Abstract
The endocannabinoid system is found in most, if not all, mammalian organs and is involved in a variety of physiological functions, ranging from the control of synaptic plasticity in the brain to the modulation of smooth muscle motility in the gastrointestinal tract. This signaling complex consists of G protein-coupled cannabinoid receptors, endogenous ligands for those receptors (endocannabinoids) and enzymes/transporters responsible for the formation and deactivation of these ligands. There are two subtypes of cannabinoid receptors, CB1 and CB2, and two major endocannabinoids, arachidonoylethanolamide (anandamide) and 2-arachidonoyl-sn-glycerol (2-AG), which are produced upon demand through cleavage of distinct phospholipid precursors. All molecular components of the endocannabinoid system are represented in the adipose organ, where endocannabinoid signals are thought to regulate critical homeostatic processes, including adipogenesis, lipogenesis and thermogenesis. Importantly, obesity was found to be associated with excess endocannabinoid activity in visceral fat depots, and the therapeutic potential of normalizing such activity by blocking CB1 receptors has been the focus of substantial preclinical and clinical research. Results have been mixed thus far, mostly owing to the emergence of psychiatric side effects rooted in the protective functions served by brain endocannabinoids in mood and affect regulation. Further studies about the roles played by the endocannabinoid system in the adipose organ will offer new insights into the pathogenesis of obesity and might help identify new ways to leverage this signaling complex for therapeutic benefit.
Keywords: 2-arachidonoyl-sn-glycerol (2-AG), adipogenesis, anandamide, cannabinoid (CB) receptors, endocannabinoid (ECB), lipogenesis, lipolysis, metabolic disorders, obesity, oleoylethanolamide (OEA), thermogenesis, trans-differentiation
1. Introduction
Δ9-tetrahydrocannabinol (THC) is a terpenophenolic constituent of cannabis and the active agent responsible for the majority of the plant’s pharmacological properties [1-3]. THC produces its effects by binding to two quasi-ubiquitous G protein-coupled receptors, CB1 and CB2 [4,5]. CB1 cannabinoid receptors mediate most of the central and peripheral actions of THC, while CB2 receptors contribute to other less-well understood effects such as those exerted on the immune system [6-8]. The indiscriminate hijacking of these receptors by THC, especially when used at high doses, contrasts with the controlled recruitment produced by its endogenous lipid-derived ligands – the endocannabinoids arachidonoylethanolamide (anandamide) and 2-arachidonoyl-sn-glycerol (2-AG) [2,9-11] – whose activities are tightly regulated by a set of enzymes and transporters that cooperate to ensure that cannabinoid receptors are recruited only when and where their activation is needed [12-14]. Collectively, these molecules constitute the endocannabinoid system, a signaling complex that serves a vast array of modulatory functions in mammalian physiology. In this mini-review, we provide a brief overview of current knowledge about the presence and role of the endocannabinoid system in the adipose organ.
2. Endocannabinoids and their receptors
As mentioned above, the endocannabinoid system is comprised of two cell-surface receptors (CB1 and CB2), two lipid-derived endocannabinoid molecules (anandamide and 2-AG), and several intracellular proteins involved in the formation, transport and deactivation of such molecules.
2.1. Cannabinoid receptors in the adipose organ
CB1 and CB2 receptors exhibit 48% identity in amino acid sequence and signal through the transducing G proteins, G1 and Go [6,7]. CB1 (encoded in humans by the CNR1 gene) is abundantly expressed in the central nervous system (CNS) where it is primarily, albeit not exclusively, localized to axon terminals of γ-amino-butyric acid (GABA)-ergic interneurons and glutamatergic projection neurons [15]. Two important consequences of neuronal CB1 receptor activation are the reduction of neurotransmitter release (via inhibition of Ca2+ channel activity) and the suppression of membrane excitability (via increase of K+ channel activity) [6,16,17]. Non-neuronal cells of the CNS, such as astrocytes and microglia, also express CB1 [18-20]. Moreover, its widespread occurrence outside the CNS – e.g., in the peripheral nervous system, vasculature, small intestine, liver, pancreas and skeletal muscle [21] – has been linked to the diverse influences exerted by endocannabinoid messengers on homeostasis [21,22]: for example, in the small intestine CB1 receptors are involved in feeding regulation and smooth muscle contractility [23] while in the liver they contribute to the control of lipogenesis and fibrogenesis [24,25].
The presence of CB1 receptors in adipocytes of both white and brown adipose tissues (abbreviated henceforth as WAT and BAT, respectively) is well established [26-28]. In vitro studies suggest that CB1 expression in white adipocytes may coincide with the differentiation of these cells from committed preadipocytes [26,29,30], raising the possibility that the endocannabinoid system might contribute to this developmental process. There is also functional evidence that noradrenergic nerve fibers and resident macrophages interspersed in WAT and BAT parenchyma might also contain CB1 [31,32], though this remains to be conclusively demonstrated.
The CB2 receptor subtype (encoded in humans by the CNR2 gene) is mainly found in cellular constituents of the innate and adaptive immune systems – including monocyte-derived cells and lymphocytes – where it exerts a broad spectrum of modulatory effects on cytokine release, apoptosis and cell migration [33]. Low levels of CB2 expression have been measured in both visceral and subcutaneous adipose tissues of mice [28] and biopsies of human adipose tissue, possibly reflecting a restricted localization to resident macrophages, pre-adipose cells or vascular elements [34]. It is still uncertain whether adipocytes contain CB2, with some evidence supporting and other negating this possibility [35-37]. Filling this knowledge gap may shed light on the positive association reported, in children, between body-mass index and expression of the hypofunctional CB2 variant CB2-Q63R [38].
2.2. Endocannabinoid messengers in the adipose organ
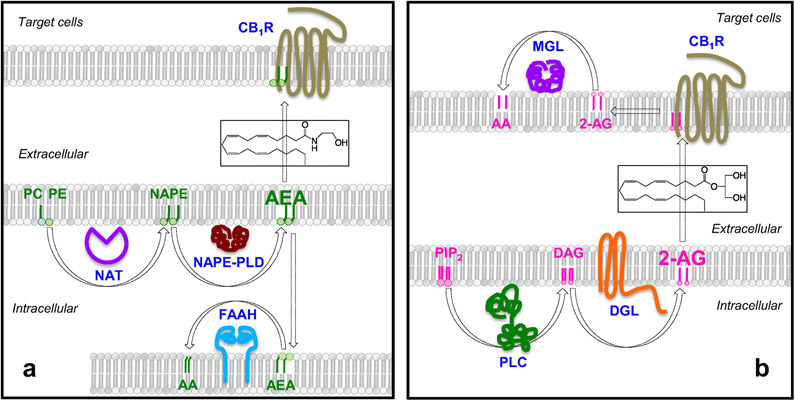
Anandamide and 2-AG are the two best-characterized endocannabinoid molecules found in mammalian tissues [2,14]. As shown in Figure 1A, anandamide formation starts with the transfer of an arachidonate group from the sn-1 position of 1,2-diarachidonoyl-phosphatidylcholine to the free amino group of phosphatidylethanolamine (PE), which generates the anandamide precursor N-arachidonoyl-PE [12,39]. This reaction occurs predominantly upon demand – for example, via ligation of certain transmitter or hormone receptors [40,41] – and is catalyzed by the calcium-dependent N-acyl transferase activity of cytosolic type-ε phospholipase A2 (encoded by PLA2G4E) [42]. Hydrolytic cleavage of membrane-bound N-arachidonoyl-PE by a unique zinc-containing phospholipase D (NAPEPLD) [43,44] releases anandamide, which diffuses out of the cell and into the external milieu [40,45]. Anandamide is deactivated through a two-step process in which the compound is first internalized by cells by an as-yet-uncharacterized facilitated-diffusion mechanism [46,47], followed by intracellular hydrolysis catalyzed by the serine amidase fatty acid amide hydrolase (FAAH) [48,49] (Figure 1A).
