Abstract
Although Borrelia theileri, the agent of bovine borreliosis, was described at the turn of the century (in 1903), its relationship with borreliae causing Lyme disease or relapsing fever remains undescribed. We tested the previously published hypothesis that spirochetes infecting Lone Star ticks (Amblyomma americanum) may comprise B. theileri by analyzing the 16S ribosomal DNAs (rDNAs) and flagellin genes of these spirochetes. B. theileri, the Amblyomma agent, and B. miyamotoi formed a natural group or clade distinct from but most closely related to that of the relapsing fever spirochetes. B. theileri and the Amblyomma agent were 97 and 98% similar at the nucleotide level within the analyzed portions of the 16S rDNA and the flagellin gene respectively, suggesting a recent divergence. The agent of bovine borreliosis might be explored as a surrogate antigen for the as-yet-uncultivatable Amblyomma agent in studies designed to explore the etiology of a Lyme disease-like infection associated with Lone Star ticks.
Ticks comprise two major natural groups, or clades: the Ixodidae (hard ticks) and the more primitive mite-like Argasidae (soft ticks) (4, 14). The spirochetes associated with these morphologically and biologically divergent kinds of ticks have generally been referred to as either hard or soft tick-borne borreliae (16). Interestingly, of the speciose hard ticks, solely the Lyme disease-like spirochetes (Borrelia burgdorferi sensu lato), transmitted by Ixodes spp. (subfamily Prostriata), have been extensively studied. Although B. theileri, associated with Rhipicephalus and Boophilus (both classified within the ixodid subfamily Metastriata), was described during the early part of the 20th century (17), its relationship to other borreliae remains poorly described. Spirochetes have been detected within the metastriate Lone Star ticks (Amblyomma americanum) (1, 2, 10, 18, 24), but only recently has their taxonomic status been analyzed. Their identity has assumed prominence because Lone Star ticks are associated with a syndrome of undescribed etiology (referred to as southern tick-associated rash infection or Masters' disease) that is confused with Lyme disease (3, 8, 22; P. M. Armstrong, L. Rosa Brunet, A. Spielman, and S. R. Telford III, submitted for publication). Because deer served as reservoir hosts for Boophilus spp. in the south central United States during the 19th and early 20th centuries, we previously suggested that spirochetes infecting Amblyomma, a deer-dependent tick, may represent a host shift of B. theileri (26). We tested this hypothesis by analyzing the nucleotide sequences of the 16S ribosomal DNAs (rDNAs) and flagellin (fla) genes of B. theileri and the Amblyomma agent, and we determined the genetic relationship of these previously uncharacterized spirochetes to others of the genus Borrelia.
MATERIALS AND METHODS
Sources of borreliae.
B. theileri was derived from naturally infected Boophilus microplus originally collected from a Mexican site, as described previously and under an existing U.S. Department of Agriculture permit (25). A colony of infected Boophilus ticks was produced by allowing nymphs to engorge on infected cattle. The resulting adult ticks were examined by the hemolymph test (5) to determine whether they were infected, allowed to engorge and oviposit to maintain the infected colony, and stored frozen at −70°C until they were analyzed.
Nymphal and adult A. americanum ticks were collected by sweeping vegetation on Gibson Island, Md., during 1994 and nondestructively assayed for spirochetal infection by dark-field microscopy of hemolymph samples taken from the amputated stump of a leg (5). Of 388 ticks, 2 contained spirochetes. Their gut contents were removed and homogenized in 100 μl of lysis buffer (4 M guanidine thiocyanate, 25 mM sodium citrate, 0.5% sarcosyl), and total DNA was extracted by a standard phenol-chloroform extraction protocol. Attempts to cultivate the agent in BSK II and Kelly's medium failed.
DNA amplification and sequencing of borrelial genes.
PCR was used to amplify an approximately 350-bp portion of the fla gene (primers FLA-1 [5′-GATGATGCTGCTGGCATGGGAGTTGCGGG-3′] and FLAG-5 [5′-CCTGAAAGTGATGCTGGTG-3′]), as well as an 886-bp fragment of the 16S rDNA (primers BOR-16SA [5′-TGTTAATTGATGAAAGGAAGCC-3′] and BOR-16SE [5′-TTTACATGCTGGTAACAGAC-3′]). Each 50-μl reaction mixture contained 5 μl of EXPAND 10× PCR buffer (Boehringer Mannheim), 0.2 μl of each deoxynucleoside triphosphate (10 μM), 0.2 μl of EXPAND Taq polymerase (Boehringer Mannheim), 1 μl of each primer (25 μM), and about 10 ng of template. Each of the 35 cycles consisted of denaturation at 94°C for 30 s, annealing at 50°C for 30 s, and extension at 72°C for 2 min in a Perkin-Elmer (model 480) thermal cycler.
