Abstract
Ehrlichia chaffeensis is an obligatory intracellular bacterium of monocytes and macrophages and the etiologic agent of human monocytic ehrlichiosis, an emerging zoonosis. The Lone Star tick (Amblyomma americanum) has been implicated as the primary vector of E. chaffeensis. The present study examined the sensitivity of the nested reverse transcription (RT)-PCR based on the 16S rRNA gene relative to that of the nested PCR for detection of E. chaffeensis in infected DH82 cells, experimentally infected dog peripheral blood mononuclear cells, or experimentally infected A. americanum tick samples. The RT-PCR was found to be approximately 100 times more sensitive than the PCR for detection of E. chaffeensis regardless of the nature of the specimens. Thus, this RT-PCR is useful for detection of E. chaffeensis when a high sensitivity is required. Positive results by RT-PCR also imply the presence of viable pathogens. This is the first demonstration of RNA of E. chaffeensis in infected blood and acquisition-fed male, nymphal, and larval A. americanum ticks.
Ehrlichia chaffeensis is a small, gram-negative, obligatory intracellular bacterium that infects monocytes and macrophages (6, 21) and that is a member of the family Rickettsiaceae. This pathogen is the etiologic agent of human monocytic ehrlichiosis (HME), which was first described in 1987 by Maeda et al. (16). E. chaffeensis is transmitted primarily by the Lone Star tick (Amblyomma americanum) (2, 10, 22). More than 700 cases of HME have been reported in the United States by the Centers for Disease Control and Prevention (18). Approximately 10 deaths have been attributed to HME. More serious cases probably develop because many physicians are still unfamiliar with HME (21). The disease is characterized by fever, malaise, headache, myalgia, rigor, arthralgia, nausea, diaphoresis, and anorexia. A rash may be present in some patients. Most patients have had hematological abnormalities such as neutropenia, lymphopenia, thrombocytopenia, and anemia, as well as elevated levels of transaminases in serum (11, 21). The diagnosis is still largely dependent on evaluation of clinical, laboratory, and epidemiological data. Most laboratory diagnosis is done by the indirect fluorescent-antibody (IFA) test with E. chaffeensis Arkansas-infected DH82 cells as antigen (21).
A PCR test based on the E. chaffeensis-specific partial sequence of the 16S rRNA gene (rDNA) was developed for greater sensitivity in detecting the infection at the early stage of the disease. PCR has been used to detect the organism in clinical samples, infected cell cultures, and tick specimens (1, 15, 20, 26). Reverse transcription (RT)-PCR is a technique similar to conventional PCR except that it detects the RNA in the samples (3). Since more rRNA is generally present than rDNA in cells, RT-PCR detection of rRNA is expected to be more sensitive than PCR, which detects rDNA. RT-PCR has an added advantage over PCR using DNA as the template for detection of viable pathogens. Since it targets RNA, which is very labile, the positive detection implies the presence of viable organisms.
In the current study the sensitivity of an RT-PCR that targets 16S rRNA was compared to that of a PCR that targets 16S rDNA in E. chaffeensis-infected DH82 cells, experimentally infected dog peripheral blood mononuclear cells (PBMCs), and experimentally infected tick samples. Various stages of tick specimens were examined due to the absence of PCR data on experimentally infected tick specimens and the reported presence of PCR inhibitors in tick specimens (10).
MATERIALS AND METHODS
Infected DH82 cell culture.
The E. chaffeensis Arkansas strain was cultivated in the DH82 dog macrophage cell line in Dulbecco's minimal essential medium (GIBCO-BRL, Grand Island, N.Y.) supplemented with 10% heat-inactivated fetal bovine serum (Atlanta Biologicals, Norcross, Ga.) and 2 mM l-glutamine–10 mM N-(2-hydroxyethylpiperazine)-N′-(4-butanesulfonic acid) buffer (GIBCO-BRL) in a humidified 37°C incubator with 5% CO2–95% air as described previously (24). The infectivity was monitored daily by Diff-Quik staining (Baxter Scientific, Obetz, Ohio) of cytocentrifuged cells.
Experimental infection of dogs.
