Abstract
The presence of the zoonotic pathogen Salmonella in the food supply chain poses a serious public health threat. This study describes the prevalence, susceptibility profiles, virulence patterns, and clonality of Salmonella from a poultry flock monitored over six weeks, using the farm-to-fork approach. Salmonella was isolated using selective media and confirmed to the genus and species level by real-time polymerase chain reaction (RT-PCR) of the invA and iroB genes, respectively. Antimicrobial susceptibility profiles were determined using Vitek-2 and the Kirby–Bauer disk diffusion method against a panel of 21 antibiotics recommended by the World Health Organisation Advisory Group on Integrated Surveillance of Antimicrobial Resistance (WHO-AGISAR). Selected virulence genes were identified by conventional PCR, and clonality was determined using enterobacterial repetitive intergenic consensus PCR (ERIC-PCR). Salmonella was present in 32.1% of the samples: on the farm (30.9%), at the abattoir (0.6%), and during house decontamination (0.6%). A total of 210 isolates contained the invA and iroB genes. Litter, faeces, and carcass rinsate isolates were classified as resistant to cefuroxime (45.2%), cefoxitin (1.9%), chloramphenicol (1.9%), nitrofurantoin (0.4%), pefloxacin (11.4%), and azithromycin (11%). Multidrug resistance (MDR) was observed among 3.8% of the isolates. All wastewater and 72.4% of carcass rinsate isolates were fully susceptible. All isolates harboured the misL, orfL, pipD, stn, spiC, hilA, and sopB virulence genes, while pefA, spvA, spvB, and spvC were absent. In addition, fliC was only present among the wastewater isolates. Various ERIC-PCR patterns were observed throughout the continuum with different subtypes, indicating the unrelated spread of Salmonella. This study concluded that poultry and the poultry environment serve as reservoirs for resistant and pathogenic Salmonella. However, there was no evidence of transmission along the farm-to-fork continuum.
1. Introduction
Salmonella infections remain one of the most common foodborne diseases, which constitutes a global public health concern [1]. As early as the 1950s, Salmonella was highlighted as an important zoonotic pathogen with economic implications by the World Health Organisation (WHO) and the Food and Agriculture Organisation (FAO) of the United Nations [2]. The genus Salmonella is divided into two species, i.e., S. enterica and S. bongori. S. enterica is also subdivided into six subspecies and comprises more than 2600 serovars [3]. The pathogenesis of Salmonella is mediated by various genes that promote host cell invasion, intracellular survival, and colonisation [4, 5].
Salmonella has been reported in intensive animal husbandry such as poultry production [6]. In poultry farms, flocks can become infected via vertical or horizontal transmission [7, 8]. Vertical transmission occurs when the reproductive tissues and organs of laying hens are infected with Salmonella, which is transmitted via the eggs to the progeny of the flock [3]. During horizontal transmission, poultry are exposed to contaminated litter, faeces, feed, water, equipment, other chickens, rodents, other animals, and farm personnel colonised/infected with Salmonella [9]. Food-producing animals are considered the primary sources of Salmonella, and their intestinal tract is the main reservoir for nontyphoidal Salmonella (NTS) strains [7]. Poultry products such as poultry meat, eggs and other poultry-derived products are common sources of NTS [1]. The sources and transmission routes of NTS in food are not well understood in low- and -middle-income countries (LMICs) due to the lack of integrated epidemiological surveillance systems [10, 11].
The extensive use of antimicrobials in poultry production and the emergence of MDR Salmonella strains, which can spread to humans via the food pathway, are a public health concern worldwide [7, 12]. In South Africa (SA), the use of antibiotics in commercial poultry farms has been associated with the development of MDR Salmonella strains in poultry intended for human consumption [2, 13]. Salmonella strains circulating among poultry have not been comprehensively investigated from the farm-to-fork approach [14, 15]. This study describes the prevalence, antimicrobial susceptibility profiles, virulence profiles, and clonality of Salmonella isolated along the farm-to-fork continuum to inform mitigation strategies.
2. Materials and Methods
2.1. Ethical Considerations
This study was part of an overarching research project on Antibiotic Resistance and One Health; ethical clearance was obtained from the Animal Research Ethics Committee (reference AREC 073/016PD) and the Biomedical Research Ethics Committee (reference BCA444/16) of the University of KwaZulu-Natal (UKZN). The study was also registered with the South African National Department of Agriculture, Forestry, and Fisheries (reference 12/11/1/5 (879)).
2.2. Study Population and Sample Strategy
2.2.1. Study Area
An intensive poultry farm located in the uMgungundlovu district in Northern KwaZulu-Natal was identified and agreed to participate in this study. The sampling strategy adopted was according to the WHO-AGISAR guidelines [16].
2.2.2. Sample Collection
A flock of one-day-old Cobb breed hatchlings, placed in a clean chicken house, was the target population. A total of 162 samples were collected using block sampling to ensure that the entire flock was represented within the poultry house over a six-week period. The sample collection included the following: weekly poultry faeces and litter, truck, and crate swabs when chickens were transported to the abattoir; carcass rinsate during slaughter at the abattoir; ceca from postslaughter and ready-to-cook packaged samples of the whole carcass, neck, and thigh. The wastewater sample was collected from the main drainpipe during house cleaning after the flock was removed. Briefly, ten litter and ten faecal samples (5 g of each sample) were collected per week (5 weeks = 50 litter, 50 faeces). On week 5, ten samples of each of the following were collected: truck swabs, crate swabs, ceca (25 g of each ceca sample), neck (25 g of each neck sample), thighs (25 g of each thigh sample), and whole carcasses. A single 40 ml sample was collected from carcass rinsate during slaughter and wastewater during the house decontamination. All samples were collected aseptically into sterile containers, labelled clearly, placed into cooler boxes, then transported immediately to the UKZN Antimicrobial Research Unit (ARU) laboratory. Samples were processed within four to six hours of collection. Salmonella prevalence was calculated as a percentage of Salmonella culture-positive samples among the total number of samples collected.
2.2.3. Sample Processing and Isolation of Salmonella
The samples were inoculated in 40 ml of nonselective tryptic soy broth (TSB) (Oxoid Ltd., Basingstoke Hampshire, UK) and incubated for two hours at 37°C with shaking at 100 rpm. Thereafter, 0.1 ml was inoculated into 9.9 ml of Rappaport-Vassiliadis broth (Oxoid Ltd., Basingstoke, UK), a selective enrichment media for Salmonella and incubated at 42°C for 24 hours [17]. A loopful of the sample was streaked onto Brilliance Salmonella Agar (Oxoid Ltd., Basingstoke Hampshire, England) and incubated at 37°C for 24 hours [18]. Single presumptive purple/pink colonies typical of Salmonella were picked and subsequently streaked onto Hektoen Enteric Agar (HEA) (Oxoid Ltd., Basingstoke Hampshire, England) and Salmonella Shigella agar (SSA) (Oxoid Ltd., Basingstoke Hampshire, England) plates and incubated at 37°C for 24 hours. Culture plates were examined for the presence of typical colonies based on morphological characteristics, i.e., clear colonies with a black centre on HEA and SSA. Single colonies were subcultured onto nutrient agar and subsequently stored in TSB supplemented with 10% glycerol (Merck, USA) at −60°C until further analysis. Salmonella enterica subsp. enterica serovar Choleraesuis ATCC 10708 (ATCC, Manassas, Virginia, USA) was included as a control strain.
2.3. Isolation and Identification of Salmonella
2.3.1. Phenotypic Confirmation of Salmonella
All presumptive stored Salmonella colonies were recovered from the freezer; subcultured and pure colonies on nutrient agar were subjected to biochemical identification by the catalase test using 3% H2O2 and the oxidase test using test strips (Sigma-Aldrich, St. Louis, USA). Further biochemical confirmation was performed using the API 20E kit (bioMérieux, Marcy I'Etoile, France) according to the manufacturer's instructions. Salmonella enterica subsp. enterica serovar Choleraesuis ATCC 10708 (ATCC, Manassas, Virginia, USA) was used as a control strain. All isolates that were phenotypically confirmed as Salmonella species (spp.) were subjected to further genotypic confirmation.
2.3.2. Molecular Confirmation of Salmonella
DNA was extracted using the boiling method [19]. Colonies from an overnight culture grown on nutrient agar were briefly suspended into 200 μl of distilled water and lysed at 100°C for 15 minutes. This was followed by centrifugation at 13000 rpm for 5 minutes; thereafter, 120 μl of supernatant was transferred to a clean microcentrifuge tube and stored at 4°C until further analysis.
