Abstract
Objective
To identify functional and structural alterations in language networks of people with temporal lobe epilepsy (TLE), who frequently present with naming and word‐finding difficulties.
Methods
Fifty‐five patients with unilateral TLE (29 left) and 16 controls were studied with auditory and picture naming functional magnetic resonance imaging (fMRI) tasks. Activation maxima in the left posterobasal temporal lobe were used as seed regions for whole‐brain functional connectivity analyses (psychophysiological interaction). White matter language pathways were investigated using diffusion tensor imaging and neurite orientation dispersion and density imaging metrics extracted along fiber bundles starting from fMRI‐guided seeds. Regression analyses were performed to investigate the correlation of functional connectivity with diffusion MRI metrics.
Results
In the whole group of patients and controls, weaker functional connectivity from the left posterobasal temporal lobe (1) to the bilateral anterior temporal lobe, precentral gyrus, and lingual gyrus during auditory naming and (2) to the bilateral occipital cortex and right fusiform gyrus during picture naming was associated with decreased neurite orientation dispersion and higher free water fraction of white matter tracts. Compared to controls, TLE patients exhibited fewer structural connections and an impaired coupling of functional and structural metrics.
Significance
TLE is associated with an impairment and decoupling of functional and structural language networks. White matter damage, as evidenced by diffusion abnormalities, may contribute to impaired functional connectivity and language dysfunction in TLE.
Keywords: diffusion MRI, DTI, functional connectivity, language network, naming function, NODDI, temporal lobe epilepsy
Key Points.
TLE is associated with impaired naming, but the underlying language network changes are poorly understood
We combine auditory and picture naming fMRI‐derived seed‐based functional connectivity with advanced diffusion MRI metrics
Weaker functional connectivity related to lower neurite orientation dispersion and higher free water fraction of white matter tracts
TLE patients showed fewer structural connections and compromised coupling of functional and structural metrics
White matter damage may contribute to impaired functional connectivity and language dysfunction in TLE
1. INTRODUCTION
Impairment of language function, in particular naming and word‐finding difficulties, is a frequent concern in patients with temporal lobe epilepsy (TLE). 1 Advanced neuroimaging techniques, such as functional magnetic resonance imaging (fMRI) 2 , 3 and diffusion MRI (dMRI), 4 , 5 are increasingly applied to study the complex organization of functional and structural language networks.
We previously showed that auditory and picture naming fMRI tasks robustly activate left temporobasal language regions, 6 , 7 which are critically involved in word‐finding and naming functions. 8 Stronger functional connectivity seeding from these regions to the bilateral anterior temporal lobe, inferior frontal lobe, and occipital cortex related to better out‐of‐scanner naming function. In left TLE patients, these functional networks were more disrupted with greater disease duration or an earlier onset of seizures. 3
dMRI and tractography are used to study structural brain connections, including language networks. Using diffusion tensor imaging (DTI), decreased fractional anisotropy (FA) and increased mean diffusivity (MD) have been described as correlates of disrupted structural language networks in various neurological and psychiatric disorders, including epilepsy and schizophrenia. 9 , 10 Interpretation of changes in FA and MD is complicated by their low specificity for specific microstructural changes, such as axon count, density, degree of myelination, or fiber organization. 11 Advanced dMRI models such as neurite orientation dispersion and density imaging (NODDI) can provide additional estimates of tissue microstructure. The NODDI model is based on the normalized signal contribution of the intraneurite compartment (axons/dendrites), free water, and the extraneurite compartment, using the following parameters: neurite density index (NDI; describing neurite volume fraction), orientation dispersion index (ODI; describing the variability of neurite orientation, ranging from 0 [all parallel] to 1 [completely isotropic]), and free water fraction (FWF). 12 , 13
Despite efforts to delineate language network changes in TLE, the interplay of functional and structural connections and their disease‐specific reorganization remain poorly understood. To improve the neurobiological understanding of language network alterations in TLE, we used auditory and picture‐naming fMRI for a seed‐based whole brain functional connectivity analysis, and analyzed the association of functional language networks with structural connectivity, derived from advanced dMRI metrics.
2. MATERIALS AND METHODS
2.1. Participants
We studied 55 patients with drug‐resistant unilateral TLE (29 left) who underwent presurgical assessment at the National Hospital for Neurology and Neurosurgery between 2014 and 2017, and 16 healthy participants with no history of epilepsy or any other chronic neurological or psychiatric disease. Exclusion criteria were nonfluency in written and spoken English, pregnancy, any contraindication to MRI, inability to give informed consent, or history of a bilateral tonic–clonic seizure within 24 h prior to the study. Demographic and clinical data are summarized in Table 1.
TABLE 1.
Demographic and clinical data in controls and patients
| Group | Sex, female/male, n | Handedness, right/left, n | Age, years | Age at onset, years | Duration, years | FS monthly | FBTCS monthly | ASMs, n | GNT score | IQ |
|---|---|---|---|---|---|---|---|---|---|---|
| LTLE, n = 29 | 15/14 | 27/2 | 37.8 ± 12.2 | 17.0 ± 10.7 | 14.0 (44.0) | 5 (59.5) | 0 (4) | 2 (2) | 14.6 ± 5.8 a | 94.7 ± 10.6 b |
| RTLE, n = 26 | 13/13 | 24/2 | 38.7 ± 10.0 | 23.5 ± 13.4 | 13.0 (41.0) | 4.5 (40.0) | 0 (3) | 2 (3) | 17.4 ± 5.1 | 96.9 ± 12.4 c |
| CTR, n = 16 | 10/6 | 15/1 | 30.0 ± 12.8 | n.a. | n.a. | n.a. | n.a. | n.a. | 20.3 ± 6.1 | 112.0 ± 8.0 |
Age, age at onset of epilepsy, McKenna GNT 15 score, and verbal IQ are shown as mean ± SD. Duration, seizure frequency (FS, FBTCS), and number of ASMs are shown as median (range).
Abbreviations: ASM, antiseizure medication; CTR, control; FBTCS, focal to bilateral tonic–clonic seizures; FS, focal seizures; GNT, Graded Naming Test; IQ, intelligence quotient; LTLE, left temporal lobe epilepsy; n.a., not applicable; RTLE, right temporal lobe epilepsy.
Naming score LTLE < CTR, p = .005.
IQ LTLE < CTR, p < .001.
IQ RTLE < CTR, p < .001.
Prolonged electroencephalographic video telemetry confirmed and lateralized areas of seizure onset to the temporal lobe. Structural MRI at 3 T identified hippocampal sclerosis (HS) in 25 patients (14 left/11 right) and normal‐appearing MRI in 13 patients (8 left/5 right). Seventeen patients had mixed pathologies, including dysembryoplastic neuroepithelial tumor in nine (5 left/4 right), focal cortical dysplasia (FCD) in two (1 left/1 right), low‐grade glioma in three (2 left/1 right), dual pathology (FCD and HS) in one (right), encephalocele in one (right), and traumatic lesion in one (right) patient. Handedness was determined using the Edinburgh Hand Preference Inventory. 14
Prior to scanning, all subjects underwent standardized clinical tests that form part of the pre‐ and postsurgical neuropsychological evaluations of TLE patients. Naming was assessed using the McKenna Graded Naming Test (GNT). 15 Estimated verbal intelligence quotient (IQ) was derived from performance on the National Adult Reading Test (NART). 16 One‐way analysis of variance (ANOVA; Tukey post hoc test) and Mann–Whitney U tests were used for continuous variables. Fisher exact test was utilized for categorical variables.
