Abstract
Objective
To provide a comprehensive description of stroke characteristics, risk factors, laboratory parameters, and treatment in a series of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)–infected patients admitted to Mayo Clinic hospitals in Rochester, Minnesota; Jacksonville, Florida; and Phoenix, Arizona, as well as the Mayo Clinic Health System.
Patients and Methods
We retrospectively identified hospitalized patients in whom stroke and SARS-CoV-2 infection were diagnosed within the same 3-month interval between September 8, 2019, and December 31, 2020. and extracted data on all available variables of interest. We further incorporated our findings into the existing body of basic science research to present a schematic model illustrating the proposed pathogenesis of ischemic stroke in SARS-CoV-2–infected patients.
Results
We identified 30 cases during the study period, yielding a 0.5% stroke rate across 6381 SARS-CoV-2–infected hospitalized patients. Strokes were ischemic in 26 of 30 individuals and hemorrhagic in 4 of 30. Traditional risk factors were common including hypertension (24 of 30), hyperlipidemia (18 of 30), smoking history (13 of 30), diabetes (11 of 30), and atrial fibrillation (8 of 30). The most common ischemic stroke mechanisms were cardioembolism (9 of 26) and cryptogenic (9 of 26). Intravenous alteplase and mechanical thrombectomy were administered to 2 of 26 and 1 of 26, respectively. The median (interquartile range) serum C-reactive protein, interleukin-6, D-dimer, fibrinogen, and ferritin levels were 66 (21-210) mg/L, 116 (8-400) pg/mL, 1267 (556-4510) ng/mL, 711 (263-772) mg/dL, and 407 (170-757) mcg/L, respectively, which were elevated in individuals with available results.
Conclusion
The high prevalence of vascular risk factors and concurrent elevation of proinflammatory and procoagulation biomarkers suggest that there is an interplay between both factors in the pathogenesis of stroke in SARS-CoV-2–infected patients.
Abbreviations and Acronyms: COVID-19, coronavirus disease 2019; EC, endothelial cell; EMR, electronic medical record; IQR, interquartile range; RAAS, renin-angiotensin-aldosterone system; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2
In March 2020, the World Health Organization declared coronavirus disease 2019 (COVID-19) a global pandemic, and since then, millions of cases and fatalities have been reported globally.1 The emergence and pervasive spread of highly transmissible mutant viral strains such as B.1.1.7 (Alpha), B.1.351 (Beta), B.1.617.2 (Delta), and B.1.1.529 (Omicron) continue to cause a global threat.2 Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the virus responsible for COVID-19 and primarily affects the respiratory system. Individuals can present with dry cough, fever, dyspnea, hypoxia, acute respiratory distress syndrome, and respiratory failure.1,3 Neurologic involvement has been reported in 30% to 50% of all individuals with SARS-CoV-2 infection,4, 5, 6 and nearly half of these cases are attributable to stroke.5,7
Severe acute respiratory syndrome coronavirus 2 viral infection has been found to considerably increase one’s risk of stroke, and this is thought to result from a dysregulated host immune response to the virus, thus triggering endothelial cell (EC) dysfunction, platelet activation, activation of the coagulation cascade, and a perturbation of the renin-angiotensin-aldosterone system (RAAS)8 (Figure 1). Data on the role of traditional vascular risk factors such as hypertension, hyperlipidemia, and diabetes in stroke pathogenesis have been mixed, with some studies reporting an association9,10 and others not.11,12 Furthermore, the temporal relationship between SARS-CoV-2 infection and stroke is unclear. Most case series have described acute stroke characteristics in those with simultaneous SARS-CoV-2 infection even though the dysregulated immune response in COVID-19 is likely to persist for several months, thus leading to various postinfectious neurologic manifestations including the COVID-19 long hauler syndrome.6,13 In this work, we aimed to provide a comprehensive description of vascular risk factors, laboratory parameters, timing of disease onset, and treatment delivery in a series of SARS-CoV-2–infected patients admitted to Mayo Clinic hospitals in Rochester, Minnesota; Jacksonville, Florida; and Phoenix, Arizona, as well as the Mayo Clinic Health System in the same 3-month interval between September 8, 2019, and December 31, 2020.
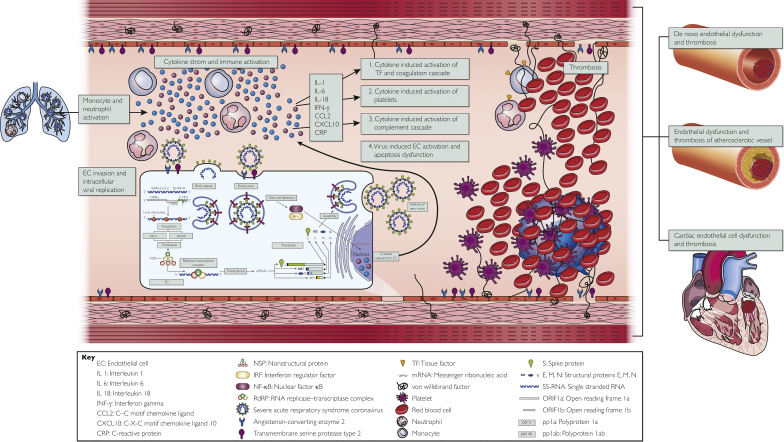
Figure 1.
