Abstract
Priming is an adaptive strategy that improves plant defenses against biotic and abiotic stresses. Stimuli from chemicals, abiotic cues, and pathogens can trigger the establishment of priming state. Priming with 5-aminolevulinic acid (ALA), a potential plant growth regulator, can enhance plant tolerance to the subsequent abiotic stresses, including salinity, drought, heat, cold, and UV-B. However, the molecular mechanisms underlying the remarkable effects of ALA priming on plant physiology remain to be elucidated. Here, we summarize recent progress made in the stress tolerance conferred by ALA priming in plants and provide the underlying molecular and physiology mechanisms of this phenomenon. Priming with ALA results in changes at the physiological, transcriptional, metabolic, and epigenetic levels, and enhances photosynthesis and antioxidant capacity, as well as nitrogen assimilation, which in turn increases the resistance of abiotic stresses. However, the signaling pathway of ALA, including receptors as well as key components, is currently unknown, which hinders the deeper understanding of the defense priming caused by ALA. In the future, there is an urgent need to reveal the molecular mechanisms by which ALA regulates plant development and enhances plant defense with the help of forward genetics, multi-omics technologies, as well as genome editing technology.
Keywords: 5-ALA, defense priming, abiotic stress, multi-omics, plant hormone
1. Introduction
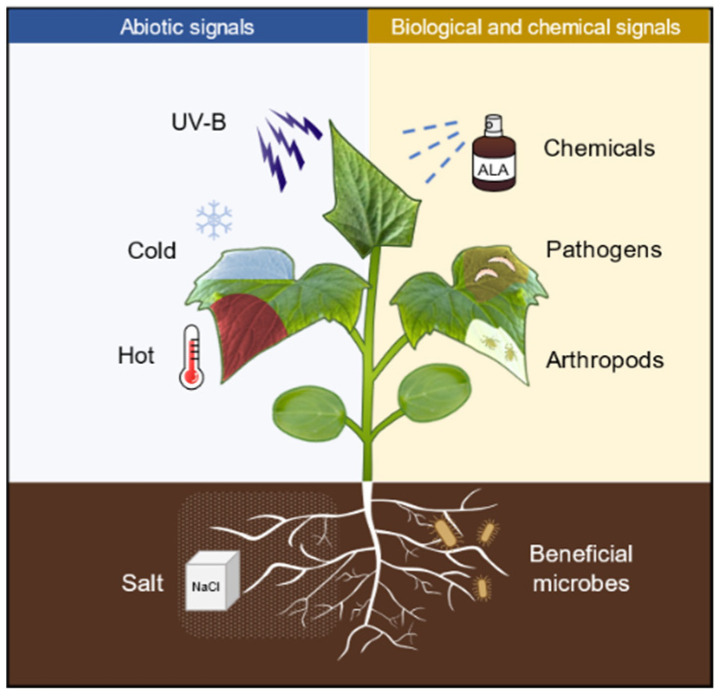
Defense priming refers to a physiological state (a state of readiness for defense) that is induced after the plants perceive a variety of stimuli, such as pathogens, arthropods, and abiotic cues, as well as chemicals (Figure 1). In this state, the defense responses are deployed in a faster, stronger, and/or more sustained manner, thus defense priming is considered an adaptive and low-cost defensive strategy [1]. The pathogen produces molecules with different biochemical natures (peptides, polysaccharides, or lipids) that are sensed by plants through the corresponding receptors, thus inducing plant priming [2]. The common bean (Phaseolus vulgaris) activates enhanced plant defense by inoculation with Rhizobium etli and develops stronger resistance to Pseudomonas syringae compared with unstimulated plants [3]. Herbivore-inducible plant volatiles (HIPVs) released in response to herbivore attack can induce priming in neighboring plants, which exhibit faster or stronger defense activation and insect resistance when subjected to insect feeding [4]. Repeated exposure of plants to mild abiotic signals, such as heat, cold, or salt, can also trigger plants into a defense priming state [5]. In addition, pretreatment with low concentrations of chemicals such as hydrogen peroxide (H2O2), sodium hydrosulfide (NaHS), sodium chloride (NaCl), sodium nitroprusside (SNP), γ-aminobutyric acid (GABA), melatonin, polyamines (PAs), as well as 5-aminolevulinic acid (ALA) also induces plants into defense priming status [6,7,8], in which plants respond to biological and abiotic stresses through faster and stronger defensive activation [9]. Furthermore, the ability of priming to enhance stress tolerance can be self-propagating. For example, defense priming occurs in roots, while transcriptional differences can be detected in both roots and leaves [10]; mobile wound signals transmitted from local damaged sites to distal undamaged sites induce the whole plant into priming [11]; infestation of plants with phloem-feeding whitefly (Bemisia tabaci) triggers local and systemic defense priming [12]. Interestingly, the priming status of plants can be inherited across generations. For example, a transgenerational priming response against pathogen attack can last for at least two generations in common beans upon treatment with the priming agent GABA [13]. This transgenerational inheritance of defense priming may involve epigenetic regulation [2,14].
Figure 1.
Numerous biotic and abiotic stress as well as defense-related chemicals are capable of inducing plants into priming status. UV-B, ultraviolet B (UVB); ALA, 5-aminolevulinic acid; NaCl, sodium chloride.
2. Biosynthesis of 5-Aminolevulinic Acid
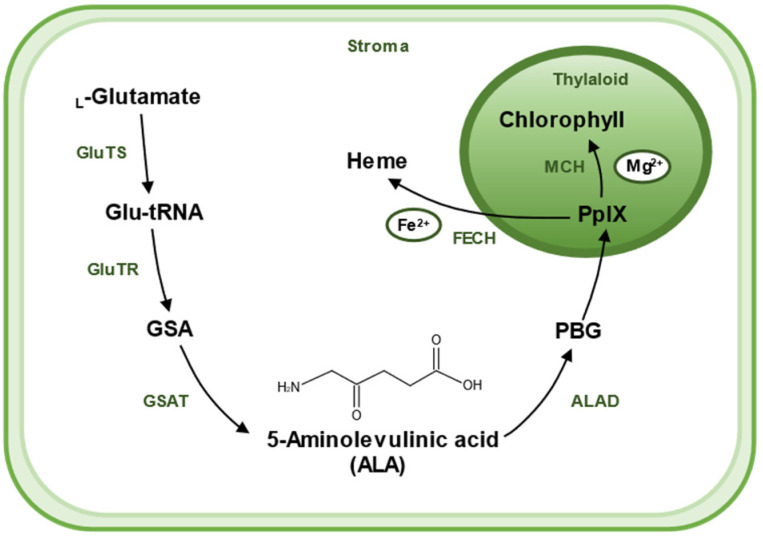
5-Aminolevulinic acid (ALA) has been found to increase tolerance to various stresses and is a promising chemical molecule for application in agriculture. ALA is widely found in various living organisms, including bacteria, algae, plants, and animals, and is a universal precursor for the synthesis of all tetrapyrroles (chlorophyll, heme, siroheme, vitamin B12, and phytochromobilin) [15,16]. Therefore, to better understand the biological role of ALA and underlying molecular mechanisms in defense priming, we firstly made a brief introduction to its biosynthesis (Figure 2). There are two pathways for the biosynthesis of ALA, the C4 pathway (or Shemin pathway) and the C5 pathway (or Beale pathway) [17]. The C4 pathway is found in animals, fungi, and some algae and bacteria. In this pathway, ALA is produced by direct condensation of succinyl-CoA and Gly catalyzed by ALA synthase. The C5 pathway is mainly found in plants and archaea, which consists of a three-step enzymatic reaction [18]. Firstly, L-glutamate is ligated to tRNAGlu, which is catalyzed by glutamyl–tRNA synthetase (GluTS) to form L-glutamy–tRNA. Secondly, the carboxyl group of Glu-tRNA is reduced to a formyl group and L-Glu-tRNA is converted to L-glutamic acid 1-semialdehyde (GSA). GluTR plays a key role during the synthesis pathway of ALA. Lastly, GSA undergoes an isomerization reaction catalyzed by glutamate-1-semialdehyde aminotransferase (GSAT) to form ALA. These reactions are located in the chloroplast stroma [19].
Figure 2.
A sketch shows the biosynthetic pathway of ALA and the use of ALA as a substrate for the synthesis of chlorophyll and heme in plants. ALA is created in stroma of chloroplast. The main biosynthetic pathway of ALA is the Beal pathway, which starts from glutamic acid. L-Glutamate is ligated to tRNAGlu, which is catalyzed by glutamyl–tRNA synthetase (GluTS) to form L-glutamy–tRNA. Then, Glu-tRNA is converted to L-glutamic acid 1-semialdehyde (GSA), a reaction catalyzed by the key rate-limiting enzyme glutamyl–tRNA reductase (GluTR). GSA then undergoes an isomerization reaction catalyzed by glutamate-1-semialdehyde aminotransferase (GSAT) to form ALA. Two molecules of ALA are catalyzed by ALA dehydratase (ALAD) and agglomerate to form a pyrrole ring called porphobilinogen (PBG). Then, after a six-step enzymatic reaction, four molecules of PBG polymerize to form a porphyrin structure, eventually forming (PpIX). PpIX combines with different enzymes and substrates to yield different products; PpIX chelates Fe2+ with Ferrochelatase (FECH) to produce heme, and Mg2+ with Mg-chelatase (MCH), and then undergoes a series of catalytic reactions to produce chlorophyll.