Figure 1. Molecular components of the endocannabinoid system.
(A) Anandamide is produced by hydrolysis of the phospholipid precursor, N-arachidonoyl-phosphatidylethanolamine (N-arachidonoyl-PE), catalyzed by a unique zinc-containing phospholipase D (PLD). N-arachidonoyl-PE is produced through a two-step reaction in which an arachidonate group is transferred from the sn-2 position of a phospholipid to the sn-1 position of lysophosphatidylcholine (PC), producing 1,2-diarachidonoyl-PC. The sn-1 arachidonoyl chain of 1,2-diarachidonoyl-PC is then transferred to the free amino group of PE generating N-arachidonoyl-PE. Anandamide is degraded by the intracellular serine amidase, fatty acid amide hydrolase (FAAH). (B) Receptor-operated phospholipase C (PLC) converts phosphatidylinositol-4,5-bisphosphate (PIP2) into 1,2-diacylglycerol (DAG). DAG is hydrolyzed by diacylglycerol lipase (DGL) forming 2-AG. 2-AG is subjected to hydrolytic cleavage catalyzed by monoacylglycerol lipase (MGL) or, to a lesser extent, α/β hydrolase domain-containing protein 6 (ABHD-6).
Like anandamide, 2-AG is also generated upon demand (Figure 1B). The membrane phospholipid that serves as its precursor, phosphatidylinositol-4,5-bisphosphate (PIP2), is hydrolyzed by a receptor-operated phospholipase C (PLC), probably PLCβ and/or PLCε [50,51], to produce 1,2-diacylglycerol (DAG), which is then cleaved by the α or β isoform of diacylglycerol lipase (DGL) to generate 2-AG [13,45,52]. In the postsynaptic spine of glutamate-sensitive neurons of the brain, PLC and DGL-α are physically linked to type-5 metabotropic glutamate receptors in a multimolecular complex (the ‘endocannabinoid signalosome’) for efficient, on-demand formation of 2-AG [53]. In contrast to classical neurotransmitters such as glutamate or GABA, 2-AG travels backwards across the synaptic cleft from the postsynaptic spine (which houses the signalosome) to the axon terminal (where the majority of CB1 receptors are localized) to mediate a retrograde signaling process that produces a variety of short- and long-term changes in synaptic efficiency [2,53]. The effects of 2-AG are terminated by the lipid hydrolases, monoacylglyceride lipase (MGL) and, to a lesser extent, α/β-hydrolase domain-6 (ABHD-6) (Figure 1B) [54,55].
The adipose organ of small mammals and humans contains the entire complement of proteins needed to produce and degrade endocannabinoid molecules [28,56,57]. In fact, the 2-AG-hydrolyzing enzyme MGL was first purified and molecularly cloned from mouse WAT, where its role in the last step of hormone-dependent lipolysis – i.e., the hydrolysis of triacylglycerol-derived monoacylglycerols into fatty acid and glycerol – has been long recognized [58]. The presence of other endocannabinoid-metabolizing enzymes has been documented in various depots of rodent and human WAT [28,59-61]. Moreover, in vivo microdialysis studies have shown that anandamide and 2-AG can be released within the parenchyma of human abdominal fat [62]. Similar to WAT, the BAT of small mammals such as mice [56] and marmots (Marmota flaviventris) [63] also contains endocannabinoids and their metabolizing enzymes.
As discussed above, the endocannabinoids are primarily generated upon demand through receptor- or activity-dependent mechanisms. What physiological stimuli control their mobilization in the adipose organ? In addition to the developmental signals mentioned in section 2.1 [64], experiments in mice suggest that cold exposure and β3 adrenergic receptor activation promote anandamide and 2-AG accumulation in WAT and enhance expression of the 2-AG-producing enzyme DGL-α [56]. The functional significance of cold-stimulated endocannabinoid production by adipose cells is discussed in the following section.
3. Physiological roles
3.1. The endocannabinoids as paracrine messengers in the adipose organ
In the CNS, a primary role of postsynaptically produced endocannabinoids is to control the release of excitatory and inhibitory neurotransmitters from axon terminals [1,2]. A similar negative-feedback mechanism may operate in white and brown cellular components of the adipose organ. As mentioned above, studies have shown that cold exposure increases endocannabinoid mobilization in WAT, presumably via norepinephrine-mediated activation of β3 adrenergic receptors [56]. Moreover, there is evidence that CB1 receptors are present on sympathetic terminals innervating the adipose organ and that their activation inhibits norepinephrine release [65-67]. A plausible interpretation of these findings is that adipocyte-derived endocannabinoids serve as negative regulators of sympathetic outflow [57] to slow down lipolysis and counter the conversion of adipocytes from a fat-storing into a heat-producing phenotype (discussed in section 3.2). This hypothesis is consistent with the broad roles played by the endocannabinoids as paracrine/autocrine mediators of energy conservation in multiple organ systems [68]. In addition to curbing autonomic outflow via their paracrine action on sympathetic nerve terminals, the endocannabinoids are also thought to modulate in an autocrine manner three processes that are critical to WAT and BAT function: adipogenesis, lipogenesis and heat production. In closing this section, it is worth noting that CB1 receptor signaling in adipose tissue might contribute, in ways that remain unclear, to the cognitive and emotional deficits produced in mice by diet-induced obesity [69].
3.2. Endocannabinoid signaling in WAT: control of adipogenesis and lipogenesis
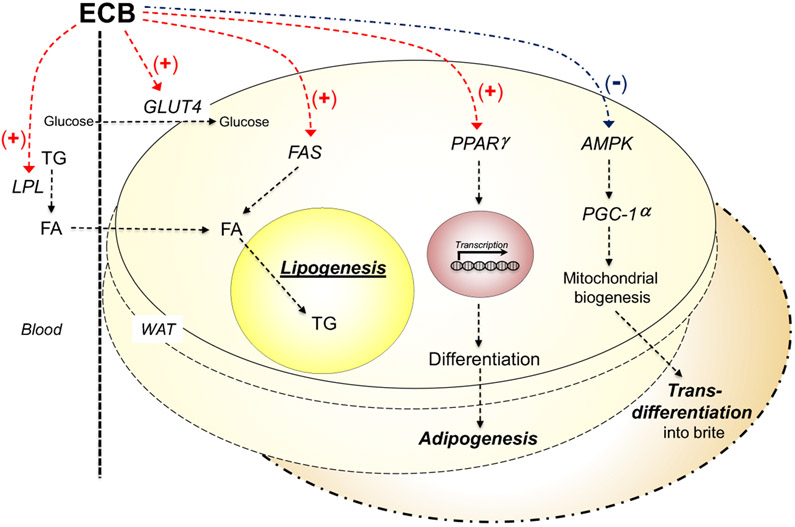
The endocannabinoid system has emerged an important regulator of adipogenesis and lipogenesis in WAT (Figure 2). Adipogenesis is the differentiation process through which preadipocytes mature into adipocytes under the control of hormonal signals such as insulin, while lipogenesis is the metabolic process through which fatty acids are synthesized from acetyl-CoA and are esterified into triglycerides [70]. Adipogenic stimuli induce terminal differentiation in committed preadipocytes by recruiting the ligand-operated transcription factor, peroxisome proliferator-activated receptor-γ (PPAR-γ) [70]. Pharmacological stimulation of CB1 receptors in cultures of mouse 3T3 adipocytes enhances PPAR-γ expression, accelerates adipocyte proliferation and promotes triglyceride accumulation in lipid droplets [30,71]. CB1 activation also increases glucose uptake in primary cultures of human white adipocytes [34] and heightens lipoprotein lipase activity [57], two events that cooperate in promoting the uptake and storage of non-esterified fatty acids by white adipocytes. Conversely, differentiation of mouse 3T3 cells is accompanied by an increase in CB1 receptor binding and other markers of endocannabinoid activity [64]. These two sets of findings are suggestive of a role for autocrine endocannabinoid signaling in adipogenesis and lipogenesis [30,72].
