Abstract
The genus Hyssopus is widespread in central Asia, East Mediterranean, and Mongolian areas. It has six main species which are used as herbal remedies, such as Hyssopus officinalis which is used as a condiment and flavoring agent in food industry. The other five species are H. ambiguus, H. cuspidatus, H. latilabiatus, H. macranthus, and H. seravschanicus. Its species are used in the treatment of various ailments such as cold, cough, loss of appetite, fungal infection, and spasmodic condition. Its constituents especially essential oils are popularly used as an additive in beverages, foods, and cosmetics. The volatile constituents are used for aroma in the food industry, cosmetic industry, and household products. The important active constituents in its essential oils are β-pinene, pinocamphone, isopinocamphone, and other terpenoids. Hyssopus genus is also bundled with other secondary metabolites including flavonoids luteolin, quercetin, apigenin, and their glucosides, as well as phenolic compounds including ferulic, p-hydroxy-benzoic acid, protocatechuic acid, chlorogenic, and caffeic acid. Combinedly, the extracts of Hyssopus are reported to have potential antiviral and antifungal activities proven using in vitro studies, whereas in vivo investigations have reported the crucial role of Hyssopus extracts in plasma membrane relaxation, cytotoxic, and sedative effects. This plant is believed to be relatively safe at levels commonly used in foods; nevertheless, more studies are needed to determine the safety profile.
1. Introduction
Hyssopus (Lamiaceae) is a genus of herbaceous or semiwoody plants that includes about 10 to 15 species, distributed mainly in the East Mediterranean to central Asia and Mongolia [1, 2]. The name Hyssopus is derived from Hebrew ezob which means “sacred herb.”
Hyssopus is linked to several folk medicinal uses, providing several biologically active constituents especially the main compounds from essential oils. The essential oil is primarily consumed for flavoring, preservation of food, and other therapeutic uses [3].
Essential oils from the plants are generally recognized as safe for consumption if consumed in limited or recommended doses. Hence, the safety profile of the hyssop extracts is important in various diseases. Hyssopus spp. possessed curative properties against cough, cold, loss of appetite, fungal infection, and spasmodic condition. Essential oil constituents also exhibited antimicrobial, antifungal, and muscle relaxant properties [4]. To the best of our knowledge, there is no literature review considering the essential oils and their health-promoting effects and safety profile of Hyssopus genus.
This comprehensive review provides insights into the chemical composition, method of extraction, pharmacological properties, adverse effects, and toxicological data of essential oils extracted from Hyssopus officinalis.
2. Review Methodology
Scientific search engines PubMed and ScienceDirect were searched to retrieve literature and cross-references using the next MeSH keywords: “Lamiaceae”, “Lamiaceae/chemistry”, “Hyssopus Plant/chemistry”, “Oils, Volatile/analysis”, “Limonene”, “beta-pinene”, “pinocamphone”, “Monoterpenes”, “Oils, Volatile/pharmacology”, “Anti-Infective Agents/isolation & purification”, “Anti-Infective Agents/pharmacology”, “Antioxidants/chemistry”, “Antioxidants/isolation & purification”, “Antioxidants/pharmacology”, “Acetylcholine/pharmacology”, “Animals”, “Muscle Relaxation/drug effects”, “Apoptosis/drug effects”, “Cell Lines”, “Antitumor”, “Oils, Volatile/adverse effects”, “Oils, Volatile/toxicity”, and “Seizures/chemically induced”.
For this comprehensive review, extenso papers written in the English language were and abstracts, communications, and research with homoeopathic preparations were excluded. The taxonomy of plants has been validated using The Plant List database (http://www.theplantlist.org/) [5, 6].
3. Botanical Description and Distribution
Among the medicinal and aromatic plants, Hyssopus spp. have not been studied extensively. The known accepted species and subspecies are H. ambiguus, H. cuspidatus, H. latilabiatus, H. macranthus, H. officinalis, H. officinalis subsp. aristatus (Godr.) Nyman, H. officinalis subsp. canescens (DC.) Nyman, H. officinalis subsp. montanus (Jord. & Fourr.) Briq., H. seravschanicus, and H. subulifolius (Rech.f.) Rech.f. [7–11].
The genus Hyssopus had six species that were used as herbal remedy like H. officinalis which is utilized as a condiment in a food factory and as a flavoring agent [12]. The other five species were Hyssopus ambiguus (Trautv.) Iljin ex Prochorov. & Lebel, Hyssopus cuspidatus Boriss, Hyssopus latilabiatus C.Y.Wu & H.W.Li, Hyssopus macranthus Boriss., and Hyssopus seravschanicus (Dubj.) Pazij.
The geographical distribution of Hyssopus spp. is shown in Table 1.
Table 1.
Species and distribution area of Hyssopus spp. [13].
| Species | Distribution area |
|---|---|
| Hyssopus ambiguus (Trautv.) Iljin ex Prochorov. & Lebel | Altai Republic, Kazakhstan |
| Hyssopus cuspidatus Boriss. | Altai Republic, Kazakhstan, Xinjiang, Mongolia |
| Hyssopus latilabiatus C.Y.Wu & http://h.w.li/ | Xinjiang |
| Hyssopus macranthus Boriss. | Altai Republic, Siberia, Kazakhstan |
| Hyssopus officinalis L. | Europe, Algeria, Morocco, Iran |
| Hyssopus seravschanicus (Dub.) Pazij | Afghanistan, Pakistan, Kyrgyzstan, Tajikistan |
Hyssopus officinalis is widespread in the Mediterranean countries [14] and central Asia and north-west India [15]. Traditionally, hyssop seedlings are transplanted into the field with the first harvest occurring two years later, immediately after bloom [16]. Several subspecies have been recorded especially for Europe and northern Africa [17]. Nevertheless, several authors do not formally recognize any intraspecific ranks within this taxon, stating that variations displayed by the species are linked to local ecological conditions [18].
The flowering tops and leaves are used as flavors in food and beverages and cosmetic product preparations [19]. Flowers are useful in attracting bees for pollination and make them an important ornamental plant. Several types, differing in flower color, flowering time, and leaf shape are available commercially: “alba” (white flowers), “grandiflora” (large flowers), “rosea” (rose flowers), and “rubia” (red flowers) [16].
The essential oil from the herb is commonly used in cosmetics, perfumes, alcoholic and nonalcoholic beverages, and food additives. Dry leaves of hyssop are consumed as spices and herbal tea [14]. The seeds are often mixed, and it is quite difficult to obtain uniform plant populations for specific requirements, such as decorative flower production, honey bee forage production, high volatile oil yield, and uniform quantitative oil composition.
The reproduction is made by dividing clumps in late spring, towards April-May, or at the beginning of autumn. Seeds may be sown in March-April in calcareous light soil.
The essential oil productivity ranges between 10 and 20 kg/ha [20]. The productivity of essential oil varies greatly depending on the variation in plant species. In fact, comparing 13 European sources of hyssop, the yield of fresh leaves ranged from 5 to 32 tons/ha, and that of dry leaves from 0.67 to 3.26 tons/ha [19].
