Abstract
A microbroth kinetic model based on turbidity measurements was developed in order to analyze the growth characteristics of three species of filamentous fungi (Rhizopus microsporus, Aspergillus fumigatus, and Scedosporium prolificans) characterized by different growth rates in five nutrient media (antibiotic medium 3, yeast nitrogen base medium, Sabouraud broth, RPMI 1640 alone, and RPMI 1640 with 2% glucose). In general, five distinct phases in the growth of filamentous fungi could be distinguished, namely, the lag phase, the first transition period, the log phase, the second transition period, and the stationary phase. The growth curves were smooth and were characterized by the presence of long transition periods. Among the different growth phases distinguished, the smallest variability in growth rates among the strains of each species was found during the log phase in all nutrient media. The different growth phases of filamentous fungi were barely distinguishable in RPMI 1640, in which the poorest growth was observed for all fungi even when the medium was supplemented with 2% glucose. R. microsporus and A. fumigatus grew better in Sabouraud and yeast nitrogen base medium than in RPMI 1640, with growth rates three to four times higher. None of the media provided optimal growth of S. prolificans. The germination of Rhizopus spores and Aspergillus and Scedosporium conidia commenced after 2 and 5 h of incubation, respectively. The elongation rates ranged from 39.6 to 26.7, 25.4 to 20.2, and 16.9 to 9.9 μm/h for Rhizopus, Aspergillus, and Scedoporium hyphae, respectively. The germination of conidia and spores and the elongation rates of hyphae were enhanced in antibiotic medium 3 and delayed in yeast nitrogen base medium. In conclusion, the growth curves provide a useful tool to gain insight into the growth characteristics of filamentous fungi in different nutrient media and may help to optimize the methodology for antifungal susceptibility testing.
In vitro susceptibility testing of filamentous fungi is becoming increasingly important because of the frequency and diversity of infections caused by them (24, 31, 37). In addition, more antifungal agents have been introduced for clinical use and other new drugs are undergoing clinical evaluation (34). Hence, standardized in vitro susceptibility tests that give reproducible results, predict the resistance of molds, and correlate with clinical outcome are required (2, 3). Better inter- and intralaboratory agreement has been achieved by standardizing various factors involved in testing filamentous fungi for their susceptibilities such as the inoculum preparation, the incubation conditions (time and temperature), the MIC determination (reading time and end points), and the nutrient medium (4, 5, 18, 27). The influence of medium on antifungal susceptibility tests of yeasts is well established (3, 11, 15, 25, 28). Although there is no consensus as to the optimal nutrient medium, by definition the nutrient medium must be able to support adequate growth of the fungus without interfering with the action of the antifungal agents and must result in reproducible results that have clinical value (19). Many studies have shown that synthetic medium RPMI 1640 gives reproducible results for the in vitro testing of the susceptibility of yeasts to various antifungal drugs (25, 28). This medium was also selected by the Subcommittee for Antifungal Susceptibility Testing of the National Committee for Clinical Laboratory Standards (NCCLS) for the in vitro susceptibility testing of conidium-forming filamentous fungi (23) although there was no evidence that this medium was suitable for filamentous fungi (3). RPMI 1640 has a number of advantages (28), but its suitability for the susceptibility testing of nonfermentative yeasts such as Cryptococcus neoformans has been questioned (10, 15, 33, 35). Therefore, the appropriateness of this medium for filamentous fungi should not be implicitly postulated. Given the greater variability in the growth rate, mechanisms of sporulation, and nutrient requirements among the filamentous fungi than among yeasts, growth characteristics of molds in relation to the medium should be studied in detail. Due to the filamentous and non homogenous growth of molds, the analysis of growth characteristics by growth curves is difficult. In the present study we developed a microbroth kinetic system in order to investigate the growth characteristics of filamentous fungi in different nutrient media. Such a system would help to select the medium that optimally supports the growth of these fungi and to establish the optimal reading time of susceptibility testing of filamentous fungi.
MATERIALS AND METHODS
Isolates.
Fifteen clinical isolates of filamentous fungi belonging to three species were selected based on their growth rates. Rhizopus microsporus var. rhizopodiformis was chosen as representative of fast-growing molds, Aspergillus fumigatus was chosen as intermediate in growth rate, and Scedosporium prolificans was chosen as representative of slow-growing fungi. For each species five strains from our private collection were tested: R. microsporus var. rhizopodiformis, AZN190, AZN410, AZN5816, AZN5805, and AZN1185; A. fumigatus, AZN9618, AZN9619, AZN9620, AZN9621, and AZN9625; S. prolificans, AZN7898, AZN7901, AZN7902, AZN7906, and AZN7918.
Isolates had been frozen in 50% glycerol at −70°C and were revived by subculturing onto Sabouraud glucose agar (SGA) tubes supplemented with 0.5% chloramphenicol and incubated at 29°C for 7 days. The isolates were subcultured again on SGA tubes and incubated for another 5 to 7 days at 37°C.
Nutrient media.
The following five nutrient media were used: RPMI 1640 medium with l-glutamine but without bicarbonate (GIBCO BRL, Life Technologies, Woerden, The Netherlands) prepared alone (RPMI) or supplemented with 2% glucose (RPMI+); yeast nitrogen base (YNB; Difco Laboratories, Amsterdam, The Netherlands); antibiotic medium 3 (AM3; Oxoid, Hampshire, United Kingdom), and Sabouraud broth (SAB; Oxoid).
All media were prepared according to the manufacturer's instructions and buffered to pH 7.0 with 0.165 M morpholinepropanesulfonic acid (MOPS) (Sigma-Aldrich Chemie GmbH, Steinheim, Germany). Double-strength media were prepared and sterilized by filtration (RPMI, RPMI+, and YNB) or by autoclavation (SAB and AM3).
Growth curves.
