SUMMARY
Background:
The ongoing UNIFI long-term extension evaluates subcutaneous ustekinumab treatment for moderate-to-severe ulcerative colitis (UC) from Weeks 44 through 220.
Aims:
To assess efficacy (through Week 92) and safety (through week 96) during the long-term extension.
Methods:
Overall, 399 intravenous ustekinumab induction responders randomized to maintenance therapy were treated in the long-term extension (subcutaneous placebo; n=115, ustekinumab 90 mg every 12 weeks [q12w]; n=141, or ustekinumab 90 mg q8w; n=143). Placebo-treated patients were discontinued at unblinding after Week 44. Partial Mayo scores were collected every 12 weeks and at each dosing visit after unblinding. Safety was evaluated throughout.
Results:
Among all patients randomized in maintenance, symptomatic remission rates (stool frequency=0/1; rectal bleeding=0) at Week 92 were, 64.5% and 67.6% in the ustekinumab q12w and q8w groups, respectively. Among randomized patients treated in the long-term extension, 78.7% and 83.2% of patients receiving q12w and q8w, respectively, attained symptomatic remission at Week 92; >95% of patients in symptomatic remission at Week 92 were corticosteroid-free. Both ustekinumab groups maintained efficacy through Week 92.
From Weeks 44–96, adverse events (AEs) per hundred patient-years of follow-up for combined ustekinumab versus placebo were: 255.68 versus 267.93; serious AEs, 9.34 versus 12.69; malignancies (including nonmelanoma skin cancers), 0.93 versus 1.49; and serious infections, 2.33 versus 2.99. One patient with multiple comorbidities who received one ustekinumab dose after dose-adjusting from placebo experienced a fatal cardiac arrest.
Conclusions:
The efficacy of ustekinumab in patients with UC was sustained through 92 weeks. No new safety signals were observed (ClinicalTrials.gov number, NCT02407236).
Keywords: ustekinumab, ulcerative colitis, long-term efficacy and safety, pharmacokinetics, immunogenicity
INTRODUCTION
Ulcerative colitis (UC) is a chronic progressive idiopathic inflammatory disease of the large bowel that is characterized by symptoms of bloody diarrhea, fecal urgency, abdominal cramps, and mucosal inflammation. Patients with UC typically require long-term therapy to control symptoms.1
Ustekinumab is an interleukin-12/23p40 antagonist that is approved for Crohn’s disease and more recently for UC. In the “Phase 3, Randomized, Double-blind, Placebo-controlled, Parallel-group, Multicenter Study to Evaluate the Safety and Efficacy of Ustekinumab Induction and Maintenance Therapy in Subjects with Moderately to Severely Active UC” study we call UNIFI (NCT02407236), ustekinumab was demonstrated to induce and maintain clinical remission and clinical response through 52 weeks of treatment in patients who had failed conventional or biologic therapy (tumor necrosis factor-α [TNF-α] antagonists and/or an α4β7 integrin mucosal addressin cell adhesion molecule-1 [MAdCAM-1] antagonist, vedolizumab) or who were biologic therapy naive.2 Here we describe the maintenance of efficacy (Week 92) and safety (Week 96) through the first year of the UNIFI long-term extension.
MATERIALS AND METHODS
Study Design
Detailed methods of the UNIFI induction and maintenance studies were previously reported.2 Briefly, adult patients with moderately-to-severely active UC (Mayo total score of 6–12, Mayo total score range 0–12, higher scores indicate more severe disease) including Mayo endoscopy subscore ≥2 (Mayo endoscopy subscore, range 0–3; determined during central review of video-endoscopy) were enrolled.3,4 Eligible patients had inadequate response or failed to tolerate TNF-antagonists, vedolizumab, or conventional (i.e., non-biologic) therapy. Stable doses of 5-aminosalicylates and immunosuppressants were maintained from induction baseline through maintenance Week 44. Oral corticosteroids were maintained at a stable dose during the induction study, but tapered during the maintenance study by 5 mg/week for patients receiving >20 mg/day prednisone or equivalent (p.eq.) or by 2.5 mg/week for patients receiving ≤20 mg/day p.eq until 0 mg/day. During the long-term extension concomitant UC medications could be changed at the discretion of the treating physician.
Eligible patients were randomly assigned to intravenous (IV) induction of placebo, or ustekinumab 130 mg or a weight-based dose approximating 6 mg/kg. Patients who responded in the induction study could enter the maintenance study and participate as randomized or nonrandomized patients.
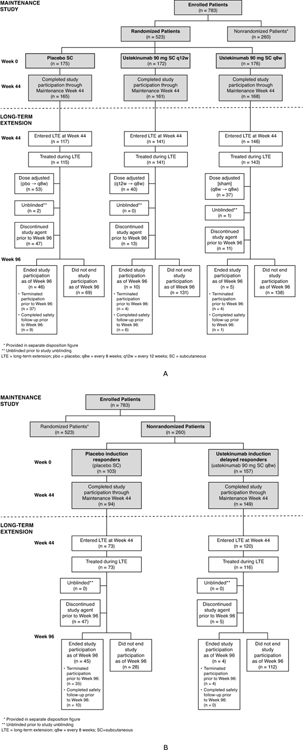
The randomized maintenance study patient population (Figure 1A) comprised patients who were in clinical response 8 weeks after ustekinumab IV induction and randomly assigned to SC maintenance treatment with placebo, or 90 mg ustekinumab every 12 weeks (q12w) or every 8 weeks (q8w).
Figure 1:
UNIFI maintenance and long-term extension study design: randomised (A) and non-randomised (B) patients
The nonrandomized population of the maintenance study (Figure 1B) included Week-16 ustekinumab induction responders and Week-8 placebo IV induction responders. Week-16 ustekinumab induction responders were patients who were not in clinical response to IV ustekinumab induction therapy at Week 8, received a SC administration of 90 mg ustekinumab and were in clinical response 8 weeks later (i.e., induction Week 16). Week-16 ustekinumab induction responders continued to receive ustekinumab 90 mg SC q8w in the maintenance study. Week-8 placebo IV induction responders continued to receive SC placebo in the maintenance study.
All patients completing Week 44 of the maintenance study were eligible to enter the long-term extension and continue their same ustekinumab or placebo maintenance regimen if in the opinion of the investigator, the patients would benefit from continued treatment. Randomized and nonrandomized patients who were still receiving placebo in the long-term extension were discontinued after unblinding of the study and analysis of the Week-44 data.
Based on the investigator’s clinical judgement of their UC disease activity, randomized patients who entered the long-term extension were eligible to receive a dose adjustment starting at Week 56 as follows: placebo to ustekinumab q8w, ustekinumab q12w to q8w, and ustekinumab q8w to q8w (sham adjustment). Dose adjustment was conducted in a blinded fashion until maintenance study unblinding, after which patients and investigators were aware of their ustekinumab maintenance regimen. Nonrandomized Week-16 ustekinumab induction responders and Week-8 placebo IV induction responders were not eligible for dose adjustment.
Efficacy endpoints
Through Week 92, efficacy data (i.e., partial Mayo scores and inflammatory biomarkers [serum C-reactive protein {CRP}, fecal calprotectin]) were collected every 12 weeks, and at each dosing visit after unblinding. Symptomatic remission, defined as a stool frequency subscore of 0 or 1 and a rectal bleeding subscore of 0, was evaluated every 12 weeks.
Pharmacokinetics and Immunogenicity
Serum blood samples for immunogenicity and ustekinumab concentration assessments were collected every 6 months. Serum ustekinumab concentrations were measured using a validated electrochemiluminescent immunoassay (ECLIA) on the MesoScale Discovery platform, in which ustekinumab was used to capture and detect induced immune responses to ustekinumab. This assay also detected anti-drug antibodies (ADAs) in the presence of up to 100 mg/mL of ustekinumab in the sample. Patients were classified as positive if antibodies were detected in their serum sample at any time.
Safety
Safety (concomitant medications, adverse events [AEs], serious AEs [SAEs], and laboratory assessments) was evaluated through Week 96.
Statistical Analysis
Demographic and baseline disease characteristic summaries, and safety analyses were based on all patients who received at least one SC study agent administration during the long-term extension.
Efficacy was evaluated in three patient populations: 1) all randomized patients in the maintenance study (inten-to-treat population), 2) randomized patients who were treated in the long-term extension, and 3) non-randomized patients who were treated in the long-term extension. Three analysis approaches were undertaken for dichotomous endpoints, nonresponder imputation with treatment failure and missing data rules applied, observed case without treatment failure and missing data rules applied, and modified observed case analyses up to the time of dose adjustment with treatment failure rules applied but not missing data rules (see Table 1 for details, including specific treatment failure rules).. Dose adjustment was considered to be part of the treatment regimen (i.e., not included in treatment failure rules) unless otherwise indicated for dichotomous endpoints. For continuous endpoints, patients with a treatment failure or dose adjustment had their induction baseline value carried forward from the time of the event onward (ie, consistent with nonresponse for dichotomous endpoints).