The inflorescence is 20-25 cm long, false spikelike, composed of 4 to 10 flowered pseudoverticils in the terminal [21]. Due to the high content of essential oils (0.3–1.0%), one of the most important species among Hyssopus is Hyssopus officinalis L. H. (hyssop), frequently used as a drug in the pharma industry and as a functional ingredient in the food industry [1, 22].
The root of H. officinalis is densely branched with a multiheaded tap root. The stems are 0.5–0.7 m in height, erect, or decumbent, dividing into many woody stems.
The leaves are opposite, shiny dark-green, entire-edged, and lanceolate or oblong, obtuse to acuminate that are 2–4 cm long and 0.5–1 cm wide.
The morphological and genetic hyssop complexity describes its high variability and the existence of numerous geographically different subspecies [23, 24]. H. officinalis is largely cultivated but also found in the wild conditions, characterized by a high heterogeneity. The high intraspecific diversity of hyssop is due to morphological differences (leaf, inflorescence, colour of flower, position, and form) and, especially, to biochemical traits (essential oils).
4. Phytochemistry and Bioactive Principles
Hyssop oil from different phenotypes or different areas shows great variability in chemical composition [25–28]. The chemical composition can be related to many factors, such as the origin, geography, climate and technological influence, stages of development, parts used, and harvesting time, as well as the presence of chemotypes [29].
The characteristics of genus Hyssopus essential oil were analyzed by gas chromatography-mass spectrometry (GC/MS) and GC analysis, and 21 compounds were identified, comprising 95.6% of oil. The compounds included six sesquiterpenes (60.5%), one phenol (0.2%), seven monoterpene hydrocarbons (32.3%), and five oxygenated monoterpenes (60.5%) [30]. Pinocamphone (49.1%), isopinocamphone (9.7%), and β-pinene (18.4%) were the major monoterpene constituents [30, 31]. Joulain [32] for the first time identified the constituents of genus Hyssopus that were cis-pinonic, hydroxyisopinocamphone, pinic acid, cis-pinic acid, myrtenic acid, methyl myrtenate, and myrtenol methyl ether. The essential oil's major components were β-pinene (10.5 and 10.8%), pinocamphone (34 and 18.5%), camphor (0.3 and 5.3%), linalool (0.2 and 7.9%), and isopinocamphone (3.2 and 29%).
GC and GC-MS analysis of H. officinalis volatile oil from Spain showed 1,8-cineole (52.89%) and β-pinene (16.82%) [26]. Özer et al. [9] performed GC analysis of H. officinalis from Turkey, with 34 components found, and they were 91% of the total detected components. The major components were isopinocamphone (5.3%), pinocamphone (19.6%), pinocarvone (36.3%), β-pinene (10.6%), and 1,8-cineole (7.2%) [9, 33]. Hyssopus officinalis from the Alps in Banon, France, was analyzed for its essential oil components, its major components were α-pinene (2.4%), β-pinene (3.0%), limonene (5.4%), linalool (49.6%), 1,8-cineole (13.3%), and β-caryophyllene (2.8%), and minor components were pinocamphone and isopinocamphone [34].
The Romanian's H. officinalis oil contains mainly aliphatic fatty acids like eicosadienoic acid (0.68%), linolenic acid (63.98%), arachidic acid (2.64%), stearic acid (10.73%), and palmitic acid (15.60%) [35]. But the plants of genus Hyssopus of Iran possessed different constituents and their concentration was found as bornyl acetate (1.42%), β-caryophyllene (2.10%), β-bourbonene (1.47%), camphor (6.76%), caryophyllene oxide (2.13%), spathulenol (2.14%), germacrene (3.39%), and myrtenyl acetate (74.08%) [36].
Chemical analysis of the H. officinalis endemic in former Yugoslavia shows that the essential oils are composed mainly of cis- and trans-pinocamphone and pinocarvone, together with lesser amounts of germacrene D, bicyclogermacrene, elemol, and spathulenol [11].
Essential oils isolated from plants of hyssop grown in two different localities near Urbino (Italy) that showed major components were pinocamphone (34% and 18.5%), isopinocamphone (3.2% and 29%), and β-pinene (10.5% and 10.8%). The major differences in the composition of the oils were detectable in the ratio of pinocamphone/isopinocamphone, in the percentage of linalool (0.2% and 7.9%) and camphor (0.3% and 5.3%) [11].
Oil from hyssop found in Poland contained 52 compounds, including 1 unidentified. The main components of hyssop essential oil were isopinocamphone (22.53–28.74%), pinocamphone (11.41–17.99%), β-pinene (6.69–12.01%), elemol (5.02–7.57%), germacrene D (3.14–6.98%), and bicyclogermacrene (2.55–4.36%) [37]. Garg et al. [30] found that the main volatile constituents of H. officinalis essential oil from a variety of locations included β-pinene, limonene, β-phellandrene, 1,8-cineole, pinocamphone, isopinocamphone, pinocarvone, germacrene-D, and methyleugenol. They also found that the oils extracted from different subspecies or plant populations of varying origin or morphology differed in percentage composition of the major volatile constituents.
Said-Al Ahl et al. [38] from Egypt found that the major constituents of H. officinalis oil were cis-pinocamphone (26.85%), β-pinene (20.43%), trans-pinocamphone (15.97%), α-elemol (7.96%), durenol (3.11%), β-phellandrene (2.41%), caryophyllene (2.34%), (E)-2,6-dimethyl-1,3,5,7-octatetraene (2.27%), 3(10)-caren-4-ol, acetoacetic acid ester (2.14%), bicyclogermacrene (1.83%), myrtenol (1.73%), germacrene D (1.68%), limonene (1.56%), γ-eudesmol (1.36%), and linalool (1.08%).
Ahmadi et al. [39] conducted a study on improving the yield of essential oil from H. officinalis after first and second harvest. It was concluded that citrulline application at the rate of 2 mM resulted in 15 and 30% of improvement in the yield after the first and second harvest, respectively. The authors found that the application of 2 mM of citrulline yielded 47% isopinocamphone as the dominant essential oil component under severe drought from H. officinalis. The principal components present in Hyssopus essential oil are presented in Table 2.
Table 2.
