Abstract
The role of various therapeutic approaches on the clinical improvement in patients with severe COVID-19 is being researched. Few published studies show positive outcomes after the use of therapeutic plasma exchange (TPE). However, additional clinical evidence is required to understand better the role of therapeutic plasma exchange in severe COVID-19 patients. Thereby, we report a case of a 57-year-old female with laboratory-confirmed COVID-19 who was included in clinical trial NCT04592705. Prompt treatment with TPE facilitated improved clinical-laboratory parameters and speedy recovery and prevented further deterioration of the condition or complications. Successful therapeutic strategies in our case suggest that TPE as a therapeutic option in critically ill COVID-19 patients could prevent the disease from worsening and reduce the need for mechanical ventilation and intensive supportive care in these patients.
Keywords: Therapeutic plasma exchange, COVID-19, Coronavirus
1. Introduction
Hypoxemia, the leading symptom of COVID-19 pneumonia, could progress to acute respiratory distress syndrome (ARDS) in some patients [1]. In addition, fulminant COVID-19 infection is associated with excessive immune dysregulation (“cytokine storm”), inflammation, hypercoagulable state, and endothelial dysfunction [2]. Lymphopenia and high levels of ferritin, C-reactive protein (CRP), lactate dehydrogenase (LDH), D-dimer, and interleukin-6 (IL-6) is also seen in severe COVID-19 [3] (see Table 1, Table 2).
Table 1.
Laboratory findings.
| DOI | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
|---|---|---|---|---|---|---|---|---|---|
| RBC ( × 106/μL) | 3.85 | 4.07 | 4.02 | 4.02 | 4.20 | 4.29 | 3.92 | 3.99 | 3.82 |
| WBC ( × 103/μL) | 4.7 | 4.6 | 2.2 | 2.7 | 6.0 | 11.9 | 14.3 | 18 | 17.5 |
| Platelet ( × 103/μL) | 203 | 217 | 258 | 298 | 367 | 409 | 255 | 438 | 458 |
| Lymphocyte ( × 103/μL) | 0.54 | 0.77 | 0.96 | 0.73 | 1.30 | 1.95 | 2.32 | 1.71 | 2.67 |
| Lymphocytes (%) | 11.6 | 16.8 | 42.9 | 27.2 | 21.6 | 16.4 | 16.2 | 9.5 | 15.2 |
| Neutrophil ( × 103/μL) | 3.99 | 3.67 | 1.14 | 1.75 | 3.92 | 8.49 | 9.97 | 14.47 | 12.34 |
| Neutrophil (%) | 85.9 | 80.0 | 50.9 | 65.3 | 65.0 | 71.6 | 69.6 | 80.3 | 70.5 |
| Creatine kinase (U/L) | 90 | - | - | - | - | - | - | - | - |
| BUN (mmol/L) | 10 | 9 | 8 | 7 | 12 | 21 | 21 | 15 | 17 |
| Serum creatinine (mg/dL) | 0.65 | 0.69 | 0.67 | 0.64 | 0.74 | 0.76 | 0.64 | 0.62 | 0.66 |
| eGFR | 125 | 116 | 121 | 127 | 107 | 104 | 127 | 132 | 123 |
| hs-CRP (mg/dL) | 14.09 | 8.92 | 2.20 | 1.36 | 0.54 | 0.34 | 0.33 | 0.26 | |
| D-dimer (mg/L FEU) | 1.55 | - | 2.33 | 3.310 | 5.470 | 3.480 | 2.360 | 2.580 | 2.030 |
| LDH (U/L) | - | - | 562 | - | 445 | 356 | 320 | 354 | 259 |
| Ferritin (ng/ml) | - | 547.1 | 654.8 | - | 465.1 | 267.6 | 189.9 | 208.0 | 184.4 |
| IL-6 (pg/ml) | - | 27.8 | - | - | - | - | 69.2 | 10.1 | 8.6 |
| VEGF, plasma (pg/ml) | - | 53 | - | - | - | - | 143 | 104 | 245 |
| TNF-α (pg/ml) | - | 1.4 | - | - | - | 0.8 | 1.2 | 0.8 | 1.2 |
| vWF Ag (IU/dL) | - | 184 | - | - | - | 159 | 167 | 192 | 191 |
DOI, Day of Illness; hs-CRP, high sensitivity C reactive protein; BUN, Blood Urea Nitrogen; eGFR, Estimated Glomerular Filtration Rate; IL-6, Interleukin-6; LDH, Lactate dehydrogenase; VEGF, Vascular Endothelial Growth Factor; TNF-α, Tumor Necrosis Factor-α; vWF Ag, von Willebrand Factor antigen.
