Summary
Background
Kidney damage in COVID-19 patients has been of special concern. The association of acute kidney injury (AKI) with post-acute kidney function among COVID-19 survivors was not sufficiently elucidated.
Methods
An ambidirectional cohort study was conducted with enrollment of COVID-19 survivors discharged from hospital between Jan 7, and May 29, 2020. Study participants were invited to follow-up visits at 6 and 12 months after symptom onset. The primary outcome was percentage of estimated glomerular filtration rate (eGFR) decreased from acute phase (between symptom onset and hospital discharge) to follow-up, and secondary outcome was reduced renal function at follow-up.
Findings
In total, 1,734 study participants were included in this study. Median follow-up duration was 342.0 days (IQR, 223.0-358.0) after symptom onset. After multivariable adjustment, percentage of eGFR decreased from acute phase to follow-up was 8.30% (95% CI, 5.99-10.61) higher among AKI participants than those without AKI at acute phase. Participants with AKI had an odds ratio (OR) of 4.60 (95% CI, 2.10-10.08) for reduced renal function at follow-up. The percentage of eGFR decreased for participants with AKI stage 1, stage 2, and stage 3 was 6.02% (95% CI, 3.48-8.57), 15.99% (95% CI, 10.77-21.22), and 17.79% (95% CI, 9.14-26.43) higher compared with those without AKI, respectively.
Interpretation
AKI at acute phase of COVID-19 was closely related to the longitudinal decline and post-acute status of kidney function at nearly one-year after symptom onset. Earlier and more intense follow-up strategies on kidney function management could be beneficial to COVID-19 survivors.
Funding
Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (CIFMS 2020-I2M-CoV19-005, 2018-I2M-1-003, and 2020-I2M-2-013); National Natural Science Foundation of China (82041011); National Key Research and Development Program of China (2018YFC1200102); Major Projects of National Science and Technology on New Drug Creation and Development of Pulmonary Tuberculosis (2020ZX09201001).
Keywords: COVID-19, Acute kidney injury, Post-acute, Renal function
Research in Context.
Evidence before this study
We searched PubMed for studies focusing on association of COVID-19 related acute kidney injury (AKI) with post-acute kidney function after discharge up to October 1, 2021 without language restriction. The search terms were (COVID-19 OR SARS-CoV-2 OR Coronavirus disease 2019 OR 2019-ncov) AND (acute kidney injury OR AKI). The study with longest follow-up duration focusing on this topic was only 6 months after discharge. The longer term effect of AKI on kidney function and whether the effect would be different as COVID-19 gradually recovered is not clear.
Added value of this study
This cohort study with nearly one-year follow-up after symptom onset is the one with longest follow-up duration conducted among COVID-19 patients discharged from hospital. Among COVID-19 survivors, percentage of estimated glomerular filtration rate (eGFR) decreased from acute phase to one year after symptom onset was found to be 8.30% (95% CI, 5.99-10.61) higher among patients with development of AKI during hospitalization than those without. The association of AKI with increased risk of reduced renal function at one-year after symptom onset was also detected (OR 4.60, 95% CI: 2.10-10.08). The lower eGFR level at acute phase (<90 mL/min per 1.73 m2) strengthened the association between AKI and kidney outcomes. Furthermore, a graded increase in percentage of eGFR decreased and risk of reduced renal function at one-year after symptom onset were found with higher AKI stage among COVID-19 survivors.
Implications of all the available evidence
The study findings lend credence to the association of AKI with longitudinal decline of kidney function from acute phase to one-year after symptom onset and post-acute status of reduced renal function at one-year after symptom onset among COVID-19 survivors, and also lend credence to the graded relationship between AKI stage at acute phase and kidney outcomes. Development of more precise and intense follow-up strategies for future kidney disease management among people recovering from COVID-19 is urgently needed.