Figure 2. Endocannabinoid signals in WAT physiology.
In WAT, CB1 receptor activation increases glucose uptake (mediated by the insulin-regulated glucose transporter 4, GLUT4) and fatty acid biosynthesis (catalyzed by fatty acid synthase, FAS). In addition, it enhances transcription of genes involved in adipocyte differentiation such as the ligand-operated transcription factor, PPARγ, and impairs mitochondrial biogenesis and WAT browning by inhibiting the 5’-AMP-activated protein kinase (AMPK) - PGC-1α pathway.
Another proposed function for white-adipose endocannabinoids is to counter mitochondrial generation and the consequent browning of WAT (Figure 2). ‘Browning’ refers to the conversion of mature fat-storing white adipocytes, which contain relatively few mitochondria, into heat-producing mitochondria-enriched ‘beige’ or ‘brite’ (brown-in-white) adipocytes [73]. These heterogeneous phenotypes are thought to arise either by trans-differentiation of mature white adipocytes or by de novo differentiation of precursor cells (for review, see [74,75]). Pharmacological stimulation of CB1 receptors in primary cultures of mouse or human white adipocytes lowers the phosphorylation state of 5’-AMP-activated protein kinase (AMPK), disabling this cellular energy sensor [76] and consequently suppressing expression of PGC-1α, master transcriptional co-activator of mitochondrial biogenesis [77,78]. Conversely, CB1 receptor blockade in cultures of SV40-immortalized mouse adipocytes suppresses PGC-1α expression and counters the development of a thermogenic phenotype [79]. Similarly, genetically modified mice that lack CB1 in adipocytes exhibit a spontaneous browning of WAT, particularly in subcutaneous fat depots, a phenomenon that is associated with enhanced energy expenditure, decreased body weight, reduced total adiposity and improved insulin sensitivity [66].
The presence of CB2 in WAT remains uncertain [35-38], though resident macrophages appear to contain it at detectable levels [80]. Its roles, if any, are also controversial. For example, administration of the CB2-selective agonist JWH-133 was found to potentiate inflammation in WAT of diet-induced obese mice [80], whereas beneficial effects on fat mass and adipocyte size were reported in the same model after administration of the less selective CB2 agonist JWH-015 [81].
3.3. Endocannabinoid signaling in BAT: control of thermogenesis
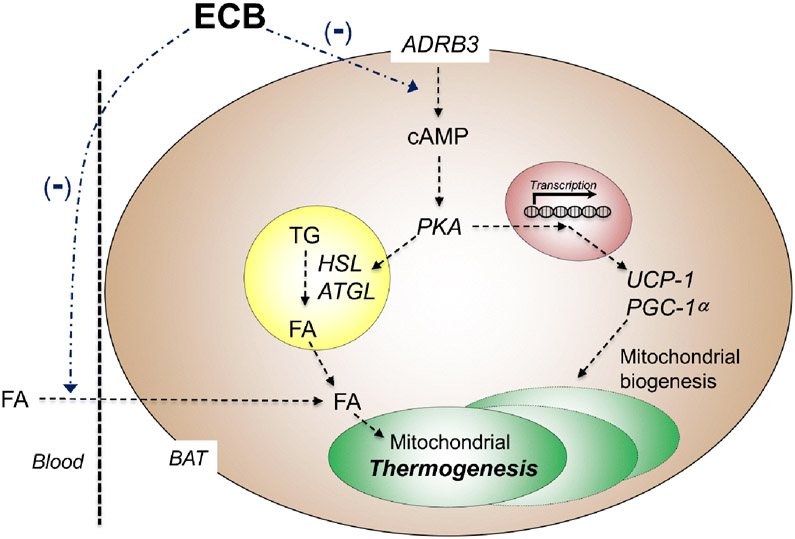
CB1 receptors are prominently expressed in rodent and human BAT [27], where their density increases following cold exposure [27]. This points to a role for CB1 in non-shivering thermogenesis [73], the metabolic process that generates heat in response to cold and other environmental stimuli [82]. Pharmacological studies support this possibility (Figure 3). Systemic administration of THC lowers the expression of uncoupling protein-1 (UCP-1) in rat BAT [83]: since the main function of UCP-1 is to dissipate the proton gradient generated by oxidative phosphorylation, its decreased activity is expected to suppress thermogenesis [84]. Conversely, incubation with the CB1 inverse agonist rimonabant heightens oxygen consumption and elevates UCP-1 expression in cultures of brown T37i adipocytes, while co-incubation of the same cells with rimonabant plus norepinephrine causes a synergistic increase in phosphorylation of the lipolytic enzyme, hormone-sensitive lipase [85]. Consistent with these in vitro results, systemic administration of rimonabant or AM6545, a peripherally restricted neutral CB1 antagonist, stimulates whole-body energy expenditure, heightens expression of genes involved in BAT thermogenesis and decreases lipid droplet size in brown adipocytes [85].
Figure 3. Endocannabinoid signals in BAT physiology.
Activation of β3 adrenergic receptors (ADRB3) in BAT initiates canonical cAMP - protein kinase A (PKA) signaling. PKA phosphorylates and activates hormone-sensitive lipase (HSL) and adipose triglyceride lipase (ATGL) to liberate fatty acids (FA) from lipid droplets. In addition, FA activate UCP-1 in the mitochondrial inner membrane, which dissipates the proton gradient and uncouples the electron transport chain from ATP synthesis, generating heat. Either central or peripheral blockade of CB1 receptors heightens thermogenesis by dampening local endocannabinoid activity and consequently boosting sympathetic outflow to the adipose organ.
3.4. Central endocannabinoid control of WAT and BAT function
Endocannabinoid signals are critical contributors to the hypothalamic regulation of adipose physiology. This topic has been thoroughly reviewed [72,86] and only two relevant examples will be highlighted here. Quarta and collaborators found that genetically modified mice in which CB1 was selectively deleted in neurons, but not in other cell types, are lean and resistant to diet-induced obesity [67]. A strikingly similar metabolic phenotype was observed in transgenic mice overexpressing the 2-AG-hydrolyzing enzyme MGL in calcium-calmodulin kinase II-containing neurons [87]. In addition to displaying reduced 2-AG levels in forebrain, these mice are lean and resistant to diet-induced obesity. Moreover, they exhibit elevated energy cost of activity, increased UCP-1 levels in BAT and hypersensitivity to β3-adrenergic-stimulated thermogenesis [87]. The converging phenotypes of these two mouse models underscore the global influence exerted by endocannabinoid signals in the regulation of adipose-organ function and body-wide energy balance.
4. The endocannabinoid system in the adipose organ: a safe target to treat obesity?
A large body of evidence indicates that obesity – a major risk factor for the development of type-2 diabetes, cardiovascular disease and liver steatosis – is associated with central and peripheral hyperactivity of the endocannabinoid system, which can be effectively (albeit not safely) corrected by administration of globally active CB1 receptor blockers. Excellent reviews of this field of research are available [88-92] but is important to point out in the present context that signs of excess endocannabinoid activity are clearly detectable both in the circulation and in visceral compartments of the adipose organ, whose pathogenic role in obesity is well recognized [73]. Circulating levels of 2-AG are significantly elevated in persons with obesity and are correlated with body mass index (BMI), visceral fat mass and fasting insulin and triglyceride concentrations [93,94]. A one-year lifestyle modification program normalized 2-AG levels [95]. In addition, Sarzani and collaborators showed that visceral fat of overweight and dysmetabolic patients contains higher levels of CB1 mRNA compared to normal-weight subjects, and that CB1 expression in this fat depot is positively correlated with local anandamide levels, perirenal and total visceral adipose tissue area, and BMI [96,97]. Reinforcing these findings, a cross-sectional study in lean and obese persons suggests that obesity may be accompanied by a relative increase in the number of CB1 receptors found in visceral compared to subcutaneous white fat depots [98].