Table 2.
Ventilatory parameters.
| DOI | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
|---|---|---|---|---|---|---|---|---|---|
| PaO2 (mm Hg) | 67 | - | 63 | - | 56 | - | 79 | - | - |
| FiO2 (%) | 28 | 28 | 40 | 40 | 35 | 35 | 28 | - | - |
| PaO2/FiO2 ratio | 239.2 | - | 157.5 | - | 160 | - | 282.1 | - | - |
DOI, Day of Illness; PaO2, Partial pressure of oxygen; FiO2, Fraction of inspired oxygen.
The role of various therapeutic approaches on the clinical improvement in patients with severe COVID-19 is being researched, and the therapeutic plasma exchange (TPE) for the management of COVID-19 has been shown to be effective in a few published reports [4,5]. However, additional clinical evidence is required to recommend TPE in severe COVID-19 patients on a larger scale.
Thereby, we report a case of laboratory-confirmed severe COVID-19 infection who presented to the Emergency Department (ED) with a 5-day history of fever, sweats, chills, fatigue, shortness of breath, palpitations, and body aches and was administered TPE. This study aims to better understand the efficacy and safety of TPE therapy in severe COVID-19 and review the literature to discuss similar studies.
2. Case report
On March 7th, 2021 (day of illness 5, DOI 5), a 57-year-old Hispanic female patient with a laboratory-confirmed COVID-19 presented to the Emergency Department (ED). She reported a 5-day history of fever, sweats, chills, fatigue, sore throat, congestion and discharge, cough, wheezing, dyspnea, pleuritic chest pain along with palpitations, joint and back pain, muscle ache, anosmia, and ageusia. Before arrival at the ED, she took two doses of Azithromycin (Zithromax®) 250 mg earlier in the morning and inhaled albuterol, but the symptoms continued to worsen, prompting her to visit the ED. She was in close contact with her daughter, who tested positive for COVID-19 around a week ago. The patient was admitted, considering the severity of the symptoms. The clinical course of the patient is demonstrated in Fig. 1.
Fig. 1.
Figure demonstrating the clinical course of the patient according to the day of illness, admission, and discharge.
The patient met the TPE clinical study eligibility criteria immediately upon evaluation and was enrolled in the randomized controlled trial (RCT) NCT04592705. Patients between 18 and 69 years of age with confirmed moderate to severe COVID-19 via nasopharyngeal swab RT-PCR were considered as eligible for the RCT. The investigation was done using the NxStage System One Plasmapheresis machine. Dialysis was provided by ARC Dialysis Center in South Miami, FL.
Exchange of 1.5 plasma volume (PV) was planned. Heparin and Fresh Frozen Plasma (FFP) were used as the anticoagulant and replacement fluid, respectively. FFP was preferred over albumin, as the replacement solution during the exchange as we found more favorable clinical and lab improvement after treatment with FFP as compared to the albumin, during the course of our study. The patients enrolled in the RCT were intended to be treated with 2–3 sessions of TPE, 36–48 hours apart, based on clinical correlation. The patient received three sessions of TPE on DOI 7,9 and 10. Treatment with TPE had to be skipped on DOI8 due to lack of plasma availability. The first and the third TPE sessions were immediately followed by administration of 1 unit of convalescent plasma from a recovered COVID-19 patient. Collectively, the patient received two units of convalescent plasma during her hospital stay.