Double-stranded amplification products were electrophoresed in a 1.5% agarose gel in 1× Tris-borate-EDTA buffer and were extracted from excised gel bands using the GeneClean kit (Bio 101). Cycle sequencing was performed using the Applied Biosystems dye-labeled dideoxy termination kit, and the products were purified and analyzed on a 6% polyacrylamide gel in an ABi 373A DNA sequencer (Applied Biosystems), as recommended by the manufacturer.
Sequences were edited using the SeqEd 675 DNA sequence editor program (Applied Biosystems). Each sequence was verified in duplicate by analyzing both strands of the amplification product.
Phylogenetic analysis.
Borrelia gene sequences were initially aligned with the CLUSTALW program (28). Corrections to alignments were made manually, particularly in the case of the fla gene, where the algorithm failed to preserve the codon reading frame. The sequences that we derived from B. theileri and the Amblyomma agent were compared with representative Borrelia 16S rDNA and fla gene sequences accessioned in GenBank (Table 1). When the Harvard laboratory submitted the sequences for the Amblyomma agent to GenBank in October 1995 (1), those for B. lonestari (2) were under embargo and unavailable for comparison. At the time we had provisionally named this spirochete B. barbouri, in honor of Alan Barbour's many contributions to the study of Borrelia biology. The sequences accessioned under the names B. lonestari and B. barbouri are identical and derive from the same entity; pending a formal designation in Bergey's Manual, we here use the term Amblyomma agent to refer to it.
TABLE 1.
Spirochete species used for phylogenetic analysis
| Species | Strain | GenBank accession no.a
|
|
|---|---|---|---|
| fla | 16S rDNA | ||
| B. afzelii | DK4 | X85194 | |
| HT61 | D63366 | ||
| B. barbouri | NAb | U42432 | U38374 |
| B. burgdorferi | B31 | X15661 | U03396 |
| B. crocidurae | NA | X75204 | |
| UESV/MER | U42291 | ||
| B. duttonii | NA | U28497 | |
| UESV/117DUT | U42288 | ||
| B. garinii | 20047 | D82846 | D67018 |
| B. hermsii | HS1 | X53940 | U42292 |
| HS1 | M86838 | ||
| B. hispanica | NA | U28498 | |
| UESV/246 | U42294 | ||
| B. japonica | IKA2 | D82853 | L40598 |
| B. lonestari | Texas20 | U26704 | U23211 |
| B. miyamotoi | HT31 | D43777 | D45192 |
| B. parkeri | NA | B82863 | U42296 |
| B. recurrentis | NA | D86618 | U42300 |
| B. theileri | NA | U42431 | U38375 |
| B. turicatae | M2007 | D82862 | U42299 |
| Cristispira sp. | CP1 | U42638 | |
New GenBank accession numbers are in boldface.
NA, not applicable or not designated.
Two 50% majority rule consensus phylograms, one for each of the two genes examined, were constructed by performing 1000 bootstrap replications of the PAUP 4.0 (27) program's “Fast” stepwise addition heuristic tree search method. In the 16S rDNA-based analysis, Cristispira sp. served as the outgroup, whereas the fla-based phylogeny is an unrooted network
Nucleotide sequence accession numbers.
The sequences determined in this study have been deposited in GenBank under accession numbers U38374, U38375, U42431, and U42432.
RESULTS
Relationships among spirochetes.