Chlorophyll and heme, with ALA as their precursors, are involved in many biochemical processes. They share a series of steps in the synthesis pathway from ALA to protoporphyrin IX (PpIX). It starts with two molecules of ALA catalyzed by ALA dehydratase (ALAD), which aggregates to form a pyrrole ring called porphobilinogen (PBG); this is followed by a six-step enzymatic reaction in which four molecules of PBG polymerize to form a porphyrin structure, eventually forming PpIX. The synthetic pathway branches off here to produce heme or chlorophyll, respectively. PpIX chelates Fe2+ by Ferrochelatase (FECH) to produce heme or chelates Mg2+ by Mg-chelatase (MCH) and undergoes a series of catalytic reactions to produce chlorophyll. ALA was originally obtained by chemical methods, which is a complex process and difficult to purify, resulting in a low yield and high price [20]. At present, ALA can be produced commercially through easier, cheaper, and sustainable microbial methods [21,22,23].
3. ALA Priming Enhances Plant Resistance to Abiotic Stresses
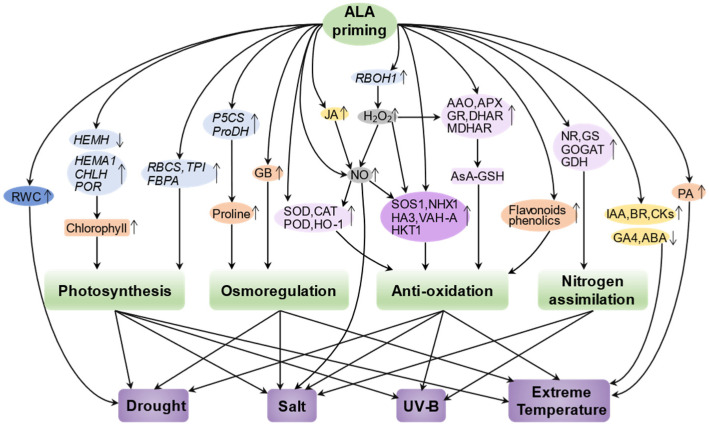
Abiotic stresses are considered to be major environmental factors limiting the yield and quality of crop plants. Developing effective strategies to mitigate the deleterious effects of abiotic stresses is critical for sustainable agriculture and food security. Recent studies have shown that exogenous treatment of plants with 5-ALA can enhance abiotic stress tolerance by inducing molecular and physiological defense mechanisms, providing a promising strategy for mitigating abiotic stress in plants. Here, we focus on reviewing the physiological and molecular mechanisms of action of ALA priming in alleviating salt stress, extreme temperature stress, drought stress, and UV stress, and construct a regulatory network to show them systematically (Figure 3; Table 1).
Figure 3.
Construction of a regulatory network of ALA priming-mediated abiotic stress tolerance. Priming with ALA enhances the ability of plants to cope with various stresses such as drought stress, salt stress, UV-B stress, and extreme temperature stress by regulating photosynthesis, osmoregulation, antioxidant capacity, and nitrogen assimilation in plants through finely tuning the activities of enzymes (light violet), channel proteins (dark violet), hormones (yellow), signaling molecules (gray), small organic molecules (orange), gene expression (light blue), or physiological levels (dark blue). The upward and downward arrows represent an upregulation or downregulation, respectively. HEMA1, glutamyl-tRNA reductase; CHLH, Mg-chelatase; POR, protochlorophyllide oxidoreductase; NR, nitrate reductase; NiR, nitrite reductase; GS, glutamine synthetase; GOGAT, glutamate synthase; CAT, catalase; SOD, superoxide dismutase; APX, ascorbate peroxidase; GR, glutathione reductase; RWC, relative water content; GDH, glutamate dehydrogenase; P5CS, delta-1-pyrroline-5-carboxylate synthase; HKT1, K+ transporter protein 1; NHX1, Na+/H+ antiporter; VHA-A, proton pump; GB, glycine betaine; DHAR, dehydroascorbic acid reductase; MDHAR, monodehydroascorbic acid reductase; AsA-GSH, ascorbate-glutathione cycle.
Table 1.
Effects of ALA priming on the tolerance to environmental stressors.
| Type of Stress | Plant Species | Stress Concentration | Mode of ALA Application and ALA Level | Effects | References |
|---|---|---|---|---|---|
| Salt stress | Asparagus (Asparagus aethiopicus L.) | 2000 and 4000 ppm NaCl | Foliar application of 3, 5, and 10 ppm | An increase in plant biomass, leaf antioxidant activity, phenolic content, proline accumulation, and photosynthetic rate | Al-Ghamdi et al., 2018 |
| Barley (Hordeum vulgare L.) | 150 mM NaCl | Hydroponics of 10, 30, and 60 mg/L | Proline content increased and ROS content decreased | Averina et al., 2010 | |
| 100 mM NaCl | Foliar application of 7 ppm | Increased chlorophyll content, antioxidant enzyme activity, and stress responsive gene expression | El-Esawi et al., 2018 | ||
| Cassia seed (Cassia obtusifolia L.) | 100 mM NaCl | Seed soaking of 5, 10, 15, and 20 mg/L; root irrigation of 10, 25, 50, and 100 mg/L | Significantly increased chlorophyll content, total soluble sugars, free proline, and soluble protein content; increased photosynthesis and antioxidant enzyme activities | Zhang et al., 2013 | |
| Cucumber (Cucumis sativus L.) | 75 mM NaCl | Foliar application of 50 mg/L | ALA might delay and counteract the upregulated expression of cucumber PIP aquaporin gene (CsPIP1:1) and cucumber NIP aquaporin gene (CsNIP) genes in cucumber seedlings under NACL stress | Yan et al., 2014 | |
| 50 mM NaCl | Foliar application of 25 mg/L | Enhancement of ascorbate-glutathione cycle; increase in shoot and root growth | Wu et al., 2019 | ||
| 50 mM NaCl | Foliar application of 25 mg/L | Increased ROS production in roots, resulting in upregulation of ion trans-porters SOS1, NHX1, and HKT1 | Baral, 2019 | ||
| 50 mM NaCl | Foliar application of 25 mg/L | Improved plant growth; upregulation of Na+/H+ antiporter SOS1 and NHX1 at the plasma and vesicle membranes, thereby reducing ion toxicity | Wu et al., 2021 | ||
| 50 mM NaCl | Foliar application of 25 mg/L | Downregulation of ferrochelatase (HEMH) gene expression; increased in chlorophyll biosynthesis pathway | Wu et al., 2018 | ||
| Date Palm (Phoenix dactylifera L.) | Seawater treatments at 1, 15, and 30 mS cm–1 | Root irrigation of 0.08% ALA based fertilizer (PentaKeep-V) | Enhanced photosynthetic assimilation by increasing chlorophyll content and stomatal conductance | Tarek et al., 2007 | |
| Maize (Zea mays L.) | 100 mM NaCl | Foliar application and seed soaking of 20 mg/L | Improved plant growth; activated the synthesis and accumulation of endogenous NO, thereby increasing the antioxidant capacity of plants | Kaya et al., 2020 | |
| Oilseed rape (Brassica napus L.) | 100 and 200 mM NaCl | Foliar application of 30 mg/L | Increased plant growth and chloroplast photosynthetic efficiency; reduced Na+ uptake and oxidative stress | Naeem et al., 2012 | |
| 200 mM NaCl | Foliar application of 30 mg/L | Increased aboveground biomass and net photosynthetic rate; promoted chlorophyll accumulation by promoting increased intermediate levels of the tetrapyrrole synthesis pathway; upregulated the expression of genes P5CS and ProDH encoding proline metabolic enzymes | Xiong et al., 2018 | ||
| 100 and 200 mM NaCl | Foliar application of 30 mg/L | Improved root and shoot growth; Enhanced plant photosynthesis, chlorophyll content; regulated the uptake of Na+ and leaf water potential | Naeem et al., 2010 | ||
| Peach (Prunnus persica L.) | 100 mM NaCl | Foliar application of 200 mg/L | Exogenous ALA treatment could improve the growth and relieve the NACL stress injury of peach seedlings by increasing photochemical efficiency, osmotic content, and antioxidant enzyme activity | Ye et al., 2016 | |
| Radix Isatidis (Isatis indigotica Fort.) | 100 mM NaCl | Foliar application of 12.5, 16.7, 25.0, and 50.0 mg/L | Increased antioxidant enzyme activity, chlorophyll content, and net photo-synthetic rate | Tang et al., 2017 | |
| Sunflower (Helianthus annuus L.) | 150 mM NaCl | Foliar application of 20, 50, and 80 mg/L | Decreased leaf H2O2 content and increased SOD activity | Akram et al., 2012 | |
| Swiss chard (Beta vulgaris L.) | 40 and 80 mM saline (molar ratio NaCl/Na2SO4 = 9:1) | Foliar application of 60 and 120 μM | The ionic toxicity was reduced by decreasing the Na+ content and Na+/K+ ratio; increased the total nitrogen and GB content | Liu et al., 2014 | |