Table 1:
List of analysis populations and analysis approaches for dichotomous endpoints
| Analysis Population | Analysis Method | Analytic Approach |
|---|---|---|
| All patients randomized in maintenance study (intent-to-treat population) | Nonresponder imputation | • All patients randomized in the maintenance study were included in the analysis • Patients who had missing data at a visit were considered not to have achieved the endpoint for that visit • Patients who had a prohibited change in medication for ulcerative colitis (UC), had undergone an ostomy or colectomy, or had used a rescue medication after a clinical flare or who had discontinued study agent owing to lack of therapeutic effect or an adverse event (AE) of worsening of UC before the Week 44 visit were considered not to have achieved the endpoint at subsequent time points. Patients who had an ostomy or colectomy or discontinued study agent owing to lack of therapeutic effect or an AE of worsening UC after the Week 44 visit were considered not to have achieved the endpoint at subsequent time points |
| Nonresponder imputation with dose adjustment as a treatment failure | Same as above, except patients who had a dose adjustment (only occurred from Week 56 onward) prior to the Week 92 visit were considered not to have achieved the endpoint at subsequent time points. | |
| Randomized patients treated in the longterm extension | Nonresponder imputation | • All randomized patients treated in the long-term extension were included in the analysis • Patients who had missing data at a visit were considered not to have achieved the endpoint for that visit • Patients who had an ostomy or colectomy or discontinued study agent owing to lack of therapeutic effect or an AE of worsening UC after the Week 44 visit were considered not to have achieved the endpoint at subsequent time points |
| Nonresponder imputation with dose adjustment as a treatment failure | Same as above, except patients who had a dose adjustment (only occurred from Week 56 onward) prior to the Week 92 visit were considered not to have achieved the endpoint at subsequent time points | |
| Observed case | • All randomized patients treated in the long-term extension, excluding patients who have missing data at a visit. • No treatment failure or missing data rules applied |
|
| Modified observed case | • Analysis of data up to the time of dose adjustment for patients treated in the LTE, excluding patients who have missing data after accounting for treatment failure rules. • Patients who had an ostomy or colectomy or discontinued study agent owing to lack of therapeutic effect or an AE of worsening UC, were considered not to have achieved the endpoint at subsequent time points |
|
| Nonrandomized patients treated in the long-term extension: Week 16 induction responders | Nonresponder imputation | • Patients included in this analysis were those who were not in clinical response 8 weeks after ustekinumab (UST) intravenous (IV) induction, received a subcutaneous (SC) dose of UST 90 mg at induction Week 8, were in clinical response at induction Week 16 entered the maintenance study in the non-randomized population, and treated in the long-term extension • Same approach as that for nonresponder imputation analysis of randomized patients treated in the long-term extension |
| Patients randomized to placebo in maintenance and dose-adjusted in the long-term extension: treatment interruption | Nonresponder imputation | • Patients included in this analysis were those who were in clinical response 8 weeks after UST IV induction, were randomized to placebo SC at maintenance study baseline, entered the long-term extension, and received dose adjustment (Week 56 or later) to SC UST 90 mg every 8 weeks • Same approach as that for nonresponder imputation analysis of randomized patients treated in the long-term extension |
Key: AE, adverse event; IV, intravenous; SC, subcutaneous; UC, ulcerative colitis; UST, ustekinumab
Serum ustekinumab concentrations were summarized over time through Week 92. The incidence of anti-drug antibodies (ADAs) was summarized through Week 96 for all treated patients who entered the long-term extension, received at least one dose of ustekinumab (either in the induction or maintenance study), and had appropriate samples for detection of antibodies to ustekinumab (i.e., patients with at least 1 sample obtained after their first dose of ustekinumab). Patients were considered positive if antibodies were detected at any timepoint.
Safety was evaluated by calculating the number of AEs, SAEs, infections, serious infections, AEs leading to discontinuation of study agent, and malignancies per one-hundred patient-years (PY) of follow-up among all patients (randomized and nonrandomized) who were treated in the long-term extension. Events per hundred PY that occurred during the maintenance study (first year of the study) and those that occurred during the second year of the study (long-term extension Weeks 44 through 96) were compared.
Descriptive statistics (e.g., mean, median, standard deviation, interquartile range, minimum, and maximum) were used to summarize continuous variables. Counts and percentages were used to summarize categorical variables.
RESULTS
Patient Demographics and Disease Characteristics
A total of 588 patients who completed safety and efficacy evaluations at Week 44 and, in the opinion of the investigator, would benefit from continued treatment were treated in the long-term extension. Among these, 399 patients were from the maintenance randomized population and 189 patients were from the non-randomized population (Figures 1A and 1B, respectively).
Demographics, UC medication history, concomitant UC medication, and UC disease characteristics among patients who were treated in the long-term extension are summarized in Table 2. Among randomized patients, 58.1% were male, 74.4% were white, and the mean age was 40.9 years (Table 2). At Week 44 of maintenance, measures of UC disease activity (e.g., Mayo scores) were generally comparable among patients randomized to ustekinumab q12w and q8w (Supplemental Table S1), with 46.1% and 52.4% in clinical remission and 56.7% and 61.5% with endoscopic improvement, respectively. Among Week 16 ustekinumab induction responders treated in the long-term extension, measures of disease activity indicated benefit from ustekinumab maintenance therapy; across measures these patients tended to have somewhat higher disease activity and inflammatory burden at Week 44 of maintenance accompanied by lower rates of clinical remission (38.8%) and endoscopic improvement (47.4%) relative to those patients in response 8 weeks after a single induction dose of IV ustekinumab and randomized to ustekinumab q8w (Supplemental Table S1).
Table 2:
Demographics, UC medication history, and concomitant UC medication at induction baseline and UC disease characteristics at maintenance baseline; patients who were treated in the long-term extension
| Randomized patients † | Non-randomized patients |
|||||||
|---|---|---|---|---|---|---|---|---|
| Ustekinumab | Responders to placebo IV induction | Week 16 responders ¶ | ||||||
|
|
|
|||||||
| Placebo SC ‡ (N=115) | 90 mg SC p12w (N=141) | 90 mg SC q8w (N=143) | Combined (N=284) | Total (N=399) | Placebo SC § (N=73) | Ustekinumab 90 mg SC q8w (N=116) | Overall total (N=588) | |
| Induction baseline | ||||||||
| Male, n (%) | 73 (63.5) | 77 (54.6) | 82 (57.3) | 159 (56.0) | 232 (58.1) | 47 (64.4) | 83 (71.6) | 362 (61.6) |
| Age, Mean (SD), years | 42.9 (14.54) | 40.4 (13.22) | 39.9 (12.92) | 40.1 (13.05) | 40.9 (13.54) | 43.1 (15.03) | 43.6 (13.20) | 41.7 (13.69) |
| Weight (kg) | ||||||||
| Median (IQ range) | 72.00 (60.00; 79.40) | 71.60 (60.00; 83.80) | 70.80 (59.00; 85.30) | 71.50 (59.55; 84.25) | 71.60 (60.00; 83.20) | 71.50 (60.00; 84.00) | 73.00 (64.10; 87.10) | 72.00 (60.25; 83.65) |
| Race, n (%) | ||||||||
| White | 79 (68.7) | 111 (78.7) | 107 (74.8) | 218 (76.8) | 297 (74.4) | 62 (84.9) | 92 (79.3) | 451 (76.7) |
| Black or African American | 1 (0.9) | 0 | 2 (1.4) | 2 (0.7) | 3 (0.8) | 0 | 0 | 3 (0.5) |
| Asian | 26 (22.6) | 19 (13.5) | 22 (15.4) | 41 (14.4) | 67 (16.8) | 8 (11.0) | 17 (14.7) | 92 (15.6) |
| Other | 3 (2.6) | 8 (5.7) | 3 (2.1) | 11 (3.9) | 14 (3.5) | 1 (1.4) | 2 (1.7) | 17 (2.9) |
| Not Reported | 6 (5.2) | 3 (2.1) | 9 (6.3) | 12 (4.2) | 18 (4.5) | 2 (2.7) | 5 (4.3) | 25 (4.3) |
| Region n (%) | ||||||||
| Asia | 24 (20.9) | 18 (12.8) | 20 (14.0) | 38 (13.4) | 62 (15.5) | 8 (11.0) | 17 (14.7) | 87 (14.8) |