Conidia and spores were collected using a cotton swab from 7- to 10-day-old cultures and suspended in 0.1% Tween 80. The suspensions were adjusted to 2 × 104 spores/ml by counting the cells in a hemacytometer cell counting chamber. Viability was confirmed by plating serial dilutions on SGA plates. One hundred microliters of each suspension containing 0.1% Tween 80 was inoculated into 100 μl of double-strength medium in 96-well flat-bottom microtitration plates. Tween 80 was used in order to prevent the growth of fungi on the surfaces of the media inside the wells. The plates were sealed and incubated at 37°C for 100 h inside a plate reader (Rosys Anthos ht3; Anthos Labtec Instruments GmbH, Salzburg, Austria). The optical density (OD) at 405 nm was recorded for each well automatically every 15 min without shaking. The reader can detect changes of 0.001 in OD. Sequential OD measurements were used to generate growth curves for each fungus and medium in triplicate. All studies were conducted two times.
Microscopic examination.
In order to correlate OD changes with the morphology of the fungi, conidia and spores were observed microscopically in microtitration plates by a reverse microscope at hourly intervals. At each time point, 100 conidia and spores were counted and the percentage of germination in each medium was estimated in triplicate. The lengths of hyphae formed by 15 germinated conidia or spores were measured, and the average length was calculated in triplicate. Furthermore, the change in hyphal length over time was computed as (average length at t2 − average length at t1)/(t2 − t1), where t1 and t2 are the times at the beginning and end of the measurement period, respectively. The mean elongation rate was calculated by averaging the changes during sequential time periods of the growth.
Kinetic parameters.
In order to compare the growth curves for each species in the five different nutrient media, various parameters were calculated based on the changes of the OD over time using the MicroWin, version 3, software (Mikrotek Laborsysteme GmbH, Overath, Germany). From the growth curve of each strain in each of the media the following parameters were calculated: the highest OD (ODmax), the average of all changes in OD (ΔOD) per minute, where ΔOD = ODfinal − ODinitial in an interval of 15 min, and the maximal slope (Smax), which was the largest increase rate in OD repeated for 25 consecutive time points. Furthermore the following time-related parameters were recorded: the time of first detectable OD change, the time when 90% of the ODmax was reached (OD90), and the time at which Smax of the growth curve was reached.
In addition, the S values for the growth curves were calculated and were used as estimates of the growth rates of the species in each nutrient medium. To visualize small changes in the growth rates during the growth curve and to determine the time point after which the growth rate changed significantly, a calculation model based on the area under the kinetic curve (AUKC) was developed. With this model the relative AUKC (rAUKC) was estimated by dividing each AUKC for each time point every 3 h by the corresponding time period. The changes in rAUKC, ΔrAUKC, were calculated for each time point by subtracting the rAUKC for each time point from the corresponding rAUKC of the previous time point. The ΔrAUKC is an estimate of changes in the slope of the growth curve and thus an estimate of the growth rate of fungus. When the ΔrAUKC value increases linearly over time, the OD increases with a constant rate, and when the ΔrAUKC value decreases or goes to zero over time, the growth rate decreases or goes to zero, respectively. Thus, increasing ΔrAUKC values corresponded to high growth rates. The ΔrAUKC values were used in order to distinguish different phases in the growth of filamentous fungi and the time employed for each phase.
RESULTS
A total of 225 growth curves based on 90,000 time points were obtained. The shapes of the growth curves were different depending on the nutrient medium used and the species tested (Fig. 1A, 2A, and 3A). However they were very reproducible among the replicates and the strains tested. Since the results of the two experiments were similar, the data of the first experiment were used for analysis.
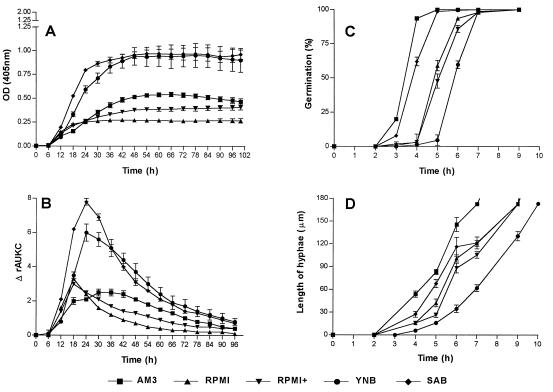
FIG. 1.
Graphical representation of the growth of R. microsporus var. rhizopodiformis in five nutrient media. (A) Changes in OD over time. (B) Changes in ΔrAUKC over time. (C) Percentages of germination of conidia over time. (D) Extension of hyphae over time. Bars, SE.
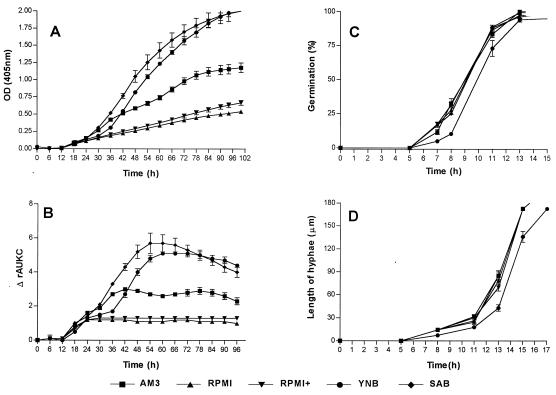
FIG. 2.
Graphical representation of the growth of A. fumigatus in five nutrient media. (A) Changes in OD over time. (B) Changes in ΔrAUKC over time. (C) Percentages of germination of conidia over time. (D) Extension of hyphae over time. Bars, SE.