| Watermelon (Citrullus lanatus L.) | 100 mM NaCl | Foliar application of 1.25 mM | Regulated nitrogen metabolism, reduced ion toxicity caused by salt stress, and increased soluble protein and proline | Chen et al., 2017 | |
| Extreme Temperature | Cucumber (Cucumis sativus L.) | 42/38 °C (day/night) | Foliar application of 3 μM | Reduced ROS content and growth inhibition under heat stress; enhanced antioxidant enzyme (SOD, CAT, and GPX) activity and proline content | Zhang et al., 2012 |
| 12 °C/8 °C (day/night) | Foliar application of 15, 30, and 45 mg/L ALA | Nutrient contents (N, P, K, Mg, Ca, Cu, Fe, Mn, and Zn) and endogenous hormones (JA, IAA, BR, IPA, and ZR) were enhanced in roots and leaves; Increased chlorophyll content, photosynthetic capacity, and antioxidant enzymes (SOD, POD, CAT, APX, and GR); reduced growth inhibition of seedlings by cold stress | Anwar et al., 2018 | ||
| 16 °C/8 °C (day/night) | Add to the culture substrate of 10, 20, or 30 mg ALA·kg−1 (ALA were mixed with a constant weight of substrate (kg)) | Significantly reduced plant growth inhibition; increased chlorophyll content, antioxidant enzymes (SOD, CAT, and POD) activity; reduced accumulation of ROS and malondialdehyde in roots and leaves | Anwar et al., 2020 | ||
| Drooping wild ryegrass (Elymus nutans Griseb.) | 5 °C | Seed soaking of 0.1, 0.5, 1, 5, 10, and 25 mg/L | Significantly increased seed respiration rate and ATP synthesis and protected germinating seeds from cold stress; increased GSH, AsA, total glutathione, and total ascorbate concentrations, as well as SOD, CAT, APX, and GR activities | Fu et al., 2014 | |
| 5 °C | Foliar application of 0.5, 1, 5, 10, and 26 mg/L | NO might be a downstream signal that mediates ALA-induced cold tolerance, thereby enhancing antioxidant defense | Fu et al., 2015 | ||
| 5 °C | Root soaking of 0.5, 1, 5, 10, and 26 mg/L | NO might act as a downstream signal to mediate ALA-induced cold resistance by activating antioxidant defense and PM H+-ATPase and maintaining Na+ and K+ homeostasis | Fu et al., 2016 | ||
| Maize (Zea mays L.) | 14 °C/5 °C (day/night) | Foliar application of 0.15 mM | Increased proline accumulation, antioxidant enzymes (SOD and CAT) and RuBP carboxylase activity; prevented reductions in maize crop yield due to low-temperature stress | Wang et al., 2018 | |
| Red pepper (Capsicum annuum cv. Sena) | 4 °C | Seed soaking of 1, 10, 25, 50, and 100 ppm | Resulted in higher germination and seedling emergence percentages, as well as faster germination and seedling emergence | Korkmaz et al., 2009 | |
| 3 °C | Seed soaking, foliar spray and soil drench of 1, 10, 25, 50, and 100 ppm | Improved plant quality, chlorophyll content, sucrose, and proline content; enhanced SOD activity | Korkmaz et al., 2010 | ||
| Rice (Oryza sativa L.) | 3 °C, 5 °C | Root soaking of 0.001, 0.1, 1, and 5 ppm | Reduced cold injury-induced tissue electrolyte leakage | Hotta et al., 1998 | |
| 10 °C | Seed soaking of 8.5 mM | Increased antioxidant enzymes (SOD, POD, APX, and GPX) activity; increased relative gene expression of enzymes of PA biosynthesis | Sheteiwy et al., 2017 | ||
| Soybean (Glycine max L.) | 4 °C | Hydroponics of 5, 10, 15, 20, 30, and 40 μM | Increased chlorophyll content and relative water content of leaves; enhanced activity of antioxidant enzymes CAT and HO-1 | Balestrasse et al., 2010 | |
| Tomato (Solanum lycopersicum) | 15 °C/8 °C (day/night) | Foliar application of 5, 10, 25, 50, and 100 mg/L | ALA induced H2O2, which in turn increased the ratio of GSH and ASA, leading to enhanced antioxidant capacity; significantly increased the activities of SOD, CAT, APX, DHAR, and GSH | Liu et al., 2018 | |
| 15 °C/8 °C (day/night) |
Foliar application of 5, 10, 25, 50, and 100 mg/L | ALA pretreatment-induced CAT and ASA-GSH reliably eliminated excess ROS under low temperature stress and maintained redox homeostasis | Liu et al., 2018 | ||
| 15 °C/8 °C (day/night) |
Foliar application of 25 mg/L | ALA triggered NO production directly, or induced H2O2 and JA signals to trigger NO production, thus NO interacted with JA to regulate cold-induced oxidative stress | Liu et al., 2019 | ||
| Drought stress | Kentucky bluegrass (Poa pratensis L.) | 10% PEG 6000 | Foliar application of 10 mg/L | Improved turf quality and leaf relative water content; enhanced antioxidant enzymes (SOD, CAT, APX, GPX, DHAR, and GR), ASA, and GSH content, thus reducing oxidative damage | Niu et al., 2017 |
| Oilseed rape (Brassica napus L.) | Drought stress (40% of water-holding capacity) | Foliar application of 30 mg/L | Maintained relatively higher leaf water status; enhanced chlorophyll content and net photosynthetic rate; increased antioxidant enzyme (POD and CAT) activity | Liu et al., 2013 | |
| Drought stress (40% of water-holding capacity) | Foliar application of 30 mg/L | Expression of photosynthetic genes (RBCS, TPI, FBP, FBPA, and TKL) was upregulated; increased leaf hexose and sucrose accumulation and maintenance of starch content | Liu et al., 2016 | ||
| Sunflower (Helianthus annuus L.) | Water stress (70% field capacity) | Foliar application of 10, 20, and 30 mg/L | Reduced oxidative damage by lowering H2O2 and MDA contents | Rasheed et al., 2020 | |
| Drought stress (40% of water-holding capacity) | Foliar application of 25, 50, 75, and 100 mg/L | Enhancement of stay green and CAT, SOD, and APX activities, thus reducing drought-induced yield losses and improving oil contents | Sher et al., 2021 | ||
| Wheat (Triticum aestivum L.) | Irrigation interval of 7, 14, and 21 days | Foliar application of 25, 50, and 100 ppm | Increased grain yield | Al-Thabet et al., 2006 | |
| Water deficit (60% and 80% of field capacity) | Foliar application of 50, 100, and 150 mg/L | Improved leaf fluorescence (qN, NPQ, and Fv/Fm), shoot and root K+, root Ca2+, proline, and GB accumulation | Akram et al., 2018 | ||
| Water stress (30% maximum water capacity) | Foliar application of 30 mg/L | Increased plant growth, photosynthesis, and chlorophyll content; reduced the degree of damage to cell membranes during early nutritional development | Ostrowska et al., 2019 | ||
| 80% (mild drought stress), and 60% (high drought stress) | Foliar application of 50, 100, and 150 mg/L | Increased fresh and dry weight of shoots and roots, chlorophyll content, GB content, and N content in leaves and roots | Kosar et al., 2015 | ||
| UV-B stress | lettuce (Lactuca sativa L.) | 3.3 W m−2 UV-B | Foliar application of 10 and 25 ppm | ALA treatment resulted in a substantial increase in phenylalanine ammonia lyase (PAL) and γ-tocopherol methyltransferase (γ-TMT) gene expression, antioxidant enzyme activity, and chlorophyll a and b concentrations. | Aksakal et al., 2017 |
| Pigeon pea (Cajanus cajan L.) | enhanced UV-B (2.2 kJ m−2d−1) | Seed soaking of 25 and 100 μM | Reduced germination time and increased germination index; upregulated photosynthesis, antioxidant enzymes (CAT, SOD, and POD), total phenolic content, and total flavonoid content to balance ROS and reduce UV-B damage to plant productivity | Gupta et al., 2021 | |
| enhanced UV-B (2.2 kJ m−2d−1) | Seed soaking of 25 and 100 μM | Increased plant growth and growth regulating parameters; increased enzyme activity and non-enzymatic antioxidant content in the plant defense system and reduced oxidative stress in seedlings | Gupta et al., 2021 |
The optimum levels of ALA are shown in bold.