2.2. Magnetic resonance data acquisition
Please refer to the Supplementary Material for details on magnetic resonance data acquisition.
2.3. Language tasks
All participants performed two overt naming fMRI tasks with active control conditions: auditory naming (naming aloud objects from their auditory description; reversed speech as control condition, duration = 5 min) and picture naming (naming aloud objects from their visual presentation; scrambled pictures and faces as control condition, duration = 6.25 min), as described in detail previously. 3 , 6 Task performance was monitored using a microphone. All study participants successfully performed the tasks (>80% accuracy).
2.4. Data analysis: fMRI activation maps
fMRI data were analyzed with Statistical Parametric Mapping 8 (SPM8; http://www.fil.ion.ucl.ac.uk/spm/). Imaging time series of each subject were realigned, normalized into standard anatomical space using a scanner‐ and acquisition‐specific template, and smoothed with a Gaussian kernel of 8 mm full‐width at half‐maximum.
At the first level, condition‐specific effects were estimated according to the general linear model. 17 Regressors of interest were formed by convolving blocks of stimuli with the canonical hemodynamic response function for conditions of interest including motion parameters as confounds. Voxelwise parameter estimates were calculated for the contrasts “auditory naming” (auditory naming minus reversed speech) and “picture naming” (picture‐naming minus scrambled pictures and faces).
At the second level, one‐sample t‐tests were used for main task effects across groups. One‐way ANOVA was used for intergroup comparisons. Activations are reported at p < .05, corrected for multiple comparisons (familywise error rate [FWE]) across the whole brain. Language laterality indices (LIs) of statistical parametric maps were calculated using the bootstrap method implemented in SPM8 18 on bilateral masks comprising temporal lobes and mesial temporal structures, as described in detail previously. 6 , 7
2.5. Data analysis: Functional connectivity (psychophysiological interaction)
We employed a psychophysiological interaction (PPI) analysis to assess task‐related functional connectivity between language activation maxima and the rest of the brain. 3 For each subject, the time series (first eigenvariate) was extracted from the normalized, smoothed echo planar images using a 12‐mm‐diameter sphere centered on the subject‐specific peak voxel within a larger 20‐mm‐diameter sphere region of interest, 19 which was centered on the groupwise peak activations in the left posterior inferior temporal gyrus (auditory naming) and the left fusiform gyrus (picture naming).
The PPI model included three regressors: (1) the main effect of the seed region (i.e., the functional time series), (2) the raw task regressor (represented by the vector of the naming or word‐generation block onsets), and (3) the interaction between the two, representing a task‐modulated change in functional connectivity, or PPI. 20
One‐sample t‐tests and one‐way ANOVA were used to investigate effects across and among groups, respectively. All PPI activations are reported at a threshold of p < .05, FWE‐corrected. Verbal IQ, task‐specific language LI, and age were used as nuisance regressors. The relation of PPI activation to out‐of‐scanner naming performance (GNT 15 scores) was explored using multiple regression. In accord with previous investigations, 2 , 3 correlational activations are shown at a threshold of p < .001 uncorrected.
2.6. Data analysis: dMRI and tractography
dMRI data were corrected for scanner drift, 21 and for motion and susceptibility‐induced distortion using FSL v5.10 eddy and topup. 22 , 23 Three‐dimensional T1 images were segmented into white matter (WM), gray matter (GM), deep GM, and cerebrospinal fluid using a probabilistic tissue segmentation approach (geodesic information flows), 24 transformed into an anatomically constrained tractography (ACT) 25 five‐tissue‐type file and rigidly registered to dMRI space. DTI metrics (FA, MD) were extracted using MRTrix3, 26 and NODDI metrics (NDI, ODI, FWF) were extracted using the NODDI toolbox v0.9 in MATLAB. 13
To create seed regions for fiber‐tracking, individual peak fMRI activations during auditory and picture naming (see Section 2.4) were registered into dMRI space, and 100‐voxel seed volumes were created in the WM region most adjacent to the GM fMRI peaks, using Euclidian distance maps. Whole‐brain probabilistic tractography was then performed in MRTrix3, 26 using a minimum curvature radius of 1 mm, a step size of 1.0 mm, and a minimum fiber orientation distribution amplitude of .1, to generate 1000 tracts from the seed region. In line with ACT, 25 tracts were terminated at the cortical GM‐WM interface. Tract maps were then registered to fMRI space and compared across groups using one‐way ANOVA in SPM at a threshold of p < .05, FWE‐corrected. Lastly, tract maps were thresholded at a probability value of .01 to create binary masks, which were averaged across groups to produce commonality maps, which indicate the degree of the spatial variability and overlap of connections. A commonality value of 1 indicates an overlap of 100% of subjects having connections in the respective voxel, and a value of 0 indicates that no subject has connections. 4 , 5 , 27 Bundle‐specific diffusion metrics were extracted for each bundle using these binary masks.
2.7. Correlation of functional and structural connectivity metrics
Correlations of functional connectivity (PPI) activations with dMRI metrics were explored using FA, MD, NDI, ODI, FWF, and tract volume as covariates of interest in multiple regression models in SPM. Task‐specific language LI, IQ, and age were used as nuisance regressors, and interaction analyses were used to assess group differences of correlations. In the absence of group differences, correlations are reported across groups. Correlation analyses were inclusively masked with the main PPI effect, derived from the group activation maps from a previous study, 3 acquired with the same sequences, paradigms, and scanner. In view of our a priori hypothesis and in accord with previous investigations, 2 , 3 correlational activations are shown at a threshold of p < .001 uncorrected.
3. RESULTS
3.1. Demographic data
The three groups did not differ in age (F 2, 68 = 3.17, p = .05), distribution of sex (Fisher exact test: p = .75), or handedness (Fisher exact test: p = .98). The two patient groups did not differ in frequency of focal seizures (Mann–Whitney U = 352.5, p = .68), focal to bilateral tonic–clonic seizures (Mann–Whitney U = 372.0, p = .90), or number of antiseizure medications (ASMs) taken at the time of the study (Mann–Whitney U = 296.0, p = .15; Table 1). There is recent evidence that ASMs may unfavorably (zonisamide, topiramate, carbamazepine) or relatively favorably (lamotrigine, levetiracetam) affect language fMRI activation patterns. 28 , 29 , 30 However, there was no statistically significant difference between left TLE and right TLE in the number of people treated with topiramate (1 left/2 right TLE, p = .60), zonisamide (7 left/6 right TLE, p = .93), carbamazepine (8 left/8 right TLE, p = .80), lamotrigine (10 left/8 right TLE, p = .77), and levetiracetam (11 left/8 right TLE, p = .58) or their respective mean daily doses (topiramate: Mann–Whitney U = 393.0, p = .49; zonisamide: Mann–Whitney U = 366.0, p = .80; carbamazepine: Mann–Whitney U = 381.0, p = .93; lamotrigine: Mann–Whitney U = 371.5, p = .91; levetiracetam: Mann–Whitney U = 341.0, p = .47). Task‐specific language LIs did not differ among left TLE, right TLE, and controls (auditory naming: H 2 = 4.156, p = .13; picture naming: H 2 = .191, p = .91; Table S3) or among the three pathology subtypes in TLE patients (auditory naming: H 2 = 3.297, p = .19; picture naming: H 2 = 1.040, p = .59; Table S3).