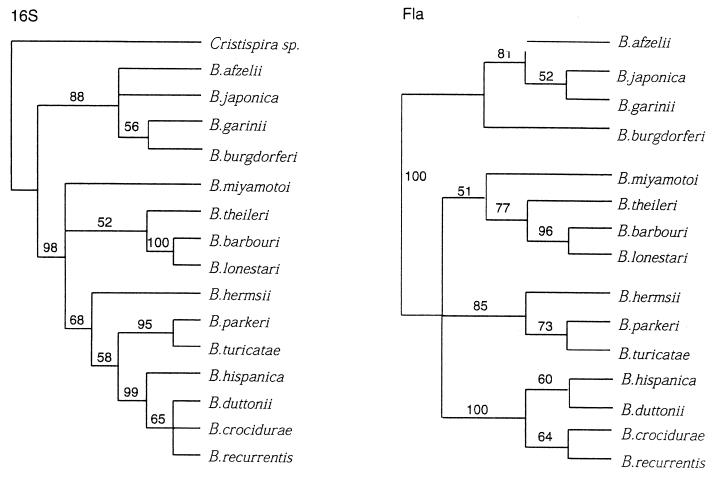
Maximum-parsimony analysis was used to determine the relatedness of B. theileri, the Amblyomma agent, and B. recurrentis to several other Borrelia species, based on the 16S rDNAs and fla genes. The low levels of nucleotide polymorphism in either the flagellin gene or 16S rDNA limits the information for resolving terminal taxa. However, reliable inferences can be drawn from the two trees for the more broadly defined groupings. Consistent with many other previous reports (9, 15, 20, 21, 23), the group of B. burgdorferi sensu lato spirochetes (B. burgdorferi sensu stricto, B garinii, and B. afzelii) group as a diverse monophyly (Fig. 1). Our analysis also places B. japonica within the clade comprised of B. burgdorferi sensu lato.
FIG. 1.
Bootstrap consensus (1,000 times) of neighbor-joining trees of Borrelia spirochetes using both 16S rDNA (left) and flagellin (right) loci. The oral spirochete Cristispira sp. was used as an outgroup. Distances were derived from the Tamura-Nei algorithm (27a). Numbers indicate the bootstrap support for each branch.
Phylograms based on both fla and 16S rDNA data sets (Fig. 1) demonstrate that B. theileri, the Amblyomma agent, and B. miyamotoi form a monophyletic group most closely related to the two soft tick-transmitted spirochete clades. Based on the fla DNA sequence, these Borrelia spp. form a monophyletic sister group to the several relapsing fever spirochetes.
DISCUSSION
Our analysis supports Filippova's hypothesis that the vectors and agents of Lyme disease comprise a monophyletic clade indicating evolution by descent within this group (11). The one exception is B. miyamotoi, originally isolated from Ixodes persulcatus (12, 13), the main Eurasian vector of B. burgdorferi sensu lato. Although this association may reflect a secondary host shift from the metastriate-associated borreliae, it may be that I. persulcatus is not the main vector but that sympatric Japanese ticks such as the metastriate Haemaphysalis longicornis serve in this capacity. Further studies on the epizootiology of B. miyamotoi may resolve this interesting exception to the otherwise strongly supported congruence between Ixodes ticks and Lyme disease-like borreliae.
The metastriate tick-transmitted B. theileri and the Amblyomma spirochete clearly group outside the other ixodid-borne borreliae, and certain features of their biology corroborate this finding. Bovine borreliosis due to B. theileri is characterized by prominent spirochetemias in the peripheral blood (7, 25), as is relapsing fever. Both B. theileri and many of the argasid-transmitted spirochetes may be efficiently maintained by transovarial transmission. In the vector, these spirochetes may easily be found in the hemolymph (25), in sharp contrast to the Ixodes-transmitted borreliae, which less frequently produce disseminated infections in the tick hosts (6). The Amblyomma agent is detectable within ticks as frequently by the hemolymph test as it is by indirect immunofluorescence of gut contents (Fisher exact test, P = 0.376) (our unpublished results), suggesting that other features of B. theileri's biology may be shared, such as the presence of spirochetemia in the as-yet-undescribed reservoir and its possible maintenance in ticks by transovarial transmission.
The degree of sequence similarity between the Amblyomma agent and B. theileri is similar to that between different isolates of B. hermsii and may reflect a very recent divergence of the two metastriate-transmitted spirochetes. Because A. americanum feeds mainly upon deer in all three instars, we previously reasoned that any spirochete maintained by this tick might be closely related to that maintained by the cattle tick Boophilus (26). Indeed, deer served as important enzootic hosts for the tick B. microplus, and the eradication program for this economically important ectoparasite of cattle (because of its role as a vector of Texas cattle fever or bovine babesiosis) targeted cervids throughout the southern United States (19). Because cattle and deer are sympatric in many sites, Boophilus and the agents that it maintained could have established parallel transmission cycles in deer.