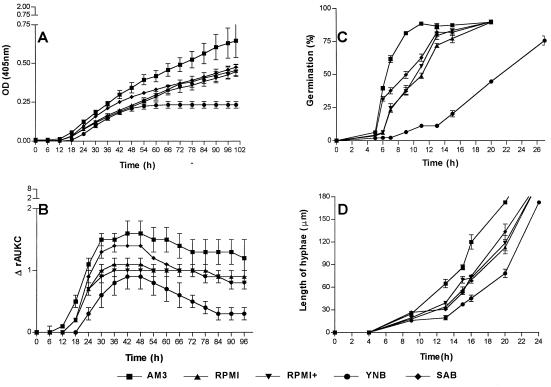
FIG. 3.
Graphical representation of the growth of S. prolificans in five nutrient media. (A) Changes in OD over time. (B) Changes in ΔrAUKC over time. (C) Percentages of germination of conidia over time. (D) Extension of hyphae over time. Bars, SE.
The growth curves were fragmented and were analyzed. In general the following phases in the growth of each of the tested genera could be distinguished based on OD changes and ΔrAUKC values (Table 1). The first phase was the lag phase in which no changes in OD were measured and the ΔrAUKC values were lower than 5% of the maximal ΔrAUKC. Microscopic examination revealed that during this phase germination of spores and conidia took place followed by elongation of hyphae to a maximal length of 60 μm (Fig. 1D, 2D, and 3D). Further elongation of hyphae was detected spectrophotometrically and resulted in a rapid increase in OD (first transition period) until 30% of the maximal ΔrAUKC was reached. After this phase, the ΔrAUKC increased and the growth curve reached the maximal slope, the maximal ΔrAUKC (log phase). Afterwards a second transition period, in which the slope of the growth curve decreased continuously until the ΔrAUKC reached 70% of its maximum and the OD tended to reach a plateau, was apparent (second transition period). The last phase was the stationary phase, where no changes in OD or negative slopes of the growth curve were observed and where values for ΔrAUKC were lower than 70% of the maximum ΔrAUKC (Fig. 1A and B, 2A and B, and 3A and B).
TABLE 1.
Time intervals at which different growth phases of R. microsporus var. rhizopodiformis, A. fumigatus, and S. prolificans were apparent in different media
| Species | Medium | Time (h) of phase end ± SD (ΔOD/min [10−5] [CVa])
|
||||
|---|---|---|---|---|---|---|
| Lagb | 1st transition | Log | 2nd transition | Stationary | ||
| R. microsporus | AM3 | 5.40 ± 1.12 (1.02 [68%]) | 6.60 ± 1.12 (14.34 [13%]) | 27.60 ± 4.18 (24.20 [14%]) | 54.00 ± 2.51 (13.14 [20%]) | >54 (−2.52 [71%]) |
| RPMI | 6.00 ± 0.95 (2.06 [23%]) | 7.80 ± 1.20 (15.70 [14%]) | 15.60 ± 0.60 (39.72 [9%]) | 24.00 ± 0.95 (8.94 [37%]) | >4 (4.36 [27%]) | |
| RPMI+ | 6.60 ± 0.60 (2.05 [23%]) | 9.00 ± 0.00 (23.60 [10%]) | 15.60 ± 0.60 (37.44 [10%]) | 27.60 ± 1.75 (14.80 [19%]) | >27.60 (2.52 [61%]) | |
| SAB | 9.00 ± 0.00 (12.41 [18%]) | 9.00 ± 0.00 (0) | 23.25 ± 0.67 (84.48 [6%]) | 34.50 ± 0.77 (25.20 [66%]) | >34.50 (8.78 [175%]) | |
| YNB | 8.40 ± 0.60 (2.26 [33%]) | 12.00 ± 0.00 (36.00 [17%]) | 25.20 ± 0.73 (65.76 [14%]) | 43.80 ± 4.31 (26.98 [49%]) | >43.80 (0.03 [126%]) | |
| A. fumigatus | AM3 | 13.20 ± 1.53 (2.90 [45%]) | 16.20 ± 1.53 (21.36 [24%]) | 54.60 ± 8.35 (25.98 [11%]) | 97.80 ± 2.24 (19.30 [21%]) | >97.80 (5.83 [17%]) |
| RPMI | 12.60 ± 1.12 (2.48 [20%]) | 14.40 ± 0.60 (17.36 [22%]) | 46.80 ± 12.24 (11.50 [5%]) | 94.80 ± 4.51 (9.16 [17%]) | >94.80 (6.60 [27%]) | |
| RPMI+ | 10.80 ± 1.20 (1.12 [29%]) | 13.80 ± 0.73 (13.92 [12%]) | 57.60 ± 12.78 (12.38 [12%]) | >99 (11.98 [11%]) | >99 (NCc) | |
| SAB | 13.80 ± 1.20 (4.41 [8%]) | 24.60 ± 1.12 (19.02 [9%]) | 56.40 ± 4.18 (55.93 [12%]) | 92.40 ± 3.47 (29.22 [28%]) | >92.40 (14.24 [22%]) | |
| YNB | 13.80 ± 0.73 (1.70 [14%]) | 30.00 ± 1.64 (17.16 [9%]) | 65.40 ± 2.58 (56.34 [5%]) | >99 (31.46 [14%]) | >99 (NC) | |
| S. prolificans | AM3 | 13.50 ± 3.19 (1.53 [40%]) | 17.40 ± 1.47 (14.58 [28%]) | 55.80 ± 9.23 (15.08 [26%]) | 87.60 ± 9.60 (9.75 [64%]) | >87.6 (7.49 [145%]) |
| RPMI | 13.20 ± 0.73 (0.59 [65%]) | 17.40 ± 0.60 (7.61 [22%]) | 49.80 ± 8.62 (11.04 [11%]) | 84.00 ± 9.90 (8.15 [42%]) | >84.00 (4.85 [24%]) | |
| RPMI+ | 15.60 ± 1.12 (1.76 [28%]) | 18.00 ± 0.95 (10.93 [26%]) | 52.20 ± 7.86 (10.06 [14%]) | 87.60 ± 10.50 (7.20 [29%]) | >87.60 (6.77 [34%]) | |
| SAB | 15.60 ± 1.12 (1.70 [29%]) | 18.60 ± 0.60 (15.44 [19%]) | 37.20 ± 1.53 (16.30 [6%]) | 75.00 ± 7.35 (7.22 [15%]) | >75.00 (6.20 [38%]) | |
| YNB | 18.75 ± 0.67 (0.91 [61%]) | 21.00 ± 1.10 (9.07 [47%]) | 41.25 ± 2.77 (13.05 [13%]) | 61.50 ± 2.32 (4.34 [47%]) | >61.50 (12.58 [171%]) | |
CV, coefficient of variation.