Schematic model illustrating the proposed pathogenesis of ischemic stroke in severe acute respiratory syndrome coronavirus 2–infected patients. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) binds to cells that express angiotensin-converting enzyme 2 and transmembrane protease serine 2 receptors such as alveolar epithelial cells and vascular endothelial cells. The infected host cells then release an initial set of cytokines and chemokines such as tumor necrosis factor, C-reactive protein, interleukin-1, and interleukin-6, which leads to the recruitment of macrophages and monocytes. These produce even more proinflammatory cytokines and eventually a cytokine storm. The resultant cytokine storm then leads to activation of tissue factor located on neutrophils, direct platelet activation, and endothelial cell damage. Endothelial cell damage leads to the release of more inflammatory cytokines, release of von Willebrand factor, and decreased production of nitrous oxide. These processes can lead to endotheliopathy and thrombosis in those with or without preexisting vascular risk factors. pp1a = polyprotein 1a; E, M, N = structural proteins E, M, N; mRNA = messenger ribonucleic acid; ORIFIa = open reading frame 1a; ORIFIb = open reading frame; pp1ab = polyprotein 1ab; S = spike protein; SS-RNA = single stranded RNA; TF = tissue factor.
Patients and Methods
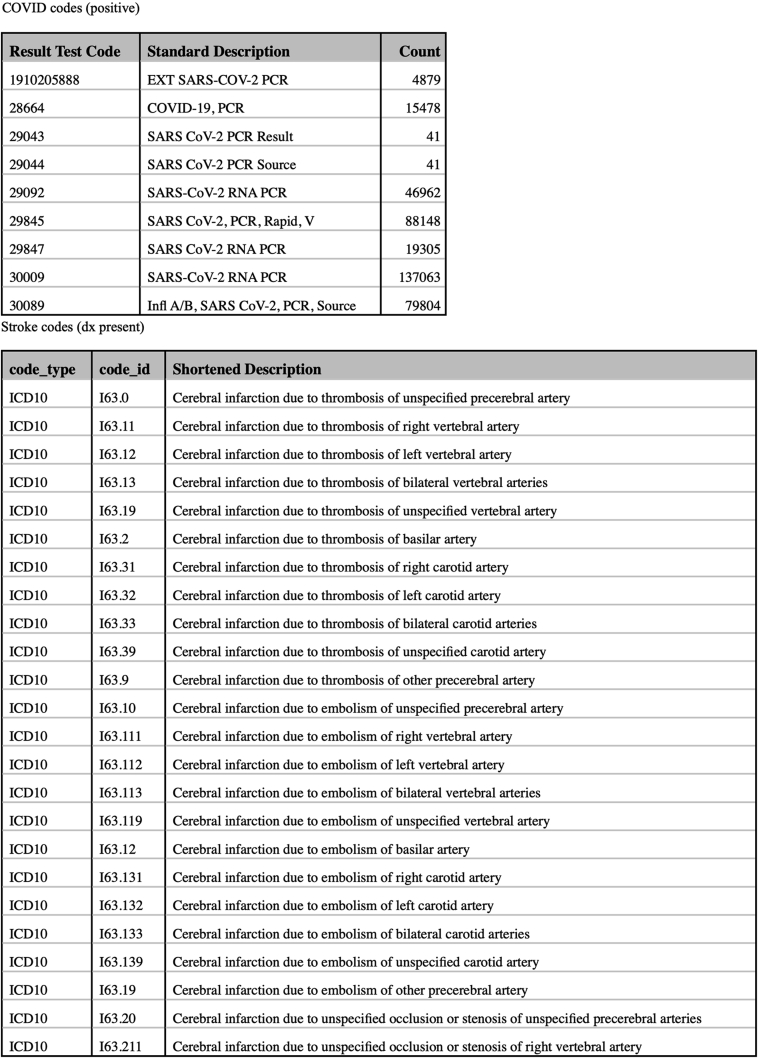
Cases were identified retrospectively from the Mayo Clinic enterprise-wide and universally adopted electronic medical record (EMR). We identified adult patients 18 years or older with computed tomography– and/or magnetic resonance imaging–confirmed acute ischemic or hemorrhagic stroke and positive SARS-CoV-2 real-time reverse transcription polymerase chain reaction test result who were admitted to any one of the Mayo Clinic hospitals in Arizona, Florida, Iowa, Minnesota, and Wisconsin. We used International Classification of Diseases, Tenth Revision codes and test result codes to identify cases (Supplemental Figure, available online at http://www.mcpiqojournal.org).