The results outlined above may have important therapeutic implications. In obese animals and humans, CB1 inverse agonists and neutral antagonists produce sustained weight loss along with significant improvements in lipid profile and insulin resistance (for review, see [99]). In a series of randomized placebo-controlled clinical trials, the CB1 inverse agonist rimonabant was shown to lower body weight and mitigate cardiovascular risk factors in persons with obesity and type-2 diabetes [100-103]. Rimonabant was approved for the treatment of obesity in some Countries but was withdrawn shortly thereafter due to the emergence of dose-dependent psychiatric side effects [99]. These included anxiety, depression and suicidal ideation and thus resulted, in all likelihood, from the interference of CNS-penetrant CB1 blockers with the protective control exerted by central endocannabinoids on affect, mood and the response to stress (for review, see [104]).
A promising alternative strategy, which is currently under preclinical investigation, is to target CB1 receptors in the adipose and other peripheral organs involved in metabolic dysfunction (e.g., pancreas, liver) [37,105,106]. The effects of two peripherally restricted compounds, AM6545 and JD5037, have been studied in detail [105,107]. Both agents have low brain penetrance, high affinity and selectivity for CB1 receptors and no overt central effects. They differ in one important property, however: AM6545 is a neutral antagonist (i.e., it has no activity in the absence of an agonist) whereas JD5037 is an inverse agonist (i.e., it produces a receptor response opposite to that of an agonist) [107,108]. AM6545 significantly reduces body-weight gain in diet-induced obese mice, though not as effectively as does the inverse agonist rimonabant [105]. In the same model, JD5037 is as effective as a globally active inverse agonist at decreasing food intake and body weight gain. The compound was also shown to improve lipid profile, glucose handling, insulin sensitivity and hepatic steatosis [107,108]. If the preclinical evidence outlined above is confirmed in the clinic, peripherally restricted CB1 blockers might have a strong positive impact on the treatment of obesity and other metabolic disorders.
5. Paracannabinoid signals in adipose organ physiology
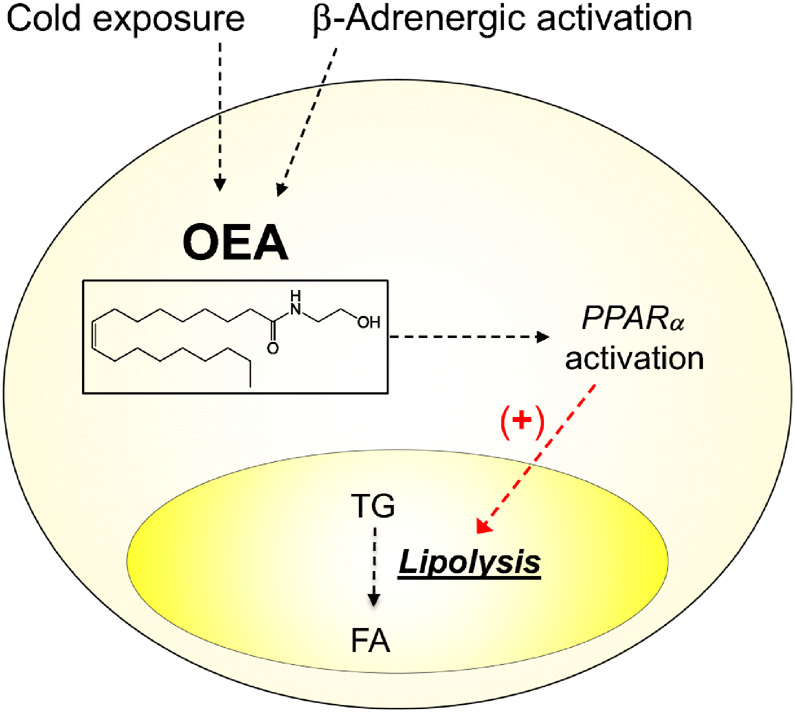
Mammalian tissues produce a family of lipid molecules that are biogenetically related to the endocannabinoids, but do not productively interact with cannabinoid receptors. In the adipose organ, the best understood member of this ‘paracannabinoid’ family is the anandamide analog, oleoylethanolamide (OEA) (Figure 4). OEA is an endogenous agonist of the ligand-operated transcription factor PPAR-α and a potent small-intestinal regulator of feeding behavior [23,109-111]. As seen with anandamide [56], OEA production in white adipose tissue is stimulated by cold exposure and β-adrenergic receptor activation [112]. Importantly, however, activation of PPAR-α by OEA causes effects that are functionally opposite to those produced by anandamide acting at CB1. OEA accelerates lipolysis in cultures of rat white adipocytes and attenuates body-weight gain and hyperlipidemia in obese mice and rats [113,114]. Even though the anti-obesity properties of OEA have been documented in a randomized, placebo-controlled trial [115-117], the physiological significance and pathological implications of this signaling lipid are still understudied.
Figure 4. Paracannabinoid signals in the adipose organ.
OEA is produced and degraded though an enzyme pathway that overlaps with anandamide’s but starts from a different phospholipid precursor (N-oleoyl-PE instead of N-arachidonoyl-PE). Unlike anandamide, however, OEA does not interact productively with CB1 receptors. In fact, its actions are opposite to those of anandamide and are mediated by activation of the ligand-operated transcription factor, PPAR-α.
6. Conclusions
There is strong preclinical and clinical evidence that endocannabinoid signaling at CB1 receptors is a critical regulator of adipose organ physiology and a potential target for anti-obesity medications. Important knowledge gaps remain, including the role of endocannabinoid signals in WAT and BAT development and their interaction with paracannabinoid messengers such as OEA. Despite these unanswered questions, it is clear that the therapeutic potential of endocannabinoid modulation calls for further basic and clinical investigation.
Acknowledgements
Research reported in this publication was supported by the National Institute on Drug Abuse (NIDA) of the National Institutes of Health (NIH) under award number P50DA044118 (to D.P.) and the UC Tobacco-Related Disease Research Program (TRDRP) under award number T29IR0618/26IP-0043 (to D.P.).
