Abstract
CXCL1 is one of the most important chemokines, part of a group of chemotactic cytokines involved in the development of many inflammatory diseases. It activates CXCR2 and, at high levels, CXCR1. The expression of CXCL1 is elevated in inflammatory reactions and also has important functions in physiology, including the induction of angiogenesis and recruitment of neutrophils. Due to a lack of reviews that precisely describe the regulation of CXCL1 expression and function, in this paper, we present the mechanisms of CXCL1 expression regulation with a special focus on cancer. We concentrate on the regulation of CXCL1 expression through the regulation of CXCL1 transcription and mRNA stability, including the involvement of NF-κB, p53, the effect of miRNAs and cytokines such as IFN-γ, IL-1β, IL-17, TGF-β and TNF-α. We also describe the mechanisms regulating CXCL1 activity in the extracellular space, including proteolytic processing, CXCL1 dimerization and the influence of the ACKR1/DARC receptor on CXCL1 localization. Finally, we explain the role of CXCL1 in cancer and possible therapeutic approaches directed against this chemokine.
Keywords: CXCL1, MGSA, Gro-α, CXCR2, inflammation, cancer, tumor, chemokine, neutrophil
1. Introduction
Intercellular signaling is an essential part of the functioning of a multicellular organism, involving the flow of information between different cells through either direct contact or via factors secreted outside the cell: simple compounds such as lactate [1], or large structures such as extracellular vesicles [2]. One group of factors responsible for intercellular signaling is chemokines, a group of about 50 chemotactic cytokines grouped according to the conserved N-terminal cysteine motif: CXC chemokines (α-chemokines), CC chemokines (β-chemokines), C chemokines (γ-chemokines) and CX3C chemokines (δ-chemokines) [3].
CXC motif chemokine ligand 1 (CXCL1) belongs to the sub-family of CXC chemokines [3,4]. Considering the number of available scientific papers, CXCL1 is one of the four most studied CXC chemokines, with more than 5000 experimental papers available on PubMed (https://pubmed.ncbi.nlm.nih.gov; accessed date: 15 November 2021), with more than 1000 of these on the role of CXCL1 in cancer. Although each year more than 400 new papers are published about this chemokine, there are no available review papers summarizing the current knowledge in this area. This paper is part of a greater project that aims to provide a comprehensive series of reviews on CXCL1; our focus is on the regulation of expression and activity of this chemokine in the intercellular space.
2. The Name ‘CXCL1’
CXCL1 was first described in the 1980s as an auto-stimulatory melanoma mitogen, to which it owes one of its first names: melanoma growth-stimulatory activity (MGSA) [5]. Another historical name of CXCL1 is neutrophil-activating peptide-3 (NAP-3), a term associated with its ability to induce neutrophil chemotaxis [6]. In subsequent years, CXCL1 was shown to be a product of the growth-regulated gene (Gro) [7,8]. Since then, CXCL1 has functioned under two names: MGSA and growth-regulated (or -related) oncogene (GRO) [8,9,10]. In the early 1990s, two more chemokines were identified that were very similar to GRO [11]. They were named GRO-β and GRO-γ, now known as CXCL2 and CXCL3 [12]. GRO itself was given the additional symbol α (GRO-α); another variant of this name is GRO-1 [9,11]. With the discovery of more CXC chemokines, a new and more systematic nomenclature was required. For this reason, a new classification was introduced based on the chemokine sub-family name and the number of the chemokine. Thus, GRO-α was named small inducible cytokine sub-family B, member 1 (SCYB1), and then CXCL1 [12].
CXCL1 has two important motifs in its sequence. One is the CXC motif (9–11 amino acids), which assigns this chemokine to CXC chemokines (or α-chemokines) [8]. The second is the ELR motif (6–8 amino acids), also located at the N-terminus, which makes CXCL1 one of the ELR+ CXC chemokines, currently also known as CXC motif chemokine receptor 2 (CXCR2) receptor agonists [13,14].
3. CXCL1: Gene and Transcriptional Regulation
3.1. 4q12–q13 CXC Chemokine Gene Cluster
The CXCL1 gene is localized in the 4q12–q13 CXC chemokine gene cluster [12,15]. This region includes the closely located loci of other CXC sub-family chemokines, including CXCL2/GRO-β, CXCL3/GRO-γ, CXCL4/PF-4, CXC motif chemokine ligand 4 variant-1 (CXCL4L1), CXCL5/ENA-78, CXCL6/GCP-2, CXCL7/PPBP/NAP-2, and CXCL8/IL-8 [4,16,17]. It also contains the CXC motif chemokine ligand 1 pseudogene 1 (CXCL1P1) previously described as a growth-regulated oncogene δ-pseudogene (GRO-δ) [18]. Near the cluster, at 4q21, are the ligands of CXCR3 and CXCL13 [4,17]. Very often the 4q12–q13 gene cluster is amplified in cancer, particularly in Barrett neoplasia [19]. In breast cancer, 7.5% of primary tumors have a CXCL1 gene amplification. The frequency of CXCL1 amplification increases with disease progression [20]. The amplification of the CXCL1 gene is observed in 20% cases of breast cancer metastasis [20]. The duplication of the 4q12–q13 CXC chemokine gene cluster is associated with a higher incidence of cancer, and is particularly found in individuals from melanoma-prone families [21].
The sequences of the chemokine genes in the 4q12–q13 CXC chemokine gene cluster are similar and located close to each other, which suggests that these genes originated as a result of duplication [4]. In general, all CXC chemokines from this gene cluster can be divided into two groups [3], CXCL6 and CXCL8 (which, at similar concentrations, activate both CXCR1 and CXCR2 receptors), and CXCL1, CXCL2, CXCL3, CXCL5, and CXCL7 (which, at about 1 nM concentration, activate CXCR2; and at a concentration about 100 times higher, activate CXCR1) [3,22,23,24,25]. Each of the groups consists of chemokines that share the same properties and activate the same receptor at similar concentrations. Nevertheless, significant differences do occur in the action of individual chemokines, as the expression and secretion of each chemokine is sometimes separately regulated and depends on the cell type [26]. For this reason, to understand the interaction of all representatives of this gene cluster, it is necessary to understand the regulation of each CXC chemokine.
At this point, it should be noted that the ancestor of today’s mammals (cretaceous period, separation of placental mammals from marsupials) had far fewer CXC chemokine genes. These genes were duplicated, and today, this gene cluster has seven CXC chemokine genes in mice and nine in humans [4]. The opossum has only three chemokine genes, and birds have between two and three depending on the species. While the accumulation of genetic changes in individual CXC chemokine genes occurred independently of each other, duplication may have occurred multiple times at different times. Therefore, mouse lipopolysaccharide-induced CXC chemokine (LIX) is as similar to rat LIX (a different species) as the human CXCL5 is to human CXCL6 (same species/genome) [27]. Therefore, a given human CXC chemokine cannot be unambiguously assigned to a mouse or rat CXC chemokine from this cluster. While it may be that in one disease or laboratory model a given chemokine in a human or mouse/rat is upregulated, when studying a second disease the expression of the given chemokine will be upregulated only in one species [28]. For this reason, the role of human CXCL1 in a given disease cannot be compared with rat cytokine-induced neutrophil chemoattractant-1 (CINC-1) [29] or mouse keratinocyte-derived chemokine (KC) [30,31] without performing experiments on samples from human patients.
Importantly, given the arrangement of the genes on chromosomes, the human CXCL1 gene does not correspond to the murine KC gene, but to the murine dendritic cell inflammatory protein-1 (DCIP-1) gene [4]. Even though the murine KC gene corresponds to the human CXCL3, CXCL5 and CXCL7 genes, in many papers, CXCL1 and KC are used interchangeably. For this reason, in this review, we distinguish murine KC from human CXCL1.
3.2. CXCL1: Promoter
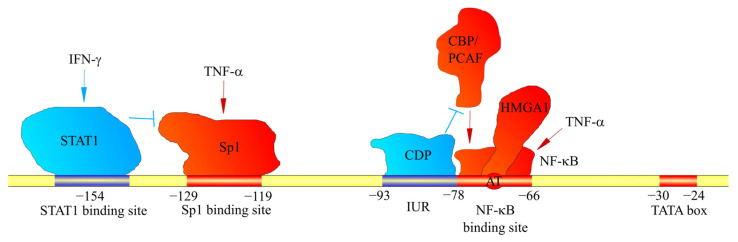
The CXCL1 gene consists of four exons and three introns. The exons and introns have a total length of 1845 bp [32]. Preceding the transcription start site is a TATA box (at a locus from −30 to −24 bp) preceded by numerous sequences to which various transcriptional activators and transcriptional repressors are attached.
Due to the significance of CXCL1 in inflammatory responses, the most important method for the induction of expression of this chemokine is the activation of NF-κB. At a locus −78 to −66 bp is the nuclear factor κB (NF-κB) binding site [33,34,35,36]. The NF-κB p50/p65 heterodimer attaches to it, inducing CXCL1 expression [37]. Therefore, through NF-κB activation, CXCL1 expression is increased by cytokines such as interleukin-1β (IL-1β) [37,38], tumor necrosis factor-α (TNF-α) [37,38] and interleukin-17 (IL-17) [39,40,41]. Importantly, the activation of NF-κB by the IL-17 receptor (IL-17R) occurs via the NF-κB activator 1 (Act1) → TNF receptor-associated factor 6 (TRAF6) → transforming growth factor β (TGF-β)-activated kinase 1 (TAK1) pathway [39,40]. The main effect of IL-17 on CXCL1 expression is an increase in the stability of CXCL1 mRNA [42,43]. In cancer cells, basal NF-κB activation is high, which results in high basal CXCL1 expression [35]. Therefore, in cancer cells, pro-inflammatory cytokines such as IL-1β and TNF-α can only increase the stability of CXCL1 mRNA [37]. CXCL1 can also increase its own expression via NF-κB activation, a mechanism that is important in cancer [8,44,45]. The effect of pro-inflammatory cytokines on CXCL1 expression is important because of inflammatory responses in malignant tumors. Depending on the type of cancer, there is an upregulation in pro-inflammatory cytokines, e.g., TNF-α [46] and IL-17 [47,48], which are involved in tumorigenesis.
In some cases, NF-κB can decrease CXCL1 expression. In hepatocytes, there is an important NF-κB p50/p50 homodimer that binds to the CXCL1 promoter to recruit the co-repressor histone deacetylase 1 (HDAC1) [49]. This results in reduced CXCL1 expression, which is important for preventing chronic liver disease.
The NF-κB binding site also contains the AT motif (the exact locus is −74 bp and −73 bp), which can attach a high-mobility group, AT-hook 1 (HMGA1) (previous name: high-mobility group-I(Y) (HMGI(Y))) [34,50]. This protein is important for the full activation of the CXCL1 promoter [34].
In the induction of CXCL1 expression by the exposure of cells to TNF-α, the formation of an NF-κB complex with cut-like homeobox 1 (CUX1) is important, and is much more intensely induced in the simultaneous exposure to IL-17 and TNF-α [51]. This complex attaches to the CXCL1 gene promoter at the NF-κB binding site in the −94 bp to −84 bp region of the CXCL1 promoter, i.e., where the binding site for CUX1 is located, leading to an increase in CXCL1 expression. This process is significant in rheumatoid arthritis, which is marked by high concentrations of IL-17 and TNF-α at disease sites [51].
The CXCL1 gene promoter also contains the immediate upstream region (IUR) at loci −93 bp to −78 bp (Figure 1) [50,52]. This region is directly upstream of the NF-κB binding site [33,34,35,36] and can attach to the human CUT homeodomain protein/CCAAT displacement protein (CDP) [53]. Then, CDP disrupts the interaction with the NF-κB of CREB-binding protein (CBP) or p300/CBP-association factor (PCAF) [52]. As CBP and PCAF are coactivators important for the function of NF-κB [54], CDP decreases the expression of CXCL1, which depends on NF-κB [52,53]. Poly(ADP-ribose) polymerase (PARP1) can also attach to the IUR [55,56], although only in the inactive state of PARP1. This inhibits the binding of NF-κB to the CXCL1 promoter, and thus the expression of this gene. In contrast, activated PARP1 loses its ability to bind to the IUR, which results in NF-κB binding to the CXCL1 promoter and an increase in CXCL1 expression [56]. The involvement of PARP1 is important in melanoma tumorigenesis; in normal melanocytes, PARP1 is inactive and inhibits CXCL1 expression, while in melanomas PARP1 is active.
Figure 1.
Factors affecting CXCL1 gene transcription. The CXCL1 promoter contains binding sites for factors that bind to these sites. They either increase (shown in red) or decrease (shown in blue) CXCL1 expression. In particular, the CXCL1 promoter contains a binding site for STAT1 and Sp1. STAT1 and Sp1 activated by IFN-γ and TNF-α, respectively, attach to these sites. Sp1 increases CXCL1 expression; however, STAT1 inhibits Sp1 binding to the CXCL1 promoter. Another mechanism regulating CXCL1 expression is IUR, a region that directly borders the NF-κB binding site. CDP binds to IUR, which inhibits the recruitment of CBP and PCAF coactivators by NF-κB. This prevents the induction of CXCL1 expression by NF-κB.
Locus −129 bp to −119 bp is the specificity protein 1 (Sp1) and specificity protein 3 (Sp3) binding site [34,50], important in the basal expression of CXCL1 and the induction of CXCL1 expression by IL-17 and TNF-α [41,57]. This site is also crucial for regulation of the expression of CXCL1 by the interferon-γ (IFN-γ) signal transducer, and the activator of transcription (STAT)1 can also inhibit CXCL1 expression. In particular, in peritoneal mesothelial cells, upon exposure of these cells to IFN-γ, STAT1 binds to the −154 bp region upstream of the transcription start site of the CXCL1 gene, which results in a decreased expression of CXCL1 [57] and reduced binding of Sp1 to the CXCL1 promoter. STAT1 may also increase the expression of CXCL1. However, its effect will depend on the selected cell model and the factor acting on these cells. In pancreatic ductal adenocarcinoma cells, there is an induction of CXCL1 expression by IL-35 [58], which is associated with the direct binding of the STAT1/STAT4 heterodimer to the CXCL1 promoter.
3.2.1. The Regulation of CXCL1 Expression by TGF-β and HGF
TGF-β reduces CXCL1 expression [59,60]. At locus −1247 bp, upstream of the transcription start site of the CXCL1 gene, is the TGF-β-inhibitory element (TIE), and at locus −560 bp is the SMAD-binding element (SBE) [61]. SMAD family member 4 (SMAD4) is the main factor in the signaling from the TGF-β receptor, but does not bind to these sites. Therefore, the effect of TGF-β on CXCL1 expression is indirect, likely reducing the activity of either NF-κB or other signaling pathways [61,62].
In contrast, in murine KC, a paralog for human CXCL1, there is a different mechanism by which TGF-β regulates the expression of this chemokine. At loci −249 bp to −246 bp and −144 bp to −141 bp, upstream of the transcription start site of the KC gene, are SBEs, which can bind SMAD2/3, reducing KC expression [63].
At the upstream transcription start site of the KC gene at loci −128 bp to −120 bp is the CCAAT/enhancer binding protein-β (C/EBP-β) binding motif. Upon activation of c-Met by the hepatocyte growth factor (HGF), C/EBP-β is activated and attaches to the C/EBP-β binding motif on the KC promoter, thus increasing the expression of this murine KC chemokine. Importantly, this mechanism only occurs in murine cells [63]. Based on the database “https://www.ncbi.nlm.nih.gov/gene”; accessed date: 15 November 2021, we did not find any C/EBP-β binding motif according to the cited paper on mouse KC, i.e., 5′-TGGAGCAAG-3′ or any sequence complementary to it up to −2 kbp upstream of the human CXCL1 gene promoter. Therefore, theoretically, C/EBP-β does not directly affect the expression of human CXCL1. In addition, studies on human cells do not show that TGF-β and SMADs directly affect human CXCL1 expression [61]. Nevertheless, further studies on the mechanisms of regulation of CXCL1 expression by HGF and C/EBP-β in humans are required.