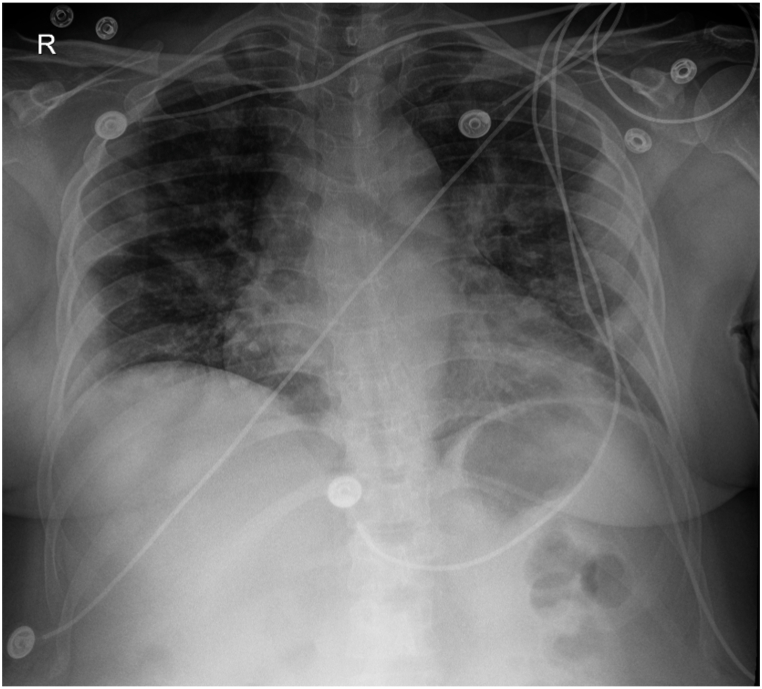
On admission (DOI 5), clinically, the patient appeared to be in mild respiratory distress with a slightly raised respiratory rate of 18/min, although SpO2 was 97% at room air. The auscultation revealed scattered crackles and rhonchi bilaterally and diminished breath sounds. Laboratory reports demonstrated severe lymphopenia (0.54 × 103 μ/L) and hypoxemia with low pO2 (67). She was put on a low-flow nasal cannula (2L). The PaO2/FiO2 ratio of 239.2 confirmed that the patient was in mild ARDS. Chest X-Ray (CXR) findings revealed mixed interstitial and patchy airspace opacities of the bilateral mid to lower lung zones compatible with multifocal, viral, or atypical pneumonia; shown in Fig. 2. The patient was prescribed empirical treatment with Azithromycin 500 mg IV QD, Vancomycin 1g IV, and Ondansetron 4 mg IV in the ED. In addition, Tylenol 650 mg q6h PRN was administered for pain/fever, Vitamin C 1000 mg PO BID, Vitamin D 400 mg PO, Zinc 220 mg PO QD, Melatonin 10 mg PO QD, Quercetin 250 mg PO BID, Z-pack regimens, Acetylsalicylic Acid 81 mg PO QD. The patient received 2L oxygen supplemental therapy via nasal cannula (2L). Methylprednisolone 250 mg IV QD was also initiated along with Enoxaparin sodium 40 mg SC sq QD for deep vein thrombosis (DVT) prophylaxis and Famotidine 40 mg IV QD for adequate GI prophylaxis.
Fig. 2.
CXR AP obtained on 03/07/2021 showing mixed interstitial and patchy airspace opacities of the bilateral mid to lower lung zones compatible with multifocal, viral, or atypical pneumonia.
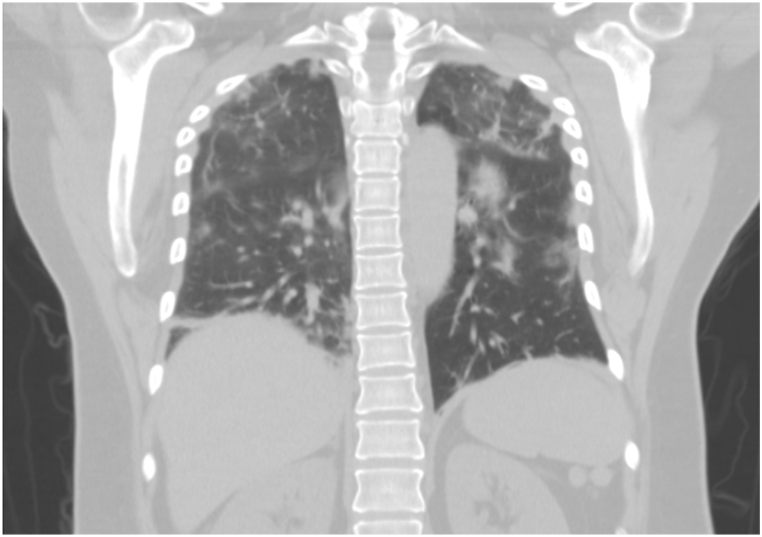
On day 2 of admission (DOI 6), the patient continued to feel short of breath but maintained oxygen saturation at 94% with a low-flow 2L nasal cannula. Pulmonary auscultation revealed crackles on mid-to-lower lung bases. Elevation in the levels of D-Dimer (1.550), CRP (14.09), ferritin (547), and Interleukin-6 (27.6) was noted. The CBC report continued to show lymphocytopenia (0.77 × 103 U/L). A temporary dialysis catheter was placed in the right femoral vein for TPE. An X-ray was done to check the dialysis catheter placement. A bilateral lower extremity venous ultrasound was performed to rule out DVT. Chest CT has been shown in Fig. 3. On the first day, the patient was started on antiviral therapy with a 5-day course of Remdesivir starting with 200 mg IV, followed by 100 mg IV on four consecutive days. In addition to the antiviral therapy, she received a single dose of Tocilizumab IV 400 mg, Ceftriaxone 1g IV QD, and Doxycycline 100 mg IV BID, 10 mg of Dexamethasone Q8h for 24 hours and continued to receive DVT and GI prophylaxis along with the supplemental treatment.