Alt-text: Unlabelled box
Introduction
The cases and deaths of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) cumulated in an unprecedented way, with 271 million and 5.32 million reported as of December 17, 2021, respectively.1 As a multi-organ disease, COVID-19 can cause both substantial respiratory pathology and extrapulmonary manifestations.2 The multi-organ damage caused by COVID-19 is not only limited to acute phase but also post-acute stage.3
Acute kidney injury (AKI) is one of the common clinical presentations of COVID-19, which was reported in 5-46% of hospitalized COVID-19 patients.4, 5, 6, 7, 8, 9 Kidney damage in COVID-19 patients has been of special concern due to the pathophysiology specific to SARS-CoV-2 that distinguishes this renal abnormality from the more general kidney damage caused by other illness.2,10 This can be reflected by the higher proportion of AKI development among hospitalized COVID-19 patients than other hospitalized patients; and also reflected by the increased risk of in-hospital mortality and requirement for renal replacement therapy, and decreased possibility to have kidney recovered function during hospitalization among AKI patients with COVID-19 compared with those without COVID-19.7,11,12
Understanding the impact of AKI among COVID-19 patients, not only focusing on acute phase but also post-acute stage, is critically important for future management of kidney disease. Previous studies mainly illustrated the effect of COVID-19 associated AKI on kidney status during hospitalization, whereas studies that focused on post-acute kidney function after discharge were very limited.13, 14, 15 The study with longest follow-up duration was only 6 months after discharge, which found COVID-19 associated AKI was associated with a greater rate of estimated glomerular filtration rate (eGFR) decrease after discharge compared with AKI patients without COVID-19.13 Considering the relatively high proportion of COVID-19 patients with AKI development, more studies are needed to comprehensively elucidate the long-term effect of AKI on kidney function.
The primary aim of this study was to assess the effect of AKI developed during hospitalization on longitudinal change of kidney function which was calculated as percentage of eGFR decreased from acute phase to one year after symptom onset among COVID-19 patients. The secondary aim was to compare the risk of reduced renal function (eGFR<60 mL/min per 1.73 m2) and proteinuria at follow-up between COVID-19 patients with and without AKI at acute phase. Furthermore, the associations of AKI stage with above kidney outcomes were also assessed. We hypothesized that AKI and higher AKI stage were associated with greater decline in kidney function and higher risk of reduced renal function and proteinuria among COVID-19 patients.
Methods
Study design and participants
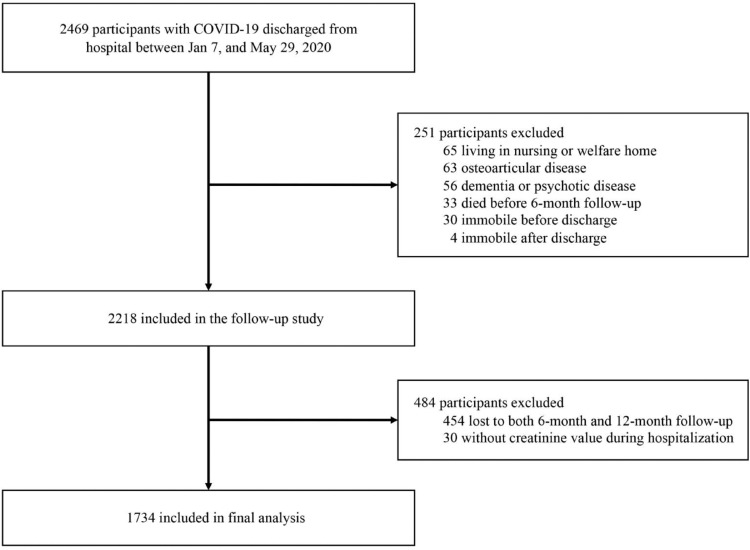
Participants for the current study were from an ambidirectional cohort study of COVID-19 survivors discharged from hospital between Jan 7, and May 29, 2020.16 Inclusion and exclusion criteria of the cohort study have been described previously.16 Briefly, all patients with laboratory confirmed COVID-19 discharged from hospital between Jan 7 and May 29, 2020, were eligible for participation. Patients were excluded if they died after discharge and before follow-up; were living in a nursing or welfare home; had psychotic disorder, dementia, or osteoarthropathy; or were immobile.
Among 2,469 participants with COVID-19, 2,218 were eligible to be enrolled in the follow-up study (Figure 1). Of these eligible participants, 1,764 (79.5%) completed the follow-up survey. After excluding 30 participants without creatinine value recorded during hospitalization, the current analysis was restricted to 1,734 participants. The study was approved by the Research Ethics Commission of hospital which enrolled the participants (KY-2020-78.01, KY-2020-78.03). Written informed consent was obtained from COVID-19 survivors who attended the follow-up visit.
Figure 1.
Flow chart of study participants.
