Abstract
In the last century, the emergence of in silico tools has improved the quality of healthcare studies by providing high quality predictions. In the case of COVID-19, these tools have been advantageous for bioinformatics analysis of SARS-CoV-2 structures, studying potential drugs and introducing drug targets, investigating the efficacy of potential natural product components at suppressing COVID-19 infection, designing peptide-mimetic and optimizing their structure to provide a better clinical outcome, and repurposing of the previously known therapeutics. These methods have also helped medical biotechnologists to design various vaccines; such as multi-epitope vaccines using reverse vaccinology and immunoinformatics methods, among which some of them have showed promising results through in vitro, in vivo and clinical trial studies. Moreover, emergence of artificial intelligence and machine learning algorithms have helped to classify the previously known data and use them to provide precise predictions and make plan for future of the pandemic condition. At this contemporary review, by collecting related information from the collected literature on valuable data sources; such as PubMed, Scopus, and Web of Science, we tried to provide a brief outlook regarding the importance of in silico tools in managing different aspects of COVID-19 pandemic infection and how these methods have been helpful to biomedical researchers.
Keywords: In silico, Virtual screening, Immunoinformatics, SARS-CoV-2, Machine learning, Drug design, Vaccine design
1. Introduction
Bioinformatics is an area of biology that focuses on the use of computer-based methods for studying the biological systems, which could provide some precise predictions that might come true in laboratory studies and clinical trials [1]. The emergence of computer-based biological methods has revolutionized the life science studies, and thanks to the bioinformatics methods, a lot of pressure related to the costs of laboratory works and animal sacrifices has been reduced from medical centers. The in silico techniques come useful for categorizing the proteins based on their structure and function, and could be helpful when we need to develop servers for assorting these molecules by using machine learning (ML) methods [[2], [3], [4]].
Moreover, the in silico methods of studying molecular interaction; such as molecular docking could be used for analyzing the potential natural therapeutics ligands and receptor complexes [5,6]. These methods could also provide information regarding the unknown molecular structures, including enzymes and their potential ligands [[7], [8], [9]], which could be important in future genetic engineering studies in different area of biotechnology. These in silico methods could also be used for optimizing the structure of biotherapy agents, like decoy ODNs, that their efficacy has been reported to suppress cancer cells in various malignancies, such as breast and colorectal cancer [10,11]. These advents come really useful for developing modern therapeutics, for example designing the multi-epitope vaccine constructs that are a new type of vaccines, with more benefits than the previous ones [12].
One of the critical conditions in which bioinformatics and in silico methods proved their importance was during the COVID-19 outbreak [13]. The universal crisis of COVID-19 is caused by Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), started at 2019 in Wuhan (China) [14]. Since the beginning of this life threatening crisis, many attempts have been devoted to study the different structures of this virus, providing molecular modeling of the viral structures, and to develop preventive and therapeutic agents against SARS-CoV-2 [15].
Since the beginning of the COVID-19 pandemic, the experts in the field of in silico studies have devoted an enormous contribution to the healthcare providers by classification of COVID-19 related data through ML methods [16], presenting precise predictions regarding the new molecular structures in SARS-CoV-2, investigating the efficacy of potential drugs against different targets in COVID-19 virus [17], developing preventive agents such as vaccines, and analyzing the potential therapeutic efficacy of the natural products and improving the efficiency of the synthesized therapeutics; such as antimicrobial peptides and even designing novel agents, like peptidomimetics, to overcome this universal human life threatening condition [18].
The use of previously designed webservers such as I-TASSER [19] and Phyre2 [20] came really helpful for molecular modeling of protein structures prior to crystallography analysis, virtual screening of potential inhibitors for different viral targets, studying SARS-CoV-2 related molecular interactions via special molecular docking tools [21], dynamics simulations [22], and the immunoinformatics tools such as VaxiJen, T-cell and B-cell epitope prediction tools for investigating the potential target sequences for vaccine development. Moreover, the availability of immune response simulation tools has come helpful for COVID-19 vaccine design. Also, the application of ML algorithms has proved useful to assort the COVID-19 related information and helped to provide timely diagnosis [23] and even designing many useful special servers for future analysis of SARS-CoV-2 [24].
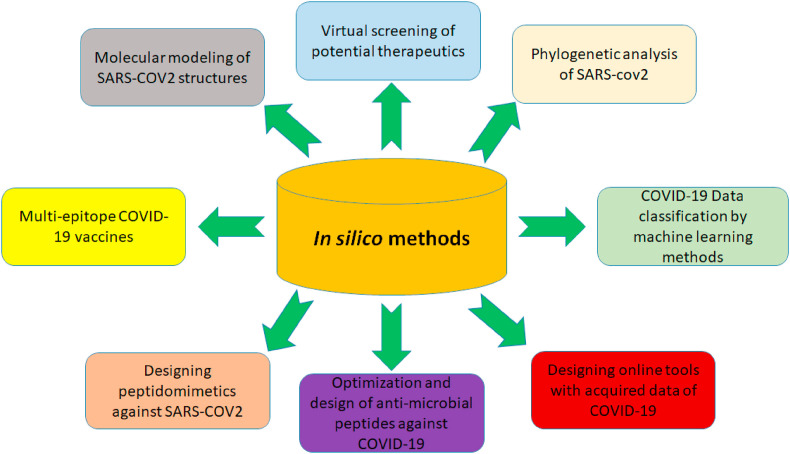
Overall, this review aims to provide a quick glance of how in silico methods have come useful for managing the different aspects of COVID-19 and their potential for application in future crisis conditions. Fig. 1 presents a schematic view of how in silico methods were useful at assisting life science experts to improve the quality of the researches at the time of COVID-19.
Fig. 1.
The critical role of in silico assays in universal battle against COVID-19 and some of their application in medicine and biology.
Through this manuscript, we tried to provide a concise review of the previous literatures of using in silico methods to battle COVID-19. The main topics are dealing with SARS-CoV-2 structure predictions and phylogenetic analysis, drug's virtual screening, natural source-derived chemicals, Anti-microbial peptides and peptidomimetics, SARS-CoV-2 vaccine design, and ML and Artificial Intelligence (AI). The last section of the article offers the potential of using some updated in slico tools to fight COVID-19, and explains how these techniques could be used against the possible future pandemics.
2. Evidence acquisition
At this study, it was aimed to provide a contemporary review of 132 related papers from the acquired published reports via searching the key words such as in silico, bioinformatics, immunoinformatics, SARS-CoV-2, Machine learning, Drug design, Vaccine design, and virtual screening, from valuable data centers including Web of Science, PubMed, and Scopus, Google scholar and the other valid data bases like preprint servers (bioRxiv, medRxiv, arXiv) to find out in what extent the in silico methods have helped researchers to handle this universal issue and to provide a perspective of possible future application of these computer-based methods.
3. Results
This section of the current review is composed of six subsections; including prediction of SARS-CoV2 structures using in silico method, virtual screening of potential drugs against this pathogen, prediction of potential natural compounds to suppress SARS-CoV2, application of anti-microbial peptides and peptidomimetics against COVID-19, designing vaccines, and artificial intelligence (AI) and different ML methods.
3.1. SARS-CoV-2 structure predictions and phylogenetic analysis
At the beginning of COVID-19 pandemic, there was not enough information regarding the structures of SARS-CoV-2 and its relation to the other viruses was not completely clear. At this condition, application of in silico methods came to assist the researchers by enriching their knowledge about this virus via providing valuable predictions such as homology modelings of this virus's structures [25]. Since the process to identify all components of SARS-CoV-2 virus by laboratory methods were time consuming, application of in silico methods helped the computer-aided researchers to be one stay ahead of those who only waited for the results of laboratory studies.
In an early study, Li et al. used in silico approaches like multiple sequence alignment, homology modeling, sequence analysis, virtual screening, reverse mutation, protein structure overlap and surface property analysis. Their study indicated that there is no significant difference in envelope protein, membrane protein, nucleocapsid protein and the key proteases in the open reading frame (ORF) 1 ab [26]. Another study by Baruah et al., used sequence analysis and structure prediction methods about SARS-CoV-2 accessory proteins 9b and ORF14. Their study suggested that there is a close relationship with bat coronavirus [27]. Another study by Vandelli et al., applied genome analysis approach on SARS-CoV-2, which provided a prediction of human interactome. For this aim, they calculated the secondary structure of >2000 coronaviruses and computed >100 000 human protein interactions with this virus [28].