3.1. ALA Priming Alleviates Salt Stress
Salt stress is one of the most deleterious environmental factors that hamper agricultural productivity worldwide by stimulating osmotic stress, ionic toxicity, nutritional disorders, and oxidative stress simultaneously [24,25]. Salt stress results in an imbalance in intracellular ionic homeostasis, triggering the increase in Na+ concentration and the decrease in K+ concentration in plant tissues [26]. Priming with ALA increases the transcripts and protein accumulations of SOS1 (Na+/H+ antiporter) and HA3 (proton pump) on the plasma membrane (PM) as well as NHX1 (Na+/H+ antiporter) and VHA-A (proton pump) on the vesicle membrane compared with the unprimed cucumber (Cucumis sativus) in response to salt stress. The ion transporter proteins SOS1 and NHX1, with the energy provided by proton pump HA3 and VAH-A, help cucumber excrete Na+ from the cytoplasm or transfer it to the vesicles, resulting in a high-low-high osmotic potential in the vesicle-protoplast-exosome, and thus alleviating ion toxicity induced by salt stress. Pretreatment with ALA upregulates the expression of high-affinity K+ transporter protein 1 (HKT1) that regulates Na+/K+ homeostasis in cucumber cells and maintains normal metabolic activities in cells under salt stress conditions [27,28]. Proline accumulates in response to salinity and is a common compatible osmolyte in higher plants. Exogenous application of ALA upregulates delta-1-pyrroline-5-carboxylate synthase (P5CS) that controls the rate-limiting step of glutamate-derived proline biosynthesis in Oilseed rape (Brassica napus) and enhances tolerance to salt stress [26,29]. In addition, priming with ALA relieves cell oxidation stress caused by salt stress by improving the activity of antioxidant enzymes, including superoxide dismutase (SOD), catalase (CAT), and peroxidase (POD), and promoting the activity of enzymes involved in the ascorbate-glutathione cycle (AsA-GSH), including ascorbic acid oxidase (AAO), ascorbate peroxidase (APX), glutathione reductase (GR), dehydroascorbic acid reductase (DHAR), and monodehydroascorbic acid reductase (MDHAR) [30,31,32,33].
In addition to coping with osmotic stress and oxidative stress caused by salt stress, priming with ALA improves plant salt tolerance by increasing photosynthetic assimilation and promoting nitrogen metabolism. Cassia seed (Cassia obtusifolia), peach (Prunnus persica), and oilseed rape treated with ALA showed an increase in the net photosynthetic rate (Pn) and transpiration rate (Tr), as well as the photochemical efficiency of photosystem II (Fv/Fm) and the non-photochemical quenching (NPQ) during salt stress [29,31,34]. Application of ALA on the leaves leads to swollen chloroplasts and dilation of thylakoid membrane under salt stress conditions, thereby modifying photosynthetic sites and increasing photosynthetic efficiency [35]. Pretreatment with ALA decreases the expression of ferrochelatase, catalyzing the insertion of Fe2+ into protoporphyrin, in Oilseed rape in response to salt stress, thereby inhibiting heme synthesis and increasing chlorophyll synthesis [36]. Additionally, ALA treatment significantly increases the activities of nitrate reductase (NR), glutamine synthetase (GS), glutamate synthase (GOGAT), and glutamate dehydrogenase (GDH) and decreases the activity of nitrite reductase (NiR) in watermelon (Citrullus lanatus). This indicates that ALA regulates nitrogen metabolism to alleviate the cellular toxicity caused by the massive accumulation of nitrate and ammonium salts due to salt stress [37].
The induction of salt tolerance in plants by ALA may be achieved through nitric oxide (NO). ALA treatment increased NO and NOS activity in leaves, suggesting that ALA triggers NO synthesis by activating NOS, and thus improves salt tolerance in maize (Zea mays) [38]. In supporting this hypothesis, ALA-induced salt tolerance is completely abolished by treatment with a scavenger of NO, 2-4-carboxyphenyl-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (cPTIO), a stable free radical compound that reacts with NO to form an imino nitroxide free radical.
3.2. ALA Priming Increases Plant Tolerance to Extreme Temperature
The rate of plant growth and development depends on the temperature surrounding the plant. Considering that plants are sessile, their survival depends on the efficient activation of resistance responses to thermal stress. No organism can withstand the full range of biosphere temperatures, and each species has a specific temperature range represented by minimum, maximum, and optimum temperatures. Extreme temperature events are expected to become more intense and frequent and last longer than those observed in recent years [39]. Extreme temperature adversely affects almost all aspects of plant growth, development, reproduction, and yield [40]. Warm temperatures cause growth restriction, increase the transpiration rate, and damage photosynthetic organs in plants [41], while extreme low temperatures lead to membrane damage, inhibition of photosynthetic properties, and oxidative stress [42]. ALA-pretreated cucumber leaves had higher antioxidant enzyme activity, higher levels of proline and soluble sugar content, and weaker growth inhibition under high-temperature stress conditions [43]. Priming with ALA increases germination and seedling emergence in red pepper (Capsicum annuum) and reduces tissue electrolyte leakage in rice (Oryza sativa) under cold stress [44,45]. Pretreatment with ALA also increases chlorophyll content and photosynthetic capacity of cucumber and enhances ribulose-1,5-bisphosphate (RuBP) carboxylase activity in maize under cold stress conditions [46,47,48]. Furthermore, ALA treatment also improved the antioxidant capacity of plants in response to cold stress by increasing the activities of SOD, APX, GR, CAT, and heme oxygenase-1 (HO-1) in red pepper, drooping wild ryegrass (Elymus nutans), and soybean plants (Glycine max) [49,50,51]. Interestingly, ALA priming upregulates the expression levels of respiratory burst oxidase homologue1 (RBOH1) in tomato (Solanum lycopersicum) and leads to the production of H2O2, which serves as a signaling molecule to activate defense against cold stress [52].
In addition, studies in drooping wild ryegrass and tomato suggest that ALA may directly trigger NO production or indirectly promote NO production by inducing jasmonic acid (JA) and H2O2 [53,54]. NO activates antioxidant enzyme activity and PM H+-ATPase and maintains Na+/K+ homeostasis, thereby reducing cold stress-induced injury [55]. Moreover, priming with ALA induces the expressions of genes involved in the biosynthesis of PA in rice [7], which enhances tolerance to cold stress [56]. Besides, studies in cucumber have shown that ALA priming increases tolerance to cold stress by regulating the biosynthesis of classic phytohormones such as JA, indole-3-acetic acid (IAA), brassinosteroid (BR), cytokinins (CKs), gibberellin (GA4), and abscisic acid (ABA) during cold stress [47]. In the future, further studies are needed to investigate how ALA regulates phytohormone synthesis under cold stress conditions and whether it regulates key components of the phytohormone signaling pathway so as to further reveal the molecular mechanisms by which ALA enhances low-temperature tolerance in plants.
3.3. ALA Priming Mitigates Drought-Induced Damage
Under natural and agricultural conditions, plants are subject to various environmental stresses during growth and development. Among them, drought is one of the most severe environmental stresses, which occurs as a result of temperature dynamics, light intensity, and low rainfall and affects plant biomass production, quality, and energy. Drought stress limits photosynthesis in plants by causing stomatal closure and reduced water content, as well as leading to excessive production of reactive oxygen species, which can inhibit plant growth [57]. ALA pretreatment can maintain moisture in the seedlings of oilseed rape and Kentucky bluegrass (Poa pratensis), thus enhancing leaf relative water content (RWC) [58,59]. It can also increase the contents of proline and foliar N in wheat (Triticum aestivum), as well as Ca2+ in the roots under drought conditions [60,61,62]. In addition, in studies with Kentucky bluegrass and sunflower (Helianthus annuus), priming with ALA increases the activities of antioxidant enzymes such as catalase (CAT), superoxide dismutase (SOD), ascorbate peroxidase (APX), and glutathione reductase (GR), which reduce the production of ROS, including H2O2 content and O2•− production, thereby improving tolerance against drought stress [58,63]. Priming with ALA also preserves plant photosynthesis in oilseed rape, wheat, and sunflower by suppressing chlorophyll degradation and increasing photosynthetic rate (Pn) during drought stress [59,61,64,65]. Furthermore, pretreatment with ALA induces the expressions of enzymes involved in the Calvin cycle such as triose-3-phosphate isomerase (TPI) and fructose-1,6-bisphosphate aldolase (FBPA) [66]. Interestingly, in addition to enhance the drought resistance of plants, ALA priming also improves waterlogging tolerance in Fig (Ficus carica), with higher levels of antioxidant enzyme activity, photosynthetic efficiency, and root respiration [67].