Although specific names have been applied to the Amblyomma agent (B. lonestari, B. barbouri, and a Borrelia sp. similar to B. nr. miyamotoi), given its genetic similarity to B. theileri, any formal description would require justifying the application of a new name. More importantly, because the Amblyomma agent is as yet uncultivatable, determining its role in the etiology of southern tick-associated rash infection (Masters' disease) may be facilitated by the use of B. theileri as a surrogate. These spirochetes attain a great density within the blood of cattle and may be purified for use as an antigen. In addition, the knowledge that B. theileri is maintained transovarially and may be found as disseminated infections within the vector, as well as producing transient spirochetemia in the ungulate host, may help advance studies that support or refute the role of borreliae in the epidemiology of Lone Star tick-associated, Lyme disease-like infections of humans.
ACKNOWLEDGMENTS
This work was supported by NIH grants AI 37993, AI 39002, and AI 19693.
REFERENCES
- 1.Armstrong P M, Rich S M, Smith R D, Hartl D L, Spielman A, Telford S R. A new Borrelia infecting Lone Star ticks. Lancet. 1996;347:67–68. doi: 10.1016/s0140-6736(96)91604-9. [DOI] [PubMed] [Google Scholar]
- 2.Barbour A G, Maupin G O, Teltow G J, Carter C J, Piesman Identification of an uncultivable Borrelia species in the hard tick Amblyomma americanum: possible agent of a Lyme disease-like illness. J Infect Dis. 1996;173:403–409. doi: 10.1093/infdis/173.2.403. [DOI] [PubMed] [Google Scholar]
- 3.Barbour A G. Does Lyme disease occur in the south? A survey of emerging tick-borne infections in the region. Am J Med Sci. 1996;311:34–40. doi: 10.1097/00000441-199601000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Black W C I, Klompen J S, Keirans J E. Phylogenetic relationships among tick subfamilies (Ixodida: Ixodidae: Argasidae) based on the 18S nuclear rDNA gene. Mol Phylogenet Evol. 1997;7:129–144. doi: 10.1006/mpev.1996.0382. [DOI] [PubMed] [Google Scholar]
- 5.Burgdorfer W. Hemolymph test. A technique for detection of rickettsiae in ticks. Am J Trop Med Hyg. 1970;19:1010–1014. [PubMed] [Google Scholar]
- 6.Burgdorfer W, Hayes S F, Corwin D. Pathophysiology of the Lyme disease spirochete, Borrelia burgdorferi, in ixodid ticks. Rev Infect Dis. 1989;11:S1442–S1450. doi: 10.1093/clinids/11.supplement_6.s1442. [DOI] [PubMed] [Google Scholar]
- 7.Callow L L. Observations on tick-transmitted spirochaetes of cattle in Australia and South Africa. Br Vet J. 1967;123:492–497. doi: 10.1016/s0007-1935(17)39704-x. [DOI] [PubMed] [Google Scholar]
- 8.Campbell G L, Paul W S, Schriefer M E, Craven R B, Robbins K E, Dennis D T. Epidemiologic and diagnostic studies of patients with suspected early Lyme disease, Missouri, 1990–1993. J Infect Dis. 1995;172:470–480. doi: 10.1093/infdis/172.2.470. [DOI] [PubMed] [Google Scholar]
- 9.Canica M M, Nato F, du Merle L, Mazie J C, Baranton G, Postic D. Monoclonal antibodies for identification of Borrelia afzelii sp. nov. associated with late cutaneous manifestations of Lyme borreliosis. Scand J Infect Dis. 1993;25:441–448. doi: 10.3109/00365549309008525. [DOI] [PubMed] [Google Scholar]
- 10.Feir D, Santanello C R B, Li W, Xie C S, Masters E, Marconi R, Weil G. Evidence supporting the presence of Borrelia burgdorferi in Missouri. Am J Trop Med Hyg. 1994;51:475–482. [PubMed] [Google Scholar]
- 11.Filippova N A. The taxonomic aspects of the transmission of the causative agent of Lyme disease. Parazitologiia. 1990;24:257–267. [PubMed] [Google Scholar]