Two 1- to 2-year-old specific-pathogen-free female dogs (dogs 30133 and 30146; weight, 18 kg) were obtained from Martin Creek Kennels (Williford, Ark.). After the dogs tested free of E. chaffeensis by the IFA test and PCR, they were intravenously inoculated with 5 × 106 E. chaffeensis-infected DH82 cells in 5 ml of Dulbecco's minimal essential medium.
Tick attachment.
For the acquisition feeding, each of three groups of uninfected laboratory-reared A. americanum ticks (100 adult males, 100 nymphs, and 100 larvae) were placed in separate feeding cells on the dogs 15 days after inoculation with E. chaffeensis. Tick attachment to the host and engorgement were monitored daily. Male ticks were removed from the dogs after 7 days, while larvae and nymphs were removed once they had engorged, and each stage of ticks from each dog were separately placed in humidified chambers at room temperature. Adult male ticks were incubated for 10 days to allow removal of E. chaffeensis from the blood meal and to ensure that the presence of this pathogen would be indicative of infection of the tick host. (D. Stiller, W. L. Goff, S. Landry, L. W. Johnson, and T. C. McGuire, Eighth Natl. Vet. Hemoparasite Dis. Conf., 1989). Engorged nymphs and larvae were allowed to molt into the subsequent stage.
IFA test.
The IFA test was performed by the procedure described elsewhere (24). E. chaffeensis Arkansas strain-infected DH82 cells were used for the preparation of antigen slides, and fluorescein isothiocyanate-conjugated goat anti-dog immunoglobulin G (Jackson ImmunoResearch Laboratories Inc., West Grove, Pa.) was used at a 1/200 dilution as a secondary antibody.
Specimens.
E. chaffeensis-infected DH82 cells were harvested when more than 90% of the cells were heavily infected. The cells were gently dislodged from the flask by scraping with a rubber policeman, centrifuged, and washed with phosphate-buffered saline (137 mM NaCl, 10 mM Na2HPO4, 2.7 mM KCl, 1.8 mM KH2PO4 [pH 7.2]). Infected DH82 cells (1 × 107) were divided into 2 equal volumes for DNA or RNA extraction (5 × 106 cells each). Heparinized blood was collected from each dog 28 days after inoculation with E. chaffeensis. The samples were centrifuged, PBMCs were isolated by overlaying the buffy coat on Histopaque 1077 (Sigma Chemical Co., St. Louis, Mo.), and the interface fraction containing mononuclear cells was collected. After being washed with phosphate-buffered saline, the cells (2 × 106) were divided into 2 equal volumes for DNA or RNA extraction (1 × 106 cells each). Five adult male ticks exposed to E. chaffeensis as adults, 5 adults exposed as nymphs, and 10 nymphs exposed as larvae from each dog were used for DNA and RNA extraction. The ticks were divided vertically from the median line with a sterile razor blade under a dissecting microscope. One half of each bisected tick was randomly placed in a pool of five ticks for DNA or RNA extraction.
Extraction of DNA.
The Qiamp Blood kit (Qiagen Inc. Valencia, Calif.) was used for extraction of DNA from infected DH82 cells and dog PBMCs. The Qiamp Tissue kit (Qiagen) was used for extraction of DNA from tick samples. DNA extraction was performed according to the manufacturer's instructions. The extracted material was eluted from the columns in 100 μl of sterile double-distilled H2O (ddH2O), and the DNA concentration and purity were determined by measuring the optical density at both 260 and 280 nm with a DNA-RNA calculator (GeneQuant II; Pharmacia Biotech, Piscataway, N.J.). The DNA template was boiled for 5 min to inactivate trace amounts of proteinase K and was immediately used for PCR analysis.
Extraction of RNA.
RNA was extracted from infected DH82 cells, experimentally infected dog PBMCs, and tick samples with the TRIzol reagent (GIBCO-BRL) according to the manufacturer's instructions. The final RNA pellet was resuspended in 10 μl of diethyl pyrocarbonate-treated sterile ddH2O. The RNA concentration and purity were determined by measuring the optical density at both 260 and 280 nm with a DNA-RNA calculator.
cDNA synthesis (RT).