Isolates were genotypically confirmed as Salmonella using RT-PCR of the genus-specific invA gene (284 bp) and the S. enterica species-specific iroB gene (606 bp), using the QuantStudio 5 RT-PCR machine (Thermo Fisher Scientific, Waltham, MA, USA). The following primer sequences were used for invA F: 5′ GTGAAATTATCGCCACGTTCGGGCAA 3′and invA R: 5′ TCATCGCACCGTCAAAGGAACC 3′; iroB F: 5′ TGCGTATTCTGTTTGTCGGTCC 3′and iroB R: 5′ TACGTTCCCACCATTCTTCCC 3' [20, 21]. A 10 ul reaction mixture containing 5 μl of Luna® Universal qPCR Master Mix (New England Biolabs, Ipswich, MA, USA), 0.5 μl of each of the forward and reverse primers (20 μM) (Inqaba Biotechnical Industries (Pty) Ltd., Pretoria, South Africa), 1 μl of nuclease-free water (Lonza Rockland, ME, USA), and 3 μl of template DNA was prepared. The following cycling conditions were used: an initial activation at 95°C for 10 minutes, followed by 50 cycles of denaturation at 95°C for 10 seconds (s), annealing at 64°C (15 s), extension at 72°C (25 s), and a final extension at 72°C for 5 min. Melting was done by ramping from 72 to 90°C, with a 0.1°C rise at each step, a premelt hold for 90 s in the first step, followed by a hold for 2 s in the next steps [22]. Real-time melt curves were obtained and the presence of the respective genes was analysed. The melting temperatures for the genes ranged from 85 to 86°C for invA and 88 to 89°C for iroB. Salmonella enterica subsp. enterica serovar Choleraesuis ATCC 10708 (ATCC, Manassas, Virginia, USA) was used as a positive control reference strain and a tube containing nuclease-free water (Thermo Fisher Scientific, Vilnius, Lithuania) instead of template DNA was included as a negative control.
2.4. Antimicrobial Susceptibility Testing (AST)
AST was carried out using the automated Vitek-2 system according to the manufacturer's instructions and the Kirby–Bauer disk diffusion method (for antibiotics absent from the Vitek-2 panel) following the established Clinical and Laboratory Standards Institute (CLSI) guidelines [23]. A panel of 21 antibiotics (Oxoid Ltd., Basingstoke Hampshire, UK) was tested following the WHO-AGISAR recommendations, as follows: ampicillin (10 μg), amoxicillin (10 μg), amoxicillin/clavulanate (10/20 μg), cefuroxime (30 μg), cefoxitin (30 μg), cefotaxime (30 μg), ceftazidime (30 μg), cefepime (30 μg), imipenem (10 μg), meropenem (10 μg), gentamicin (10 μg), amikacin (30 μg), chloramphenicol (30 μg), azithromycin (15 μg), nitrofurantoin (300 μg), nalidixic acid (30 μg), ciprofloxacin (5 μg), pefloxacin (5 μg), tetracycline (30 μg), tigecycline (15 μg), and trimethoprim/sulfamethoxazole (1.25/23.75 µg). The WHO-AGISAR panel of antibiotics recommended for testing is based on well-established monitoring and surveillance systems on antimicrobial resistance in foodborne bacteria. The selection of antibiotics provides a harmonised standard that allows for data comparison between laboratories and countries [16]. In brief, a bacterial inoculum matched to a 0.5 McFarland standard was prepared from a pure overnight culture. For Vitek-2, the inoculum and AST-N255 cartridges were loaded into the instrument and programmed to run overnight. For disk diffusion, the suspension was evenly spread on a Mueller–Hinton agar plate using a sterile cotton swab and allowed to air-dry before dispensing the antibiotic disks onto the surface. Plates were incubated at 37°C for 18–24 hours. The minimum inhibitory concentrations (MIC) were determined by Vitek-2 and inhibition zones were measured for disk diffusion. The results were interpreted as susceptible, intermediate, or resistant according to the CLSI breakpoints [23]. E. coli ATCC 25922 (ATCC, Manassas, Virginia, USA) was used as a quality control strain. Isolates were classified as MDR if they displayed resistance to one or more antibiotics belonging to three or more different antibiotic classes [24, 25].
2.5. Molecular Detection of Virulence Factors
2.5.1. DNA Extraction
Genomic DNA was purified from bacterial cultures grown overnight in 1 ml of TSB. The genomic DNA GeneJET extraction kit (Thermo Fisher Scientific, Vilnius, Lithuania) was used, following the manufacturer's instructions for Gram-negative bacteria. All DNA was stored at −20°C until further analysis by conventional PCR for virulence genes and clonality by ERIC-PCR.
2.5.2. Detection of Virulence Genes
Twelve virulence genes were analysed by conventional PCR using specific primer pairs as described previously [4, 26, 27]. The primer sequences, cycling conditions, and product sizes are listed in Table 1. All PCR reactions were performed in a final volume of 15 μl containing the following: 8 μl of DreamTaq Master Mix (Thermo Fisher Scientific, Vilnius, Lithuania), 0.5 μl of each the forward and reverse primers (20 μM), 3.5 μl of nuclease-free water (Thermo Fisher Scientific, Vilnius, Lithuania), and 2.5 μl of the template DNA. Salmonella enterica subsp. enterica serovar Choleraesuis ATCC 10708 was used as quality control. The amplified PCR products were electrophoresed on a 1.5% agarose (Lonza Rockland, ME, USA) gel, containing 5 μl of ethidium bromide (Sigma-Aldrich, St. Louis, MO, USA) in 1X TAE buffer (BioConcept Ltd., Allschwil, Switzerland), at 100 volts (V) for 1 hour. The bands were visualised using the Bio-Rad gel documentation system (Bio-Rad, Hercules, CA, USA) and Image software (Bio-Rad, Hercules, CA, USA). A 100 bp molecular weight ladder (New England Biolabs, Ipswich, MA, USA) was used to determine the size of the PCR fragments. Salmonella enterica subsp. enterica serovar Choleraesuis ATCC 10708 (ATCC, Manassas, Virginia, USA) was used as a positive control strain and a tube containing nuclease-free water (Thermo Fisher Scientific, Vilnius, Lithuania), instead of template DNA, was included as a negative control.
Table 1.
Primers used for the amplification of Salmonella virulence genes.