3.2. Neuropsychological results
Estimated verbal IQ derived from performance on the NART 16 significantly differed among groups (F 2, 68 = 14.77, p < .001; Table 1). Post hoc analysis (Tukey) indicated that IQ was lower in both patient groups compared to controls (left TLE vs. controls: mean IQ difference = 17.3 points, p < .001; right TLE vs. controls: mean IQ difference = 15.1 points, p < .001), whereas there was no difference between left TLE and right TLE (p = .72). Out‐of‐scanner naming test scores 15 also differed significantly among groups (F 2, 68 = 5.36, p = .007; Table 1); with lower naming scores by a mean of 5.7 in left TLE versus controls (p = .005), whereas there was no difference between left and right TLE (p = .17) or between right TLE and controls (p = .24). In TLE patients, there was no significant difference in naming scores (F 2, 52 = 1.40, p = .26) or IQ (F 2, 52 = .62, p = .55) among the three pathology subtypes.
3.3. Main fMRI effects
During auditory naming, main activations across groups were seen in the left posterior inferior temporal gyrus, left anterior middle temporal gyrus, left inferior and middle frontal gyrus and supplementary motor region, right lingual gyrus, right parahippocampal gyrus, and left cerebellum. During picture naming, main activations across all subjects were seen in the left fusiform gyrus, right posterior inferior temporal gyrus, and middle occipital gyrus, as well as the bilateral inferior occipital gyrus.
Deactivations during auditory and picture naming were observed in the right precuneus, bilateral inferior parietal lobe (angular gyrus/supramarginal gyrus), bilateral middle frontal gyrus, and bilateral anterior superior/middle temporal gyrus (Table S1, Figure 1). There were no significant intergroup differences in activations or deactivations among left TLE, right TLE, and controls, or among the three pathology subtypes in TLE patients.
FIGURE 1.
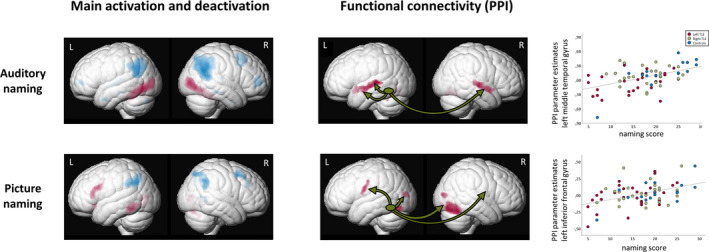
Left column: Main functional magnetic resonance imaging activations (red) and deactivations (blue) across all three groups (left temporal lobe epilepsy [TLE], right TLE, controls) for auditory naming (upper row) and picture naming (lower row). Right column: Functional connectivity (psychophysiological interaction [PPI]) from the left temporal lobe seed region across all three groups for auditory naming and picture naming. Green ellipses represent the left temporal lobe seed region. All activations are shown rendered at p < .05, corrected for multiple comparisons (familywise error). Scatterplots show correlation of PPI parameter estimates extracted from the left anterior middle temporal gyrus (auditory naming; upper row) and left inferior frontal gyrus (picture naming; lower row) with out‐of‐scanner naming scores. L, left; R, right
3.4. Functional connectivity (PPI)
3.4.1. PPI: Auditory naming
Across groups, the left posterior inferior temporal seed region was coupled to the bilateral anterior superior and middle temporal gyrus and the left cerebellum (Table S2, Figure 1), without intergroup differences among left TLE, right TLE, and controls, or among the three pathology subtypes in TLE patients. Stronger connectivity to the left middle temporal gyrus was related to better out‐of‐scanner naming performance (Table S2, Figure 1). Furthermore, later age at onset related to stronger connectivity to the right parahippocampal gyrus, left cerebellum, and bilateral angular gyrus, and patients with shorter disease duration had stronger connectivity to the left thalamus and left angular gyrus (Table S4).
3.4.2. PPI: Picture naming
Across groups, functional coupling was observed from the left fusiform seed to the bilateral precentral gyrus, posterior fusiform gyrus, cerebellum, and middle occipital gyrus as well as the right inferior occipital gyrus (Table S2, Figure 1), without intergroup differences among left TLE, right TLE, and controls, or among the three pathology subtypes in TLE patients. Stronger connectivity to the left inferior frontal gyrus related to better out‐of‐scanner naming performance (Table S2, Figure 1). Patients with a later age at onset had stronger connectivity to the left middle temporal gyrus, left angular gyrus, and left cerebellum. Stronger connectivity to the right fusiform gyrus, left middle temporal gyrus, and bilateral cerebellum was observed with shorter disease duration (Table S4).
3.5. Fiber‐tracking results
Visual assessment of the group commonality maps of structural connections demonstrated consistent connectivity patterns from the seed regions in the left posterior inferior temporal gyrus (auditory naming; Figure 2A,B) and left fusiform gyrus (picture naming; Figure 2A,B). For both naming tasks, connections extended to the left temporal pole, angular gyrus, and middle and inferior occipital gyri, as well as left frontal and prefrontal regions. Less extensive connections were observed to the right precuneus. Group comparisons of the number of streamlines per voxel indicated that whereas there were no differences between left and right TLE, both patient groups had fewer connections to the left anterior temporal lobe (auditory naming seed) and to the left precentral gyrus (picture naming seed) compared to controls (Figure 2C). There were no differences in connections among the three pathology subtypes in TLE patients.
FIGURE 2.