In silico methods have also provided phylogenetic analysis of the SARS-CoV-2, which is an important technique for realizing the relationship of various structures with peer ones in the other viruses. A phylogenetic supertree investigation by Li et al. reported that matrix representation with parsimony (MRP) pseudo-sequence supertree could provide more information regarding the SARS-CoV-2 evolution inference compared to the normal phylogenetic tree analysis based on full-length genomic sequences [29]. Table 1 provides some examples of in silico structural predictions and phylogenetic studies regarding COVID-19.
Table 1.
Examples of in silico structural predictions and phylogenetic studies regarding COVID-19.
| Author and Reference | Aim of study | Results |
|---|---|---|
| Tabibzadeh et al. [30] | Study and tracking SARS-CoV-2 in Iranian COVID-19 sufferers by molecular and phylogenetic methods | Isolates showed to be closely related with Chinese and reference sequences. No considerable differences were detected between Iranian isolates and those of other countries. |
| Zhang et al. [31] | Reanalysis of protein structure and sequence of COVID-19 genome | Suggesting that snakes are the intermediate hosts of SARS-CoV-2 and spike protein insertions share a high similarity with HIV-1 |
| Zhang et al. [32] | Genomic characterization and phylogenetic evolution of SARS-CoV-2 | SARS-CoV-2 is closely related (88% identity) to bat-SARS-like coronavirus |
| Sacco et al. [33] | Developing dual inhibitors against Mpro and cathepsin L | The structure of Mpro with calpain inhibitor II proved that S1 pocket could accommodate a hydrophobic methionine side chain |
| Sakkiah et al. [34] | Using homology modeling to construct a trimeric form of the spike protein complexed with h.ACE2 | Interactions between ACE2 and the tertiary structure of the full-length S protein trimer are different from those of ACE2-truncated monomer of RBD |
3.2. Drug's virtual screening
One of the area in which in silico methods came helpful is virtual screening of potential anti-viral drugs for SARS-CoV-2. These in silico techniques have prevented a considerable time and expenses by removing the less likely effective drugs from considerations. Without application of these in silico methods, one should have tried all of the potential drugs in lab, that could be time consuming and very expensive. In this regard, so many studies have been carried out; such as an investigation by Pundir et al. who used the famous 5-steps rules of Chou for virtual screening of SARS-CoV-2 Mpro inhibitors. In that study, they used molecular docking, toxicity, pharmacophore analysis, and MD simulation which showed that two potent inhibitors of the Mpro (PubChem3408741 and PubChem4167619) act as anti-viral candidates against COVID-19 [35].
Repurposing of a previously known and safe drug against new target could help the researchers to use a previously known agent against a new target and avoid all of the unnecessary researches required for introducing a new drug which also faces a delay for medical application prior to all of the safety studies and approvals from medical resources. In this regard, a recent study by Mahdian et al. reported that application of in silico methods could be helpful for targeting SARS-CoV-2 RdRp enzyme and host cell receptors (ACE2, CD147) [36].
Another in silico study by Marinho et al. evaluated the molecular interactions of various drugs for treatment of COVID-19 (Azithromycin, Baricitinib and Hydroxychloroquine) and drugs with similar structures (Chloroquine, Quinacrine and Ruxolitinib) via molecular docking with SARS-CoV-2 main protease (Mpro) protein. Their study shed light of the fact that all of the inhibitors bind to the same enzyme site, among which domain III of the SARS-CoV-2 main protease was a more specific target [37]. Moreover, a study by Hu et al. claimed that virtual screening for human host cell transmembrane protease serine 2 (TMPRSS2) could provide a potential treatment through in silico step [38].
Application of QSAR modeling for drugs screening is another area that has considerably helped scientists to develop therapeutics, such as the study by Ishula et al. who used QSAR modeling and pharmacoinformatics of SARS coronavirus to investigate the 3C-like protease inhibitors [39]. Table 2 provides five more examples of using in silico methods for COVID-19 drugs screening.
Table 2.
Examples of in silico virtual drugs screening against COVID-19.
| Author and Reference | Molecular target | Potential drugs |
|---|---|---|
| Chen et al. [40] | C-like protease (3CL pro) | Yelpatasvir, and ledipasvir |
| Rahman et al. [41] | Main protease (Mpro) | Simeprevir, Ergotamine, Bromocriptine and Tadalafil, |
| Hosseini et al. [42] | Mpro, PLpro, and RdRp | Antiemetics rolapitant and ondansetron, labetalol and levomefolic acid, leucal and antifungal natamycin |
| Senathilake et al. [43] | Spike Glycoprotein | Dgitoxin, zorubicin and aclarubicin, rolitetracycline, cefoperazone and E−155 |
| Alibakhshi et al. [44] | Envelope (E) and Membrane (M) Proteins | Conivaptan, Ecamsule, Conivaptan, etc. |
3.3. Natural source-derived chemicals
In silico methods provide a platform for screening the activity of potential therapeutics against the molecular targets, which helps to select the ones with the highest potential activity for further in vitro and in vivo experiments. Focusing on only selected targets will reduce the cost for laboratory trial that requires financial and human resources [45]. One of the fields in which in silico methods have proved to be useful is investigating the efficacy of natural products-derived compounds against COVID-19 [46]. Regarding this area of biomedicine, in silico assays have come very helpful by providing simple and effective assays, such as molecular docking. From this perspective, a novel study by Xu et al., investigated the efficiency of flavonoid inhibitors against COVID-19 3CL protein by screening 2030 natural compounds via six ML algorithms. Their study indicated that compound Rutin presents the most satisfactory results compared to the other candidates [47].
Another study by Majumder et al. used molecular docking and dynamics assay to screen plan-based natural compounds against COVID-19. Their study showed that Peonidin 3-O-glucoside, Kaempferol 3-O-β–rutinoside, 4-(3,4-Dihydroxyphenyl)-7-methoxy-5-[(6-O-β-d-xylopyranosyl-β-d-glucopyranosyl)oxy]-2H-1-benzopyran-2-one, Quercetin-3-D-xyloside, and Quercetin 3-O-α-l-arabinopyranoside present high molecular docking scores, along with providing high stability and flexibility, therefore they could be regarded as suitable candidates for future COVID-19 studies [48]. A more recent study by Moradi et al. investigated the activity of plant-derived protease inhibitors to suppress the activity of Papain-like protease of SARS-CoV-2. That study was performed by using molecular docking for selecting the potential agents for suppressing the target enzyme and the results were confirmed through molecular dynamics assay which indicated that VcTI from Veronica hederifolia provides a suppressive activity against both Zn-site and the classic active site of this enzyme [49]. Table 3 presents some of the other studies that used in silico approaches for analysis of natural compounds against COVID-19.
Table 3.
Examples of studies that used in silico approaches against COVID-19.
| Author and Reference | Source of compound | Results |
|---|---|---|
| Nouadi et al. [50] | Moroccan Plants | Taxol, Rutin, Genkwanine, and Luteolin-glucoside showed to a high affinity with ACE2 and 3CLpro |
| Nikunj et al. [51] | Red Algae | n-Decanoic acid and 9-dodecenoic acid, methyl ester,(E) showed 81.90% and 81.81% affinity on RBD, respectively |
| Joseph et al. [52] | Green tea and Spirulina extracts | Blocking the cell entry of SARS-CoV-2 |
| Marwal et al. [53] | Piperine (Black Pepper), Eugenol (Clove), Alliin (Garlic), Gingerol (Ginger) and Curcumin (Turmeric | All compounds showed good docking scores with their respective receptor, ranging from −8.195 to −5.263 via DockThor |
| Beirami et al. [54] | 6570 molecules from different herbal plants | Sodwanone B, Cyclomulberrin, and a glycosylated derivative of kaempferol were chosen for future studies based on their docking score |
3.4. Anti-microbial peptides and peptidomimetics
Anti-microbial peptides (AMPs) are by nature a part from the innate immune response of various organisms that provide immunity toward a wide variety of infectious agents; such as viruses, bacteria and fungi. These agents prevent the infection via different patterns, such as membrane disruption or physical blocking of the molecular receptors. In the case of COVI-19, one of the initial therapeutics candidates were these peptides that provide a precise viral life cycle inhibition and the virus could not easily develop resistance toward these agents [55,56]. Application of the natural existing AMPs without any information regarding their influence on a specific target requires to use all of these components, hoping that maybe one provides a good response against a target, but application of in silico methods not only helps to screen these components prior to laboratory application, but also provides a platform to make desired modifications in their construct.
