation, the difference in parameter is equivalent to the difference in treatment effect size. The palmtree15 procedure was used to fit these models using R version 3.6.0. Because of the exploratory nature of this analysis, we did not adjust for multiple comparisons.
RESULTS
Participants’ characteristics
A total of 761 participants from the ICATA and PROSE studies were included in this analysis consisting of 465 participants treated with omalizumab and 296 participants on placebo treatment. The baseline demographics of the cohort are presented in Table I. Ages ranged from 6 to 20 years (median age of 10 years), and 62% were male. Participants were commonly from families with low income (55% with family income <$15,000 per year), minority race or ethnicity (59% African American and 36% Hispanic), and overweight (median body mass index [BMI] percentile of 86%). The mean FEV1 was 91% of predicted and the mean FEV1 to FVC ratio was 0.77.
Table I:
Baseline Characteristics by Treatment Group (pooled studies)
| All (N = 761) |
Omalizumab (N = 465) |
Placebo (N = 296) |
p-value | |
|---|---|---|---|---|
| Study 1 | ||||
| ICATA – no. (%) | 413 (54.3%) | 206 (44.3%) | 207 (69.9%) | <0.01 |
| PROSE – no. (%) | 348 (45.7%) | 259 (55.7%) | 89 (30.1%) | |
| Demographic | ||||
| Age – yr. – median [25th%; 75th%] | 10.0 [8.0; 13.0] | 10.0 [8.0; 13.0] | 10.0 [8.0; 13.0] | 0.49 |
| Male sex – no. (%) | 472 (62.0%) | 294 (63.2%) | 178 (60.1%) | 0.44 |
| Race or ethnic group – no. (%): | ||||
| African American | 449 (59.0%) | 275 (59.1%) | 174 (58.8%) | 0.12 |
| Hispanic | 271 (35.6%) | 159 (34.2%) | 112 (37.8%) | |
| Other or missing | 41 (5.39%) | 31 (6.67%) | 10 (3.38%) | |
| Caretaker completed high school – no. (%) | 540 (72.0%) | 329 (71.8%) | 211 (72.3%) | 0.97 |
| Household income < $15,000 – no. (%) | 416 (55.2%) | 254 (55.2%) | 162 (55.1%) | 1.00 |
| ≥1 household member employed – no. (%) | 533 (70.0%) | 313 (67.3%) | 220 (74.3%) | 0.05 |
| BMI Percentile – median [25th%; 75th%] | 85.7 [59.8; 96.4] | 85.8 [61.8; 96.2] | 85.3 [58.1; 96.6] | 0.80 |
| Clinical | ||||
| Maximum symptom days (in prior 2 weeks) mean ± SD | 2.78 ±3.39 | 2.71 ±3.38 | 2.90 ±3.41 | 0.47 |