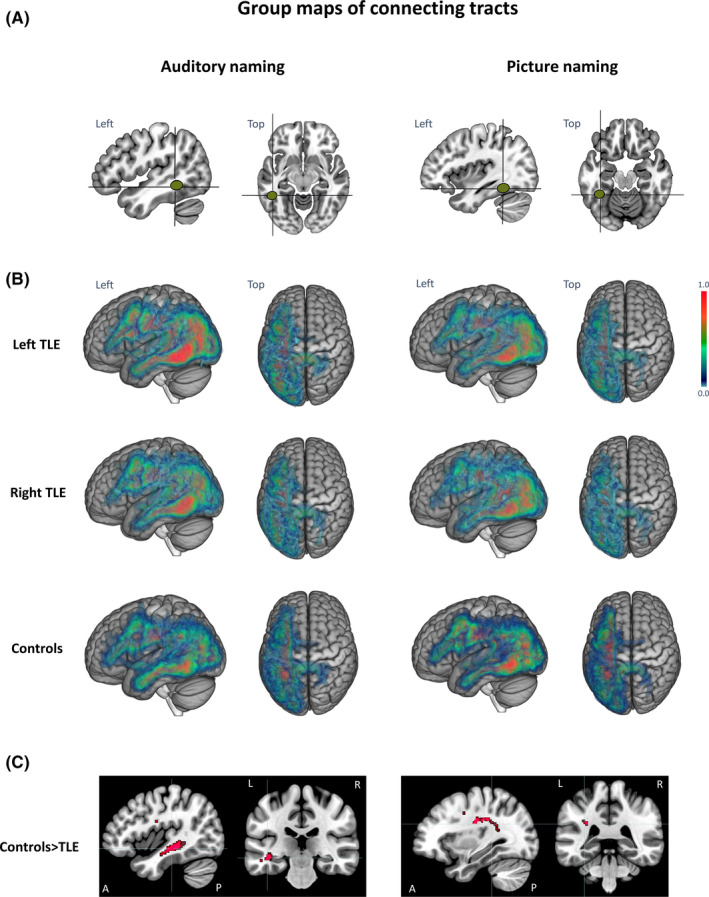
(A) Seed regions for tractography derived from functional magnetic resonance imaging (fMRI) activation maxima during auditory naming (left two columns) and picture naming (right two columns). (B) Group commonality maps (at a probability threshold of .01) of connecting tracts seeding from the fMRI‐guided seeds in the left posterobasal temporal lobe for each of the three groups (left temporal lobe epilepsy [TLE], right TLE, controls) for auditory naming (left two columns) and picture naming (right two columns). Images show left and top view surface renderings with embedded spatial distribution of tracts. The color scale indicates the degree of overlap of tracts among subjects, expressed as a commonality value. A commonality value of 1 indicates an overlap of 100% of subjects having connections in the respective voxel, and a value of 0 indicates that no subject has connections. Groups generally showed similar connection patterns, with consistent connections to the left anterior temporal lobe, left angular gyrus, and occipital cortex, as well as left frontal and prefrontal regions. (C) Group differences of connecting tracts superimposed on sagittal and coronal T1 images (displayed at p < .001 uncorrected for better visualization). Controls had more connections to the left anterior middle temporal gyrus during auditory naming (Z = 4.63, Montreal Neurological Institute [MNI] coordinates: −42 −35 −7, p < .05 familywise error rate [FWE]‐corrected) and to the left precentral gyrus during picture naming (Z = 4.32, MNI coordinates: −32 −23 −30, p < .05 FWE‐corrected) compared to both left TLE and right TLE patients. A, anterior; L, left; P, posterior; R, right
3.6. Relation of functional connectivity (PPI) to dMRI metrics
Regression analyses were performed to investigate the correlation of functional connectivity (PPI) seeding from the left posterior inferior temporal gyrus (auditory naming) or left fusiform gyrus (picture naming) with dMRI metrics (FA, MD, FWF, NDI, ODI) derived from tractography starting from WM seeds adjacent to the PPI seeds. Stronger functional connectivity during both auditory naming and picture naming related to higher ODI and lower FWF. No suprathreshold correlations were observed with FA, MD, NDI, or tract volumes. Further analyses (F‐tests for intergroup differences in correlations and subsequent pairwise comparisons) were therefore restricted to the metrics FWF and ODI. Notably, mean FWF and ODI values did not differ among left TLE, right TLE, and controls, or among the three pathology subtypes in TLE patients (p > .05). There was not a significant correlation of FWF and ODI values with out‐of‐scanner naming tasks (p > .05).
3.6.1. Auditory naming
Across groups, stronger functional connectivity correlated with lower FWF in the bilateral anterior superior temporal gyurs, transverse temporal (Heschl's) gyrus, and bilateral precentral gyrus. Group comparisons indicated group differences in correlations in the left middle temporal gyrus, left cerebellum, and left lingual gyrus. Pairwise comparisons showed that controls had stronger correlations than right TLE and left TLE, whereas there was no difference between left TLE and right TLE (Figure 3, Table 2). Within the group of TLE patients, nonlesional patients had stronger correlations than patients with HS or those with mixed pathologies in the left superior temporal gyrus and left transverse temporal (Heschl's) gyrus (Figure S1, Table S5).
FIGURE 3.
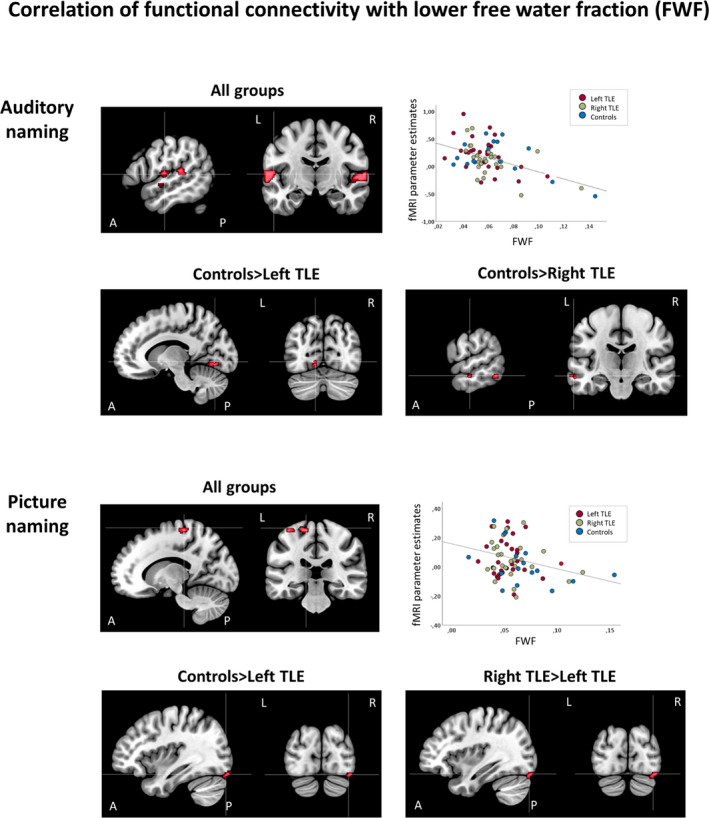
Correlations of functional connectivity of auditory naming (seed region: left inferior temporal gyrus) and picture naming (seed region: left fusiform gyrus) with lower free water fraction (FWF) of underlying white matter tracts. Areas of significant connectivity are shown superimposed on sagittal and coronal T1 images masked for the group effects at p < .001 uncorrected. Top row and second row: Auditory naming. Stronger functional connectivity correlated with lower FWF in the bilateral anterior superior temporal gyrus, transverse temporal (Heschl's) gyrus, and bilateral precentral gyrus in all groups. Scatterplot shows correlation of functional magnetic resonance imaging (fMRI) parameter estimates extracted from the left anterior superior temporal gyrus with mean FWF values. Controls had stronger correlations compared to left temporal lobe epilepsy (TLE; lingual gyrus) and right TLE (left middle temporal gyrus, left cerebellum [not shown on slice], bilateral lingual gyrus [not shown on slice]). Third row and bottom row: Picture naming. All groups had correlations of stronger functional connectivity with lower FWF in the left precentral gyrus. Scatterplot shows correlation of fMRI parameter estimates extracted from the left precentral gyrus with mean FWF values. Controls and right TLE patients had stonger correlations in the right inferior occipital gyrus compared to left TLE. A, anterior; L, left; P, posterior; R, right
TABLE 2.