| Eastern Europe | 50 (43.5) | 68 (48.2) | 56 (39.2) | 124 (43.7) | 174 (43.6) | 35 (47.9) | 55 (47.4) | 264 (44.9) |
| Rest of World | 41 (35.7) | 55 (39.0) | 67 (46.9) | 122 (43.0) | 163 (40.9) | 30 (41.1) | 44 (37.9) | 237 (40.3) |
| North America | 12 (10.4) | 27 (19.1) | 28 (19.6) | 55 (19.4) | 67 (16.8) | 11 (15.1) | 12 (10.3) | 90 (15.3) |
| Western Europe | 23 (20.0) | 20 (14.2) | 32 (22.4) | 52 (18.3) | 75 (18.8) | 16 (21.9) | 26 (22.4) | 117 (19.9) |
| Other | 6 (5.2) | 8 (5.7) | 7 (4.9) | 15 (5.3) | 21 (5.3) | 3 (4.1) | 6 (5.2) | 30 (5.1) |
| Any UC medication at induction baseline | 105 (91.3) | 128 (90.8) | 128 (89.5) | 256 (90.1) | 361 (90.5) | 71 (97.3) | 110 (94.8) | 542 (92.2) |
| Immunosuppressants | 39 (33.9) | 37 (26.2) | 41 (28.7) | 78 (27.5) | 117 (29.3) | 25 (34.2) | 43 (37.1) | 185 (31.5) |
| Aminosalicylates | 86 (74.8) | 115 (81.6) | 94 (65.7) | 209 (73.6) | 295 (73.9) | 56 (76.7) | 91 (78.4) | 442 (75.2) |
| Corticosteroids use | 57 (49.6) | 69 (48.9) | 74 (51.7) | 143 (50.4) | 200 (50.1) | 40 (54.8) | 52 (44.8) | 292 (49.7) |
| Corticosteroid use (excl. budesonide and beclomethasone dipropionate) | 46 (40.0) | 58 (41.1) | 65 (45.5) | 123 (43.3) | 169 (42.4) | 34 (46.6) | 41 (35.3) | 244 (41.5) |
| Budesonide | 12 (10.4) | 11 (7.8) | 9 (6.3) | 20 (7.0) | 32 (8.0) | 7 (9.6) | 10 (8.6) | 49 (8.3) |
| Beclomethasone dipropionate | 1 (0.9) | 2 (1.4) | 0 | 2 (0.7) | 3 (0.8) | 0 | 2 (1.7) | 5 (0.9) |
|
| ||||||||
| Induction baseline | ||||||||
| Biologic naïve | 63 (54.8) | 82 (58.2) | 67 (46.9) | 149 (52.5) | 212 (53.1) | 36 (49.3) | 54 (46.6) | 302 (51.4) |
| Biologic refractory | 52 (45.2) | 53 (37.6) | 71 (49.7) | 124 (43.7) | 176 (44.1) | 35 (47.9) | 58 (50.0) | 269 (45.7) |
| Only TNF antagonist | 38 (33.0) | 36 (25.5) | 54 (37.8) | 90 (31.7) | 128 (32.1) | 24 (32.9) | 40 (34.5) | 192 (32.7) |
| Vedolizumab regardless of TNF antagonist | 14 (12.2) | 17 (12.1) | 17 (11.9) | 34 (12.0) | 48 (12.0) | 11 (15.1) | 18 (15.5) | 77 (13.1) |
| ≥1 TNF antagonist regardless of vedolizumab | 52 (45.2) | 53 (37.6) | 70 (49.0) | 123 (43.3) | 175 (43.9) | 34 (46.6) | 58 (50.0) | 267 (45.4) |
| TNF antagonist and vedolizumab | 14 (12.2) | 17 (12.1) | 16 (11.2) | 33 (11.6) | 47 (11.8) | 10 (13.7) | 18 (15.5) | 75 (12.8) |
| Maintenance baseline | ||||||||
| Mayo score (0–12) | ||||||||
| Mean (SD) | 3.2 (2.52) | 2.6 (2.07) | 2.4 (1.95) | 2.5 (2.01) | 2.7 (2.19) | 3.6 (2.48) | 3.2 (2.33) | 2.9 (2.28) |
| Clinical remission, †† n (%) | 32 (27.8) | 35 (24.8) | 32 (22.4) | 67 (23.6) | 99 (24.8) | 13 (17.8) | 15 (12.9) | 127 (21.6) |
| Endoscopic improvement, n (%) | 49 (42.6) | 57 (40.4) | 47 (32.9) | 104 (36.6) | 153 (38.3) | 28 (38.4) | 25 (21.6) | 206 (35.0) |
| C-Reactive Protein (mg/L) | ||||||||
| N | 114 | 140 | 143 | 283 | 397 | 72 | 116 | 585 |
| Median (IQ range) | 1.03 (0.47; 3.20) | 1.49 (0.51; 3.18) | 1.75 (0.73; 5.23) | 1.59 (0.62; 4.04) | 1.50 (0.56; 3.83) | 1.78 (0.58; 6.09) | 1.86 (0.74; 4.71) | 1.56 (0.60; 4.13) |
| Abnormal CRP‡‡, n (%) | 32 (28.1) | 37 (26.4) | 52 (36.4) | 89 (31.4) | 121 (30.5) | 29 (40.3) | 39 (33.6) | 189 (32.3) |
| Fecal calprotectin (mg/kg) | ||||||||
| N | 111 | 131 | 130 | 261 | 372 | 69 | 113 | 554 |
| Median (IQ range) | 316.0 (92.0; 900.0) | 431.0 (84.0; 1171.0) | 450.5 (157.0; 1515.0) | 450.0 (138.0; 1253.0) | 422.5 (109.5; 1176.0) | 332.0 (95.0; 1234.0) | 428.0 (165.0; 1183.0) | 421.5 (114.0; 1183.0) |
| Abnormal fecal calprotectin, ‡‡ n (%) | 59 (53.2) | 78 (59.5) | 85 (65.4) | 163 (62.5) | 222 (59.7) | 38 (55.1) | 76 (67.3) | 336 (60.6) |
| Maintenance baseline | ||||||||
| Fecal lactoferrin (μg/g) | ||||||||
| N | 110 | 132 | 133 | 265 | 375 | 72 | 112 | 559 |
| Median (IQ range) | 28.79 (4.30; 176.80) | 37.93 (3.78; 139.89) | 50.03 (14.25; 174.33) | 43.14 (9.17; 164.22) | 41.74 ((8.24; 169.65) | 32.13 (3.80; 184.85) | 52.58 (13.14; 171.91) | 42.88 (8.28; 172.00) |
| Abnormal fecal lactoferrin‡‡, n (%) | 78 (70.9) | 93 (70.5) | 111 (83.5) | 204 (77.0) | 282 (75.2) | 50 (69.4) | 90 (80.4) | 422 (75.5) |
Patients who were in clinical response to ustekinumab IV induction dosing based on the treatment assignment by IWRS on entry into the maintenance study, regardless whether patients had a dose adjustment during the long-term extension.
Patients who were in clinical response to ustekinumab IV induction dosing and were randomized to placebo SC on entry into the maintenance.
Patients who were in clinical response to placebo IV induction dosing and received placebo SC on entry into the maintenance study.
Patients who were not in clinical response to ustekinumab at I-8 but were in clinical response at I-16 after a SC administration of ustekinumab at I-8.
Mayo score ≤2 points, with no individual subscore >1.
Abnormal values for: CRP, >3 mg/L; fecal calprotectin, > 250 mg/kg; fecal lactoferrin, >7.24 μg/g.
Key: CRP, C-reactive protein; IQ, interquartile; I-8, induction Week 8; I-16, induction Week 16; IV, intravenous; IWRS, interactive web response system; q8w, every 8 weeks; q12w, every 12 weeks; SC, subcutaneous; SD, standard deviation; TNF, tumor necrosis factor; UC, ulcerative colitis.
Of the patients randomized in maintenance who were treated in the long-term extension, 55.9% (223/399) had no history of biologic failure of whom 95.1% (212/223) were biologic-naïve and 4.9% (11/223) were biologic experienced without documentation of failure (Table 2). Among 44.1% (176/399) patients with a history of biologic failure, 99.4% (175/176) failed ≥1 TNF antagonist (regardless of vedolizumab) and 26.7% (47/176) failed both vedolizumab and a TNF antagonist at induction baseline (Table 2).
Among all patients receiving ustekinumab during the long-term extension, 338/399 (84.7%) of randomized patients and 112/120 (93.3%) of nonrandomized ustekinumab induction Week 16 responders completed study participation through Week 96. Through Week 96, the placebo group (i.e., ustekinumab IV induction responders randomized to placebo SC maintenance therapy) had 12 fewer weeks of follow-up on average (37.1 weeks) as compared to the combined ustekinumab groups (49.1 weeks), which is primarily attributed to the protocol-specified discontinuation of placebo-treated patients at the time of study unblinding. The proportions of patients from the randomized and nonrandomized populations who discontinued ustekinumab prior to Week 96 were 8.5% (24 patients) and 4.3% (5 patients), respectively (Table 3).
Table 3:
Patients who discontinued study agent before Week 96; patients who were treated in the long-term
| Randomized patients † | Non-randomized patients | |||||
|---|---|---|---|---|---|---|
| Ustekinumab | Responders to placebo IV induction | Week 16 responders ¶ | ||||
| Placebo SC‡ (N=115) | 90 mg SC q12w (N=141) | 90 mg SC q8w (N=143) | Combined (N=284) | Placebo SC § (N=73) | Ustekinumab 90 mg SC q8w (N=116) | |
| Patients who discontinued study agent, n (%) | 47 (40.9) | 13 (9.2) | 11 (7.7) | 24 (8.5) | 47 (64.4) | 5 (4.3) |
| Reason for discontinuation, n (%) | ||||||
| Adverse event | 5 (4.3) | 9 (6.4) | 2 (1.4) | 11 (3.9) | 7 (9.6) | 2 (1.7) |
| Worsening of UC | 5 (4.3) | 6 (4.3) | 1 (0.7) | 7 (2.5) | 7 (9.6) | 1 (0.9) |
| Other than worsening of UC | 0 | 3 (2.1) | 1 (0.7) | 4 (1.4) | 0 | 1 (0.9) |
| Lack of efficacy | 4 (3.5) | 1 (0.7) | 2 (1.4) | 3 (1.1) | 6 (8.2) | 1 (0.9) |
| Did not show improvement in UC disease activity 16 weeks following dose adjustment, n (%) | 1 (0.9) | 1 (0.7) | 2 (1.4) | 3 (1.1) | 1 (1.4) | 0 |
| Lost to follow up | 0 | 0 | 0 | 0 | 0 | 0 |
| Placebo patients discontinued after study unblinding, n (%) | 34 (29.6) | 0 | 0 | 0 | 29 (39.7) | 0 |
| Death | 0 | 0 | 0 | 0 | 0 | 0 |
| Other | 3 (2.6) | 2 (1.4) | 5 (3.5) | 7 (2.5) | 4 (5.5) | 2 (1.7) |
Patients who were in clinical response to ustekinumab IV induction dosing based on the treatment assignment by IWRS on entry into the maintenance study, regardless whether patients had a dose adjustment during the long-term extension.
Patients who were in clinical response to ustekinumab IV induction dosing and were randomized to placebo SC on entry into the maintenance.
Patients who were in clinical response to placebo IV induction dosing and received placebo SC on entry into the maintenance study.
Patients who were not in clinical response to ustekinumab at I-8 but were in clinical response at I-16 after a SC administration of ustekinumab at I-8
Key: I-8, induction week 8; I-16, induction week 16; IV, intravenous; IWRS, interactive web response system; q8w, every 8 weeks; q12w, every 12 weeks; SC, subcutaneous; UC, ulcerative colitis.