Fig. 3.
Chest CT without contrast obtained on 03/08/2021 showing diffuse bilateral ground-glass pulmonary patchy opacities with diffuse lymphadenopathy.
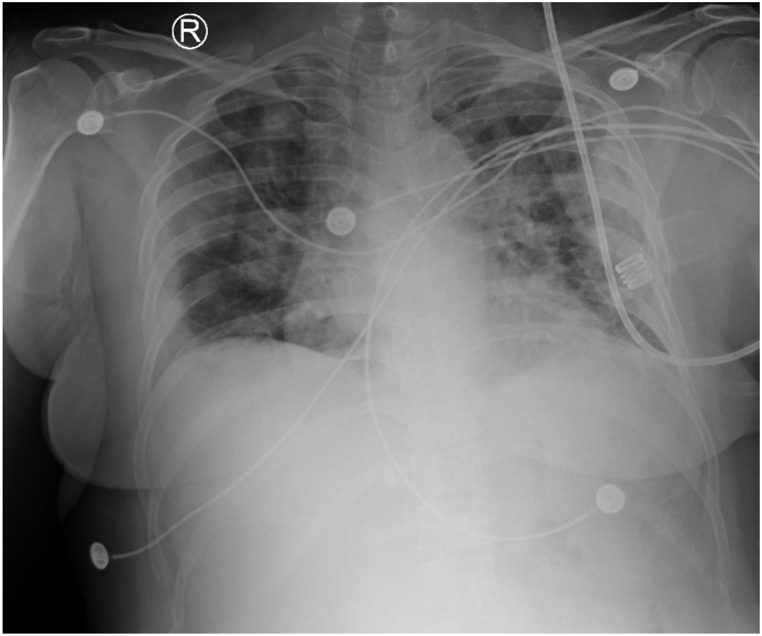
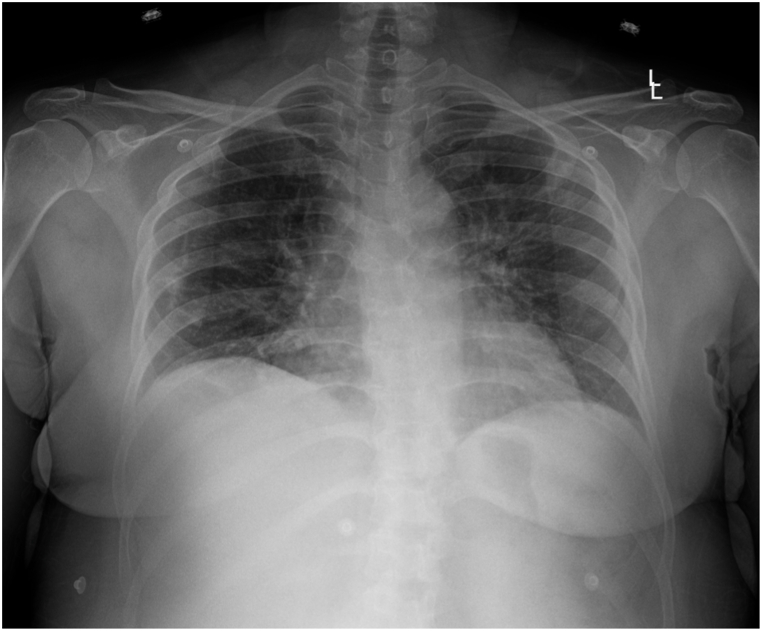
On day 3 of admission (DOI 7), the COVID-19 diagnosis was confirmed based on the RT-PCR reports. The patient's condition deteriorated overnight with a drop in oxygen saturation levels to the 80s. She complained of sudden onset acute dyspnea and palpitations that lasted for about a minute. The respiratory rate was elevated to 21/min at the time of exacerbation. She was immediately placed on supplemental oxygen therapy with the venturi mask with 50% FiO2, which improved her oxygenation to 96%. She could maintain the oxygen saturation of 96% after titrating her to 40% FiO2 with the venturi mask. The patient had progressed to moderate respiratory distress based on her symptoms and with a PaO2/FiO2 of 157 despite the treatment with Dexamethasone and Tocilizumab, although CRP level decreased to 8.92. Lab reports revealed leukocytopenia (2.2 × 103/μL) with low lymphocyte (0.96 × 103/μL) and neutrophil (1.14 × 103/μL) count. In addition, D-dimer and Ferritin levels elevated (1.55–2.23 and 547.1 to 654.8, respectively). The patient was transferred to the ICU for further evaluation; additional CXR was performed upon ICU transfer. CXR has been shown in Fig. 4. Ultrasound-guided catheter placement in the left femoral vein was performed due to right femoral vein catheter malfunction. TPE (1.5 plasma volume/4.5 L) with FFP (17 units) was initiated, followed by 1 unit of convalescent plasma administration. No adverse reactions were noted during and after the procedure. Ultrasound-guided Trialysis catheter placement in the left femoral vein was performed.