| Gene | Primer sequence | Initial denaturation | # of cycles | Cycling conditions | Final extension | Product size (bp) | References | ||
|---|---|---|---|---|---|---|---|---|---|
| Denaturation | Annealing | Extension | |||||||
| hilA | F: 5′ CGGAAGCTTATTTG CGCCATGCTGAGGTAG 3′ |
94°C, 5 m | 30 | 94°C, 1 m | 65°C, 1 m | 72°C, 1 m | 72°C, 10 m | 854 | [26] |
| R: 5′ GCATGGATCCCCG CGGCGAGATTGTG 3′ | |||||||||
| spiC | F: 5′ CCTGGATAATGAC TATTGAT 3′ |
94°C, 3 m | 30 | 94°C, 1 m | 55°C, 1 m | 72°C, 1 m | 72°C, 5 m | 309 | [21, 28] |
| R: 5′ AGTTTATGGTG ATTGCGTAT 3′ | |||||||||
| misL | F:5′ GTCGGCGAATGC CGCGAATA 3′ |
94°C, 3 m | 30 | 94°C, 1 m | 55°C, 1 m | 72°C, 1 m | 72°C, 5 m | 550 | [21, 28] |
| R: 5′ GCGCTGTTAAC GCTAATAGT 3′ | |||||||||
| orfL | F: 5′ GGAGTATCGAT AAAGATGTT 3′ |
94°C, 3 m | 30 | 94°C, 1 m | 55°C, 1 m | 72°C, 1 m | 72°C, 5 m | 350 | [21, 28] |
| R: 5′ GCGCGTAACGTC AGAATCAA 3′ | |||||||||
| pipD | F: 5′ CGGCGATTCATG ACTTTGAT 3′ |
94°C, 5 m | 34 | 94°C, 25°s | 56°C, 30°s | 72°C, 50°s | 72°C, 5 m | 400 | [21, 28] |
| R: 5′ CGTTATCATTCG GATCGTAA 3′ | |||||||||
| sopB | F: 5′ TCAGAAGRCGTC TAACCACTC 3′ |
94°C, 3 m | 30 | 94°C, 1 m | 55°C, 1 m | 72°C, 1 m | 72°C, 5 m | 517 | [27] |
| R: 5′ TACCGTCCTCA TGCACACTC 3′ | |||||||||
| stn | F: 5′TTGTGTCGCTATCA CTGGCAACC 3′ |
94°C, 5 m | 25 | 94°C, 1 m | 59°C, 1 m | 72°C, 1 m | 72°C, 10 m | 617 | [26] |
| R: 5′ ATTCGTAACCCG CTCTCGTCC 3′ | |||||||||
| fliC | F: 5′ CGGTGTTGCCCA GGTTGGTAAT 3′ |
94°C, 3 m | 30 | 94°C, 1 m | 55°C, 1 m | 72°C, 1.5 m | 72°C, 10 m | 620 | [26] |
| R: 5′ ACTGGTAAAGAT GGCT 3′ | |||||||||
| pefA | F: 5′ TGTTTCCGGGCT TGTGCT 3′ |
94°C, 5 m | 25 | 94°C, 55°s | 55°C, 55°s | 72°C, 55°s | 72°C, 10 m | 700 | [26] |
| R: 5′ CAGGGCATTTGC TGATTCTTCC 3′ | |||||||||
| spvA | F: 5′GTCAGACCCGT AAACAGT 3′ |
94°C, 5 m | 30 | 94°C, 30°s | 60°C, 30°s | 72°C, 1 m | 72°C, 5 m | 604 | [29] |
| R: 5′ GCACGCAGAG TACCCGCA 3′ | |||||||||
| spvB | F: 5′ ACGCCTCAGCG ATCCGCA 3′ |
94°C, 5 m | 30 | 94°C, 30 s | 60°C, 30°s | 72°C, 1 m | 72°C, 5 m | 1063 | [29] |
| R: 5′ GTACAACATCT CCGAGTA 3′ | |||||||||
| spvC | F: 5′ CGGAAATACCA TCAAATA 3′ |
94°C, 5 m | 30 | 93°C, 1 m | 42°C, 1 m | 72°C, 2 m | 72°C, 4°m | 669 | [26] |
| R: 5′ CCCAAACCCAT ACTTACTCTG 3′ | |||||||||
2.6. Determination of the Clonal Relationship Using Enterobacterial Repetitive Intergenic Consensus (ERIC-PCR)
For ERIC-PCR, universal primers ERIC-1R (5' -ATGTAAGCTCCTGGGGATTCAC- 3′) and ERIC-2 (5' -AAGTAAGTGACTGGGGTGAGCG- 3′) (Inqaba Biotechnical Industries (Pty) Ltd., Pretoria, South Africa) were used. The 25 μl reaction mixture consisted of 12.5 μl DreamTaq master Mix (Thermo Fisher Scientific, Vilnius, Lithuania), 0.1 μl ERIC-R1 primer (100 μM), 0.1 μl ERIC-2 primer (100 μM), 9.3 μl nuclease-free water (Thermo Fisher Scientific, Vilnius, Lithuania), and 3 μl template DNA. The following cycling conditions were used: initial denaturation at 95°C for 2 minutes followed by 34 cycles of denaturation at 90°C for 30 s, annealing at 52°C for 1 minute, extension at 65°C for 8 minutes, and a final extension at 65°C for 16 minutes [30]. Salmonella enterica subsp. enterica serovar Choleraesuis ATCC 10708 (ATCC, Manassas, Virginia, USA) was included as a quality control strain. An aliquot of the amplified products was separated on a 1.5% agarose gel, containing 5 μl of ethidium bromide (Sigma-Aldrich, St. Louis, MO, USA) in 1X TAE buffer (BioConcept Ltd., Allschwil, Switzerland), set at 75 V for 3 hours. The bands were visualised using the Bio-Rad gel documentation system (Bio-Rad, Hercules, CA, USA) and image software (Bio-Rad, Hercules, CA, USA). Moreover, 100 bp and 1 Kb molecular weight ladders (New England Biolabs, Ipswich, MA, USA) were included on each gel. BioNumerics software version 6.6 (Applied Maths, Sint-Martens-Latem, Belgium) was used to construct a dendrogram using the unweighted pair group method with arithmetic mean (UPGMA) and Dice's coefficient set at 0.5% optimisation and 1% tolerance. Isolates were grouped based on a similarity index of 70% [31].
3. Results
3.1. Salmonella Prevalence
Salmonella was isolated from 32.1% (52/162) of the samples collected along the farm-to-fork continuum (Table 2). Salmonella was isolated as follows: on the farm: week 2, litter (10/10) and faeces (10/10); week 3, litter (10/10); week 5, litter (10/10) and faeces (10/10); at the abattoir during slaughter: carcass rinsate (1/1); week 6 during house decontamination, wastewater (1/1). Salmonella was not isolated from the following samples: week 1: litter (0/10) and faeces (0/10); week 3, faeces (0/10); week 4, litter (0/10) and faeces (0/10); week 5: trucks (0/10), crates (0/10), and ceca (0/10) and retail: neck (0/10), thigh (0/10), and whole carcass (0/10). The overall prevalence of Salmonella on the farm was 30.9% (50/162), consisting of 18.5% for litter (30/162) and 12.4% faeces (20/162). Salmonella also isolated from carcass rinsate was 0.6% (1/162) and wastewater 0.6% (1/162) (Table 2). A total of 210 isolates (Table S1) were confirmed as Salmonella enterica spp. by biochemical tests and using RT-PCR.
Table 2.
Prevalence of Salmonella along the farm-to-fork continuum.
| Week | Production stage | Type of sample | # of samples | # Salmonella-positive | Prevalence (%) |
|---|---|---|---|---|---|
| 1 to 5 | Farm: growth period | Litter | 50 | 30 | 18.5 |
| Faeces | 50 | 20 | 12.4 | ||
| 5 | Transport and handling | Truck swabs | 10 | 0 | |
| Crate swabs | 10 | 0 | |||
| 5 | Slaughter | Abattoir: carcass rinsate | 40 ml = 1 | 1 | 0.6 |
| Postslaughter | Caeca | 10 | 0 | ||
| Retail meat | Neck | 10 | 0 | ||
| Thigh | 10 | 0 | |||
| Whole carcass | 10 | 0 | |||
| 6 | House decontamination | Wastewater | 40 ml = 1 | 1 | 0.6 |
| Total | 162 | 52 | 32.1 | ||
#: number.
3.2. Antimicrobial Susceptibility Tests (ASTs)
The antimicrobial susceptibility profiles of the Salmonella isolates are shown in Table 3. Of the total isolates, 51% (107/210) were resistant to at least one antibiotic and of these, 3.8% (8/210) were categorised as MDR (Table 4). MDR isolates exhibited resistance among 2–4 different antibiotic classes (Tables 3 and 4). Of the total isolates, 24.3% (51/210) were classified as intermediate and 24.8% (52/210) were susceptible to all antibiotics (Table 3).
Table 3.
Number of isolates with varying susceptibility profiles stratified by source.
| Susceptibility profile | Week 2 | Week 3 | Week 5 | Week 6 | Total | ||||
|---|---|---|---|---|---|---|---|---|---|
| Resistant | Intermediate | Litter | Faeces | Litter | Litter | Faeces | Carcass rinsate | Wastewater | |
| CXM | FOX | 3 | 7 | 3 | 16 | 17 | 1 | 47 | |
| CXM | FOX-CHL | 1 | 2 | 1 | 4 | ||||
| CXM | FOX-NIT | 1 | 1 | 5 | 9 | 16 | |||
| CXM | FOX-NIT-CHL | 1 | 1 | ||||||
| CXM | FOX-NIT-NA | 1 | 1 | ||||||
| AZM | CXM-FOX-CHL | 2 | 2 | ||||||
| AZM | CXM-FOX-NIT-CHL | 1 | 1 | ||||||
| PEF | CXM-FOX-CHL | 1 | 1 | ||||||
| PEF | CXM-FOX-NIT-CHL | 1 | 1 | ||||||
| CXM-FOX | — | 1 | 1 | 2 | |||||
| CXM-FOX | NIT | 2 | 2 | ||||||
| CXM-CHL | FOX | 1 | 1 | ||||||
| CXM-AZM | FOX-CHL | 1 | 3 | 4 | |||||
| CXM-AZM | FOX-NIT-CHL | 1 | 1 | ||||||
| CXM-PEF | FOX-CHL | 3 | 3 | ||||||
| CXM-PEF | FOX-CCHL-AMX | 1 | 1 | ||||||
| CXM-PEF | FOX-CHL-NA | 2 | 2 | ||||||
| CXM-PEF | FOX-CHL-NA-AMX | 1 | 1 | ||||||
| CXM-NIT | FOX-CHL | 1 | 1 | ||||||
| PEF-AZM | CXM-FOX-CHL | 4 | 4 | ||||||
| PEF-AZM | CXM-FOX-CHL-NA | 2 | 2 | ||||||
| PEF-AZM | CXM-FOX-NIT-CHL | 1 | 1 | ||||||
| CXM-PEF-AZM | FOX-CHL | 2 | 2 | ||||||
| CXM-PEF-AZM | FOX-NIT-CHL | 1 | 1 | 2 | |||||
| CXM-PEF-AZM | FOX-NIT-CHL-NA-AMX | 1 | 1 | ||||||
| CXM-CHL-PEF-AZM | FOX-NA | 1 | 1 | 1 | 3 | ||||
| — | CXM | 2 | 2 | ||||||
| — | CXM-NIT | 1 | 1 | ||||||
| — | CXM-FOX | 12 | 2 | 8 | 5 | 2 | 5 | 34 | |
| — | CXM-FOX-CHL | 3 | 3 | ||||||
| — | CXM-FOX-NIT | 10 | 10 | ||||||
| — | CXM-FOX-NIT-CHL | 1 | 1 | ||||||
| Susceptible | 21 | 31 | 52 | ||||||
| Total | 30 | 30 | 30 | 30 | 30 | 29 | 31 | 210 | |
CXM: cefuroxime; FOX: cefoxitin; CHL: chloramphenicol; NIT: nitrofurantoin; AZM: azithromycin; PEF: pefloxacin.