The regulation of CXCL1 expression by TGF-β is an important mechanism observed in cancer [61,63]. For example, SMAD4 expression is reduced in colorectal cancer cells, as it interferes with the action of TGF-β, and thus leads to an increase in CXCL1 expression in the cancer cell [61]. Notably, in a prostate cancer tumor, in cancer-associated fibroblasts (CAF), there is a decrease in TGF-β type II receptor (TβRII) expression [64]. This decreases the effect of TGF-β on these cells, and thus leads to the increased expression of CXCL1 in these cells. As CXCL1 is a chemotactic factor for neutrophils [65], an increase in CXCL1 expression in colorectal cancer tumors results in the recruitment of tumor-associated neutrophils (TAN), cells with pro-cancer properties [61,66]. Additionally, CXCL1 causes tumor cell migration, and consequently, metastasis [60].
3.2.2. Significance of p53 Transcription Factor Family in CXCL1 Expression
Gain-of-function and loss-of-function mutations of the TP53 gene are very common in cancer cells and lead to changes in the expression of various genes [67,68]. Loss-of-function TP53 mutations cause an increase in CXCL1 expression [69] due to reduced p53-induced inhibition of NF-κB activation [70,71]. If p53 loses its functions, there is an increase in NF-κB activity and in the expression of genes dependent on this transcription factor. This mechanism is not universal in all cells because in monocytes and macrophages, p53 together with NF-κB increase the expression of pro-inflammatory cytokines [72]. In addition, the interaction of CXCL1 with p53 is complicated, as CXCR2 activation reduces p53 expression, this action is associated with protein kinase B (PKB)/Akt activation, which in turn activates murine double minute 2 (Mdm2) [73].
The gain-of-function TP53 mutations cause mutated p53 proteins to directly bind to the CXCL1 promoter, which results in an increased CXCL1 expression in the tumor cell [74,75]. The exact mechanism of the increase in CXCL1 expression depends on the type of p53 mutation. DNA-contact p53 mutants (for example R248Q and R273H) bind directly to the CXCL1 promoter [75]. Some of the single-nucleotide polymorphisms (SNP) of p53 may increase the ability of p53 with the aforementioned mutation to attach to the CXCL1 promoter. An example of this is the P72R SNP polymorphism of p53 (rs1042522) [75]. The R72 p53 mutant has a higher binding capacity to the CXCL1 promoter than the P72 p53 mutant. DNA-contact p53 mutants also increase NF-κB activation, which increases the expression of genes dependent on this transcription factor, including CXCL1 [69].
Another type of mutation is the Zn2+ region conformational p53 mutant. Examples of such p53 mutants are R175H and H179R, which attach directly to the CXCL1 promoter, and thus increase CXCL1 expression [74]. It appears that p53 with this type of mutation also increases CXCL1 expression by increasing H-Ras activity [69]. This effect is related to the interaction of mutant p53 with B-cell translocation gene 2 (BTG2). Significantly, NF-κB is not required in the induction of CXCL1 expression by Zn2+ region conformational p53 mutants [74].
The last type of mutation is the L3 loop conformational p53 mutant [69]. An example of such a mutation is G245S p53. Although this type of p53 mutation increases CXCL1 expression, the effect is much smaller than when p53 expression is significantly reduced [69]. This is likely due to the reduced effect of p53 with such a mutation, relative to wild-type (WT) p53.
Another protein that attaches near the CXCL1 gene is p63, a transcription factor from the p53 transcription factor family [76,77]. Both proteins (p53 and p63) induce the expression of different genes, but can also cooperate in the expression of the same genes. p63 attaches to the −3 kb region of the upstream transcription start site of the CXCL1 gene, thus increasing its expression [77]. This transcription factor may also cooperate with NF-κB in increasing CXCL1 expression. The significance of p63 in CXCL1 expression was found in tumors, particularly in pancreatic ductal adenocarcinoma cells.
3.2.3. CXCL1 Expression and Hypoxia
The promoter of murine KC, a paralog for human CXCL1, contains five hypoxia response element (HRE) sequences at loci −1309 bp, −433 bp, −313 bp, −302 bp and −289 bp [78]. The expression of this gene is induced by hypoxia-inducible factor (HIF)-2, as shown by studies in mouse epithelial cells [79] and murine chondrocytes [78]. HIF-1 also increases KC expression in murine myeloid-derived suppressor cells (MDSCs) [80]. CXCL1 expression may depend indirectly on HIF-1; for example, in human aortic endothelial cells and mouse aortic endothelial cells, HIF-1 increases miR-19a expression [81], which indirectly increases the expression of CXCL1. Based on “https://www.ncbi.nlm.nih.gov/gene; accessed date: 15 November 2021”, we identified seven sequences thought to be an HRE, i.e., 5′-A/GCGTG-3′ and complementary ones in the region up to the −2 kbp upstream transcription start site of the human CXCL1 gene [82,83]. HRE is the binding site of HIF-1 and HIF-2, transcription factors activated by hypoxia. Therefore, it seems theoretically possible that hypoxia increases CXCL1 expression—this was already shown by a study involving hepatocellular carcinoma cells [84]. Nevertheless, this effect may depend on the particular research model, as hypoxia did not affect CXCL1 expression in lung adenocarcinoma cells [85].
3.2.4. Other Mechanisms That Alter CXCL1 Promoter Activity
In addition to the effects on CXCL1 expression of the aforementioned factor binding sites near the transcription start site, there are also regulatory sequences located at various distances from the CXCL1 gene.
CXCL1 expression is increased in an AP-1-dependent manner, as shown by experiments using IL-17 [41] and TNF-α [86,87].
The transcription factors responsible for the induction of CXCL1 expression by TNF-α also include early growth response gene 1 (Egr-1) [50], which binds directly to the CXCL1 promoter at two loci: −367 bp and −134 bp. This process is significant in cancer, particularly in esophageal cancer, where Erg-1 is frequently overexpressed [88].
Studies on breast cancer cells showed that a breast cancer susceptibility gene 1 (BRCA1) complex with GATA-binding protein 3 (GATA3) reduced CXCL1 expression [89]. Nevertheless, further studies are required to determine whether this effect is direct or indirect.
Other transcription factors also attach to the CXCL1 promoter. In particular, the activation of transcription factor 2 (ATF2) and acute myeloid leukemia 1 (AML1) is responsible for the increase in CXCL1 transcription by IL-17, which attaches to the CXCL1 promoter [41].
At locus −277 bp, upstream of the transcription start site of the CXCL1 gene, a binding site for myeloid ecotropic viral integration site 1 (MEIS1) was identified [90]. MEIS1 is a transcription factor that is overexpressed in cancer cells, particularly in ovarian cancer, increasing the expression of many chemokines including CCL18, CCL4, CXCL7, and indirectly increasing the expression of CXCL1, as the identified binding site of this transcription factor appears to be non-functional.
At −375 bp upstream of the transcription start site of the CXCL1 gene, a binding site for the microphthalmia-associated transcription factor (MITF) was identified [91]. This is a transcription factor important for melanocyte differentiation. It also undergoes overexpression in melanoma. Therefore, the increase in CXCL1 expression in melanoma depends partly on MITF.
The −551 bp to −517 bp region upstream of the transcription start site region of the KC gene contains a binding site for Y-box protein-1 (YB-1) [92]. KC expression is induced upon the binding of this protein; this process is relevant in a murine bile duct ligation model, where the expression of KC increases in the liver in a YB-1-dependent manner. However, further studies are required to confirm the role of YB-1 in humans.
The −984 bp to −301 bp region, upstream of the transcription start site of the CXCL1 gene, contains a binding site for Snail, a transcription factor crucial for epithelial–mesenchymal transition (EMT) [93,94], a process important in cancer cell migration, invasion and metastasis. After EMT, the tumor cell begins to migrate. The increased expression of CXCL1 in this cell plays an important role in migration [95] and metastasis formation [64,96] by stimulating the tumor cell to migrate, and causing the supporting adhesion of the tumor cell to target tissues, as well as in the recruitment of different cells in metastasis.
Further from the transcription start site of the CXCL1 gene at the locus −2 kbp, a Hey-like (HeyL) binding site is located [97,98]. This factor belongs to Notch signaling, which is overexpressed in many cancers, such as in breast cancer. Because CXCL1 is an angiogenic chemokine [13,14], Notch signaling in cancer increases CXCL1 expression, and thus causes angiogenesis.
Some proteins regulating the expression of CXCL1 can be bound very far from the CXCL1 gene. In particular, avian v-maf musculoaponeurotic fibrosarcoma oncogene homolog (MAF)F binds to three sites, precisely at the −15 kpb, −12.5 kpb and −7.5 kbp upstream of the transcription start site of CXCL1 gene [99]. MAFF increases CXCL1 expression in human term myometrium [99,100]. However, to date, the function of CXCL1 during labor is unknown. The mechanism of induction of CXCL1 expression in human term myometrium is not related to inflammatory factors, which means that CXCL1 expression is induced by a specific transcription factor independent of inflammatory responses [99]. This shows that CXCL1 is not only a mediator of inflammatory responses but may also have its own physiological functions unrelated to inflammation.
Histone methylation is another important means of regulating CXCL1 expression. A −2.0 to −1.5 kbp fragment upstream of the transcription start site of the CXCL1 gene undergoes histone H3 Lys36 trimethylation (H3K36me3) by histone H3 lysine 36 methyltransferase SET-domain-containing 2 (SETD2) [101]. This leads to a reduction in CXCL1 expression. This process is important because, in cancers such as breast cancer [102], glioblastoma multiforme [103], hepatocellular carcinoma [104] and lung adenocarcinoma [101] the SETD2 gene is either mutated or there is a decreased expression of the product of this gene. However, in castration-resistant prostate cancer, SETD2 is an oncogene that supports tumor growth [105]. The precise regulation of CXCL1 expression by SETD2 is not known. As this enzyme modifies a fragment very far from the CXCL1 gene, it is possible that it alters the ability to bind some proteins that are important for the regulation of gene expression (Table 1).
Table 1.
Proteins that bind to the CXCL1 gene promoter, leading to a change in its expression.
| Name of the Factor | BINDING SITE | Effect on Expression | Notes | References |
|---|---|---|---|---|
| p50:p65 NF-κB | −78 bp to −66bp | ↑ | High basal NF-κB activity in cancer conditions; high basal CXCL1 expression in tumors. Activated in inflammation |
[33,34,35,36] |
| p50:p50 NF-κB | ? | ↓ | Prevention of chronic liver disease | [49] |
| HMGA1 | From −74 bp to −73 bp | ↑ | Essential in the full activation of the CXCL1 promoter by NF-κB | [34] |
| CDP | from −93 bp to −78 bp | ↓ | Reduction in CXCL1 expression by disruption of NF-κB function | [50,52,53] |
| PARP1 | from −93 bp to −78 bp | ↓ | PARP1 binding in the inactive state. Inhibition of NF-κB binding to the CXCL1 promoter | [55,56] |
| CUX1 | −94 bp to −84 bp | ↑ | Enhancement of CXCL1 expression by the joint action of IL-17 and TNF-α | [51] |
| Sp1 | −129 bp to −119 bp | ↑ | Significant in basal CXCL1 expression and in upregulation of CXCL1 expression by IL-17 or TNF-α | [34,50] |
| STAT1 | −154 bp | ↓ | Reduction in CXCL1 expression by IFN-γ through disruption of Sp1 function | [57] |
| STAT1/STAT4 | ? | ↑ | Enhancement of CXCL1 expression by IL-35 | [58] |
| HIF-1 and HIF-2 | ? | (↑) | Increased expression of CXCL1 in hypoxia. No precise studies on the direct effect | [84] |
| MEIS1 | −277 bp | (↑) | Sequence identified as potential binding site but non-functional. Factor influence indirect. Relevant in cancer, particularly in ovarian cancer | [90] |
| Erg-1 | −367 bp and −134 bp | ↑ | Important in cancer, especially in esophageal cancer | [88] |
| MITF | −375 bp | ↑ | Important in cancer, especially in melanoma cancer | [91] |
| Snail | from −984 bp to −301 bp | ↑ | Increased CXCL1 expression during EMT, important in cancer during metastasis formation | [94] |
| SMAD4 | −1247 bp and −560 bp |
(↓) | Sequences identified as potential binding sites but non-functional. Theoretically, when TGF-β action is reduced, the effect of SMAD4 is abolished, and thus CXCL1 expression increases | [61] |
| SETD2 | from −2.0 to −1.5 kbp | ↓ | This is the enzyme that causes histone methylation. The exact mechanisms of how epigenetic changes in this region affect CXCL1 expression are not known | [101] |
| HeyL | −2 kbp | ↑ | Notch signaling element. Relevant for cancer | [97,98] |
| Mutated p53 | ? | ↑ | Relevant in cancers with TP53 gene mutation | [74,75] |
| p63 | −3 kb | ↑ | Relevant in cancer, especially in pancreatic ductal adenocarcinoma cells | [77] |
| MAFF | −15 kpb, −12.5 kpb and −7.5 kbp |
↑ | Induction of CXCL1 expression in human term myometrium, immediately before birth. The exact functions of CXCL1 in labor are unknown | [99] |
↑—a factor that increases the expression of CXCL1; ↓—a factor that reduces the expression of CXCL1; (↓)—A factor that reduces CXCL1 expression with an identified direct binding site in the CXCL1 promoter, but the effect of the indicated factor is only indirect.
3.3. Regulation during Transcription
The induction of signaling pathways and attachment of all transcription factors to the promoter of the CXCL1 gene is followed by transcription. RNA polymerase II (Pol II) begins to transcribe the CXCL1 mRNA fragment with a length of approximately 50 nucleotides [106]. Then, transcription elongation requires the phosphorylation on Ser2 of Pol II by positive transcription elongation factor b (P-TEFb). Hairy and Enhancer of Split-1 (Hes1) prevent this phosphorylation, and therefore inhibit CXCL1 transcription [106]. This regulation occurs particularly in macrophages treated with pro-inflammatory agents such as lipopolysaccharide (LPS) [107].
4. CXCL1 mRNA Stability as a Method to Regulate CXCL1 Expression
The production of a 1.1–1.2 kb-long transcript [8] is followed by the next step: the regulation of CXCL1 expression, associated with changes in the stability of CXCL1 mRNA [108]. CXCL1 mRNA is a transcript with a low half-life; if the cell is not influenced by any factors that increase its stability, then this transcript is degraded within 1 to 4 h [109,110]. The half-life of CXCL1 mRNA is estimated to be approximately 15 min [37]. After this time, from the ends of the 3′-untranslated region (UTR) of CXCL1 mRNA, an approximately 130-nucleotide fragment is removed [109,110]. CXCL1 mRNA is transformed into a 0.9 kb transcript. However, there are many mechanisms, some of which increase and some of which decrease the half-life of CXCL1 mRNA. In particular, regions 6–23 and 632–651 on the 3′-UTR of CXCL1 mRNA are responsible for increasing the stability of the transcript. Region 562–581 on the 3′-UTR of CXCL1 mRNA is responsible for decreasing the stability of CXCL1 mRNA [111]. The AUUUA motifs on the 3′-UTR also act to reduce the stability of CXCL1 mRNA [42].
Various proteins that are activated by factors such as IL-1 [37,109,110], TNF-α [37] and IL-17 [43] are responsible for increasing CXCL1 mRNA stability. This effect depends on the cell type and tissue selected as the research model, as these factors can also increase CXCL1 transcription, e.g., depending on NF-κB activation [37,39,57]. Notably, in non-cancer cells, where there is no high basal NF-κB activation, transcriptional regulation plays a major role in regulating CXCL1 expression [37]. Additionally, no less important for CXCL1 mRNA stability are miRNAs, which cause the decay of this transcript [112,113,114,115,116,117]. All of the factors mentioned in this section lead to the degradation or improvement in the stability of CXCL1 mRNA. This alters the half-life of CXCL1 mRNA, and consequently, the number of these transcripts in the cytoplasm, at unchanged CXCL1 gene transcription levels.
4.1. Role of Cytokines in Regulating CXCL1 Expression by Altering mRNA Stability. The Mechanisms of IL-17-Induced Effects on CXCL1 Expression
CXCL1 mRNA decay is IL-17-sensitive. In unstimulated cells, splicing factor 2 (SF2)/alternative splicing factor (ASF) attaches to the 3′-UTR of CXCL1 mRNA, which reduces the stability of this transcript [118]. This factor attaches to CXCL1 mRNA sites other than tristetraprolin (TTP). The activation of the receptor for IL-17 leads to activation of Act1, TRAF2 and TRAF5. Act1 causes the phosphorylation of SF2/ASF via the inhibitor of NF-κB kinase ε (IKKε) [43] and forms the Act1-TRAF2-TRAF5-SF2/ASF complex [118,119]. In this complex, SF2/ASF is no longer bound to CXCL1 mRNA, which increases the stability of this transcript. At the same time, Act1 causes K63-linked polyubiquitination of the human antigen R (HuR) [108]. This process is dependent on Ubc13-Uev1A E2 complex. Therefore, modified HuR binds to the AU-rich element (ARE) on CXCL1 mRNA, causing its stabilization; HuR also promotes the translation of CXCL1.