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Jung KM, Piomelli D. Cannabinoids and Endocannabinoids. In: Pfaff D, Volkow N (eds) Neuroscience in the 21st Century. 2015; Springer, New York, NY. 10.1007/978-1-4614-6434-1_136-1. [DOI] [Google Scholar]
- 2.Piomelli D The molecular logic of endocannabinoid signalling. Nat Rev Neurosci. 2003;4(11):873–884. doi: 10.1038/nrn1247 [DOI] [PubMed] [Google Scholar]
- 3.Gaoni Y, Mechoulam R. Isolation, structure and partial synthesis of an active constituent of hashish. J Am Chem Soc. 1964, 86:1646–47. [Google Scholar]
- 4.Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346(6284):561–564. doi: 10.1038/346561a0 [DOI] [PubMed] [Google Scholar]
- 5.Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365(6441):61–65. doi: 10.1038/365061a0 [DOI] [PubMed] [Google Scholar]
- 6.Mackie K Cannabinoid receptors as therapeutic targets. Annu Rev Pharmacol Toxicol. 2006;46:101–122. doi: 10.1146/annurev.pharmtox.46.120604.141254 [DOI] [PubMed] [Google Scholar]
- 7.Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83(3):1017–1066. doi: 10.1152/physrev.00004.2003 [DOI] [PubMed] [Google Scholar]
- 8.Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M. Endocannabinoid-mediated control of synaptic transmission. Physiol Rev. 2009;89(1):309–380. doi: 10.1152/physrev.00019.2008 [DOI] [PubMed] [Google Scholar]
- 9.Devane WA, Hanus L, Breuer A, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258(5090):1946–1949. doi: 10.1126/science.1470919 [DOI] [PubMed] [Google Scholar]
- 10.Sugiura T, Kondo S, Sukagawa A, et al. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun. 1995;215(1):89–97. doi: 10.1006/bbrc.1995.2437 [DOI] [PubMed] [Google Scholar]
- 11.Mechoulam R, Ben-Shabat S, Hanus L, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50(1):83–90. doi: 10.1016/0006-2952(95)00109-d [DOI] [PubMed] [Google Scholar]
- 12.Di Marzo V, Fontana A, Cadas H, et al. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature. 1994;372(6507):686–691. doi: 10.1038/372686a0 [DOI] [PubMed] [Google Scholar]
- 13.Stella N, Schweitzer P, Piomelli D. A second endogenous cannabinoid that modulates long-term potentiation. Nature. 1997;388(6644):773–778. doi: 10.1038/42015 [DOI] [PubMed] [Google Scholar]
- 14.Piomelli D, Astarita G, Rapaka R. A neuroscientist's guide to lipidomics. Nat Rev Neurosci. 2007;8(10)743–754. doi: 10.1038/nrn2233 [DOI] [PubMed] [Google Scholar]
- 15.Howlett AC, Barth F, Bonner TI, et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54(2):161–202. doi: 10.1124/pr.54.2.161 [DOI] [PubMed] [Google Scholar]
- 16.Mackie K, Lai Y, Westenbroek R, Mitchell R. Cannabinoids activate an inwardly rectifying potassium conductance and inhibit Q-type calcium currents in AtT20 cells transfected with rat brain cannabinoid receptor. J Neurosci. 1995;15(10):6552–6561. doi: 10.1523/JNEUROSCI.15-10-06552.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Twitchell W, Brown S, Mackie K. Cannabinoids inhibit N- and P/Q-type calcium channels in cultured rat hippocampal neurons. J Neurophysiol. 1997;78(1):43–50. doi: 10.1152/jn.1997.78.1.43 [DOI] [PubMed] [Google Scholar]
- 18.Waksman Y, Olson JM, Carlisle SJ, Cabral GA. The central cannabinoid receptor (CB1) mediates inhibition of nitric oxide production by rat microglial cells. J Pharmacol Exp Ther. 1999;288(3):1357–1366. [PubMed] [Google Scholar]
- 19.Gómez Del Pulgar T, De Ceballos ML, Guzmán M, Velasco G. Cannabinoids protect astrocytes from ceramide-induced apoptosis through the phosphatidylinositol 3-kinase/protein kinase B pathway. J Biol Chem. 2002;277(39):36527–36533. doi: 10.1074/jbc.M205797200 [DOI] [PubMed] [Google Scholar]
- 20.Cannabinoid Stella N. and cannabinoid-like receptors in microglia, astrocytes, and astrocytomas. Glia. 2010;58(9):1017–1030. doi: 10.1002/glia.20983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maccarrone M, Bab I, Bíró T, et al. Endocannabinoid signaling at the periphery: 50 years after THC. Trends Pharmacol Sci. 2015;36(5):277–296. doi: 10.1016/j.tips.2015.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kunos G, Tam J. The case for peripheral CB1 receptor blockade in the treatment of visceral obesity and its cardiometabolic complications. Br J Pharmacol. 2011;163(7):1423–1431. doi: 10.1111/j.1476-5381.2011.01352.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DiPatrizio NV, Piomelli D. Intestinal lipid-derived signals that sense dietary fat. J Clin Invest. 2015;125(3):891–898. doi: 10.1172/JCI76302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kunos G, Osei-Hyiaman D. Endocannabinoids and liver disease. IV. Endocannabinoid involvement in obesity and hepatic steatosis. Am J Physiol Gastrointest Liver Physiol. 2008;294(5):G1101–G1104. doi: 10.1152/ajpgi.00057.2008 [DOI] [PubMed] [Google Scholar]
- 25.Tam J, Liu J, Mukhopadhyay B, Cinar R, Godlewski G, Kunos G. Endocannabinoids in liver disease. Hepatology. 2011;53(1):346–355. doi: 10.1002/hep.24077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cota D, Marsicano G, Tschöp M, et al. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J Clin Invest. 2003; 112(3):423–431. doi: 10.1172/JCI17725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eriksson O, Mikkola K, Espes D, et al. The Cannabinoid Receptor-1 Is an Imaging Biomarker of Brown Adipose Tissue. J Nucl Med. 2015;56(12):1937–1941. doi: 10.2967/jnumed.115.156422 [DOI] [PubMed] [Google Scholar]
- 28.Starowicz KM, Cristino L, Matias I, et al. Endocannabinoid dysregulation in the pancreas and adipose tissue of mice fed with a high-fat diet. Obesity (Silver Spring). 2008;16(3):553–565. doi: 10.1038/oby.2007.106 [DOI] [PubMed] [Google Scholar]
- 29.Bensaid M, Gary-Bobo M, Esclangon A, et al. The cannabinoid CB1 receptor antagonist SR141716 increases Acrp30 mRNA expression in adipose tissue of obese fa/fa rats and in cultured adipocyte cells. Mol Pharmacol. 2003;63(4):908–914. doi: 10.1124/mol.63.4.908 [DOI] [PubMed] [Google Scholar]
- 30.Matias I, Gonthier MP, Orlando P, et al. Regulation, function, and dysregulation of endocannabinoids in models of adipose and beta-pancreatic cells and in obesity and hyperglycemia. J Clin Endocrinol Metab. 2006;91(8):3171–3180. doi: 10.1210/jc.2005-2679 [DOI] [PubMed] [Google Scholar]
- 31.Mehrpouya-Bahrami P, Miranda K, Singh NP, Zumbrun EE, Nagarkatti M, Nagarkatti PS. Role of microRNA in CB1 antagonist-mediated regulation of adipose tissue macrophage polarization and chemotaxis during diet-induced obesity. J Biol Chem. 2019;294(19):7669–7681. doi: 10.1074/jbc.RA118.005094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ishac EJ, Jiang L, Lake KD, Varga K, Abood ME, Kunos G. Inhibition of exocytotic noradrenaline release by presynaptic cannabinoid CB1 receptors on peripheral sympathetic nerves. Br J Pharmacol. 1996;118(8):2023–2028. doi: 10.1111/j.1476-5381.1996.tb15639.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller AM, Stella N. CB2 receptor-mediated migration of immune cells: it can go either way. Br J Pharmacol. 2008;153(2):299–308. doi: 10.1038/sj.bjp.0707523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pagano C, Pilon C, Calcagno A, et al. The endogenous cannabinoid system stimulates glucose uptake in human fat cells via phosphatidylinositol 3-kinase and calcium-dependent mechanisms. J Clin Endocrinol Metab. 2007;92(12):4810–4819. doi: 10.1210/jc.2007-0768 [DOI] [PubMed] [Google Scholar]