Table 4.
Single and multiple antibiotic resistance patterns of Salmonella isolates.
| Number of antibiotics | Resistance pattern (#) | # of isolates (%) | MDR (%) |
|---|---|---|---|
| 1 | CXM (69), AZM (3), PEF (2) | 74 (35.2%) | |
| 2 | CXM-FOX (4), CXM-CHL (1), CXM-AZM (5), CXM-PEF (7), CXM-NIT (1), PEF-AZM (7) | 25 (11.9%) | |
| 3 | CXM-PEF-AZM | 5 (2.4%) | 5 (2.4%) |
| 4 | CXM-CHL-PEF-AZM | 3 (1.4%) | 3 (1.4%) |
| Total | 107 (51%) | 8 (3.8%) | |
#: number; CXM: cefuroxime; FOX : cefoxitin; CHL : chloramphenicol; NIT : nitrofurantoin; AZM : azithromycin; PEF : pefloxacin.
The isolates were resistant to the following antibiotics: cefuroxime (45.2%, 95/210), cefoxitin (1.9%, 4/210), chloramphenicol (1.9%, 4/210), nitrofurantoin (0.4%, 1/210), pefloxacin (11.4%, 24/210), and azithromycin (11%, 23/210). The isolates were also classified as intermediate to the following antibiotics: 30% (63/210) to cefuroxime, 71.9% (151/210) to cefoxitin, 18.6% (39/210) to nitrofurantoin, 1.4% (3/210) to amoxicillin, 18.6% (39/210) to chloramphenicol, and 4.8% (10/210) to nalidixic acid (Table 5). The distribution of resistant isolates obtained from different sources is shown in Figure 1. All isolates were susceptible to tetracycline, cefotaxime, ceftazidime, cefepime, gentamicin, ampicillin, amikacin, meropenem, imipenem, tigecycline, ciprofloxacin, trimethoprim/sulfamethoxazole, and amoxicillin/clavulanic acid (Table 5).
Table 5.
Antimicrobial susceptibility results for Salmonella isolates from poultry.
| Antimicrobial class | Antimicrobials | # of isolates | Susceptibility profile | ||
|---|---|---|---|---|---|
| S (%) | I (%) | R (%) | |||
| Aminoglycosides | AMK | 210 | 210 (100%) | 0 | 0 |
| GEN | 210 | 210 (100%) | 0 | 0 | |
| Carbapenems | MER | 210 | 210 (100%) | 0 | 0 |
| IMP | 210 | 210 (100%) | 0 | 0 | |
| Cephalosporins | FOX (II) | 210 | 55 (26.2%) | 151 (71.9%) | 4 (1.9%) |
| CXM (II) | 210 | 52 (24.8%) | 63 (30%) | 95 (45.2%) | |
| CTX (III) | 210 | 210 (100%) | 0 | 0 | |
| CFZ (III) | 210 | 210 (100%) | 0 | 0 | |
| FEP (IV) | 210 | 210 (100%) | 0 | 0 | |
| Macrolides | AZM | 210 | 187 (89%) | 0 (0%) | 23 (11%) |
| Nitrofurans | NIT | 210 | 170 (81%) | 39 (18.6%) | 1 (0.4%) |
| Penicillins | AMX | 210 | 207 (98.6%) | 3 (1.4%) | 0 |
| AMP | 210 | 210 (100%) | 0 | 0 | |
| Phenicols | CHL | 210 | 167 (79.5%) | 39 (18.6%) | 4 (1.9%) |
| Quinolones | NA | 210 | 200 (95.2%) | 10 (4.8%) | 0 |
| PEF | 210 | 186 (88.6%) | 0 (0%) | 24 (11.4%) | |
| CIP | 210 | 210 (100%) | 0 | 0 | |
| Tetracyclines | TET | 210 | 210 (100%) | 0 | 0 |
| TGC | 210 | 210 (100%) | 0 | 0 | |
| Other | AMC | 210 | 210 (100%) | 0 | 0 |
| SXT | 210 | 210 (100%) | 0 | 0 | |
S: susceptible; I: intermediate; R: resistant; AMC: amoxicillin/clavulanic acid; AMK: amikacin; AMP: ampicillin; AZM: azithromycin; CIP: ciprofloxacin; CXM: cefuroxime; FOX: cefoxitin; CHL: chloramphenicol; CTX: cefotaxime; CFZ: ceftazidime; FEP: cefepime; GEN: gentamicin; IMP: imipenem; MER: meropenem; NIT: nitrofurantoin; PEF: pefloxacin; TET: tetracycline; TGC: tigecycline; SXT: trimethoprim/sulfamethoxazole.
Figure 1.
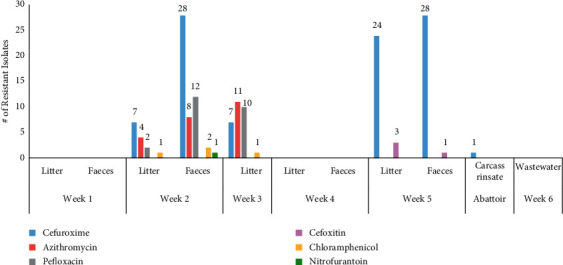
Distribution of resistant isolates stratified by source over the study period: on the farm, weeks 1–5 isolates consisted of litter and faeces; at the abattoir, carcass rinsate isolates were present.
3.3. Virulence Genes
Eight of the 12 virulence genes were detected among the isolates. The misL, orfL, pipD, stn, spiC, hilA, and sopB genes were present in all isolates from all sources, whereas pefA, spvA, spvB, and spvC were absent. The chromosomal virulence gene fliC was only present in the week 6 wastewater isolates.
3.4. ERIC-PCR
The ERIC-PCR yielded patterns consisting of 3–13 bands, with sizes ranging from 150 bp to 3 Kb. Diverse patterns were observed along the continuum. Clusters were source-specific based on a similarity index of 70% (Figure S1).
4. Discussion
The contamination of poultry and poultry products with Salmonella can occur at various stages of the production process. Although there are food safety standards, Salmonella continues to persist in the poultry processing industry [32].
4.1. Prevalence
In this study, an overall Salmonella prevalence of 32.1% was detected. The results are in agreement with those in previous studies conducted in broiler poultry farms in other countries that have reported a similar prevalence of Salmonella of 35% in Morocco and 31.25% in Bangladesh [33, 34]. The results of this study are also higher than those reported in the European Union (EU) (Finland and Sweden: <1%; Ireland: 19.8%), Asia (China: 12.6%; Korea: 15.3%), and South America (Columbia: 17.8%) [35–38]. Studies done in the USA and India along the farm-to-fork pathway reported an overall lower prevalence of 18.1% and 25.8%, respectively, than that in the current study [39, 40]. The proportion of Salmonella isolated from poultry can be influenced and varies by geographical location, environmental contamination, nature of farm management systems, type of production systems, breed of the flock, age of birds, sample size, sampling procedures, and the methods used for isolation [6, 11]. The biological nature of Salmonella in an infected host and its shedding pattern can also influence its isolation frequency, which may be seasonal and determined by environmental factors [25]. After contamination in poultry farms, Salmonella can colonise the intestines of chickens and result in contamination of the carcass during the slaughtering and meat processing stages [32].