Isolated RNA was heated to 70°C for 10 min and cooled on ice before 1 to 5 μg of RNA was reverse transcribed in a 20-μl reaction mixture (10 mM random hexamer, 0.5 mM deoxynucleoside triphosphate mixture, 1 U of RNase inhibitor [GIBCO-BRL], 200 U of SuperScript II reverse transcriptase [GIBCO-BRL]) at 42°C for 50 min. The reaction was terminated by heating the mixture to 70°C for 15 min. The final total cDNA volume was adjusted to 100 μl and was immediately used in the PCR.
PCR.
Extracted DNA or cDNA was used as the template for nested PCR amplification of 16S rRNA or rDNA, respectively, of E. chaffeensis. Nested PCR was performed as described previously (7). A DNA template known to be positive for E. chaffeensis was used as a positive control, and ddH2O was used as the template for the negative control.
The PCR was performed with a 10-fold dilution series of DNA and cDNA templates. In the first PCR, 10 μl of each template sample was amplified in a 50-μl reaction mixture containing 5 μl of 10× PCR buffer (10 mM Tris-HCl [pH 8.4], 50 mM KCl), 5 μl of 50 mM MgCl2, 1 μl of 10 mM deoxynucleoside triphosphate mixture, 1.5 U of Taq polymerase (GIBCO-BRL), and 5 pmol each of primers ECB and ECC, which are specific for all ehrlichial species (25). Amplification was performed in a GeneAmp PCR system 9700 thermal cycler (Perkin-Elmer Applied Biosystems, Norwalk, Conn.) with a three-step program (5 min of denaturation at 94°C; 40 cycles of 1 min of denaturation at 94°C, 1 min of annealing at 60°C, and 1 min of extension at 72°C; and a final extension for 10 min).
For the nested PCR, 1 μl of the first PCR product was amplified in a second 50-μl reaction mixture assembled as described above, except that primers HE1 and HE3, which are specific for E. chaffeensis 16S rDNA, were used (1). The same temperature cycle used for the first PCR was used. To prevent false-positive PCR results, in addition to the use of filtered tips for all PCR reagents and templates, reagent mixing, reagent addition, DNA purification, etc., were done in a Biosafety II laminar-flow hood dedicated only for the PCR. In each PCR run a negative control (reaction mixtures without template) was included. A positive control E. chaffeensis DNA (known amount) prevents a false-negative result or inferior sensitivity of the PCR test due to reagent or equipment failure.
Ten microliters of the nested PCR products was separated by electrophoresis on a 1.5% agarose gel (Sigma), stained with ethidium bromide, and visualized with UV transillumination. An HaeIII-digested φX174 replicative-form DNA marker (GIBCO-BRL) was included in each agarose gel electrophoresis run to identify accurately the sizes of the amplified bands.
RESULTS
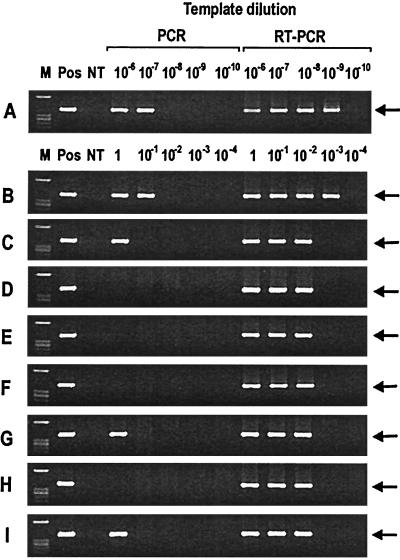
The amplicon with a distinct single band of 389 bp was detected in positive control and several experimental specimens by PCR and RT-PCR. Negative controls run simultaneously with the same reaction mixture were all negative. The sensitivities of RT-PCR and PCR for detection of E. chaffeensis in DH82 cells were 5 × 10−5 cells (3.5 fg of RNA) and 5 × 10−3 cells (48 fg of DNA), respectively (Table 1; Fig. 1). RT-PCR and PCR detected E. chaffeensis in as few as 100 and 10,000 PBMCs, respectively, from dog 30133. RT-PCR and PCR also detected E. chaffeensis in as few as 1,000 and 100,000 PBMCs, respectively, from dog 30146. Only two pooled specimens, adult A. americanum ticks infected as nymphs on dog 30146 and nymphal A. americanum ticks infected as larvae on dog 30146, were positive, and the remaining four specimens were negative by PCR. However, all of these pooled specimens tested positive up to a dilution of 102 by RT-PCR (Table 1; Fig. 1). The final total volumes of both cDNA and DNA were adjusted to 100 μl to eliminate dilution factor differences and the extraction of RNA and DNA, and PCR and RT-PCR were performed in a time frame which does not introduce differences in storage effects. This study demonstrated for the first time E. chaffeensis RNA in infected blood and acquisition-fed male, nymphal, and larval A. americanum ticks.