The beginning of the lag phase is at time zero in each case.
NC, not calculated.
R. microsporus.
The strains of R. microsporus var. rhizopodiformis showed the shortest lag phase, with the first significant change in OD after 4.8 to 6.5 h of incubation at 37°C, and the OD90s for the five strains ranged between 20 and 40 h (Table 2). The OD90 was reached earlier (after 20 h) when RPMI was used as the nutrient medium; however in this medium the growth rate was very low, with an ODmax of 0.29 and a slope of 0.61 × 10−4 (Table 2). Supplementation of RPMI with 2% glucose resulted in a slight increase of the growth rate of the fungus. By contrast the growth rates in SAB and YNB media were the highest, with ODmaxs of 1.01 and 1.02 and slopes of 2.28 × 10−4 and 2.17 × 10−4, respectively. The Smaxs in these media were observed after 15.75 and 18.60 h, respectively. After 66 h of incubation a decrease in the OD was observed in AM3 but not in the other media (Fig. 1A). The microscopical observations showed that the germination of spores started after 2 h in AM3 and SAB, after 4 h in RPMI and RPMI+, and after 5 h in YNB (Fig. 1C). The highest elongation rates of hyphae were observed in AM3 (39.6 μm/h), and the lowest were observed in YNB (26.7 μm/h) (Fig. 1D). The highest rate of increase in OD (ΔOD per minute) was observed during the log phase (0.5 to 3 times higher than those in the other growth phases (Table 1). Based on the ΔrAUKC model the log phase was from 9 to 15.6 h in RMPI and RPMI+, from 12 to 25.2 h in YNB, from 9 to 23.3 h in SAB, and from 6.6 to 27 h in AM3 (Fig. 1B and Table 1). Based on ΔOD-per-minute values the lowest interstrain variation for all media was found during the log phase. The mean variations ± standard errors (SE) of ΔOD-per-minute values in the five media were 11% ± 2% for the log phase, 14% ± 1% for the first transition period, 33% ± 9% for the lag phase, 38% ± 9% for the second transition period, and 92% ± 26% for the stationary phase (Table 1).
TABLE 2.
Parameters calculated from the growth curves of the five strains from each fungal species in five nutrient media based on changes of ODa
| Species | Medium | ODmax | S (10−4) | Smax (10−4) | OD0b (h) | OD90 (h) | Time of Smax (h) |
|---|---|---|---|---|---|---|---|
| R. microsporus | AM3 | 0.55 ± 0.05 | 1.17 ± 0.11 | 1.17 ± 0.11 | 4.80 ± 0.21 | 39.35 ± 5.10 | 12.06 ± 6.57 |
| RPMI | 0.29 ± 0.03 | 0.61 ± 0.08 | 0.61 ± 0.08 | 4.95 ± 0.33 | 20.30 ± 6.10 | 11.15 ± 0.93 | |
| RPMI+ | 0.41 ± 0.06 | 0.84 ± 0.06 | 0.84 ± 0.06 | 5.15 ± 0.29 | 37.15 ± 8.86 | 10.65 ± 1.04 | |
| SAB | 1.01 ± 0.10 | 2.28 ± 0.24 | 2.28 ± 0.24 | 5.38 ± 0.14 | 29.24 ± 6.04 | 15.75 ± 2.19 | |
| YNB | 1.02 ± 0.24 | 2.17 ± 0.60 | 2.17 ± 0.60 | 6.50 ± 0.35 | 40.18 ± 9.47 | 18.60 ± 1.15 | |
| A. fumigatus | AM3 | 1.17 ± 0.15 | 2.49 ± 0.32 | 2.49 ± 0.32 | 10.02 ± 0.78 | 73.33 ± 3.78 | 31.17 ± 0.76 |
| RPMI | 0.53 ± 0.06 | 1.08 ± 0.11 | 1.08 ± 0.11 | 9.65 ± 0.38 | 76.05 ± 2.33 | 17.30 ± 5.04 | |
| RPMI+ | 0.65 ± 0.08 | 1.27 ± 0.15 | 1.27 ± 0.15 | 9.70 ± 0.27 | 81.21 ± 0.53 | 17.13 ± 4.61 | |
| SAB | 2.00 ± 0.26 | 4.23 ± 0.53 | 4.23 ± 0.53 | 9.65 ± 0.76 | 72.80 ± 3.56 | 42.44 ± 4.79 | |
| YNB | 2.01 ± 0.12 | 4.28 ± 0.31 | 4.28 ± 0.31 | 11.35 ± 0.34 | 80.90 ± 4.03 | 43.63 ± 0.48 | |
| S. prolificans | AM3 | 0.64 ± 0.23 | 1.31 ± 0.45 | 1.31 ± 0.45 | 11.35 ± 0.89 | 71.49 ± 13.46 | 40.25 ± 14.15 |
| RPMI | 0.45 ± 0.08 | 0.97 ± 0.14 | 0.97 ± 0.14 | 13.30 ± 1.01 | 74.05 ± 3.45 | 43.69 ± 19.90 | |
| RPMI+ | 0.44 ± 0.06 | 0.87 ± 0.14 | 0.87 ± 0.14 | 12.95 ± 0.93 | 75.44 ± 7.36 | 39.63 ± 23.43 | |
| SAB | 0.47 ± 0.04 | 0.97 ± 0.06 | 0.97 ± 0.06 | 12.95 ± 0.72 | 69.10 ± 8.62 | 22.50 ± 1.62 | |
| YNB | 0.24 ± 0.04 | 0.53 ± 0.10 | 0.53 ± 0.10 | 17.75 ± 2.28 | 45.21 ± 5.41 | 26.31 ± 3.37 |
Values are means ± standard deviations.