In this study, Salmonella was not detected on the farm during week 1 and week 4. Sporadic isolation may be associated with various factors on the farm. Salmonella colonisation in chickens can be influenced by factors such as the age of the chicken, physiological and environmental stresses, survival of Salmonella through the gastric barrier, health and disease status of the chickens, vaccination, use of antimicrobials as growth promoters and prophylaxis, diet, and genetic background of the chickens [8, 41, 42]. In a previous study conducted in Ethiopia, young chicks were infected with 107−9 CFU/ml of S. typhimurium. Salmonella was detected two weeks postinfection, suggesting that the immunity of young chicks can provide early protection. However, over time, at four weeks, a decline was observed, indicating that resistance to Salmonella infection by older chickens may be associated with the activation of cellular and humoral immunity [43]. This scenario may provide information on the immune status of the poultry flock evaluated in our study.
4.2. Antimicrobial Susceptibility
Salmonella strains found in poultry have also developed resistance to antibiotics, which are crucial for treating human infections [7]. In SA, antibiotics among livestock are used mainly in intensively farmed poultry and pigs. The AST results revealed that 51% (107/210) of the isolates were resistant to one or more antibiotics, and 3.8% (8/210) were classified as MDR. Resistance was detected for six of the 21 antibiotics tested, which belonged to classes of antibiotics (cephalosporins, macrolides, and quinolones) that are used in the SA poultry production. In comparison to our study, higher rates of MDR Salmonella among poultry isolates have previously been reported in Africa (12.1%–100%), Asia (34.72–43.1%), the USA (9.5–18%), and the EU (38.2%) [12, 33, 37, 44–48].
In this study, resistance to the second-generation cephalosporins, cefuroxime (45.2%) and cefoxitin (1.9%), among isolates that were susceptible to other β-lactam antibiotics such as ampicillin was observed. A similar resistance phenotype was reported in Saudi Arabia, where clinical and environmental samples showed resistance to cefuroxime (90.9%) and cefoxitin (87.9%), while most isolates were susceptible to β-lactams [49]. Previous studies in Africa, Australia, and the USA have reported a higher frequency of resistance to cefuroxime or cefoxitin among Salmonella-positive poultry isolates [14, 39, 46, 50].
In 2015, pefloxacin was recommended as a reliable surrogate marker to identify susceptibility to fluoroquinolones, as tests with nalidixic acid and ciprofloxacin disks do not reliably detect resistance at low levels in Salmonella spp. [51–55]. This was observed in this study where there was 100% susceptibility to ciprofloxacin and 4.8% (10/210) intermediate to nalidixic acid compared with 11.4% (24/210) resistance to pefloxacin (Table 5), indicating that antibiotic panels may impact the resistance profiles observed. A similar scenario was reported in India by Prabhurajan et al. [56], where a higher rate of resistance to pefloxacin than that of nalidixic acid was observed among clinical Salmonella isolates. It was concluded that different mechanisms and mutations associated with fluoroquinolone resistance can influence atypical resistance phenotypes [56]. A systematic review of fluoroquinolone resistance in Salmonella in Africa by Taddesse et al. [57] also reported that Salmonella strains susceptible to nalidixic acid and resistant to ciprofloxacin have been identified among NTS serovars. The review also indicated that although reports on the distribution of Salmonella strains with unusual AST phenotypes are scarce, these strains occur in Africa [57].
Previous studies have reported varying levels of resistance or susceptibility to nalidixic acid, ciprofloxacin, and pefloxacin among isolates, attributed to the varying resistance mechanisms of fluoroquinolones. Different AST methodologies or reagents have also been identified as possible causes of this variation. Antibiotic discs from different manufacturers have given varying results and the narrow range to classify isolates as resistant or susceptible may introduce subjectivity when reading plates. The CLSI M100 guidelines indicate that no single antibiotic test can accurately detect all types of resistance associated with fluoroquinolones [7, 54, 58–60]. Automated systems used in laboratories may also have challenges in implementing new lower breakpoints for ciprofloxacin, and therefore, low-level resistance in isolates may be undetected [61].
In comparison to the results of the current study, higher rates of fluoroquinolone resistance (ciprofloxacin and nalidixic) have been reported in poultry in the EU (64.7% and 61.5%), Brazil (86.5% and 89%), and China (25.7% and 46.7%) [37, 45, 60]. However, Salmonella isolated from broilers in Canada (3% and <1%) and in the USA (0% and <1%) have shown a low resistance frequency to fluoroquinolones, which can be attributed to the restricted use of fluoroquinolones in poultry [39, 60, 62, 63].
In this study, low resistance frequencies were also observed for chloramphenicol (1.9%), nitrofurantoin (0.4%), and azithromycin (11%). Similarly, low rates of chloramphenicol resistance were reported among poultry isolates in Brazil (0.6%) and the USA (0.3%) [39, 60]. The low chloramphenicol and nitrofurantoin resistance in this study can be attributed to the prohibited use of these antibiotics in food animals in SA [64]. In South Korea, a slightly higher but comparable azithromycin resistance (17.9%) was reported compared to the results in the present study. In contrast, in the EU, USA, and Brazil, azithromycin resistance in food-producing animals and food products was either not detected or detected at a very low frequency (<1%) [12, 48, 60, 65]. Azithromycin is used for the treatment of MDR Salmonellosis in humans. Resistance to azithromycin observed in the present study may be associated with the authorised use of other macrolides such as tylosin on poultry farms in SA compared to the EU where macrolides are banned [12, 64, 66].
However, based on the resistant results obtained in this study associated with second-generation cephalosporins, quinolones, and fluoroquinolones, further molecular analysis of the genes that confer resistance and the mechanisms involved would be needed to explain the unusual AST phenotypes observed. This study also identified the presence of MDR Salmonella strains specifically at the farm level (Table 2). Antibiotic resistance continues to increase in LMICs, due to the injudicious use of antimicrobials as growth promoters and feed additives to strengthen the intestinal microflora and for the control, prophylaxis, and treatment of infectious diseases in animal husbandry [41]. In this study, Salmonella isolates were resistant to antibiotic classes that are listed as critically and highly important antimicrobials for human medicine by the WHO and as antimicrobials of veterinary importance by the World Organisation for Animal Health (OIE) [67, 68]. In Europe and the USA, many of these antibiotic classes are restricted or prohibited for use in food animals [63, 66]. In some African countries, antibiotics are used in food animals without strict regulations [14, 46]. However, in SA, regulations and policies exist, although enforcement remains challenging [64, 69]. The resistant Salmonella strains in food animals and food products can be transmitted to humans through the food chain, and this poses a health risk to consumers by causing infections that are more severe and difficult to treat [60, 70].
4.3. Virulence
The virulence genes present in Salmonella can determine the pathogenicity among the strains and serotypes circulating in poultry and the environment [26, 71]. In SA, information on the prevalence of Salmonella virulence genes found in poultry is limited, and only a few studies have investigated virulence in chickens [4, 21]. Gene clusters known as Salmonella pathogenicity islands (SPIs) are regions located on chromosomes that are distributed in the Salmonella genome, which are associated with virulence and enable efficient bacterial colonisation in the host. Genes such as hilA are found in SPI-1 that encode proteins involved in cell invasion [27]. Other genes located in the following SPIs, the SPI-2 gene spiC, the SPI-3 gene misL, and the SPI-4 gene orfL, are responsible for replication within macrophages [21, 28]. Genes located in SPI-5, such as pipD and sopB, promote enteritis and macrophage invasion accordingly [21, 27, 28, 72].
In this study, all Salmonella isolates possessed these virulence genes, indicating that healthy chickens and their environment serve as carriers of pathogenic Salmonella strains, which can cause systemic infection and enteric infection in the host [28]. Previous studies conducted in SA, UK, Egypt, and China have also reported the presence of these virulence genes in poultry [21, 26, 28, 72].
Other genes present on the Salmonella chromosomes found in this study were stn and fliC, which play a role in enterotoxin production and encode phase-1 flagellin, respectively [26]. All Salmonella isolates contained the stn gene, which has also been reported in all poultry isolates from studies conducted in China, Benin, and India [72–74]. However, the fliC gene in our study was only detected in the wastewater isolates obtained during the house decontamination. The fliC gene encodes for the flagellin protein and has been used as a target gene to determine antigenic specificity and genetic diversity in Salmonella. Studies done in Egypt, Malaysia, and Saudi Arabia described that fliC was serovar-specific for S. typhimurium, S. Kentucky, S. Aberdeen, S. Bergen, and S. Kedougou [26, 27, 75]. Serovars were not determined in this study.