TABLE 1.
Sensitivities of RT-PCR and PCR in detecting E. chaffeensis in infected DH82 cells, experimentally infected dog PBMCs, and experimentally infected A. americanum tick specimens
| Specimens (no. of cells or ticks/10 μl of template) | Maximum dilution of specimens found positive (minimum amt of DNA or RNA detectable per reaction mixture)
|
|
|---|---|---|
| PCR | RT-PCR | |
| Infected DH82 cells (500,000 cells) | 1/108 (48 fg) | 1/1010 (3.5 fg) |
| Dog 30133 PBMCs (100,000 cells) | 1/101 (0.19 μg) | 1/103 (6.8 ng) |
| Dog 30146 PBMCs (100,000 cells) | 1/1 (1.26 μg) | 1/102 (3.6 ng) |
| Adult tick infected as adult on dog 30133 (2.5 ticks) | UDa (0.59 μg) | 1/102 (67 ng) |
| Adult tick infected as adult on dog 30146 (2.5 ticks) | UD (1.15 μg) | 1/102 (86 ng) |
| Adult tick infected as nymph on dog 30133 (2.5 ticks) | UD (0.87 μg) | 1/102 (130 ng) |
| Adult tick infected as nymph on dog 30146 (2.5 ticks) | 1/1 (1.48 μg) | 1/102 (190 ng) |
| Nymph infected as larvae on dog 30133 (5 ticks) | UD (0.18 μg) | 1/102 (11.8 ng) |
| Nymph infected as larvae on dog 30146 (5 ticks) | 1/1 (0.11 μg) | 1/102 (8.7 ng) |
UD, undetectable.
FIG. 1.
Agarose gel electrophoresis of the nested PCR and RT-PCR products according to the template dilutions. DNA and cDNA templates were E. chaffeensis-infected DH82 cells (A) dog 30133 PBMCs (B), dog 30146 PBMCs (C), adult male A. americanum ticks infected as adults on dog 30133 (D), adult male A. americanum ticks infected as adults on dog 30146 (E), adult male A. americanum ticks infected as nymphs on dog 30133 (F), adult male A. americanum ticks infected as nymphs on dog 30146 (G), A. americanum nymphs infected as larvae on dog 30133 (H), and A. americanum nymphs infected as larvae on dog 30146 (I). Lanes M, HaeIII-digested φX174 as a molecular marker (GIBCO-BRL); Pos, positive control; NT, no template. Horizontal arrows indicate the amplicons (389 bp).
DISCUSSION
Although the isolation and culture of E. chaffeensis is the “gold standard” for the diagnosis of HME, successful isolation E. chaffeensis is rare, and only 16 primary isolates have been reported to date (23). Thus, PCR and other diagnostic tests are important for the diagnosis of HME. Several studies of other Ehrlichia species have been performed by conventional one-step PCR. Iqbal and Rikihisa (14) reported that PCR can detect as little as 15 pg of DNA from purified Ehrlichia canis. Iqbal et al. (13) reported that 20 pg of DNA extracted from E. canis-infected DH82 cells could be detected by PCR. Chu (5) reported that PCR can detect as little as 10 copies of ehrlichial 16S rDNA and as few as 0.3 human granulocytic ehrlichiosis agent-infected horse neutrophils.