- 35.Roche R, Hoareau L, Bes-Houtmann S, et al. Presence of the cannabinoid receptors, CB1 and CB2, in human omental and subcutaneous adipocytes. Histochem Cell Biol. 2006;126(2):177–187. doi: 10.1007/s00418-005-0127-4 [DOI] [PubMed] [Google Scholar]
- 36.Spoto B, Fezza F, Parlongo G, et al. Human adipose tissue binds and metabolizes the endocannabinoids anandamide and 2-arachidonoylglycerol. Biochimie. 2006;88(12):1889–1897. doi: 10.1016/j.biochi.2006.07.019 [DOI] [PubMed] [Google Scholar]
- 37.Pagano C, Rossato M, Vettor R. Endocannabinoids, adipose tissue and lipid metabolism. J Neuroendocrinol. 2008;20 Suppl 1:124–129. doi: 10.1111/j.1365-2826.2008.01690.x [DOI] [PubMed] [Google Scholar]
- 38.Rossi F, Bellini G, Luongo L, et al. Cannabinoid Receptor 2 as Antiobesity Target: Inflammation, Fat Storage, and Browning Modulation. J Clin Endocrinol Metab. 2016;101 (9):3469–3478. doi: 10.1210/jc.2015-4381 [DOI] [PubMed] [Google Scholar]
- 39.Cadas H, di Tomaso E, Piomelli D. Occurrence and biosynthesis of endogenous cannabinoid precursor, N-arachidonoyl phosphatidylethanolamine, in rat brain. J Neurosci. 1997;17(4):1226–1242. doi: 10.1523/JNEUROSCI.17-04-01226.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giuffrida A, Parsons LH, Kerr TM, Rodriguez de Fonseca F, Navarro M, Piomelli D. Dopamine activation of endogenous cannabinoid signaling in dorsal striatum. Nat Neurosci. 1999;2(4):358–363. doi: 10.1038/7268 [DOI] [PubMed] [Google Scholar]
- 41.Wei D, Lee D, Cox CD, et al. Endocannabinoid signaling mediates oxytocin-driven social reward. Proc Natl Acad Sci U S A. 2015;112(45):14084–14089. doi: 10.1073/pnas.1509795112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ogura Y, Parsons WH, Kamat SS, Cravatt BF. A calcium-dependent acyltransferase that produces N-acyl phosphatidylethanolamines. Nat Chem Biol. 2016;12(9):669–671. doi: 10.1038/nchembio.2127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Okamoto Y, Morishita J, Tsuboi K, Tonai T, Ueda N. Molecular characterization of a phospholipase D generating anandamide and its congeners. J Biol Chem. 2004;279(7):5298–5305. doi: 10.1074/jbc.M306642200 [DOI] [PubMed] [Google Scholar]
- 44.Tsuboi K, Ikematsu N, Uyama T, Deutsch DG, Tokumura A, Ueda N. Biosynthetic pathways of bioactive N-acylethanolamines in brain. CNS Neurol Disord Drug Targets. 2013;12(1):7–16. doi: 10.2174/1871527311312010005 [DOI] [PubMed] [Google Scholar]
- 45.Serrano A, Pavon FJ, Buczynski MW, et al. Deficient endocannabinoid signaling in the central amygdala contributes to alcohol dependence-related anxiety-like behavior and excessive alcohol intake. Neuropsychopharmacology. 2018;43(9):1840–1850. doi: 10.1038/s41386-018-0055-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beltramo M, Stella N, Calignano A, Lin SY, Makriyannis A, Piomelli D. Functional role of high-affinity anandamide transport, as revealed by selective inhibition. Science. 1997;277(5329):1094–1097. doi: 10.1126/science.277.5329.1094 [DOI] [PubMed] [Google Scholar]
- 47.Nicolussi S, Gertsch J. Endocannabinoid transport revisited. Vitam Horm. 2015;98:441–485. doi: 10.1016/bs.vh.2014.12.011 [DOI] [PubMed] [Google Scholar]
- 48.Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384(6604):83–87. doi: 10.1038/384083a0 [DOI] [PubMed] [Google Scholar]
- 49.McKinney MK, Cravatt BF. Structure and function of fatty acid amide hydrolase. Annu Rev Biochem. 2005;74:411–432. [DOI] [PubMed] [Google Scholar]
- 50.Seal SN, Rose ZB. Characterization of a phosphoenzyme intermediate in the reaction of phosphoglycolate phosphatase. J Biol Chem. 1987;262(28):13496–13500. [PubMed] [Google Scholar]
- 51.Bennett CF, Balcarek JM, Varrichio A, Crooke ST. Molecular cloning and complete amino-acid sequence of form-I phosphoinositide-specific phospholipase C. Nature. 1988;334(6179):268–270. doi: 10.1038/334268a0 [DOI] [PubMed] [Google Scholar]
- 52.Bisogno T, Howell F, Williams G, et al. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J Cell Biol. 2003;163(3):463–468. doi: 10.1083/jcb.200305129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jung KM, Sepers M, Henstridge Cm, et al. Uncoupling of the endocannabinoid signalling complex in a mouse model of fragile X syndrome. Nat Commun. 2012;3:1080. doi: 10.1038/ncomms2045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dinh TP, Carpenter D, Leslie FM, et al. Brain monoglyceride lipase participating in endocannabinoid inactivation [published correction appears in Proc Natl Acad Sci U S A 2002 Oct 15;99(21):13961]. Proc Natl Acad Sci U S A. 2002;99(16):10819–10824. doi: 10.1073/pnas.152334899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Blankman JL, Simon GM, Cravatt BF. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem Biol. 2007;14(12):1347–1356. doi: 10.1016/j.chembiol.2007.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Krott LM, Piscitelli F, Heine M, et al. Endocannabinoid regulation in white and brown adipose tissue following thermogenic activation. J Lipid Res. 2016;57(3):464–473. doi: 10.1194/jlr.M065227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Eenige R, van der Stelt M, Rensen PCN, Kooijman S. Regulation of Adipose Tissue Metabolism by the Endocannabinoid System. Trends Endocrinol Metab. 2018;29(5):326–337. doi: 10.1016/j.tem.2018.03.001 [DOI] [PubMed] [Google Scholar]
- 58.Karlsson M, Contreras JA, Hellman U, Tornqvist H, Holm C. cDNA cloning, tissue distribution, and identification of the catalytic triad of monoglyceride lipase. Evolutionary relationship to esterases, lysophospholipases, and haloperoxidases. J Biol Chem. 1997;272(43):27218–27223. doi: 10.1074/jbc.272.43.27218 [DOI] [PubMed] [Google Scholar]
- 59.Spoto B, Fezza F, Parlongo G, et al. Human adipose tissue binds and metabolizes the endocannabinoids anandamide and 2-arachidonoylglycerol. Biochimie. 2006;88(12):1889–1897. doi: 10.1016/j.biochi.2006.07.019 [DOI] [PubMed] [Google Scholar]
- 60.Nahon KJ, Kantae V, den Haan R, et al. Gene Expression of Endocannabinoid System Components in Skeletal Muscle and Adipose Tissue of South Asians and White Caucasians with Overweight. Obesity (Silver Spring). 2018;26(8):1332–1337. doi: 10.1002/oby.22245 [DOI] [PubMed] [Google Scholar]
- 61.You T, Disanzo BL, Wang X, Yang R, Gong D. Adipose tissue endocannabinoid system gene expression: depot differences and effects of diet and exercise. Lipids Health Dis. 2011;10:194. Published 2011 Oct 28. doi: 10.1186/1476-511X-10-194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zoerner AA, Rakers C, Engeli S, et al. Peripheral endocannabinoid microdialysis: in vitro characterization and proof-of-concept in human subjects. Anal Bioanal Chem. 2012;402(9):2727–2735. doi: 10.1007/s00216-012-5729-9 [DOI] [PubMed] [Google Scholar]