Data collection at acute phase
Acute phase was defined as the time between symptom onset and hospital discharge. Data at acute phase was retrieved from electronic medical records, including demographic characteristics (age, sex, education, and cigarette smoking); clinical characteristics (self-reported comorbidities and symptom onset time); laboratory test results (serum creatinine); disease severity characterized by highest seven-category scale during hospital stay (3, hospitalized, not requiring supplemental oxygen; 4, hospitalized, requiring supplemental oxygen; 5, hospitalized, requiring high-flow nasal cannula (HFNC), noninvasive mechanical ventilation (NIV), or both; 6, hospitalized, requiring extracorporeal membrane oxygenation (ECMO), invasive mechanical ventilation (IMV), or both); and treatment (corticosteroids, antivirals including lopinavir-ritonavir, arbidol, chloroquine phosphate, and hydroxychloroquine, antibiotics, thymosin, and intravenous immunoglobulin).
Follow-up assessment
Eligible study participants were invited to attend two face-to-face follow-up visits at hospital at 6 and 12 months after symptom onset. The 6-month and 12-month follow-up visits were conducted from June 16 to Sept 3, 2020, and from Dec 16, 2020 to Feb 7, 2021, respectively. The detailed 6-month and 12-month follow-up procedures have been described previously.16,17 Briefly, at each visit, study participants underwent detailed interview and physical examination; completed a series of questionnaires, including a self-reported symptom questionnaire, the modified British Medical Research Council (mMRC) dyspnea scale, et al.18, 19, 20, 21 Venous blood samples were drawn for the measurement of creatinine and other laboratory indicators. Spot urine samples were collected for measurement of proteinuria and hematuria. In light of the emergency state of hospital at the early stage of COVID-19 pandemic, demographic information and self-reported comorbidity collected at admission may not be that accurate. To confirm demographic information and self-reported comorbidity collected at baseline, a standard questionnaire was designed and administered to obtain information including age, gender, education, cigarette smoking, alcohol consumption, personal medical history, and family history face-to-face by trained staff at 12-month follow-up visit. All data collected was checked for completeness by the staff who collected information unless study participants were unwilling to provide the information. The data was finally recoded to reflect baseline characteristics of study participants according to time variables collected for some variables such as comorbidity and admission date.
Measurement of acute kidney injury and other variables
According to Kidney Disease: Improving Global Outcomes (KDIGO) guideline, AKI was defined as either at least 50% increase in serum creatinine from the baseline creatinine to maximum creatinine level during hospitalization or a 0.3 mg/dL increase in serum creatinine within 48 hours.22 Due to the lack of baseline creatinine level which was defined as the mean creatinine value between 7 and 365 days before hospitalization, the minimum creatinine value during hospitalization was used as the baseline creatinine.23 AKI stages were defined according to KDIGO criteria as following: stage 1 as an increase in serum creatinine to 1.5-1.9 times baseline serum creatinine or an increase in serum creatinine of ≥0.3 mg/dl within 48 hours, stage 2 as an increase to 2-2.9 times baseline creatinine, and stage 3 as an increase to at least 3 times baseline creatinine. Urine output was not used for definition of AKI due to missingness of this variable. Hematuria was defined as 1+ or higher on urinalysis.8
Measurement of outcomes
The primary outcome was the percentage of eGFR decreased from acute phase to follow-up, which was calculated as (highest eGFR at acute phase-eGFR at follow-up)/ highest eGFR at acute phase × 100%. The calculation of eGFR was based on Asian modified Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.24 The secondary outcomes were reduced renal function and proteinuria. Reduced renal function was defined as an eGFR of less than 60 mL/min per 1.73 m2. Proteinuria was defined as 1+ proteinuria or higher on dipstick due to the lack of spot urine albumin-to-creatinine ratio and protein-to-creatinine ratio.8
Statistical analysis
Demographic and clinical characteristics, and laboratory findings of study participants were presented as median (IQR) for continuous variables according to Shapiro-Wilk testing results for normality (all p value<0.0001) and expressed as absolute values along with percentages for categorical variables. Participants were categorized into AKI and no AKI group according to serum creatinine level during hospitalization. For the comparison of demographic and clinical characteristics, and laboratory findings between study participants with and without AKI, χ2 test, Fisher's exact test, or Mann-Whitney U test was used where appropriate. Furthermore, study participants were also categorized into four groups according to percentage of eGFR decreased from acute phase to follow-up (<15%, 15-24.9%, 25-34.9%, and ≥35%). The comparisons of demographic and clinical characteristics across these groups were performed with χ2 test, Fisher's exact test, or Kruskal-Wallis test where appropriate.