Act1 can also cause the phosphorylation of decapping 1 (Dcp1) via TANK-binding kinase 1 (TBK1) [43]. This inhibits the decapping of CXCL1 mRNA, and of other transcripts. On the 3′-UTR of CXCL1 mRNA, there is a fragment at locus 780–900 that forms a secondary structure with four stem loops. This is a “similar expression to fibroblast growth factor genes + IL-17R” (SEFIR)-binding element. Act1 binds directly to it, which increases the stability of the transcript in question by competing with SF2/ASF binding [43].
IL-17 increases CXCL1 mRNA stability, and thus CXCL1 expression. Nevertheless, IL-17 can also increase CXCL1 transcription, but this effect is not as significant as the change in CXCL1 mRNA stability and may also depend on the research model chosen [41]. An increase in CXCL1 transcription is associated with NF-κB activation by the IL-17R receptor [39,40,41]. Additionally, other transcription factors increase CXCL1 expression, including AP1, ATF2, AML1 and SP1 [41,57].
In macrophages [120], keratinocytes [36] and fibroblasts [121], tristetraprolin (TTP) is responsible for reducing the stability of KC mRNA, as shown by experiments on mouse cells. TTP binds to AREs and, more specifically, to AUUUA motifs on the 3′-UTR of KC mRNA. This leads to a recruitment of the deadenylases, and consequently, to a decay of KC mRNA. However, activated p38 MAPK by LPS can activate the MAPK-activated protein kinase 2 (MK2) [122]. This kinase phosphorylates TTP, which does not affect the binding of TTP to mRNA but prevents the recruitment of deadenylases by this factor and prevents the decay of KC mRNA [123]. However, research on human cells showed that TTP does not affect the stability of CXCL1 mRNA [42].
4.2. The Role of miRNAs in the Regulation of CXCL1 Expression
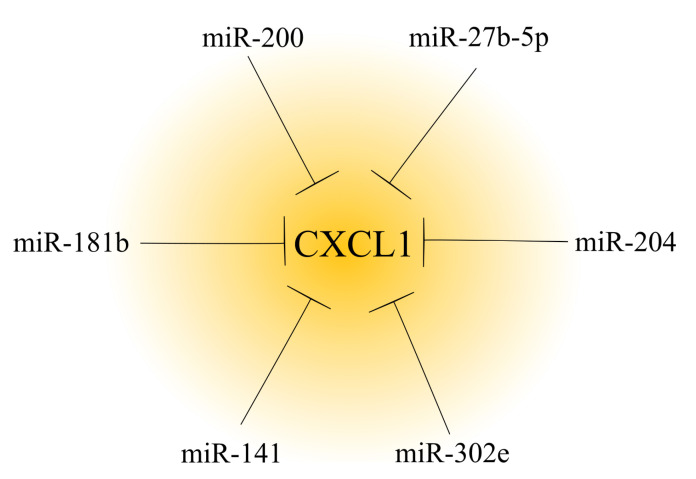
To date, many miRNAs have been found to be involved in reducing CXCL1 expression. In particular, many of them have important roles in cancer due to the pro-tumorigenic properties of CXCL1 (Figure 2) [5,114,124,125]. The development of cancer is associated with a decrease in the expression of miRNAs regulating CXCL1 expression in the tumor, and consequently, is associated with an increase in the expression of this chemokine. In ovarian cancer, renal cancer [112] and hepatocellular carcinoma [114], there is a downregulation of the miR-200 family [112]; in ovarian cancer there is a downregulation of miR-27b-5p [116]; and in gastric cancer, there is a downregulation of miR-204 [115]. Other examples include miR-302e in colorectal cancer [117] and miR-141 in non-small cell lung cancer [113].
Figure 2.
Effect of microRNAs on CXCL1 expression in a tumor. In cancer tumors, CXCL1 expression is regulated by microRNAs. Based on the available literature, six different microRNAs that decrease CXCL1 expression have been identified to date.
A change in miRNA expression may also account for the effects of some anticancer substances. An example of this is the increased expression of miR-181b in breast cancer cells by curcumin [126]. This miRNA directly decreases CXCL1 expression, which is one of the anticancer mechanisms of curcumin.
It is also possible that miRNAs affect signaling pathways that increase the expression of chemokines, which would mean that such miRNAs indirectly decrease CXCL1 expression. An example of this is miR-155 in tumor-infiltrating MDSCs [80]. This miRNA decreases HIF-1 levels, and thus the expression of genes dependent on the transcription factor HIF-1, such as CXCL1.
CXCL1 expression can also be indirectly increased by miRNAs. Examples of this are miR-155, miR-193b and miR-210 [127]. These miRNAs are secreted in extracellular vesicles by cancer cells, and then increase CXCL1 expression in fibroblasts. This mechanism was demonstrated in gastric cancer [127].
It is not only in cancers that the regulation of CXCL1 expression by miRNAs occurs. During cerebral ischemia–reperfusion injury, there is a downregulation of miR-429 in brain microvascular endothelial cells [128] and miR-532-5p in brain tissues [129]. At least for miR-532-5p, this was shown to be related to the promoter of this miRNA: hypermethylation. Both miRNAs directly reduce the expression of CXCL1. Therefore, when the levels of these miRNAs are decreased, there is an increase in CXCL1 expression, which leads to brain tissue damage during cerebral ischemia–reperfusion injury.
Additionally, studies of extracellular vesicles from murine, adipose-derived mesenchymal stem cells showed that they contain miR-150-5p [130]. This miRNA reduces the expression of KC. This chemokine is important in the development of hepatic fibrosis. Therefore, adipose-derived mesenchymal stem cells may inhibit the development of hepatic fibrosis.
Another miRNA that reduces CXCL1 expression is miR-7641 [131]. The expression of this miRNA occurs in human embryonic stem cells and decreases during endothelial cell biogenesis. This increases the expression of CXCL1, a chemokine also important in angiogenesis.
5. CXCL1: From Translation to Extracellular Factor
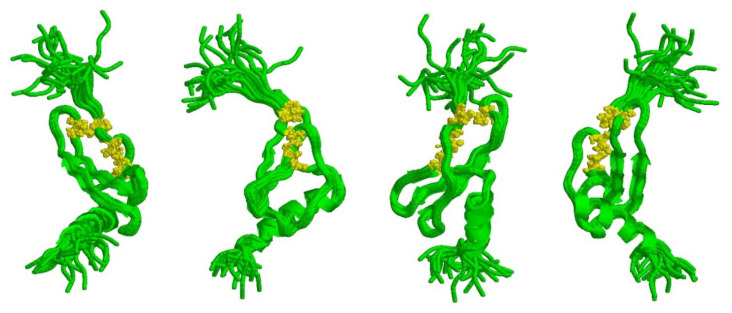
After translation, the synthesized CXCL1 precursor is 107aa long [132]. A signal peptide is removed from its N-terminus, which shortens the precursor to 73aa. Two other amino acids can also be removed from the C-terminus. In addition, two disulfide bridges are formed from all four cysteine residues in CXCL1 (Figure 3) [8,133,134]. The disulfide bridges give the appropriate structure to CXCL1, which determines the properties of this chemokine [135]. Apart from the aforementioned modifications, this chemokine does not undergo other post-translational modifications such as glycosylation, sulfation or phosphorylation [132].
Figure 3.
Structure of CXCL1. The tertiary structure of CXCL1 with the two disulfide bridges is highlighted in yellow. The N-terminal structure of CXCL1 is not stabilized; therefore, the figure shows many different possible conformations of this, as well as the other terminus of the CXCL1 chain. The graphic was created using RasMol 2.7.4.2 [136,137], and the CXCL1 structure was deposited in the Protein Data Bank (PDB) [138] under the identifier 1MGS [133,134].
Following the production of CXCL1 in the cytoplasm, this chemokine is sorted to vesicles [139]. It was shown that, at least in endothelial cells, these are histamine-responsive intracellular compartments located throughout the cytoplasm [140]. CXCL1 is not sorted by the Weibel–Palade body (WPB), i.e., it occurs in different locations than CXCL8 [140]. The localization of CXCL1 allows for a rapid release of CXCL1 under the influence of pro-inflammatory factors. An example of this is TNF-α, which causes a p38 MAPK- and PI3K-dependent release of CXCL1 [86]. Additionally, in endothelial cells, IL-1β stimulation results in the sorting of serglycin and CXCL1 into the same vesicles, and then a simultaneous secretion of both biomolecules [139].
6. CXCL1 as an Extracellular Factor
6.1. CXCL1 and Glycosaminoglycans
Following its secretion outside the cell, CXCL1 binds to glycosaminoglycans (GAGs), in particular heparan sulfate, chondroitin sulfate, and dermatan sulfate [141]. This mechanism is also important in the removal of excess CXCL1, which inhibits an overly intense pro-inflammatory response [142,143]. CXCL1 attached to GAG can be readily released by enzymes that degrade GAG. An example of such an enzyme is the matrix metalloproteinase (MMP)7/matrilysin during colon injury in mice [143,144,145]. This enzyme causes the shedding of syndecan-1, which results in the release of syndecan-1/CXCL1 complexes. This complex is responsible for the neutrophil influx to pro-inflammatory response sites. In addition, GAGs are important for the full activation of CXCR2 receptor by CXCL1 [146]. Without GAGs, CXCL1 binds poorly to the aforementioned receptor.
6.2. Proteolytic Processing as One of the Mechanisms Regulating CXCL1 Activity
After secretion, CXCL1 undergoes further proteolytic processing, which regulates the activity of this chemokine. From the N-terminus, three, four or five amino acids are removed, which produce CXCL1(4–73), CXCL1(5–73), and CXCL1(6–73), respectively [147]. This increases CXCL1 activity 30 times, as measured by its ability to induce the chemotaxis of treated cells. The process of amino acid removal may involve cathepsins, in particular cathepsin K, cathepsin L, and cathepsin S [148]. These proteases show their activity at a neutral pH and remove four amino acids from the N-terminus of CXCL1, resulting in the formation of CXCL1(5–73). Nevertheless, no proteases responsible for the formation of CXCL1(4–73) and CXCL1(6–73) have been identified so far. The identification of proteases responsible for processing CXCL1 would allow new pathways regulating the activity of this chemokine to be demonstrated. However, studies on metalloproteinases, MMP1 and MMP9, showed that they do not exhibit any activity against CXCL1 [149]. Instead, it was shown that CXCL1 could be sliced inside the ELR sequence by MMP12, which resulted in the formation of CXCL1(7–73) and the inactivation of CXCL1.
6.3. Dimerization of CXCL1
CXCL1 can be dimerized [150] and tends to form oligomers [132,151], especially at high concentrations. At concentrations from around 5 μM [152] to 20 μM [153], depending on the literature cited, half of the CXCL1 molecules occur in the form of dimers. However, for mouse KC, which is the paralog of human CXCL1, this concentration can be higher: 36 μM [154]. This concentration is more than 100 times higher than the concentration of CXCL1 in normal- and cancer-tissue homogenates [155,156]. At high concentrations, CXCL1 proteins are dimerized during biochemical isolation [132,151]. Therefore, in living organisms, dimers can only exist locally. Surface GAG binds to various chemokines, causing them to aggregate [157]. Importantly, surface GAG binds CXCL1 homodimers, for which it has a higher affinity than for the monomers of this chemokine [154,158]. That is, at low concentrations, CXCL1 homodimers are bound to surface glycosaminoglycans, and the monomers are soluble forms of CXCL1. However, the arrangement of GAG-bound CXCL1 prevents the activation of CXCR2 [158]. The activation of this receptor must be carried out by soluble CXCL1. GAGs, particularly heparan sulfate proteoglycans, appear to play an important role in CXCR2 receptor activation by CXCL1 [146].
Both CXCL1 monomers and homodimers have a similar ability to activate CXCR2 [153,159]. It appears that the synergistic action of both CXCL1 monomers and homodimers is required for full CXCL1 activity [160,161]. Therefore, at low concentrations, unmodified CXCL1 has a greater activity than stable CXCL1 homodimers and a greater activity than modified CXCL1 monomers, so that they do not dimerize [159,160,161]. In contrast, at very high concentrations, both forms have similar properties because unmodified CXCL1 undergoes homodimerization.
The mixture of monomers and homodimers of CXCL1 is only a simplified model, as CXCL1 can also form heterodimers with other CXC chemokines. In particular with CXCL4 [150], CXCL7 [162] and CXCL8 [150]. Such a heterodimer binds to GAGs in a different arrangement than the CXCL1 homodimer [162,163]. However, heterodimers appear to have the same activity in CXCR2 activation as the monomers of individual chemokines [162].
The formation of CXCL1 heterodimers with other CXC chemokines has been poorly studied. Usually, the role of only a single selected chemokine is studied in a given process, even though the demonstration of interactions between different CXC chemokines may provide a better understanding of therapeutic methods against a selected chemokine, particularly in cancers, associated with the increased expressions of many various CXC chemokines, particularly in colorectal cancer [164], head and neck squamous cell carcinoma [165] and skin cutaneous melanoma [166].
6.4. CXCL1 Receptors
To date, three CXCL1 receptors have been discovered—CXCR1, CXCR2 and atypical chemokine receptor 1 (ACKR1) [167]. Of these, CXCL1 binds with high affinity to just two of them—CXCR2 and ACKR1. ACKR1 binds CXCL1 with a dissociation constant (Kd) of 1.81 nM [167], similar to the binding parameters of CXCL1 to the CXCR2 receptor. Depending on the study, the half maximal effective concentration (EC50) for CXCR2 and 5.0 ± 1.7 nM [24] or 0.4 nM [168], with Kd of 3 nM [23].
CXCL1 can bind to and activate CXCR1, but the binding parameters to this receptor are 300 to 1000 times weaker than for CXCR2 [23]. The EC50 for CXCL1 to activate CXCR1 is 65 ± 13 nM [24] or 220 nM [168], while Kd is 300 nM [23] or 450–880 nM [169], depending on the study. The properties of CXCL1 seem to mainly associate with CXCR2, especially in the light of its much higher affinity to CXCL1 than that observed for CXCR1, even though some papers show that CXCR1 in some models is important for the action of CXCL1 [170,171].
6.5. ACKR1 and CXCL1
ACKR1 was first described as a Duffy antigen, which forms the Duffy blood-group system, hence its original name: Duffy antigen receptor for chemokines (DARC) [172]. With a molecular mass of 47 kDa [173], ACKR1 is expressed on erythrocytes, and in the absence of expression in these cells, it is found in the capillaries and postcapillary venular endothelial cells [174,175]. ACKR1 is also an important receptor for malaria parasites, Plasmodium vivax and Plasmodium knowlesi, to infect erythrocytes [172,176,177]. For this reason, in tropical areas where these parasites occur, selection pressure results in the prevalence of ACKR1 alleles whose expression is not found on erythrocytes [172].
ACKR1 is not only a receptor for CXCL1 but also for CXCL8 and CC chemokines, CCL2 and CCL5, although not for CCL3 or CXCR3 receptor agonists [167,178]. The ACKR1 sequence does not contain a DRY motif [179], a motif associated with G-protein coupling [180,181]. Therefore, G-protein-dependent signal transduction does not occur after ligand binding. However, ACKR1 can activate extracellular signal-regulated kinase (ERK) mitogen-activated protein kinase (MAPK), which interfere with CXCR2 receptor function [182].
ACKR1 has an important role in chemokine action. On erythrocytes, it binds chemokines (including CXCL1) from the blood, which leads to the maintenance of CXCL1 levels and regulation of the intensity of the inflammatory response [173,183,184].
In addition, on endothelial cells, ACKR1 is involved in immune cell migration. After it binds chemokines, it is endocytosed with the bound chemokine via a macropinocytosis-like process, dependent on cholesterol and dynamin II [185]. Significantly, the chemokine is not degraded in this process but translocated across the endothelium, which is followed by the migration of the immune cell through the endothelium. [175,185].