OD0, Time of the first significant change in OD.
A. fumigatus.
The first detectable growth for the strains of A. fumigatus was after 9.65 h (Table 2) although the germination of conidia was completed after 13 h (Fig. 2C). In Fig. 2A the differences in the shapes of the growth curves for each nutrient medium are shown. The slopes of the growth curves in RPMI medium, even when supplemented with 2% glucose, were fourfold lower than those in SAB and YNB (Table 2). By contrast to that of Rhizopus strains, the growth of the Aspergillus strains failed to reach the stationary phase within 100 h. Smax was reached after 30 h for all media except RPMI, in which Smax occurred after 17 h (Table 2). The germination of conidia started after 5 h of incubation in all media although it was delayed for 1.5 h in YNB (Fig. 2C). Similar rates of elongation of Aspergillus hyphae occurred in all media (≈25 μm/h) except in YNB, in which a lower elongation rate (20.2 μm/h) was observed (Fig. 2D). The ΔOD-per-minute values in the log phase were higher than those in the other growth phases except in RPMI, where the ΔOD per minute was comparable to that in the first transition period. The log phase based on ΔrAUKC values was between 14.4 and 46.8 h in RPMI and RPMI+, between 24.6 and 56.4 h in SAB, between 30 and 65.4 h in YNB, and between 16.2 and 54.6 h in AM3 (Fig. 2B and Table 1). The lowest variation in ΔOD per minute among the five strains and among the growth phases was found during the log phase. The mean variations ± SE of ΔOD per minute in the five media were 9% ± 2% for the log phase, 15% ± 3% for the first transition period, 18% ± 3% for the second transition period, 22% ± 2% for the stationary phase, and 23% ± 6% for the lag phase (Table 1).
S. prolificans.
The growth of this fungus was the slowest among the species tested since after 100 h of incubation the ODmax ranged from 0.27 in YNB to 0.64 in AM3 (Table 2). The highest growth rate occurred in AM3, and the lowest occurred in YNB, with slopes of the growth curves of 1.31 × 10−4 and 0.53 × 10−4, respectively. The Smax in these media occurred after 40 and 26 h, respectively (Table 2). In all the nutrient media the fungus continued to grow until 100 h, except for YNB, in which the plateau was reached within 50 h (Fig. 3A). The germination of Scedosporium conidia started after 4 h of incubation in AM3 and SAB, after 5 h of incubation in RPMI and RPMI+, and after 7 h of incubation in YNB, in which the delay in germination increased during the incubation. Complete germination was not achieved in any of the media after 20 h of incubation (Fig. 3C). The elongation rates ranged from 16.9 to 9.9 μm/h, with the highest in AM3 and the lowest in YNB (Fig. 3D). The ΔOD-per-minute values were higher during the log phase but were comparable to those of the first transition period (Fig. 3B and Table 1). The log phase based on the ΔrAUKC model was between 17.4 and 49.8 h in RPMI and RPMI+, between 18 and 55.8 h in AM3, between 18.6 and 37.2 h in SAB, and between 21 and 41.3 h in YNB. The lowest interstrain variation was found during the log phase, with a mean ± SE of 14% ± 3% compared with those during the first transition period (28% ± 5%), the second transition period (39% ± 8%), the lag phase (44% ± 8%), and the stationary phase (82% ± 31%) (Table 1).
DISCUSSION
The nutrient medium is a major factor that influences the results of susceptibility tests (3, 30). According to clinical laboratory standards an optimal nutrient medium should provide good or adequate growth of the microorganisms (19). The relativity of this definition is clear since all the media tested here supported the growth of filamentous fungi to various degrees. Moreover the definition of an adequate medium is really a minimal requirement that has to be fulfilled in order for a medium to be considered as a candidate for the susceptibility tests. An optimal nutrient medium should provide not simply adequate growth but the best possible growth in order to allow molds to grow without restriction and express all phenotypes. Under these growth conditions, any failure of the fungus to grow in the presence of antifungal drugs should be considered as a true inability, i.e., lack of proper genetic predisposition to resist the antifungal drugs or interaction of the drug with the target.
The importance of the nutrient medium and the growth rate of the fungus in relation to in vitro susceptibility testing has been shown previously. The in vitro susceptibility of Candida albicans to fluconazole and miconazole depends on the stage of the growth of the fungus and the nutrient medium used (6, 17, 36). Yeasts in the exponential growth phase were more susceptible to fluconazole than those in the lag phase when they were cultivated in YNB–2% glucose medium (6, 17), and the in vitro activity of miconazole in CYG (0.5% casein gydrolase, 0.5% yeast extract, 0.5% glucose), NG (1% neopeptone, 0.5% glucose), and YNB–4% glucose media was greater when richer media were used (36). Differences in MICs for filamentous fungi were also observed when conidia (fungus in the lag phase) and hyphae (fungus in the log or stationary phase) were cultivated in RPMI 1640 (12). In another study where RPMI and YNB were employed the interaction between antimicrobial agents and fungi depended on the type of medium used (22).