To compare the long-term kidney function status between study participants with and without AKI, the distributions of kidney function status at follow-up (both eGFR<60 mL/min per 1.73 m2 and proteinuria; only eGFR<60 mL/min per 1.73 m2; only proteinuria; eGFR 60-89.9 mL/min per 1.73 m2; eGFR ≥90 mL/min per 1.73 m2) were shown. The absolute value and percentage of eGFR decreased from acute phase to follow-up by AKI were also shown. χ2 test or Fisher's exact test was used for the comparison where appropriate.
The association between AKI and percentage of eGFR decreased from acute phase to follow up was assessed with multivariable adjusted generalized linear regression model. Multivariable adjusted logistic regression analysis was used to explore association of AKI with secondary outcomes including reduced renal function and proteinuria. For association of AKI with outcomes, age, sex, cigarette smoking (never-smoker, current smoker, former smoker), education (college or higher vs. middle school or lower), disease severity (scale 3, scale 4, scale 5-6), comorbidity including hypertension, diabetes, CKD, cardiovascular disease, and rheumatoid arthritis, and duration from symptom onset to follow-up were adjusted. In addition, we conducted several subgroup analyses by a range of variables, including age, sex (20-64 years; ≥65 years), education (college or higher; middle school or lower), comorbidity including hypertension, diabetes, and cardiovascular disease, disease severity (scale 3-4; scale 5-6), and highest eGFR at acute phase (≥90 mL/min per 1.73 m2; <90 mL/min per 1.73 m2). The potential interaction was further evaluated by adding a multiplicative interaction term between AKI and the above categorical variable. Furthermore, the associations of different AKI stage with primary outcome and secondary outcomes were assessed with generalized linear regression model and logistic regression models, respectively. The covariables adjusted for association of AKI with outcomes were also included in the models for AKI stage with outcomes.
Sensitivity analyses were also conducted for the association of AKI and AKI stage with primary and secondary outcomes after excluding participants with self-reported CKD before SARS-CoV-2 infection, or with further adjustment for potential confounding effect of antibiotics use at acute phase.
We also explored risk factors associated with AKI. For association of factors including sex, intensive care unit (ICU) admission, corticosteroid, antivirals, antibiotics, and intravenous immunoglobulin with AKI, the variables including age, sex, cigarette smoking, disease severity, comorbidities including hypertension, diabetes, CKD, and cardiovascular disease, ICU admission, corticosteroid, antivirals, antibiotics, and intravenous immunoglobulin were all included in the model. When exploring associations of comorbidities including hypertension, diabetes, CKD, and cardiovascular disease with AKI, the aforementioned variables except for disease severity and ICU admission were included due to the potential mediation. Only sex and cigarette smoking were adjusted for association between age and AKI due to the potential mediation of other factors. For association of cigarette smoking with AKI, the aforementioned variables except for disease severity and comorbidities including hypertension, diabetes, CKD, and cardiovascular disease were all included, while ICU admission, corticosteroid, antivirals, antibiotics, and intravenous immunoglobulin were not included for the association of disease severity with AKI.
Our analysis used all participants for whom the variables of interest were available without imputing missing data. All significance tests were two-sided, and a p value less than 0.05 was considered statistically significant. All statistical analyses were done with SAS, version 9.4.
Role of funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Baseline characteristics of study participants
The demographic and clinical characteristics of 1,734 study participants included in this study are presented in Table 1. Median follow-up duration was 342.0 days (IQR, 223.0-358.0) after symptom onset. The median age of study participants was 57.0 (IQR, 47.0-65.0) years and 53% of them were men. Of these participants, 6.1% were found to have AKI during hospitalization. Compared with study participants without AKI, those with AKI were more likely to have history of hypertension (p<0.0001) and diabetes (p=0.0017), more severely ill (p<0.0001), more likely to receive corticosteroids (p<0.0001), lopinavir-ritonavir (p=0.0004), antibiotics (p<0.0001), and intravenous immunoglobulin (p<0.0001), and also more likely to admit to ICU (p<0.0001). Furthermore, the length of hospital stay (p<0.0001) and ICU stay (p=0.0082) were also longer among AKI participants compared with no AKI participants. The proportion of men and participants who received oxygen therapy during hospital stay were slightly higher in patients who were included in final analysis than those who were not (p=0.017 and <0.0001, respectively; Table S1). There was no statistically significant difference in age, smoking status, comorbidities except for previous history of malignancy, and ICU admission between both groups (all p>0.05).