Principal bioactive compounds of Hyssopus essential oil.
| Plant material | Essential oil yield | Principal component of essential oil | Region | Reference |
|---|---|---|---|---|
| Air-dried aerial parts | 1.2% | Cis-pinocamphone (21.59%), trans-pinocamphone (7.93%), elemol (7.12%), bicyclogermacrene (6.58%), germacrene D (6.52%), limonene (6.36%), β-pinene (5.20%), trans-caryophyllene (4.65%) | Iran | [40] |
| Aerial parts | 0.18% | Myrtenyl acetate (74.08%), camphor (6.76%), germacrene (3.39%), spathulenol (2.14%), caryophyllene oxide (2.13%), β-caryophyllene (2.10%), cis- sabinol (1.75%), β-bourbonene (1.47%), bornyl acetate (1.42%) | Iran | [39] |
| Fresh aerial part (stem with inflorescence) | 0.47% | Pinocamphone 19.8%, β − pinene ≤ 20.5%, iso-pinocamphone 24.6% | Spain | [3, 41] |
| Aerial part (blue flowers) | 0.36% | Hedycaryol ≤ 9.1%, germacrene D ≤ 5.5%, β − pinene ≤ 11.4%, isopinocamphone ≤ 33.6%, pinocarvone ≤ 28.1% | East Lithuania | [41, 42] |
| Aerial parts | 0.4% | Limonene ≤ 9.8%, germacrene D ≤ 11.3%, 1, 8 − cineole ≤ 11.9%, pinocamphone ≤ 64.9%, β − pinene ≤ 32.4% | India | [41, 43] |
The main constituents of Hyssopus essential oils and their chemical structures are shown in Figure 1.
Figure 1.
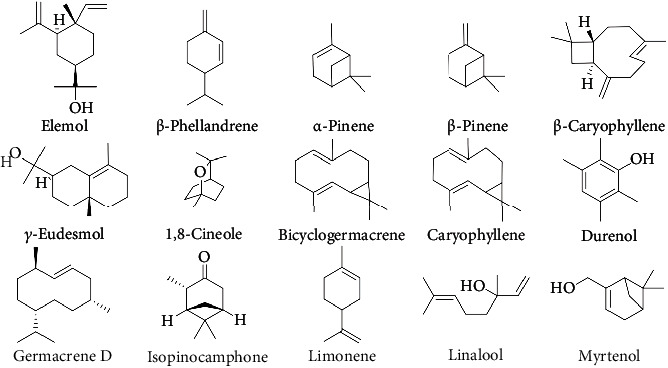
Chemical structures of the main bioactive constituents of Hyssopus essential oil.
5. Extraction Methods of Essential Oil from Hyssopus Genus
Hyssopus essential oils are usually extracted using traditional method such as Soxhlet extraction and hydrodistillation whereas nonconventional methods such as supercritical fluid extraction (SFE), ultrasound-assisted extraction (UAE), and instant controlled pressure drop process (DIC) have been utilized recently to improve the yield of essential oils from Hyssopus aerial plant parts [23].
In a recent study, the essential oil components were extracted using supercritical carbon dioxide (SC-CO2) extraction and Soxhlet extraction from 4 species of genus Hyssopus (differentiated by the corolla color). The extraction showed that the major components of genus Hyssopus species were pinocarvone, pinocamphone, and isopinocamphone. SC-CO2 results showed a lesser presence of monoterpene hydrocarbons and a high amount of oxygenated hydrocarbons [44].
In a recent study, supercritical fluid extraction (SFE) was used for the extraction of essential oil components from hyssop and yielded pinocamphone in the concentration of 0.7 to 13.6%, sabinene (4.2 to 17.1%), and iso-pinocamphone (0.9 to 16.5%). For the highest extraction of isopinocamphone and pinocamphone, the optimized conditions were 45°C temperature; 100 atmospheric pressure, with methanol 4.5 μL (0.14% w/w); 25 min static extraction time; and 20 min dynamic extraction time [45]. The authors concluded that input factors such as pressure, temperature, static and dynamic time, and modifier affect the extraction of essential oil components from Hyssopus.
Kazazi and Rezaei [46] collected hyssop from Iran which after analysis showed the presence of a high concentration of sabinene (11.04%). The major compounds of both extractions SFE and Soxhlet extraction in comparison were β-pinene (1 to 6%), terpinene-4-ol (4 to 10%), and 1, 8-cineol (eucalyptol) (60 to 75%) [47, 48]. The extraction methods, agrotechnical factors, climatic parameters, and from one growing season to another were the factors that vary the essential oil content of extract [27, 49–51].
In another study, essential oil from H. officinalis was extracted using an instant controlled pressure drop process (DIC). GC-MS analysis showed that 65% of total constituents of chromatogram were cis-pinocamphone (21.59%), trans-pinocamphone (7.93%), elemol (7.12%), bicyclogermacrene (6.58%), germacrene D (6.52%), limonene (6.36%), β-pinene (5.20%), and trans-caryophyllene (4.65%). Essential oil components such as linalool, 3-octanone, α-pinene, and α-thujene were detected in abundance in DIC compared to the hydrodistillation and ultrasound-assisted extraction.
6. Biological Activities
The essential oil and the extracts isolated from plants demonstrated average in vitro antibacterial activity, antimycotic insect killing, and antiviral properties [52, 53]. Hyssop essential oil extracts showed excellent antioxidant and anticancer activities [28, 54], although there is significant potential that has yet to be investigated [55, 56].
6.1. Antimicrobial, Antifungal, and Antiviral
The essential oil of Hyssopus contains β-pinene, iso pinocamphone, terpinene-4-ol, carvacrol, pinocarvone, pinocamphone, and p-cymene compounds with antimicrobial properties, which could be used to new drug development for therapy or prophylaxis against human infectious diseases [57]. In a recent study, hyssop essential oil demonstrated activity against pathogenic microbes against Staphylococcus aureus, Escherichia coli, Bacillus cereus, Proteus hauseri, Listeria monocytogenes, Rhodococcus equi, Listeria ivanovii, Salmonella enteritidis, Enterococcus faecalis, Listeria innocua, and Bacillus spizizenii [58].
Hyssopus officinalis essential oil holds antifungal activity which is particularly efficient in the reduction of the expansion of fungal species such as Candida krusei, Candida albicans, and Candida tropicalis [10, 58]. The MIC of H. officinalis essential oil in a liquid medium for yeast was between 0.15 and 0.3%. The essential oil also exhibited antimycotic activity against 10 yeast and fungi and C. albicans in the range of 15.625 to 250 μL/mL [59]. In Serbia, the mushroom Agaricus bisporus (Lange) Imbach was destroyed by the fungus Mycogone perniciosa (Mang); in 2005, the research was conducted to find out the antifungal agent to inhibit its growth.
Then, the essential oil of H. officinalis was tested for its antiperniciosa activity; the results showed minimal fungicidal activity of about 15 to 20 μL/mL and MIC about 5 μL/mL [60]. Then, aromatic plants were tested for their antifungal activity, to enhance the growth of mushroom [60, 61]. Then, from the aromatic plants of Mediterranean area, 12 essential oils were tested against four fungi, which were Penicillium Italicum, Botrytis cinerea, Rhizopus stolonifer, and Phytophthora citrophthora. The conclusion revealed that the hyssop oil exhibited weak to moderate fungicide activity but they could act as a food product preservative [4]. By using the agar diffusion method, H. officinalis essential oil showed complete growth inhibition of Aspergillus niger in concentrations between 0.5 and 1.5% [62].