4.4. Clonality
In Columbia, Herrera-Sanchez et al. [76] reported similar ERIC banding patterns consisting of 2—13 bands that ranged from 200 to 4000 bp in size whilst contrasting ERIC results of band sizes 15—1500 bp and 190—1430 bp were observed by Fendri et al. [77] in France and Oliveria et al. [78] in Brazil, respectively. Among the 210 isolates, there were various patterns and clusters. Isolates obtained from litter and faeces over the collection period clustered, indicating clonal spread on the farm. However, the carcass rinsate and wastewater isolates formed separate clusters. Salmonella transmission along the farm-to-fork continuum was not evident as unrelated strains from different sources belonged to different clusters based on a 70% similarity index. Of note, although ERIC-PCR has a shorter turnaround time, it is less discriminatory. Therefore, further studies involving more resolute typing approaches, such as serotyping and whole-genome sequencing (WGS), are recommended to elucidate the population structure of the strains.
5. Conclusion
Our study showed sporadic Salmonella contamination along the farm-to-fork pathway and demonstrated that healthy chickens could serve as a reservoir of resistant and pathogenic Salmonella strains, which harbour virulence factors and exhibit resistance to various antibiotics. The presence of MDR Salmonella strains among litter and faeces in this study indicates that the imprudent use of antibiotics on poultry farms could contribute to the increasing development and spread of antibiotic resistance in food animals and animal food products. Therefore, the initial steps to reduce and control Salmonella contamination of poultry meat should be taken at the farm level. SA has developed and implemented an Antimicrobial Resistance National Strategy Framework for 2018–2024 with the strategic objective of promoting the appropriate use of antibiotics in humans and animals using regulations. However, interventions to address antimicrobial use and resistance in food animals need to be expedited.
Acknowledgments
The authors express their gratitude to Professor Olarniran Ademola of the School of Life Sciences, University of KwaZulu-Natal (UKZN), Durban, South Africa, for granting them access to the BioNumerics software version 6.6 (Applied Maths NV, Belgium) to construct dendrograms and Dr. Sumayya Haffejee from Northdale Hospital and Mrs. Sharmla Lutchman from Lancet Laboratories for their assistance with AST. This research project was funded by the WHO Advisory Group on Integrated Surveillance of Antimicrobial Resistance (AGISAR) Research Project: “Triangulation of Antibiotic Resistance from Humans, the Food Chain, and Associated Environments—A One Health Project” (Reference ID: 204517) and the South African Research Chairs Initiative of the Department of Science and Technology and National Research Foundation of South Africa (Grant no. 98342).
Data Availability
The data used to support the findings of this study are included within the article.
Disclosure
Any opinions, findings, conclusions, or recommendations expressed in this material are those of the authors and do not necessarily reflect the organizations or agencies' views that provided support for the project. The funders had no role in the study design or the decision to submit the work for publication.
Conflicts of Interest
Professor Sabiha Y. Essack is the chairperson of the Global Respiratory Infection Partnership and a member of the Global Hygiene Council, both sponsored by unconditional educational grants from Reckitt, UK. All other authors declare that they have no conflicts of interest.
Authors' Contributions
All authors co-conceptualised the study, vetted the results, and undertook a critical review of the manuscript. M. A. R. performed the laboratory work. M. A. R., D. G. A., A. M. S., L. A. B., and A. L. K. A. conducted data analysis. S. Y. E., D. G. A., A. L. K. A., and L. A. B. supervised the work. M. A. R. wrote the original draft.
Supplementary Materials
Table S1: source of Salmonella isolates. Figure S1: dendrogram of ERIC-PCR patterns constructed of Salmonella enterica isolates recovered from the farm-to-fork continuum. Salmonella enterica subsp. enterica serovar Choleraesuis ATCC 10708 was used as the quality control strain. .
References
- 1.Eguale T. Non-typhoidal Salmonella serovars in poultry farms in central Ethiopia: prevalence and antimicrobial resistance. BMC Veterinary Research . 2018;14:217–218. doi: 10.1186/s12917-018-1539-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akinola S. A., Mwanza M., Ateba C. N. Occurrence, genetic diversities and antibiotic resistance profiles of Salmonella serovars isolated from chickens. Infection and Drug Resistance . 2019;12:3327–3342. doi: 10.2147/idr.s217421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jajere S. M. A review of Salmonella enterica with particular focus on the pathogenicity and virulence factors, host specificity and antimicrobial resistance including multidrug resistance. Veterinary World . 2019;12(4):504–521. doi: 10.14202/vetworld.2019.504-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mthembu T. P., Zishiri O. T., El Zowalaty M. E. Detection and molecular identification of Salmonella virulence genes in livestock production systems in South Africa. Pathogens . 2019;8:1–17. doi: 10.3390/pathogens8030124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh Y., Saxena A., Kumar R., Saxena M. K. Salmonella - A Re-emerging Pathogen . West Palm, FL, USA: InTech; 2018. Virulence system of Salmonella with special reference to Salmonella enterica. [Google Scholar]
- 6.Asfaw Ali D., Tadesse B., Ebabu A. Prevalence and antibiotic resistance pattern of Salmonella isolated from caecal contents of exotic chicken in Debre Zeit and Modjo, Ethiopia. The Internet Journal of Microbiology . 2020;2020:6. doi: 10.1155/2020/1910630.1910630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antunes P., Mourão J., Campos J., Peixe L. Salmonellosis: the role of poultry meat. Clinical Microbiology and Infections . 2016;22(2):110–121. doi: 10.1016/j.cmi.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Cosby D. E., Cox N. A., Harrison M. A., Wilson J. L., Buhr R. J., Fedorka-Cray P. J. Salmonella and antimicrobial resistance in broilers: a review. The Journal of Applied Poultry Research . 2015;24(3):408–426. doi: 10.3382/japr/pfv038. [DOI] [Google Scholar]
- 9.Poppe C. Salmonella in Domestic animals., Salmonella in Domestic Animals . Wallingford, UK: CABI; 2000. [Google Scholar]
- 10.Dione M. M., Ikumapayi U. N., Saha D., et al. Clonal differences between Non-Typhoidal Salmonella (NTS) recovered from children and animals living in close contact in the Gambia. PLoS Neglected Tropical Diseases . 2011;5(5):p. e1148. doi: 10.1371/journal.pntd.0001148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kagambèga A., Lienemann T., Aulu L., et al. Prevalence and characterization of Salmonella enterica from the faeces of cattle, poultry, swine and hedgehogs in Burkina Faso and their comparison to human Salmonella isolates. BMC Microbiology . 2013;13:1–9. doi: 10.1186/1471-2180-13-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shang K., Wei B., Kang M. Distribution and dissemination of antimicrobial-resistant Salmonella in broiler farms with or without enrofloxacin use. BMC Veterinary Research . 2018;14:1–14. doi: 10.1186/s12917-018-1590-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eagar H., Swan G., Van Vuuren M. A survey of antimicrobial usage in animals in South Africa with specific reference to food animals. Journal of the South African Veterinary Association . 2012;83:p. 16. doi: 10.4102/jsava.v83i1.16. [DOI] [PubMed] [Google Scholar]
- 14.Abdi R. D., Mengstie F., Beyi A. F., et al. Determination of the sources and antimicrobial resistance patterns of Salmonella isolated from the poultry industry in Southern Ethiopia. BMC Infectious Diseases . 2017;17:352–412. doi: 10.1186/s12879-017-2437-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barbour E. K., Ayyash D. B., Alturkistni W., et al. Impact of sporadic reporting of poultry Salmonella serovars from selected developing countries. Journal of infection in developing countries . 2015;9:1–7. doi: 10.3855/jidc.5065. [DOI] [PubMed] [Google Scholar]
- 16.Who. WHO Integrated Surveillance of Antimicrobial Resistance in Foodborne Bacteria . Geneva, Switzerland: WHO; 2017. [Google Scholar]
- 17.Akeem A. O., Mamman P. H., Raji M. A., Kwanashie C. N., Raufu I. A., Aremu A. Distribution of virulence genes in Salmonella serovars isolated from poultry farms in Kwara State, Nigeria. Ceylon Journal of Science . 2017;46(4):p. 69. doi: 10.4038/cjs.v46i4.7469. [DOI] [Google Scholar]