3.4. ALA Priming Attenuates UV-B-Induced Damage
Ultraviolet-B (UV-B) radiation is a component of sunlight that induces several plant photomorphogenic responses, including hypocotyl growth inhibition and cotyledon curling [68]. High-intensity UV-B injures plants by damaging DNA, impaired photosynthesis, and cell death, and triggering the accumulation of ROS [69]. Priming with ALA was reported to significantly reduce plant damage from UV-B radiation by promoting photosynthesis, enhancing antioxidant capacity, and improving nitrogen metabolism. As a key precursor of chlorophyll biosynthesis, ALA alleviated the deficiency of chlorophyll biosynthesis during UV-B stress; ALA pretreatment upregulates the expression of genes involved in chlorophyll biosynthesis such as glutamyl-tRNA reductase (HEMA1), Mg-chelatase (CHLH), and protochlorophyllide oxidoreductase (POR) in pigeon pea (Cajanus cajan), thus promoting plant photosynthesis during UV-B stress [8,70]. In addition, ALA priming-increased activities of antioxidant enzymes are essential for lettuce (Lactuca sativa) resistance to UV-B stress [71]. In addition to enzymatic antioxidants, ALA also increases the content of non-enzymatic antioxidants such as flavonoids and phenolics [8]. Under UV-B stress conditions, ALA priming significantly improves the activities of nitrate reductase (NR), nitrite reductase (NiR), glutamine synthetase (GS), and glutamate synthase (GOGAT), and then increases the levels of NO3− and NO2− in the seedlings of pigeon pea [70]. Collectively, ALA priming contributes to UV-B tolerance by regulating photosynthesis, antioxidant, and nitrogen metabolism in plants.
4. Application of ALA in Agriculture and Medicine
ALA is a growth regulator that regulates the growth and development of plants at different growth stages. Soaking seeds with ALA reduces the germination time and increases the germination index of pigeon pea, and promotes Arabidopsis thaliana root elongation by regulating auxin transport [8,72]. ALA significantly increased yields through root or foliar fertilization in grapevines (Vitis vinifera) [73]. The shoot application of ALA stimulates the growth of maize seedlings [74]. ALA-based fertilizer can significantly increase the growth of tomato and date palm (Phoenix dactylifera) [75,76,77]. Rhizospheric application of ALA significantly increases the endogenous ALA content and improves fruit coloration and interior qualities in apple cultivar (Malus domestica) [78].
A dose-effect was observed in the application of ALA in plants, implying that high concentrations of ALA are harmful to plants. For example, low concentrations of ALA (0.5 or 1 mg/L) increase the biomass and the content of various bioactive compounds in oilseed rape, whereas high concentrations of ALA (5 or 10 mg/L) cause oxidative stress, which is detrimental to the growth of oilseed rape [79]. Using this property, ALA is also used as a “photodynamic herbicide” in agriculture [15]. Plants treated with high concentrations of ALA accumulate excess PpIX, which produces ROS in the light, thus causing damage to the plants [80]. Foliar spray of 5 mM ALA resulted in the formation of white necrotic spots on the leaves of rice plants [81]. More interestingly, some herbicides such as diphenyl ether herbicides kill plants by stimulating excessive ALA production, which leads to the accumulation of PpIX in plant cells [82]. As expected, these herbicides are more toxic when used in combination with ALA and enhance weed control [83].
In medicine, ALA is widely used in photodynamic therapy (PDT) [84,85,86], a treatment for certain cancers and precancerous diseases using photosensitizers and lasers. ALA itself is not a photosensitizer, but a precursor substance for the photosensitizer PpIX [87]. When excessive ALA is applied, it is selectively absorbed by proliferating active cells and converted intracellularly into porphyrins such as PpIX. Intracellular PpIX can be activated, leading to ROS generation after exposure to lasers, which is ultimately toxic to tumor cells [88]. Topical ALA-PDT has also been used to treat the tumor or non-tumor dermatoses, which is non-invasive and has better therapeutic effects compared with conventional treatments [89,90,91].
5. Conclusions and Future Perspectives
Dramatic global climate change has triggered high temperatures and droughts in some regions and floods in others, seriously threatening crop growth and food security worldwide. Priming significantly improves plant tolerance to various biotic and abiotic stresses, which provides a strategy for improving crop yield and quality in a non-optimal environment [1]. ALA, as a plant growth regulator and a precursor substance of chlorophyll, plays an important role in inducing defense priming [15,16]. Here, we review the remarkable role of ALA priming in plant tolerance. Priming with ALA can activate NO, H2O2, and hormone signals and strengthen photosynthesis, antioxidant capacity, osmoregulation, and nitrogen assimilation, thus helping plants to obtain a stronger ability to cope with abiotic stresses. High concentrations of ALA function as an herbicide because it causes oxidative stress. Curiously, different plant species have different adaptations to high concentrations of ALA, which may be owing to differences in photosynthetic patterns.
Currently, the use of ALA to activate plant resistance is mainly through seed dips, foliar sprays, and soil watering. The use of operable promoters to construct transgenic plants to produce ALA will facilitate the study of the molecular mechanisms by which ALA regulates plant development and increases plant stress tolerance. Regretfully, most of the current studies on ALA priming have focused on the measurement of physiological parameters; we still know very little about its molecular or biochemical mechanisms. One of the reasons may be that we do not yet dissect the signaling pathways of ALA. For example, is there an ALA receptor? and what are the key components of the ALA signaling pathway? We could address these issues in two ways. (i) Identification of the key components of ALA signaling by screening for mutants with an altered response to ALA through a forward genetic approach. (ii) Identification of ALA-binding proteins (ABP) through biochemical pathways, and then investigation of their functions through a reverse genetic strategy using genome editing techniques. The availability of these genetic materials will help to reveal the physiological functions of ALA and the molecular basis of ALA priming-enhanced plant resistance against numerous environmental stresses.
Acknowledgments
We sincerely apologize to those authors whose work is not included in this review because of space limitations.
Author Contributions
Conceptualization, Z.L.; writing—original draft preparation, S.T.; writing—review and editing, Z.L.; visualization, J.C.; supervision, Z.L. and X.X.; project administration, Z.L.; funding acquisition, Z.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by grants from the National Natural Science Foundation of China (32170345, 31970196, and 32011540381 to Z.L.), and the startup funding for plant aging research from Beijing Forestry University (BJFU2021YJRC00600K).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Martinez-Medina A., Flors V., Heil M., Mauch-Mani B., Pieterse C.M.J., Pozo M.J., Ton J., van Dam N.M., Conrath U. Recognizing Plant Defense Priming. Trends Plant Sci. 2016;21:818–822. doi: 10.1016/j.tplants.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 2.Mauch-Mani B., Baccelli I., Luna E., Flors V. Defense Priming: An Adaptive Part of Induced Resistance. Annu. Rev. Plant Biol. 2017;68:485–512. doi: 10.1146/annurev-arplant-042916-041132. [DOI] [PubMed] [Google Scholar]