ACKR1 activation can also cause the signal transduction of ERK MAPK, which interferes with the function of CXCR2 on the same cell as ACKR1 [182]. This process leads to the inhibition of CXCR2-dependent cell migration, e.g., airway smooth muscle cells [182]. The interaction between ACKR1 and CXCR2 is also important in cancer. The high expression of ACKR1, associated with the inhibition of CXCR2, results in a better prognosis for patients with pancreatic ductal adenocarcinoma [186].
7. Significance of CXCL1 in Tumors
CXCL1 expression in cancer tumors is upregulated by numerous mechanisms at almost all possible regulatory steps: gene amplification [19], the activation of transcription by high basal NF-κB activation [35], effects of pro-inflammatory cytokines on CXCL1 transcription and CXCL1 mRNA stability [46,47,48], as well as miRNAs involved in regulating CXCL1 expression in the tumor cell [112,114].
After CXCL1 expression is induced by carcinogens, it participates in inflammatory responses by recruiting neutrophils. This leads to chronic inflammation, which causes tumor formation as demonstrated in the models of carcinogen-induced skin cancer [187,188]. CXCL1 is produced in the tumor by cancer cells [189,190], and also by CAFs [189,191,192,193], MDSCs [80], mesenchymal stem cells (MSCs) [194] and tumor-associated macrophages (TAMs) [189,195,196]. Since the first experiments on CXCL1, this chemokine is considered an important factor in cancer development. It was first classified as a factor produced by cancer cells that increased their proliferation [5], hence its original names: “growth regulated oncogene” and “melanoma growth stimulatory activity factor” [5,7,8]. Over time, other pro-tumor properties of this chemokine were discovered. In addition to increasing proliferation [5,197], CXCL1 also induces cancer cell migration, particularly EMT [193,196,197]. Produced by lymphatic endothelial cells (LECs), CXCL1 enables tumor cell migration into the lymphatic vessels during lymphangiogenesis, leading to lymph node metastasis.
CXCL1 is a chemotactic factor for neutrophils [65]. Additionally, it causes the mobilization of these cells from the bone marrow [198]. These processes lead to the recruitment of TAN [199,200] and granulocytic, myeloid-derived suppressor cells (G-MDSC) [195,201,202] into the tumor niche. In doing so, this chemokine also increases the expansion of monocytic, myeloid-derived suppressor cells (Mo-MDSCs) in the bone marrow, which increases the number of these cells in the body, and thus in the tumor after the recruitment of these cells by other chemokines [203]. CXCL1 can also induce recruitment of regulatory T cells (Treg) [113] and MSCs [204] into the tumor niche.
Another no-less-important property of CXCL1 is its ability to induce angiogenesis [13,14]. In particular, the expression of CXCL1 is increased by the vascular endothelial growth factor (VEGF) [205,206,207,208] in an interaction that induces and supports angiogenesis.
All the pro-tumor properties of CXCL1 are confirmed by clinical tumor studies, which showed that CXCL1 expression increased with cancer progression. This was also shown in studies of patients with cancers such as bladder cancer [189], colorectal cancer [209], gastric cancer [210], hepatocellular carcinoma [114], laryngeal squamous cell carcinoma [211], prostate cancer [212] and renal cell carcinoma [213].
The high importance of CXCL1 in tumorigenesis allows for the development of effective therapies targeting this chemokine. An example of this is HL2401—an anti-CXCL1 monoclonal antibody, which shows promising results against bladder cancer and prostate cancer in animal experiments [214]. Additionally, CXCR2 inhibitors such as SB225002 are currently being tested [215,216,217]. This compound has anti-tumor effects on various cancer cell lines as well as animal models. Another possibility is the use of dual CXCR1/CXCR2 inhibitors such as repertaxin [218], ladarixin [219], SCH-479833 or SCH-527123 [220]. They inhibit not only CXCL1 activity but also other CXCR2 agonists.
Anti-cancer therapies do not just have to be associated with a reduction in the expression and effects of CXCL1. For example, as T cells do not express CXCR2 [221,222], if the expression of the receptor on these cells was increased, then such T cells would be specifically recruited to sites with a high concentration of CXCR2 ligands, including CXCL1. Therefore, such modified T cells could be recruited to solid tumors with an elevated concentration of CXCL1, which would result in the initiation of an anti-tumor immune response [221,222]. This process can be exploited in immunotherapy, e.g., using modified autologous chimeric antigen receptor (CAR)-T cells.
8. Perspective for Further Research
The regulation of CXCL1 expression and activity is very well researched. In particular, we already know much about the regulation of CXCL1 gene transcription and the role of miRNAs in CXCL1 mRNA stability. However, there are areas of knowledge that are poorly understood and should be further investigated in the near future. In particular, little is known about the following aspects of the regulation of CXCL1 expression and activity:
-
-
The regulation of CXCL1 mRNA stability: The effect of IL-17 on this process is fairly well established, but the role of IL-1 and TNF-α requires further investigation. Additionally, the exact mechanism causing the low stability of CXCL1 mRNA in cells not stimulated by any cytokines are unknown;
-
-
The mechanisms of sorting the CXCL1 to vesicles and its release outside the cell: CXCL1 is an important component of cellular responses to dangerous agents. For this reason, intercellular signaling involving CXCL1 must sometimes be very rapid, and in some cases, the release of CXCL1 from the cell must be immediate. However, very little research is devoted to the regulation of CXCL1 levels outside cells;
-
-
Proteolytic processing: Until now, not all proteases involved in proteolytic processing of CXCL1 have been identified. The investigation of this mechanism could indicate new therapeutic avenues for diseases in which CXCL1 plays an important role;
-
-
CXCL1 heterodimerization with other CXC chemokines and interactions between CXC chemokines: A characteristic feature of CXC subfamily chemokines is the eight different CXC chemokines that cause the same ability to activate CXCR2 at similar concentrations. Evolutionary pressures caused many duplications of the ancestral gene of all ELR+ CXC chemokines. However, it is not entirely clear why, perhaps due to the different regulation of expression for each chemokine in this group. This argument can be linked to the heterodimerization of ELR+ CXC chemokines. With 8 ELR+ CXC chemokines, we have 28 different heterodimers, 8 homodimers and 8 monomers—a total of 44 different molecules that can activate CXCR2 in slightly different ways. This is almost 5.5 times more than the number of chemokines. The interaction with GAG is also important in this model.
Author Contributions
J.K., writing—original draft preparation; K.B., investigation; I.G., investigation; D.C., supervision, funding acquisition; I.B.-B., writing—original draft preparation, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the statutory budget of the Department of Biochemistry and Medical Chemistry Pomeranian Medical University in Szczecin, Poland.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pérez-Tomás R., Pérez-Guillén I. Lactate in the Tumor Microenvironment: An Essential Molecule in Cancer Progression and Treatment. Cancers. 2020;12:3244. doi: 10.3390/cancers12113244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Han L., Lam E.W., Sun Y. Extracellular vesicles in the tumor microenvironment: Old stories, but new tales. Mol. Cancer. 2019;18:59. doi: 10.1186/s12943-019-0980-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hughes C.E., Nibbs R.J.B. A guide to chemokines and their receptors. FEBS J. 2018;285:2944–2971. doi: 10.1111/febs.14466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nomiyama H., Osada N., Yoshie O. Systematic classification of vertebrate chemokines based on conserved synteny and evolutionary history. Genes Cells. 2013;18:1–16. doi: 10.1111/gtc.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richmond A., Lawson D.H., Nixon D.W., Chawla R.K. Characterization of autostimulatory and transforming growth factors from human melanoma cells. Cancer Res. 1985;45:6390–6394. [PubMed] [Google Scholar]
- 6.Schröder J.M., Persoon N.L., Christophers E. Lipopolysaccharide-stimulated human monocytes secrete, apart from neutrophil-activating peptide 1/interleukin 8, a second neutrophil-activating protein. NH2-terminal amino acid sequence identity with melanoma growth stimulatory activity. J. Exp. Med. 1990;171:1091–1100. doi: 10.1084/jem.171.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anisowicz A., Bardwell L., Sager R. Constitutive overexpression of a growth-regulated gene in transformed Chinese hamster and human cells. Proc. Natl. Acad. Sci. USA. 1987;84:7188–7192. doi: 10.1073/pnas.84.20.7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Richmond A., Balentien E., Thomas H.G., Flaggs G., Barton D.E., Spiess J., Bordoni R., Francke U., Derynck R. Molecular characterization and chromosomal mapping of melanoma growth stimulatory activity, a growth factor structurally related to beta-thromboglobulin. EMBO J. 1988;7:2025–2033. doi: 10.1002/j.1460-2075.1988.tb03042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y., Wu J.Z., Yang Y.Q., Ma R., Zhang J.Y., Feng J.F. Expression of growth-regulated oncogene-1, hepatocyte growth factor, platelet-derived growth factor-AA and soluble E-selectin and their association with high-risk human papillomavirus infection in squamous cell carcinoma of the uterine cervix. Mol. Med. Rep. 2014;10:1013–1024. doi: 10.3892/mmr.2014.2293. [DOI] [PubMed] [Google Scholar]
- 10.Heim C.E., Yamada K.J., Fallet R., Odvody J., Schwarz D.M., Lyden E.R., Anderson M.J., Alter R., Vidlak D., Hartman C.W., et al. Orthopaedic Surgery Elicits a Systemic Anti-Inflammatory Signature. J. Clin. Med. 2020;9:2123. doi: 10.3390/jcm9072123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haskill S., Peace A., Morris J., Sporn S.A., Anisowicz A., Lee S.W., Smith T., Martin G., Ralph P., Sager R. Identification of three related human GRO genes encoding cytokine functions. Proc. Natl. Acad. Sci. USA. 1990;87:7732–7736. doi: 10.1073/pnas.87.19.7732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zlotnik A., Yoshie O. Chemokines: A new classification system and their role in immunity. Immunity. 2000;12:121–127. doi: 10.1016/S1074-7613(00)80165-X. [DOI] [PubMed] [Google Scholar]
- 13.Strieter R.M., Polverini P.J., Kunkel S.L., Arenberg D.A., Burdick M.D., Kasper J., Dzuiba J., Van Damme J., Walz A., Marriott D., et al. The functional role of the ELR motif in CXC chemokine-mediated angiogenesis. J. Biol. Chem. 1995;270:27348–27357. doi: 10.1074/jbc.270.45.27348. [DOI] [PubMed] [Google Scholar]
- 14.Addison C.L., Daniel T.O., Burdick M.D., Liu H., Ehlert J.E., Xue Y.Y., Buechi L., Walz A., Richmond A., Strieter R.M. The CXC chemokine receptor 2, CXCR2, is the putative receptor for ELR+ CXC chemokine-induced angiogenic activity. J. Immunol. 2000;165:5269–5277. doi: 10.4049/jimmunol.165.9.5269. [DOI] [PubMed] [Google Scholar]
- 15.Tunnacliffe A., Majumdar S., Yan B., Poncz M. Genes for beta-thromboglobulin and platelet factor 4 are closely linked and form part of a cluster of related genes on chromosome 4. Blood. 1992;79:2896–2900. doi: 10.1182/blood.V79.11.2896.bloodjournal79112896. [DOI] [PubMed] [Google Scholar]
- 16.O’Donovan N., Galvin M., Morgan J.G. Physical mapping of the CXC chemokine locus on human chromosome 4. Cytogenet Cell Genet. 1999;84:39–42. doi: 10.1159/000015209. [DOI] [PubMed] [Google Scholar]
- 17.Nomiyama H., Mera A., Ohneda O., Miura R., Suda T., Yoshie O. Organization of the chemokine genes in the human and mouse major clusters of CC and CXC chemokines: Diversification between the two species. Genes Immun. 2001;2:110–113. doi: 10.1038/sj.gene.6363742. [DOI] [PubMed] [Google Scholar]
- 18.Shattuck-Brandt R.L., Wood L.D., Richmond A. Identification and characterization of an MGSA/GRO pseudogene. DNA Seq. 1997;7:379–386. doi: 10.3109/10425179709034060. [DOI] [PubMed] [Google Scholar]
- 19.Alvarez H., Opalinska J., Zhou L., Sohal D., Fazzari M.J., Yu Y., Montagna C., Montgomery E.A., Canto M., Dunbar K.B., et al. Widespread hypomethylation occurs early and synergizes with gene amplification during esophageal carcinogenesis. PLoS Genet. 2011;7:e1001356. doi: 10.1371/annotation/8dcded85-a924-40f4-a7ea-56961b87447f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Acharyya S., Oskarsson T., Vanharanta S., Malladi S., Kim J., Morris P.G., Manova-Todorova K., Leversha M., Hogg N., Seshan V.E., et al. A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell. 2012;150:165–178. doi: 10.1016/j.cell.2012.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang X.R., Brown K., Landi M.T., Ghiorzo P., Badenas C., Xu M., Hayward N.K., Calista D., Landi G., Bruno W., et al. Duplication of CXC chemokine genes on chromosome 4q13 in a melanoma-prone family. Pigment Cell Melanoma Res. 2012;25:243–247. doi: 10.1111/j.1755-148X.2012.00969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schumacher C., Clark-Lewis I., Baggiolini M., Moser B. High- and low-affinity binding of GRO alpha and neutrophil-activating peptide 2 to interleukin 8 receptors on human neutrophils. Proc. Natl. Acad. Sci. USA. 1992;89:10542–10546. doi: 10.1073/pnas.89.21.10542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loetscher P., Seitz M., Clark-Lewis I., Baggiolini M., Moser B. Both interleukin-8 receptors independently mediate chemotaxis. Jurkat cells transfected with IL-8R1 or IL-8R2 migrate in response to IL-8, GRO alpha and NAP-2. FEBS Lett. 1994;341:187–192. doi: 10.1016/0014-5793(94)80454-0. [DOI] [PubMed] [Google Scholar]
- 24.Ahuja S.K., Murphy P.M. The CXC chemokines growth-regulated oncogene (GRO) alpha, GRObeta, GROgamma, neutrophil-activating peptide-2, and epithelial cell-derived neutrophil-activating peptide-78 are potent agonists for the type B, but not the type A, human interleukin-8 receptor. J. Biol. Chem. 1996;271:20545–20550. doi: 10.1074/jbc.271.34.20545. [DOI] [PubMed] [Google Scholar]
- 25.Fan X., Patera A.C., Pong-Kennedy A., Deno G., Gonsiorek W., Manfra D.J., Vassileva G., Zeng M., Jackson C., Sullivan L., et al. Murine CXCR1 is a functional receptor for GCP-2/CXCL6 and interleukin-8/CXCL8. J. Biol. Chem. 2007;282:11658–11666. doi: 10.1074/jbc.M607705200. [DOI] [PubMed] [Google Scholar]