A nested PCR greatly enhances the sensitivity and specificity of detection of target nucleic acid sequences (12). This method has been used to increase the sensitivity for detection of E. chaffeensis in infected cell cultures and in blood, tissue, and naturally infected tick specimens (8, 15, 26, 27). Wen et al. (25) reported that, by using serially diluted DNA from purified E. canis, as little as 0.2 pg of E. canis DNA could be detected by the nested PCR. Mott et al. (19) reported that nested PCR can be used to detect the Ehrlichia risticii 16S rRNA gene at a level of 0.02 pg of purified E. risticii. We have used the nested 16S rDNA-based PCR in this study. The sensitivity of the nested PCR was 48 fg of DNA from E. chaffeensis-infected DH82 cells (5 × 10−3 DH82-infected cells), and it detected E. chafeensis in as few as 10,000 PBMCs from an infected dog. Stich et al. (R. W. Stich, D. L. Grover, Y. Rikihisa, G. R. Needham, S. A. Ewing, and S. Jittapalapong, submitted for publication) found that a nested PCR assay for E. canis based on a copy of p30 from the omp-1 multigene family was more sensitive than the nested 16S rDNA-based assay. McBride et al. (17) reported that chemiluminescent hybridization improved the PCR assay sensitivity 1,000-fold compared with visualization on ethidium bromide-stained agarose gels. By this method, PCR can detect as little as 30 fg of E. canis genomic DNA from purified E. canis.
The need for expression of individual genes changes in response to physiologic stimuli, and requirements for flexible gene expression are reflected in the rapid metabolic turnover of most mRNAs. There are thousands of molecules of mRNA or rRNA copies in bacteria (3). For that reason, it is expected that RT-PCR must be more sensitive than PCR. In this study, we found that RT-PCR based on 16S rRNA is at least 100 times more sensitive than PCR regardless of the type of specimen used. Clinical diagnosis of HME may be improved by using this sensitive RT-PCR. The cost of the assay and the requirement for stringent controls to prevent DNA contamination may make the nested RT-PCR prohibitive in some cases. However, by targeting RNA, which is very labile, RT-PCR is more likely to detect viable organisms, thus providing biologically relevant information for the pathogen than PCR, which targets stable DNA.
In an experimental transmission study, Ewing et al. (10) reported that adult A. americanum ticks fed as nymphs or larvae on deer were negative by the nested 16S rDNA-based PCR 10 to 61 days after exposure. They concluded that reliable amplification of E. chaffeensis 16S rDNA in infected ticks by PCR was not possible due to the presence of PCR inhibitors in the tick host. In our study, we found that nested PCR was negative for four of six tick samples. In these samples, however, all RT-PCR results were positive at 102 dilutions (or 11.8 to 130 ng) of RNA.
The PCR has been used for detection of E. chaffeensis DNA in field-collected tick specimens in several epidemiologic studies. In those studies, the prevalence of infected ticks was reported to be 0 to 29% in the United States (2, 4, 22, 26). The results of our current study, however, suggest that the prevalence rates of positive ticks may be greater if this nested RT-PCR is used.
It was reported that dogs can be naturally and experimentally infected with E. chaffeensis (8, 9). In the present study we found that both dogs were infected by the intravenous inoculation of E. chaffeensis-infected DH82 cells. The previous study suggested that transovarial transmission of E. chaffeensis is uncommon, but ticks acquire the infection during the nymphal blood meal, before molting to the adult stage, and that human infection occurs by the bite of infected adults (2, 21). Ewing et al. (10), however, demonstrated transstadial transmission of E. chaffeensis to white-tailed deer but not to dogs by experimentally infected nymphal or adult A. americanum ticks (10). Our current study, which showed infection with E. chaffeensis of all three stages of the A. americanum ticks engorged on infected dogs, supports the observation of Ewing et al. (10) and further suggests the potential role of intrastadially infected adult ticks in the transmission of E. chaffeensis.
In conclusion, RT-PCR based on 16S rRNA is highly sensitive for detection of E. chaffeensis in both blood and tick specimens and is expected to be especially useful when the sensitivity is critical for examination of samples with low levels of infection.
ACKNOWLEDGMENTS
This study was supported by grants R01 AI40934 and R01 AI47407 from the National Institutes of Health. A. Unver is the recipient of a Turkish government fellowship.
We thank D. L. Grover for technical assistance with the tick study.