The previous findings can be correlated with the results of this study. Figures 1A, 2A, and 3A show that RPMI poorly supports the growth of the three species of filamentous fungi tested. Supplementation with glucose essentially had little effect, despite being proposed as a means of improving the characteristics of this medium (32), and resulted only in a slight increase in the fungal growth even after incubation for 100 h. SAB and YNB were the more nutritious media and provided the highest growth for R. microsporus and A. fumigatus, with growth rates three to four times higher than those achieved in RPMI. None of the media supported the growth of S. prolificans sufficiently. Apparent were other effects of the media, such as the delay of germination of spores and conidia as well as the lower elongation rates of hyphae of all species in YNB medium, processes which were enhanced in AM3. By contrast with the growth of yeasts, where the stationary phase is reached within 30 h (15, 36), the growth of filamentous fungi is characterized by smoother curves and long transition periods although it depended on the medium and species. Interestingly, during the growth of R. microsporus in AM3, an OD decrease after 66 h of incubation, which could be correlated with the death phase of bacterial growth, occurred (20).
The use of a poor medium such as RPMI, in which fungi grow slowly, might result in erroneous MICs. In an extreme situation a fungus unable to grow in a certain medium would seem to be susceptible despite the fact that the inhibition of growth is not due to the action of the antifungal agent but due to the medium. There might be other situations, which are difficult to prove experimentally, in which a poor medium, although it supports fungal growth, acts synergistically with the drug in inhibiting growth, resulting in an appearance of better activity. The discrepancy in the interaction of a fluoroquinolone with amphotericin B against A. fumigatus in YNB (synergistic) and in RPMI (antagonistic) (22), as well as the higher activity of miconazole in richer media (36), could be explained by the growth curves. The high growth in YNB and the poor growth in RPMI correlate with different levels of metabolic activity. Thus, in YNB the drugs might penetrate better into the intracellular site of action in sufficient concentrations to exhibit an effect.
RPMI has been evaluated extensively for in vitro susceptibility testing of yeasts and has been shown to provide reproducible results (2, 25, 28, 30). Therefore, the NCCLS has proposed to use this medium as the standard medium for antifungal susceptibility testing of filamentous fungi (23). Like YNB, RPMI is a synthetic and completely defined medium and is characterized by small lot-to-lot variation, resulting in high reproducibility of susceptibility tests, unlike AM3, which has considerable variation from lot to lot and source to source. By contrast the use of chemically complex undefined media such as SAB medium is not recommended (19, 28) since undefined components that they may contain might interact with antifungal drugs (13, 14, 19, 26). Furthermore, acidic media such as YNB and SAB, if they were used unbuffered, may inactivate amphotericin B, which is not stable at low pH (16, 21).
Another important variable in susceptibility testing of filamentous fungi is the reading time. It is well known that prolonged incubation elevates the MICs of antifungal drugs (8, 29). The NCCLS addressed this by recommending different incubation periods for each species based on visual growth. Although the growth curves of filamentous fungi have not been previously studied in order to characterize the growth over time, the NCCLS recommended incubation periods of 24 h for the fast-growing species, usually belonging to the Zygomycetes, 72 h for the slow-growing species such as the black fungi, and 48 h for the other species (23).
In the field of antibacterial susceptibility testing, the MICs should be read when the growth control is in the log phase and not in the transition periods, i.e., between lag phase and log phase or between log phase and stationary phase, where unbalanced growth exists (20). Since the growth curves of filamentous fungi are characterized by long transition periods, the precise determination of these periods is a crucial parameter in order to obtain balanced growth. The same conclusion was made by Galgiani and Stevens (7) in terms of variability of MICs, when they observed that yeasts showed increased variation in the concentration of a drug producing 50% of the growth seen in a drug-free well when the MIC determination was made beyond 48 h of incubation. Beyond this incubation period, the drug-free culture reached the stationary phase and stopped growing resulting in higher variation in MICs (7). This effect is more obvious for fungistatic drugs since in the stationary phase the fungi in the drug-free control stop growing but those in drug-containing wells do not, which increases the difference in optical density between these two wells (unpublished observations). These findings could be correlated with our finding of high variation in the stationary phase compared with that in the other growth phases. That the growth phase is an important variable is supported by the findings that the inhibitory effects of ketoconazole and miconazole against C. albicans were indistinguishable when the yeasts were tested in the stationary phase (1).
Based on the results of this study (Table 1), the growth curves for filamentous fungi during the transition periods were characterized by rapid changes in slope and high variation compared with other parts of the growth curve. The slopes in the transition periods were either continuously decreasing until a plateau was reached (second transition period) or increasing until the log phase was reached (first transition period). Since the log phase is located between these two transition periods, precise determination of these periods is required in order to determine the boundaries of this growth phase. Due to long and smooth transition periods of the growth curves of filamentous fungi, the visualization of the start and end points of these periods by using the OD changes is difficult. Therefore, a calculation model based on ΔrAUKC was developed. With the ΔrAUKC model even small changes of the slopes, which might be an indication of transition between phases, can be observed. The log phase of the growth curve is that part of the curve where ΔrAUKC values increase linearly over time and the growth rate is constant at its highest value. This model can be used to describe the different growth phases of filamentous fungi and to determine the boundaries of each phase. The level of 30% (increase until 30% and decrease until 70%) of the maximal ΔrAUKC seems to be the crucial breakpoint of the growth curves of filamentous fungi indicating the presence of symmetry.
In summary, the above-mentioned studies indicate that during the log phase balanced growth takes place (20) and a greater distribution of MICs is obtained (1) and the reproducibility of MICs is higher (7) than in the other growth phases. In addition, this study shows that the lowest interstrain variation was observed during the log phase of the growth curve. Thus, the optimal reading time of antifungal susceptibility testing of filamentous fungi could be during the log phase. Therefore the precise knowledge of the growth phases during the growth of filamentous fungi would help to find the optimal reading time of the MICs.