Montreal Neurological Institute coordinates and Z‐scores of correlations (regression analysis) of cluster‐level functional connectivity (PPI) during auditory naming and picture naming with structural connectivity measures (FWF, ODI), shown at p < .001 uncorrected
| fMRI task | Correlation of functional connectivity (PPI) with structural connectivity measures | ||||||
|---|---|---|---|---|---|---|---|
| Regressor | Group | Region | LH | RH | |||
| Z | Coordinates | Z | Coordinates | ||||
| Auditory naming | FWF | All groups | Sup temp G | 3.64 | −50 −36 8 | 4.20 | 60 −20 8 |
| Heschl G | 4.14 | −62 −8 10 | 4.07 | 60 −8 6 | |||
| Precentral G | 3.18 | −54 −8 6 | 3.27 | 48 −10 36 | |||
| CTR > LTLE | Lingual G | 3.16 | −12 −74 −8 | ||||
| 3.12 | −14 −70 −8 | ||||||
| CTR > RTLE | Lingual G | 3.53 | 6 −72 −2 | ||||
| Cerebellum | 3.42 | −14 −54 −12 | |||||
| Mid temp G | 3.97 | −60 −52 −14 | |||||
| 3.40 | −62 −20 −12 | ||||||
| ODI | All groups | Sup temp G | 3.61 | −52 10 −14 | |||
| CTR > LTLE | Sup temp G | 3.12 | −52 6 −12 | 3.31 | 60 0 −4 | ||
| 3.10 | −52 −2 −10 | ||||||
| Mid temp G | 3.68 | −58 −24 4 | 3.67 | 58 −20 −12 | |||
| CTR > RTLE | Mid temp G | 3.25 | 54 −22 −10 | ||||
| RTLE > LTLE | Precentral G | 3.19 | −56 −6 46 | ||||
| Picture naming | FWF | All groups | Precentral G | 3.27 | −34 −30 66 | ||
| CTR > LTLE | Inf occ G | 3.27 | 38 −86 −18 | ||||
| RTLE > LTLE | Inf occ G | 3.37 | 38 −84 −20 | ||||
| ODI | All groups | Precentral G | 3.45 | −40 −6 34 | |||
| Cerebellum | 3.24 | −48 −62 −32 | |||||
| CTR > LTLE | Lingual G | 4.05 | −34 −88 −16 | 3.16 | 20 −96 −16 | ||
| Cerebellum | 4.25 | −26 −82 −22 | 3.13 | 10 −74 −16 | |||
| RTLE > LTLE | Lingual G | 3.61 | −36 −88 −16 | 3.27 | 10 −98 −14 | ||
| Fusiform G | 3.74 | 42 −72 −20 | |||||
| 3.74 | 18 −94 −18 | ||||||
| Cerebellum | 4.53 | −24 −78 −20 | 3.46 | 16 −78 −18 | |||
Abbreviations: CTR, control; FWF, free water fraction; G, gyrus; Inf, inferior; LH, left hemisphere; LTLE, left temporal lobe epilepsy; Mid, middle; occ, occipital; ODI, orientation dispersion index; PPI, psychophysiological interaction; RH, right hemisphere; RTLE, right temporal lobe epilepsy; Sup, superior; temp, temporal.
In all groups, stronger functional connectivity correlated with higher ODI in the left temporal pole. Correlations differed among groups in the bilateral temporal pole and left precentral gyrus. Pairwise comparisons indicated that correlations in the anterior temporal lobes were stronger in controls than both left TLE and right TLE. Additionally, right TLE had stronger correlations in the left precentral gyrus compared to left TLE (Figure 4, Table 2). Within TLE patients, nonlesional patients had stronger correlations than patients with HS in the left fusiform gyrus (Figure S1, Table S5).
FIGURE 4.
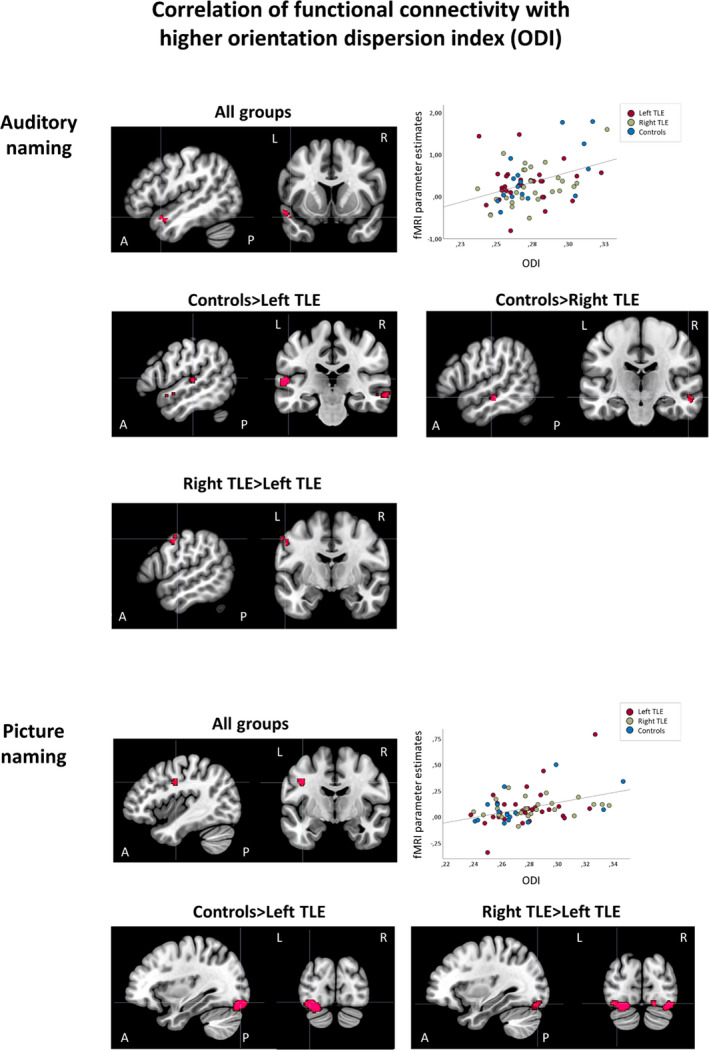
Correlations of functional connectivity of auditory naming (seed region: left inferior temporal gyrus) and picture naming (seed region: left fusiform gyrus) with higher orientation dispersion index (ODI) of underlying white matter tracts. Areas of significant connectivity are shown superimposed on sagittal and coronal T1 images masked for the group effects at p < .001 uncorrected. Top three rows: Auditory naming. Stronger functional connectivity correlated with higher ODI in the left anterior superior temporal gyrus in all groups. Scatterplot shows correlation of functional magnetic resonance imaging (fMRI) parameter estimates extracted from the left anterior superior temporal gyrus with mean ODI values. Controls had stronger correlations compared to both left temporal lobe epilepsy (TLE; bilateral anterior superior and middle temporal gyrus) and right TLE (right anterior superior temporal gyrus). Bottom two rows: Picture naming. Across groups, correlation of stronger functional connectivity with higher ODI was seen in the left precentral gyrus. Scatterplot shows correlation of fMRI parameter estimates extracted from the left precentral gyrus with mean ODI values. Compared to left TLE, stronger correlations were seen in controls (bilateral lingual gyrus, left cerebellum) and right TLE (bilateral lingual gyrus, left cerebellum, right fusiform gyrus). A, anterior; L, left; P, posterior; R, right
3.6.2. Picture naming
Across left TLE, right TLE, and controls, stronger functional connectivity related to lower FWF in the left precentral gyrus. Correlations differed among groups in the right inferior occipital gyrus, and pairwise comparisons showed that both controls and right TLE patients had stonger correlations than left TLE (Figure 3). There were no differences in correlations among pathology subtypes in TLE patients.