We included all those in whom stroke and SARS-CoV-2 infection were diagnosed within the same 3-month interval (comorbidity window) between September 8, 2019, and December 31, 2020. We specifically defined the comorbidity window as stroke or SARS-CoV-2 detection having preceded the other by an interval period of less than 92 days (3 months). Only those in whom the SARS-CoV-2 test result was available for confirmation in our EMR system were included. We subsequently reviewed all identified cases resulting from this initial search and included only those with a confirmed new diagnosis of acute ischemic or hemorrhagic stroke. We excluded individuals with a history of stroke or those in whom stroke imaging characteristics were consistent with chronic infarct. For all included participants, we extracted data on sociodemographic characteristics, vascular risk factors, clinical presentation, timing of disease onset, laboratory markers, radiologic findings, and treatment strategies. Ischemic stroke features on imaging were summarized by anatomic location. We recorded the proposed stroke mechanism, as documented in the clinical notes, and data on mortality outcomes.
Data Entry and Statistical Analyses
All relevant information was entered directly into a pretested REDCap (Research Electronic Data Capture) database questionnaire hosted on the Mayo Clinic server. Categorical data from all participants were summarized using proportions and percentages, while continuous data were summarized using medians and interquartile ranges (IQRs). Mortality outcome was summarized using proportions and percentages, categorized by stroke type. The Fisher exact test was used to compare the risk of mortality between those with ischemic and hemorrhagic stroke. As an exploratory analysis, the Fisher exact test and Kruskal Wallis test were used, where appropriate, to compare demographic and clinical characteristics between patients with cryptogenic ischemic stroke and all other patients with ischemic stroke.
Ethical Considerations
This study was approved by the Mayo Clinic Institutional Review Board. We excluded individuals who did not provide research authorization, as required by Minnesota state laws. Only de-identified data were extracted from patient charts and entered into REDCap.
Results
Inclusions and Exclusions
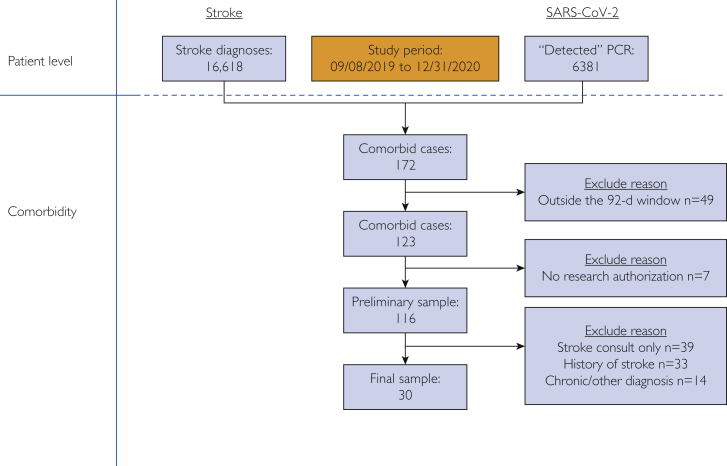
During the period between September 8, 2019, and December 31, 2020, we identified 16,618 unique patients with a diagnosis of stroke and 6381 cases with a positive SARS-CoV-2 test result. Of these, 172 had comorbid stroke and SARS-CoV-2 infection (Figure 2). We excluded 49 cases who were outside our 92-day comorbidity window and 7 cases in whom research authorization was not sought at the time of hospitalization. We then individually reviewed all relevant clinical notes and imaging findings for the remaining 116 cases for inclusion. We excluded 39 individuals in whom a stroke consult or acute stroke code was requested but evaluation and imaging results were negative for stroke. We excluded 33 individuals with a history of stroke but without evidence of acute stroke and 14 individuals with other diagnoses such as traumatic subarachnoid hemorrhage, subdural hematoma, and brain tumor (Figure 2). A total of 30 cases were included for data extraction and final analysis. Therefore, during the study interval and within a 3-month comorbidity window, there was a 0.5% stroke rate (n=30) across 6381 SARS-CoV-2–infected patients.
Figure 2.
Flow diagram showing all included and excluded participants. SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
Sociodemographic Features and Prevalence of Vascular Risk Factors
The median (IQR) age for all those included was 73 (63-80) years, and most were men (67% [20 of 30]). Those with cryptogenic ischemic stroke were significantly younger (median age (IQR), 69 (61-72) years vs 79 (75-82) years; P=.0081) (Supplemental Table 1, available online at http://www.mcpiqojournal.org).