Considering the therapeutics limitation for remedy of COVID-19 sufferers, application of AMPs, anti-viral peptides (AVPs), and the other peptide-like compounds such as peptidomimetics, that are more tolerant toward digestion, are justified to battle SARS-CoV2 pandemic conditions [57]. So far, many different AMPs have been used against COVID-19, and the future studies on some of these natural peptides; such as Lactoferrin (LF) has been recommended [58].
In silico studies have been helpful in respect of repurposing and designing and optimizing of the AMPs. Another example is a study by Ahmadi et al. has reported the efficacy of Enfuvirtide (an effective inhibitor against HIV-1) to be useful against SARS-CoV-2 [59]. Another in silico study by Al-Rabia the repurposing of sitagliptin-melittin optimized nanoformula against SARS-CoV-2 via anti-viral screening and molecular docking assays [60].
In the case of designing peptidomimetics, a study by Alagumuthu et al. reported the structure-based design of novel peptidomimetics for targeting spike protein of SARS-CoV-2 which were bound at the ACE2 binding site of the receptor-binding domain (RBD), effectively [61]. Table 4 shows more examples of in silico studies at developing peptide-based therapeutics against COVID-19.
Table 4.
Some in silico studies for developing peptide-based therapeutics against SARS-CoV2.
| Authors and Reference | Name of peptide | Viral target | Results |
|---|---|---|---|
| Ling et al. [62] | HR1-P and HR2-P | Spike | Binding energy of HR2-based antiviral peptide to HR1 was−43.0 kcal/mol, stronger than the natural fusion compound |
| Baig et al. [63] | Two novel 23aa and 18aa peptides | Spike | 18aa peptide showed a stable and effective blocking of SARS-CoV-2 cell entry |
| Barh et al. [64] | cnCoVP-3, cnCoVP-4, | Spike | Optimal blockade of the Spike RBD and hACE2 interaction which potentially leads to preventing the cell entry |
| and cnCoVP-7 chimeric peptides | |||
| Balmeh et al. [65] | glycocin F from Lactococcus lactis and lactococcine G from Lactobacillus plantarum | Spike, RdRp, 3CL, and N protein | Efficient structural suppression of the viral structures by peptides derived from probiotic bacteria |
| Mohammadi et al. [66] | Pacific oyster Antiviral Polypeptides | Main Protease | HIV–1PIP-1 (Leu-Leu-Glu-Tyr-Ser-Leu) polypeptide could be a potential inhibitory compound for Mpro |
3.5. SARS-CoV-2 vaccine design
Designing an inclusive vaccine that could cover more subtypes of SARS-CoV-2 is a serious issue at the time of this universal crisis, when we see new subtypes are coming from each corner of the world [67]. In the past, vaccine design studies were only based on laboratory and dead pathogens or weakened ones were injected to the subjects, that was along with a life treating hazards such as acute immune response and being time consuming and expensive were among the disadvantages of those vaccines. Although, the previous methods of vaccine design; such as virus inactivations are still used and present some good results, the modern generation of vaccines such as multi-epitope ones, DNA, and RNA vaccines have emerged that show promising results.
Application of in silico methods help the scientists to present a more potent vaccine with adjusted components that does not pose hazards such as those of earlier ones. In silico approaches have shown to be useful for designing various multi-epitope vaccines for viruses such as influenza A [12]. In this regard, application of reverse vaccinology (RV) which is a common approach for identifying potential vaccine constructs via screening of the proteome of the target pathogen by in silico analysis [68], has been the subject of many SARS-CoV-2 vaccine studies. For example, a study by Qamar et al. used the reverse vaccinology technique to design a multiepitope-based subunit vaccine (MESV) against SARS-CoV-2. They investigated the presence of the epitopes suitable for potential vaccine candidate design using AllerTOP and VaxiJen to selected their epitopes, and made a construct that could provide B-cell and T-cell esponse.
There are many online in silico tools that come helpful for designing multi-epitope peptide vaccines; such as IEDB MHC-I prediction tool (http://tools.immuneepitope.org/analyze/html/mhc_binding.html) [69], IEDB MHC-II prediction online server (http://tools.immuneepitope.org/mhcii) [70], VaxiJen RV tool (http://ww.ddg-pharmfac.net/vaxijen/VaxiJen/VaxiJen.html) [71], ElliPro (http://crdd.osdd.net/raghava/bcepred) [72], and IEDB population coverage tool (http://tools.iedb.org/population) [73] that are used or screening the potential epitopes from protein sequences.
For predicting the antigenicity, there are various program such as Predicting Antigenic Peptides (http://imed.med.ucm.es/Tools/antigenic.Pl) [74], and AlgPred (http://crdd.osdd.net/ragha va/algpred/) [75]. In the case of toxicity, ToxinPred (http://crdd.osdd.net/raghava/toxinpred/) [76] is one of the common tools, and C-IMMSIM tool was helpful for simulating the immune responses (http://www.cbs.dtu.dk/services/C-ImmSim-10.1/) [77].
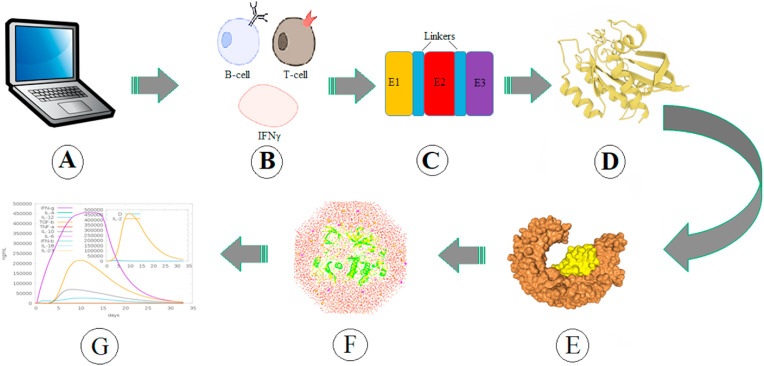
Fig. 2 presents a simple schematic workflow of the multi-epitope vaccine design, and one should consider that there are always new steps could be added to improve the novelty of study and increase the potential efficacy of the designed vaccine. In the case of COVID-19, attempts for designing multi-epitope peptide vaccines were initiated at the beginning of the crisis, and since then, many studies have been performed regarding such a vaccine design [78].
Fig. 2.
Schematic workflow of in silico designing a multi-epitope vaccine. A. Collecting the required information regarding the potential vaccine target structures B. Predicting the potential T-cell and B-cell and IFN-γ epitopes in the structure of the molecular target, such as spike protein C. Joining the selected epitopes, and peptide adjuvants by using suitable linkers D. structure prediction and analyzing the modeled multi-epitope peptide E. Molecular docking with potential immune receptors, such as TLRs F. Molecular dynamics simulation to investigate the stability of the complex G.In silico simulation of immune response toward the designed multi-epitope construct.
A study by Pourseif et al. reported designing of two domain-based vaccine constructs against SARS-CoV-2 on the basis of two different vaccine production and delivery systems; including an adjuvanted domain-based protein vaccine construct (DPVC), and a self-amplifying mRNA vaccine (SAMV) by using the target of spike glycoprotein, which require future in vivo and in vitro investigations [79]. Another study by Kar et al. also designed a multi-epitope vaccine candidate based on the structure of spike glycoprotein of SARS-CoV-2 and verified the quality of the designed vaccine candidate based on in silico assays; such as molecular docking, molecular dynamics (using the steepest descent algorithm of GROMACS) and in silico immune response simulation. They further optimized the vaccine candidate structure for expression by E. coli expression system [80].
Moreover, application of ML and deep learning algorithms has also come useful for multi-epitope vaccine design against SARS-CoV-2. In this regard, a study by Yang et al. used an in silico deep learning method to predict and design of a multi-epitope vaccine candidate (DeepVacPred). Their study predicted 26 potential vaccine subunits from the available SARS-CoV-2 spike protein sequence, which could be used in a multi-epitope vaccine. They used the best 11 of the epitopes (694 amino acids) to construct a multi-epitope peptide as a potential vaccine candidate for SARS-CoV-2 virus [81]. Another study by Behmard et al., provided a multi epitope vaccine constructed by epitopes from whole set of viral structural proteins which proved its safety and efficacy at in vivo level, too [82]. It also has been proven that application of reverse vaccinology could provide a better outcome, when it is accompanied with ML method, such as VaxiJen-ML application by Ong et al. [83]. Table 5 presents more examples of in silico designing of multi-epitope SARS-CoV-2 vaccine.