- 3.Díaz-Valle A., López-Calleja A.C., Alvarez-Venegas R. Enhancement of Pathogen Resistance in Common Bean Plants by Inoculation With Rhizobium etli. Front. Plant Sci. 2019;10:1317. doi: 10.3389/fpls.2019.01317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim J.I., Baek D., Park H.C., Chun H.J., Oh D.H., Lee M.K., Cha J.Y., Kim W.Y., Kim M.C., Chung W.S., et al. Overexpression of Arabidopsis YUCCA6 in potato results in high-auxin developmental phenotypes and enhanced resistance to water deficit. Mol. Plant. 2013;6:337–349. doi: 10.1093/mp/sss100. [DOI] [PubMed] [Google Scholar]
- 5.Singh P., Yekondi S., Chen P.-W., Tsai C.-H., Yu C.-W., Wu K., Zimmerli L. Environmental History Modulates Arabidopsis Pattern-Triggered Immunity in a HISTONE ACETYLTRANSFERASE1–Dependent Manner. Plant Cell. 2014;26:2676–2688. doi: 10.1105/tpc.114.123356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Savvides A., Ali S., Tester M., Fotopoulos V. Chemical Priming of Plants Against Multiple Abiotic Stresses: Mission Possible? Trends Plant Sci. 2016;21:329–340. doi: 10.1016/j.tplants.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Mohamed S., Hangqi S., Jungui X., Yajing G., Wenjian S., Jin H. Seed polyamines metabolism induced by seed priming with spermidine and 5-aminolevulinic acid for chilling tolerance improvement in rice (Oryza sativa L.) seedlings. Environ. Exp. Bot. 2017;137:58–72. [Google Scholar]
- 8.Gupta D., Prasad S.M. Priming with 5-aminolevulinic acid (ALA) attenuates UV-B induced damaging effects in two varieties of Cajanus cajan L. seedlings by regulating photosynthetic and antioxidant systems. S. Afr. J. Bot. 2021;138:129–140. doi: 10.1016/j.sajb.2020.12.009. [DOI] [Google Scholar]
- 9.Conrath U., Beckers G.J.M., Langenbach C.J.G., Jaskiewicz M.R. Priming for Enhanced Defense. Annu. Rev. Phytopathol. 2015;53:97–119. doi: 10.1146/annurev-phyto-080614-120132. [DOI] [PubMed] [Google Scholar]
- 10.Tanou G., Filippou P., Belghazi M., Job D., Diamantidis G., Fotopoulos V., Molassiotis A. Oxidative and nitrosative-based signaling and associated post-translational modifications orchestrate the acclimation of citrus plants to salinity stress. Plant J. 2012;72:585–599. doi: 10.1111/j.1365-313X.2012.05100.x. [DOI] [PubMed] [Google Scholar]
- 11.Li M., Yu G., Ma J., Liu P. Interactions of importers in long-distance transmission of wound-induced jasmonate. Plant Signal. Behav. 2021;16:1886490. doi: 10.1080/15592324.2021.1886490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee H.-R., Lee S., Park S., van Kleeff P.J.M., Schuurink R.C., Ryu C.-M. Transient Expression of Whitefly Effectors in Nicotiana benthamiana Leaves Activates Systemic Immunity Against the Leaf Pathogen Pseudomonas syringae and Soil-Borne Pathogen Ralstonia solanacearum. Front. Ecol. Evol. 2018;6:90. doi: 10.3389/fevo.2018.00090. [DOI] [Google Scholar]
- 13.Ramírez-Carrasco G., Martínez-Aguilar K., Alvarez-Venegas R. Transgenerational Defense Priming for Crop Protection against Plant Pathogens: A Hypothesis. Front. Plant Sci. 2017;8:696. doi: 10.3389/fpls.2017.00696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Antoniou C., Savvides A., Christou A., Fotopoulos V. Unravelling chemical priming machinery in plants: The role of reactive oxygen–nitrogen–sulfur species in abiotic stress tolerance enhancement. Curr. Opin. Plant Biol. 2016;33:101–107. doi: 10.1016/j.pbi.2016.06.020. [DOI] [PubMed] [Google Scholar]
- 15.Sasaki K., Watanabe M., Tanaka T., Tanaka T. Biosynthesis, biotechnological production and applications of 5-aminolevulinic acid. Appl. Microbiol. Biotechnol. 2002;58:23–29. doi: 10.1007/s00253-001-0858-7. [DOI] [PubMed] [Google Scholar]
- 16.Agrawal S., Karcher D., Ruf S., Bock R. The Functions of Chloroplast Glutamyl-tRNA in Translation and Tetrapyrrole Biosynthesis1 [OPEN] Plant Physiol. 2020;183:263–276. doi: 10.1104/pp.20.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iida K., Kajiwara M. Carbon Source Dependence of the Ratio of δ-Aminolevulinic Acid Biosynthesis via the C5 and Shemin Pathways in Euglena Gracilis (Euglenophyceae)1. J. Phycol. 2008;44:292–298. doi: 10.1111/j.1529-8817.2008.00466.x. [DOI] [PubMed] [Google Scholar]
- 18.Wu Y., Liao W., Dawuda M.M., Hu L., Yu J. 5-Aminolevulinic acid (ALA) biosynthetic and metabolic pathways and its role in higher plants: A review. Plant Growth Regul. 2019;87:357–374. doi: 10.1007/s10725-018-0463-8. [DOI] [Google Scholar]
- 19.Senge M.O., Ryan A.A., Letchford K.A., MacGowan S.A., Mielke T. Chlorophylls, Symmetry, Chirality, and Photosynthesis. Symmetry. 2014;6:781–843. doi: 10.3390/sym6030781. [DOI] [Google Scholar]
- 20.Kang Z., Zhang J., Zhou J., Qi Q., Du G., Chen J. Recent advances in microbial production of δ-aminolevulinic acid and vitamin B12. Biotechnol. Adv. 2012;30:1533–1542. doi: 10.1016/j.biotechadv.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 21.Yang P., Liu W., Cheng X., Wang J., Wang Q., Qi Q., Liu S.-J. A New Strategy for Production of 5-Aminolevulinic Acid in Recombinant Corynebacterium glutamicum with High Yield. Appl. Environ. Microbiol. 2016;82:2709–2717. doi: 10.1128/AEM.00224-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noh M.H., Lim H.G., Park S., Seo S.W., Jung G.Y. Precise flux redistribution to glyoxylate cycle for 5-aminolevulinic acid production in Escherichia coli. Metab. Eng. 2017;43:1–8. doi: 10.1016/j.ymben.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 23.Chen B., Li J., Feng Y., Le K., Zai Y., Tang X., Sun Y., Zeng X., Lin L. Green and mild production of 5-aminolevulinic acid from algal biomass. Korean J. Chem. Eng. 2021;38:899–905. doi: 10.1007/s11814-021-0774-8. [DOI] [Google Scholar]
- 24.Mohsin T., Adnan Noor S. An insight into salt stress tolerance mechanisms of Chenopodium album. Environ. Sci. Pollut. Res. 2017;24:16531–16535. doi: 10.1007/s11356-017-9337-2. [DOI] [PubMed] [Google Scholar]
- 25.Zhao S., Zhang Q., Liu M., Zhou H., Ma C., Wang P. Regulation of Plant Responses to Salt Stress. Int. J. Mol. Sci. 2021;22:4609. doi: 10.3390/ijms22094609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liyun L., Nguyen Tran N., Akihiro U., Hirofumi S. Effects of 5-aminolevulinic acid on Swiss chard (Beta vulgaris L. subsp. cicla) seedling growth under saline conditions. Plant Growth Regul. 2014;74:219–228. [Google Scholar]
- 27.Anirban B. Strawberries under salt stress: ALA and ROS to the rescue. Physiol. Plant. 2019;167:2–4. doi: 10.1111/ppl.13010. [DOI] [PubMed] [Google Scholar]
- 28.Yue W., Na L., Linli H., Weibiao L., Zhongqi T., Xuemei X., Jian L., Jianming X., Alejandro C.-U., Jihua Y. 5-Aminolevulinic Acid Improves Morphogenesis and Na+ Subcellular Distribution in the Apical Cells of Cucumis sativus L. under Salinity Stress. Front. Plant Sci. 2021;12:404. doi: 10.3389/fpls.2021.636121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jun-Lan X., Hang-Chao W., Xiao-Yu T., Chun-Lei Z., Muhammad Shahbaz N. 5-aminolevulinic acid improves salt tolerance mediated by regulation of tetrapyrrole and proline metabolism in Brassica napus L. seedlings under NaCl stress. Plant Physiol. Biochem. 2018;124:88–99. doi: 10.1016/j.plaphy.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 30.Nudrat Aisha A., Muhammad A., Al-Qurainy F. Aminolevulinic acid-induced changes in some key physiological attributes and activities of antioxidant enzymes in sunflower (Helianthus annuus L.) plants under saline regimes. Sci. Hortic-Amst. 2012;142:143–148. [Google Scholar]
- 31.Jia Y., Qiang C., Ting T., Gui W., Feng X. Promotive effects of 5-Aminolevulinic acid on growth, photosynthetic gas exchange, chlorophyll, and antioxidative enzymes under salinity stress in Prunnus persica (L.) Batseh Seedling. Emir. J. Food Agric. 2016:786–795. doi: 10.9755/ejfa.2016-06-647. [DOI] [Google Scholar]