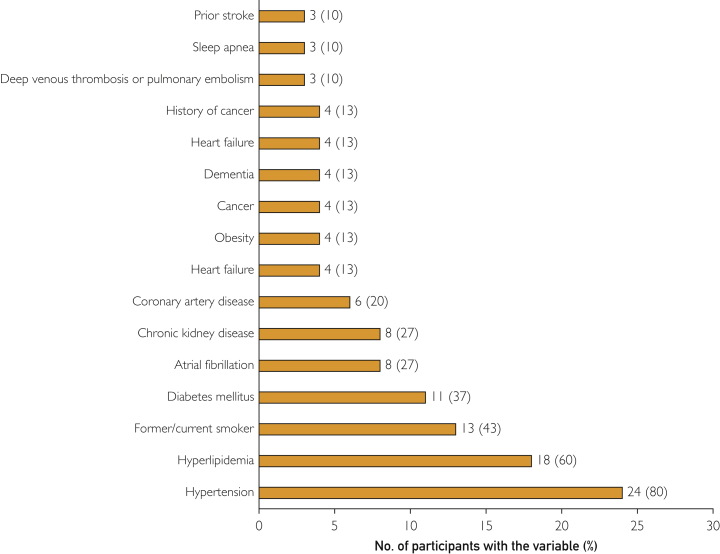
Participants were predominantly white (67% [20 of 30]), followed by black or African American individuals (13% [4 of 30]) and Asian Americans (7% [2 of 30]) (Table 1). Traditional stroke risk factors were highly prevalent in the cohort. Hypertension was the most common risk factor (80% [24 of 30]), followed by hyperlipidemia (60% [18 of 30]), smoking history (45% [13 of 30]), diabetes (39% [12 of 30]), atrial fibrillation (27% [8 of 30]), and chronic kidney disease (27% [8 of 30]) (Figure 3). Moreover, 90% (27 of 30) of individuals had more than 1 vascular risk factor.
Table 1.
| Variable | Value |
|---|---|
| Age at diagnosis (y) | 73 (63-80) |
| Male sex | 20 (67) |
| Race | |
| White | 20 (67) |
| Black or African American | 4 (13) |
| Asian | 2 (7) |
| American Indian | 1 (3) |
| Others/choose not to disclose | 3 (10) |
| Stroke/COVID-19 timing | |
| Stroke preceded SARS-CoV-2 infection | 6 (20) |
| COVID-19 preceded stroke | 10 (33) |
| Simultaneous stroke and SARS-CoV-2 infection | 14 (47) |
| Days from stroke onset to diagnosis | 0 (0-1) |
| Days from symptom onset to positive SARS-CoV-2 test result | 2 (0-4) |
| Stroke/COVID-19 interval (absolute days) | 2 (0-26) |
| NIHSS score | 6 (3-10) |
| Stroke treatment strategies | |
| Antithrombotic treatment | 17 (57) |
| Intravenous alteplase treatment | 2 (7) |
| Mechanical thrombectomy | 1 (3) |
| Osmolar therapy (mannitol) | 1 (3) |
| Therapeutic anticoagulation | 4 (13) |
| Documented reasons for not receiving intravenous alteplase for acute stroke treatment | |
| Presentation out of the 4.5-h window | 17 (55) |
| Subacute infarct on imaging done for altered mental status | 2 (7) |
| Subacute infarct on imaging done for not waking up despite lightening sedation | 3 (10) |
| COVID-19 severity grade at stroke diagnosis | |
| Asymptomatic | 16 (53) |
| Mild disease | 3 (10) |
| Moderate disease | 2 (7) |
| Severe disease | 2 (7) |
| Critical disease | 7 (23) |
| COVID-19 treatment strategies | |
| Dexamethasone | 10 (33) |
| Remdesivir | 14 (47) |
| Tocilizumab | 1 (3) |
| Convalescent plasma | 3 (10) |
| Therapeutic anticoagulation | 6 (20) |
| Intubation and mechanical ventilation for respiratory failure | 9 (30) |
COVID-19, coronavirus disease 2019; NIHSS, National Institutes of Health Stroke Scale; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Data are presented as median (interquartile range) or No. (percentage).
Figure 3.
Prevalence of vascular risk factors.
Stroke Type, Severity, Mechanism, Imaging Characteristics, and Treatment
Strokes were ischemic in 87% (26 of 30) and hemorrhagic in 13% (4 of 30) of participants. The median (IQR) National Institutes of Health Stroke Scale score was 6 (3-10). Anterior circulation was the most common ischemic stroke location, accounting for 80% (21 of 26) of cases. In terms of stroke mechanisms, cardioembolism (35% [9 of 26]) and cryptogenic (35% [9 of 26]) were the most documented (Supplemental Table 2, available online at http://www.mcpiqojournal.org).
Intravenous alteplase for acute stroke treatment was administered in only 7% (2 of 26) of ischemic stroke cases, and the most documented reasons for not treating with thrombolysis were presentation outside the 4.5-hour window and/or subacute infarct noted on head imaging (Table 1). Only 1 patient underwent mechanical thrombectomy.
Hemorrhagic strokes were mostly lobar (75% [3 of 4]), with a median (IQR) hematoma volume of 33 (21-61) cm3. Of those with hemorrhagic stroke, 33% (1 of 4) were receiving therapeutic doses of anticoagulation therapy.