- 63.Mulawa EA, Kirkwood JS, Wolfe LM, et al. Seasonal Changes in Endocannabinoid Concentrations between Active and Hibernating Marmots (Marmota flaviventris). J Biol Rhythms. 2018;33(4):388–401. doi: 10.1177/0748730418777660 [DOI] [PubMed] [Google Scholar]
- 64.Gasperi V, Fezza F, Pasquariello N, et al. Endocannabinoids in adipocytes during differentiation and their role in glucose uptake. Cell Mol Life Sci. 2007;64(2):219–229. doi: 10.1007/s00018-006-6445-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ishac EJ, Jiang L, Lake KD, Varga K, Abood ME, Kunos G. Inhibition of exocytotic noradrenaline release by presynaptic cannabinoid CB1 receptors on peripheral sympathetic nerves. Br J Pharmacol. 1996;118(8):2023–2028. doi: 10.1111/j.1476-5381.1996.tb15639.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ruiz de Azua I, Mancini G, Srivastava RK, et al. Adipocyte cannabinoid receptor CB1 regulates energy homeostasis and alternatively activated macrophages. J Clin Invest. 2017;127(11):4148–4162. doi: 10.1172/JCI83626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Quarta C, Bellocchio L, Mancini G, et al. CB(1) signaling in forebrain and sympathetic neurons is a key determinant of endocannabinoid actions on energy balance. Cell Metab. 2010;11(4):273–285. doi: 10.1016/j.cmet.2010.02.015 [DOI] [PubMed] [Google Scholar]
- 68.DiPatrizio NV, Piomelli D. The thrifty lipids: endocannabinoids and the neural control of energy conservation. Trends Neurosci. 2012;35(7):403–411. doi: 10.1016/j.tins.2012.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Suárez J, Rivera P, Aparisi Rey A, et al. Adipocyte cannabinoid CB1 receptor deficiency alleviates high fat diet-induced memory deficit, depressive-like behavior, neuroinflammation and impairment in adult neurogenesis. Psychoneuroendocrinology. 2019;110:104418. doi: 10.1016/j.psyneuen.2019.104418 [DOI] [PubMed] [Google Scholar]
- 70.Cristancho AG, Lazar MA. Forming functional fat: a growing understanding of adipocyte differentiation. Nat Rev Mol Cell Biol. 2011;12(11):722–734. Published 2011 Sep 28. doi: 10.1038/nrm3198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bellocchio L, Cervino C, Vicennati V, Pasquali R, Pagotto U. Cannabinoid type 1 receptor: another arrow in the adipocytes' bow. J Neuroendocrinol. 2008;20 Suppl 1:130–138. doi: 10.1111/j.1365-2826.2008.01682.x [DOI] [PubMed] [Google Scholar]
- 72.Di Marzo V, Matias I. Endocannabinoid control of food intake and energy balance. Nat Neurosci. 2005;8(5):585–589. doi: 10.1038/nn1457 [DOI] [PubMed] [Google Scholar]
- 73.Giordano A, Frontini A, Cinti S. Convertible visceral fat as a therapeutic target to curb obesity. Nat Rev Drug Discov. 2016;15(6):405–424. doi: 10.1038/nrd.2016.31 [DOI] [PubMed] [Google Scholar]
- 74.Shapira SN, Seale P. Transcriptional Control of Brown and Beige Fat Development and Function. Obesity (Silver Spring). 2019;27(1):13–21. doi: 10.1002/oby.22334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Maurer S, Harms M, Boucher J. The colorful versatility of adipocytes: white-to-brown transdifferentiation and its therapeutic potential in humans. FEBS J. 2020. Jul 3. doi: 10.1111/febs.15470. Epub ahead of print. PMID: 32621398. [DOI] [PubMed] [Google Scholar]
- 76.Hardie DG, Schaffer BE, Brunet A. AMPK: An Energy-Sensing Pathway with Multiple Inputs and Outputs. Trends Cell Biol. 2016;26(3):190–201. doi: 10.1016/j.tcb.2015.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tedesco L, Valerio A, Dossena M, et al. Cannabinoid receptor stimulation impairs mitochondrial biogenesis in mouse white adipose tissue, muscle, and liver: the role of eNOS, p38 MAPK, and AMPK pathways. Diabetes. 2010;59(11):2826–2836. doi: 10.2337/db09-1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cheng CF, Ku HC, Lin H. PGC-1α as a Pivotal Factor in Lipid and Metabolic Regulation. Int J Mol Sci. 2018;19(11):3447. Published 2018 Nov 2. doi: 10.3390/ijms19113447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Perwitz N, Wenzel J, Wagner I, et al. Cannabinoid type 1 receptor blockade induces transdifferentiation towards a brown fat phenotype in white adipocytes. Diabetes Obes Metab. 2010;12(2):158–166. doi: 10.1111/j.1463-1326.2009.01133.x [DOI] [PubMed] [Google Scholar]
- 80.Deveaux V, Cadoudal T, Ichigotani Y, et al. Cannabinoid CB2 receptor potentiates obesity-associated inflammation, insulin resistance and hepatic steatosis. PLoS One. 2009;4(6):e5844. Published 2009 Jun 9. doi: 10.1371/journal.pone.0005844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Verty AN, Stefanidis A, McAinch AJ, Hryciw DH, Oldfield B. Anti-Obesity Effect of the CB2 Receptor Agonist JWH-015 in Diet-Induced Obese Mice. PLoS One. 2015;10(11):e0140592. Published 2015 Nov 20. doi: 10.1371/journal.pone.0140592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Silva JE. Physiological importance and control of non-shivering facultative thermogenesis. Front Biosci (Schol Ed). 2011;3:352–371. Published 2011 Jan 1. doi: 10.2741/s156 [DOI] [PubMed] [Google Scholar]
- 83.Verty AN, Evetts MJ, Crouch GJ, McGregor IS, Stefanidis A, Oldfield BJ. The cannabinoid receptor agonist THC attenuates weight loss in a rodent model of activity-based anorexia. Neuropsychopharmacology. 2011;36(7): 1349–1358. doi: 10.1038/npp.2011.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Krauss S, Zhang CY, Lowell BB. The mitochondrial uncoupling-protein homologues. Nat Rev Mol Cell Biol. 2005;6(3):248–261. doi: 10.1038/nrm1592 [DOI] [PubMed] [Google Scholar]
- 85.Boon MR, Kooijman S, van Dam AD, et al. Peripheral cannabinoid 1 receptor blockade activates brown adipose tissue and diminishes dyslipidemia and obesity. FASEB J. 2014;28(12):5361–5375. doi: 10.1096/fj.13-247643 [DOI] [PubMed] [Google Scholar]
- 86.Matias I, Di Marzo V. Endocannabinoids and the control of energy balance. Trends Endocrinol Metab. 2007;18(1):27–37. doi: 10.1016/j.tem.2006.11.006 [DOI] [PubMed] [Google Scholar]
- 87.Jung KM, Clapper JR, Fu J, et al. 2-arachidonoylglycerol signaling in forebrain regulates systemic energy metabolism. Cell Metab. 2012;15(3):299–310. doi: 10.1016/j.cmet.2012.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cinar R, Iyer MR, Kunos G. The therapeutic potential of second and third generation CB1R antagonists. Pharmacol Ther. 2020;208:107477. doi: 10.1016/j.pharmthera.2020.107477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hirsch S, Tam J. Cannabis: From a Plant That Modulates Feeding Behaviors toward Developing Selective Inhibitors of the Peripheral Endocannabinoid System for the Treatment of Obesity and Metabolic Syndrome. Toxins (Basel). 2019;11(5):275. Published 2019 May 15. doi: 10.3390/toxins11050275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tam J, Hinden L, Drori A, Udi S, Azar S, Baraghithy S. The therapeutic potential of targeting the peripheral endocannabinoid/CB1 receptor system. Eur J Intern Med. 2018;49:23–29. doi: 10.1016/j.ejim.2018.01.009 [DOI] [PubMed] [Google Scholar]