| ACT / Childhood ACT no. (%) | ||||
| Well controlled | 535 (70.3%) | 332 (71.4%) | 203 (68.6%) | 0.42 |
| Not well controlled or poorly controlled | 226 (29.7%) | 133 (28.6%) | 93 (31.4%) | |
| FEV1 (% predicted) – mean ± SD | 90.8 ±17.7 | 90.5 ±17.1 | 91.3 ±18.8 | 0.55 |
| FEV1/FVC (x100) – mean ± SD | 77.2 ±9.81 | 77.2 ±9.75 | 77.3 ±9.93 | 0.93 |
| Total IgE (kU/L) – median [25th%; 75th%] | 242 [123; 436] | 243 [130; 450] | 242 [118; 415] | 0.27 |
| Eosinophils Count (cells/mcL) – median [25th%, 75th%] | 280 [170; 450] | 290 [180; 450] | 270 [160; 430] | 0.30 |
| Treatment step level2 | 3.88 (1.41) | 3.96 (1.36) | 3.76 (1.49) | 0.06 |
| Sensitization to Aeroallergens3 – no. (%) | ||||
| Cat | 438 (57.7%) | 260 (56.2%) | 178 (60.1%) | 0.31 |
| Dog | 369 (48.6%) | 229 (49.5%) | 140 (47.3%) | 0.61 |
| Rodent (mouse or rat) | 343 (45.4%) | 212 (46.1%) | 131 (44.3%) | 0.68 |
| Dust mite (Der f or Der p) | 511 (67.4%) | 307 (66.5%) | 204 (68.9%) | 0.53 |
| Cockroach | 494 (65.4%) | 292 (63.3%) | 202 (68.7%) | 0.15 |
| Mold (Alternaria or Aspergillus) | 468 (62.0%) | 272 (59.1%) | 196 (66.4%) | 0.05 |
| Ragweed | 319 (42.3%) | 202 (44.1%) | 117 (39.5%) | 0.24 |
| Number of sensitizations (out of 7)4 mean ± SD | 3.88 ±1.69 | 3.84 ±1.71 | 3.95 ±1.65 | 0.37 |
Randomization in ICATA was 1:1 omalizumab to placebo. Randomization in PROSE was 3:1 omalizumab to placebo
Based on NHLBI NAEPP EPR-3 Guidelines
Sensitization = positive skin test or specific IgE ≥ 0.35 kU/L
Sum of: cat, dog, rodent, dust mite, cockroach, mold, ragweed
Participants’ sensitization profiles and IgE levels at baseline
Per protocols, all of the participants were sensitized to at least one aeroallergen. Sensitization to dust mite (67.4%), cockroach (65.4%), and mold (62.0%) were most common (Table I). A total of 9.4% of participants were sensitized to only 1 group of aeroallergens and 6.6% were sensitized to all 7 groups of aeroallergens with a median number of 4 group sensitizations (Figure 1A). Total serum IgE ranged from 30.1 IU/mL to 1286 IU/mL with a median of 242 IU/mL (Figure 1B). Total serum eosinophil count ranged from 0 cells/mcL to 1900 cells/mcL with a median of 280 cells/mcL (Figure 1C).