Coronavirus Disease 2019 Severity and Treatment Strategies
Nearly half of the individuals had asymptomatic SARS-CoV-2 infection (53% [16 of 30]). Those with severe or critical disease were also common, collectively accounting for 30% (9 of 30) of all cases (Table 1). Remdesivir (47% [14 of 30]) and dexamethasone (33% [10 of 30]) were commonly administered to patients. Approximately one-third (30% [9 of 30]) of individuals were mechanically ventilated in the intensive care unit for acute respiratory failure. Convalescent plasma was used in 10% (3 of 30) of cases (Table 1).
Timing Between SARS-CoV-2 Infection and Stroke
Simultaneous SARS-CoV-2 infection and acute stroke occurred in 47% (14 of 30) of cases. Severe acute respiratory syndrome coronavirus 2 infection preceded stroke in 33% (10 of 30) of cases, whereas stroke was the preceding diagnosis in 20% (6 of 30) of cases. The median (IQR) interval time from stroke diagnosis to SARS-CoV-2 diagnosis was 2 (0-26) days. The median (IQR) time from stroke symptom onset to stroke diagnosis was 0 (0-1) days, and the median (IQR) time from COVID-19–related symptom onset to SARS-CoV-2 detection was 0 (0-4) days. Nine individuals with SARS-CoV-2 infection did not have symptom duration recorded, as they remained asymptomatic throughout their entire hospitalization.
Proinflammatory and Procoagulation Serum Biomarkers
Laboratory testing was not uniformly done in all cases (Table 2). More than half of the individuals had complete blood count, international normalized ratio, and D-dimer, serum lipid, ferritin, and troponin levels tested. Table 2 provides summary statistics (median [IQR]) for all individuals in whom a test result was available. Serum inflammatory and procoagulant biomarkers were elevated in the subset of individuals with available results (Table 2). The median (IQR) C-reactive protein, ferritin, and interleukin-6 levels were 66 (21- 210) mg/dL, 407 (170-757) ng/mL, and 116 (8-400) pg/mL, respectively. The median (IQR) serum fibrinogen and D-dimer levels were 711 (263-772) mg/dL and 1267 (556-4510) ng/mL, respectively.
Table 2.
| Test | Number with available results/total | Median (IQR) |
|---|---|---|
| White blood cell count (cells/L) | 28/30 | 9 (6-11) × 109 |
| Platelet count (cells/L) | 28/30 | 243 (151-295) × 109 |
| C-reactive protein level (mg/L) | 18/30 | 66 (21-210) |
| INR | 25/30 | 1 (1-1) |
| APTT (s) | 21/30 | 31 (27-34) |
| Troponin level (ng/L) | 15/30 | 21 (13-47) |
| Interleukin-6 level (pg/mL) | 6/30 | 116 (8-400) |
| LDL level (mg/dL) | 15/30 | 57 (45-68) |
| HDL level (mg/dL) | 16/30 | 42 (34-46) |
| Triglyceride level (mg/dL) | 16/30 | 103 (94-139) |
| D-dimer level (ng/mL) | 20/30 | 1267 (556-4510) |
| Fibrinogen level (mg/dL) | 13/30 | 711 (263-772) |
| Ferritin level (mcg/L) | 16/30 | 407 (170-757) |
APTT, Activated partial thromboplastin time; COVID-19, coronavirus disease 2019; INR, international normalized ratio; IQR, interquartile range; LDL, low-density lipoprotein; HDL, high-density lipoprotein.
SI conversion factors: To convert mg/dL values to mmol/L, multiply by 0.0259; to convert ng/mL values to nmol/L, multiply by 2.5; to convert pg/mL values to pmol/L, multiply by 3.671.
Mortality Outcomes
Overall, 11 of 30 (37%) individuals died: 9 with ischemic stroke (9 of 26 [34%]) and 4 with hemorrhagic stroke (2 of 4 [50%]). The difference in mortality rates was not statistically significant (P=.611), although the event rate and sample size were low for this analysis.
Discussion
There are several notable findings from our study. First, we found a low prevalence (0.5%) of acute stroke in SARS-CoV-2–infected patients. Second, traditional stroke risk factors were highly prevalent in our cohort, with more than 80% of individuals having had at least 1 documented risk factor such as hypertension, hyperlipidemia, smoking history, diabetes, or atrial fibrillation. Third, serum biomarkers for inflammation and coagulation were elevated in individuals in whom these laboratory values were available. Fourth, only a small minority of acute ischemic stroke cases were eligible for reperfusion therapy mostly because of late presentation. Lastly, in-hospital outcomes were poor in our individuals with stroke and SARS-CoV-2 infection, as evidenced by the high mortality rate. Our findings are relevant as they add to the mounting body of evidence that is aimed at helping to improve our understanding of the unique interaction between stroke and SARS-CoV-2 infection, thus providing avenues by which critical stroke prevention and treatment strategies can be derived.