Table 5.
In silico studies of vaccine design against COVID-19.
| Authors and Reference | Viral antigens | Results of study |
|---|---|---|
| Enayatkhani et al. [84] | Nucleocapsid, ORF3a, and Membrane protein | Designed chimeric protein showed to elicit humoral and cell-mediated immune responses |
| Dong et al. [85] | ORF7a protein, ORF8 protein, nsp9, nsp6, nsp3, endoRNAse, ORF3a protein, membrane glycoprotein, and nucleocapsid phosphoprotein | In silico assays validated the efficacy of the designed multi-epitope vaccine |
| Rahman et al. [86] | S, M, and E proteins | Vaccine candidate showed a significant potential in silico |
| Arshad Dar et al. [87] | Spike | Proposed multiepitope vaccine could provide protective immunity against COVID-19 |
| Kumar et al. [88] | Spike | Eliciting a strong immune response for vaccine in silico |
Another type of vaccines that were provided by the help of in silico methods are nucleic acid base vaccines. In this regard, designing a SARS-CoV-2 spike-based DNA vaccine by Alamri et al. could be noted, who used these computational methods for designing the codon-optimized synthetic consensus S protein [89]. A similar study by Prompetchara et al. reported a DNA vaccine that showed strong humoral and T helper type 1 (Th1) cell-mediated immune responses in mice [90]. Regarding the potential of RNA vaccines against COVID-19 and how in silico methods could be helpful, the readers are suggested to refer the study by Borah et al. [91]. Moreover, the in silico methods were also have been used for designing an RNA-Peptide fusion vaccine candidate against COVID-19 [92].
3.6. Machine learning (ML) and artificial intelligence (AI)
ML is a novel technology that has been provided by advancements of in silico approaches. ML methods are useful for classification of data and help to provide an accurate prediction of the unknown queries using different algorithms [2]. These methods could be useful for identifying patients at high risk, analyzing the death rate, and the other abnormalities. These technologies use different algorithms, which could help to learn more about the nature of this virus. Moreover, ML also could help to analyze the risk factors as per age, social habits, location, and climate [93]. Some of ML methods such as Support Vector Machine (SVM), Convolutional Neural Network (CNN), and Generative Adversarial Network (GAN), are among the broadly used ones for diagnosis of SARS-CoV-2. AI methods have helped to provide high quality studies on drug repurposing, analysis of dissemination patterns, and clinical diagnosis this virus [94].
Regarding COVID-19 pandemic condition, AI methods have showed efficiency to predict specific properties, potential risks, and results of a pandemic condition of COVID-19. Application of deep learning has provided opportunities to detect various aspect of this pandemic situation. In this regard, the use of deep learning and reinforcement learning showed useful to predict some of the COVID-19 related problems [95].
Due to the advances in the field of AI and emergence of valuable in silico assays, it is possible to provide an accurate diagnosis model of COVID-19 based on patient symptoms and routine test results via application of ML and reanalysis of collected COVID-19 related data, such the study by Li et al. who trained an XGBoost model to provide a sensitivity of 92.5% and a specificity of 97.9% for discrimination of COVID-19 patients from influenza patients [96].
Various ML algorithms; such as SVM, RF, Covariance Discrimination (CD) and Optimized Evidence-Theoretic K-nearest Neighbor (OET-KNN) have shown to be useful for assorting the data provided by pseudo amino acid composition, which is really helpful to provide high accuracy predictions about the protein structures of different enzymes [2,3], and such predictions are very important for the designing online tools for future analysis of similar proteins [21].
One of the most critical points while using modern ML algorithms is to define the right question about how these algorithms could be helpful to provide an optimum clinical outcome. An issue that must be considered in this regard is acting without expert clinical oversight. The wrong used AI research could end up in solutions' looking for problems: a form of supply that tries to find a demand, instead of the pre-thought application [97].
A previous meta-analysis by Khalid Raza demonstrated that state-of-the-art AI applications against COVID-19 could be useful at four major areas of applications; including diagnosis and prediction of infection, epidemiology of viral infection namely viral forecasting, control, and spread dynamics, molecular studies such as categorizing the viral structures, designing drugs and remedy for sufferers and social aspects such as commerce, business, governance, and education and training [98]. Table 6 presents a list of highlighted ML studies for battling COVID-19.
Table 6.
Some of the highlighted studies that used ML.
| Authors and year | Aim of study | The algorithms used | The outcome of study |
|---|---|---|---|
| Ong et al. [83] | COVID-19 Vaccine Design | Five supervised classification algorithms, including logistic regression, SVM, k-nearest neighbor, RF, and extreme gradient boosting (XGB) | The designed construct showed to trigger immune response, and the Sp/Nsp cocktail vaccine could stimulate effective complementary immune responses |
| Magar et al. [99] | Discovering Potential neutralizing antibodies | XGBoost, Random Forest, Multilayer perceptron, SVM, and Logistic Regression | Screening of thousands antibody sequences and finding nine stable ones that potentially inhibit SARS-CoV-2 |
| Khalifa et al. [100] | Classification of potential coronavirus treatments on a single human cell | SVM, decision trees, ensemble and DCNN (Deep convolutional neural networks) | DCNN provided 98.05% testing accuracy, and was more effective than classical ML methods |
| Wu et al. [101] | Rapid and accurate identification of COVID-19 infection based on clinical available blood test results | RF | Developing a tool for preliminary assessment of suspected patients and help them to get timely treatment and quarantine suggestion |
| Mohapatra et al. [102] | Predicting the efficacy of commercially available drugs against COVID-19 | Naive Bayes | Amprenavir (DrugBank ID–DB00701) could probably be an effective drug for COVID-19 |
4. Future perspective
Since the beginning of COVID-19, experts in the field of bioinformatics have been dedicated to find a solution for SARS-CoV-2 pandemics and have made a considerable contribution by reducing the workload of laboratorians, diminishing the costs and preventing the unnecessary animal sacrifices. In this regard, application of drug screening tools such as QSAR, molecular docking methods, molecular dynamics simulation tools; such as GROMACS came helpful to discriminate the potential drug candidates [103,104].
In the case of COVID-19, many innovations at the field of bioinformatics came out; such as the study by Russo et al. who used Universal Immune System Simulator (UISS) in silico platform for trial investigation of the COVID-19 candidate vaccines, and such a platform showed to be an effective way for discovery pipeline of vaccine against SARS-CoV-2 [105]. These innovative in silico platform applications could come very helpful in the face of possible pandemics in future and will assist to provide a timely, and cost effective therapeutics design.
Some of the bioinformatics assays could be really useful for prediction of the possible dangers that are invisible to the naked eyes, for example to investigate the potential long term effects of viruses on the nervous system due to the prion-like domains in the viruses that show their effect years after infection. In this regard, bioinformatics methods could be applied for analysis of these structures [106]. Regarding these concerns, there are some reports that predict the potential presence of these structures at the spike protein of SARS-CoV-2 at the site of interaction with human ACE2, which might be a reason for higher virulence of this pathogen, and be a sign of caution for those who study in this field for drug and vaccine design against COVID-19 [107]. Another study by Mohabatkar et al. reported the prediction of a potential prion-like domain in SARS-CoV2 polyprotein at the nonstructural protein 3 (Nsp3) site, by using in silico tools [108].
Considering the importance of early patient's diagnosis, which helps to the better outcome for provided therapy, ML methods have been a blessing to the medical experts. In this regard, modified Manta-Ray Foraging Optimization used by Elaziz et al. showed to be effective for image-based diagnosis of COVID-19 [109]. Moreover, the model provided by Goodman-Meza et al. showed to provide sensitivity of 0.93 (95% CI 0.85–0.98), and specificity of 0.64 (95% CI 0.58–0.69) for inpatient diagnosis [110]. Furthermore, emergence of neural networking showed promising results regarding COVID-19, such as the study by Hartono who used pairwise predictions and similarity maps for analyzing the transmission dynamics of COVID-19 [111].
In silico tools have also been used for designing and optimization of the molecular constructs that could be used for early diagnosis of the COVID-19 viruses. An example in this regard is the in silico study of designing quadruplex aptamers against the spike protein of SARS-CoV-2 [112]. These agents are really useful for molecular detection of SARS-CoV-2, and could be used in immediate responding tools such as biosensors. Regarding the importance of designing aptamers, its necessary to note that ML tools, and genetic algorithms could come helpful to discriminate the optimum structures [113].