Hyssopus officinalis dried leaf extract also showed efficacy in the replication inhibition of HIV (human immunodeficiency virus) [63].
6.2. Antioxidant
2,2-Diphenyl-1-picrylhydrazyl (DPPH) and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid (ABTS) assays are generally used for measuring the antioxidant activity of the plant extracts. Hyssop essential oil reported lower antioxidant activity against DPPH (1.21 μmol Trolox equivalent (TE)/g) and reported approximately ten times higher scavenging activity towards ABTS (10.6 μmol TE/g) [15].
In another study, the hyssop extracts from Serbia and Turkey showed an IC50 value of 156.60 mg/mL and 16.37 mg/mL [8, 57]. The differences in the antioxidant properties are due to genetic factors, environmental conditions, and geographic origin [28]. Antioxidant activity of the hyssop essential oil attributed towards the chelating power of transition metals. The antioxidative properties of hyssop essential oil can find important application in preservation of the food items such as ground beef by inhibiting the growth of pathogenic bacteria [64].
6.3. Anticancer/Cytotoxic
Natural products are a rich source of compounds with many applications in cancer chemotherapy [65–67]. Moreover, the broad spectrum of natural compounds provides important compounds for therapeutic refinement through molecular modifications [68, 69].
Hyssop extracts were evaluated for anticancer activity against human cancer cell lines using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay [15]. Authors utilized human breast cancer (MDA-MB 231), malignant melanoma (A375), and human colon cancer (HCT116 lines) cell lines for their investigation. The essential oil extracts from hyssop showed a concentration-dependent inhibitory effect against cancer cell lines. They reported IC50 values of 62.66, 35.16, and 29.91 μg/mL MDS-MB 231, A375, and HCT116 cell lines, respectively. High antioxidant activity was suggested to be due to the components of hyssop essential oil such as linalool and methyl eugenol. In another study, Hyssopus essential oils showed antitumor activity against tumor cells lines (HeLa, MRC-5, and MDA-MB231) [28]. On the same way, H. officinalis subsp. aristatus shows slight cytotoxicity towards the A549 cell line (adenocarcinomic human alveolar basal epithelial cells) [70].
The cytotoxicity of marrubiin was studied in 66 cancer cell lines without showing toxic activity; however, in vivo, an LD50 of 370 mg/kg body weight for marrubiin had been documented [71]. Recent studies have shown a safety limit of up to 100 mg/kg body weight when injected into mice [72].
6.4. Mosquito Larvicidal Activity
Mosquitoes are one of the emerging causes of death in developing and underdeveloped countries. Control of mosquito vectors is a challenging task due to environmental concerns and the development of pesticide resistance. Hence, researchers are in search of new botanicals for the control of mosquito-related diseases such as malaria, chikungunya, Zika virus, and dengue [73].
Essential oil of H. officinalis demonstrated lethal concentration (LC50) values more than 90 μL/L in an acute toxicity research study against double blends of Culex quinquefasciatus agent, a vector of lymphatic filariasis, maintaining it as environment-friendly, efficient, and inexpensive mosquito larva killer [74]. The study is crucial for the development of newer and safer mosquitocidal biopesticides for the control of mosquito-borne diseases.
6.5. Miorelaxation Activity
An experiment was carried out for testing the sedative and simulative effect of essential oil by inhaling in mouse forced swimming test [75]. The hyssop essential oil inhalation increased the sessile state, and this condition was artificially overagitated by caffeine parenteral injection. The result showed that hyssop essential oil could produce a sedative effect [75].
Lu et al. [76] designed an experiment for evaluation of the myorelaxant effect of H. officinalis essential oil on the intestinal muscle sample of rabbit and guinea pigs. The myorelaxing effect of essential oil was due to its component isopinocamphone. BaCl2 was involved in myocontraction in the ileum (IC50 = 48.3 and 70.4 μg/mL), and isopinocamphone did myorelaxation by the inhibition of acetylcholine on ileum muscles (IC50 42.4 and 61.9 μg/mL).
H. officinalis oil contains limonene and β-pinene which produced no effect on muscle contraction, but the synergic effect of components could not be excluded. The researchers believed that hyssop oil could induce myorelaxation after the alteration of ion channels and interaction with plasma membrane. The synergic activity of components of essential oil depended upon chemical structure, lipophilic nature, and subcomponents of essential oil such as interaction of β-pinene and limonene [76].
A recent study of extract of H. officinalis on seizures induced by pentylenetetrazole and hippocampus mRNA level of iNOS in rats found that 100 mg/kg dose of hyssop extract might have anticonvulsant effects. However, these anticonvulsant effects might not occur through the iNOS gene expression [77].
The biological activities of Hyssopus spp. essential oils are summarized in Figure 2.
Figure 2.
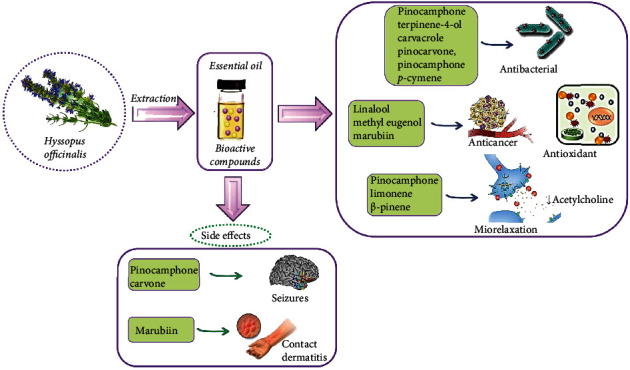
Schematic representation of biological actions, side effects, and the correlation with the most representative bioactive compounds isolated from Hyssopus officinalis essential oil. Biological activities of Hyssopus essential oils
7. Safety Data and Adverse Effects
A survey of the literature documented essential oils of hyssop and other 10 plants: eucalyptus, fennel, hyssop, pennyroyal, rosemary, sage, savin, tansy, thuja, turpentine, and wormwood, to be powerful convulsants due to their content of highly reactive monoterpene ketones, such as camphor, pinocamphone, thujone, cineole, pulegone, sabinyl acetate, and fenchone. The hyssop essential oil contains one or more oxygenated monoterpenes, such as pinocamphone.
The epileptogenic properties of these convulsants have been well established and studied in animals [78]. They may interact with and exert pharmacological action on central nervous system targets involved in epilepsy [79, 80]. It was shown by electrocorticographic records that Wistar rats, after injection of 80 mg/kg hyssop essential oil, develop subclinical generalized sustained high-voltage spikes. Increasing the dose to 1.25 g/kg leads to rhythmic myoclonus, subsequently to one or several tonics, clonic, ortonic-clonic seizures, and eventually lethal convulsive status. Similar results were obtained with half these doses of pinocamphone that produced seizures when used both internally and topically [81]. Also, a carvone derivative, hydroxy dihydrocarvone that increases seizure latency at high doses, may come with negative side effects, including palpebral ptosis, decreased response to touch, and decreased motor activity [82].