Figure 1A:
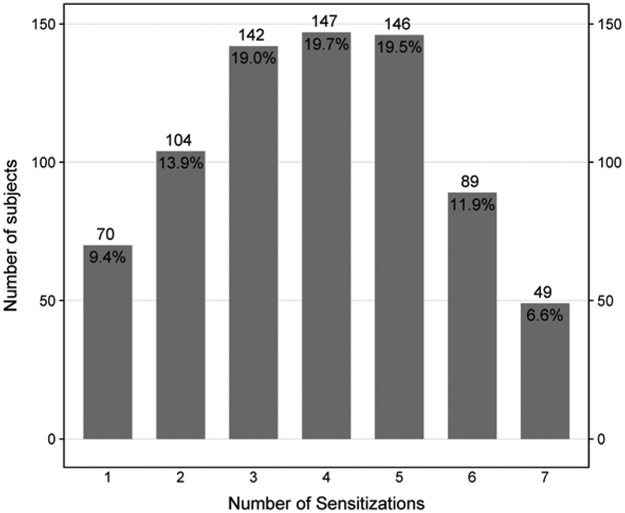
Distribution of Number of Sensitizations to Specified Aeroallergen Groupings
Figure 1B:
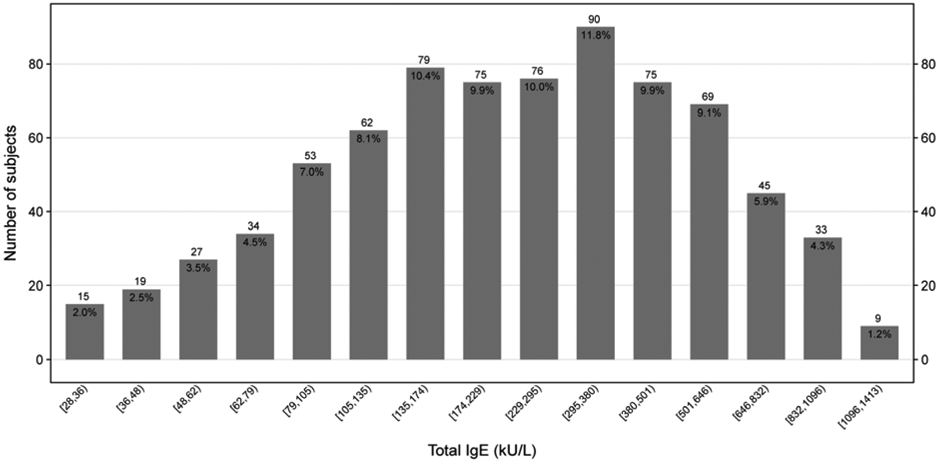
Distribution of Total IgE
Figure 1C:
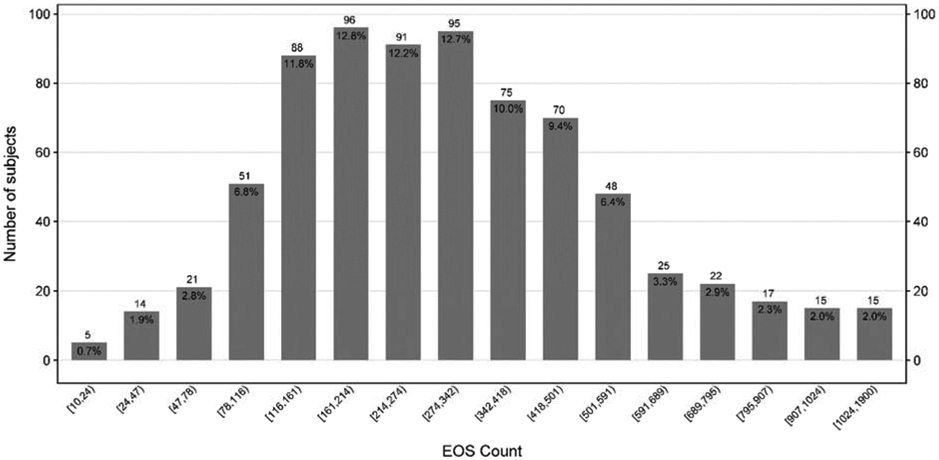
Distribution of Total Eosinophils Count
Bar chart displaying the total number of subjects for each aeroallergen sensitization category (A), Total IgE category (B) and Eosinophils count (C), with n and (%) annotated above and below each bar respectively. Number of sensitizations is defined as the sum of a positive skin test OR a positive specific IgE to the following aeroallergen groupings: cat, dog, rodent, dust mite, cockroach, mold, and ragweed. The numbers below the columns indicate the range of IgE levels and Eosinophils count for participants within that column.
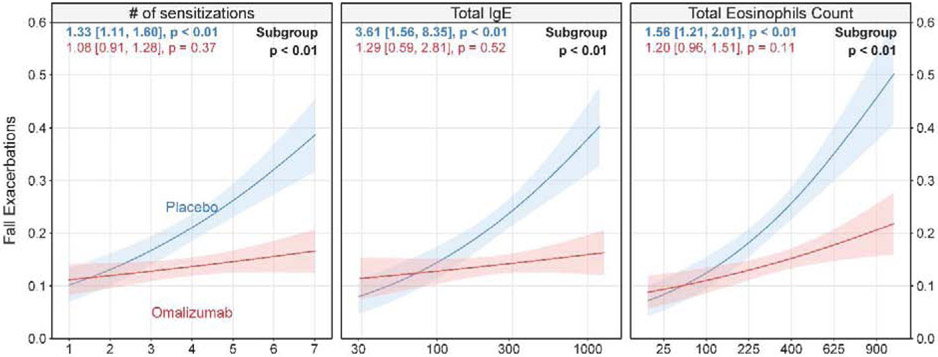
Fall season asthma exacerbations based on sensitization status, IgE level, eosinophil count, and treatment group
The left panel of Figure 2 shows exacerbation rates based on the total number of grouped sensitizations by treatment arm. In the combined analyses, fall asthma exacerbations were significantly higher in children with greater numbers of aeroallergen-specific sensitizations in the placebo group (OR 1.33, 95% CI 1.11–1.60, p<0.01), but not in the omalizumab-treated children (OR 1.08, 95% CI 0.91–1.28, p=0.37), indicating a significant differential effect (p<0.01). This trend was also seen in our raw data on exacerbation rates as presented in Supplemental Table EI. Additionally, significant findings were demonstrated when sensitization was analyzed using all available tested allergens, including non-fall season aeroallergens, and when the allergens were not defined by groupings as seen in Supplemental Figure E1. Furthermore, these findings were consistent when sensitization was defined solely by skin testing results (p=0.02) or solely by blood testing results (p=0.01). In addition, we examined the relationship between the ratio of specific IgE to total IgE and exacerbation rate in the placebo group; however, there was not a significant relationship.