In silico methods could be used for diagnosis of possible other viral epidemics, since they have shown their effectiveness in the case COVID-19 [114]. In this regard, many studies have emphasized the importance of these tools; such as the in silico detection of SARS-CoV-2 specific B-cell epitopes by Phan et al. [115] or in silico discovery of antigenic proteins and epitopes COVID-19 for diagnostic purpose by Can et al. [116]. These computational methods have also aided researchers to present diagnostic devices such as nanocarbon biosensors for accelerated diagnosis of COVID-19 [117], and still there are much more potential for developing other tools by the use of bioinformatics.
Application of computer methods have also come very useful in managing other aspects of COVID-19 crisis, for example helping the healthcare providers to design and develop web‐based registry systems for suffers, which help to better handle the condition and plan for patients [118]. In silico methods have also been used for programming the diet for COVID-19 patients, and to provide an optimum diet, which could act as both preventive and even co-therapeutics agents. Considering this issue, a study by Matteo et al. has provided useful insights regarding the importance of in silico methods for programing of foods that help individuals for battling COVID-19 pandemic condition [119].
These ML methods have also shown to be useful for psychological distress during COVID-19, which is an important area of managing the health of people [120]. Also, there is a high potential for application of these in silico methods for personalized medicine in the case of COVID-19, which could be an area of study for future researches, that could revolutionize the healthcare system [121]. Regarding the importance of such an issue, a study by Voutouri et al. reported a comprehensive mathematical model that considers factors such as innate and adaptive immune responses, rates of viral replication, and inflammatory cytokines. This model indicated divergent treatment responses and clinical outcomes by accounting the dynamics of COVID-19 phenotypes, which helps to improve the clinical management and provide a framework to understand trajectories for individual patients [122].
Moreover, application of ML has proved its efficiency to predict the severity of disease and its outcome in COVID-19 suffers [123], which could be used against the future possible tragic crisis similar to that of SARS-CoV-2 or perhaps controlling the new strains of COVID-19 that are being reported from different corners of the world due to mutations [124,125]. Such predictions about these health crisis's could be really helpful, especially when it comes to planning for the health of these sufferers by governments [126].
Another area in which the in silico methods have shown their efficiency against COVID-19 was single-cell transcriptomics. These assays investigate the levels of gene expression at individual cells in a specified population via simultaneous measuring of the messenger RNA (mRNA) concentration of many genes. The discovery of heterogenous cell populations, reconstruction of cellular developmental trajectories, and transcriptional dynamics modeling are possible via studying these data [[127], [128], [129]]. Regarding COVID-19 condition, so many studies were performed in this area, such as the study by Wang et al. who investigated the single-cell transcriptomic of COVID-19 patients’ lungs, which provided an extensive cellular and molecular atlas that could facilitate the identification of biomarkers and developing the symptomatic treatments [130].
A study by Shi et al. revealed that Mucosal-associated invariant T (MAIT) cells could be involved in the host immune response against this virus and their transcriptomic data presented a better knowledge of the immune pathogenesis of SARS-CoV-2 [131]. Moreover, it has been suggested that considering the high similarity between the transcriptome of SARS-CoV and SARS-CoV-2, the data of immunological regulations, signaling pathways, and proinflammatory cytokines in SARS-CoV infection could be expanded to COVID-19 to provide a better platform for future medical investigations [132]. This field of study showed to be useful to battle COVID-19, and perhaps could be used against the other potential pandemics in future.
5. Conclusion
From reviewing of contemporary publications regarding the applications of in silico methods and managing different aspects of COVID-19 infection, it could be concluded that in silico methods have been very helpful for studying unrecognized molecular structures, classification of the COVID-19 related data via AI and deep learning methods, analyzing the interaction of potential inhibitors and their viral targets, and designing novel preventive and therapeutic agents. The in silico platform also showed to assist the experts in the bioinformatics field to develop novel and specific web servers for various scientific applications for SARS-CoV-2 management.
Research involving human participants and/or animals
No human or animal was involved in this study.
Informed consent
There was no human participant and consent was not required.
Funding
None.
Data availability statement
The data supporting this study are available from the corresponding author upon reasonable request.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors wish to thank Baqiyatallah University of medical sciences.
References
- 1.Mehmood M.A., Sehar U., Ahmad N. Use of bioinformatics tools in different spheres of life sciences. J Data Min Genom Proteonomics. 2014;5(2):1. [Google Scholar]
- 2.Behbahani M., et al. Using Chou's general pseudo amino acid composition to classify laccases from bacterial and fungal sources via Chou's five-step rule. Appl Biochem Biotechnol. 2020;190(3):1035–1048. doi: 10.1007/s12010-019-03141-8. [DOI] [PubMed] [Google Scholar]
- 3.Mohabatkar H., Ebrahimi S., Moradi M. Using Chou's five-steps Rule to Classify and predict glutathione S-Transferases with different machine learning Algorithms and pseudo amino acid composition. Int J Pept Res Therapeut. 2021;27(1):309–316. [Google Scholar]
- 4.Vellido A. The importance of interpretability and visualization in machine learning for applications in medicine and health care. Neural Comput Appl. 2019:1–15. [Google Scholar]
- 5.Nabati F., Moradi M., Mohabatkar H. In silico analyzing the molecular interactions of plant-derived inhibitors against E6AP, p53, and c-Myc binding sites of HPV type 16 E6 oncoprotein. Mol Biol Res Commun. 2020;9(2):71. doi: 10.22099/mbrc.2020.36522.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sadeghi M., et al. In silico study of garlic (Allium sativum L.)-derived compounds molecular interactions with α-glucosidase. In Silico Pharmacol. 2021;9(1):1–8. doi: 10.1007/s40203-020-00072-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haghighi O., Moradi M. In silico study of the structure and ligand interactions of alcohol dehydrogenase from cyanobacterium Synechocystis Sp. PCC 6803 as a key enzyme for biofuel production. Appl Biochem Biotechnol. 2020;192(4):1346–1367. doi: 10.1007/s12010-020-03400-z. [DOI] [PubMed] [Google Scholar]
- 8.Haghighi O., et al. Homology modeling and molecular docking studies of glutamate dehydrogenase (GDH) from cyanobacterium Synechocystis sp. PCC 6803. Int J Pept Res Therapeut. 2020;26(2):783–793. [Google Scholar]
- 9.Haghighi O. In silico study of the structure and ligand preference of pyruvate kinases from cyanobacterium Synechocystis sp. PCC 6803. Appl Biochem Biotechnol. 2021;193(11):3651–3671. doi: 10.1007/s12010-021-03630-9. [DOI] [PubMed] [Google Scholar]
- 10.Bigdelou Z., et al. Investigation of specific binding of designed oligodeoxynucleotide decoys to transcription factors in HT29 cell line undergoing epithelial–mesenchymal transition (EMT) J Cell Physiol. 2019;234(12):22765–22774. doi: 10.1002/jcp.28841. [DOI] [PubMed] [Google Scholar]
- 11.Rahmati M., et al. Suppressing the metastatic properties of the breast cancer cells using STAT3 decoy oligodeoxynucleotides: a promising approach for eradication of cancer cells by differentiation therapy. J Cell Physiol. 2020;235(6):5429–5444. doi: 10.1002/jcp.29431. [DOI] [PubMed] [Google Scholar]