- 18.McWhorter A. R., Chousalkar K. K. From hatch to egg grading: monitoring of Salmonella shedding in free-range egg production systems. Veterinary Research . 2019;50(1):p. 58. doi: 10.1186/s13567-019-0677-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Z., Zhang K., Yin H., Li Q., Wang L., Liu Z. Detection of Salmonella and several common Salmonella serotypes in food by loop-mediated isothermal amplification method. Food Science and Human Wellness . 2015;4(2):75–79. doi: 10.1016/j.fshw.2015.05.001. [DOI] [Google Scholar]
- 20.Malorny B., Hoorfar J., Bunge C., Helmuth R. Multicenter validation of the analytical accuracy of salmonella PCR: towards an international standard. Applied and Environmental Microbiology . 2003;69(1):290–296. doi: 10.1128/aem.69.1.290-296.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zishiri O. T., Mkhize N., Mukaratirwa S. Prevalence of virulence and antimicrobial resistance genes in Salmonella spp. isolated from commercial chickens and human clinical isolates from South Africa and Brazil. Onderstepoort Journal of Veterinary Research . 2016;83:1–11. doi: 10.4102/ojvr.v83i1.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abia A. L. K., Ubomba-Jaswa E., Momba M. N. B. Prevalence of pathogenic microorganisms and their correlation with the abundance of indicator organisms in riverbed sediments. International journal of Environmental Science and Technology . 2016;13(12):2905–2916. doi: 10.1007/s13762-016-1116-y. [DOI] [Google Scholar]
- 23.Clsi. Performance Standards for Antimicrobial Susceptibility Testing . 27th. Wayne, PA, USA: Clinical and Laboratory Standards Institute; 2017. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; twenty-seventh informational supplement. CLSI Document M100-S27. [Google Scholar]
- 24.Elkenany R., Elsayed M. M., Zakaria A. I., El-Sayed S. A., Rizk M. A. Antimicrobial resistance profiles and virulence genotyping of Salmonella enterica serovars recovered from broiler chickens and chicken carcasses in Egypt. BMC Veterinary Research . 2019;15:124–129. doi: 10.1186/s12917-019-1867-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phiri N., Mainda G., Mukuma M., et al. Antibiotic-resistant Salmonella species and Escherichia coli in broiler chickens from farms, abattoirs, and open markets in selected districts of Zambia. Journal of Epidemiological Research . 2020;6:1–22. doi: 10.5430/jer.v6n1p13. [DOI] [Google Scholar]
- 26.Ammar A. M., Mohamed A. A., Abd El-Hamid M. I., El-Azzouny M. M. Virulence genotypes of clinical SalmonellaSerovars from broilers in Egypt. The Journal of Infection in Developing Countries . 2016;10(04):337–346. doi: 10.3855/jidc.7437. [DOI] [PubMed] [Google Scholar]
- 27.Thung T. Y., Radu S., Mahyudin N. A., et al. Prevalence, virulence genes and antimicrobial resistance profiles of Salmonella serovars from retail beef in Selangor, Malaysia. Frontiers in Microbiology . 2018;8:1–8. doi: 10.3389/fmicb.2017.02697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hughes L. A., Shopland S., Wigley P., et al. Characterisation of Salmonella enterica serotype Typhimurium isolates from wild birds in northern England from 2005 - 2006. BMC Veterinary Research . 2008;4:4–10. doi: 10.1186/1746-6148-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith S., Braun S., Akintimehin F., et al. Serogenotyping and antimicrobial susceptibility testing of Salmonella spp. isolated from retail meat samples in Lagos, Nigeria. Molecular and Cellular Probes . 2016;30:189–194. doi: 10.1016/j.mcp.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 30.McIver K. S., Amoako D. G., Abia A. L. K., Bester L. A., Chenia H. Y., Essack S. Y. Molecular epidemiology of antibiotic-resistant escherichia coli from farm-to-fork in intensive poultry production in Kwazulu-Natal, South Africa. Antibiotics . 2020;9:1–16. doi: 10.3390/antibiotics9120850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Molechan C., Amoako D. G., Abia A. L. K., Somboro A. M., Bester L. A., Essack S. Y. Molecular epidemiology of antibiotic-resistant Enterococcus spp. from the farm-to-fork continuum in intensive poultry production in KwaZulu-Natal, South Africa. The Science of the Total Environment . 2019;692:868–878. doi: 10.1016/j.scitotenv.2019.07.324. [DOI] [PubMed] [Google Scholar]
- 32.Thames H. T., Sukumaran A. T. A review of Salmonella and Campylobacter in broiler meat: emerging challenges and food safety measures. Foods . 2020;9:1–22. doi: 10.3390/foods9060776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.El Allaoui A., Rhazi Filali F., Ameur N., Bouchrif B. Contamination des élevages de dinde de chair par Salmonella spp. au Maroc: prévalence, antibiorésistances et facteurs de risque associés. Revue Scientifique et Technique de l’OIE . 2017;36(3):935–946. doi: 10.20506/rst.36.3.2726. [DOI] [PubMed] [Google Scholar]
- 34.Mridha D., Uddin M. N., Alam B., et al. Identification and characterization of Salmonella spp. from samples of broiler farms in selected districts of Bangladesh. February-2020 . 2020;13(2):275–283. doi: 10.14202/vetworld.2020.275-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gutierrez M., Fanning J., Murphy A., et al. Salmonella in broiler flocks in the republic of Ireland. Foodborne Pathogens and Disease . 2009;6(1):111–120. doi: 10.1089/fpd.2008.0163. [DOI] [PubMed] [Google Scholar]
- 36.Ha J. S., Seo K. W., Kim Y. B., Kang M. S., Song C.-S., Lee Y. J. Prevalence and characterization of Salmonella in two integrated broiler operations in Korea. Irish Veterinary Journal . 2018;71(1):p. 3. doi: 10.1186/s13620-018-0114-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuang X., Hao H., Dai M., et al. Serotypes and antimicrobial susceptibility of Salmonella spp. isolated from farm animals in China. Frontiers in Microbiology . 2015;6:p. 602. doi: 10.3389/fmicb.2015.00602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodríguez-Hernández R., Bernal J. F., Cifuentes J. F., et al. Prevalence and molecular characterization of salmonella isolated from broiler farms at the Tolima region—Colombia. Animals . 2021;11:1–11. doi: 10.3390/ani11040970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rothrock M. J., Guard J. Y., Oladeinde A. Salmonella diversity along the farm-to-fork continuum of pastured poultry flocks in the southeastern United States. Frontiers in Animal Science . 2021;2 doi: 10.3389/fanim.2021.761930. [DOI] [Google Scholar]
- 40.Sohail M. N., Rathnamma D., Priya S. C., et al. Salmonella from farm to table: isolation, characterization, and antimicrobial resistance of Salmonella from commercial broiler supply chain and its environment. BioMed Research International . 2021;2021:12. doi: 10.1155/2021/3987111.3987111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Agada G. Prevalence and antibiotic resistance profile of Salmonella isolates from commercial poultry and poultry farm-handlers in Jos, Plateau State, Nigeria. British Microbiology Research Journal . 2014;4(4):462–479. doi: 10.9734/bmrj/2014/5872. [DOI] [Google Scholar]
- 42.Dórea F. C., Cole D. J., Hofacre C., et al. Effect of Salmonella vaccination of breeder chickens on contamination of broiler chicken carcasses in integrated poultry operations. Applied and Environmental Microbiology . 2010;76(23):7820–7825. doi: 10.1128/aem.01320-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gebrerufael G., Mahendra P., Tadelle D., Tesfaye S., Alehegn W. Evaluating the relative resistance of different poultry breeds to Salmonella Typhimurium. African Journal of Agricultural Research . 2015;10(30):2928–2939. doi: 10.5897/ajar2014.9425. [DOI] [Google Scholar]
- 44.Andoh L. A., Dalsgaard A., Obiri-Danso K., Newman M. J., Barco L., Olsen J. E. Prevalence and antimicrobial resistance ofSalmonellaserovars isolated from poultry in Ghana. Epidemiology and Infection . 2016;144(15):3288–3299. doi: 10.1017/s0950268816001126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Efsa E. F. S. A., Ecdc E. C. The European Union Summary Report on Antimicrobial Resistance in zoonotic and indicator bacteria from humans, animals and food in 2018/2019. EFSA Journal . 2021;19 doi: 10.2903/j.efsa.2021.6490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elkenany R. M., Eladl A. H., El-Shafei R. A. Genetic characterisation of class 1 integrons among multidrug-resistant Salmonella serotypes in broiler chicken farms. Journal of Global Antimicrobial Resistance . 2018;14:202–208. doi: 10.1016/j.jgar.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 47.Makaya P. V., Matope G., Pfukenyi D. M. Distribution ofSalmonellaserovars and antimicrobial susceptibility ofSalmonellaEnteritidis from poultry in Zimbabwe. Avian Pathology . 2012;41(2):221–226. doi: 10.1080/03079457.2012.667558. [DOI] [PubMed] [Google Scholar]