Fig. 4.
CXR AP obtained on 03/09/2021 showing interval worsening of lung findings as compared to prior imaging done on the previous day.
On day 4 of admission (DOI 8), the cough was persistent in addition to post-tussive sternal pain. SpO2 was 96% on a Venturi Mask with 40% FiO2. No improvement in blood examination reports was noted, although the CRP level declined to 2.20. D-dimer continued to rise despite administering Enoxaparin sodium 40 mg subcutaneously.
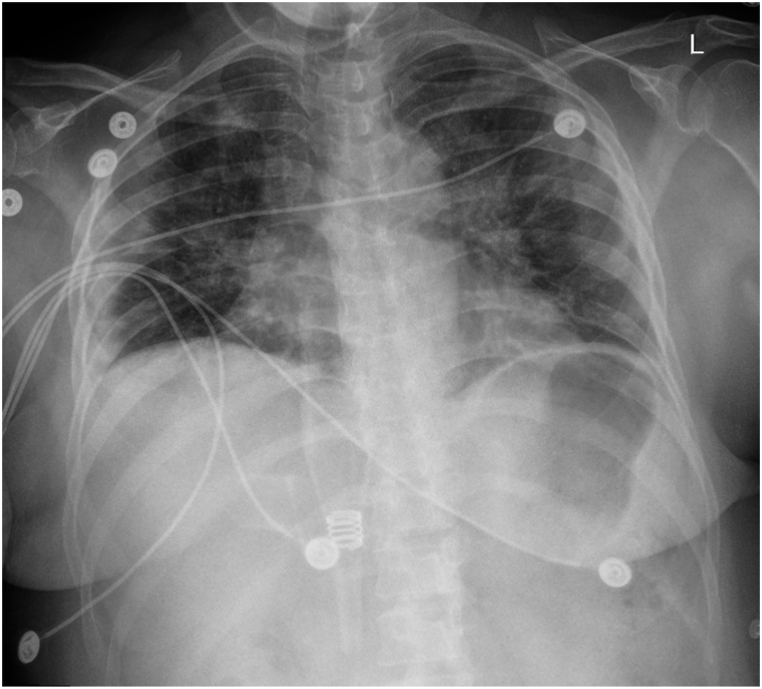
On day 5 of admission (DOI 9), the patient reported mild improvement in cough. Clinically, the patient continued to be in respiratory distress (PaO2/FiO2 ratio of 140, respiratory rate of 20/min). SpO2 was maintained at 96% with the help of a venturi mask with 35% FiO2. However, the patient developed an allergic rash on both the lower extremities which could be attributed to either Remdesivir or TPE (performed on DOI 7). Diphenhydramine was administered, and plasma exchange was held until the resolution of the allergic reaction. Another daily CXR was performed and is demonstrated in Fig. 5. Lab reports showed marked improvement in WBC (6.0 × 10⁹/L), lymphocyte (1.30 × 10⁹/L), and neutrophil count (3.92 × 10⁹/L) as well CRP level (1.36). However, D-dimer levels peaked at 5.47. Based on the patient's clinical status, IV antibiotic therapy with Ceftriaxone 1g IV q24h for seven days and Doxycycline 100mg IV q12h for five days was commenced along with a reduction in the dose of Dexamethasone 6 mg TID. She received TPE (1.5 plasma volume) with 17 units of FFP.
Fig. 5.
CXR AP obtained on 03/11/2021 showing slight radiographic improvement over the right midlung zone.