- 12.Behbahani M., Moradi M., Mohabatkar H. In silico design of a multi-epitope peptide construct as a potential vaccine candidate for Influenza A based on neuraminidase protein. In silico Pharmacol. 2021;9(1):1–13. doi: 10.1007/s40203-021-00095-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hufsky F., et al. Computational strategies to combat COVID-19: useful tools to accelerate SARS-CoV-2 and coronavirus research. Briefings Bioinf. 2021;22(2):642–663. doi: 10.1093/bib/bbaa232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Q., et al. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dehelean C.A., et al. SARS-CoV-2: repurposed drugs and novel therapeutic approaches—insights into chemical structure—biological activity and toxicological screening. J Clin Med. 2020;9(7):2084. doi: 10.3390/jcm9072084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alimadadi A., et al. 52(4) American Physiological Society Bethesda; MD: 2020. Artificial Intelligence and Machine Learning to Fight COVID-19; pp. 200–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guedes I.A., et al. Drug design and repurposing with DockThor-VS web server focusing on SARS-CoV-2 therapeutic targets and their non-synonym variants. Sci Rep. 2021;11(1):5543. doi: 10.1038/s41598-021-84700-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alagumuthu M., Rajpoot S., Baig M.S. Structure-based Design of novel peptidomimetics Targeting the SARS-CoV-2 spike protein. Cell Mol Bioeng. 2020;14(2):1–9. doi: 10.1007/s12195-020-00658-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng W., et al. Folding non-homologous proteins by coupling deep-learning contact maps with I-TASSER assembly simulations. Cell Rep Methods. 2021 doi: 10.1016/j.crmeth.2021.100014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelley L.A., et al. The Phyre2 web portal for protein modeling, prediction and analysis. Nat Protoc. 2015;10(6):845–858. doi: 10.1038/nprot.2015.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kong R., et al. COVID-19 Docking Server: a meta server for docking small molecules, peptides and antibodies against potential targets of COVID-19. Bioinformatics. 2020;36(20):5109–5111. doi: 10.1093/bioinformatics/btaa645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arantes P.R., Saha A., Palermo G. ACS Publications; 2020. Fighting covid-19 using molecular dynamics simulations. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zoabi Y., Deri-Rozov S., Shomron N. Machine learning-based prediction of COVID-19 diagnosis based on symptoms. NPJ Digit Med. 2021;4(1):3. doi: 10.1038/s41746-020-00372-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lalmuanawma S., Hussain J., Chhakchhuak L. Applications of machine learning and artificial intelligence for Covid-19 (SARS-CoV-2) pandemic: a review. Chaos, Solit. Fractals. 2020;139 doi: 10.1016/j.chaos.2020.110059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dong S., et al. A guideline for homology modeling of the proteins from newly discovered betacoronavirus, 2019 novel coronavirus (2019-nCoV) J Med Virol. 2020;92(9):1542–1548. doi: 10.1002/jmv.25768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Q., Peng W., Ou Y. Prediction and analysis of key protein structures of 2019-nCoV. Future Virol. 2020;15(6):349–357. [Google Scholar]
- 27.Baruah C., Devi P., Sharma D.K. Sequence analysis and structure prediction of SARS-CoV-2 accessory proteins 9b and ORF14: evolutionary analysis indicates close relatedness to bat coronavirus. BioMed Res Int. 2020;2020:7234961. doi: 10.1155/2020/7234961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vandelli A., et al. Structural analysis of SARS-CoV-2 genome and predictions of the human interactome. Nucleic Acids Res. 2020;48(20):11270–11283. doi: 10.1093/nar/gkaa864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li T., et al. Phylogenetic supertree reveals detailed evolution of SARS-CoV-2. Sci Rep. 2020;10(1):22366. doi: 10.1038/s41598-020-79484-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tabibzadeh A., et al. SARS-CoV-2 Molecular and Phylogenetic analysis in COVID-19 patients: a preliminary report from Iran. Infect Genet Evol. 2020;84 doi: 10.1016/j.meegid.2020.104387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang C., et al. Protein structure and sequence reanalysis of 2019-nCoV genome refutes snakes as its intermediate host and the unique similarity between its spike protein insertions and HIV-1. J Proteome Res. 2020;19(4):1351–1360. doi: 10.1021/acs.jproteome.0c00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang R.H., et al. Genomic characterization and phylogenetic evolution of the SARS-CoV-2. Acta Virol. 2020;64(4):496–500. doi: 10.4149/av_2020_403. [DOI] [PubMed] [Google Scholar]
- 33.Sacco M.D., et al. Structure and inhibition of the SARS-CoV-2 main protease reveal strategy for developing dual inhibitors against Mpro and cathepsin L. Sci Adv. 2020;6(50) doi: 10.1126/sciadv.abe0751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sakkiah S., et al. Elucidating interactions between SARS-CoV-2 trimeric spike protein and ACE2 using homology modeling and molecular dynamics simulations. Front Chem. 2021;8(1247) doi: 10.3389/fchem.2020.622632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pundir H., et al. Using Chou's 5-steps rule to study pharmacophore-based virtual screening of SARS-CoV-2 Mpro inhibitors. Mol Divers. 2020:1–14. doi: 10.1007/s11030-020-10148-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mahdian S., et al. Repurposing FDA-approved drugs to fight COVID-19 using in silico methods: targeting SARS-CoV-2 RdRp enzyme and host cell receptors (ACE2, CD147) through virtual screening and molecular dynamic simulations. Inform Med. 2021;23 doi: 10.1016/j.imu.2021.100541. Unlocked. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marinho E.M., et al. Virtual screening based on molecular docking of possible inhibitors of Covid-19 main protease. Microb Pathog. 2020;148:104365. doi: 10.1016/j.micpath.2020.104365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu X., et al. Discovery of TMPRSS2 Inhibitors from virtual Screening as a potential Treatment of COVID-19. ACS Pharmacol Transl Sci. 2021;4(3):1124–1135. doi: 10.1021/acsptsci.0c00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ishola A.A., et al. QSAR modeling and pharmacoinformatics of SARS coronavirus 3C-like protease inhibitors. Comput Biol Med. 2021;134 doi: 10.1016/j.compbiomed.2021.104483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen Y.W., Yiu C.-P.B., Wong K.-Y. Prediction of the SARS-CoV-2 (2019-nCoV) 3C-like protease (3CL (pro)) structure: virtual screening reveals velpatasvir, ledipasvir, and other drug repurposing candidates. F1000Research. 2020;9:129. doi: 10.12688/f1000research.22457.1. 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rahman M.M., et al. Virtual screening, molecular dynamics and structure–activity relationship studies to identify potent approved drugs for Covid-19 treatment. J Biomol Struct Dyn. 2020:1–11. doi: 10.1080/07391102.2020.1794974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hosseini M., et al. Computational molecular docking and virtual screening revealed promising SARS-CoV-2 drugs. Precis Clin Med. 2021;4(1):1–16. doi: 10.1093/pcmedi/pbab001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Senathilake K., Samarakoon S., Tennekoon K. 2020. Virtual screening of inhibitors against spike glycoprotein of 2019 novel corona virus: a drug repurposing approach. [Google Scholar]
- 44.Alibakhshi A., et al. Virtual screening for the identification of potential candidate molecules against envelope (e) and membrane (m) proteins of SARS-CoV-2. J Comput Biophys Chem. 2021;20(3):209–224. [Google Scholar]
- 45.Sadeghi M., et al. Screening cyclooxygenase-2 inhibitors from Allium sativum L. compounds: in silico approach. J Mol Model. 2021;28(1):24. doi: 10.1007/s00894-021-05016-4. [DOI] [PubMed] [Google Scholar]
- 46.Sattari A., Ramazani A., Aghahosseini H. Repositioning therapeutics for COVID-19: virtual screening of the potent synthetic and natural compounds as SARS-CoV-2 3CLpro inhibitors. J Iran Chem Soc. 2021:1–21. [Google Scholar]
- 47.Xu Z., et al. Discovery of potential flavonoid inhibitors against COVID-19 3CL proteinase based on virtual screening strategy. Front Mol Biosci. 2020;7(247) doi: 10.3389/fmolb.2020.556481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Majumder R., Mandal M. Screening of plant-based natural compounds as a potential COVID-19 main protease inhibitor: an in silico docking and molecular dynamics simulation approach. J Biomol Struct Dyn. 2020:1–16. doi: 10.1080/07391102.2020.1817787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moradi M., et al. In silico analysis of inhibiting papain-like protease from SARS-CoV-2 by using plant-derived peptides. Int J Pept Res Therapeut. 2021;28(1):24. doi: 10.1007/s10989-021-10331-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nouadi B., et al. Prediction of anti-COVID 19 therapeutic Power of medicinal Moroccan plants using molecular docking. Bioinf Biol Insights. 2021;15 doi: 10.1177/11779322211009199. 11779322211009199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alam M., et al. Algae-derived bioactive molecules for the potential treatment of sars-cov-2. Molecules. 2021;26(8):2134. doi: 10.3390/molecules26082134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Joseph J., et al. Bentham Science; 2020. The Use of Pseudotyped Coronaviruses for the Screening of Entry Inhibitors: Green Tea Extract Inhibits the Entry of SARS-CoV-1, MERS-CoV, and SARS-CoV-2 by Blocking Receptor-Spike Interaction. Current Pharmaceutical Biotechnology. In press. [DOI] [PubMed] [Google Scholar]