Hyssop essential oil modulates structures involved in neurotransmitter release and metabolism such as N-methyl-D-aspartate receptor (NMDA), gamma-aminobutyric acid type A (GABAA) and type B (GABAB), glycine, and acetylcholine receptors, as well as the acetylcholine esterase and GABA transaminase enzymes. Hyssop essential oils also modulate voltage-gated sodium and calcium channels and affect neuronal excitability and action potential dynamics. Another key property is the inability to cross the blood-brain barrier (BBB) of hyssop essential oil [79].
Other possible side effects of hyssop include allergic reactions. It can irritate the respiratory tract due to vexation of mucous and bronchospasm by marrubiin—another component of hyssop essential oil.
Hyssop oils are often used in medical aromatherapy, like other plant oils from the Lamiaceae: basil, lavender, peppermint, rosemary, or thyme. However, the side effects of aromatherapy have been suppressed or refuted by many inexpert aromatherapists to date. According to Yoo [83], the most common complication among 3000 aromatherapy patients recorded was a cutaneous adverse reaction, occurring in 0.99% of all participants. Most of these cases are deemed contact dermatitis caused by an essential oil. The second most common complication was asthmatic symptoms, early menstruation, arrhythmia, and oliguria.
No clinical trials have been conducted on hyssop essential oil adverse effects in humans. Reports are only from clinical research data obtained from hospitals [79]. The metabolites of principal active ingredients of hyssop: cis- and trans-3-pinanones, were examined from mouse and human liver microsomes by Höld et al. [33]. The major metabolites of cis-3-pinanone were 2-hydroxy-cis-3-pinanone and two minor metabolites. These compounds exhibited reduced toxicity but also acted as GABAA receptor antagonists. The urine from oral cis-3-pinanone treatment contained conjugates of metabolites and 2,10-dehydro-3-pinanone. Trans-3-pinanone has slower metabolization than cis-isomer and produces different hydroxy derivatives.
8. Limitations
In general, at the right dose, hyssop does not have adverse effects and toxicity. However, most of the herbs are safe when used in moderation, but excessive consumption can produce unknown effects [84]. For this fact, hyssop is no exception. This herb is believed to be relatively safe at levels commonly used in foods. The appropriate dose of hyssop depends on several factors such as the user's age, health, and several other conditions. At this time, there is not enough scientific information to determine an appropriate range of doses for this plant. However, at high doses, hyssop oil may be dangerous due to various other side effects, mainly drowsiness, seizures, and sensory effects associated with psychological disorders.
Although herbal products may offer a benefit, it is important to detect even small risks that would significantly affect the risk-benefit ratio in pregnancy. There is very little research on how hyssop products may affect pregnancy (it might cause the uterus to contract or start menstruation).
Although hyssop products may offer a benefit, it is important to detect even small risks that would significantly affect the risk. In addition, herb interactions with conventional medications may provoke undesirable effects. Several interactions have been described between herbal drugs and antidepressants or psycholeptics [85].
Despite the promising medical effects achieved in various researches, yet further detailed studies are required to better clarify the side effect of hyssop. The use of herbal medications does not have strict regulations. This problem is still poorly recognized and underestimated [78]. Since herbal medicines are a part of traditional medicine, they are not included in the FDA or EFSA categories giving a false impression of safety.
Therefore, in general, there is a need for the regulation of hyssop drugs to ensure their safety and determine the efficacy and constituents of the preparations [84]. Physicians should consider balancing the risks and benefits of complementary and alternative medicine using hyssop and its active compounds [86].
9. Overall Conclusions
Hyssopus essential oils are usually extracted using conventional methods such as hydrodistillation. However, recently, nonconventional methods such as SFE, UAE, and DIC are getting popularized due to higher extraction efficiencies compared to conventional methods. Most of the recent and older studies used GC and GC-MS techniques for qualitative and quantitative characterization of the essential oil from the Hyssopus genus. The genus Hyssopus had essential constituents which perform multiple actions. Its essential oil constituents possessed curative properties against cough, loss of appetite, fungal infection, spasmodic condition, and potent antimicrobial activities. The other in vivo actions are the sedative effects, plasma membrane relaxation, and cytotoxic activity. Biological activities and aroma from essential oil suggest its use as a potential antioxidant food ingredient and other pharmaceutical drugs.
At the same time, well-designed clinical trials examining the described characteristics of hyssop extracts and possible adverse reactions are still not investigated and need more studies to complete whether biological differences in results of the researches reverse the various types of plant material used, different procedures of isolation, different chemotypes, collection time, and locations. Further, molecular insights into various biological activities of essential oil from Hyssopus genus need to be explored in more depth.
Contributor Information
Javad Sharifi-Rad, Email: javad.sharifirad@gmail.com.
Miquel Martorell, Email: martorellpons@gmail.com.
Monica Butnariu, Email: monicabutnariu@yahoo.com.
Daniela Calina, Email: calinadaniela@gmail.com.
William C. Cho, Email: chocs@ha.org.hk.
Conflicts of Interest
The authors declare no conflict of interest.
Authors' Contributions
JSR and WCC conceived the review. CQ, MK, MA, MA, MI, NK, OS, DK, SN, AE, MV, NKD, MMA, MP, and LSR collected and analyzed studies. All authors contribute to the writings of the manuscript. JSR, MM, NCM, MB, DC, and WCC provide comments for the manuscript. JSR, WCC, DC contribute to the final revision and edit of the manuscript. All authors read and approved the final version of the manuscript.