Of 399 randomized patients who entered the long-term extension, 43.5% (50/115), 46.8% (66/141), and 46.9% (67/143) of placebo, and ustekinumab q12w, and q8w groups, respectively completed Week 96 assessments before study unblinding.
Efficacy
Because 95.1% of the biologic nonfailure population was comprised of biologic-naïve patients, only results for subgroups of patients who were biologic-naïve and those with a history of biologic failure are presented for each analysis population.
All patients randomized to maintenance treatment at Week 0 (intent-to-treat population): nonresponder imputation analysis
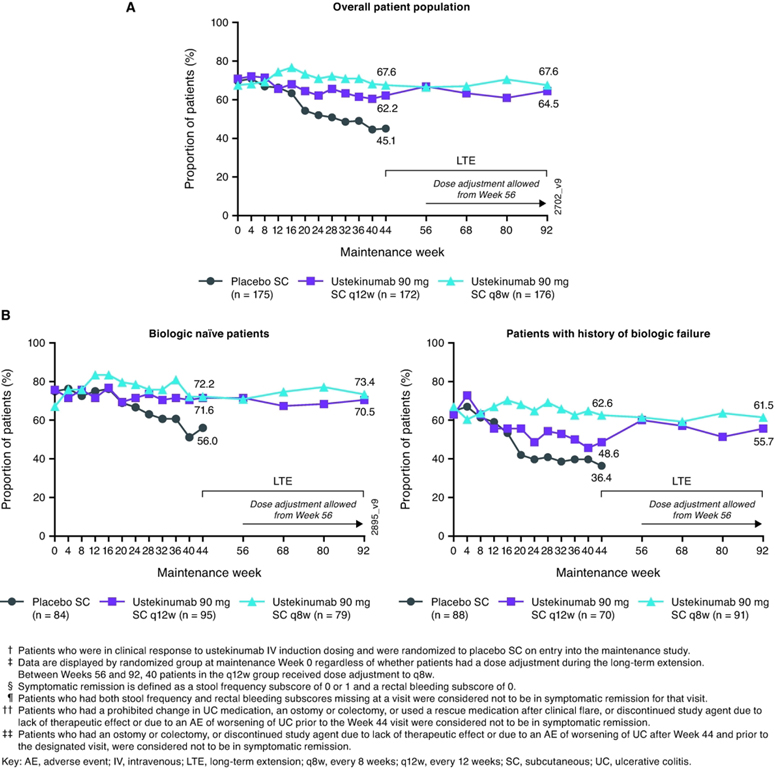
Symptomatic remission rates were generally sustained through Week 92 among patients who initially responded to ustekinumab induction and were randomized to ustekinumab maintenance (Figure 2A). At Week 44, 62.2% of patients randomized to 90 mg q12w and 67.6% of patients randomized to 90 mg q8w were in symptomatic remission. At Week 92, 64.5% and 67.6% of randomized patients, respectively, were in symptomatic remission. Proportions of patients in symptomatic remission were also sustained in biologic naïve patients (70.5% and 73.4% at Week 92, respectively) and in those with a history of biologic failure (55.7% and 61.5% at Week 92, respectively) (Figure 2B). Supplemental Figure S1 summarizes proportions of patients in symptomatic remission over time when dose-adjustment was considered a treatment failure.
Figure 2:
Nonresponder imputation analysis of all patients randomized in maintenance study (intent-to-treat population) for symptomatic remission†, ‡, §, ¶, ††, ‡‡ through Week 92 (A) and by biologic treatment history subgroup (B)
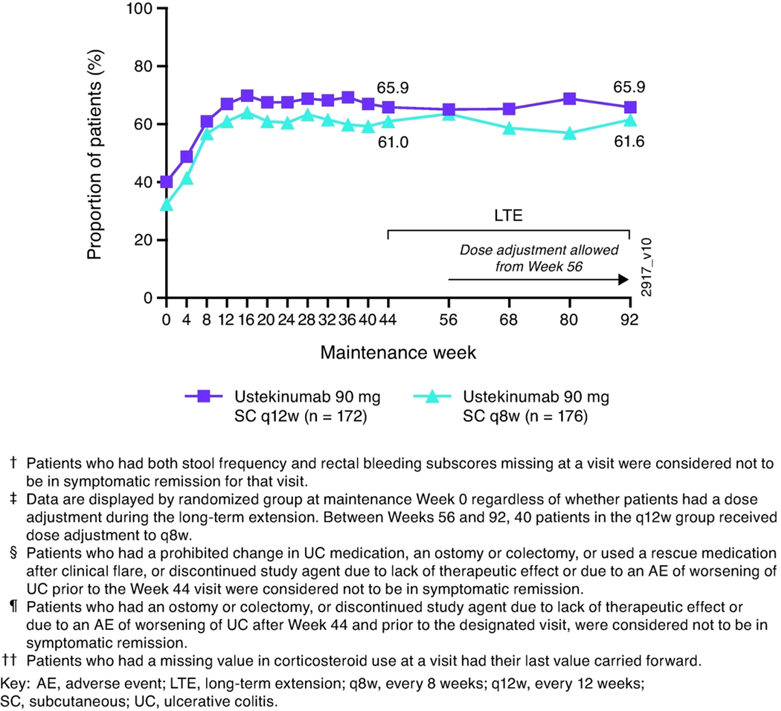
As steroids were tapered per protocol, proportions of patients achieving corticosteroid-free symptomatic remission increased during the maintenance study and were 61.6% and 65.9% at Week 92 for patients randomized to ustekinumab 90 mg q12w and q8w, respectively (Figure 3).
Figure 3:
Nonresponder imputation analysis of corticosteroid-free symptomatic remission through Week 92†, ‡, §, ¶, ††, ‡‡ for all patients randomized to ustekinumab in the maintenance study (intent-to-treat population)
Randomized patients treated in the long-term extension: nonresponder imputation analysis
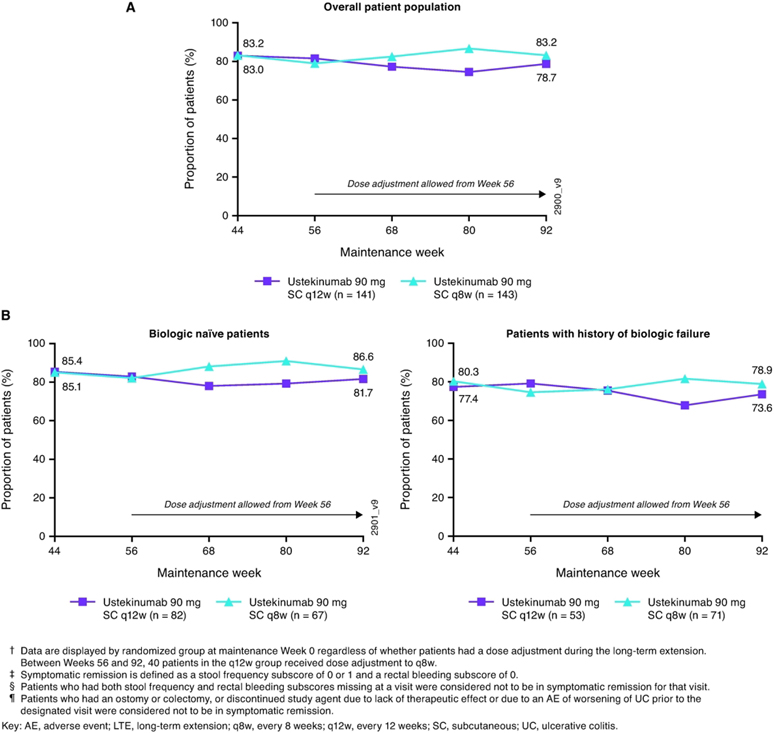
Proportions of patients in symptomatic remission were generally sustained through Week 92 (Figure 4A). At Week 44, 83.0% of patients in the q12w group and 83.2% of patients in the q8w group were in symptomatic remission. At Week 92, 78.7% and 83.2%, respectively, were in symptomatic remission. Proportions of patients in symptomatic remission were also sustained in biologic naïve patients (81.7% and 86.6% at Week 92, respectively) and in those with a history of biologic failure (73.6% and 78.9% at Week 92, respectively; Figure 4B). Supplemental Figure S2 summarizes proportions of patients who were in symptomatic remission from Week 44 through Week 92 when dose-adjustment was considered a treatment failure.
Figure 4:
Nonresponder imputation analysis of symptomatic remission†, ‡, §, ¶ from Week 44 through Week 92 for all patients randomized to ustekinumab in the maintenance study and treated in the long-term extension (A) and by biologic treatment history subgroup (B)
The proportions of patients from the ustekinumab q12w and q8w groups who had achieved symptomatic clinical remission at Week 44 were 83.0% and 83.2%, respectively. Among these patients, 72.6% and 70.6%, respectively, maintained symptomatic remission at Week 92.
Maintenance baseline median partial Mayo scores (2.0 in the ustekinumab q12w and q8w groups) were generally maintained from Week 44 (median change, −1.0 and −1.0 in the ustekinumab q12w and q8w groups, respectively) through Week 92 (median change, 0.0 and −1.0 in the ustekinumab q12w and q8w groups, respectively).