Figure 2:
Relationship between number of aeroallergen sensitizations, total serum IgE and serum eosinophil count with fall exacerbations
Probability of fall exacerbation vs. number of aeroallergen sensitizations (left panel), total IgE (middle panel) and Total Eosinophils Count (right panel) by treatment group. Pink and blue lines represent omalizumab and placebo-treated subjects, respectively. Lines and associated confidence bands (+/− 1 standard error) are least squares estimates from logistic regression models. Colored numbers are odds ratio [95% confidence interval] for a unit, log-unit and five square root unit increase in each variable respectively, followed by the associated p-value within treatment group. Subgroup p-value < 0.10 indicates significantly different subgroups. Results represent pooled ICATA and PROSE participants.
The middle panel of Figure 2 shows exacerbation rates based on serum IgE level by treatment arm. In the combined analyses, fall asthma exacerbations were significantly higher in children with greater total serum IgE levels in the placebo group (OR 3.61, 95% CI 1.56-8.35, p<0.01), but not among participants treated with omalizumab (OR 1.29, 95% CI 0.59–2.81, p=0.52; differential effect p<0.01). In agreement with the serum IgE findings, the right panel of Figure 2 shows a differential effect (p<0.01) for children based on greater serum eosinophil counts. These patterns for all three predictors were not only seen in combined analyses, but also in analyses focused on each study individually as seen in Supplementary Figure 2.
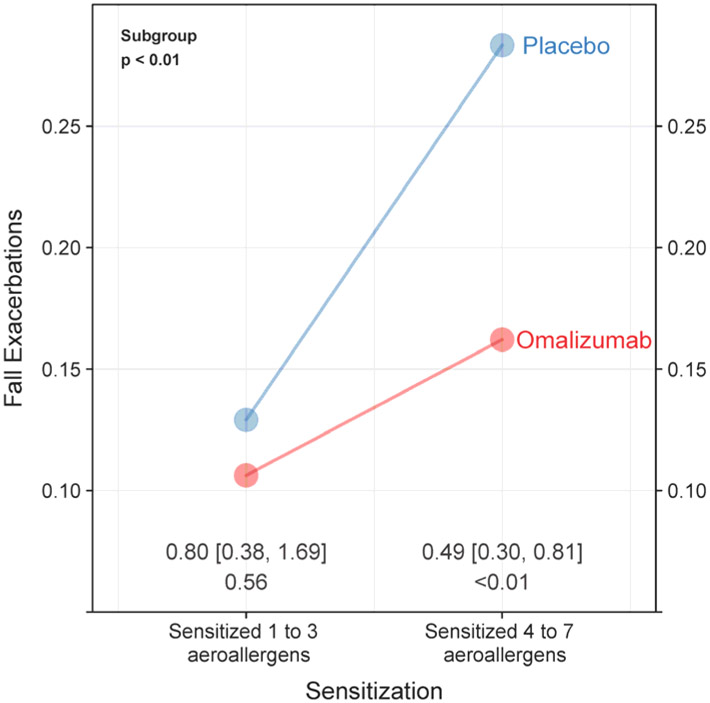
Number of sensitizations as the optimal predictor of response to omalizumab
Recursive partitioning was then used to investigate which of these three biomarkers was the preferred predictor of a successful differential response to omaliuzumab therapy. It was determined that the number of sensitizations was the best predictor (p<0.01) as seen in Figure 3. Among children who were sensitized to at least 4 of the defined sensitization groups, those treated with omalizumab had a 51% reduction in the odds of fall asthma exacerbations (OR 0.49, 95% CI 0.30–0.81, p<0.01) compared to controls. In contrast, there was not a significant differential response to omalizumab in the children sensitized to less than 4 of the defined sensitization groups (OR 0.80, 95% CI 0.38–1.69, p=0.41). Total serum IgE and eosinophils did not emerge as significant predictors in the final model once number of sensitizations was accounted for.