References
- 1.Fatemeh F., Hamedeyazdan S. A review on Hyssopus officinalis L.: composition and biological activities. African Journal of Pharmacy and Pharmacology . 2011;5(17):1959–1966. doi: 10.5897/ajpp11.527. [DOI] [Google Scholar]
- 2.Satyal P. Composition and antimicrobial activity of the essential oil of Hyssopus seravschanicus growing wild in Tajikistan. Der Pharma Chemica . 2012;4(3):961–966. [Google Scholar]
- 3.Moro A. Effects of agronomic practices on volatile composition of Hyssopus officinalis L. Essential Oils. Molecules . 2011;16(5):4131–4139. doi: 10.3390/molecules16054131. [DOI] [Google Scholar]
- 4.Camele I. An attempt of postharvest orange fruit rot control using essential oils from Mediterranean plants. Journal of Medicinal Food . 2010;13(6):1515–1523. doi: 10.1089/jmf.2009.0285. [DOI] [PubMed] [Google Scholar]
- 5.PlantList T. 2021. http://www.theplantlist.org/
- 6.Heinrich M., Appendino G., Efferth T., et al. Best practice in research - Overcoming common challenges in phytopharmacological research. Journal of Ethnopharmacology . 2020;246:p. 112230. doi: 10.1016/j.jep.2019.112230. [DOI] [PubMed] [Google Scholar]
- 7. The Plant List, Version 1.1 . 2020. http://www.theplantlist.org/
- 8.Džamić A. M., Soković M. D., Novaković M., et al. Composition, antifungal and antioxidant properties of Hyssopus officinalis L. subsp. _pilifer_ (Pant.) Murb. essential oil and deodorized extracts. Industrial Crops and Products . 2013;51:401–407. doi: 10.1016/j.indcrop.2013.09.038. [DOI] [Google Scholar]
- 9.Özer H., Şahin F., Kılıç H., Güllüce M. Essential oil composition of Hyssopus officinalis L. subsp.angustifolius(Bieb.) Arcangeli from Turkey. Flavour and Fragrance Journal . 2005;20(1):42–44. doi: 10.1002/ffj.1421. [DOI] [Google Scholar]
- 10.Mazzanti G., Battinelli L., Salvatore G. Antimicrobial properties of the linalol-rich essential oil of Hyssopus officinalis L. vardecumbens (Lamiaceae) Flavour and Fragrance Journal . 1998;13(5):289–294. doi: 10.1002/(SICI)1099-1026(1998090)13:5<289::AID-FFJ750>3.0.CO;2-A. [DOI] [Google Scholar]
- 11.Chalchat J.-C., Adamovic D., Gorunovic M. S. Composition of oils of three cultivated forms of Hyssopus officinalis Endemic in Yugoslavia: f. albus Alef., F. cyaneus Alef and f. ruber Mill. Journal of Essential Oil Research . 2001;13(6):419–421. doi: 10.1080/10412905.2001.9699712. [DOI] [Google Scholar]
- 12.Dragland S., Senoo H., Wake K., Holte K., Blomhoff R. Several culinary and medicinal herbs are important sources of dietary antioxidants. The Journal of Nutrition . 2003;133(5):1286–1290. doi: 10.1093/jn/133.5.1286. [DOI] [PubMed] [Google Scholar]
- 13.Govaerts R. World Checklist of Selected Plant Families Database in ACCESS: 1-216203 . Kew: The Board of Trustees of the Royal Botanic Gardens; 2003. [Google Scholar]
- 14.Kara N., Baydar H. Morphogenetic, ontogenetic and diurnal variabilities of hyssop (of Hyssopus officinalis L.) Research on Crops . 2012;13:661–668. [Google Scholar]
- 15.Venditti A. Essential oil composition, polar compounds, glandular trichomes and biological activity of Hyssopus officinalis subsp. aristatus (Godr.) Nyman from central Italy. Industrial Crops and Products . 2015;77:353–363. doi: 10.1016/j.indcrop.2015.09.002. [DOI] [Google Scholar]
- 16.Simon J. E., Chadwick A. F., Craker L. E. Archon Books . 1984. Herbs: An Indexed Bibliography, 1971-1980 : the Scientific Literature on Selected Herbs, and Aromatic and Medicinal Plants of the Temperate Zone. The Scientific Literature on Selected Herbs. (Aromatic and Medicinal Plants of the Temperate Zone Series). [Google Scholar]
- 17.Govaerts R. World Checklist of Monocotyledons Database in ACCESS: 1-71827 . Kew: The Board of Trustees of the Royal Botanic Gardens; 2003. [Google Scholar]
- 18.Tison J., de Foucault B. Flora Gallica: Flore de France . France: Biotope Editions; 2014. [Google Scholar]
- 19.Galambosi B. Agronomical and phytochemical investigation of Hyssopus officinalis. Agricultural Science in Finland . 1993;2(4):293–302. doi: 10.23986/afsci.72652. [DOI] [Google Scholar]
- 20.Dzhumaev K. D. Dynamics of essential oil accumulation in the hyssop Hyssopus seravschanicus. Uzb. Biol. Zh. . 1986;6:31–33. [Google Scholar]
- 21.Ankovský M. J., Anda T. L. Genus Hyssopus L.–Recent Knowledge. Horticultural Science (Prague) . 2002;29(3):119–123. [Google Scholar]
- 22.Pirbalouti A. G. Chemical compositions and antioxidant activity of essential oils from inflorescences of two landraces of hyssop Hyssopus officinalis L. subsp.angustifolius Bieb. cultivated in southwestern, Iran. Journal of Essential Oil Bearing Plants . 2019;22(4):1074–1081. doi: 10.1080/0972060X.2019.1641431. [DOI] [Google Scholar]
- 23.Borrelli F. Hyssopus officinalis subsp. aristatus: an unexploited wild-growing crop for new disclosed bioactives. Industrial Crops and Products . 2019;140, article 111594 doi: 10.1016/j.indcrop.2019.111594. [DOI] [Google Scholar]
- 24.Mutu A. In: Intraspecific genetic variability of Hyssopus officinalis L . Cuza A. I., editor. Secţiunea Genetică şi Biologie Moleculară: Analele Ştiinţifice ale Universităţii; 2014. [Google Scholar]
- 25.Gorunovic M. S. Essential oil of Hyssopus officinalis L., Lamiaceae of Montenegro origin. Journal of Essential Oil Research . 1995;7(1):39–43. doi: 10.1080/10412905.1995.9698459. [DOI] [Google Scholar]
- 26.Vallejo M. C. G., Herraiz J. G., Pérez-Alonso M. J., Velasco-Negueruela A. Volatile oil of Hyssopus officinalis L. from Spain. Journal of Essential Oil Research . 1995;7(5):567–568. doi: 10.1080/10412905.1995.9698590. [DOI] [Google Scholar]
- 27.Ben Hamida N. Effect of salinity on the antiparasitic activity of hyssop essential oil. Journal of Essential Oil Research . 2020;32(1):69–78. doi: 10.1080/10412905.2019.1656677. [DOI] [Google Scholar]
- 28.Mićović T. Antioxidant, antigenotoxic and cytotoxic activity of essential oils and methanol extracts of Hyssopus officinalis L. Subsp. aristatus (Godr.) Nyman (Lamiaceae) Subsp. aristatus (godr.) nyman (lamiaceae) Plants . 2021;10(4):p. 711. doi: 10.3390/plants10040711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salehi B., Rescigno A., Dettori T., et al. Avocado–soybean unsaponifiables: a panoply of potentialities to be exploited. Biomolecules . 2020;10(1):p. 130. doi: 10.3390/biom10010130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garg S. Composition of essential oil from an annual crop of Hyssopus officinalis grown in Indian plains. Flavour and Fragrance Journal . 1999;14(3):170–172. doi: 10.1002/(SICI)1099-1026(199905/06)14:3<170::AID-FFJ808>3.0.CO;2-Q. [DOI] [Google Scholar]