Nevertheless, many factors are involved in the standardization of antifungal susceptibility testing. Unequivocally intercenter and intracenter reproducibility, as underlined by NCCLS, is a major issue for antifungal susceptibility testing of filamentous fungi. However another primary goal of susceptibility tests is the correlation of in vitro results with clinical response, and this does not favor necessarily and absolutely simplified methodologies. Although an ultimate challenge would be to find a common medium that would be suitable for as many fungi as possible, this study indicates that the standardization of susceptibility testing of filamentous fungi may require different nutrient media for each species and consequently different reading times of the MICs of antifungal drugs. Furthermore, findings for one species are not readily extrapolated to others, particularly for filamentous fungi, where significant morphological and physiological variations exist. Therefore, based on the results obtained from the growth curves further studies are required to investigate the effect of nutrient media and growth phases on MICs and ultimately to determine which approach correlates best with the clinical outcome.
ACKNOWLEDGMENTS
This study was supported by the EC-TMR-EUROFUNG network (ERBFMXR-CT970145) and by the Mycology Research Center of Nijmegen.
REFERENCES
- 1.Beggs W. Growth phase in relation to ketoconazole and miconazole susceptibilities of Candida albicans. Antimicrob Agents Chemother. 1984;25:316–318. doi: 10.1128/aac.25.3.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cormican M G, Pfaller M A. Standardization of antifungal susceptibility testing. J Antimicrob Chemother. 1996;38:561–578. doi: 10.1093/jac/38.4.561. [DOI] [PubMed] [Google Scholar]
- 3.Espinel-Ingroff A, Barchiesi F, Hazen K C, Martinez-Suarez J V, Scalise G. Standardization of antifungal susceptibility testing and clinical relevance. Med Mycol. 1998;36(Suppl. 1):68–78. [PubMed] [Google Scholar]
- 4.Espinel-Ingroff A, Bartlett M, Bowden R, Chin N X, Cooper C, Jr, Fothergill A, McGinnis M R, Menezes P, Messer S A, Nelson P W, Odds F C, Pasarell L, Peter J, Pfaller M A, Rex J H, Rinaldi M G, Shankland G S, Walsh T J, Weitzman I. Multicenter evaluation of proposed standardized procedure for antifungal susceptibility testing of filamentous fungi. J Clin Microbiol. 1997;35:139–143. doi: 10.1128/jcm.35.1.139-143.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Espinel-Ingroff A, Dawson K, Pfaller M, Anaissie E, Breslin B, Dixon D, Fothergill A, Paetznick V, Peter J, Rinaldi M G, Walsh T. Comparative and collaborative evaluation of standardization of antifungal susceptibility testing for filamentous fungi. Antimicrob Agents Chemother. 1995;39:314–319. doi: 10.1128/aac.39.2.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gale E F, Johnson A M, Kerridge D, Koh T Y. Factors affecting the changes in amphotericin B sensitivity of Candida albicans during growth. J Gen Microbiol. 1975;87:20–36. doi: 10.1099/00221287-87-1-20. [DOI] [PubMed] [Google Scholar]
- 7.Galgiani J N, Stevens D A. Antimicrobial susceptibility testing of yeasts: a turbidimetric technique independent of inoculum size. Antimicrob Agents Chemother. 1976;10:721–728. doi: 10.1128/aac.10.4.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gehrt A, Peter J, Pizzo P A, Walsh T J. Effect of increasing inoculum sizes of pathogenic filamentous fungi on MICs of antifungal agents by broth microdilution method. J Clin Microbiol. 1995;33:1302–1307. doi: 10.1128/jcm.33.5.1302-1307.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghannoum M A. Susceptibility testing of fungi and correlation with clinical outcome. J Chemother. 1997;9(Suppl. 1):19–24. [PubMed] [Google Scholar]
- 10.Ghannoum M A, Ibrahim A S, Fu Y, Shafiq M C, Edwards J E, Jr, Criddle R S. Susceptibility testing of Cryptococcus neoformans: a microdilution technique. J Clin Microbiol. 1992;30:2881–2886. doi: 10.1128/jcm.30.11.2881-2886.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghannoum M A, Rice L B. Antifungal agents: mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin Microbiol Rev. 1999;12:501–517. doi: 10.1128/cmr.12.4.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guarro J, Llop C, Agular C, Pujol I. Comparison of in vitro antifungal susceptibilities of conidia and hyphae of filamentous fungi. Antimicrob Agents Chemother. 1997;41:2760–2762. doi: 10.1128/aac.41.12.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoeprich P D, Finn P D. Obfuscation of the activity of antifungal antimicrobics by culture media. J Infect Dis. 1972;126:353–361. doi: 10.1093/infdis/126.4.353. [DOI] [PubMed] [Google Scholar]
- 14.Hoeprich P D, Huston A C. Effect of culture media on the antifungal activity of miconazole and amphotericin B methyl ester. J Infect Dis. 1976;134:336–341. doi: 10.1093/infdis/134.4.336. [DOI] [PubMed] [Google Scholar]
- 15.Hoeprich P D, Merry J M. Influence of culture medium on susceptibility testing with BAY n 7133 and ketoconazole. J Clin Microbiol. 1986;24:269–271. doi: 10.1128/jcm.24.2.269-271.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson B, White R J, Williamson G M. Factors influencing the susceptibility of Candida albicans to the polyenic antibiotics nystatin and amphotericin B. J Gen Microbiol. 1978;104:325–333. doi: 10.1099/00221287-104-2-325. [DOI] [PubMed] [Google Scholar]