- 32.Mohamed E.-E., Ibrahim A., Abdulaziz A., Hayssam A., Aisha A., Jacques W., Margaret A. Genetic Variation and Alleviation of Salinity Stress in Barley (Hordeum vulgare L.) Molecules. 2018;23:2488. doi: 10.3390/molecules23102488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yue W., Linli H., Weibiao L., Mohammed Mujitaba D., Jian L., Jianming X., Zhi F., Alejandro C.-U., Jihua Y. Foliar application of 5-aminolevulinic acid (ALA) alleviates NaCl stress in cucumber (Cucumis sativus L.) seedlings through the enhancement of ascorbate-glutathione cycle. Sci. Hortic-Amst. 2019;257:108761. [Google Scholar]
- 34.Chun-Ping Z., Yi-Cun L., Feng-Gang Y., Shi-Jun H., Hai-Ying L., Ping H. Role of 5-aminolevulinic acid in the salinity stress response of the seeds and seedlings of the medicinal plant Cassia obtusifolia L. Bot. Stud. 2013;54:1–13. doi: 10.1186/1999-3110-54-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muhammad S.N., Hasitha W., Hongbo L., Dan L., Rashid A., Ejaz Ahmad W., Ling X., Weijun Z. 5-Aminolevulinic acid alleviates the salinity-induced changes in Brassica napus as revealed by the ultrastructural study of chloroplast. Plant Physiol. Biochem. 2012;57:84–92. doi: 10.1016/j.plaphy.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 36.Yue W., Xin J., Weibiao L., Linli H., Mohammed M.D., Xingjie Z., Zhongqi T., Tingyu G., Jihua Y. 5-Aminolevulinic Acid (ALA) Alleviated Salinity Stress in Cucumber Seedlings by Enhancing Chlorophyll Synthesis Pathway. Front. Plant Sci. 2018;9:635. doi: 10.3389/fpls.2018.00635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen G., Fan P.S., Feng W.M., Guan A.Q., Lu Y.Y., Wan Y.L. Effects of 5-aminolevulinic acid on nitrogen metabolism and ion distribution of watermelon seedlings under salt stress. Russ. J. Plant Physiol. 2017;64:116–123. doi: 10.1134/S1021443717010046. [DOI] [Google Scholar]
- 38.Cengiz K., Muhammad A. Nitric Oxide is Required for Aminolevulinic Acid-Induced Salt Tolerance by Lowering Oxidative Stress in Maize (Zea mays) J. Plant Growth Regul. 2020;40:617–627. [Google Scholar]
- 39.Hatfield J.L., Prueger J.H. Temperature extremes: Effect on plant growth and development. Weather Clim. Extrem. 2015;10:4–10. doi: 10.1016/j.wace.2015.08.001. [DOI] [Google Scholar]
- 40.Liu Q., Yan S., Yang T., Zhang S., Chen Y.-Q., Liu B. Small RNAs in regulating temperature stress response in plants. J. Integr. Plant Biol. 2017;59:774–791. doi: 10.1111/jipb.12571. [DOI] [PubMed] [Google Scholar]
- 41.Fahad S., Bajwa A.A., Nazir U., Anjum S.A., Farooq A., Zohaib A., Sadia S., Nasim W., Adkins S., Saud S., et al. Crop Production under Drought and Heat Stress: Plant Responses and Management Options. Front. Plant Sci. 2017;8:1147. doi: 10.3389/fpls.2017.01147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khan T.A., Fariduddin Q., Yusuf M. Low-temperature stress: Is phytohormones application a remedy? Environ. Sci. Pollut. Res. 2017;24:21574–21590. doi: 10.1007/s11356-017-9948-7. [DOI] [PubMed] [Google Scholar]
- 43.Zhang J., Li D.M., Gao Y., Yu B., Xia C.X., Bai J.G. Pretreatment with 5-aminolevulinic acid mitigates heat stress of cucumber leaves. Biol. Plant. 2012;56:780–784. doi: 10.1007/s10535-012-0136-9. [DOI] [Google Scholar]
- 44.Ahmet K., Yakup K. Promotion by 5-aminolevulenic acid of pepper seed germination and seedling emergence under low-temperature stress. Sci. Hortic-Amst. 2009;119:98–102. [Google Scholar]
- 45.Hotta Y., Tanaka T., Luo B., Takeuchi Y., Konnai M. Improvement of Cold Resistance in Rice Seedlings by 5-Aminolevulinic Acid. J. Pestic. Sci. 1998;23:29–33. doi: 10.1584/jpestics.23.29. [DOI] [Google Scholar]
- 46.Wang Y., Li J., Gu W., Zhang Q., Tian L., Guo S., Wei S. Exogenous application of 5-aminolevulinic acid improves low-temperature stress tolerance of maize seedlings. Crop Pasture Sci. 2018;69:587–593. doi: 10.1071/CP17401. [DOI] [Google Scholar]
- 47.Ali A., Yan Y., Yumei L., Yansu L., Xianchang Y. 5-Aminolevulinic Acid Improves Nutrient Uptake and Endogenous Hormone Accumulation, Enhancing Low-Temperature Stress Tolerance in Cucumbers. Int. J. Mol. Sci. 2018;19:3379. doi: 10.3390/ijms19113379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ali A., Jun W., Xianchang Y., Chaoxing H., Yansu L. Substrate Application of 5-Aminolevulinic Acid Enhanced Low-temperature and Weak-light Stress Tolerance in Cucumber (Cucumis sativus L.) Agronomy. 2020;10:472. [Google Scholar]
- 49.Karina B.B., María L.T., Alcira B., Guillermo O.N. The role of 5-aminolevulinic acid in the response to cold stress in soybean plants. Phytochemistry. 2010;71:2038–2045. doi: 10.1016/j.phytochem.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 50.Ahmet K., Yakup K., Ali Rıza D. Enhancing chilling stress tolerance of pepper seedlings by exogenous application of 5-aminolevulinic acid. Environ. Exp. Bot. 2010;67:495–501. [Google Scholar]
- 51.Juanjuan F., Yongfang S., Xitong C., Yuefei X., Tianming H. Exogenous 5-aminolevulenic acid promotes seed germination in Elymus nutans against oxidative damage induced by cold stress. PLoS ONE. 2014;9:e107152. doi: 10.1371/journal.pone.0107152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu T., Xu J., Zhang J., Li J., Hu X. Exogenous 5-aminolevulinic acid pretreatment ameliorates oxidative stress triggered by low-temperature stress of Solanum lycopersicum. Acta Physiol Plant. 2018;40:210. doi: 10.1007/s11738-018-2788-3. [DOI] [Google Scholar]
- 53.Juanjuan F., Xitong C., Yongfang S., Yanjun M., Yuefei X., Tianming H. Nitric Oxide Mediates 5-Aminolevulinic Acid-Induced Antioxidant Defense in Leaves of Elymus nutans Griseb. Exposed to Chilling Stress. PLoS ONE. 2015;10:e0130367. doi: 10.1371/journal.pone.0130367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tao L., Jiaojiao X., Jianming L., Xiaohui H. NO is involved in JA- and H2O2-mediated ALA-induced oxidative stress tolerance at low temperatures in tomato. Environ. Exp. Bot. 2019;161:334–343. [Google Scholar]
- 55.Fu J.J., Chu X.T., Sun Y.F., Xu Y.F., Hu T.M. Involvement of nitric oxide in 5-aminolevulinic acid-induced antioxidant defense in roots of Elymus nutans exposed to cold stress. Biol. Plant. 2016;60:585–594. doi: 10.1007/s10535-016-0635-1. [DOI] [Google Scholar]
- 56.Megha S., Basu U., Kav N.N.V. Metabolic engineering of cold tolerance in plants. Biocatal. Agric. Biotechnol. 2014;3:88–95. doi: 10.1016/j.bcab.2013.11.007. [DOI] [Google Scholar]
- 57.Yuan S., Cuiting W., Han Y.H.C., Honghua R. Response of Plants to Water Stress: A Meta-Analysis. Front. Plant Sci. 2020;11:978. doi: 10.3389/fpls.2020.00978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kuiju N., Xiang M., Guoling L., Huiling M., Zhifeng J., Wenhui L., Qianqian Y. 5-Aminolevulinic acid modulates antioxidant defense systems and mitigates drought-induced damage in Kentucky bluegrass seedlings. Protoplasma. 2017;254:2083–2094. doi: 10.1007/s00709-017-1101-4. [DOI] [PubMed] [Google Scholar]
- 59.Dan L., Lingtong W., Muhammad Shahbaz N., Hongbo L., Xiangqin D., Ling X., Fan Z., Weijun Z. 5-Aminolevulinic acid enhances photosynthetic gas exchange, chlorophyll fluorescence and antioxidant system in oilseed rape under drought stress. Acta Physiol. Plant. 2013;35:2747–2759. [Google Scholar]
- 60.Al-Thabet S.S. Promotive Effect of 5-amino Levulinic Acid on Growth and Yield of Wheat Grown under Dry Conditions. J. Agron. 2006;5:45–49. doi: 10.3923/ja.2006.45.49. [DOI] [Google Scholar]
- 61.Nudrat Aisha A., Shamim K., Naila F., Muhammad A., Fahad A.-Q. 5−Aminolevulinic Acid Induces Regulation in Growth, Yield and Physio-Biochemical Characteristics of Wheat under Water Stress. Sains Malays. 2018;47:661–670. [Google Scholar]