REFERENCES
- 1.Anderson B E, Sumner J W, Dawson J E, Tzianabos T, Greene C, Olson J G, Fishbein D B, Olsen-Ramussen M, Holloway B P, George E H, Azad A F. Detection of the etiologic agent of human ehrlichiosis by polymerase chain reaction. J Clin Microbiol. 1992;30:775–780. doi: 10.1128/jcm.30.4.775-780.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson B E, Simms K G, Olson J G, Childs J E, Piesman J F, Happ C M, Maupin G O, Johnson B J. Amblyomma americanum: a potential vector of human ehrlichiosis. Am J Trop Med Hyg. 1993;49:239–244. doi: 10.4269/ajtmh.1993.49.239. [DOI] [PubMed] [Google Scholar]
- 3.Brooks G F, Butel J S, Morse S A. Jawetz, Melnick, & Adelberg's medical microbiology. 21st ed. Stanford, Conn: Appleton & Lange; 1998. Microbial genetics; pp. 90–109. [Google Scholar]
- 4.Burket C T, Vann C N, Pinger R R, Chatot C L, Steiner F E. Minimum infection rate of Amblyomma americanum (Acari: Ixodidae) by Ehrlichia chaffeensis (Rickettsiales: Ehrlichieae) in southern Indiana. J Med Entomol. 1998;35:653–659. doi: 10.1093/jmedent/35.5.653. [DOI] [PubMed] [Google Scholar]
- 5.Chu F K. Rapid and sensitive PCR-based detection and differentiation of aetiologic agents of human garnulocytotropic and monocytotropic ehrlichiosis. Mol Cell Probes. 1998;12:93–99. doi: 10.1006/mcpr.1998.0150. [DOI] [PubMed] [Google Scholar]
- 6.Dawson J E, Anderson B E, Fishbein D B, Sanchez J L, Goldsmith C S, Wilson K H, Duntley C W. Isolation and characterization of an Ehrlichia sp. from a patient diagnosed with human ehrlichiosis. J Clin Microbiol. 1991;29:2838–2842. doi: 10.1128/jcm.29.12.2741-2745.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dawson J E, Stallknecht D E, Howerth E W, Warner C, Biggie K, Davidson W R, Lockhart J M, Nettles V F, Olson J G, Childs J E. Susceptibility of white tailed deer (Odocoileus virginianus) to infection with Ehrlichia chaffeensis, the etiologic agent of human ehrlichiosis. J Clin Microbiol. 1994;32:2725–2728. doi: 10.1128/jcm.32.11.2725-2728.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dawson J E, Biggie K L, Warner C K, Cookson K, Jenkins S, Levine J F, Olson J G. Polymerase chain reaction evidence of Ehrlichia chaffeensis, an etiologic agent of human ehrlichiosis, in dogs from southeast Virginia. Am J Vet Res. 1996;57:1175–1179. [PubMed] [Google Scholar]
- 9.Dawson J E, Ewing S A. Susceptibility of dogs to infection with Ehrlichia chaffeensis, causative agent of human ehrlichiosis. Am J Vet Res. 1992;53:1322–1327. [PubMed] [Google Scholar]
- 10.Ewing S A, Dawson J E, Kocan A A, Barker R W, Warner C K, Panciera R J, Fox J C, Kocan K M, Blouin E F. Experimental transmission of Ehrlichia chaffeensis (Rickettsiales: Ehrlichieae) among white-tailed deer by Amblyomma americanum (Acari: Ixodidae) J Entomol. 1995;32:368–374. doi: 10.1093/jmedent/32.3.368. [DOI] [PubMed] [Google Scholar]
- 11.Fishbein D B, Dawson J E, Robinson L E. Human ehrlichiosis in the United States, 1985 to 1990. Ann Intern Med. 1994;120:736–743. doi: 10.7326/0003-4819-120-9-199405010-00003. [DOI] [PubMed] [Google Scholar]
- 12.Haff L A. Improved quantitative PCR using nested primers. PCR Methods Appl. 1994;3:332–337. doi: 10.1101/gr.3.6.332. [DOI] [PubMed] [Google Scholar]
- 13.Iqbal Z, Chaichanasiriwithaya W, Rikihisa Y. Comparison of PCR with other tests for early diagnosis of canine ehrlichiosis. J Clin Microbiol. 1994;32:1658–1662. doi: 10.1128/jcm.32.7.1658-1662.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iqbal Z, Rikihisa Y. Application of the polymerase chain reaction for the detection of Ehrlichia canis in tissues of dogs. Vet Microbiol. 1994;42:281–287. doi: 10.1016/0378-1135(94)90059-0. [DOI] [PubMed] [Google Scholar]