Stronger functional connectivity also correlated with higher ODI in the left precentral gyrus and left cerebellum in all groups. Correlations differed among groups in the bilateral lingual gyrus, right fusiform gyrus, and cerebellum. Pairwise comparisons identified stronger correlations in controls versus left TLE and in right TLE versus left TLE (Figure 4, bottom row; Table 2), without differences in controls versus right TLE. Within TLE patients, nonlesional patients had stronger correlations than patients with HS or with mixed pathologies in the left lingual gyrus (Figure S1, Table S5).
4. DISCUSSION
Auditory and picture naming fMRI visualizes functional language networks with a particular focus on posterobasal temporal lobe regions, which are critically involved in naming performance. We report a novel combination of naming fMRI‐derived seed‐based functional connectivity with advanced dMRI metrics to better understand the alterations of language networks in TLE. We demonstrate that weaker functional connectivity during auditory and picture naming is accompanied by decreased neurite orientation dispersion and increased free water fraction of underlying WM connections. Compared to controls, TLE patients showed compromised coupling of functional and structural metrics.
4.1. Naming performance in TLE: fMRI and functional connectivity
Naming and word‐finding difficulties are a frequent and burdensome concern in TLE. Posterobasal temporal regions are strongly involved in clinical naming performance, 3 , 7 which highlights the importance of using fMRI tasks that reliably activate these brain regions in the evaluation of TLE. 2 , 6
We used our previously validated overt auditory and picture naming fMRI tasks with active control conditions. Monitoring task execution demonstrated excellent task performance in all subjects, supporting the interpretation that our findings relate to disease‐specific network alterations rather than intergroup differences in task performance. We show that left posterobasal temporal regions are functionally coupled to the bilateral anterior temporal lobe during auditory naming, and to the bilateral precentral gyrus, contralateral fusiform gyrus, and bilateral occipital cortex during picture‐naming. Stronger connectivity to the left middle temporal gyrus (auditory naming) and to the left inferior frontal gyrus (picture naming) related to better out‐of‐scanner clinical naming performance. In line with previous findings, 3 these connectivity patterns support conceptual models of a widespread left‐lateralized functional language network, particularly in regard to semantic processing. 31 Using conservative statistical thresholds, there were no significant intergroup differences in functional connection patterns.
4.2. Structural language networks: dMRI and relation to functional networks
The use of dMRI to study the structure of the brain and its connections is a fundamental tool in neuroscientific research, and is increasingly applied in clinical settings, including epilepsy surgery planning. 32 Because there is evidence that axon count and density as well as myelination and fiber organization may impact upon diffusion measures, 11 we analyzed both conventional DTI (FA, MD) as well as advanced NODDI 12 , 13 metrics (NDI, ODI, FWF).
We used fMRI‐guided seeds for whole‐brain tractography, and group variability maps of the connection patterns showed consistent connections to the left anterior temporal lobe, frontal and prefrontal regions, and angular and occipital gyri for both auditory naming and picture naming, which were partly impaired in patients compared to controls. Visual inspection of the connection patterns suggested an overlap of our tractography results with well‐defined language tracts such as arcuate fasciculus (AF), inferior longitudinal fasciculus (ILF), middle longitudinal fasciculus, uncinate fasciculus, and inferior fronto‐occipital fasciculus (IFOF). This supports previous findings in TLE patients comparing naming fMRI‐guided to automated tractography, showing high overlap of the resulting tracts with AF, IFOF, and ILF. 33
We demonstrated a spatial overlap of functional and structural language networks. Within these networks, stronger functional connectivity related to higher ODI and lower FWF, suggesting that more efficient functional language networks are associated with distinct features of underlying WM tracts. Higher FA values were previously shown to correlate with stronger left frontal fMRI activation during verb generation, and with stronger left temporal activation during reading comprehension in TLE patients. 4 Our naming fMRI‐derived functional connectivity maps were unrelated to standard DTI metrics (FA and MD), but showed strong and group‐dependent associations with NODDI metrics. This suggests that although DTI measures relate to fMRI activation, they might be less sensitive to detect structural changes that affect functional connectivity, 34 whereas NODDI increases the specificity of microstructural correlates of functional language connections by considering neurite orientation dispersion and density.
Reduced neurite orientation dispersion has previously been found in the affected hippocampus of TLE patients with HS, 35 which may relate to the histopathological features of abnormal dendritic branching in HS tissue. 36 Conversely, increased neurite orientation dispersion has been reported to relate to increased dendritic arborization. 37 Interestingly, neurite density was unrelated to functional connectivity in our cohort. This may suggest that the functional relevance of WM tracts subserving language is more dependent on neurite orientation dispersion/organization than neurite density/volume, although further comprehensive studies are needed to allow for more specific inferences. Reductions in neurite density or dispersion may be accompanied by an increase in FWF as a possible surrogate of tissue atrophy or altered extracellular organization. 38 Our finding of a correlation of stronger functional connectivity with lower FWF parallels the correlation with increased ODI, and further supports the view that functional coupling of language regions is supported by distinct features of critical WM pathways. A recent study in left TLE patients showed an overlap of PPI‐derived functional connectivity maps during a semantic decision fMRI task with structural connectivity to Broca's area through the AF. 39 We demonstrated the association of whole‐brain functional and structural connectivity measures, rather than restricting our analyses to a predefined anatomic tract like AF and provide a more specific assessment of WM microstructure using NODDI metrics.
On the out‐of‐scanner naming tasks, left TLE patients also performed worse than controls, whereas there was no statistically significant difference between right TLE and left TLE. We interpret this as reflecting bilateral temporal lobe involvement in auditory naming and picture naming. This supports the view that the language system is particularly vulnerable to effects of epileptic activity, and neuronal damage resulting from ongoing seizures 40 may contribute to language impairment, 41 along with effects of underlying pathology. In patients without identifiable lesions on MRI, coupling of functional and structural connections within language networks was relatively preserved compared to patients with discrete lesions, especially HS, whereas there was no difference between patients with HS and patients with mixed lesions. This confirms previous reports of most pronounced language network changes in TLE patients with lesions including HS, 42 , 43 emphasizing differences across distinct brain networks between MRI‐negative and lesional TLE. 44
4.3. Strengths, limitations, and future work
This is the first study to combine seed‐based functional connectivity maps with advanced diffusion‐based analyses to delineate the structural basis of the language network and its alterations in TLE. We used overt language tasks that allow controlling for task performance, 6 and applied active control conditions that diminish the activations caused by type of stimulus presentation (auditory/visual) or motor cortex activations. 6 Verbal IQ and language LI were included as nuisance regressors, supporting the view that decoupling of functional and structural language networks in TLE may be independent of intelligence level or atypical language representation.