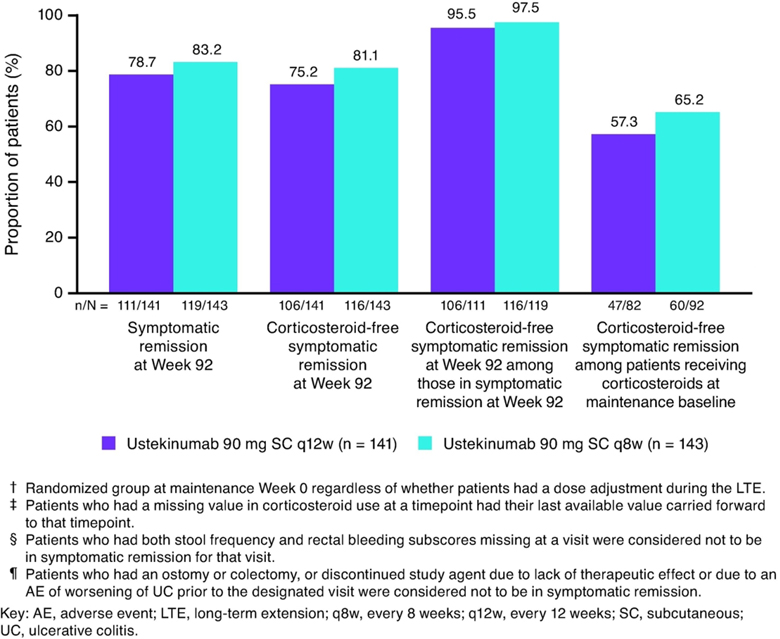
Greater than 95% of patients who were in symptomatic remission at Week 92 were corticosteroid-free (Figure 5).
Figure 5:
Nonresponder imputation analysis of symptomatic remission and corticosteroid-free symptomatic remission†, ‡, §, ¶ at Week 92; patients randomized to ustekinumab in the maintenance study and treated in the long-term extension
The mean daily prednisone-equivalent (P.Eq.) corticosteroid dose (excluding budesonide and beclomethasone dipropionate) among patients receiving corticosteroids at maintenance baseline was 15.4 mg/day in both ustekinumab groups. At Week 44, the mean daily doses in the ustekinumab q12w and q8w groups were 1.2 mg/day and 1.7 mg/day, respectively. Reductions observed by Week 44 were generally maintained through Week 92 (Supplemental Figure S3). Among patients receiving corticosteroids at maintenance baseline (including budesonide and beclomethasone dipropionate), 91.2% (62/68) and 94.4% (67/71) in the ustekinumab q12w and q8w groups, respectively, were not receiving corticosteroids at Week 92 on the basis of the nonresponder imputation analysis with dose adjustment as a treatment failure.
C reactive protein and fecal calprotectin
At maintenance baseline, median CRP concentrations were 1.5 mg/L and 1.8 mg/L in the ustekinumab q12w and q8w groups, respectively. Over time through Week 92, the median CRP concentrations at Week 44 were generally maintained (mean change 0.1 mg/L and 0.0 mg/L for the q12w and q8w groups, respectively; Supplemental Figure S4).
At maintenance baseline, median fecal calprotectin concentrations were 431.0 mg/kg and 450.5 mg/kg in the ustekinumab q12w and q8w groups, respectively. Through Week 92, median fecal calprotectin concentrations observed at Week 44 were generally maintained (Supplemental Figure S5).
Dose adjustment of patients randomized to ustekinumab and treated in the long-term extension
Patients randomized to ustekinumab maintenance who in the opinion of the investigator had a worsening of disease activity during the long-term extension could receive a dose adjustment after Week 56 as follows: ustekinumab q12w→ustekinumab q8w (n=40) and ustekinumab q8w→ustekinumab q8w (sham dose adjustment; n=37). Of these, 20 and 28 patients, respectively, had ≥16 weeks of follow-up. The majority of these patients were in symptomatic remission at the time of dose adjustment, 55.0% (11/20 patients) and 64.3% (18/28 patients), respectively (Supplemental Table S2). The proportion of patients in symptomatic remission ≥16 weeks after dose adjustment was 70.0% (14/20 patients) and 71.4% (20/28 patients), respectively. Among patients who were not in symptomatic remission at the time of dose adjustment, 44.4% (4/9 patients) and 60.0% (6/10 patients), respectively, were in symptomatic remission after dose adjustment. Among biologic naïve patients and those with a history of biologic failure, ≥70% of patients were in symptomatic remission after dose adjustment. Partial Mayo scores, and CRP and fecal calprotectin concentrations generally improved among patients who received dose adjustment although the sample sizes were limited (Supplemental Table S2).
Randomized patients treated in the long-term extension: observed case analysis
Among randomized patients with data at Week 44, 83.0% and 83.9% of patients in the ustekinumab q12w and q8w groups, respectively, were in symptomatic remission. Over time, proportions of patients in symptomatic remission were sustained from Week 44 through Week 92 in both ustekinumab groups and were 86.0% and 88.9% in the ustekinumab q12w and q8w groups at Week 92, respectively (Supplemental Figure S6A).
Proportions of patients in symptomatic remission were sustained from Week 44 through Week 92 in the ustekinumab q12w and q8w groups at similar rates using modified observed case analysis (Supplemental Figure S7A).
In the biologic treatment history subgroups, proportions of patients in symptomatic remission were sustained from Week 44 through Week 92 in both ustekinumab groups in biologic naïve patients and patients with a history of biologic failure for the observed case and modified observed case analyses (Supplemental Figures 6B and 7B, respectively).
Nonrandomized patients: nonresponder imputation analysis
Week-16 ustekinumab induction responders
Patients who received ustekinumab IV induction, who were not in clinical response 8 weeks later, received a single dose of SC ustekinumab 90 mg at induction Week 8, and were in clinical response 8 weeks later (induction Week 16) were identified as Week 16 responders and continued to receive 90 mg ustekinumab q8w through the maintenance study and long-term extension (Figure 1B). Among all ustekinumab Week 16 responders (n=157) enrolled at maintenance Week 0, the proportions of patients in symptomatic remission were sustained from Weeks 44 through Week 92, with 58.6% in symptomatic remission at Week 92. Of ustekinumab Week 16 responders treated in the long-term extension (n=116), the proportions of patients in symptomatic remission were sustained from Week 44 through Week 92 with 79.3% in symptomatic remission at Week 92.
Treatment interruption after ustekinumab IV induction
Patients who were ustekinumab IV induction responders, re-randomized to SC placebo at maintenance baseline, and treated with placebo during the long-term extension were eligible for dose adjustment to ustekinumab 90 mg q8w after Week 56 if according to the investigator’s judgement they had worsening disease (Figure 1B). Among placebo-treated patients, 46.1% (53/115) (Figure 1A) had a dose adjustment to ustekinumab q8w, and 42 patients had data ≥16 weeks after dose adjustment (Supplemental Table S3). Overall, 71.4% (30/42 patients) were in symptomatic remission at the first visit ≥16 weeks after dose adjustment, including 82.4% (14/17 patients) and 64.0% (16/25 patients) with and without symptomatic remission at the time of dose adjustment, respectively. Mean partial Mayo score (3.2, 1.5), and median CRP (3.6 mg/L, 2.0 mg/L) and median fecal calprotectin (1016.5 mg/kg, 355.0 mg/kg) concentrations at the time of treatment interruption improved ≥16 weeks after treatment re-introduction, respectively (Supplemental Table S3).
Pharmacokinetics and Immunogenicity
Randomized patients who continued receiving either the ustekinumab q12w or q8w dose regimen in the long-term extension had sustained and consistent levels of ustekinumab through Week 92 of the long-term extension that were generally comparable with serum ustekinumab levels observed during the maintenance phase of the study (Supplemental Table S4).
Between induction Week 0 and maintenance Week 96 of the long-term extension, 5.5% (22/400) of patients who received ustekinumab in maintenance and continued on ustekinumab in the long-term extension were positive for ADAs, including patients who were Week 8 responders to ustekinumab IV induction and randomized to ustekinumab SC maintenance, and those who were Week 16 responders who received SC maintenance thereafter; 18.2% (4/22) of these patients were positive for neutralizing antibodies. The proportions of randomized patients in symptomatic remission at Week 92 were comparable between those who were positive and those who were negative for antibodies to ustekinumab (Supplemental Table S5).
Safety
Among all patients treated in the long-term extension from maintenance Week 0 through long-term extension Week 96, the patient-years of follow-up was nearly 2.5 times greater for those receiving ustekinumab than placebo. The average duration of follow-up was comparable for those receiving placebo and ustekinumab. Rates of AEs, SAEs, AEs leading to discontinuation, serious infections, malignancies (excluding non-melanoma skin cancer [NMSC]), and deaths per hundred PYs of follow-up were generally similar for combined ustekinumab versus placebo (Table 4).