- 48.NARMS. The National Antimicrobial Resistance Monitoring System: NARMS Integrated Report, 2016-2017 . Laurel, MD, USA: U.S. Department of Health and Human Services, FDA; 2019. https://www.fda.gov/animal-veterinary/national-antimicrobial-resistance-monitoring-system/2016-2017-narms-integrated-summary . [Google Scholar]
- 49.El-Tayeb M. A., Ibrahim A. S. S., Al-Salamah A. A., Almaary K. S., Elbadawi Y. B. Prevalence, serotyping and antimicrobials resistance mechanism of Salmonella enterica isolated from clinical and environmental samples in Saudi Arabia. Brazilian Journal of Microbiology . 2017;48(3):499–508. doi: 10.1016/j.bjm.2016.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abraham S., O’Dea M., Sahibzada S., et al. Escherichia coli and Salmonella spp. isolated from Australian meat chickens remain susceptible to critically important antimicrobial agents. PLoS One . 2019;14(10):p. e0224281. doi: 10.1371/journal.pone.0224281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clsi. Performance Standards for Antimicrobial Susceptibility Testing . 25th. Wayne, PA, USA: Clinical and Laboratory Standards Institute; 2015. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; twenty-fifth informational supplement. CLSI Document M100-S25. [Google Scholar]
- 52.Deak E., Skov R., Hindler J. A., Humphries R. M. Evaluation of surrogate disk tests for detection of ciprofloxacin and levofloxacin resistance in clinical isolates of salmonella enterica. Journal of Clinical Microbiology . 2015;53(11):3405–3410. doi: 10.1128/jcm.01393-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eucast. The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters . Chicago, IL, USA: Eucast; 2015. [Google Scholar]
- 54.Skov R., Matuschek E., Sjölund-Karlsson M., et al. Development of a pefloxacin disk diffusion method for detection of fluoroquinolone-resistant Salmonella enterica. Journal of Clinical Microbiology . 2015;53:3411–3417. doi: 10.1128/jcm.01287-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Veeraraghavan B., Anandan S., Sethuvel D. P. M., Ragupathi N. K. D. Pefloxacin as a surrogate marker for fluoroquinolone susceptibility for Salmonella Typhi: problems and prospects. Journal of Clinical and Diagnostic Research . 2016;10 doi: 10.7860/JCDR/2016/17022.8306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Prabhurajan R., Selvam R. M. E., Padmavathy K. Unusual antibiotic resistance pattern among blood culture isolates of Salmonella Paratyphi A. The Journal of Infection in Developing Countries . 2021;15:579–583. doi: 10.3855/jidc.12336. [DOI] [PubMed] [Google Scholar]
- 57.Tadesse G., Tessema T., Beyene G., Aseffa A. Molecular epidemiology of fluoroquinolone resistant Salmonella in Africa: a systematic review and meta-analysis. PLoS One . 2018;13:1–30. doi: 10.1371/journal.pone.0192575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gupta V., Datta P., Mohi G., Chander J. Pefloxacin as a surrogate marker for ciprofloxacin resistance in Salmonella: study from north India. International Journal of Pharmacy and Pharmaceutical Sciences . 2016;10:272–275. doi: 10.5281/zenodo.1124451. [DOI] [Google Scholar]
- 59.Gupta V., Pal K., Bhagat A., Goel A., Chander J. Quinolone susceptibility in Salmonella isolates based on minimum inhibitory concentration determination. Journal of Laboratory Physicians . 2020;12(4):263–267. doi: 10.1055/s-0040-1721163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rau R. B., Ribeiro A. R., dos Santos A., Barth A. L. Antimicrobial resistance of Salmonella from poultry meat in Brazil: results of a nationwide survey. Epidemiology and Infection . 2021;149:p. e228. doi: 10.1017/s0950268821002156. [DOI] [Google Scholar]
- 61.Van T. T., Minejima E., Chiu C. A., Butler-Wu S. M. Don’t get wound up: revised fluoroquinolone breakpoints for Enterobacteriaceae and Pseudomonas aeruginosa. Journal of Clinical Microbiology . 2019;57:1–11. doi: 10.1128/JCM.02072-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Caffrey N., Agunos A., Gow S., Liljebjelke K., Mainali C., Checkley S. L. Salmonella spp. prevalence and antimicrobial resistance in broiler chicken and Turkey flocks in Canada from 2013 to 2018. Zoonoses and Public Health . 2021;68(7):719–736. doi: 10.1111/zph.12769. [DOI] [PubMed] [Google Scholar]
- 63.Fda. Extralabel Use and Antimicrobials . Rockville, MD, USA: U.S. Department of Health and Human Services; 2018. https://www.fda.gov/animal-veterinary/antimicrobial-resistance/extralabel-use-and-antimicrobials . [Google Scholar]
- 64.Henton M. M., Eagar H. A., Swan G. E., van Vuuren M. Part VI. Antibiotic management and resistance in livestock production. South African medical journal = Suid-Afrikaanse tydskrif vir geneeskunde . 2011;101:583–586. [PubMed] [Google Scholar]
- 65.Efsa E. F. S. A., Ecdc E. C. The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2016. EFSA Journal . 2018;16 doi: 10.2903/j.efsa.2018.5182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.European Commission. Ban on Antibiotics as Growth Promoters in Animal Feed Enters into Effect . Brussels, Belgium: European Commission; 2005. https://ec.europa.eu/commission/presscomer/detail/en/IP_05_1687 . [Google Scholar]
- 67.Oie. World Organisation for Animal Health (OIE), OIE List of Antimicrobial Agents of Veterinaty Importance . Paris, France: Oie; 2018. [Google Scholar]
- 68.Who. World Health Organisation (WHO) | WHO List of Critically Important Antimicrobials (CIA) Geneva, Switzerland: WHO; 2018. [Google Scholar]
- 69.Eagar H., Naidoo V. Veterinary antimicrobial stewardship in South Africa. Int. Biol. Rev. . 2017;1:309–311. [Google Scholar]
- 70.Ma F., Xu S., Tang Z., Li Z., Zhang L. Use of antimicrobials in food animals and impact of transmission of antimicrobial resistance on humans. Biosafety and Health . 2021;3(1):32–38. doi: 10.1016/j.bsheal.2020.09.004. [DOI] [Google Scholar]
- 71.Kim J. E., Lee Y. J. Molecular characterization of antimicrobial resistant non-typhoidal Salmonella from poultry industries in Korea. Irish Veterinary Journal . 2017;70:1–9. doi: 10.1186/s13620-017-0095-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen Z., Bai J., Wang S., et al. Prevalence, antimicrobial resistance, virulence genes and genetic diversity of Salmonella isolated from retail duck meat in Southern China. Microorganisms . 2020;8:1–12. doi: 10.3390/microorganisms8030444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Deguenon E., Dougnon V., Lozes E., et al. Resistance and virulence determinants of faecal Salmonella spp. isolated from slaughter animals in Benin. BMC Research Notes . 2019;12:317–7. doi: 10.1186/s13104-019-4341-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Karabasanavar N., Madhavaprasad C., Gopalakrishna S., Hiremath J., Patil G., Barbuddhe S. Prevalence of Salmonella serotypes S . Enteritidis and S . Typhimurium in poultry and poultry products. Journal of Food Safety . 2020;40 doi: 10.1111/jfs.12852. [DOI] [Google Scholar]
- 75.Magdy O. S., Moussa I. M., Hussein H. A., et al. Genetic diversity of Salmonella enterica recovered from chickens farms and its potential transmission to human. Journal of Infection and Public Health . 2020;13(4):571–576. doi: 10.1016/j.jiph.2019.09.007. [DOI] [PubMed] [Google Scholar]
- 76.Herrera-Sánchez M. P., Rodríguez-Hernández R., Rondón-Barragán I. S. Molecular characterization of antimicrobial resistance and enterobacterial repetitive intergenic consensus-PCR as a molecular typing tool for Salmonella spp. isolated from poultry and humans. Veterinary World . 2020;13(9):1771–1779. doi: 10.14202/vetworld.2020.1771-1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fendri I., Ben Hassena A., Grosset N., et al. Genetic diversity of food-isolated Salmonella strains through pulsed field gel electrophoresis (PFGE) and enterobacterial repetitive intergenic consensus (ERIC-PCR) PLoS One . 2013;8:e81315–8. doi: 10.1371/journal.pone.0081315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Oliveira S. D. d., Bessa M. C., Santos L. R. D., Cardoso M. R. de I., Cardoso M. R. d. I., Brandelli A. Phenotypic and genotypic characterization of Salmonella Enteritidis isolates. Brazilian Journal of Microbiology . 2007;38:720–728. doi: 10.1590/s1517-83822007000400025. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: source of Salmonella isolates. Figure S1: dendrogram of ERIC-PCR patterns constructed of Salmonella enterica isolates recovered from the farm-to-fork continuum. Salmonella enterica subsp. enterica serovar Choleraesuis ATCC 10708 was used as the quality control strain. .
Data Availability Statement
The data used to support the findings of this study are included within the article.