MRI findings in our cohort were heterogeneous; hence, we performed a subgroup analysis in TLE patients, comparing nonlesional TLE to HS or other discrete lesions. However, detailed effects of certain pathologies (e.g., FCD, low‐grade glioma) could not be assessed, and subgroup analyses in adequate samples should be addressed in future investigations, including detailed effects of medication on fMRI activation. Naming performance was assessed using a visual confrontation naming task, which is widely used in clinical practice. 45 , 46 However, it has been postulated that performance on auditory naming tasks particularly reflects word‐finding difficulties in conversational speech with its multiple neuropsychological demands, 47 which should be addressed in future studies. Longitudinal studies including patients who undergo temporal lobe resection will help to clarify the relation of language network decoupling to the extent of naming decline following epilepsy surgery, with the potential to improve risk stratification and to aid surgical planning. The NODDI method used in this work estimates microstructural parameters through mathematical model fitting procedures using multiple b‐values. Although more complex diffusion‐weighting schemes, such as b‐tensor encoding, 48 allow better specificity to the microstructural organization, NODDI provides an excellent balance between ease of acquisition on routine clinical scanners and microstructural sensitivity. 13
5. CONCLUSIONS
Word‐finding difficulties and impaired naming are a frequent and disabling concern in TLE, particularly in patients with left hemisphere seizure onset. Combining auditory and picture naming fMRI‐derived whole brain functional connectivity seeding from the left posterobasal temporal lobe (PPI) with advanced microstructural tissue properties (NODDI), we demonstrate that TLE patients had impairment of both functional and structural language networks. Impairment of critical WM pathways in TLE may contribute to impaired functional connectivity and language performance.
CONFLICT OF INTEREST
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
We thank Monika Czech for helping with patient recruitment; the radiographers at the Epilepsy Society, Jane Burdett and Andrea Hill; and all our participants and our colleagues for their enthusiastic cooperation. This study was supported by the National Institute for Health Research University College London Hospitals Biomedical Research Centre. We are grateful to the Wolfson Foundation and the Epilepsy Society for supporting the Epilepsy Society MRI scanner; and the European Academy of Neurology and Austrian Society of Neurology, which each supported KT with a 1‐year fellowship. L.C. acknowledges support from a PhD scholarship by Brain Research UK (award 14181). G.P.W. was funded by the Medical Research Council (G0802012, MR/M00841X/1). This work was supported by Epilepsy Research UK (grant number P1904).
Trimmel K, Vos SB, Caciagli L, Xiao F, van Graan LA, Winston GP, et al. Decoupling of functional and structural language networks in temporal lobe epilepsy. Epilepsia. 2021;62:2941–2954. 10.1111/epi.17098
REFERENCES
- 1. Davey D, Thompson P. Interictal language functioning in chronic epilepsy. J Neurolinguistics. 1991;6:381–99. [Google Scholar]
- 2. Trimmel K, Caciagli L, Xiao F, van Graan LA, Koepp MJ, Thompson PJ, et al. Impaired naming performance in temporal lobe epilepsy: language fMRI responses are modulated by disease characteristics. J Neurol. 2020;268:147–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Trimmel K, van Graan AL, Caciagli L, Haag A, Koepp MJ, Thompson PJ, et al. Left temporal lobe language network connectivity in temporal lobe epilepsy. Brain. 2018;141:2406–18. [DOI] [PubMed] [Google Scholar]
- 4. Powell HWR, Parker GJM, Alexander DC, Symms MR, Boulby PA, Wheeler‐Kingshott CAM, et al. Abnormalities of language networks in temporal lobe epilepsy. Neuroimage. 2007;36:209–21. [DOI] [PubMed] [Google Scholar]
- 5. Powell HWR, Parker GJM, Alexander DC, Symms MR, Boulby PA, Wheeler‐Kingshott CAM, et al. Hemispheric asymmetries in language‐related pathways: a combined functional MRI and tractography study. Neuroimage. 2006;32:388–99. [DOI] [PubMed] [Google Scholar]
- 6. Gonzálvez GG, Trimmel K, Haag A, van Graan LA, Koepp MJ, Thompson PJ, et al. Activations in temporal areas using visual and auditory naming stimuli: a language fMRI study in temporal lobe epilepsy. Epilepsy Res. 2016;128:102–12. [DOI] [PubMed] [Google Scholar]
- 7. Trimmel K, Graan LA, Gonzálvez GG, Haag A, Caciagli L, Vos SB, et al. Naming fMRI predicts the effect of temporal lobe resection on language decline. Ann Clin Transl Neurol. 2019;6:2186–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fonseca A‐D, Guedj E, Alario F‐X, Laguitton V, Mundler O, Chauvel P, et al. Brain regions underlying word finding difficulties in temporal lobe epilepsy. Brain. 2009;132:2772–84. [DOI] [PubMed] [Google Scholar]
- 9. McDonald CR, Ahmadi ME, Hagler DJ, Tecoma ES, Iragui VJ, Gharapetian L, et al. Diffusion tensor imaging correlates of memory and language impairments in temporal lobe epilepsy. Neurology. 2008;71:1869–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Leroux E, Delcroix N, Dollfus S. Left fronto‐temporal dysconnectivity within the language network in schizophrenia: an fMRI and DTI study. Psychiatry Res. 2014;223:261–7. [DOI] [PubMed] [Google Scholar]
- 11. Winston GP. The physical and biological basis of quantitative parameters derived from diffusion MRI. Quant Imaging Med Surg. 2012;2:254–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Winston GP, Vos SB, Caldairou B, Hong S‐J, Czech M, Wood TC, et al. Microstructural imaging in temporal lobe epilepsy: diffusion imaging changes relate to reduced neurite density. Neuroimage Clin. 2020;26:102231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang H, Schneider T, Wheeler‐Kingshott CA, Alexander DC. NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage. 2012;61:1000–16. [DOI] [PubMed] [Google Scholar]
- 14. Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. [DOI] [PubMed] [Google Scholar]
- 15. McKenna P, Warrington E. Graded naming test: manual. Windsor, UK: NFER‐Nelson Publishing Co; 1983. [Google Scholar]
- 16. Nelson HE, Wilson J. National adult reading test (NART). Windsor, UK: NFER‐Nelson Publishing Co; 1991. [Google Scholar]
- 17. Friston KJ, Holmes AP, Worsley KJ, Poline J‐P, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1995;2:189–210. [Google Scholar]
- 18. Wilke M, Lidzba K. LI‐tool: a new toolbox to assess lateralization in functional MR‐data. J Neurosci Methods. 2007;163:128–36. [DOI] [PubMed] [Google Scholar]