The low prevalence (0.5%) of stroke in our study is similar to findings from 1 prior study from New York that reported an ischemic stroke prevalence rate of 0.9%.14 However, larger studies and meta-analyses have reported slightly higher prevalence rates of around 1.3% to 1.5%,15,16 which are more than double the rates reported in our study. Several other studies have reported even higher stroke rates. One study from a large university health system in the United States that enrolled a significantly younger population than ours, most of whom were African Americans (68% black; mean age, 59 years), reported a stroke prevalence rate of 2.5%, 5 times more than ours.15 Further, a retrospective study conducted at the peak of the COVID-19 pandemic in China reported a high stroke prevalence rate of 5.7% in those with severe SARS-CoV-2 infection, therein supporting the theory that individuals with severe infection are at a heightened risk of stroke.7 Therefore, the differences in prevalence rates between the various studies can be explained by a difference in participant sociodemographic characteristics, severity of SARS-CoV-2 infection in enrolled participants, timing of the conducted study with regard to the community prevalence of SARS-CoV-2 infection, and the background prevalence of stroke in the community studied.
Regarding stroke risk factors, we found that proinflammatory and procoagulation biomarkers were elevated in individuals in our cohort. This unsurprising finding can be corroborated by data from numerous prior studies reporting similar laboratory findings in SARS-CoV-2–infected patients with acute stroke.15,17, 18, 19 In addition, we found that there was a concurrently high rate of traditional stroke risk factors in our cohort, also similar to what others have previously noted.16,20 In one US study, 95% of patients had a history of hypertension and 60% had diabetes mellitus.20 In fact, both diabetes mellitus and hypertension have also been associated with increased COVID-19 severity, acute respiratory distress, and poor outcomes.21, 22, 23 Diabetes and hypertension are hypothesized to lead to severe SARS-CoV-2 infection through a number of mechanisms such as impaired glucose homeostasis, impaired immune response, and activation of RAAS.24 Thus, the increased risk of stroke in SARS-CoV-2–infected patients is likely a result of the close interaction between traditional vascular risk factors and the dysregulated immune response seen in SARS-CoV-2–infected individuals.
We have incorporated our findings into the existing basic science research data to present a comprehensive schematic model to illustrate the proposed pathogenesis of ischemic stroke in SARS-CoV-2 infection (Figure 1). The inciting event appears to be the binding of SARS-CoV-2 to cells that express angiotensin-converting enzyme 2 (ACE2) and transmembrane protease serine 2 receptors, such as alveolar epithelial cells and vascular ECs.25,26 The infected host cells then release an initial set of cytokines and chemokines such as tumor necrosis factor, C-reactive protein, interleukin-1, and interleukin-6, which leads to the recruitment of macrophages and monocytes. These produce even more proinflammatory cytokines and eventually a cytokine storm. The resultant cytokine storm leads to activation of tissue factor located on neutrophils and other immune cells, which in turn interacts with factor VII to stimulate the extrinsic coagulation cascade.27,28 The hyperinflammatory response also independently leads to direct platelet activation and EC damage, which both contribute to thrombosis.29 In addition, EC ubiquitously express ACE2 and transmembrane protease serine 2, thus making them a target for direct viral invasion, viral proliferation, and eventually EC activation/damage (Figure 1).30, 31, 32 Endothelial activation by itself is a major orchestrator of thrombosis and stroke occurrence, as it leads to a number of downstream deleterious effects as summarized in Figure 1, including extensive release of inflammatory cytokines and chemokines further fueling the cytokine storm,30,31,33 release of von Willebrand factor that further promotes coagulopathy, decreased production of nitrous oxide through suppression of nitric oxide synthase (necessary for vasodilation), and release of oxygen reactive species.34,35 Another negative consequence of EC damage is the resultant decrease in the amount of ACE2 available to counter the effects of the classical RAAS pathway, leading to further endotheliopathy and end-organ damage.34 All the processes described above can lead to either de novo endotheliopathy (particularly in younger individuals without a history of preexisting vascular risk factors) or endothelial damage in those with underlying atherosclerosis and comorbid vascular risk factors (Figure 1).
Another key finding in our study was the revelation that there was an extremely low rate of reperfusion therapies offered to patients with ischemic stroke at the time of stroke diagnosis. The most common documented reason for not treating with thrombolysis was presentation outside the 4.5-hour treatment window. This is likely due to individuals being hesitant to go to the hospital in the midst of the COVID-19 pandemic, a phenomenon that has been seen across several regions of the world with a worldwide decline in stroke admissions during the COVID-19 pandemic observed.36,37 This likely contributes to poor stroke outcomes in patients with COVID-19. Therefore, considerable efforts are needed to combat hesitancy toward hospital presentation to improve the number of patients eligible for acute stroke therapy.