Table 4:
Key safety findings per hundred patient-years of follow-up from maintenance Week 0 through Week 96; patients who were treated in the long-term extension
| Ustekinumab | |||||
|---|---|---|---|---|---|
| Placebo SC† (N=188) |
90 mg SC q12w‡ (N-141) |
90 mg SC q8w§ (N=353) |
Combined (N=454) |
All Ustekinumab ¶ (N=516) |
|
| Avg duration of follow-up (weeks) | 81.3 | 88.8 | 77.7 | 80.8 | 79.2 |
| Total patient-years of follow-up | 293.9 | 240.7 | 527.3 | 768.0 | 786.1 |
| No. events per hundred patient-years of follow-up (95% CI) †† | |||||
| Adverse events | 272.54 | 257.61 | 295.47 | 290.19 | 285.71 |
| (253.99, 292.08) | (237.73, 278.71) | (280.98, 310.52) | (278.13, 302.64) | (274.01, 297.77) | |
| Serious adverse events | 8.85 | 7.06 | 8.91 | 8.53 | 8.14 |
| (5.78, 12.96) | (4.11, 11.31) | (6.55, 11.85) | (6.57, 10.89) | (6.27, 10.40) | |
| Infections ‡‡ | 81.66 | 83.51 | 91.03 | 90.73 | 89.17 |
| (71.65, 92.67) | (72.37, 95.89) | (83.07, 99.55) | (84.05, 97.81) | (82.69, 96.03) | |
| Serious infections ‡‡ | 2.38 | 4.15 | 1.71 | 2.53 | 2.42 |
| (0.96, 4.91) | (1.99, 7.64) | (0.78, 3.24) | (1.52, 3.95) | (1.46, 3.77) | |
| Adverse events leading to discontinuation of study agent | 4.08 | 2.49 | 2.47 | 2.53 | 2.42 |
| (2.11, 7.13) | (0.91, 5.43) | (1.31, 4.22) | (1.52, 3.95) | (1.46, 3.77) | |
| Death | 0.00 | 0.00 | 0.19 | 0.13 | 0.13 |
| (0.00, 1.02) | (0.00, 1.24) | (0.00, 1.06) | (0.00, 0.74) | (0.00, 0.71) | |
| All malignancies | 0.68 | 0.42 | 0.95 | 0.80 | 0.76 |
| (0.08, 2.46) | (0.01, 2.31) | (0.31, 2.21) | (0.29, 1.74) | (0.28, 1.66) | |
| Excluding nonmelanoma skin cancer | 0.34 | 0.00 | 0.00 | 0.00 | 0.00 |
| (0.01, 1.90) | (0.00, 1.24) | (0.00, 0.57) | (0.00, 0.40) | (0.00, 0.38) | |
| Nonmelanoma skin cancer | 0.34 | 0.42 | 0.95 | 0.80 | 0.76 |
| (0.01, 1.90) | (0.01, 2.31) | (0.31, 2.21) | (0.29, 1.74) | (0.28, 1.66) | |
Includes 1) data from M-8 onward for patients who were in clinical response to ustekinumab IV induction dosing and were randomized to placebo SC on entry into the maintenance study, up to the dose adjustment for patients who had dose adjustment during LTE; and 2) data from Week 0 of maintenance for patients who were in clinical response to placebo IV induction dosing and received placebo SC on entry into the maintenance study.
Includes data from M-0 through Week 96, or up to the dose adjustment if patients had a dose adjustment during the long-term extension (LTE), for patients who were in clinical response to ustekinumab IV induction dosing and were randomized to ustekinumab 90 mg SC q12w on entry into the maintenance study.
Includes: 1) Patients who were in clinical response to ustekinumab IV induction dosing and were randomized to receive ustekinumab 90 mg SC q8w on entry into the maintenance study, with data from M-0 through Week 96; 2) Patients who were in clinical response to ustekinumab IV induction dosing, randomized to receive placebo SC or ustekinumab 90 mg SC q12w on entry into the maintenance study, and had a dose adjustment to ustekinumab SC 90 mg q8w, with data from the time of dose adjustment onward; 3) Patients who were not in clinical response to ustekinumab at I-8 but were in clinical response at I-16 after a SC administration of ustekinumab at I-8 and received ustekinumab 90 mg SC q8w on entry into the maintenance study with data from M-0 through Week 96.
Includes: 1) data from M-0 to M-8 for patients who were in clinical response to ustekinumab IV induction dosing and received placebo SC on entry into the maintenance study; 2) data from the first ustekinumab dose through Week 96 for patients who were treated with ustekinumab 90 mg SC (q12w or q8w) on entry into the maintenance study or for patients who were in clinical response to ustekinumab IV induction dosing and were randomized to placebo SC on entry into the maintenance study and had a dose adjustment during the LTE with data from the time of dose adjustment onward.
Confidence intervals based on an exact method assuming that the observed number of events follows a Poisson distribution.
Infection as assessed by the investigator.
Note: The “combined” summarizes data for patients who received either ustekinumab SC 90 mg q12w or q8w. The “all ustekinumab” summarized data from “combined” plus the data from induction IV ustekinumab groups.
Key CI, confidence interval; IV, intravenous; I-8, induction Week 8; I-16, induction Week 16; M-0, maintenance Week 0; LTE, long-term extension; M-8, maintenance Week 8; q8w, every 8 weeks; q12w, every 12 weeks; SC, subcutaneous; UC, ulcerative colitis.
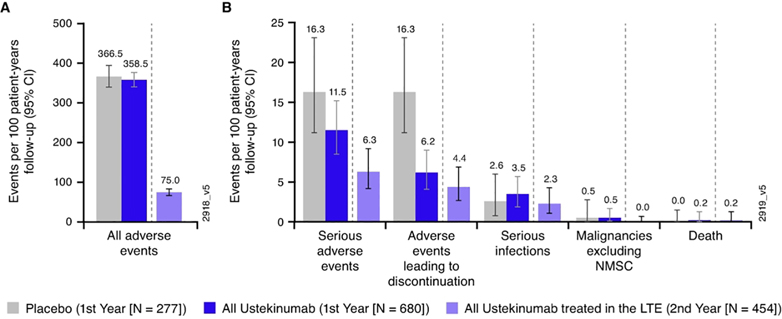
From Week 44 through Week 96, the average duration of follow-up for patients in the placebo group (37.1 weeks) was shorter than that in the ustekinumab q12w (44.5 weeks) and q8w (45.3 weeks) groups, largely due to patients on placebo being discontinued at the time of study unblinding; duration of follow-up was comparable in the ustekinumab groups (Supplemental Table S6). The number of AEs per hundred PYs of follow-up were 267.93, 223.82, and 268.17 in the placebo, ustekinumab q12w, and ustekinumab q8w groups, respectively. Ulcerative colitis, nasopharyngitis, upper respiratory tract infection, influenza, and headache were the most frequently reported AEs (Supplemental Table S6). There was no increase in the overall rates of AEs, SAEs, AEs leading to discontinuation, serious infections, malignancies (excluding non-melanoma skin cancer [NMSC]), or deaths from Week 44 through Week 96 with increased exposure to ustekinumab (Figure 6).
Figure 6:
All adverse events (A) and key safety events (B) during ustekinumab exposure†, ‡,§, ¶,††
†. Number of treatment-emergent adverse events per 100 patient-years of follow-up and 95% confidence interval (rates by each year of follow-up) in the pooled ustekinumab ulcerative colitis safety cohort. Confidence intervals based on an exact method assuming that the observed number of events follows a Poisson distribution.
‡. Infection as assessed by the investigator.
§. Placebo (First Year) includes 1) Patients who were in clinical response to ustekinumab IV induction dosing and were randomized to placebo SC on entry into this maintenance study and were followed after Week 8; and 2) Patients who were in clinical response to placebo IV induction dosing and received placebo SC on entry into this maintenance study. Only includes data from Week 8 onward for patients who were in clinical response to ustekinumab IV induction dosing and were randomized to placebo SC on entry into this maintenance study.
¶. All Ustekinumab (First Year) includes 1) patients who received ustekinumab SC (q8w or q12w) in this maintenance study; and 2) patients who were in clinical response to ustekinumab IV induction dosing and received placebo SC on entry into this maintenance study; 2)data from Week 0 to Week 8 for patients who were in clinical response to ustekinumab IV induction dosing and were randomized to placebo SC on entry into this maintenance study.
††. All Ustekinumab-treated in the LTE (Second Year) includes: 1) Patients who were in clinical response to ustekinumab IV induction dosing and were randomized to receive ustekinumab 90 mg SC q12w or q8w on entry into the maintenance study, with data from Week 44 through Week 96; 2) Patients who were in clinical response to ustekinumab IV induction dosing, randomized to receive placebo SC on entry into the maintenance study, and had a dose adjustment to ustekinumab 90 mg SC q8w, with data from the time of dose adjustment onward; 3) Patients who were not in clinical response to ustekinumab at I-8 but were in clinical response at I-16 after a SC administration of ustekinumab at I-8 and received ustekinumab 90 mg SC q8w on entry into the maintenance study with data from Week 44 through Week 96.
Key: CI, confidence interval; IV, intravenous; NMSC, nonmelanoma skin cancer; q8w, every 8 weeks; 12w, every 12 weeks; SC, subcutaneous
As previously reported,2 one patient died during the long-term extension. The patient had responded to ustekinumab IV induction, was randomized to placebo SC maintenance, and received one 90 mg ustekinumab dose after dose-adjusting from placebo. The immediate cause of death was attributed to cardiac arrest, but the patient had previously reported multiple AEs including cytomegalovirus (CMV) colitis, worsening UC, and failure to thrive. The patient also had multiple comorbidities, including prior myocardial infarction and coronary artery disease with placement of two stents.
Among patients treated with ustekinumab from Week 44 through Week 96, three patients had NMSCs. One patient each receiving ustekinumab q12w or q8w had basal cell carcinoma (BCC), one patient receiving ustekinumab q8w had a concurrent squamous cell carcinoma and BCC. Two patients who received placebo had one each: BCC and lentigo malignant melanoma. No other malignancies were reported.
Among all treated patients, serious major adverse cardiovascular events from Week 44 through Week 96 were reported in three patients: nonfatal myocardial infarction in one ustekinumab IV Week 16 responder, nonfatal stroke in one ustekinumab IV induction responder randomized to placebo SC maintenance therapy who dose-adjusted to ustekinumab q8w, and one cardiovascular death (reported above).
Two serious infections considered to be opportunistic were reported between Week 44 and Week 96. One patient was hospitalized for diarrhea secondary to UC complicated by CMV colitis as identified by the presence of CMV inclusion bodies on biopsy (>60 years old, ustekinumab IV induction responder randomized to placebo SC maintenance therapy who dose-adjusted to ustekinumab q8w); this patient as reported above expired due to cardiac arrest. Another patient (>60 years old, placebo SC maintenance therapy who dose-adjusted to ustekinumab q8w) was hospitalized for symptoms associated with worsening UC and fever, received treatment including ceftriaxone and tacrolimus, and had a blood culture positive for Listeria monocytogenes.