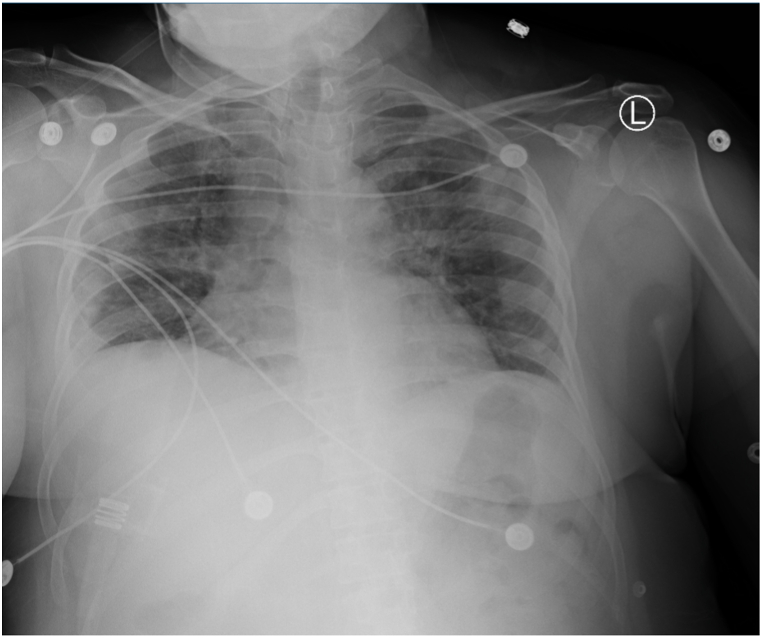
On day 6 of admission (DOI 10), the patient appeared to be responding well to the treatment as we found her with stable vitals. Lab reports revealed steroid-induced neutrophilic leukocytosis along with normal CRP levels (0.54). CXR has been shown in Fig. 6. She received the last dose of Remdesivir, and the 3rd round of TPE (1.5 PV) was replaced with 17 units of FFP and received 1 unit of convalescent plasma immediately after the TPE session. The patient was maintaining oxygen saturation of 95% with the venturi mask at 35% FiO2. Accordingly, she was titrated to a low-flow 2L nasal cannula.
Fig. 6.
CXR AP without contrast obtained on 03/12/2021 showing worsening of multifocal pneumonia along with trace right-sided pleural effusion or consolidation or atelectasis blunting the right costophrenic recess.
On day 7 of admission (DOI 11), PaO2/FiO2 ratio (282.1) tremendously improved, which could be correlated with remarkable clinical improvement. Lab reports revealed a marked elevation in the lymphocyte count (2.32 × 10⁹/L), and downregulation of the inflammatory markers such as ferritin (189.9) and TNF-α (1.2) was noted. Also, the patient could maintain an oxygen saturation of 97% at room air. CXR and CT have been shown in Fig. 7 and Fig. 8.
Fig. 7.
CXR AP without contrast obtained on 03/13/2021 showing moderate levels of improvement in diffuse bilateral ground-glass pulmonary patchy opacities with diffuse lymphadenopathy.
Fig. 8.
Chest CT without contrast obtained on 03/13/2021 showing moderate interval improvement in diffuse degree bilateral ground-glass pulmonary patchy opacities with diffuse lymphadenopathy. Although nonspecific, these findings are commonly reported in patients with COVID-19 pneumonia.
The patient was asymptomatic for two consecutive days and maintained SpO2 of 99% at room air, consistent with the improvement seen in the imaging and lab reports. Also, the nasal swab collected on 03/14/21 (DOI 12) and 03/15/21 (DOI 13) for COVID-19 RT-PCR was negative. Therefore, she was discharged.
3. Discussion
Our patient was a severe case of COVID-19 who presented to the hospital in respiratory distress after five days of symptom onset and was included in the randomized controlled trial NCT04592705. Speedy recovery and prevention of further complications such as hypoxic respiratory failure and multi-organ dysfunction during the course of the disease could possibly be attributed to the prompt initiation of TPE along with on-going medical therapy with antivirals, antibiotics and corticosteroids. This case supports and adds to the pre-existing studies on improvements in severe COVID-19 patients treated with TPE discussed in the next section.
The early phase of COVID-19 is associated with tissue damage owing to viral replication. On the other hand, the late phase dysregulation of immune cellular response leading to cytokine storm [6]. Increasing evidence suggests that patients afflicted with severe COVID-19 have elevated levels of pro-inflammatory cytokines - IL-1β, IL-6, and TNFα [7].
In this case, the patient received three TPE sessions on DOI 7, 9, and 10, and two units of convalescent plasma on DOI 7 and 10 were administered, which improved the patient's condition. In addition, marked improvements were seen on the imaging, PaO2/FiO2 ratio, lymphocyte count, and the inflammatory markers (hs-CRP, LDH, ferritin, TNFα). Moreover, the patient's oxygenation status improved with 97% SpO2 at ambient air. The gradual decrease in D-dimer and normal IL-6 levels was seen two days after completing the treatment with TPE. Prompt initiation of TPE was beneficial in reducing the viral load and mitigating the cytokine storm, which stopped the patient from escalating to acute respiratory failure, thereby preventing mechanical ventilation and reducing the hospital stay. TPE could also be beneficial in severe COVID-19 patients with a higher thrombosis risk [8].