A major strength of our study lies in the fact that this study was conducted in one of the single largest North American academic medical centers focused on integrated health care, education, and research with hospitals in 5 states, all universally adopting a single EMR. We included all eligible hospitalized patients in whom stroke and SARS-CoV-2 infection were diagnosed within a comorbidity window, facilitating more rapid study completion and timely dissemination of results. Data fields and analysis plans were prespecified.
Limitations
This observational study was retrospective, and the small sample size did not allow us to perform further characterization and subgroup analyses for a better understanding of the interaction between traditional vascular risk factors and proinflammatory/procoagulation factors in stroke pathogenesis. Several participants were missing some of the key laboratory data of interest, which could have affected our results. It is possible that we might have included individuals at the extremes of our comorbidity window in whom there was less of an interaction between stroke and SARS-CoV-2 infection. However, the median interval time between SARS-CoV-2 detection and stroke diagnosis in our cohort was 2 days, with a 75th percentile value of 26 days which is well within the window of expected delayed effects of COVID-19.
Conclusion
We have found that traditional vascular risk factors and concurrent elevation of proinflammatory and procoagulation biomarkers are common in patients with stroke and SARS-CoV-2 infection, suggesting that there is an interplay between both factors in the pathogenesis of stroke in these patients. Further research aimed at improving our understanding of this interaction will greatly improve our ability to prevent and treat stroke in this specific group of individuals.
Footnotes
Grant Support: The work was supported by the Department of Neurology and Department of Clinical Research, Mayo Clinic College of Medicine and Science.
Potential competing interests: The authors report no competing interests.
Supplemental material can be found online at: http://www.mcpiqojournal.org. Supplemental material attached to journal articles has not been edited, and the authors take responsibility for the accuracy of all data.
Supplemental Online Material
Supplementary figure 1.
References
- 1.Zhu N., Zhang D., Wang W., et al. for the China Novel Coronavirus Investigating and Research Team. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Washington N.L., Gangavarapu K., Zeller M., et al. Emergence and rapid transmission of SARS-CoV-2 B.1.1.7 in the United States. Cell. 2021;184(10):2587–2594.e2587. doi: 10.1016/j.cell.2021.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. Published correction appears in JAMA. 2021;325(11):1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paterson R.W., Brown R.L., Benjamin L., et al. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain. 2020;143(10):3104–3120. doi: 10.1093/brain/awaa240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Varatharaj A., Thomas N., Ellul M.A., et al. CoroNerve Study Group Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry. 2020;7(10):875–882. doi: 10.1016/S2215-0366(20)30287-X. Published correction appears in Lancet Psychiatry. 2020;7(10):e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taquet M., Geddes J.R., Husain M., Luciano S., Harrison P.J. 6-Month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry. 2021;8(5):416–427. doi: 10.1016/S2215-0366(21)00084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mao L., Jin H., Wang M., et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wijeratne T., Crewther S.G., Sales C., Karimi L. COVID-19 pathophysiology predicts that ischemic stroke occurrence is an expectation, not an exception—a systematic review. Front Neurol. 2021;11:607221. doi: 10.3389/fneur.2020.607221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mohamud A.Y., Griffith B., Rehman M., et al. Intraluminal carotid artery thrombus in COVID-19: another danger of cytokine storm? AJNR Am J Neuroradiol. 2020;41(9):1677–1682. doi: 10.3174/ajnr.A6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morassi M., Bagatto D., Cobelli M., et al. Stroke in patients with SARS-CoV-2 infection: case series. J Neurol. 2020;267(8):2185–2192. doi: 10.1007/s00415-020-09885-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahmadi Karvigh S., Vahabizad F., Banihashemi G., et al. Ischemic stroke in patients with COVID-19 disease: a report of 10 cases from Iran. Cerebrovasc Dis. 2021;50(2):239–244. doi: 10.1159/000513279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oxley T.J., Mocco J., Majidi S., et al. Large-vessel stroke as a presenting feature of Covid-19 in the young. N Engl J Med. 2020;382(20) doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davido B., Seang S., Tubiana R., de Truchis P. Post–COVID-19 chronic symptoms: a postinfectious entity? Clin Microbiol Infect. 2020;26(11):1448–1449. doi: 10.1016/j.cmi.2020.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yaghi S., Ishida K., Torres J., et al. SARS-CoV-2 and stroke in a New York healthcare system. Stroke. 2020;51(7):2002–2011. doi: 10.1161/STROKEAHA.120.030335. Published correction appears in Stroke. 2020;51(8):e179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katsanos A.H., Palaiodimou L., Zand R., et al. The impact of SARS-CoV-2 on stroke epidemiology and care: a meta-analysis. Ann Neurol. 2021;89(2):380–388. doi: 10.1002/ana.25967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qureshi A.I., Baskett W.I., Huang W., et al. Acute ischemic stroke and COVID-19: an analysis of 27 676 patients. Stroke. 2021;52(3):905–912. doi: 10.1161/STROKEAHA.120.031786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benussi A., Pilotto A., Premi E., et al. Clinical characteristics and outcomes of inpatients with neurologic disease and COVID-19 in Brescia, Lombardy, Italy. Neurology. 2020;95(7):e910–e920. doi: 10.1212/WNL.0000000000009848. [DOI] [PubMed] [Google Scholar]