- 12.Fukunaga M, Sohnaka M, Nakao M, Miyamoto K. Evaluation of genetic divergence of borrelial isolates from Lyme disease patients in Hokkaido, Japan, by rRNA gene probes. J Clin Microbiol. 1993;31:2044–2048. doi: 10.1128/jcm.31.8.2044-2048.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukunaga M, Sohnaka M, Takahashi Y, Nakao M, Miyamoto K. Antigenic and genetic characterization of Borrelia species isolated from Ixodes persulcatus in Hokkaido, Japan. J Clin Microbiol. 1993;31:1388–1391. doi: 10.1128/jcm.31.5.1388-1391.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoogstraal H, Aeschlimann A. Tick-host specificity. Bull Soc Entomol Suisse. 1982;55:5–32. [Google Scholar]
- 15.Kawabata H, Masuzawa T, Yanagihara Y. Genomic analysis of Borrelia japonica sp. nov. isolated from Ixodes ovatus in Japan. Microbiol Immunol. 1993;37:843–848. doi: 10.1111/j.1348-0421.1993.tb01714.x. [DOI] [PubMed] [Google Scholar]
- 16.Korenberg E I. Infections of the Lyme borreliosis group—ixodid tick-borne borrelioses in Russia. Med Parazitol (Mosk) 1996;3:14–18. [PubMed] [Google Scholar]
- 17.Laveran A. Sur la spirillose des bovides. C R Acad Sci Paris. 1903;136:939. [Google Scholar]
- 18.Levine J F, Apperson C S, Nicholson W L. The occurrence of spirochetes in ixodid ticks in North Carolina. J Entomol Sci. 1989;24:594–602. [Google Scholar]
- 19.MacKellar W M. Keeping livestock healthy. Yearbook of Agriculture 1942. USDA, 77th Congress, 2nd Session, House Document 527. Washington, D.C.: United States Government Printing Office; 1942. [Google Scholar]
- 20.Marconi R T, Liveris D, Schwartz I. Identification of novel insertion elements, restriction fragment length polymorphism patterns, and discontinuous 23S rRNA in Lyme disease spirochetes: phylogenetic analyses of rRNA genes and their intergenic spacers in Borrelia japonica sp. nov. and genomic group 21038 (Borrelia andersonii sp. nov.) isolates. J Clin Microbiol. 1995;33:2427–2434. doi: 10.1128/jcm.33.9.2427-2434.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marti Ras N, Postic D, Foretz M, Baranton G. Borrelia burgdorferi sensu stricto, a bacterial species “made in the U.S.A.”? Int J Syst Bacteriol. 1997;47:1112–1117. doi: 10.1099/00207713-47-4-1112. [DOI] [PubMed] [Google Scholar]
- 22.Masters E J, Donnell H D. Lyme and/or Lyme-like disease in Missouri. Missouri Med. 1995;92:346–353. [PubMed] [Google Scholar]
- 23.Postic D, Assous M V, Grimont P A, Baranton G. Diversity of Borrelia burgdorferi sensu lato evidenced by restriction fragment length polymorphism of rrf (5S)−rrl (23S) intergenic spacer amplicons. Int J Syst Bacteriol. 1994;44:743–752. doi: 10.1099/00207713-44-4-743. [DOI] [PubMed] [Google Scholar]
- 24.Schulze T L, Bowen G S, Bosler E M, Lakat M F, Parkin W E, Altman R, Ormiston B G, Shisler J K. Amblyomma americanum: a potential vector of Lyme disease in New Jersey. Science. 1984;224:601–603. doi: 10.1126/science.6710158. [DOI] [PubMed] [Google Scholar]
- 25.Smith R D, Miranpuri G S, Adams J H, Ahrens E H. Borrelia theileri: isolation from ticks (Boophilus microplus) and tick-borne transmission between splenectomized calves. Am J Vet Res. 1985;46:1396–1398. [PubMed] [Google Scholar]
- 26.Spielman A, Pollack R J, Telford S R., III . The origins and course of the present outbreak of Lyme disease. In: Ginsberg H S, editor. Ecology and environmental management of Lyme disease. New Brunswick, N.J: Rutgers University Press; 1992. pp. 83–96. [Google Scholar]
- 27.Swofford D. Phylogenetic analysis using parsimony, paup4d61.ppc ed. Washington, D.C.: Smithsonian Institution; 1998. [Google Scholar]
- 27a.Tamura K, Nei M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol. 1993;10:512–526. doi: 10.1093/oxfordjournals.molbev.a040023. [DOI] [PubMed] [Google Scholar]
- 28.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, positions-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]