4. Review of literature
Prior studies have demonstrated that one plasma volume plasma exchange removes approximately 66% of the intravascular constituents. TPE is seldom used as monotherapy and it is mainly used in combination with other treatment modalities [[9], [10], [11]]. Also, the exact therapeutic window for administering TPE to severe COVID-19 patients is yet to be determined [6]. The majority of the deaths in COVID-19 patients occur due to ARDS followed by multi-organ failure owing to the cytokine storm.
In a recent study, fourteen confirmed cases of severe COVID-19, COVID-19 patients with ARDS, sepsis, septic shock, requiring invasive mechanical ventilation underwent treatment with one session of TPE followed by transfusion of ABO-compatible convalescent plasma. Following the treatment, all the patients observed decreased levels of inflammatory markers, including LDH, CRP, ferritin, D-dimer, and subsidence of fever [12].
Several studies have shown improved clinical outcomes on administering TPE to critically-ill COVID-19 patients [13,14]. A recently published article reported improvement in the clinical status of a severe COVID-19 patient with ARDS and multiple organ failure, following successful treatment with TPE [15].
In a randomized controlled trial, eighty-seven patients were treated with daily TPE along with empirical treatment (antivirals, antibiotics, steroids, anti-coagulation). Marked improvement in lymphocyte count and inflammatory markers such as IL-6, ADAMTS-13 was noted. Also, significant reduction in the duration of the hospital ICU stay and mortality on the 35th day of ICU admission after receiving sequential TPE were documented. However, the risk of pulmonary embolism was higher in the TPE group than in the control group treated with only an empirical regimen [16]. In another case series, seven critically ill COVID-19 patients were treated with consecutive TPE sessions after unsuccessful attempts of improving oxygenation status and mitigating cytokine storms by administering antivirals and corticosteroids. Treatment with TPE showed improvement in the symptoms and imaging and laboratory reports [17].
In another study, five elderly patients with severe COVID-19 (ARDS, acute kidney injury, and multiple organ failure) were treated with TPE along with multi-drug therapy with antibiotics, antivirals, antifungals, steroids, and immunomodulators. A significant reduction in levels of IL-6, CRP, ferritin, and LDH in addition to improved symptoms after two consecutive sessions of TPE was reported [18].
In a retrospective propensity matched control study, it was demonstrated that the 28 days survival was significantly higher in the TPE group vs. the control group. The median hospitalization in the TPE treated group was significantly lower than the control group (10 vs. 15 days, p < 0.001). Overall survival (OS) was higher in the TPE group in 21 patients without comorbidities than 24 patients with comorbidities demonstrating the association of the use of TPE with overall improved survival [19].
These studies demonstrate the potential benefits of using TPE as a treatment modality in severe COVID-19 patients with ARDS and multi-organ failure. The limitations of our study cannot be overlooked. Although remarkable improvement was mainly seen after receiving the third session of TPE, it is unclear if the clinical improvement was solely due to TPE or attributable to the convalescent plasma and combination of other drugs administered to the patient. It should be noted that, in cohort pilot study [20], ten COVID-19 patients with severe cytokine storm (Penn Class 3 and 4 according to the Penn classification) were treated with five consecutive TPE sessions. A remarkable decrease of the inflammatory markers and improvement in the oxygenation status of the patients immediately after receiving the second session of TPE was seen. Unlike several other studies discussed earlier, the majority of the patients (8 out of 10) in the pilot study did not receive any other drugs apart from TPE, which showcases the influence of TPE in reducing the cytokine storm [20].
5. Conclusions
In conclusion, timely initiation of TPE in severe COVID-19 may prevent the disease from worsening and reduce the requirements for mechanical ventilation and intensive supportive care. Further, extensive studies are mandated to understand better the efficacy of TPE in severe COVID-19.