- 31.Shah N. C. Gas chromatographic examination of oil of Hyssopus officinalis. Parfuemerie und Kosmetik . 1986;67:116–118. [Google Scholar]
- 32.Joulain D. Study of the chemical composition of hyssop (Hyssopusofficinalis Linnaeus) essential oil. Rivista Italiana Essenze . 1976;58:479–485. [Google Scholar]
- 33.Höld K. M., Sirisoma N. S., Sparks S. E., Casida J. E. Metabolism and mode of action of cis- and trans-3-pinanones (the active ingredients of hyssop oil) Xenobiotica . 2002;32(4):251–265. doi: 10.1080/00498250110095745. [DOI] [PubMed] [Google Scholar]
- 34.Salvatore G., D'Andrea A., Nicoletti M. A pinocamphone poor oil of Hyssopus officinalis L. var.decumbensfrom France (Barton) Journal of Essential Oil Research . 1998;10(5):563–567. doi: 10.1080/10412905.1998.9700972. [DOI] [Google Scholar]
- 35.Benedec D., et al. The presence of the superior aliphatic acids in Hyssopus officinalis L. (Lamiaceae). Farmacia-Bucuresti . 2002;50:61–64. [Google Scholar]
- 36.Fathiazad F., Mazandarani M., Hamedeyazdan S. Phytochemical analysis and antioxidant activity of Hyssopus officinalis L. from Iran. Adv. Pharmaceutical Bulletin . 2011;1(2):63–67. doi: 10.5681/apb.2011.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zawislak G. Essential oil composition of Hyssopus officinalis L. grown in Poland. Journal of Essential Oil-Bearing Plants . 2016;19(3):699–705. doi: 10.1080/0972060X.2014.935034. [DOI] [Google Scholar]
- 38.Said-Al Ahl H. Essential oil composition of Hyssopus officinalis L. cultivated in Egypt. International Journal of Plant Research . 2015;1(2):49–53. [Google Scholar]
- 39.Ahmadi H., Babalar M., Sarcheshmeh M. A. A., Morshedloo M. R., Shokrpour M. Effects of exogenous application of citrulline on prolonged water stress damages in hyssop (Hyssopus officinalis L.): Antioxidant activity, biochemical indices, and essential oils profile. Food Chemistry . 2020;333, article 127433 doi: 10.1016/j.foodchem.2020.127433. [DOI] [PubMed] [Google Scholar]
- 40.Rashidi S., Eikani M. H., Ardjmand M. Extraction of Hyssopus officinalis L. essential oil using instant controlled pressure drop process. Journal of Chromatography A . 2018;1579:9–19. doi: 10.1016/j.chroma.2018.10.020. [DOI] [PubMed] [Google Scholar]
- 41.Judžentienė A. Hyssop (Hyssopus officinalis L.) oils, in Essential Oils in Food Preservation, Flavor and Safety . Elsevier; 2016. [Google Scholar]
- 42.Bernotiene G., Butkiene R. Essential oils of Hyssopus officinalis L. cultivated in East Lithuania. Chemija . 2010;21(2-4):135–138. [Google Scholar]
- 43.Khan R., Shawl A. S., Tantry M. A. Determination and seasonal variation of chemical constituents of essential oil of Hyssopus officinalis growing in Kashmir valley as incorporated species of Western Himalaya. Chemistry of Natural Compounds . 2012;48(3):502–505. doi: 10.1007/s10600-012-0290-5. [DOI] [Google Scholar]
- 44.Kerrola K., Galambosi B., Kallio H. Volatile components and odor intensity of four phenotypes of hyssop (Hyssopus officinalis L.) Journal of Agricultural and Food Chemistry . 1994;42(3):776–781. doi: 10.1021/jf00039a035. [DOI] [Google Scholar]
- 45.Kazazi H. Supercriticial fluid extraction of flavors and fragrances from Hyssopus officinalis L. cultivated in Iran. Food Chemistry . 2007;105(2):805–811. doi: 10.1016/j.foodchem.2007.01.059. [DOI] [Google Scholar]
- 46.Kazazi H., Rezaei K. Effect of various parameters on the selective extraction of main components from hyssop using supercritical fluid extraction (SFE) Food Science and Technology Research . 2009;15(6):645–652. doi: 10.3136/fstr.15.645. [DOI] [Google Scholar]
- 47.Toth N. F., Schwirtlich E. B., Mironovic I. Isolation of antioxidants bysupercritical extraction of hyssop and sage with carbon dioxide. HemijskaIndustrija . 1989;43:450–453. [Google Scholar]
- 48.Langa E. The evolution of hyssop oil composition in the supercritical extraction curve: modelling of the oil extraction process. The Journal of Supercritical Fluids . 2009;49(1):37–44. doi: 10.1016/j.supflu.2008.11.022. [DOI] [Google Scholar]
- 49.Benhammou N., Bekkara F., Panovska T. K. Antioxidant and antimicrobial activities of the Pistacia lentiscus and Pistacia atlantica extracts. African Journal of Pharmacy and Pharmacology . 2007;2:22–28. [Google Scholar]
- 50.Xu L. Recent advances on supercritical fluid extraction of essential oils. African Journal of Pharmacy and Pharmacology . 2011;5(9):1196–1211. doi: 10.5897/AJPP11.228. [DOI] [Google Scholar]
- 51.Ghalem B. R., Mohamed B. Antimicrobial activity evaluation of the oleoresin oil of Pistacia vera L. African Journal of Pharmacy and Pharmacology . 2008;3:92–96. [Google Scholar]
- 52.Salehi B. Veronica plants-drifting from farm to traditional healing, food application, and phytopharmacology. Molecules . 2019;24(13):p. 2454. doi: 10.3390/molecules24132454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Salehi B. Ficus plants: state of the art from a phytochemical, pharmacological, and toxicological perspective. Phytotherapy Research . 2021;35(3):1187–1217. doi: 10.1002/ptr.6884. [DOI] [PubMed] [Google Scholar]
- 54.Oalđe M. A comprehensive assessment of the chemical composition, antioxidant, genoprotective and antigenotoxic activities of Lamiaceae species using different experimental models in vitro. Food & Function . 2021;12(7):3233–3245. doi: 10.1039/D1FO00447F. [DOI] [PubMed] [Google Scholar]
- 55.Zvezdina Е. V. Members of the family Lamiaceae Lindl. as sources of medicinal plant raw materials to obtain neurotropic drugs. Pharmacy & Pharmacology . 2020;8(1):4–28. doi: 10.19163/2307-9266-2020-8-1-4-28. [DOI] [Google Scholar]
- 56.Popov I. V. Biologically active substances exhibiting antioxidant activity, some representatives of the Lamiaceae family cultivated in the stavropol region. Chemistry of plant raw material . 2020;4:163–172. [Google Scholar]
- 57.Kizil S. Chemical composition, antimicrobial and antioxidant activities of Hyssop (Hyssopus officinalis L.) essential oil. Notulae Botanicae Horti Agrobotanici Cluj-Napoca . 2010;38:99–103. [Google Scholar]
- 58.Proškovcová M. Antibiofilm activity of selected plant essential oils from the family against Candida albicans clinical isolates. Annals of Agricultural and Environmental Medicine . 2021;28(2):260–266. doi: 10.26444/aaem/135892. [DOI] [PubMed] [Google Scholar]