Figure 3:
Effect of treatment on fall exacerbations rates within key sensitization subgroups based on results of recursive partitioning
Probability of a fall exacerbation by treatment group within key subgroups. Subgroups are based on level of aeroallergen sensitization, total IgE and total eosinophils count. The points are least squares estimates from the site adjusted logistic models. Odds ratio, confidence interval, and associated p-value comparing omalizumab vs. placebo are annotated. Subgroup p-value <0.10 indicates significantly differential effect between 1 to 3 and ≥ 4 aeroallergen sensitizations.
DISCUSSION
This analysis of inner-city children demonstrates that, a greater number of aeroallergen sensitizations, a greater total serum IgE level, and a greater total serum eosinophil count were all associated with differential omalizumab treatment responses. This highlights the greater beneficial effect of omalizumab in children with increased levels of common markers of allergic inflammation. These results suggest that omalizumab therapy is more likely to reduce fall season asthma exacerbations in children with greater degrees of atopy. These findings may influence providers’ decision-making when considering omalizumab for a specific patient.
Omalizumab has been shown to reduce exacerbations and prevent hospitalizations.(6) In an era of precision medicine,(17) the question arises as to which patients with persistent allergic asthma will benefit the most from treatment with omalizumab. This question is even more important given the high expense of biologic medications, such as omalizumab.(18) Currently, omalizumab is FDA approved for usage in moderate to severe persistent allergic asthma with at least one aeroallergen sensitization and serum IgE levels between 30 and 700 IU/mL for patients greater than 12 years of age or up to 1300 IU/mL for patients between the ages of 6-11 years. Our current analysis evaluated the total number of aeroallergen sensitizations, total serum IgE level, and serum eosinophil count as possible predictors of differential response to omalizumab. Not unexpectedly, we found a statistically significant positive association between these three biomarkers and the rate of fall asthma exacerbations within the control participants. This association in control children was demonstrated in a previous study(19) and indicates that, in the absence of omalizumab therapy, children with a higher degree of allergic inflammation have a greater number of fall asthma exacerbations.
The important new finding of our current analysis stems from the comparison between control participants and participants treated with omalizumab. In the omalizumab-treated group, the relationships between aeroallergen sensitization, total IgE, and eosinophil counts and fall asthma exacerbations were blunted as evidenced by the relatively flat lines for the omalizumab-treated groups in Figure 2. These findings indicate that omalizumab treatment may be more successful and beneficial in children with greater levels of these biomarkers. The most effective approach, from a cost and health perspective, may be to direct therapy to these children.
The results of our recursive partitioning analysis demonstrate that children who are sensitized to four or more of the pre-defined aeroallergen groups had a significantly greater response to omalizumab, as evaluated by reductions in exacerbations. In the children with less than four aeroallergen group sensitizations, omalizumab did not significantly reduce fall exacerbations. While our analysis considered both aeroallergen sensitization profiles and total IgE levels as possible predictors, the number of sensitizations was a more significant predictor suggesting that this may be the preferred biomarker for precision medicine and treatment response.
Bousquet et al. investigated possible predictors of omalizumab response in adolescent and adult patients with severe persistent asthma.(9) The group used pooled data from multiple studies and found baseline serum IgE was a predictor of omalizumab success with lower baseline IgE being associated with smaller treatment benefits; however, the benefits of omalizumab were seen across a range of IgE levels.(9) This same study did not find the number of positive allergens to be a predictor of omalizumab success.(9) Sorkness et al. reported omalizumab efficacy was predicted by high exhaled nitric oxide, blood eosinophils, and body mass index.(13) In that study, total serum IgE was not a predictor of differential rates of exacerbations; however, serum IgE was evaluated as a dichotomous variable (less than or greater than 700 IU/mL) and not a continuous variable as in our current analysis.(13) Another study found that a greater number of positive allergens was associated with omalizumab success, but only for a quality of life outcome and not for any asthma-specific measures.(8) In contrast, we found the number of positive aeroallergens to be associated with improved exacerbations on omalizumab. Furthermore, the number of aeroallergens was predominant over serum IgE levels in our recursive partitioning analyses. The differences in these results may be due to our focus on inner-city children. Additionally, we evaluated sensitization only for perennial and seasonal allergens that are prevalent in the fall and it is possible that biomarkers predicting success with omalizumab may vary depending on season of the year similar to the way that predictors of asthma exacerbation vary by season.(19) Finally, approved usage of omalizumab includes children, ages 6-11 years, with IgE levels up to 1300 IU/mL while approved usage in adolescents and adults caps at 700 IU/mL. Our analysis included participants with levels up to 1300 IU/mL. While our data cannot make a conclusion on differential responses of children with levels above this threshold, the positive relationship between total or specific IgE and efficacy suggests that children with higher levels of IgE should be included in future studies; however, investigating omalizumab usage in patients with higher levels of IgE will present other hurdles including appropriate higher dosing strategies and more emphasis on body weight as a possible prohibitive factor.