Among ustekinumab-treated patients from Week 44 through Week 96, proportions of injections with injection site reactions were 0.5% among all ustekinumab injections and 0.3% among all placebo injections administered. There were no cases of anaphylactic or delayed hypersensitivity reactions reported from Week 44 through Week 96. No relationship between the development of ADAs and injections with injection-site reactions was identified in this study (Supplemental Table S7).
DISCUSSION
The objectives of this long-term extension study were to assess the efficacy, PK and immunogenicity, and safety, of one additional year of ustekinumab maintenance treatment in patients originally manifesting moderate-to-severe UC activity who had completed the 44-week maintenance study. Of the 523 randomized patients who participated in the maintenance study, 399 (76.3%) patients continued treatment in the long-term extension. Greater than 90% of patients with no history of biologic failure were biologic naïve. Approximately 25% of patients with a history of biologic failure had failed both vedolizumab and a TNF antagonist.
In the long-term extension, a majority of randomized patients who continued to receive ustekinumab completed study participation through Week 96. Additionally, among all ustekinumab-treated randomized patients and Week 16-responder nonrandomized patients, 95.3% (388/400) of patients completed study participation through Week 96.
Our findings show that among all ustekinumab induction responders who were randomized to ustekinumab in the maintenance study (intent-to-treat population), approximately two-thirds were in symptomatic remission at Week 92. Among randomized patients who continued treatment in the long-term extension, greater than 80% were in symptomatic remission at Week 92. Rates of symptomatic remission were maintained from Week 44 through Week 92. Rates for symptomatic remission over time are supported by sustained improvements in partial Mayo scores, and reductions in CRP and fecal calprotectin concentrations. The majority (>95%) of patients who achieved symptomatic remission at Week 92 were also not receiving corticosteroids. Similar and consistent efficacy was observed for ustekinumab q12w and q8w dosing.
Sustained efficacy was observed under varying clinical scenarios including biologic treatment history (e.g., those with documented biologic failure or those naïve to biologic therapy), when dose adjustment was considered part of the treatment regimen, and with those patients with a delayed response to their initial treatment with ustekinumab. Also, in a limited subset of patients who responded to the ustekinumab IV induction dose but delayed initiation of the SC ustekinumab maintenance therapy (ie, were randomized to placebo maintenance and dose adjusted to ustekinumab maintenance), ustekinumab was still effective after dose interruption.
Following continued treatment with ustekinumab q12w or q8w, sustained serum ustekinumab concentrations were observed through Week 92 that were generally consistent with serum ustekinumab concentrations observed during the maintenance study. Through two years of ustekinumab maintenance therapy, rates of ADAs were low with no notable impact on proportions of patients in symptomatic remission at Week 92 or injections with injection-site reactions.
Nasopharyngitis was the most frequently reported infection and UC flare was the most frequently reported gastrointestinal AE. Malignancy rates were low and similar between groups, and primarily NMSC. Age, prior immunomodulator use, and sun exposure were confounding factors in these patients. One death due to cardiac arrest occurred in the long-term extension and was reported previously.2 No cases of tuberculosis were reported in ustekinumab-treated patients. Two patients developed serious infections considered to be opportunistic infections (CMV colitis and L. monocytogenes infection detected by blood culture). Proportions of injections with injection-site reactions remained low from Week 44 through Week 96, with no reports of serious reactions, anaphylaxis, or serum sickness-like reactions.
The efficacy and safety findings reported here are consistent with those reported in patients with moderately-to-severely active Crohn’s disease who also had their clinical benefits maintained through two years with ustekinumab 90 mg q12w and q8w.5
With the introduction of biologic therapies including TNF-α,6–9 α4β7 integrin–MAdCAM-1,10 and Janus kinase antagonists,11 management of UC has greatly improved. Efficacy and safety reported from study extensions of phase 3, randomized clinical trials, showed that an additional year of treatment with infliximab,12 golimumab,13 adalimumab,14 vedolizumab,15 or tofacitinib16 maintained efficacy and safety profiles of the respective therapies.
It should be noted that the patients receiving placebo in the long-term extension originated from both randomized and nonrandomized populations with different treatment histories in the study. Patients randomized to placebo maintenance had responded to IV ustekinumab induction, and efficacy shown by these patients in the maintenance study extension may reflect an as yet identified prolonged pharmacodynamic effect as median serum ustekinumab levels in this group were below the level of detection 20 weeks following the single IV administration. Patients who were in clinical response after IV placebo induction received SC placebo in the maintenance study and were followed in the nonrandomized population. All patients and investigators were blinded to induction and maintenance study treatments into the long-term extension, including placebo, until the Week 44 database was locked, and the study was unblinded. Patients who were still receiving placebo at the time of unblinding were discontinued from the long-term extension per protocol. Because patients were enrolled in the study at different times but stopped the study at the same time, their treatment duration during the long-term extension varied. Of note, from Week 44 through Week 96, the placebo groups had 12 fewer weeks of follow-up on average (37.1 weeks) as compared with the combined ustekinumab groups (49.1 weeks). Therefore, we did not use efficacy data from the placebo group after Week 44 for efficacy comparisons; however, they were included in the safety analyses that were normalized by summarizing events per 100 PY of follow up.
Placebo-treated patients in the randomized maintenance population who dose adjusted to ustekinumab maintenance provided an opportunity to evaluate ustekinumab treatment interruption, since they responded to ustekinumab IV induction and started ustekinumab maintenance after initially being randomized to placebo maintenance for at least one year. The results showed that patients improved after starting ustekinumab maintenance. While delaying the start of ustekinumab maintenance after induction may not be a recommended treatment regimen in patients with inflammatory bowel diseases, these results suggest that patients may still show benefit if such a delay is unavoidable.
Our results should be interpreted in light of the limitations associated with the design of the long-term extension. Patients were selected by the investigator to participate because, in their opinion, they might benefit from continued treatment, which may limit the generalizability of the results of analyses based solely on the cohort of patients treated in the long-term extension. Unlike the rigorously-controlled maintenance study where concomitant UC medication dosages except for oral corticosteroids remained constant through Week 44, during the long-term extension patients could change concomitant medications at any time which mimics real world practice. Also, when considering the data for dose adjustment within the randomized population, it should be noted that the decision to dose adjust was based on the clinical judgement of the investigator regarding a patient’s disease activity; no protocol-specified criteria (e.g., clinical flare based on partial Mayo score) were applied and some patients were in symptomatic remission at the time of the dose adjustment, thereby limiting the interpretability of these data.
The results reported here in patients with moderately-to-severely active UC, together with both clinical trial and registry data confirm the positive long-term efficacy and safety profile of ustekinumab-treated patients with other immune-mediated inflammatory diseases such as psoriasis17–19 and Crohn’s disease.5,20 In summary, patients with moderately-to-severely active UC treated with ustekinumab 90 mg SC q12w or q8w maintained symptomatic remission measured through the second year of maintenance treatment (Week 44 through Week 96). The safety profile observed for ustekinumab in the second year of maintenance treatment was consistent with that reported through the first year during the maintenance study and with the established ustekinumab safety profile; no new safety signals were identified.
Supplementary Material
Acknowledgments:
Editorial support was provided by James P. Barrett, BS an employee of Janssen Scientific Affairs, LLC.
Declaration of funding interests:
This study was funded by Janssen Research & Development, LLC. William Sandborn supported in part by NIDDK-funded San Diego Digestive Diseases Research Center (P30 DK120515).
Data were presented in part at United European Gastroenterology Week 2019 (oral presentation, Barcelona, Spain), 2020 European Crohn’s and Colitis Organisation (poster, digital oral, Vienna, Austria), and Digestive Disease Week 2020 (poster, on-line).
Disclosure of Financial Relationships
Remo Panaccione, MD reports having received consulting fees from AbbVie, Janssen Research & Development, LLC., Takada California, Inc.
Silvio Danese, MD, PhD reports receiving consulting fees from Allergan, Amgen, AstraZeneca, Biogen, Boehringer Ingelheim, Celgene, Celltrion Healthcare, Ferring, Gilead Sciences, Hospira, Inc., Janssen Research & Development, LLC., Johnson & Johnson Health Care Systems, Inc., Pfizer, Sandoz, UCB, Inc., Vifor International Inc.
William J Sandborn, MD reports: research grants from Atlantic Healthcare Limited, Amgen, Genentech, Gilead Sciences, AbbVie, Janssen Research & Development, LLC., Takeda, Lilly, Celgene/Receptos, Pfizer, Prometheus Laboratories (now Prometheus Biosciences); consulting fees from AbbVie, Allergan, Amgen, Arena Pharmaceuticals, Avexegen Therapeutics, BeiGene, Boehringer Ingelheim, Celgene, Celltrion, Conatus, Cosmo, Escalier Biosciences, Ferring, Forbion, Genentech, Gilead Sciences, Gossamer Bio, Incyte, Janssen Research & Development, LLC., Kyowa Kirin Pharmaceutical Research, Landos Biopharma, Lilly, Oppilan Pharma, Otsuka, Pfizer, Progenity, Prometheus Biosciences (merger of Precision IBD and Prometheus Laboratories), Reistone, Ritter Pharmaceuticals, Robarts Clinical Trials (owned by Health Academic Research Trust, HART), Series Therapeutics, Shire, Sienna Biopharmaceuticals, Sigmoid Biotechnologies, Sterna Biologicals, Sublimity Therapeutics, Takeda, Theravance Biopharma, Tigenix, Tillotts Pharma, UCB Pharma, Ventyx Biosciences, Vimalan Biosciences, Vivelix Pharmaceuticals; and stock or stock options from BeiGene, Escalier Biosciences, Gossamer Bio, Oppilan Pharma, Prometheus Biosciences (merger of Precision IBD and Prometheus Laboratories), Progenity, Ritter Pharmaceuticals, Ventyx Biosciences, Vimalan Biosciences. Spouse: Iveric Bio - consultant, stock options; Progenity - consultant, stock; Oppilan Pharma - consultant, stock options; Escalier Biosciences – prior employee, stock options; Prometheus Biosciences (merger of Precision IBD and Prometheus Laboratories) - employee, stock options; Ventyx Biosciences – stock options; Vimalan Biosciences – stock options.