- 26.Girbl T., Lenn T., Perez L., Rolas L., Barkaway A., Thiriot A., Del Fresno C., Lynam E., Hub E., Thelen M., et al. Distinct Compartmentalization of the Chemokines CXCL1 and CXCL2 and the Atypical Receptor ACKR1 Determine Discrete Stages of Neutrophil Diapedesis. Immunity. 2018;49:1062–1076.e6. doi: 10.1016/j.immuni.2018.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith J.B., Rovai L.E., Herschman H.R. Sequence similarities of a subgroup of CXC chemokines related to murine LIX: Implications for the interpretation of evolutionary relationships among chemokines. J. Leukoc. Biol. 1997;62:598–603. doi: 10.1002/jlb.62.5.598. [DOI] [PubMed] [Google Scholar]
- 28.Belperio J.A., Keane M.P., Burdick M.D., Gomperts B.N., Xue Y.Y., Hong K., Mestas J., Zisman D., Ardehali A., Saggar R., et al. CXCR2/CXCR2 ligand biology during lung transplant ischemia-reperfusion injury. J. Immunol. 2005;175:6931–6939. doi: 10.4049/jimmunol.175.10.6931. [DOI] [PubMed] [Google Scholar]
- 29.Zagorski J., DeLarco J.E. Rat CINC (cytokine-induced neutrophil chemoattractant) is the homolog of the human GRO proteins but is encoded by a single gene. Biochem. Biophys. Res. Commun. 1993;190:104–110. doi: 10.1006/bbrc.1993.1017. [DOI] [PubMed] [Google Scholar]
- 30.Deng Z.W., Denkinger D.J., Peterson K.E., Deuel T.F., Kawahara R.S. Glucocorticoids negatively regulate the transcription of KC, the mouse homolog of MGSA/GRO. Biochem. Biophys. Res. Commun. 1994;203:1809–1814. doi: 10.1006/bbrc.1994.2397. [DOI] [PubMed] [Google Scholar]
- 31.Bozic C.R., Kolakowski L.F., Jr., Gerard N.P., Garcia-Rodriguez C., von Uexkull-Guldenband C., Conklyn M.J., Breslow R., Showell H.J., Gerard C. Expression and biologic characterization of the murine chemokine KC. J. Immunol. 1995;154:6048–6057. [PubMed] [Google Scholar]
- 32.Baker N.E., Kucera G., Richmond A. Nucleotide sequence of the human melanoma growth stimulatory activity (MGSA) gene. Nucleic Acids Res. 1990;18:6453. doi: 10.1093/nar/18.21.6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anisowicz A., Messineo M., Lee S.W., Sager R. An NF-kappa B-like transcription factor mediates IL-1/TNF-alpha induction of gro in human fibroblasts. J. Immunol. 1991;147:520–527. [PubMed] [Google Scholar]
- 34.Wood L.D., Farmer A.A., Richmond A. HMGI(Y) and Sp1 in addition to NF-kappa B regulate transcription of the MGSA/GRO alpha gene. Nucleic Acids Res. 1995;23:4210–4219. doi: 10.1093/nar/23.20.4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Devalaraja M.N., Wang D.Z., Ballard D.W., Richmond A. Elevated constitutive IkappaB kinase activity and IkappaB-alpha phosphorylation in Hs294T melanoma cells lead to increased basal MGSA/GRO-alpha transcription. Cancer Res. 1999;59:1372–1377. [PubMed] [Google Scholar]
- 36.Wu Z., Neufeld H., Torlakovic E., Xiao W. Uev1A-Ubc13 promotes colorectal cancer metastasis through regulating CXCL1 expression via NF-кB activation. Oncotarget. 2018;9:15952–15967. doi: 10.18632/oncotarget.24640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shattuck R.L., Wood L.D., Jaffe G.J., Richmond A. MGSA/GRO transcription is differentially regulated in normal retinal pigment epithelial and melanoma cells. Mol. Cell Biol. 1994;14:791–802. doi: 10.1128/mcb.14.1.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cuenca R.E., Azizkhan R.G., Haskill S. Characterization of GRO alpha, beta and gamma expression in human colonic tumours: Potential significance of cytokine involvement. Surg. Oncol. 1992;1:323–329. doi: 10.1016/0960-7404(92)90094-2. [DOI] [PubMed] [Google Scholar]
- 39.Huang F., Kao C.Y., Wachi S., Thai P., Ryu J., Wu R. Requirement for both JAK-mediated PI3K signaling and ACT1/TRAF6/TAK1-dependent NF-kappaB activation by IL-17A in enhancing cytokine expression in human airway epithelial cells. J. Immunol. 2007;179:6504–6513. doi: 10.4049/jimmunol.179.10.6504. [DOI] [PubMed] [Google Scholar]
- 40.You Z., Ge D., Liu S., Zhang Q., Borowsky A.D., Melamed J. Interleukin-17 Induces Expression of Chemokines and Cytokines in Prostatic Epithelial Cells but Does Not Stimulate Cell Growth In Vitro. Int. J. Med. Biol. Front. 2012;18:629–644. [PMC free article] [PubMed] [Google Scholar]
- 41.Wu H.H., Hwang-Verslues W.W., Lee W.H., Huang C.K., Wei P.C., Chen C.L., Shew J.Y., Lee E.Y., Jeng Y.M., Tien Y.W., et al. Targeting IL-17B-IL-17RB signaling with an anti-IL-17RB antibody blocks pancreatic cancer metastasis by silencing multiple chemokines. J. Exp. Med. 2015;212:333–349. doi: 10.1084/jem.20141702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Datta S., Novotny M., Pavicic P.G., Jr., Zhao C., Herjan T., Hartupee J., Hamilton T. IL-17 regulates CXCL1 mRNA stability via an AUUUA/tristetraprolin-independent sequence. J. Immunol. 2010;184:1484–1491. doi: 10.4049/jimmunol.0902423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Herjan T., Hong L., Bubenik J., Bulek K., Qian W., Liu C., Li X., Chen X., Yang H., Ouyang S., et al. IL-17-receptor-associated adaptor Act1 directly stabilizes mRNAs to mediate IL-17 inflammatory signaling. Nat. Immunol. 2018;19:354–365. doi: 10.1038/s41590-018-0071-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang D., Richmond A. Nuclear factor-kappa B activation by the CXC chemokine melanoma growth-stimulatory activity/growth-regulated protein involves the MEKK1/p38 mitogen-activated protein kinase pathway. J. Biol. Chem. 2001;276:3650–3659. doi: 10.1074/jbc.M006115200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilson C., Purcell C., Seaton A., Oladipo O., Maxwell P.J., O’Sullivan J.M., Wilson R.H., Johnston P.G., Waugh D.J. Chemotherapy-induced CXC-chemokine/CXC-chemokine receptor signaling in metastatic prostate cancer cells confers resistance to oxaliplatin through potentiation of nuclear factor-kappaB transcription and evasion of apoptosis. J. Pharmacol. Exp. Ther. 2008;327:746–759. doi: 10.1124/jpet.108.143826. [DOI] [PubMed] [Google Scholar]
- 46.Szlosarek P.W., Grimshaw M.J., Kulbe H., Wilson J.L., Wilbanks G.D., Burke F., Balkwill F.R. Expression and regulation of tumor necrosis factor alpha in normal and malignant ovarian epithelium. Mol. Cancer Ther. 2006;5:382–390. doi: 10.1158/1535-7163.MCT-05-0303. [DOI] [PubMed] [Google Scholar]
- 47.Liu Z., Yang L., Xu J., Zhang X., Wang B. Enhanced expression and clinical significance of chemokine receptor CXCR2 in hepatocellular carcinoma. J. Surg. Res. 2011;166:241–246. doi: 10.1016/j.jss.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 48.Tsai Y.F., Huang C.C., Lin Y.S., Hsu C.Y., Huang C.P., Liu C.Y., Chiu J.H., Tseng L.M. Interleukin 17A promotes cell migration, enhances anoikis resistance, and creates a microenvironment suitable for triple negative breast cancer tumor metastasis. Cancer Immunol. Immunother. 2021;70:2339–2351. doi: 10.1007/s00262-021-02867-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wilson C.L., Jurk D., Fullard N., Banks P., Page A., Luli S., Elsharkawy A.M., Gieling R.G., Chakraborty J.B., Fox C., et al. NFκB1 is a suppressor of neutrophil-driven hepatocellular carcinoma. Nat. Commun. 2015;6:6818. doi: 10.1038/ncomms7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shin S.Y., Lee J.M., Lim Y., Lee Y.H. Transcriptional regulation of the growth-regulated oncogene α gene by early growth response protein-1 in response to tumor necrosis factor α stimulation. Biochim. Biophys. Acta. 2013;1829:1066–1074. doi: 10.1016/j.bbagrm.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 51.Slowikowski K., Nguyen H.N., Noss E.H., Simmons D.P., Mizoguchi F., Watts G.F.M., Gurish M.F., Brenner M.B., Raychaudhuri S. CUX1 and IκBζ (NFKBIZ) mediate the synergistic inflammatory response to TNF and IL-17A in stromal fibroblasts. Proc. Natl. Acad. Sci. USA. 2020;117:5532–5541. doi: 10.1073/pnas.1912702117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ueda Y., Su Y., Richmond A. CCAAT displacement protein regulates nuclear factor-kappa beta-mediated chemokine transcription in melanoma cells. Melanoma Res. 2007;17:91–103. doi: 10.1097/CMR.0b013e3280a60888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nirodi C., Hart J., Dhawan P., Moon N.S., Nepveu A., Richmond A. The role of CDP in the negative regulation of CXCL1 gene expression. J. Biol. Chem. 2001;276:26122–26131. doi: 10.1074/jbc.M102872200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sheppard K.A., Rose D.W., Haque Z.K., Kurokawa R., McInerney E., Westin S., Thanos D., Rosenfeld M.G., Glass C.K., Collins T. Transcriptional activation by NF-kappaB requires multiple coactivators. Mol. Cell Biol. 1999;19:6367–6378. doi: 10.1128/MCB.19.9.6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nirodi C., NagDas S., Gygi S.P., Olson G., Aebersold R., Richmond A. A role for poly(ADP-ribose) polymerase in the transcriptional regulation of the melanoma growth stimulatory activity (CXCL1) gene expression. J. Biol. Chem. 2001;276:9366–9374. doi: 10.1074/jbc.M009897200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Amiri K.I., Ha H.C., Smulson M.E., Richmond A. Differential regulation of CXC ligand 1 transcription in melanoma cell lines by poly(ADP-ribose) polymerase-1. Oncogene. 2006;25:7714–7722. doi: 10.1038/sj.onc.1209751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Catar R.A., Chen L., Cuff S.M., Kift-Morgan A., Eberl M., Kettritz R., Kamhieh-Milz J., Moll G., Li Q., Zhao H., et al. Control of neutrophil influx during peritonitis by transcriptional cross-regulation of chemokine CXCL1 by IL-17 and IFN-γ. J. Pathol. 2020;251:175–186. doi: 10.1002/path.5438. [DOI] [PubMed] [Google Scholar]
- 58.Huang C., Li Z., Li N., Li Y., Chang A., Zhao T., Wang X., Wang H., Gao S., Yang S., et al. Interleukin 35 Expression Correlates With Microvessel Density in Pancreatic Ductal Adenocarcinoma, Recruits Monocytes, and Promotes Growth and Angiogenesis of Xenograft Tumors in Mice. Gastroenterology. 2018;154:675–688. doi: 10.1053/j.gastro.2017.09.039. [DOI] [PubMed] [Google Scholar]
- 59.Bierie B., Stover D.G., Abel T.W., Chytil A., Gorska A.E., Aakre M., Forrester E., Yang L., Wagner K.U., Moses H.L. Transforming growth factor-beta regulates mammary carcinoma cell survival and interaction with the adjacent microenvironment. Cancer Res. 2008;68:1809–1819. doi: 10.1158/0008-5472.CAN-07-5597. [DOI] [PubMed] [Google Scholar]
- 60.Bernard S., Myers M., Fang W.B., Zinda B., Smart C., Lambert D., Zou A., Fan F., Cheng N. CXCL1 Derived from Mammary Fibroblasts Promotes Progression of Mammary Lesions to Invasive Carcinoma through CXCR2 Dependent Mechanisms. J. Mammary Gland. Biol. Neoplasia. 2018;23:249–267. doi: 10.1007/s10911-018-9407-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ogawa R., Yamamoto T., Hirai H., Hanada K., Kiyasu Y., Nishikawa G., Mizuno R., Inamoto S., Itatani Y., Sakai Y., et al. Loss of SMAD4 Promotes Colorectal Cancer Progression by Recruiting Tumor-Associated Neutrophils via the CXCL1/8-CXCR2 Axis. Clin. Cancer Res. 2019;25:2887–2899. doi: 10.1158/1078-0432.CCR-18-3684. [DOI] [PubMed] [Google Scholar]
- 62.Ijichi H., Chytil A., Gorska A.E., Aakre M.E., Bierie B., Tada M., Mohri D., Miyabayashi K., Asaoka Y., Maeda S., et al. Inhibiting Cxcr2 disrupts tumor-stromal interactions and improves survival in a mouse model of pancreatic ductal adenocarcinoma. J. Clin. Investig. 2011;121:4106–4117. doi: 10.1172/JCI42754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fang W.B., Mafuvadze B., Yao M., Zou A., Portsche M., Cheng N. TGF-β Negatively Regulates CXCL1 Chemokine Expression in Mammary Fibroblasts through Enhancement of Smad2/3 and Suppression of HGF/c-Met Signaling Mechanisms. PLoS ONE. 2015;10:e0135063. doi: 10.1371/journal.pone.0135063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li X., Sterling J.A., Fan K.H., Vessella R.L., Shyr Y., Hayward S.W., Matrisian L.M., Bhowmick N.A. Loss of TGF-β responsiveness in prostate stromal cells alters chemokine levels and facilitates the development of mixed osteoblastic/osteolytic bone lesions. Mol. Cancer Res. 2012;10:494–503. doi: 10.1158/1541-7786.MCR-11-0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moser B., Clark-Lewis I., Zwahlen R., Baggiolini M. Neutrophil-activating properties of the melanoma growth-stimulatory activity. J. Exp. Med. 1990;171:1797–1802. doi: 10.1084/jem.171.5.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hurt B., Schulick R., Edil B., El Kasmi K.C., Barnett C., Jr. Cancer-promoting mechanisms of tumor-associated neutrophils. Am. J. Surg. 2017;214:938–944. doi: 10.1016/j.amjsurg.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 67.O’Connor P.M., Jackman J., Bae I., Myers T.G., Fan S., Mutoh M., Scudiero D.A., Monks A., Sausville E.A., Weinstein J.N., et al. Characterization of the p53 tumor suppressor pathway in cell lines of the National Cancer Institute anticancer drug screen and correlations with the growth-inhibitory potency of 123 anticancer agents. Cancer Res. 1997;57:4285–4300. [PubMed] [Google Scholar]
- 68.Leroy B., Girard L., Hollestelle A., Minna J.D., Gazdar A.F., Soussi T. Analysis of TP53 mutation status in human cancer cell lines: A reassessment. Hum. Mutat. 2014;35:756–765. doi: 10.1002/humu.22556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Solomon H., Buganim Y., Kogan-Sakin I., Pomeraniec L., Assia Y., Madar S., Goldstein I., Brosh R., Kalo E., Beatus T., et al. Various p53 mutant proteins differently regulate the Ras circuit to induce a cancer-related gene signature. J. Cell Sci. 2012;125:3144–3152. doi: 10.1242/jcs.099663. [DOI] [PubMed] [Google Scholar]