We focused on the fall season given that the effects of omalizumab are most striking during this season(5) when exacerbations are most common.(19) The PROSE study was a fall-only trial.(7) While the ICATA trial was a yearlong trial,(5) only its fall outcome data were included to ensure consistency when combining the PROSE and ICATA data. We chose exacerbations given the significant consequences of this outcome including greater morbidity, increased health care utilization costs, and possibly disease progression.(20, 21)
The strengths of this analysis include that the data were evaluated from two different randomized, double-blind, parallel-group, multicenter trials that compared omalizumab with placebo added to standard guideline-based therapy. The pattern of results was replicated across both studies individually and strengthened by the combination of the two studies. Furthermore, this study focused on children who are known to have higher rates of asthma exacerbations than adults. The majority of previous studies have focused on predictors of omalizumab success in adolescents or adults.
Limitations of the study were the inclusion only of children and adolescents living in inner-city low-income environments. These children have especially high rates of exacerbations, and results could differ in other non-urban populations. Additionally, our analyses were limited by focusing on asthma outcomes during the fall season only and treatment with omalizumab at standard doses only. It is uncertain if there are differential effects if use of omalizumab were to be analyzed in other seasons or at doses lower than or higher than standard regimens. Furthermore, children with moderate or severe eczema were not excluded from our study unless they had had total IgE levels prohibiting the use of omalizumab. It is possible that children with more severe eczema may have differential responses given that they typically have higher levels of total IgE and eosinophils. Finally, this is a post hoc analysis of previous studies and thus these findings need to be confirmed in a prospective trial.
In conclusion, these results indicate that highly allergic children, defined by sensitization to multiple aeroallergens, elevated total serum IgE levels, or greater serum eosinophil counts, are at the highest risk for asthma morbidity and would most benefit from treatment with omalizumab. If these findings are confirmed in other populations, these results could alter prescribing practices in that omalizumab could be prioritized for children with asthma that is highly allergic in nature. Moreover, the correlation between total or specific IgE and efficacy suggests that children with IgE levels in excess of the current upper limit for omalizumab dosing would be excellent treatment candidates and should be included in future studies. These results are another important step towards optimal precision medicine based on patient biomarkers for the treatment of difficult-to-control asthma in an era of biologic therapies.
Supplementary Material
HIGHLIGHTS BOX:
What is already known about this topic? Omalizumab is an effective therapy to reduce the frequency of asthma exacerbations in inner-city children; however, there is a paucity of studies investigating biomarkers to identify which children may benefit the most from this intervention.
What does this article add to our knowledge? In preventing fall season asthma exacerbations, treatment with omalizumab was most beneficial in children and adolescents with a greater degree of allergic inflammation defined by either aeroallergen sensitization status, serum IgE, or serum eosinophils.
How does this study impact current management guidelines? In an era of precision medicine with high costs for biologic therapies, these findings will assist providers’ decision-making when considering omalizumab for a specific patient.