- 59.Ozer H. In vitro antimicrobial and antioxidant activities of the essentialoils and methanol extracts of Hyssopus officinalis L. ssp. angustifolius. Italian Journal of Food Science . 2006;18:73–83. [Google Scholar]
- 60.Glamoclija J. Antifungal activity of essential oil Hyssopus officinalis L. against micopathogen Mycogone perniciosa (Mang) Matica Srpska Proceedings for Natural Sciences . 2005;109(109):123–128. doi: 10.2298/ZMSPN0519123G. [DOI] [Google Scholar]
- 61.Raila A. Different drying technologies and alternation of mycobiots in the raw material of Hyssopus officinalis L. Annals of Agricultural and Environmental Medicine . 2009;16(1):93–101. [PubMed] [Google Scholar]
- 62.Motiejunaite O., Kalediene L. Antimicrobial activity of Lamiaceaeplant essential oils on Aspergillus niger growth. Biol. Sci. . 2003;51:237–242. [Google Scholar]
- 63.Gollapudi S. Isolation of a Previously Unidentified Polysaccharide (MAR-10) from Hyssop officinalis That Exhibits Strong Activity Against Human Immunodeficiency Virus Type 1. Biochemical and Biophysical Research Communications . 1995;210(1):145–151. doi: 10.1006/bbrc.1995.1639. [DOI] [PubMed] [Google Scholar]
- 64.Michalczyk M. Effect of adding essential oils of coriander (Coriandrum sativum L) and hyssop (Hyssopus officinalis L.) on the shelf life of ground beef. Meat Science . 2012;90(3):842–850. doi: 10.1016/j.meatsci.2011.11.026. [DOI] [PubMed] [Google Scholar]
- 65.Sharifi-Rad J. Paclitaxel: application in modern oncology and nanomedicine-based cancer therapy. Oxidative Medicine and Cellular Longevity . 2021;2021:24. doi: 10.1155/2021/3687700.3687700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sharifi-Rad J. Chitosan nanoparticles as a promising tool in nanomedicine with particular emphasis on oncological treatment. Cancer Cell International . 2021;21(1):318–318. doi: 10.1186/s12935-021-02025-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sharifi-Rad J. Genistein: an integrative overview of its mode of action, pharmacological properties, and health benefits. Oxidative Medicine and Cellular Longevity . 2021;2021:36. doi: 10.1155/2021/3268136.3268136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sani T. A. Cytotoxic and apoptogenic properties of Dracocephalum kotschyi aerial part different fractions on Calu-6 and Mehr-80 lung cancer cell lines. Farmácia . 2017;65(2):189–199. [Google Scholar]
- 69.Salehi B. Pharmacological properties of chalcones: a review of preclinical including molecular mechanisms and clinical evidence. Frontiers in Pharmacology . 2021;11 doi: 10.3389/fphar.2020.592654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Guerrini A. Wild Italian Hyssopus officinalis subsp. aristatus (Godr.) Nyman: from morphological and phytochemical evidences to biological activities. Plants (Basel) . 2021;10(4):p. 631. doi: 10.3390/plants10040631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.National Cancer Institutev. National Center for Biotechnology Information, PubChem Database . Marrubiin, CID=7340; 2020. [Google Scholar]
- 72.Popoola O. Marrubiin. Molecules (Basel, Switzerland) . 2013;18(8):9049–9060. doi: 10.3390/molecules18089049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dahmana H., Mediannikov O. Mosquito-borne diseases emergence/resurgence and how to effectively control it biologically. Pathogens (Basel, Switzerland) . 2020;9(4):p. 310. doi: 10.3390/pathogens9040310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Benelli G. Acute larvicidal toxicity of five essential oils (Pinus nigra, Hyssopus officinalis, Satureja montana, Aloysia citrodora and Pelargonium graveolens) against the filariasis vector Culex quinquefasciatus : Synergistic and antagonistic effects. Parasitology International . 2017;66(2):166–171. doi: 10.1016/j.parint.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 75.Lim W. C. Stimulative and sedative effects of essential oils upon inhalation in mice. Archives of Pharmacal Research . 2005;28(7):770–774. doi: 10.1007/BF02977341. [DOI] [PubMed] [Google Scholar]
- 76.Lu M. Muscle relaxing activity of Hyssopus officinalis essential oil on isolated intestinal preparations. Planta Medica . 2002;68(3):213–216. doi: 10.1055/s-2002-23139. [DOI] [PubMed] [Google Scholar]
- 77.Gholami M. Effects of aqueous extract of Hyssopus officinalis on seizures induced by pentylenetetrazole and hippocampus mRNA level of iNOS in rats. Avicenna Journal of Phytomedicine . 2019;10 [PMC free article] [PubMed] [Google Scholar]
- 78.Burkhard P. R. Plant-induced seizures: reappearance of an old problem. Journal of Neurology . 1999;246(8):667–670. doi: 10.1007/s004150050429. [DOI] [PubMed] [Google Scholar]
- 79.Bahr T. A. The effects of various essential oils on epilepsy and acute seizure: a systematic review. Evidence-based Complementary and Alternative Medicine . 2019;2019:14. doi: 10.1155/2019/6216745.31239862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stappen I. Chemical composition and biological activity of essential oils of Dracocephalum heterophyllum and Hyssopus officinalis from Western Himalaya. Natural Product Communications . 2015;10(1):133–138. doi: 10.1177/1934578X1501000131. [DOI] [PubMed] [Google Scholar]
- 81.Millet Y. Toxicity of some essential plant oils. Clinical and experimental study. Clin Toxicol . 1981;18(12):1485–1498. doi: 10.3109/15563658108990357. [DOI] [PubMed] [Google Scholar]
- 82.de Sousa D. P., de Sousa Oliveira F., de Almeida R. N. Evaluation of the central activity of hydroxydihydrocarvone. Biological & Pharmaceutical Bulletin . 2006;29(4):811–812. doi: 10.1248/bpb.29.811. [DOI] [PubMed] [Google Scholar]
- 83.Yoo K. Statistical analysis of aromatherapy preferences and complications for 3000 otolaryngology patients. International Journal of Aromatherapy . 2006;16:181–185. [Google Scholar]
- 84.John L. J., Shantakumari N. Herbal medicines use during pregnancy: a review from the Middle East. Oman Medical Journal . 2015;30(4):229–236. doi: 10.5001/omj.2015.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Holst L., Nordeng H., Haavik S. Use of herbal drugs during early pregnancy in relation to maternal characteristics and pregnancy outcome. Pharmacoepidemiology and Drug Safety . 2008;17(2):151–159. doi: 10.1002/pds.1527. [DOI] [PubMed] [Google Scholar]
- 86.Javid A. Short-course administration of a traditional herbal mixture ameliorates asthma symptoms of the common cold in children. Avicenna J Phytomed . 2019;9(2):126–133. [PMC free article] [PubMed] [Google Scholar]