- 91.Jourdan T, Godlewski G, Kunos G. Endocannabinoid regulation of β-cell functions: implications for glycaemic control and diabetes. Diabetes Obes Metab. 2016;18(6):549–557. doi: 10.1111/dom.12646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Silvestri C, Di Marzo V. The endocannabinoid system in energy homeostasis and the etiopathology of metabolic disorders. Cell Metab. 2013;17(4):475–490. doi: 10.1016/j.cmet.2013.03.001 [DOI] [PubMed] [Google Scholar]
- 93.Blüher M, Engeli S, Klöting N, et al. Dysregulation of the peripheral and adipose tissue endocannabinoid system in human abdominal obesity. Diabetes. 2006;55(11):3053–3060. doi: 10.2337/db06-0812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Côté M, Matias I, Lemieux I, et al. Circulating endocannabinoid levels, abdominal adiposity and related cardiometabolic risk factors in obese men. Int J Obes (Lond). 2007;31(4):692–699. doi: 10.1038/sj.ijo.0803539 [DOI] [PubMed] [Google Scholar]
- 95.Di Marzo V, Côté M, Matias I, Lemieux I, Arsenault BJ, Cartier A, Piscitelli F, Petrosino S, Alméras N, Després JP. Changes in plasma endocannabinoid levels in viscerally obese men following a 1 year lifestyle modification programme and waist circumference reduction: associations with changes in metabolic risk factors. Diabetologia. 2009. Feb;52(2):213–7. doi: 10.1007/s00125-008-1178-6. PMID: 18972095. [DOI] [PubMed] [Google Scholar]
- 96.Sarzani R, Bordicchia M, Marcucci P, et al. Altered pattern of cannabinoid type 1 receptor expression in adipose tissue of dysmetabolic and overweight patients. Metabolism. 2009;58(3):361–367. doi: 10.1016/j.metabol.2008.10.009 [DOI] [PubMed] [Google Scholar]
- 97.Bordicchia M, Battistoni I, Mancinelli L, et al. Cannabinoid CB1 receptor expression in relation to visceral adipose depots, endocannabinoid levels, microvascular damage, and the presence of the Cnr1 A3813G variant in humans. Metabolism. 2010;59(5):734–741. doi: 10.1016/j.metabol.2009.09.018 [DOI] [PubMed] [Google Scholar]
- 98.Bennetzen MF. Investigations of the endocannabinoid system in adipose tissue: effects of obesity/ weight loss and treatment options. Dan Med Bull. 2011;58(4):B4269. [PubMed] [Google Scholar]
- 99.Engeli S. Central and peripheral cannabinoid receptors as therapeutic targets in the control of food intake and body weight. Handb Exp Pharmacol. 2012;(209):357–381. doi: 10.1007/978-3-642-24716-3_17 [DOI] [PubMed] [Google Scholar]
- 100.Van Gaal LF, Rissanen AM, Scheen AJ, Ziegler O, Rössner S; RIO-Europe Study Group. Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study [published correction appears in Lancet. 2005 Jul 30-Aug 5;366(9483):370]. Lancet. 2005;365(9468):1389–1397. doi: 10.1016/S0140-6736(05)66374-X [DOI] [PubMed] [Google Scholar]
- 101.Pi-Sunyer FX, Aronne LJ, Heshmati HM, Devin J, Rosenstock J; RIO-North America Study Group. Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients: RIO-North America: a randomized controlled trial [published correction appears in JAMA. 2006 Mar 15;295(11):1252]. JAMA. 2006;295(7):761–775. doi: 10.1001/jama.295.7.761 [DOI] [PubMed] [Google Scholar]
- 102.Scheen AJ, Finer N, Hollander P, Jensen MD, Van Gaal LF; RIO-Diabetes Study Group. Efficacy and tolerability of rimonabant in overweight or obese patients with type 2 diabetes: a randomised controlled study [published correction appears in Lancet. 2006 Nov 11;368(9548):1650]. Lancet. 2006;368(9548):1660–1672. doi: 10.1016/S0140-6736(06)69571-8 [DOI] [PubMed] [Google Scholar]
- 103.Després JP, Golay A, Sjöström L; Rimonabant in Obesity-Lipids Study Group. Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med. 2005;353(20):2121–2134. doi: 10.1056/NEJMoa044537 [DOI] [PubMed] [Google Scholar]
- 104.Clapper JR, Mangieri RA, Piomelli D. The endocannabinoid system as a target for the treatment of cannabis dependence. Neuropharmacology. 2009;56 Suppl 1(Suppl 1):235–243. doi: 10.1016/j.neuropharm.2008.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tam J, Vemuri VK, Liu J, et al. Peripheral CB1 cannabinoid receptor blockade improves cardiometabolic risk in mouse models of obesity [published correction appears in J Clin Invest. 2010 Oct 1;120(10):3735]. J Clin Invest. 2010;120(8):2953–2966. doi: 10.1172/JCI42551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.DiPatrizio NV, Joslin A, Jung KM, Piomelli D. Endocannabinoid signaling in the gut mediates preference for dietary unsaturated fats. FASEB J. 2013;27(6):2513–2520. doi: 10.1096/fj.13-227587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tam J, Cinar R, Liu J, et al. Peripheral cannabinoid-1 receptor inverse agonism reduces obesity by reversing leptin resistance. Cell Metab. 2012;16(2):167–179. doi: 10.1016/j.cmet.2012.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cinar R, Godlewski G, Liu J, et al. Hepatic cannabinoid-1 receptors mediate diet-induced insulin resistance by increasing de novo synthesis of long-chain ceramides. Hepatology. 2014;59(1):143–153. doi: 10.1002/hep.26606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rodríguez de Fonseca F, Navarro M, Gómez R, et al. An anorexic lipid mediator regulated by feeding. Nature. 2001;414(6860):209–212. doi: 10.1038/35102582 [DOI] [PubMed] [Google Scholar]
- 110.Fu J, Gaetani S, Oveisi F, et al. Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-alpha. Nature. 2003;425(6953):90–93. doi: 10.1038/nature01921 [DOI] [PubMed] [Google Scholar]
- 111.Schwartz GJ, Fu J, Astarita G, et al. The lipid messenger OEA links dietary fat intake to satiety. Cell Metab. 2008;8(4):281–288. doi: 10.1016/j.cmet.2008.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.LoVerme J, Guzmán M, Gaetani S, Piomelli D. Cold exposure stimulates synthesis of the bioactive lipid oleoylethanolamide in rat adipose tissue. J Biol Chem. 2006;281(32):22815–22818. doi: 10.1074/jbc.M604751200 [DOI] [PubMed] [Google Scholar]
- 113.Guzmán M, Lo Verme J, Fu J, Oveisi F, Blázquez C, Piomelli D. Oleoylethanolamide stimulates lipolysis by activating the nuclear receptor peroxisome proliferator-activated receptor alpha (PPAR-alpha). J Biol Chem. 2004;279(27):27849–27854. doi: 10.1074/jbc.M404087200 [DOI] [PubMed] [Google Scholar]
- 114.Fu J, Oveisi F, Gaetani S, Lin E, Piomelli D. Oleoylethanolamide, an endogenous PPAR-alpha agonist, lowers body weight and hyperlipidemia in obese rats. Neuropharmacology. 2005;48(8):1147–1153. doi: 10.1016/j.neuropharm.2005.02.013 [DOI] [PubMed] [Google Scholar]
- 115.Laleh P, Yaser K, Abolfazl B, et al. Oleoylethanolamide increases the expression of PPAR-A and reduces appetite and body weight in obese people: A clinical trial. Appetite. 2018;128:44–49. doi: 10.1016/j.appet.2018.05.129 [DOI] [PubMed] [Google Scholar]
- 116.Payahoo L, Khajebishak Y, Asghari Jafarabadi M, Ostadrahimi A. Oleoylethanolamide Supplementation Reduces Inflammation and Oxidative Stress in Obese People: A Clinical Trial. Adv Pharm Bull. 2018;8(3):479–487. doi: 10.15171/apb.2018.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tutunchi H, Ostadrahimi A, Saghafi-Asl M, et al. Oleoylethanolamide supplementation in obese patients newly diagnosed with non-alcoholic fatty liver disease: Effects on metabolic parameters, anthropometric indices, and expression of PPAR-α, UCP1, and UCP2 genes. Pharmacol Res. 2020;156:104770. doi: 10.1016/j.phrs.2020.104770 [DOI] [PubMed] [Google Scholar]