- 70.Son D.S., Kabir S.M., Dong Y.L., Lee E., Adunyah S.E. Inhibitory effect of tumor suppressor p53 on proinflammatory chemokine expression in ovarian cancer cells by reducing proteasomal degradation of IκB. PLoS ONE. 2012;7:e51116. doi: 10.1371/journal.pone.0051116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Carrà G., Lingua M.F., Maffeo B., Taulli R., Morotti A. P53 vs NF-κB: The role of nuclear factor-kappa B in the regulation of p53 activity and vice versa. Cell Mol. Life Sci. 2020;77:4449–4458. doi: 10.1007/s00018-020-03524-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lowe J.M., Menendez D., Bushel P.R., Shatz M., Kirk E.L., Troester M.A., Garantziotis S., Fessler M.B., Resnick M.A. p53 and NF-κB coregulate proinflammatory gene responses in human macrophages. Cancer Res. 2014;74:2182–2192. doi: 10.1158/0008-5472.CAN-13-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ignacio R.M.C., Dong Y.L., Kabir S.M., Choi H., Lee E.S., Wilson A.J., Beeghly-Fadiel A., Whalen M.M., Son D.S. CXCR2 is a negative regulator of p21 in p53-dependent and independent manner via Akt-mediated Mdm2 in ovarian cancer. Oncotarget. 2018;9:9751–9765. doi: 10.18632/oncotarget.24231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yan W., Chen X. Identification of GRO1 as a critical determinant for mutant p53 gain of function. J. Biol. Chem. 2009;284:12178–12187. doi: 10.1074/jbc.M900994200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.De Souza C., Madden J.A., Minn D., Kumar V.E., Montoya D.J., Nambiar R., Zhu Z., Xiao W.W., Tahmassebi N., Kathi H., et al. The P72R Polymorphism in R248Q/W p53 Mutants Modifies the Mutant Effect on Epithelial to Mesenchymal Transition Phenotype and Cell Invasion via CXCL1 Expression. Int. J. Mol. Sci. 2020;21:8025. doi: 10.3390/ijms21218025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Woodstock D.L., Sammons M.A., Fischer M. p63 and p53: Collaborative Partners or Dueling Rivals? Front. Cell Dev. Biol. 2021;9:701986. doi: 10.3389/fcell.2021.701986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Somerville T.D., Biffi G., Daßler-Plenker J., Hur S.K., He X.Y., Vance K.E., Miyabayashi K., Xu Y., Maia-Silva D., Klingbeil O., et al. Squamous trans-differentiation of pancreatic cancer cells promotes stromal inflammation. eLife. 2020;9:e53381. doi: 10.7554/eLife.53381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Huh Y.H., Lee G., Lee K.B., Koh J.T., Chun J.S., Ryu J.H. HIF-2α-induced chemokines stimulate motility of fibroblast-like synoviocytes and chondrocytes into the cartilage-pannus interface in experimental rheumatoid arthritis mouse models. Arthritis Res. Ther. 2015;17:302. doi: 10.1186/s13075-015-0816-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Triner D., Xue X., Schwartz A.J., Jung I., Colacino J.A., Shah Y.M. Epithelial Hypoxia-Inducible Factor 2α Facilitates the Progression of Colon Tumors through Recruiting Neutrophils. Mol. Cell Biol. 2017;37:e00481-16. doi: 10.1128/MCB.00481-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang J., Yu F., Jia X., Iwanowycz S., Wang Y., Huang S., Ai W., Fan D. MicroRNA-155 deficiency enhances the recruitment and functions of myeloid-derived suppressor cells in tumor microenvironment and promotes solid tumor growth. Int. J. Cancer. 2015;136:E602–E613. doi: 10.1002/ijc.29151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Akhtar S., Hartmann P., Karshovska E., Rinderknecht F.A., Subramanian P., Gremse F., Grommes J., Jacobs M., Kiessling F., Weber C., et al. Endothelial Hypoxia-Inducible Factor-1α Promotes Atherosclerosis and Monocyte Recruitment by Upregulating MicroRNA-19a. Hypertension. 2015;66:1220–1226. doi: 10.1161/HYPERTENSIONAHA.115.05886. [DOI] [PubMed] [Google Scholar]
- 82.Wang D., Wang L.H., Zhao Y., Lu Y.P., Zhu L. Hypoxia regulates the ferrous iron uptake and reactive oxygen species level via divalent metal transporter 1 (DMT1) Exon1B by hypoxia-inducible factor-1. IUBMB Life. 2010;62:629–636. doi: 10.1002/iub.363. [DOI] [PubMed] [Google Scholar]
- 83.Salvi A., Thanabalu T. Expression of N-WASP is regulated by HiF1α through the hypoxia response element in the N-WASP promoter. Biochem. Biophys. Rep. 2016;9:13–21. doi: 10.1016/j.bbrep.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ye L.Y., Chen W., Bai X.L., Xu X.Y., Zhang Q., Xia X.F., Sun X., Li G.G., Hu Q.D., Fu Q.H., et al. Hypoxia-Induced Epithelial-to-Mesenchymal Transition in Hepatocellular Carcinoma Induces an Immunosuppressive Tumor Microenvironment to Promote Metastasis. Cancer Res. 2016;76:818–830. doi: 10.1158/0008-5472.CAN-15-0977. [DOI] [PubMed] [Google Scholar]
- 85.Huang G., Tao L., Shen S., Chen L. Hypoxia induced CCL28 promotes angiogenesis in lung adenocarcinoma by targeting CCR3 on endothelial cells. Sci. Rep. 2016;6:27152. doi: 10.1038/srep27152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lo H.M., Lai T.H., Li C.H., Wu W.B. TNF-α induces CXCL1 chemokine expression and release in human vascular endothelial cells in vitro via two distinct signaling pathways. Acta Pharmacol. Sin. 2014;35:339–350. doi: 10.1038/aps.2013.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shieh J.M., Tsai Y.J., Tsou C.J., Wu W.B. CXCL1 regulation in human pulmonary epithelial cells by tumor necrosis factor. Cell Physiol. Biochem. 2014;34:1373–1384. doi: 10.1159/000366344. [DOI] [PubMed] [Google Scholar]
- 88.Wang B., Khachigian L.M., Esau L., Birrer M.J., Zhao X., Parker M.I., Hendricks D.T. A key role for early growth response-1 and nuclear factor-kappaB in mediating and maintaining GRO/CXCR2 proliferative signaling in esophageal cancer. Mol. Cancer Res. 2009;7:755–764. doi: 10.1158/1541-7786.MCR-08-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tkocz D., Crawford N.T., Buckley N.E., Berry F.B., Kennedy R.D., Gorski J.J., Harkin D.P., Mullan P.B. BRCA1 and GATA3 corepress FOXC1 to inhibit the pathogenesis of basal-like breast cancers. Oncogene. 2012;31:3667–3678. doi: 10.1038/onc.2011.531. [DOI] [PubMed] [Google Scholar]
- 90.Karapetsas A., Tokamani M., Evangelou C., Sandaltzopoulos R. The homeodomain transcription factor MEIS1 triggers chemokine expression and is involved in CD8+ T-lymphocyte infiltration in early stage ovarian cancer. Mol. Carcinog. 2018;57:1251–1263. doi: 10.1002/mc.22840. [DOI] [PubMed] [Google Scholar]
- 91.Botton T., Puissant A., Cheli Y., Tomic T., Giuliano S., Fajas L., Deckert M., Ortonne J.P., Bertolotto C., Tartare-Deckert S., et al. Ciglitazone negatively regulates CXCL1 signaling through MITF to suppress melanoma growth. Cell Death Differ. 2011;18:109–121. doi: 10.1038/cdd.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hermert D., Martin I.V., Reiss L.K., Liu X., Breitkopf D.M., Reimer K.C., Alidousty C., Rauen T., Floege J., Ostendorf T., et al. The nucleic acid binding protein YB-1-controlled expression of CXCL-1 modulates kidney damage in liver fibrosis. Kidney Int. 2020;97:741–752. doi: 10.1016/j.kint.2019.10.024. [DOI] [PubMed] [Google Scholar]
- 93.Georgakopoulos-Soares I., Chartoumpekis D.V., Kyriazopoulou V., Zaravinos A. EMT Factors and Metabolic Pathways in Cancer. Front. Oncol. 2020;10:499. doi: 10.3389/fonc.2020.00499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Taki M., Abiko K., Baba T., Hamanishi J., Yamaguchi K., Murakami R., Yamanoi K., Horikawa N., Hosoe Y., Nakamura E., et al. Snail promotes ovarian cancer progression by recruiting myeloid-derived suppressor cells via CXCR2 ligand upregulation. Nat. Commun. 2018;9:1685. doi: 10.1038/s41467-018-03966-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Xiang Z., Zhou Z.J., Xia G.K., Zhang X.H., Wei Z.W., Zhu J.T., Yu J., Chen W., He Y., Schwarz R.E., et al. A positive crosstalk between CXCR4 and CXCR2 promotes gastric cancer metastasis. Oncogene. 2017;36:5122–5133. doi: 10.1038/onc.2017.108. [DOI] [PubMed] [Google Scholar]
- 96.Sharma B., Nannuru K.C., Saxena S., Varney M.L., Singh R.K. CXCR2: A Novel Mediator of Mammary Tumor Bone Metastasis. Int. J. Mol. Sci. 2019;20:1237. doi: 10.3390/ijms20051237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Heisig J., Weber D., Englberger E., Winkler A., Kneitz S., Sung W.K., Wolf E., Eilers M., Wei C.L., Gessler M. Target gene analysis by microarrays and chromatin immunoprecipitation identifies HEY proteins as highly redundant bHLH repressors. PLoS Genet. 2012;8:e1002728. doi: 10.1371/journal.pgen.1002728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Han L., Korangath P., Nguyen N.K., Diehl A., Cho S., Teo W.W., Cope L., Gessler M., Romer L., Sukumar S. HEYL Regulates Neoangiogenesis Through Overexpression in Both Breast Tumor Epithelium and Endothelium. Front. Oncol. 2021;10:581459. doi: 10.3389/fonc.2020.581459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Saliba J., Coutaud B., Solovieva V., Lu F., Blank V. Regulation of CXCL1 chemokine and CSF3 cytokine levels in myometrial cells by the MAFF transcription factor. J. Cell Mol. Med. 2019;23:2517–2525. doi: 10.1111/jcmm.14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chan Y.W., van den Berg H.A., Moore J.D., Quenby S., Blanks A.M. Assessment of myometrial transcriptome changes associated with spontaneous human labour by high-throughput RNA-seq. Exp. Physiol. 2014;99:510–524. doi: 10.1113/expphysiol.2013.072868. [DOI] [PubMed] [Google Scholar]
- 101.Zhou Y., Zheng X., Xu B., Deng H., Chen L., Jiang J. Histone methyltransferase SETD2 inhibits tumor growth via suppressing CXCL1-mediated activation of cell cycle in lung adenocarcinoma. Aging. 2020;12:25189–25206. doi: 10.18632/aging.104120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Al Sarakbi W., Sasi W., Jiang W.G., Roberts T., Newbold R.F., Mokbel K. The mRNA expression of SETD2 in human breast cancer: Correlation with clinico-pathological parameters. BMC Cancer. 2009;9:290. doi: 10.1186/1471-2407-9-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Patil V., Pal J., Somasundaram K. Elucidating the cancer-specific genetic alteration spectrum of glioblastoma derived cell lines from whole exome and RNA sequencing. Oncotarget. 2015;6:43452–43471. doi: 10.18632/oncotarget.6171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lee M., Kim K., Kim S.Y., Jung S.H., Yoon J., Kim M.S., Park H.C., Jung E.S., Chung Y.J., Lee S.H. Genomic structures of dysplastic nodule and concurrent hepatocellular carcinoma. Hum. Pathol. 2018;81:37–46. doi: 10.1016/j.humpath.2018.06.026. [DOI] [PubMed] [Google Scholar]
- 105.Zhang Y., Yan L., Yao W., Chen K., Xu H., Ye Z. Integrated Analysis of Genetic Abnormalities of the Histone Lysine Methyltransferases in Prostate Cancer. Med. Sci. Monit. 2019;25:193–239. doi: 10.12659/MSM.912294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shang Y., Coppo M., He T., Ning F., Yu L., Kang L., Zhang B., Ju C., Qiao Y., Zhao B., et al. The transcriptional repressor Hes1 attenuates inflammation by regulating transcription elongation. Nat. Immunol. 2016;17:930–937. doi: 10.1038/ni.3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hu X., Chung A.Y., Wu I., Foldi J., Chen J., Ji J.D., Tateya T., Kang Y.J., Han J., Gessler M., et al. Integrated regulation of Toll-like receptor responses by Notch and interferon-gamma pathways. Immunity. 2008;29:691–703. doi: 10.1016/j.immuni.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Herjan T., Novotny M., Hamilton T.A. Diversity in sequence-dependent control of GRO chemokine mRNA half-life. J. Leukoc. Biol. 2013;93:895–904. doi: 10.1189/jlb.0812370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Stoeckle M.Y. Removal of a 3’ non-coding sequence is an initial step in degradation of gro alpha mRNA and is regulated by interleukin-1. Nucleic Acids Res. 1992;20:1123–1127. doi: 10.1093/nar/20.5.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Stoeckle M.Y., Guan L. High-resolution analysis of gro alpha mRNA poly(A) shortening: Regulation by interleukin-1 beta. Nucleic Acids Res. 1993;21:1613–1617. doi: 10.1093/nar/21.7.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhao W., Siegel D., Biton A., Tonqueze O.L., Zaitlen N., Ahituv N., Erle D.J. CRISPR-Cas9-mediated functional dissection of 3’-UTRs. Nucleic Acids Res. 2017;45:10800–10810. doi: 10.1093/nar/gkx675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pecot C.V., Rupaimoole R., Yang D., Akbani R., Ivan C., Lu C., Wu S., Han H.D., Shah M.Y., Rodriguez-Aguayo C., et al. Tumour angiogenesis regulation by the miR-200 family. Nat. Commun. 2013;4:2427. doi: 10.1038/ncomms3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lv M., Xu Y., Tang R., Ren J., Shen S., Chen Y., Liu B., Hou Y., Wang T. miR141-CXCL1-CXCR2 signaling-induced Treg recruitment regulates metastases and survival of non-small cell lung cancer. Mol. Cancer Ther. 2014;13:3152–3162. doi: 10.1158/1535-7163.MCT-14-0448. [DOI] [PubMed] [Google Scholar]
- 114.Cui X., Li Z., Gao J., Gao P.J., Ni Y.B., Zhu J.Y. Elevated CXCL1 increases hepatocellular carcinoma aggressiveness and is inhibited by miRNA-200a. Oncotarget. 2016;7:65052–65066. doi: 10.18632/oncotarget.11350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shrestha S., Yang C.D., Hong H.C., Chou C.H., Tai C.S., Chiew M.Y., Chen W.L., Weng S.L., Chen C.C., Chang Y.A., et al. Integrated MicroRNA-mRNA Analysis Reveals miR-204 Inhibits Cell Proliferation in Gastric Cancer by Targeting CKS1B, CXCL1 and GPRC5A. Int. J. Mol. Sci. 2017;19:87. doi: 10.3390/ijms19010087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liu C.H., Jing X.N., Liu X.L., Qin S.Y., Liu M.W., Hou C.H. Tumor-suppressor miRNA-27b-5p regulates the growth and metastatic behaviors of ovarian carcinoma cells by targeting CXCL1. J. Ovarian Res. 2020;13:92. doi: 10.1186/s13048-020-00697-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chen B., Song L., Nie X., Lin F., Yu Z., Kong W., Qi X., Wang W. CXCL1 Regulated by miR-302e Is Involved in Cell Viability and Motility of Colorectal Cancer via Inhibiting JAK-STAT Signaling Pathway. Front. Oncol. 2021;10:577229. doi: 10.3389/fonc.2020.577229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sun D., Novotny M., Bulek K., Liu C., Li X., Hamilton T. Treatment with IL-17 prolongs the half-life of chemokine CXCL1 mRNA via the adaptor TRAF5 and the splicing-regulatory factor SF2 (ASF) Nat. Immunol. 2011;12:853–860. doi: 10.1038/ni.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Herjan T., Yao P., Qian W., Li X., Liu C., Bulek K., Sun D., Yang W.P., Zhu J., He A., et al. HuR is required for IL-17-induced Act1-mediated CXCL1 and CXCL5 mRNA stabilization. J. Immunol. 2013;191:640–649. doi: 10.4049/jimmunol.1203315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Datta S., Biswas R., Novotny M., Pavicic P.G., Jr., Herjan T., Mandal P., Hamilton T.A. Tristetraprolin regulates CXCL1 (KC) mRNA stability. J. Immunol. 2008;180:2545–2552. doi: 10.4049/jimmunol.180.4.2545. [DOI] [PubMed] [Google Scholar]