- 17.Kerridge D, Koh T Y, Marriott M S, Gale E F. Microbiology and plant protoplasts. In: Peberdy J F, Rose A H, Rodger H J, Cocking E C, editors. Microbiology and plant protoplasts. London, England: Churchill Livingston; 1976. pp. 23–28. [Google Scholar]
- 18.Llop C, Pujol I, Aguilar C, Sala J, Riba D, Guarro J. Comparison of three methods of determining MICs for filamentous fungi using different end point criteria and incubation periods. Antimicrob Agents Chemother. 2000;44:239–242. doi: 10.1128/aac.44.2.239-242.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGinnis M R, Rinaldi M G. Antifungal drugs: mechanisms of action, drug resistance, susceptibility testing, and assays of activity in biological fluids. In: Lorian V, editor. Antibiotics in laboratory medicine. 3rd ed. Baltimore, Md: Williams & Wilkins; 1991. pp. 198–251. [Google Scholar]
- 20.McGinnis M R, Rinaldi M G. Determining the effects of antibiotics on bacterial growth by optical and electrical methods. In: Lorian V, editor. Antibiotics in laboratory medicine. 3rd ed. Baltimore, Md: Williams & Wilkins; 1991. pp. 64–75. [Google Scholar]
- 21.Minagawa H, Kitaura K, Nakamizo N. Effects of pH on the activity of ketoconazole against Candida albicans. Antimicrob Agents Chemother. 1983;23:105–107. doi: 10.1128/aac.23.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakajima R, Kitamura A, Someya K, Tanaka M, Sato K. In vitro and in vivo antifungal activities of DU-6859a, a fluoroquinolone, in combination with amphotericin B and fluconazole against pathogenic fungi. Antimicrob Agents Chemother. 1995;39:1517–1521. doi: 10.1128/aac.39.7.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of conidium forming filamentous fungi. Proposed standard M38-P. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1998. [Google Scholar]
- 24.Perfect J R, Schell W A. The new fungal opportunists are coming. Clin Infect Dis. 1996;22(Suppl. 2):S112–S118. doi: 10.1093/clinids/22.supplement_2.s112. [DOI] [PubMed] [Google Scholar]
- 25.Pfaller M A, Rinaldi M G, Galgiani J N, Bartlett M S, Body B A, Espinel-Ingroff A, Fromtling R A, Hall G S, Hughes C E, Odds F C. Collaborative investigation of variables in susceptibility testing of yeasts. Antimicrob Agents Chemother. 1990;34:1648–1654. doi: 10.1128/aac.34.9.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Polak A, Scholer H J. Fungistatic activity, uptake and incorporation of 5-fluorocytosine in Candida albicans, as influenced by pyrimidines and purines. I. Reversal experiments. Pathol Microbiol. 1973;39:148–159. doi: 10.1159/000162642. [DOI] [PubMed] [Google Scholar]
- 27.Pujol I, Guarro J, Sala J, Riba M D. Effects of incubation temperature, inoculum size, and time of reading on broth microdilution susceptibility test results for amphotericin B against Fusarium. Antimicrob Agents Chemother. 1997;41:808–811. doi: 10.1128/aac.41.4.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Radetsky M, Wheeler R C, Roe M H, Todd J K. Microtiter broth dilution method for yeast susceptibility testing with validation by clinical outcome. J Clin Microbiol. 1986;24:600–606. doi: 10.1128/jcm.24.4.600-606.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reuben A, Anaissie E, Nelson P E, Hashem R, Legrand C, Ho D H, Bodey G P. Antifungal susceptibility of 44 clinical isolates of Fusarium species determined by using a broth microdilution method. Antimicrob Agents Chemother. 1989;33:3290–3295. doi: 10.1128/aac.33.9.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rex J H, Pfaller M A, Rinaldi M G, Polak A, Galgiani J N. Antifungal susceptibility testing. Clin Microbiol Rev. 1993;6:367–381. doi: 10.1128/cmr.6.4.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Richardson M D. Opportunistic and pathogenic fungi. J Antimicrob Chemother. 1991;28(Suppl. A):1–11. doi: 10.1093/jac/28.suppl_a.1. [DOI] [PubMed] [Google Scholar]
- 32.Rodriguez-Tudela J L, Martinez-Suarez J V. Defining conditions for microbroth antifungal susceptibility tests: influence of RPMI and RPMI-2% glucose on the selection of endpoint criteria. J Antimicrob Chemother. 1995;35:739–749. doi: 10.1093/jac/35.6.739. [DOI] [PubMed] [Google Scholar]
- 33.Rodriguez-Tudela J L, Martin-Diez F, Cuenca-Estrella M, Rodero L, Carpintero Y, Gorgojo B. Influence of shaking on antifungal susceptibility testing of Cryptococcus neoformans: a comparison of the NCCLS standard M27A medium, buffered yeast nitrogen base, and RPMI-2% glucose. Antimicrob Agents Chemother. 2000;44:400–404. doi: 10.1128/aac.44.2.400-404.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sheehan D J, Hitchcock C A, Sibley C M. Current and emerging azole antifungal agents. Clin Microbiol Rev. 1999;12:40–79. doi: 10.1128/cmr.12.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.St.-Germain G, Dion C. Effect of media on growth rate and susceptibility testing of Cryptococcus neoformans. Mycoses. 1996;39:201–206. doi: 10.1111/j.1439-0507.1996.tb00125.x. [DOI] [PubMed] [Google Scholar]
- 36.van den Bossche H, Willemsens G, van Cutsem J M. The action of miconazole on the growth of Candida albicans. Sabouraudia. 1975;13:63–73. [PubMed] [Google Scholar]
- 37.Vartivarian S E, Anaissie E J, Bodey G P. Emerging fungal pathogens in immunocompromised patients: classification, diagnosis, and management. Clin Infect Dis. 1993;17(Suppl. 2):S487–S491. doi: 10.1093/clinids/17.supplement_2.s487. [DOI] [PubMed] [Google Scholar]