- 62.Kosar F., Akram N.A., Ashraf M. Exogenously-applied 5-aminolevulinic acid modulates some key physiological characteristics and antioxidative defense system in spring wheat (Triticum aestivum L.) seedlings under water stress. S. Afr. J. Bot. 2015;96:71–77. doi: 10.1016/j.sajb.2014.10.015. [DOI] [Google Scholar]
- 63.Ahmad S., Anum Samreen T., Abdul S., Ahmad N., Abdul Q., Shabir H., Abdul M. Foliage application of 5-aminolevulinic acid alleviates drought stress in sunflower (Helianthus annuus L.) through improving stay green and antioxidant enzymes activities. Acta Physiol. Plant. 2021;43:1–7. [Google Scholar]
- 64.Ostrowska A., Biesaga-Kościelniak J., Grzesiak M.T., Hura T. Physiological responses of spring wheat to 5-aminolevulinic acid under water stress applied at seedling stage. Cereal Res. Commun. 2019;47:32–41. doi: 10.1556/0806.46.2018.060. [DOI] [Google Scholar]
- 65.Rizwan R., Humaira Y., Iqbal H., Muhammad I., Muhammad Arslan A., Abida P. Exogenously applied 5-aminolevulinic acid modulates growth, secondary metabolism and oxidative defense in sunflower under water deficit stress. Physiol. Mol. Biol. Plants. 2020;26:489–499. doi: 10.1007/s12298-019-00756-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu D., Hu L.Y., Ali B., Yang A.G., Wan G.L., Xu L., Zhou W.J. Influence of 5-aminolevulinic acid on photosynthetically related parameters and gene expression in Brassica napus L. under drought stress. Soil Sci. Plant Nutr. 2016;62:254–262. doi: 10.1080/00380768.2016.1198216. [DOI] [Google Scholar]
- 67.Yuyan A., Lin Q., Liangju W. ALA Pretreatment Improves Waterlogging Tolerance of Fig Plants. PLoS ONE. 2016;11:e0147202. doi: 10.1371/journal.pone.0147202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dotto M., Casati P. Developmental reprogramming by UV-B radiation in plants. Plant Sci. 2017;264:96–101. doi: 10.1016/j.plantsci.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 69.Shi C., Liu H. How plants protect themselves from ultraviolet-B radiation stress. Plant Physiol. 2021;187:1096–1103. doi: 10.1093/plphys/kiab245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Divya G., Sheo Mohan P. 5-aminolevulinic acid (ALA) regulates photosynthetic performance and nitrogen metabolism status in UV-B challenged Cajanus cajan L. seedlings. J. Plant Biochem. Biot. 2021 doi: 10.1007/s13562-021-00672-2. [DOI] [Google Scholar]
- 71.Ozkan A., Omer Faruk A., Feyza Icoglu A., Ferhunde A. Exogenous 5-aminolevulinic acid alleviates the detrimental effects of UV-B stress on lettuce (Lactuca sativa L) seedlings. Acta Physiol Plant. 2017;39:55. [Google Scholar]
- 72.An Y.Y., Cheng D.X., Rao Z.X., Sun Y.P., Tang Q., Wang L.J. 5-Aminolevulinic acid (ALA) promotes primary root elongation through modulation of auxin transport in Arabidopsis. Acta Physiol. Plant. 2019;41:6. doi: 10.1007/s11738-019-2878-x. [DOI] [Google Scholar]
- 73.Watanabe K., Nishihara E., Watanabe S., Tanaka T., Takahashi K., Takeuchi Y. Enhancement of growth and fruit maturity in 2-year-old grapevines cv. Delaware by 5-aminolevulinic acid. Plant Growth Regul. 2006;49:35–42. doi: 10.1007/s10725-006-0024-4. [DOI] [Google Scholar]
- 74.Yonezawa T., Sunohara Y., Matsumoto H. Involvement of heme synthesis in the growth stimulation of maize seedlings by 5-aminolevulinic acid. Weed Biol. Manag. 2015;15:53–60. doi: 10.1111/wbm.12064. [DOI] [Google Scholar]
- 75.Tarek Y., Mohamed A.A. Mechanisms of Enhancing Photosynthetic Gas Exchange in Date Palm Seedlings (Phoenix dactylifera L.) under Salinity Stress by a 5-Aminolevulinic Acid-based Fertilizer. J. Plant Growth Regul. 2007;27:1–9. [Google Scholar]
- 76.Mohamed A.A. Promotive effects of a 5-aminolevulinic acid-based fertilizer on growth of tissue culture-derived date palm plants (Phoenix dactylifera L.) during acclimatization. Sci. Hortic-Amst. 2008;118:48–52. [Google Scholar]
- 77.Mahmoud A., Ali A. Influence of Pentakeep-V on the nutrient interaction and availability for tomato production. Emir. J. Food Agric. 2010;22:174–188. [Google Scholar]
- 78.Zheng J., An Y.Y., Feng X.X., Wang L.J. Rhizospheric application with 5-aminolevulinic acid improves coloration and quality in ‘Fuji’ apples. Sci. Hortic-Amst. 2017;224:74–83. doi: 10.1016/j.scienta.2017.06.004. [DOI] [Google Scholar]
- 79.Maodzeka A., Wang Q., Chen X.Y., Hussain N., Wu D.Z., Jiang L. Effects of 5-aminolevulinic Acid on the Bioactive Compounds and Seedling Growth of Oilseed Rape (Brassica napus L.) J. Plant Biol. 2019;62:181–194. doi: 10.1007/s12374-018-0299-9. [DOI] [Google Scholar]
- 80.Rebeiz C.A., Montazer-Zouhoor A., Hopen H.J., Wu S.M. Photodynamic herbicides: 1. Concept and phenomenology. Enzym. Microb. Technol. 1984;6:390–396. doi: 10.1016/0141-0229(84)90012-7. [DOI] [Google Scholar]
- 81.Phung T.-H., Jung S. Perturbed porphyrin biosynthesis contributes to differential herbicidal symptoms in photodynamically stressed rice (Oryza sativa) treated with 5-aminolevulinic acid and oxyfluorfen. Pestic. Biochem. Physiol. 2014;116:103–110. doi: 10.1016/j.pestbp.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 82.Hiroyuki K., Tatsuru M., Shooichi M. Action mechanism of diphenyl ether herbicides; Stimulation of 5-aminolevulinic acid-synthesizing system activities. Pestic. Biochem. Physiol. 1989;33:230–238. [Google Scholar]
- 83.Ling X., Wenfang Z., Basharat A., Faisal I., Jinwen Z., Weijun Z. Synergism of herbicide toxicity by 5-aminolevulinic acid is related to physiological and ultra-structural disorders in crickweed (Malachium aquaticum L.) Pestic. Biochem. Physiol. 2015;125:53–61. doi: 10.1016/j.pestbp.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 84.Koizumi N., Harada Y., Minamikawa T., Tanaka H., Otsuji E., Takamatsu T. Recent advances in photodynamic diagnosis of gastric cancer using 5-aminolevulinic acid. World J. Gastroentero. 2016;22:1289–1296. doi: 10.3748/wjg.v22.i3.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Keiji I. 5-Aminolevulinic acid-mediated photodynamic therapy for bladder cancer. Int. J. Urol. 2017;24:97–101. doi: 10.1111/iju.13291. [DOI] [PubMed] [Google Scholar]
- 86.Peiru W., Guolong Z., Linglin Z., Zhongxia Z., Lei S., Qingyu Z., Lude Z., Xiuli W. 5-Aminolevulinic acid photodynamic therapy for early-stage lip squamous cell carcinoma. Photodiagn. Photodyn. Ther. 2021;35:102321. doi: 10.1016/j.pdpdt.2021.102321. [DOI] [PubMed] [Google Scholar]
- 87.Di Venosa G., Fukuda H., Batlle A., MacRobert A., Casas A. Photodynamic therapy: Regulation of porphyrin synthesis and hydrolysis from ALA esters. J. Photochem. Photobiol. B Biol. 2006;83:129–136. doi: 10.1016/j.jphotobiol.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 88.Chi Z., Ting G., Jiajia W., Adriana C., Jack J.J. Topical Application of 5-Aminolevulinic Acid Is Sufficient for Photodynamic Therapy on Vocal Folds. Laryngoscope. 2018;129:E80–E86. doi: 10.1002/lary.27437. [DOI] [PubMed] [Google Scholar]
- 89.Cappugi P., Campolmi P., Mavilia L., Prignano F., Rossi R. Topical 5-aminolevulinic acid and photodynamic therapy in dermatology: A minireview. J. Chemother. 2002;13:494–502. doi: 10.1179/joc.2001.13.5.494. [DOI] [PubMed] [Google Scholar]
- 90.Amit K.J., Chang Hyun L., Harvinder S.G. 5-Aminolevulinic acid coated microneedles for photodynamic therapy of skin tumors. J. Control. Release. 2016;239:72–81. doi: 10.1016/j.jconrel.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 91.Wei Z., Xiao-Feng S., Chang-Liang W., Xin-Zhou L., Zhen L., Hai-Lu X., Zhong-Wei L., Rong-Tao Z., Jian-Ling H., Hong-Qing T. Topical 5-aminolevulinic acid photodynamic therapy for intra anal-rectal warts. J. Dermatol. Treat. 2019;31:241–244. doi: 10.1080/09546634.2019.1594670. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.