Declaration of competing interest
The author(s) declare(s) that there is no conflict of interest regarding the publication of this article.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.rmcr.2022.101587.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Osuchowski M.F., Winkler M.S., Skirecki T., et al. The COVID-19 puzzle: deciphering pathophysiology and phenotypes of a new disease entity. Lancet Respir. Med. 2021;9(6):622–642. doi: 10.1016/S2213-2600(21)00218-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khamis F., Al-Zakwani I., Hashmi S al, et al. Therapeutic plasma exchange in adults with severe COVID-19 infection. Int. J. Infect. Dis. 2020;99:214. doi: 10.1016/J.IJID.2020.06.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu X., Han M., Li T., et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc. Natl. Acad. Sci. U.S.A. 2020;117(20):10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi H., Zhou C., He P., et al. Successful treatment with plasma exchange followed by intravenous immunoglobulin in a critically ill patient with COVID-19. Int. J. Antimicrob. Agents. 2020;56(2):105974. doi: 10.1016/j.ijantimicag.2020.105974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang L., Zhai H., Ma S., Chen J., Gao Y. Efficacy of therapeutic plasma exchange in severe COVID-19 patients. Br. J. Haematol. 2020;190(4):e181–e183. doi: 10.1111/bjh.16890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balcells M.E., Rojas L., le Corre N., et al. Early versus deferred anti-SARS-CoV-2 convalescent plasma in patients admitted for COVID-19: a randomized phase II clinical trial. PLoS Med. 2021;18(3) doi: 10.1371/journal.pmed.1003415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gilcher R.O., Smith J.W. In: Rossi's Principles of Transfusion Medicine. fourth ed. Simon T.L., Snyder E.L., Stowell C.P., Strauss R.G., Solheim B.G., Petrides M., editors. Wiley-Blackwell; 2011. Apheresis: principles and technology of hemapheresis; pp. 617–628.https://www.wiley.com/en-us/Rossi%27s+Principles+of+Transfusion+Medicine%2C+4th+Edition-p-9781444358865 [Google Scholar]
- 8.Gucyetmez B., Atalan H.K., Sertdemir I., Cakir U., Telci L., COVID-19 Study Group Therapeutic plasma exchange in patients with COVID-19 pneumonia in intensive care unit: a retrospective study. Crit. Care. 2020;24(1):492. doi: 10.1186/s13054-020-03215-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 10.Keith P.D., Wells A.H., Hodges J., Fast S.H., Adams A., Scott L.K. The therapeutic efficacy of adjunct therapeutic plasma exchange for septic shock with multiple organ failure: a single-center experience. Crit. Care. 2020;24(1):518. doi: 10.1186/s13054-020-03241-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patten E., Berkman E.M. Therapeutic Plasmapheresis and plasma exchange. CRC Crit. Rev. Clin. Lab. Sci. 1986;23(2):147–175. doi: 10.3109/10408368609165798. [DOI] [PubMed] [Google Scholar]
- 12.Jaiswal V., Nasa P., Raouf M., et al. Therapeutic plasma exchange followed by convalescent plasma transfusion in critical COVID-19-An exploratory study. Int. J. Infect. Dis. : IJID : official publication of the International Society for Infectious Diseases. 2021;102:332–334. doi: 10.1016/j.ijid.2020.10.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Altmayer V., Saheb S., Rohaut B., et al. Therapeutic plasma exchange in a critically ill Covid-19 patient. J. Clin. Apher. 2021;36(1):179–182. doi: 10.1002/jca.21830. [DOI] [PubMed] [Google Scholar]
- 14.Lin J.-H., Chen Y.-C., Lu C.-L., Hsu Y.-N., Wang W.-J. Application of plasma exchange in association with higher dose CVVH in Cytokine Storm Complicating COVID-19. J. Formos. Med. Assoc. = Taiwan yi zhi. 2020;119(6):1116–1118. doi: 10.1016/j.jfma.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keith P., Day M., Choe C., et al. vol. 8. SAGE open medical case reports; 2020. (The Successful Use of Therapeutic Plasma Exchange for Severe COVID-19 Acute Respiratory Distress Syndrome with Multiple Organ Failure). 2050313X20933473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Faqihi F., Alharthy A., Abdulaziz S., et al. Therapeutic plasma exchange in patients with life-threatening COVID-19: a randomised controlled clinical trial. Int. J. Antimicrob. Agents. 2021;57(5):106334. doi: 10.1016/j.ijantimicag.2021.106334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adeli S.H., Asghari A., Tabarraii R., et al. Therapeutic plasma exchange as a rescue therapy in patients with coronavirus disease 2019: a case series. Pol. Arch. Intern. Med. 2020;130(5):455–458. doi: 10.20452/pamw.15340. [DOI] [PubMed] [Google Scholar]
- 18.Morath C., Weigand M.A., Zeier M., Speer C., Tiwari-Heckler S., Merle U. Plasma exchange in critically ill COVID-19 patients. Crit. Care. 2020;24(1):481. doi: 10.1186/s13054-020-03171-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamran S.M., Mirza Z.e.H., Naseem A., et al. Therapeutic plasma exchange for coronavirus disease-2019 triggered cytokine release syndrome; A retrospective propensity matched control study. PLoS One. 2021;16(1 January) doi: 10.1371/journal.pone.0244853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gluck W.L., Callahan S.P., Brevetta R.A., et al. Efficacy of therapeutic plasma exchange in the treatment of penn class 3 and 4 cytokine release syndrome complicating COVID-19. Respir. Med. 2020;175:106188. doi: 10.1016/j.rmed.2020.106188. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.