- 53.Marwal A., Meena M., Gaur R. Molecular docking studies of coronavirus proteins with medicinal plant-based phytochemicals. Defence Life Sci J. 2021:57–63. [Google Scholar]
- 54.Dowlati Beirami A., et al. In silico identification of potentially effective herbal inhibitors of SARS-cov-2 main protease by virtual screening method: potential anti-COVID-19 molecules. School Med Stud J. 2020;2(3):2–6. [Google Scholar]
- 55.Huan Y., et al. Antimicrobial peptides: classification, design, application and research progress in multiple fields. Front Microbiol. 2020;11:2559. doi: 10.3389/fmicb.2020.582779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Heydari H., et al. Antiviral peptides against Coronaviridae family: a review. Peptides. 2021:170526. doi: 10.1016/j.peptides.2021.170526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mousavi Maleki M.S., Rostamian M., Madanchi H. Antimicrobial peptides and other peptide-like therapeutics as promising candidates to combat SARS-CoV-2. Expert Rev Anti-infect Ther. 2021:1–13. doi: 10.1080/14787210.2021.1912593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Elnagdy S., AlKhazindar M. The potential of antimicrobial peptides as an antiviral therapy against COVID-19. ACS Pharmacol Transl Sci. 2020;3(4):780–782. doi: 10.1021/acsptsci.0c00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ahmadi K., et al. Enfuvirtide, an HIV-1 fusion inhibitor peptide, can act as a potent SARS-CoV-2 fusion inhibitor: an in silico drug repurposing study. J Biomol Struct Dyn. 2021:1–11. doi: 10.1080/07391102.2021.1871958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Al-Rabia M.W., et al. Repurposing of sitagliptin- melittin optimized nanoformula against SARS-CoV-2; antiviral screening and molecular docking studies. Pharmaceutics. 2021;13(3):307. doi: 10.3390/pharmaceutics13030307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alagumuthu M., Rajpoot S., Baig M.S. Structure-based Design of novel peptidomimetics Targeting the SARS-CoV-2 spike protein. Cell Mol Bioeng. 2021;14(2):177–185. doi: 10.1007/s12195-020-00658-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ling R., et al. In silico design of antiviral peptides targeting the spike protein of SARS-CoV-2. Peptides. 2020;130:170328. doi: 10.1016/j.peptides.2020.170328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Baig M.S., et al. Identification of a potential peptide inhibitor of SARS-CoV-2 targeting its entry into the host cells. Drugs R. 2020;20(3):161–169. doi: 10.1007/s40268-020-00312-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barh D., et al. Potential chimeric peptides to block the SARS-CoV-2 spike receptor-binding domain. F1000Research. 2020:9. doi: 10.12688/f1000research.24074.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Balmeh N., Mahmoudi S., Fard N.A. Manipulated bio antimicrobial peptides from probiotic bacteria as proposed drugs for COVID-19 disease. Inform Med Unlocked. 2021;23 doi: 10.1016/j.imu.2021.100515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mohammadi M., et al. In silico study of Pacific oyster antiviral polypeptides as potential inhibitory compounds for SARS-CoV-2 main protease. Jentashapir J Cell Mol Biol. 2020;11(3) [Google Scholar]
- 67.Andreadakis Z., et al. The COVID-19 vaccine development landscape. Nat Rev Drug Discov. 2020;19(5):305–306. doi: 10.1038/d41573-020-00073-5. [DOI] [PubMed] [Google Scholar]
- 68.Dalsass M., et al. Comparison of open-source reverse vaccinology Programs for bacterial vaccine antigen discovery. Front Immunol. 2019;10(113) doi: 10.3389/fimmu.2019.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lundegaard C., et al. NetMHC-3.0: accurate web accessible predictions of human, mouse and monkey MHC class I affinities for peptides of length 8–11. Nucleic Acids Res. 2008;36(suppl_2):W509–W512. doi: 10.1093/nar/gkn202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang P., et al. Peptide binding predictions for HLA DR, DP and DQ molecules. BMC Bioinf. 2010;11(1):1–12. doi: 10.1186/1471-2105-11-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Doytchinova I.A., Flower D.R. VaxiJen: a server for prediction of protective antigens, tumour antigens and subunit vaccines. BMC Bioinf. 2007;8(1):1–7. doi: 10.1186/1471-2105-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ponomarenko J., et al. ElliPro: a new structure-based tool for the prediction of antibody epitopes. BMC Bioinf. 2008;9(1):1–8. doi: 10.1186/1471-2105-9-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bui H.-H., et al. Predicting population coverage of T-cell epitope-based diagnostics and vaccines. BMC Bioinf. 2006;7(1):1–5. doi: 10.1186/1471-2105-7-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kolaskar A.S., Tongaonkar P.C. A semi-empirical method for prediction of antigenic determinants on protein antigens. FEBS Lett. 1990;276(1–2):172–174. doi: 10.1016/0014-5793(90)80535-q. [DOI] [PubMed] [Google Scholar]
- 75.Saha S., Raghava G. AlgPred: prediction of allergenic proteins and mapping of IgE epitopes. Nucleic Acids Res. 2006;34(suppl_2):W202–W209. doi: 10.1093/nar/gkl343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gupta S., et al. In silico approach for predicting toxicity of peptides and proteins. PLoS One. 2013;8(9) doi: 10.1371/journal.pone.0073957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rapin N., et al. Computational immunology meets bioinformatics: the use of prediction tools for molecular binding in the simulation of the immune system. PLoS One. 2010;5(4) doi: 10.1371/journal.pone.0009862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rezaei S., Sefidbakht Y., Uskoković V. Tracking the pipeline: immunoinformatics and the COVID-19 vaccine design. Briefings Bioinf. 2021;22(6):1–20. doi: 10.1093/bib/bbab241. [DOI] [PubMed] [Google Scholar]
- 79.Pourseif M.M., et al. A domain-based vaccine construct against SARS-CoV-2, the causative agent of COVID-19 pandemic: development of self-amplifying mRNA and peptide vaccines. Bioimpacts: BI. 2021;11(1):65. doi: 10.34172/bi.2021.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kar T., et al. A candidate multi-epitope vaccine against SARS-CoV-2. Sci Rep. 2020;10(1):10895. doi: 10.1038/s41598-020-67749-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang Z., Bogdan P., Nazarian S. An in silico deep learning approach to multi-epitope vaccine design: a SARS-CoV-2 case study. Sci Rep. 2021;11(1):3238. doi: 10.1038/s41598-021-81749-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Behmard E., et al. Immunoinformatic design of a COVID-19 subunit vaccine using entire structural immunogenic epitopes of SARS-CoV-2. Sci Rep. 2020;10(1):20864. doi: 10.1038/s41598-020-77547-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ong E., et al. COVID-19 coronavirus vaccine design using reverse Vaccinology and machine learning. Front Immunol. 2020;11(1581) doi: 10.3389/fimmu.2020.01581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Enayatkhani M., et al. Reverse vaccinology approach to design a novel multi-epitope vaccine candidate against COVID-19: an in silico study. J Biomol Struct Dyn. 2021;39(8):2857–2872. doi: 10.1080/07391102.2020.1756411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dong R., et al. Contriving multi-epitope Subunit of Vaccine for COVID-19: immunoinformatics approaches. Front Immunol. 2020;11(1784) doi: 10.3389/fimmu.2020.01784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rahman M.S., et al. Epitope-based chimeric peptide vaccine design against S, M and E proteins of SARS-CoV-2, the etiologic agent of COVID-19 pandemic: an in silico approach. PeerJ. 2020;8 doi: 10.7717/peerj.9572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dar H.A., et al. Multiepitope subunit vaccine design against COVID-19 based on the spike protein of SARS-CoV-2: an in silico analysis. J Immunol Res. 2020 doi: 10.1155/2020/8893483. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kumar N., Sood D., Chandra R. Design and optimization of a subunit vaccine targeting COVID-19 molecular shreds using an immunoinformatics framework. RSC Adv. 2020;10(59):35856–35872. doi: 10.1039/d0ra06849g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Alamri S.S., et al. Synthetic SARS-CoV-2 spike-based DNA vaccine elicits Robust and long-lasting Th1 Humoral and cellular Immunity in mice. Front Microbiol. 2021;12(2412) doi: 10.3389/fmicb.2021.727455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Prompetchara E., et al. DNA vaccine candidate encoding SARS-CoV-2 spike proteins elicited potent humoral and Th1 cell-mediated immune responses in mice. PLoS One. 2021;16(3) doi: 10.1371/journal.pone.0248007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Borah P., et al. Perspectives on RNA vaccine Candidates for COVID-19. Front Mol Biosci. 2021;8(30) doi: 10.3389/fmolb.2021.635245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cruz-Rodriguez L., et al. A RNA-Peptide fusion as a vaccine candidate against the novel Coronavirus (COVID-19) J Diabetes Endocrinol Res. 2020;1(1):1–11. [Google Scholar]