- 121.Qiu L.Q., Lai W.S., Bradbury A., Zeldin D.C., Blackshear P.J. Tristetraprolin (TTP) coordinately regulates primary and secondary cellular responses to proinflammatory stimuli. J. Leukoc. Biol. 2015;97:723–736. doi: 10.1189/jlb.3A0214-106R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Smallie T., Ross E.A., Ammit A.J., Cunliffe H.E., Tang T., Rosner D.R., Ridley M.L., Buckley C.D., Saklatvala J., Dean J.L., et al. Dual-Specificity Phosphatase 1 and Tristetraprolin Cooperate To Regulate Macrophage Responses to Lipopolysaccharide. J. Immunol. 2015;195:277–288. doi: 10.4049/jimmunol.1402830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Clement S.L., Scheckel C., Stoecklin G., Lykke-Andersen J. Phosphorylation of tristetraprolin by MK2 impairs AU-rich element mRNA decay by preventing deadenylase recruitment. Mol. Cell Biol. 2011;31:256–266. doi: 10.1128/MCB.00717-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lian S., Zhai X., Wang X., Zhu H., Zhang S., Wang W., Wang Z., Huang J. Elevated expression of growth-regulated oncogene-alpha in tumor and stromal cells predicts unfavorable prognosis in pancreatic cancer. Medicine. 2016;95:e4328. doi: 10.1097/MD.0000000000004328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang L., Zhang C., Xu J., Wu H., Peng J., Cai S., He Y. CXCL1 gene silencing inhibits HGC803 cell migration and invasion and acts as an independent prognostic factor for poor survival in gastric cancer. Mol. Med. Rep. 2016;14:4673–4679. doi: 10.3892/mmr.2016.5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kronski E., Fiori M.E., Barbieri O., Astigiano S., Mirisola V., Killian P.H., Bruno A., Pagani A., Rovera F., Pfeffer U., et al. miR181b is induced by the chemopreventive polyphenol curcumin and inhibits breast cancer metastasis via down-regulation of the inflammatory cytokines CXCL1 and -2. Mol. Oncol. 2014;8:581–595. doi: 10.1016/j.molonc.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Naito Y., Yamamoto Y., Sakamoto N., Shimomura I., Kogure A., Kumazaki M., Yokoi A., Yashiro M., Kiyono T., Yanagihara K., et al. Cancer extracellular vesicles contribute to stromal heterogeneity by inducing chemokines in cancer-associa.a.a.ated fibroblasts. Oncogene. 2019;38:5566–5579. doi: 10.1038/s41388-019-0832-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Leng J., Liu W., Li L., Wei F.Y., Tian M., Liu H.M., Guo W. MicroRNA-429/Cxcl1 Axis Protective Against Oxygen Glucose Deprivation/Reoxygenation-Induced Injury in Brain Microvascular Endothelial Cells. Dose Response. 2020;18:1559325820913785. doi: 10.1177/1559325820913785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shi Y., Yi Z., Zhao P., Xu Y., Pan P. MicroRNA-532-5p protects against cerebral ischemia-reperfusion injury by directly targeting CXCL1. Aging. 2021;13:11528–11541. doi: 10.18632/aging.202846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Du Z., Wu T., Liu L., Luo B., Wei C. Extracellular vesicles-derived miR-150-5p secreted by adipose-derived mesenchymal stem cells inhibits CXCL1 expression to attenuate hepatic fibrosis. J. Cell Mol. Med. 2021;25:701–715. doi: 10.1111/jcmm.16119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yoo J.K., Jung H.Y., Kim C.H., Son W.S., Kim J.K. miR-7641 modulates the expression of CXCL1 during endothelial differentiation derived from human embryonic stem cells. Arch. Pharm. Res. 2013;36:353–358. doi: 10.1007/s12272-013-0067-9. [DOI] [PubMed] [Google Scholar]
- 132.Balentien E., Han J.H., Thomas H.G., Wen D.Z., Samantha A.K., Zachariae C.O., Griffin P.R., Brachmann R., Wong W.L., Matsushima K., et al. Recombinant expression, biochemical characterization, and biological activities of the human MGSA/gro protein. Biochemistry. 1990;29:10225–10233. doi: 10.1021/bi00496a011. [DOI] [PubMed] [Google Scholar]
- 133.Fairbrother W.J., Reilly D., Colby T.J., Hesselgesser J., Horuk R. The solution structure of melanoma growth stimulating activity. J. Mol. Biol. 1994;242:252–270. doi: 10.1006/jmbi.1994.1577. [DOI] [PubMed] [Google Scholar]
- 134.Fairbrother W.J. The Solution Structure of Melanoma Growth Stimulating Activity. 1994. [(accessed on 25 November 2021)]. Available online: https://www.rcsb.org/structure/1MGS. [DOI] [PubMed]
- 135.Richmond A., Thomas H.G. Purification of melanoma growth stimulatory activity. J. Cell Physiol. 1986;129:375–384. doi: 10.1002/jcp.1041290316. [DOI] [PubMed] [Google Scholar]
- 136.Sayle R.A., Milner-White E.J. RASMOL: Biomolecular graphics for all. Trends Biochem. Sci. 1995;20:374. doi: 10.1016/S0968-0004(00)89080-5. [DOI] [PubMed] [Google Scholar]
- 137.Bernstein H.J. Recent changes to RasMol, recombining the variants. Trends Biochem. Sci. 2000;25:453–455. doi: 10.1016/S0968-0004(00)01606-6. [DOI] [PubMed] [Google Scholar]
- 138.Berman H.M., Westbrook J., Feng Z., Gilliland G., Bhat T.N., Weissig H., Shindyalov I.N., Bourne P.E. The Protein Data Bank. Nucleic Acids Res. 2000;28:235–242. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Meen A.J., Øynebråten I., Reine T.M., Duelli A., Svennevig K., Pejler G., Jenssen T., Kolset S.O. Serglycin is a major proteoglycan in polarized human endothelial cells and is implicated in the secretion of the chemokine GROalpha/CXCL1. J. Biol. Chem. 2011;286:2636–2647. doi: 10.1074/jbc.M110.151944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Øynebråten I., Bakke O., Brandtzaeg P., Johansen F.E., Haraldsen G. Rapid chemokine secretion from endothelial cells originates from 2 distinct compartments. Blood. 2004;104:314–320. doi: 10.1182/blood-2003-08-2891. [DOI] [PubMed] [Google Scholar]
- 141.Sepuru K.M., Rajarathnam K. Structural basis of chemokine interactions with heparan sulfate, chondroitin sulfate, and dermatan sulfate. J. Biol. Chem. 2019;294:15650–15661. doi: 10.1074/jbc.RA119.009879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hayashida K., Parks W.C., Park P.W. Syndecan-1 shedding facilitates the resolution of neutrophilic inflammation by removing sequestered CXC chemokines. Blood. 2009;114:3033–3043. doi: 10.1182/blood-2009-02-204966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gill S.E., Nadler S.T., Li Q., Frevert C.W., Park P.W., Chen P., Parks W.C. Shedding of Syndecan-1/CXCL1 Complexes by Matrix Metalloproteinase 7 Functions as an Epithelial Checkpoint of Neutrophil Activation. Am. J. Respir. Cell Mol. Biol. 2016;55:243–251. doi: 10.1165/rcmb.2015-0193OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Li Q., Park P.W., Wilson C.L., Parks W.C. Matrilysin shedding of syndecan-1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell. 2002;111:635–646. doi: 10.1016/S0092-8674(02)01079-6. [DOI] [PubMed] [Google Scholar]
- 145.Swee M., Wilson C.L., Wang Y., McGuire J.K., Parks W.C. Matrix metalloproteinase-7 (matrilysin) controls neutrophil egress by generating chemokine gradients. J. Leukoc. Biol. 2008;83:1404–1412. doi: 10.1189/jlb.0108016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wang D., Sai J., Richmond A. Cell surface heparan sulfate participates in CXCL1-induced signaling. Biochemistry. 2003;42:1071–1077. doi: 10.1021/bi026425a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wuyts A., Govaerts C., Struyf S., Lenaerts J.P., Put W., Conings R., Proost P., Van Damme J. Isolation of the CXC chemokines ENA-78, GRO alpha and GRO gamma from tumor cells and leukocytes reveals NH2-terminal heterogeneity. Functional comparison of different natural isoforms. Eur. J. Biochem. 1999;260:421–429. doi: 10.1046/j.1432-1327.1999.00166.x. [DOI] [PubMed] [Google Scholar]
- 148.Repnik U., Starr A.E., Overall C.M., Turk B. Cysteine Cathepsins Activate ELR Chemokines and Inactivate Non-ELR Chemokines. J. Biol. Chem. 2015;290:13800–13811. doi: 10.1074/jbc.M115.638395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Dean R.A., Cox J.H., Bellac C.L., Doucet A., Starr A.E., Overall C.M. Macrophage-specific metalloelastase (MMP-12) truncates and inactivates ELR+ CXC chemokines and generates CCL2, -7, -8, and -13 antagonists: Potential role of the macrophage in terminating polymorphonuclear leukocyte influx. Blood. 2008;112:3455–3464. doi: 10.1182/blood-2007-12-129080. [DOI] [PubMed] [Google Scholar]
- 150.Nesmelova I.V., Sham Y., Gao J., Mayo K.H. CXC and CC chemokines form mixed heterodimers: Association free energies from molecular dynamics simulations and experimental correlations. J. Biol. Chem. 2008;283:24155–24166. doi: 10.1074/jbc.M803308200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kim K.S., Clark-Lewis I., Sykes B.D. Solution structure of GRO/melanoma growth stimulatory activity determined by 1H NMR spectroscopy. J. Biol. Chem. 1994;269:32909–32915. doi: 10.1016/S0021-9258(20)30077-6. [DOI] [PubMed] [Google Scholar]
- 152.Rajarathnam K., Kay C.M., Dewald B., Wolf M., Baggiolini M., Clark-Lewis I., Sykes B.D. Neutrophil-activating peptide-2 and melanoma growth-stimulatory activity are functional as monomers for neutrophil activation. J. Biol. Chem. 1997;272:1725–1729. doi: 10.1074/jbc.272.3.1725. [DOI] [PubMed] [Google Scholar]
- 153.Ravindran A., Sawant K.V., Sarmiento J., Navarro J., Rajarathnam K. Chemokine CXCL1 dimer is a potent agonist for the CXCR2 receptor. J. Biol. Chem. 2013;288:12244–12252. doi: 10.1074/jbc.M112.443762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Poluri K.M., Joseph P.R.B., Sawant K.V., Rajarathnam K. Molecular basis of glycosaminoglycan heparin binding to the chemokine CXCL1 dimer. J. Biol. Chem. 2013;288:25143–25153. doi: 10.1074/jbc.M113.492579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Baier P.K., Eggstein S., Wolff-Vorbeck G., Baumgartner U., Hopt U.T. Chemokines in human colorectal carcinoma. Anticancer. Res. 2005;25:3581–3584. [PubMed] [Google Scholar]
- 156.Cao Y., Huang H., Wang Z., Zhang G. The Inflammatory CXC Chemokines, GROαhigh, IP-10low, and MIGlow, in Tumor Microenvironment Can Be Used as New Indicators for Non-small Cell Lung Cancer Progression. Immunol. Investig. 2017;46:361–374. doi: 10.1080/08820139.2017.1280052. [DOI] [PubMed] [Google Scholar]
- 157.Hoogewerf A.J., Kuschert G.S., Proudfoot A.E., Borlat F., Clark-Lewis I., Power C.A., Wells T.N. Glycosaminoglycans mediate cell surface oligomerization of chemokines. Biochemistry. 1997;36:13570–13578. doi: 10.1021/bi971125s. [DOI] [PubMed] [Google Scholar]
- 158.Sepuru K.M., Rajarathnam K. CXCL1/MGSA Is a Novel Glycosaminoglycan (GAG)-binding Chemokine: STRUCTURAL EVIDENCE FOR TWO DISTINCT NON-OVERLAPPING BINDING DOMAINS. J. Biol. Chem. 2016;291:4247–4255. doi: 10.1074/jbc.M115.697888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sawant K.V., Xu R., Cox R., Hawkins H., Sbrana E., Kolli D., Garofalo R.P., Rajarathnam K. Chemokine CXCL1-Mediated Neutrophil Trafficking in the Lung: Role of CXCR2 Activation. J. Innate Immun. 2015;7:647–658. doi: 10.1159/000430914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sawant K.V., Poluri K.M., Dutta A.K., Sepuru K.M., Troshkina A., Garofalo R.P., Rajarathnam K. Chemokine CXCL1 mediated neutrophil recruitment: Role of glycosaminoglycan interactions. Sci. Rep. 2016;6:33123. doi: 10.1038/srep33123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sawant K.V., Sepuru K.M., Lowry E., Penaranda B., Frevert C.W., Garofalo R.P., Rajarathnam K. Neutrophil recruitment by chemokines Cxcl1/KC and Cxcl2/MIP2: Role of Cxcr2 activation and glycosaminoglycan interactions. J. Leukoc. Biol. 2021;109:777–791. doi: 10.1002/JLB.3A0820-207R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Brown A.J., Joseph P.R., Sawant K.V., Rajarathnam K. Chemokine CXCL7 Heterodimers: Structural Insights, CXCR2 Receptor Function, and Glycosaminoglycan Interactions. Int. J. Mol. Sci. 2017;18:748. doi: 10.3390/ijms18040748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sepuru K.M., Rajarathnam K. Structural basis of a chemokine heterodimer binding to glycosaminoglycans. Biochem. J. 2021;478:1009–1021. doi: 10.1042/BCJ20200927. [DOI] [PubMed] [Google Scholar]
- 164.Yu L., Yang X., Xu C., Sun J., Fang Z., Pan H., Han W. Comprehensive analysis of the expression and prognostic value of CXC chemokines in colorectal cancer. Int. Immunopharmacol. 2020;89:107077. doi: 10.1016/j.intimp.2020.107077. [DOI] [PubMed] [Google Scholar]
- 165.Li Y., Wu T., Gong S., Zhou H., Yu L., Liang M., Shi R., Wu Z., Zhang J., Li S. Analysis of the Prognosis and Therapeutic Value of the CXC Chemokine Family in Head and Neck Squamous Cell Carcinoma. Front. Oncol. 2021;10:570736. doi: 10.3389/fonc.2020.570736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhou X., Peng M., He Y., Peng J., Zhang X., Wang C., Xia X., Song W. CXC Chemokines as Therapeutic Targets and Prognostic Biomarkers in Skin Cutaneous Melanoma Microenvironment. Front. Oncol. 2021;11:619003. doi: 10.3389/fonc.2021.619003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Szabo M.C., Soo K.S., Zlotnik A., Schall T.J. Chemokine class differences in binding to the Duffy antigen-erythrocyte chemokine receptor. J. Biol. Chem. 1995;270:25348–25351. doi: 10.1074/jbc.270.43.25348. [DOI] [PubMed] [Google Scholar]
- 168.Ludwig A., Petersen F., Zahn S., Götze O., Schröder J.M., Flad H.D., Brandt E. The CXC-chemokine neutrophil-activating peptide-2 induces two distinct optima of neutrophil chemotaxis by differential interaction with interleukin-8 receptors CXCR-1 and CXCR-2. Blood. 1997;90:4588–4597. doi: 10.1182/blood.V90.11.4588. [DOI] [PubMed] [Google Scholar]
- 169.Geiser T., Dewald B., Ehrengruber M.U., Clark-Lewis I., Baggiolini M. The interleukin-8-related chemotactic cytokines GRO alpha, GRO beta, and GRO gamma activate human neutrophil and basophil leukocytes. J. Biol. Chem. 1993;268:15419–15424. doi: 10.1016/S0021-9258(18)82274-8. [DOI] [PubMed] [Google Scholar]
- 170.Kuo P.L., Shen K.H., Hung S.H., Hsu Y.L. CXCL1/GROα increases cell migration and invasion of prostate cancer by decreasing fibulin-1 expression through NF-κB/HDAC1 epigenetic regulation. Carcinogenesis. 2012;33:2477–2487. doi: 10.1093/carcin/bgs299. [DOI] [PubMed] [Google Scholar]
- 171.Zhang T., Tseng C., Zhang Y., Sirin O., Corn P.G., Li-Ning-Tapia E.M., Troncoso P., Davis J., Pettaway C., Ward J., et al. CXCL1 mediates obesity-associated adipose stromal cell trafficking and function in the tumour microenvironment. Nat. Commun. 2016;7:11674. doi: 10.1038/ncomms11674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Höher G., Fiegenbaum M., Almeida S. Molecular basis of the Duffy blood group system. Blood Transfus. 2018;16:93–100. doi: 10.2450/2017.0119-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Horuk R., Colby T.J., Darbonne W.C., Schall T.J., Neote K. The human erythrocyte inflammatory peptide (chemokine) receptor. Biochemical characterization, solubilization, and development of a binding assay for the soluble receptor. Biochemistry. 1993;32:5733–5738. doi: 10.1021/bi00073a002. [DOI] [PubMed] [Google Scholar]
- 174.Peiper S.C., Wang Z.X., Neote K., Martin A.W., Showell H.J., Conklyn M.J., Ogborne K., Hadley T.J., Lu Z.H., Hesselgesser J., et al. The Duffy antigen/receptor for chemokines (DARC) is expressed in endothelial cells of Duffy negative individuals who lack the erythrocyte receptor. J. Exp. Med. 1995;181:1311–1317. doi: 10.1084/jem.181.4.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lee J.S., Frevert C.W., Wurfel M.M., Peiper S.C., Wong V.A., Ballman K.K., Ruzinski J.T., Rhim J.S., Martin T.R., Goodman R.B. Duffy antigen facilitates movement of chemokine across the endothelium in vitro and promotes neutrophil transmigration in vitro and in vivo. J. Immunol. 2003;170:5244–5251. doi: 10.4049/jimmunol.170.10.5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hans D., Pattnaik P., Bhattacharyya A., Shakri A.R., Yazdani S.S., Sharma M., Choe H., Farzan M., Chitnis C.E. Mapping binding residues in the Plasmodium vivax domain that binds Duffy antigen during red cell invasion. Mol. Microbiol. 2005;55:1423–1434. doi: 10.1111/j.1365-2958.2005.04484.x. [DOI] [PubMed] [Google Scholar]