- 19. Caciagli L, Allen LA, He X, Trimmel K, Vos SB, Centeno M, et al. Thalamus and focal to bilateral seizures: a multiscale cognitive imaging study. Neurology. 2020;95:e2427–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Friston KJ. Functional and effective connectivity in neuroimaging: a synthesis. Hum Brain Mapp. 1994;2:56–78. [Google Scholar]
- 21. Vos SB, Tax CMW, Luijten PR, Ourselin S, Leemans A, Froeling M. The importance of correcting for signal drift in diffusion MRI. Magn Reson Med. 2017;77:285–99. [DOI] [PubMed] [Google Scholar]
- 22. Andersson JLR, Skare S, Ashburner J. How to correct susceptibility distortions in spin‐echo echo‐planar images: application to diffusion tensor imaging. Neuroimage. 2003;20:870–88. [DOI] [PubMed] [Google Scholar]
- 23. Andersson JLR, Sotiropoulos SN. An integrated approach to correction for off‐resonance effects and subject movement in diffusion MR imaging. Neuroimage. 2016;125:1063–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cardoso MJ, Modat M, Wolz R, Melbourne A, Cash D, Rueckert D, et al. Geodesic information flows: spatially‐variant graphs and their application to segmentation and fusion. IEEE Trans Med Imaging. 2015;34:1976–88. [DOI] [PubMed] [Google Scholar]
- 25. Smith RE, Tournier J‐D, Calamante F, Connelly A. Anatomically‐constrained tractography: improved diffusion MRI streamlines tractography through effective use of anatomical information. Neuroimage. 2012;62:1924–38. [DOI] [PubMed] [Google Scholar]
- 26. Tournier J‐D, Smith R, Raffelt D, Tabbara R, Dhollander T, Pietsch M, et al. MRtrix3: a fast, flexible and open software framework for medical image processing and visualisation. Neuroimage. 2019;202:116137. [DOI] [PubMed] [Google Scholar]
- 27. Ciccarelli O, Parker G, Toosy AT, Wheeler‐Kingshott C, Barker GJ, Boulby PA, et al. From diffusion tractography to quantitative white matter tract measures: a reproducibility study. Neuroimage. 2003;18:348–59. [DOI] [PubMed] [Google Scholar]
- 28. Wandschneider B, Burdett J, Townsend L, Hill A, Thompson PJ, Duncan JS, et al. Effect of topiramate and zonisamide on fMRI cognitive networks. Neurology. 2017;88:1165–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xiao F, Caciagli L, Wandschneider B, Sander JW, Sidhu M, Winston G, et al. Effects of carbamazepine and lamotrigine on functional magnetic resonance imaging cognitive networks. Epilepsia. 2018;59:1362–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yasuda CL, Centeno M, Vollmar C, Stretton J, Symms M, Cendes F, et al. The effect of topiramate on cognitive fMRI. Epilepsy Res. 2013;105:250–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Middlebrooks EH, Yagmurlu K, Szaflarski JP, Rahman M, Bozkurt B. A contemporary framework of language processing in the human brain in the context of preoperative and intraoperative language mapping. Neuroradiology. 2017;59:69–87. [DOI] [PubMed] [Google Scholar]
- 32. Winston GP, Daga P, White MJ, Micallef C, Miserocchi A, Mancini L, et al. Preventing visual field deficits from neurosurgery. Neurology. 2014;83:604–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mancini M, Vos SB, Vakharia VN, O'Keeffe AG, Trimmel K, Barkhof F, et al. Automated fiber tract reconstruction for surgery planning: extensive validation in language‐related white matter tracts. Neuroimage Clin. 2019;23:101883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Alexander AL, Hasan KM, Lazar M, Tsuruda JS, Parker DL. Analysis of partial volume effects in diffusion‐tensor MRI. Magn Reson Med. 2001;45:770–80. [DOI] [PubMed] [Google Scholar]
- 35. Sone D, Sato N, Ota M, Maikusa N, Kimura Y, Matsuda H. Abnormal neurite density and orientation dispersion in unilateral temporal lobe epilepsy detected by advanced diffusion imaging. Neuroimage Clin. 2018;20:772–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Thom M. Hippocampal sclerosis in epilepsy: a neuropathology review. Neuropathol Appl Neurobiol. 2014;40:520–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Batalle D, O'Muircheartaigh J, Makropoulos A, Kelly CJ, Dimitrova R, Hughes EJ, et al. Different patterns of cortical maturation before and after 38 weeks gestational age demonstrated by diffusion MRI in vivo. Neuroimage. 2019;185:764–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kamiya K, Hori M, Aoki S. NODDI in clinical research. J Neurosci Methods. 2020;346:108908. [DOI] [PubMed] [Google Scholar]
- 39. Takaya S, Liu H, Greve DN, Tanaka N, Leveroni C, Cole AJ, et al. Altered anterior‐posterior connectivity through the arcuate fasciculus in temporal lobe epilepsy. Hum Brain Mapp. 2016;37:4425–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Caciagli L, Bernasconi A, Wiebe S, Koepp MJ, Bernasconi N, Bernhardt BC. A meta‐analysis on progressive atrophy in intractable temporal lobe epilepsy: time is brain? Neurology. 2017;89:506–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang W‐H, Liou H‐H, Chen C‐C, Chiu M‐J, Chen T‐F, Cheng T‐W, et al. Neuropsychological performance and seizure‐related risk factors in patients with temporal lobe epilepsy: a retrospective cross‐sectional study. Epilepsy Behav. 2011;22:728–34. [DOI] [PubMed] [Google Scholar]
- 42. Hamberger MJ, Seidel WT, Goodman RR, Williams A, Perrine K, Devinsky O, et al. Evidence for cortical reorganization of language in patients with hippocampal sclerosis. Brain J Neurol. 2007;130:2942–50. [DOI] [PubMed] [Google Scholar]
- 43. Foesleitner O, Nenning K‐H, Bartha‐Doering L, Baumgartner C, Pataraia E, Moser D, et al. Lesion‐specific language network alterations in temporal lobe epilepsy. AJNR Am J Neuroradiol. 2020;41:147–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Koubeissi M. Resting connectivity in non‐lesional temporal lobe epilepsy. Epilepsy Curr. 2017;17:219–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Davies KG, Bell BD, Bush AJ, Hermann BP, Dohan FC, Jaap AS. Naming decline after left anterior temporal lobectomy correlates with pathological status of resected hippocampus. Epilepsia. 1998;39:407–19. [DOI] [PubMed] [Google Scholar]
- 46. Hermann BP, Perrine K, Chelune GJ, Barr W, Loring DW, Strauss E, et al. Visual confrontation naming following left anterior temporal lobectomy: a comparison of surgical approaches. Neuropsychology. 1999;13:3–9. [DOI] [PubMed] [Google Scholar]
- 47. Bell BD, Seidenberg M, Hermann BP, Douville K. Visual and auditory naming in patients with left or bilateral temporal lobe epilepsy. Epilepsy Res. 2003;55:29–37. [DOI] [PubMed] [Google Scholar]
- 48. Westin C‐F, Knutsson H, Pasternak O, Szczepankiewicz F, Özarslan E, van Westen D, et al. Q‐space trajectory imaging for multidimensional diffusion MRI of the human brain. Neuroimage. 2016;135:345–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