- 18.Szegedi I., Orbán-Kálmándi R., Csiba L., Bagoly Z. Stroke as a potential complication of COVID-19-associated coagulopathy: a narrative and systematic review of the literature. J Clin Med Res. 2020;9(10):3137. doi: 10.3390/jcm9103137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan Y.K., Goh C., Leow A.S.T., et al. COVID-19 and ischemic stroke: a systematic review and meta-summary of the literature. J Thromb Thrombolysis. 2020;50(3):587–595. doi: 10.1007/s11239-020-02228-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rothstein A., Oldridge O., Schwennesen H., Do D., Cucchiara B.L. Acute cerebrovascular events in hospitalized COVID-19 patients. Stroke. 2020;51(9):e219–e222. doi: 10.1161/STROKEAHA.120.030995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu C., Chen X., Cai Y., et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934–943. doi: 10.1001/jamainternmed.2020.0994. Published correction appears in JAMA Intern Med. 2020;180(7):1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pranata R., Lim M.A., Huang I., Raharjo S.B., Lukito A.A. Hypertension is associated with increased mortality and severity of disease in COVID-19 pneumonia: a systematic review, meta-analysis and meta-regression. J Renin Angiotensin Aldosterone Syst. 2020;21(2) doi: 10.1177/1470320320926899. 1470320320926899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang I., Lim M.A., Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia—a systematic review, meta-analysis, and meta-regression. Diabetes Metab Syndr. 2020;14(4):395–403. doi: 10.1016/j.dsx.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lim S., Bae J.H., Kwon H.S., Nauck M.A. COVID-19 and diabetes mellitus: from pathophysiology to clinical management. Nat Rev Endocrinol. 2021;17(1):11–30. doi: 10.1038/s41574-020-00435-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou P., Yang X.L., Wang X.G., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agbuduwe C., Basu S. Haematological manifestations of COVID-19: from cytopenia to coagulopathy. Eur J Haematol. 2020;105(5):540–546. doi: 10.1111/ejh.13491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DiNicolantonio J.J., McCarty M. Thrombotic complications of COVID-19 may reflect an upregulation of endothelial tissue factor expression that is contingent on activation of endosomal NADPH oxidase. Open Heart. 2020;7(1) doi: 10.1136/openhrt-2020-001337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bautista-Vargas M., Bonilla-Abadía F., Cañas C.A. Potential role for tissue factor in the pathogenesis of hypercoagulability associated with in COVID-19. J Thromb Thrombolysis. 2020;50(3):479–483. doi: 10.1007/s11239-020-02172-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zaid Y., Puhm F., Allaeys I., et al. Platelets can associate with SARS-Cov-2 RNA and are hyperactivated in COVID-19. Circ Res. 2020;127(11):1404–1418. doi: 10.1161/CIRCRESAHA.120.317703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teuwen L.A., Geldhof V., Pasut A., Carmeliet P. COVID-19: the vasculature unleashed. Nat Rev Immunol. 2020;20(7):389–391. doi: 10.1038/s41577-020-0343-0. Published correction appears in Nat Rev Immunol. 2020;20(7):448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huertas A., Montani D., Savale L., et al. Endothelial cell dysfunction: a major player in SARS-CoV-2 infection (COVID-19)? Eur Respir J. 2020;56(1):2001634. doi: 10.1183/13993003.01634-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nishiga M., Wang D.W., Han Y., Lewis D.B., Wu J.C. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat Rev Cardiol. 2020;17(9):543–558. doi: 10.1038/s41569-020-0413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pober J.S., Sessa W.C. Evolving functions of endothelial cells in inflammation. Nat Rev Immunol. 2007;7(10):803–815. doi: 10.1038/nri2171. [DOI] [PubMed] [Google Scholar]
- 34.Liu Y., Yang Y., Zhang C., et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63(3):364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Green S.J. Covid-19 accelerates endothelial dysfunction and nitric oxide deficiency. Microbes Infect. 2020;22(4-5):149–150. doi: 10.1016/j.micinf.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nogueira R.G., Qureshi M.M., Abdalkader M., et al. SVIN COVID-19 Global Stroke Registry; SVIN COVID-19 Global Stroke Registry. Global impact of COVID-19 on stroke care and IV thrombolysis. Neurology. 2021;96(23):e2824–e2838. doi: 10.1212/WNL.0000000000011885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Demaerschalk B.M. Where in the world have all the strokes gone? Neurology. 2021;96(23):1069–1070. doi: 10.1212/WNL.0000000000011886. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.