- 177.Lim K.L., Amir A., Lau Y.L., Fong M.Y. The Duffy binding protein (PkDBPαII) of Plasmodium knowlesi from Peninsular Malaysia and Malaysian Borneo show different binding activity level to human erythrocytes. Malar. J. 2017;16:331. doi: 10.1186/s12936-017-1984-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Neote K., Mak J.Y., Kolakowski L.F., Jr., Schall T.J. Functional and biochemical analysis of the cloned Duffy antigen: Identity with the red blood cell chemokine receptor. Blood. 1994;84:44–52. doi: 10.1182/blood.V84.1.44.44. [DOI] [PubMed] [Google Scholar]
- 179.Chaudhuri A., Polyakova J., Zbrzezna V., Williams K., Gulati S., Pogo A.O. Cloning of glycoprotein D cDNA, which encodes the major subunit of the Duffy blood group system and the receptor for the Plasmodium vivax malaria parasite. Proc. Natl. Acad. Sci. USA. 1993;90:10793–10797. doi: 10.1073/pnas.90.22.10793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lu Z.H., Wang Z.X., Horuk R., Hesselgesser J., Lou Y.C., Hadley T.J., Peiper S.C. The promiscuous chemokine binding profile of the Duffy antigen/receptor for chemokines is primarily localized to sequences in the amino-terminal domain. J. Biol. Chem. 1995;270:26239–26245. doi: 10.1074/jbc.270.44.26239. [DOI] [PubMed] [Google Scholar]
- 181.Koenen A., Babendreyer A., Schumacher J., Pasqualon T., Schwarz N., Seifert A., Deupi X., Ludwig A., Dreymueller D. The DRF motif of CXCR6 as chemokine receptor adaptation to adhesion. PLoS ONE. 2017;12:e0173486. doi: 10.1371/journal.pone.0173486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Al-Alwan L.A., Chang Y., Rousseau S., Martin J.G., Eidelman D.H., Hamid Q. CXCL1 inhibits airway smooth muscle cell migration through the decoy receptor Duffy antigen receptor for chemokines. J. Immunol. 2014;193:1416–1426. doi: 10.4049/jimmunol.1302860. [DOI] [PubMed] [Google Scholar]
- 183.Dawson T.C., Lentsch A.B., Wang Z., Cowhig J.E., Rot A., Maeda N., Peiper S.C. Exaggerated response to endotoxin in mice lacking the Duffy antigen/receptor for chemokines (DARC) Blood. 2000;96:1681–1684. doi: 10.1182/blood.V96.5.1681. [DOI] [PubMed] [Google Scholar]
- 184.Fukuma N., Akimitsu N., Hamamoto H., Kusuhara H., Sugiyama Y., Sekimizu K. A role of the Duffy antigen for the maintenance of plasma chemokine concentrations. Biochem. Biophys. Res. Commun. 2003;303:137–139. doi: 10.1016/S0006-291X(03)00293-6. [DOI] [PubMed] [Google Scholar]
- 185.Zhao Y., Mangalmurti N.S., Xiong Z., Prakash B., Guo F., Stolz D.B., Lee J.S. Duffy antigen receptor for chemokines mediates chemokine endocytosis through a macropinocytosis-like process in endothelial cells. PLoS ONE. 2011;6:e29624. doi: 10.1371/journal.pone.0029624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Maeda S., Kuboki S., Nojima H., Shimizu H., Yoshitomi H., Furukawa K., Miyazaki M., Ohtsuka M. Duffy antigen receptor for chemokines (DARC) expressing in cancer cells inhibits tumor progression by suppressing CXCR2 signaling in human pancreatic ductal adenocarcinoma. Cytokine. 2017;95:12–21. doi: 10.1016/j.cyto.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 187.Finegan K.G., Perez-Madrigal D., Hitchin J.R., Davies C.C., Jordan A.M., Tournier C. ERK5 is a critical mediator of inflammation-driven cancer. Cancer Res. 2015;75:742–753. doi: 10.1158/0008-5472.CAN-13-3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kunisada M., Hosaka C., Takemori C., Nakano E., Nishigori C. CXCL1 Inhibition Regulates UVB-Induced Skin Inflammation and Tumorigenesis in Xpa-Deficient Mice. J. Investig. Dermatol. 2017;137:1975–1983. doi: 10.1016/j.jid.2017.04.034. [DOI] [PubMed] [Google Scholar]
- 189.Miyake M., Hori S., Morizawa Y., Tatsumi Y., Nakai Y., Anai S., Torimoto K., Aoki K., Tanaka N., Shimada K., et al. CXCL1-Mediated Interaction of Cancer Cells with Tumor-Associated Macrophages and Cancer-Associated Fibroblasts Promotes Tumor Progression in Human Bladder Cancer. Neoplasia. 2016;18:636–646. doi: 10.1016/j.neo.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Bardi G.T., Al-Rayan N., Richie J.L., Yaddanapudi K., Hood J.L. Detection of Inflammation-Related Melanoma Small Extracellular Vesicle (sEV) mRNA Content Using Primary Melanocyte sEVs as a Reference. Int. J. Mol. Sci. 2019;20:1235. doi: 10.3390/ijms20051235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Öhlund D., Handly-Santana A., Biffi G., Elyada E., Almeida A.S., Ponz-Sarvise M., Corbo V., Oni T.E., Hearn S.A., Lee E.J., et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J. Exp. Med. 2017;214:579–596. doi: 10.1084/jem.20162024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kim E.K., Moon S., Kim D.K., Zhang X., Kim J. CXCL1 induces senescence of cancer-associated fibroblasts via autocrine loops in oral squamous cell carcinoma. PLoS ONE. 2018;13:e0188847. doi: 10.1371/journal.pone.0188847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Lu Y., Dong B., Xu F., Xu Y., Pan J., Song J., Zhang J., Huang Y., Xue W. CXCL1-LCN2 paracrine axis promotes progression of prostate cancer via the Src activation and epithelial-mesenchymal transition. Cell Commun. Signal. 2019;17:118. doi: 10.1186/s12964-019-0434-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Avnet S., Di Pompo G., Chano T., Errani C., Ibrahim-Hashim A., Gillies R.J., Donati D.M., Baldini N. Cancer-associated mesenchymal stroma fosters the stemness of osteosarcoma cells in response to intratumoral acidosis via NF-κB activation. Int. J. Cancer. 2017;140:1331–1345. doi: 10.1002/ijc.30540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Wang D., Sun H., Wei J., Cen B., DuBois R.N. CXCL1 Is Critical for Premetastatic Niche Formation and Metastasis in Colorectal Cancer. Cancer Res. 2017;77:3655–3665. doi: 10.1158/0008-5472.CAN-16-3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wang N., Liu W., Zheng Y., Wang S., Yang B., Li M., Song J., Zhang F., Zhang X., Wang Q., et al. CXCL1 derived from tumor-associated macrophages promotes breast cancer metastasis via activating NF-κB/SOX4 signaling. Cell Death Dis. 2018;9:880. doi: 10.1038/s41419-018-0876-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Chen X., Chen R., Jin R., Huang Z. The role of CXCL chemokine family in the development and progression of gastric cancer. Int. J. Clin. Exp. Pathol. 2020;13:484–492. [PMC free article] [PubMed] [Google Scholar]
- 198.Martin C., Burdon P.C., Bridger G., Gutierrez-Ramos J.C., Williams T.J., Rankin S.M. Chemokines acting via CXCR2 and CXCR4 control the release of neutrophils from the bone marrow and their return following senescence. Immunity. 2003;19:583–593. doi: 10.1016/S1074-7613(03)00263-2. [DOI] [PubMed] [Google Scholar]
- 199.Jablonska J., Wu C.F., Andzinski L., Leschner S., Weiss S. CXCR2-mediated tumor-associated neutrophil recruitment is regulated by IFN-β. Int. J. Cancer. 2014;134:1346–1358. doi: 10.1002/ijc.28551. [DOI] [PubMed] [Google Scholar]
- 200.SenGupta S., Hein L.E., Xu Y., Zhang J., Konwerski J.R., Li Y., Johnson C., Cai D., Smith J.L., Parent C.A. Triple-Negative Breast Cancer Cells Recruit Neutrophils by Secreting TGF-β and CXCR2 Ligands. Front. Immunol. 2021;12:659996. doi: 10.3389/fimmu.2021.659996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Varikuti S., Oghumu S., Elbaz M., Volpedo G., Ahirwar D.K., Alarcon P.C., Sperling R.H., Moretti E., Pioso M.S., Kimble J., et al. STAT1 gene deficient mice develop accelerated breast cancer growth and metastasis which is reduced by IL-17 blockade. Oncoimmunology. 2017;6:e1361088. doi: 10.1080/2162402X.2017.1361088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Gibson J.T., Orlandella R.M., Turbitt W.J., Behring M., Manne U., Sorge R.E., Norian L.A. Obesity-Associated Myeloid-Derived Suppressor Cells Promote Apoptosis of Tumor-Infiltrating CD8 T Cells and Immunotherapy Resistance in Breast Cancer. Front. Immunol. 2020;11:590794. doi: 10.3389/fimmu.2020.590794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Han X., Shi H., Sun Y., Shang C., Luan T., Wang D., Ba X., Zeng X. CXCR2 expression on granulocyte and macrophage progenitors under tumor conditions contributes to mo-MDSC generation via SAP18/ERK/STAT3. Cell Death Dis. 2019;10:598. doi: 10.1038/s41419-019-1837-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Senst C., Nazari-Shafti T., Kruger S., Höner Zu Bentrup K., Dupin C.L., Chaffin A.E., Srivastav S.K., Wörner P.M., Abdel-Mageed A.B., Alt E.U., et al. Prospective dual role of mesenchymal stem cells in breast tumor microenvironment. Breast Cancer Res. Treat. 2013;137:69–79. doi: 10.1007/s10549-012-2321-0. [DOI] [PubMed] [Google Scholar]
- 205.Karl E., Warner K., Zeitlin B., Kaneko T., Wurtzel L., Jin T., Chang J., Wang S., Wang C.Y., Strieter R.M., et al. Bcl-2 acts in a proangiogenic signaling pathway through nuclear factor-kappaB and CXC chemokines. Cancer Res. 2005;65:5063–5069. doi: 10.1158/0008-5472.CAN-05-0140. [DOI] [PubMed] [Google Scholar]
- 206.Kaneko T., Zhang Z., Mantellini M.G., Karl E., Zeitlin B., Verhaegen M., Soengas M.S., Lingen M., Strieter R.M., Nunez G., et al. Bcl-2 orchestrates a cross-talk between endothelial and tumor cells that promotes tumor growth. Cancer Res. 2007;67:9685–9693. doi: 10.1158/0008-5472.CAN-07-1497. [DOI] [PubMed] [Google Scholar]
- 207.Karl E., Zhang Z., Dong Z., Neiva K.G., Soengas M.S., Koch A.E., Polverini P.J., Núñez G., Nör J.E. Unidirectional crosstalk between Bcl-xL and Bcl-2 enhances the angiogenic phenotype of endothelial cells. Cell Death Differ. 2007;14:1657–1666. doi: 10.1038/sj.cdd.4402174. [DOI] [PubMed] [Google Scholar]
- 208.Huang Z., Zhang M., Chen G., Wang W., Zhang P., Yue Y., Guan Z., Wang X., Fan J. Bladder cancer cells interact with vascular endothelial cells triggering EGFR signals to promote tumor progression. Int. J. Oncol. 2019;54:1555–1566. doi: 10.3892/ijo.2019.4729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Zhuo C., Wu X., Li J., Hu D., Jian J., Chen C., Zheng X., Yang C. Chemokine (C-X-C motif) ligand 1 is associated with tumor progression and poor prognosis in patients with colorectal cancer. Biosci. Rep. 2018;38:BSR20180580. doi: 10.1042/BSR20180580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Yamamoto Y., Kuroda K., Sera T., Sugimoto A., Kushiyama S., Nishimura S., Togano S., Okuno T., Yoshii M., Tamura T., et al. The Clinicopathological Significance of the CXCR2 Ligands, CXCL1, CXCL2, CXCL3, CXCL5, CXCL6, CXCL7, and CXCL8 in Gastric Cancer. Anticancer. Res. 2019;39:6645–6652. doi: 10.21873/anticanres.13879. [DOI] [PubMed] [Google Scholar]
- 211.Han L., Liu W., Chen Y., Wu H., Zhang Y., Jiang B. GROα expression and its prognostic implications in laryngeal squamous cell carcinoma. Neoplasma. 2015;62:152–158. doi: 10.4149/neo_2015_020. [DOI] [PubMed] [Google Scholar]
- 212.Wang D., Luo Y., Guo Y., Li G., Li F. A-kinase interacting protein 1, a potential biomarker associated with advanced tumor features and CXCL1/2 in prostate cancer. Int. J. Biol. Markers. 2020;35:74–81. doi: 10.1177/1724600820914944. [DOI] [PubMed] [Google Scholar]
- 213.Zeng Q., Sun S., Li Y., Li X., Li Z., Liang H. Identification of Therapeutic Targets and Prognostic Biomarkers Among CXC Chemokines in the Renal Cell Carcinoma Microenvironment. Front. Oncol. 2020;9:1555. doi: 10.3389/fonc.2019.01555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Miyake M., Furuya H., Onishi S., Hokutan K., Anai S., Chan O., Shi S., Fujimoto K., Goodison S., Cai W., et al. Monoclonal Antibody against CXCL1 (HL2401) as a Novel Agent in Suppressing IL6 Expression and Tumoral Growth. Theranostics. 2019;9:853–867. doi: 10.7150/thno.29553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Romanini J., Mielcke T.R., Leal P.C., Figueiredo C.P., Calixto J.B., Morrone F.B., Batista E.L., Jr., Campos M.M. The role of CXCR2 chemokine receptors in the oral squamous cell carcinoma. Investig. New Drugs. 2012;30:1371–1378. doi: 10.1007/s10637-011-9701-x. [DOI] [PubMed] [Google Scholar]
- 216.Xu M., Jiang H., Wang H., Liu J., Liu B., Guo Z. SB225002 inhibits prostate cancer invasion and attenuates the expression of BSP, OPN and MMP-2. Oncol. Rep. 2018;40:726–736. doi: 10.3892/or.2018.6504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Liu L., Sun H., Wu S., Tan H., Sun Y., Liu X., Si S., Xu L., Huang J., Zhou W., et al. IL-17A promotes CXCR2-dependent angiogenesis in a mouse model of liver cancer. Mol. Med. Rep. 2019;20:1065–1074. doi: 10.3892/mmr.2019.10310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Wang J., Hu W., Wang K., Yu J., Luo B., Luo G., Wang W., Wang H., Li J., Wen J. Repertaxin, an inhibitor of the chemokine receptors CXCR1 and CXCR2, inhibits malignant behavior of human gastric cancer MKN45 cells in vitro and in vivo and enhances efficacy of 5-fluorouracil. Int. J. Oncol. 2016;48:1341–1352. doi: 10.3892/ijo.2016.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Kemp D.M., Pidich A., Larijani M., Jonas R., Lash E., Sato T., Terai M., De Pizzol M., Allegretti M., Igoucheva O., et al. Ladarixin, a dual CXCR1/2 inhibitor, attenuates experimental melanomas harboring different molecular defects by affecting malignant cells and tumor microenvironment. Oncotarget. 2017;8:14428–14442. doi: 10.18632/oncotarget.14803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Singh S., Sadanandam A., Nannuru K.C., Varney M.L., Mayer-Ezell R., Bond R., Singh R.K. Small-molecule antagonists for CXCR2 and CXCR1 inhibit human melanoma growth by decreasing tumor cell proliferation, survival, and angiogenesis. Clin. Cancer Res. 2009;15:2380–2386. doi: 10.1158/1078-0432.CCR-08-2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Idorn M., Skadborg S.K., Kellermann L., Halldórsdóttir H.R., Holmen Olofsson G., Met Ö., Thor Straten P. Chemokine receptor engineering of T cells with CXCR2 improves homing towards subcutaneous human melanomas in xenograft mouse model. Oncoimmunology. 2018;7:e1450715. doi: 10.1080/2162402X.2018.1450715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Liu G., Rui W., Zheng H., Huang D., Yu F., Zhang Y., Dong J., Zhao X., Lin X. CXCR2-modified CAR-T cells have enhanced trafficking ability that improves treatment of hepatocellular carcinoma. Eur. J. Immunol. 2020;50:712–724. doi: 10.1002/eji.201948457. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.