ABBREVIATIONS USED:
- IgE
Immunoglobulin E
- PROSE
Preventative Omalizumab or Step-Up Therapy for Fall Exacerbations study
- ICATA
Inner-City Anti-IgE Therapy for Asthma study
- BMI
Body Mass Index
- OR
Odds Ratio
- CI
Confidence Interval
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Gergen PJ, Togias A. Inner city asthma. Immunol Allergy Clin North Am. 2015;35(1):101–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abrams EM, Becker AB, Szefler SJ. Current State and Future of Biologic Therapies in the Treatment of Asthma in Children. Pediatr Allergy Immunol Pulmonol. 2018;31(3):119–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strunk RC, Bloomberg GR. Omalizumab for asthma. N Engl J Med. 2006;354(25):2689–95. [DOI] [PubMed] [Google Scholar]
- 4.Humbert M, Beasley R, Ayres J, Slavin R, Hebert J, Bousquet J, et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy. 2005;60(3):309–16. [DOI] [PubMed] [Google Scholar]
- 5.Busse WW, Morgan WJ, Gergen PJ, Mitchell HE, Gern JE, Liu AH, et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N Engl J Med. 2011;364(11):1005–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Normansell R, Walker S, Milan SJ, Walters EH, Nair P. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev. 2014(1):CD003559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teach SJ, Gill MA, Togias A, Sorkness CA, Arbes SJ Jr., Calatroni A, et al. Preseasonal treatment with either omalizumab or an inhaled corticosteroid boost to prevent fall asthma exacerbations. J Allergy Clin Immunol. 2015;136(6):1476–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bousquet J, Wenzel S, Holgate S, Lumry W, Freeman P, Fox H. Predicting response to omalizumab, an anti-IgE antibody, in patients with allergic asthma. Chest. 2004;125(4):1378–86. [DOI] [PubMed] [Google Scholar]
- 9.Bousquet J, Rabe K, Humbert M, Chung KF, Berger W, Fox H, et al. Predicting and evaluating response to omalizumab in patients with severe allergic asthma. Respir Med. 2007;101(7):1483–92. [DOI] [PubMed] [Google Scholar]
- 10.Hanania NA, Wenzel S, Rosen K, Hsieh HJ, Mosesova S, Choy DF, et al. Exploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. Am J Respir Crit Care Med. 2013;187(8):804–11. [DOI] [PubMed] [Google Scholar]
- 11.Busse W, Spector S, Rosen K, Wang Y, Alpan O. High eosinophil count: a potential biomarker for assessing successful omalizumab treatment effects. J Allergy Clin Immunol. 2013;132(2):485–6 e11. [DOI] [PubMed] [Google Scholar]
- 12.Casale TB, Chipps BE, Rosen K, Trzaskoma B, Haselkorn T, Omachi TA, et al. Response to omalizumab using patient enrichment criteria from trials of novel biologics in asthma. Allergy. 2018;73(2):490–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sorkness CA, Wildfire JJ, Calatroni A, Mitchell HE, Busse WW, O'Connor GT, et al. Reassessment of omalizumab-dosing strategies and pharmacodynamics in inner-city children and adolescents. J Allergy Clin Immunol Pract. 2013;1(2):163–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Asthma E, Prevention P. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007;120(5 Suppl):S94–138. [DOI] [PubMed] [Google Scholar]
- 15.Reddel HK, Taylor DR, Bateman ED, Boulet LP, Boushey HA, Busse WW, et al. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. 2009;180(1):59–99. [DOI] [PubMed] [Google Scholar]
- 16.Seibold H, Zeileis A, Hothorn T. Model-Based Recursive Partitioning for Subgroup Analyses. Int J Biostat. 2016;12(1):45–63. [DOI] [PubMed] [Google Scholar]
- 17.Slob EM, Maitland-Van der Zee AH, Koppelman GH, Pijnenburg MW. Precision medicine in childhood asthma. Curr Opin Allergy Clin Immunol. 2019;19(2):141–7. [DOI] [PubMed] [Google Scholar]
- 18.Campbell JD, Spackman DE, Sullivan SD. The costs and consequences of omalizumab in uncontrolled asthma from a USA payer perspective. Allergy. 2010;65(9):1141–8. [DOI] [PubMed] [Google Scholar]
- 19.Teach SJ, Gergen PJ, Szefler SJ, Mitchell HE, Calatroni A, Wildfire J, et al. Seasonal risk factors for asthma exacerbations among inner-city children. J Allergy Clin Immunol. 2015;135(6):1465–73 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bai TR, Vonk JM, Postma DS, Boezen HM. Severe exacerbations predict excess lung function decline in asthma. Eur Respir J. 2007;30(3):452–6. [DOI] [PubMed] [Google Scholar]
- 21.Ivanova JI, Bergman R, Birnbaum HG, Colice GL, Silverman RA, McLaurin K. Effect of asthma exacerbations on health care costs among asthmatic patients with moderate and severe persistent asthma. J Allergy Clin Immunol. 2012;129(5):1229–35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.