- 15.Lockhart J M, Davidson W R, Stallknecht D E, Dawson J E, Little S E. Natural history of Ehrlichia chaffeensis (Rickettsiales: Ehrlichieae) in the Piedmont physiographic province of Georgia. J Parasitol. 1997;85:887–894. [PubMed] [Google Scholar]
- 16.Maeda K, Markowitz N, Hawley R C, Ristic M, Cox D, McDade J E. Human infection with Ehrlichia canis a leukocytic rickettsia. N Engl J Med. 1987;316:853–857. doi: 10.1056/NEJM198704023161406. [DOI] [PubMed] [Google Scholar]
- 17.McBride J W, Corstvet R E, Gaunt S D, Chinsangaram J, Akita G Y, Osburn B I. PCR detection of acute Ehrlichia canis infection in dogs. J Vet Diagn Investig. 1996;8:441–447. doi: 10.1177/104063879600800406. [DOI] [PubMed] [Google Scholar]
- 18.McQuiston J H, Paddock C D, Holman R C, Childs J E. The human ehrlichioses in the United States. Emerg Infect Dis. 1999;5:635–642. doi: 10.3201/eid0505.990504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mott J, Rikihisa Y, Zhang Y, Reed S M, Yu C Y. Comparison of PCR and culture to the indirect fluorescent antibody test for diagnosis of Potomac horse fever. J Clin Microbiol. 1997;35:2215–2219. doi: 10.1128/jcm.35.9.2215-2219.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Persing D H. In vitro nucleic acid amplification techniques. In: Persing D H, Smith T F, Tevover F C, White T J, editors. Diagnostic microbiology: principles and applications. Washington, D.C.: American Society for Microbiology; 1993. pp. 51–87. [Google Scholar]
- 21.Rikihisa Y. Clinical and biological aspects of infection caused by Ehrlichia chaffeensis. Microb Infect. 1999;1:367–376. doi: 10.1016/s1286-4579(99)80053-7. [DOI] [PubMed] [Google Scholar]
- 22.Roland W E, Everett E D, Cyr T L, Hasan S Z, Dommaraju C B, McDonald G A. Ehrlichia chaffeensis in Missouri Ticks. Am J Trop Med Hyg. 1998;59:641–643. doi: 10.4269/ajtmh.1998.59.641. [DOI] [PubMed] [Google Scholar]
- 23.Standaert S M, Yu T, Scott M A, Childs J E, Paddock C D, Nicholson W L, Singleton J, Blaser M J. Primary isolation of Ehrlichia chaffeensis from patients with febrile illness: clinical and molecular characteristics. J Infect Dis. 2000;181:1082–1088. doi: 10.1086/315346. [DOI] [PubMed] [Google Scholar]
- 24.Unver A, Rikihisa Y, Ohashi N, Cullman L C, Buller R, Storch G A. Western and dot blotting assay analysis of Ehrlichia chaffeensis indirect fluorescent-antibody assay-positive and -negative human sera by using native and recombinant E. chaffeensis and E. canis antigens. J Clin Microbiol. 1999;37:3888–3895. doi: 10.1128/jcm.37.12.3888-3895.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wen B, Rikihisa Y, Mott J, Greene R, Kim H Y, Zhi N, Couto G C, Unver A, Bartsch R. Comparison of nested PCR with immunofluorescent antibody assay for detection of Ehrlichia canis infection in dogs treated with doxycycline. J Clin Microbiol. 1997;35:1852–1855. doi: 10.1128/jcm.35.7.1852-1855.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whitlock J E, Fang Q Q, Durden L A, Oliver J H. Prevalence of Ehrlichia chaffeensis (Rickettsiales: Rickettsiaceae) in Amblyomma americanum (Acari: Ixodidae) from the Georgia Coast and Barrier Islands. J Med Entomol. 2000;37:276–280. doi: 10.1603/0022-2585-37.2.276. [DOI] [PubMed] [Google Scholar]
- 27.Yu X, Piesman J F, Olson J G, Walker D H. Short report: geographic distribution of different genetic types of Ehrlichia chaffeensis. Am J Trop Med Hyg. 1997;56:679–680. doi: 10.4269/ajtmh.1997.56.679. [DOI] [PubMed] [Google Scholar]