Stephan Targan, MD reports having received consulting fees from: Robarts Clinical Trials, Prometheus Biosciences and owns stock in Prometheus Biosciences.
Maria T Abreu, MD reports having received grant support from Prometheus Bioscience, Takeda, and Pfizer; consulting fees from Janssen Research & Development, LLC., Prometheus Bioscience, Takeda, Focus Medical Communications, Pfizer, Boehringer Ingelheim Pharmaceuticals, Gilead, Imedex, Cornerstone Health, Inc, Landos Biophama, UCB Biopharma SRL, Eli Lilly, and Cosmo Biopharma.
Tadakazu Hisamatsu, MD, PhD reports having received grant support from AbbVie, Daiichi-Sankyo, EA Pharma Co, Ltd. JIMRO, Mitsubishi Tanabe Pharma Corporation, Mochida Pharmacuetical Co., Ltd., Nippon Kayaku Co., Ltd., Pfizer Inc., Takeda Pharmaceutical Co., Ltd., consulting fees from EA Pharma Co, Ltd., Janssen Research & Development, LLC.,, and lecture fees from AbbVie, EA Pharma Co, Ltd, Mitsubishi Tanabe Pharma Corporation, Takeda Pharmaceutical Co., Ltd.
Ellen J Scherl, MD reports having received grant support from AbbVie, AstraZeneca, Crohn’s and Colitis Foundation, Janssen Research & Development, LLC., New York Crohn’s Foundation, Pfizer, UCB, Genentech, Seres Therapeutics, and Celgene; consulting fees: AbbVie, Abgenomics, Crohn’s and Colitis Foundation, Evidera, GI Health Foundation, Janssen, Protagonist, Seres, Takeda Stock: Gilead; speaker fees from GI Health Foundation, Prime Therapeutics.
Rupert W Leong, MD reports grant support from NHMRC, GESA, Janssen, Shire, Endochoice, Ferring; and consulting fees from Janssen Research & Development, LLC. Aspen, AbbVie, Celgene, Ferring, Pfizer, Hospira, Novartis, Dr Falk Pharma, MSD, Takeda.
David S Rowbotham, MD reports grant support from NIL, consulting fees from NIL, lecture fees from AbbVie; Janssen Research & Development, LLC., Baxter; Pharmaco; bioCSL; Given imaging; Emerge Health, and has served on the advisory board of AbbVie; Janssen Research & Development, LLC.; Baxter; Pharmaco; Hospira.
Ramesh P Arasaradnam, PhD reports grant support and consulting fees from Janssen Research & Development, LLC.
Bruce E Sands, MD reports personal fees from AbbVie, personal fees from Amgen, personal fees from AstraZeneca, personal fees from Bristol-Myers Squibb, grants and personal fees from Celgene, personal fees and non-financial support from Janssen Research & Development, LLC.,, personal fees and non-financial support from MedImmune, grants, personal fees and non-financial support from Takeda, personal fees from Akros Pharma, personal fees from Arena Pharmaceuticals, personal fees from Boehringer-Ingelheim, personal fees from Forward Pharma, personal fees from Immune Pharmaceuticals, personal fees and non-financial support from Lilly, personal fees from Shire, personal fees from Synergy Pharmaceuticals, personal fees from Theravance Biopharma R&D, personal fees from TiGenix, personal fees from TopiVert Pharma, personal fees from Receptos, personal fees from Allergan, personal fees from EnGene, personal fees from Target PharmaSolutions, personal fees from Lycera, personal fees from Lyndra, personal fees from Ironwood Pharmaceuticals, personal fees from Salix, personal fees from Vivelix Pharmaceuticals, personal fees from UCB, personal fees from Oppilan Pharmaceuticals, personal fees from Gilead, personal fees from Rheos Medicines, personal fees from Seres Therapeutics, personal fees from 4D Pharma, personal fees from Capella Bioscience, personal fees from Otsuka, personal fees from Ferring, personal fees from Protagonist Therapeutics, personal fees from Palatin Technologies, grants, personal fees and non-financial support from Pfizer, personal fees from Hoffman-La Roche, personal fees from Prometheus Laboratories.
Christopher D O’Brien, Yiying Zhou, Hongyan Zhang, Omoniyi J Adedokun, Ilia Tikhonov, and Colleen Marano are employees of Janssen Research & Development, LLC. and own stock in Johnson & Johnson, of which Janssen is a subsidiary.
The Ethics Committee or Institutional Review Board at each participating site reviewed and approved the protocol. All patients provided informed consent before participating in the study.
REFERENCES
- 1.Danese S, Fiocchi C. Ulcerative colitis. N Engl J Med 2011;365:1713–1725. [DOI] [PubMed] [Google Scholar]
- 2.Sands BE, Sandborn WJ, Panaccione R, et al. Ustekinumab as induction and maintenance therapy for ulcerative colitis. New Engl J Med. 2019;381:1201–1214. [DOI] [PubMed] [Google Scholar]
- 3.Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis: a randomized study. N Engl J Med 1987;317:1625–1629. [DOI] [PubMed] [Google Scholar]
- 4.D’Haens G, Sandborn WJ, Feagan BG, et al. A review of activity indices and efficacy end points for clinical trials of medical therapy in adults with ulcerative colitis. Gastroenterology 2007;132:763–786. [DOI] [PubMed] [Google Scholar]
- 5.Sandborn WJ, Rutgeerts P, Gasink C, et al. Long-term efficacy and safety of ustekinumab for Crohn’s disease through the second year of therapy. Aliment Pharmacol Ther 2018;48:65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med 2005;353:2462–2476. [DOI] [PubMed] [Google Scholar]
- 7.Sandborn WJ, Feagan BG, Marano C, et al. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2014; 146:85–95. [DOI] [PubMed] [Google Scholar]
- 8.Sandborn WJ, Feagan BG, Marano C, et al. Subcutaneous golimumab maintains clinical response in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2014; 146:96–109. [DOI] [PubMed] [Google Scholar]
- 9.Reinisch W, Sandborn WJ, Hommes DW, et al. Adalimumab for induction of clinical remission in moderately to severely active ulcerative colitis: results of a randomised controlled trial. Gut 2011;60:780–787 [DOI] [PubMed] [Google Scholar]
- 10.Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013; 369:699–710. [DOI] [PubMed] [Google Scholar]
- 11.Sandborn WJ, Su C, Sands BE, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2017;376:1723–1736. [DOI] [PubMed] [Google Scholar]
- 12.Reinisch W, Sandborn WJ, Rutgeerts P, et al. Long-term infliximab maintenance therapy for ulcerative colitis: the ACT-1 and −2 extension studies. Inflamm Bowel Dis 2012;18:201–211. [DOI] [PubMed] [Google Scholar]
- 13.Gibson PR, Feagan BG, Sandborn WJ, et al. Maintenance of efficacy and continuingsafety of golimumab for active ulcerative colitis: PURSUIT-SC maintenance study extension through 1 year. Clin Translation Gastroenterol 2016;7:e168; doi: 10.1038/ctg.2016.24; published online 28 April 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colombel J-F, Sandborn WJ, Gosh S, et al. Four-year maintenance treatment with adalimumab in patients with moderately to severely active ulcerative colitis: data from ULTRA 1, 2, and 3. Am J Gastroenterol 2014; 109:1771–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaser A, James A, et al. Long-term effectiveness and safety of vedolizumab in patients with ulcerative colitis: 5-year cumulative exposure of Gemini 1 completers rolling into the Gemini open-label extension study. Gut 2017;66(Suppl 2):A120 [abstract]. [Google Scholar]
- 16.Chiorean M, Su C, Matsuoka K, et al. Efficacy and safety of open-label treatment with tofacitinib 10 mg twice daily in patients with ulcerative colitis with clinical response, but not remission, after 52 weeks of maintenance therapy: data from the OCTAVE studies. J Crohns Colitis 2019;13(Suppl 1):S049–S050 [abstract]. [Google Scholar]
- 17.Kimball AB, Papp KA, Wasfi Y, et al. Long-term efficacy of ustekinumab in patients with moderate-to-severe psoriasis treated for up to 5 years in the PHOENIX 1 study. J Eur Acad Dermatol Venereol 2013;27:1535–1545. [DOI] [PubMed] [Google Scholar]
- 18.Menter A, Papp KA, Gooderham M, et al. Drug survival of biologic therapy in a large, disease-based registry of patients with psoriasis: results from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Eur Acad Dermatol Venereol. 2016;30:1148–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kimball AB, Gordon KB, Fakharzadeh S, et al. Long-term efficacy of ustekinumab in patients with moderate-to-severe psoriasis: results from the PHOENIX 1 trial through up to 3 years. Br J Dermatol. 2012;166:861–872 [DOI] [PubMed] [Google Scholar]
- 20.Feagan BG, Sandborn WJ, Gasink C, et al. Ustekinumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med 2016;375:1946–1960. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.