- 93.Kushwaha S., et al. Significant applications of machine learning for COVID-19 pandemic. J Ind Integrat Manage. 2020;5(4) [Google Scholar]
- 94.Mottaqi M.S., Mohammadipanah F., Sajedi H. Contribution of machine learning approaches in response to SARS-CoV-2 infection. Inform Med Unlocked. 2021;23:100526. doi: 10.1016/j.imu.2021.100526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kumar R.L., et al. Recurrent neural Network and reinforcement learning model for COVID-19 prediction. Front Public Health. 2021;9(1437) doi: 10.3389/fpubh.2021.744100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li W.T., et al. medrxiv; 2020. Using Machine Learning of Clinical Data to Diagnose Covid-19; pp. 1–21. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bachtiger P., Peters N.S., Walsh S.L. Machine learning for COVID-19—asking the right questions. Lancet Digit Health. 2020;2(8):e391–e392. doi: 10.1016/S2589-7500(20)30162-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Raza K. In: Big data analytics and artificial intelligence against COVID-19: innovation vision and approach. Hassanien A.-E., Dey N., Elghamrawy S., editors. Springer International Publishing; Cham: 2020. Artificial intelligence against COVID-19: a meta-analysis of current research; pp. 165–176. [Google Scholar]
- 99.Magar R., Yadav P., Barati Farimani A. Potential neutralizing antibodies discovered for novel corona virus using machine learning. Sci Rep. 2021;11(1):5261. doi: 10.1038/s41598-021-84637-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Khalifa N.E.M., et al. A deep learning model and machine learning methods for the classification of potential coronavirus treatments on a single human cell. J Nanoparticle Res. 2020;22(11):313. doi: 10.1007/s11051-020-05041-z. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 101.Wu J., et al. medRxiv; 2020. Rapid and Accurate Identification of COVID-19 Infection through Machine Learning Based on Clinical Available Blood Test Results. In press. [Google Scholar]
- 102.Mohapatra S., et al. Repurposing therapeutics for COVID-19: rapid prediction of commercially available drugs through machine learning and docking. PLoS One. 2020;15(11) doi: 10.1371/journal.pone.0241543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Amin S.A., et al. Chemical-informatics approach to COVID-19 drug discovery: Monte Carlo based QSAR, virtual screening and molecular docking study of some in-house molecules as papain-like protease (PLpro) inhibitors. J Biomol Struct Dyn. 2020:1–10. doi: 10.1080/07391102.2020.1780946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hosseini M., Chen W., Wang C. Precision Clinical Medicine. 4 (1) Oxford academics; 2020. Computational Molecular Docking and Virtual Screening Revealed Promising SARS-CoV-2 Drugs; pp. 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Russo G., et al. In silico trial to test COVID-19 candidate vaccines: a case study with UISS platform. BMC Bioinf. 2020;21(17):527. doi: 10.1186/s12859-020-03872-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tetz G., Tetz V. Prion-like domains in eukaryotic viruses. Sci Rep. 2018;8(1):1–10. doi: 10.1038/s41598-018-27256-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tetz G., Tetz V. 2020. SARS-CoV-2 prion-like domains in spike proteins enable higher affinity to ACE2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mohabatkar H., Behbahani M., Moradi M. A concise IN silico prediction report OF a potential PRION-like domain IN SARS-COV-2 polyprotein. Journal of microbiology. Biotechnol Food Sci. 2021:e4813. [Google Scholar]
- 109.Elaziz M.A., et al. New machine learning method for image-based diagnosis of COVID-19. PLoS One. 2020;15(6) doi: 10.1371/journal.pone.0235187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Goodman-Meza D., et al. A machine learning algorithm to increase COVID-19 inpatient diagnostic capacity. PLoS One. 2020;15(9) doi: 10.1371/journal.pone.0239474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hartono P. Similarity maps and pairwise predictions for transmission dynamics of COVID-19 with neural networks. Inform Med. 2020;20:100386. doi: 10.1016/j.imu.2020.100386. Unlocked. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Behbahani M., Mohabatkar H., Hosseini B. In silico design of quadruplex aptamers against the spike protein of SARS-CoV-2. Inform Med Unlocked. 2021;26:100757. doi: 10.1016/j.imu.2021.100757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Torkamanian-Afshar M., et al. In silico design of novel aptamers utilizing a hybrid method of machine learning and genetic algorithm. Mol Divers. 2021;25(3):1395–1407. doi: 10.1007/s11030-021-10192-9. [DOI] [PubMed] [Google Scholar]
- 114.Chen J., See K.C. Artificial intelligence for COVID-19: rapid review. J Med Internet Res. 2020;22(10) doi: 10.2196/21476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Phan I.Q., et al. In silico detection of SARS-CoV-2 specific B-cell epitopes and validation in ELISA for serological diagnosis of COVID-19. Sci Rep. 2021;11(1):4290. doi: 10.1038/s41598-021-83730-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Can H., et al. In silico discovery of antigenic proteins and epitopes of SARS-CoV-2 for the development of a vaccine or a diagnostic approach for COVID-19. Sci Rep. 2020;10(1):22387. doi: 10.1038/s41598-020-79645-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Harismah K., et al. In silico investigation of nanocarbon biosensors for diagnosis of COVID-19. Eurasian Chem Commun. 2021;3(2):95–102. [Google Scholar]
- 118.Kazemi-Arpanahi H., Moulaei K., Shanbehzadeh M. Design and development of a web-based registry for Coronavirus (COVID-19) disease. Med J Islam Repub Iran. 2020;34:68. doi: 10.34171/mjiri.34.68. 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Di Matteo G., et al. Food and COVID-19: preventive/Co-therapeutic strategies Explored by current clinical Trials and in silico studies. Foods. 2020;9(8):1036. doi: 10.3390/foods9081036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Prout T.A., et al. Identifying predictors of psychological distress during COVID-19: a machine learning approach. Front Psychol. 2020;11(3063) doi: 10.3389/fpsyg.2020.586202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dopazo J., et al. Implementing personalized medicine in COVID-19 in Andalusia: an opportunity to transform the healthcare system. J Personalized Med. 2021;11(6):475. doi: 10.3390/jpm11060475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Voutouri C., et al. In silico dynamics of COVID-19 phenotypes for optimizing clinical management. Proc Natl Acad Sci Unit States Am. 2021;118(3) doi: 10.1073/pnas.2021642118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Aljameel S.S., et al. Machine learning-based Model to Predict the disease Severity and Outcome in COVID-19 patients. Sci Program. 2021 2021. [Google Scholar]
- 124.Robinson J., et al. COVID-19 and mutations a threat level assessment. Nepal J Epidemiol. 2021;11(1):983. doi: 10.3126/nje.v11i1.35659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.van Oosterhout C., et al. Taylor & Francis; 2021. COVID-19 evolution during the pandemic–Implications of new SARS-CoV-2 variants on disease control and public health policies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Painuli D., et al. 2021. Forecast and Prediction of COVID-19 using Machine Learning. Data Science for COVID-19; pp. 381–397. [Google Scholar]
- 127.Kanter I., Kalisky T. Single cell transcriptomics: methods and applications. Front Oncol. 2015;5:53. doi: 10.3389/fonc.2015.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Liu S., Trapnell C. Single-cell transcriptome sequencing: recent advances and remaining challenges. F1000Research. 2016:5. doi: 10.12688/f1000research.7223.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hasan M.Z., et al. Meta-analysis of single-cell RNA-seq data reveals phenotypic switching of immune cells in severe COVID-19 patients. Comput Biol Med. 2021;137:104792. doi: 10.1016/j.compbiomed.2021.104792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang S., et al. A single-cell transcriptomic landscape of the lungs of patients with COVID-19. Nat Cell Biol. 2021;23(12):1314–1328. doi: 10.1038/s41556-021-00796-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Shi J., et al. Single-cell transcriptomic Profiling of MAIT Cells in patients with COVID-19. Front Immunol. 2021;12(3112) doi: 10.3389/fimmu.2021.700152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zolfaghari Emameh R., et al. Expansion of single cell transcriptomics data of SARS-CoV infection in human bronchial epithelial cells to COVID-19. Biol Proced Online. 2020;22(1):16. doi: 10.1186/s12575-020-00127-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting this study are available from the corresponding author upon reasonable request.