Table 1.
Baseline characteristics of study participants according to acute kidney injury status at acute phase.
| Characteristics | Total (n=1734) | No AKI (n=1629) | AKI (n=105) | p value |
|---|---|---|---|---|
| Age, years | 57.0 (47.0-65.0) | 57.0 (47.0-65.0) | 59.0 (50.0-68.0) | 0.049 |
| Sex | 0.57 | |||
| Men | 911 (53%) | 853 (52%) | 58 (55%) | |
| Women | 823 (47%) | 776 (48%) | 47 (45%) | |
| Education | 0.51 | |||
| College or higher | 501/1680 (30%) | 467/1576 (30%) | 34/104 (33%) | |
| Middle school or lower | 1179/1680 (70%) | 1109/1576 (70%) | 70/104 (67%) | |
| Cigarette smoking | 0.96 | |||
| Never-smoker | 1474/1730 (85%) | 1385/1625 (85%) | 89 (85%) | |
| Current smoker | 105/1730 (6%) | 98/1625 (6%) | 7 (7%) | |
| Former smoker | 151/1730 (9%) | 142/1625 (9%) | 9 (9%) | |
| Comorbidity | ||||
| Hypertension | 579/1732 (33%) | 520/1627 (32%) | 59 (56%) | <0.0001 |
| Diabetes | 236/1732 (14%) | 211/1627 (13%) | 25 (24%) | 0.0017 |
| Coronary heart diseases | 153/1731 (9%) | 139/1627 (9%) | 14/104 (13%) | 0.09 |
| Cerebrovascular diseases | 80/1732 (5%) | 74/1627 (5%) | 6 (6%) | 0.59 |
| Malignancy | 43/1733 (2%) | 41/1628 (3%) | 2 (2%) | 0.68 |
| Chronic obstructive pulmonary disease | 29/1732 (2%) | 27/1627 (2%) | 2 (2%) | 0.85 |
| Chronic kidney disease | 62/1733 (4%) | 59/1628 (4%) | 3 (3%) | 0.67 |
| Highest seven-category scale during hospital stay | <0.0001 | |||
| 3: hospitalization, not requiring supplemental oxygen | 435 (25%) | 426 (26%) | 9 (9%) | |
| 4: hospitalization, requiring supplemental oxygen | 1180 (68%) | 1128 (69%) | 52 (50%) | |
| 5: hospitalization, requiring HFNC or non-IMV, or both | 110 (6%) | 75 (5%) | 35 (33%) | |
| 6: hospitalization, requiring ECMO or IMV, or both | 9 (1%) | 0 (0%) | 9 (9%) | |
| Treatment received during hospital stay | ||||
| Corticosteroids | 399 (23%) | 338 (21%) | 61 (58%) | <0.0001 |
| Antivirals | 946 (55%) | 878 (54%) | 68 (65%) | 0.030 |
| Lopinavir-ritonavir | 233 (13%) | 207 (13%) | 26 (25%) | 0.0004 |
| Arbidol | 833 (48%) | 774 (48%) | 59 (56%) | 0.08 |
| Chloroquine phosphate | 4 (0%) | 4 (0%) | 0 (0%) | 1.00 |
| Hydroxychloroquine | 2 (0%) | 1 (0%) | 1 (1%) | 0.12 |
| Antibiotics | 1340 (77%) | 1237 (76%) | 103 (98%) | <0.0001 |
| Beta lactams | 932 (54%) | 844 (52%) | 88 (84%) | <0.0001 |
| Quinolones | 975 (56%) | 895 (55%) | 80 (76%) | <0.0001 |
| Sulfonamides | 229 (13%) | 213 (13%) | 16 (15%) | 0.53 |
| Macrolides | 26 (1%) | 24 (1%) | 2 (2%) | 0.73 |
| Tetracycline | 7 (0%) | 0 (0%) | 7 (7%) | <0.0001 |
| Aminoglycosides | 2 (0%) | 1 (0%) | 1 (1%) | 0.12 |
| Others* | 45 (3%) | 25 (2%) | 20 (19%) | <0.0001 |
| Thymosin | 289 (17%) | 271 (17%) | 18 (17%) | 0.89 |
| Intravenous immunoglobulin | 347 (20%) | 298 (18%) | 49 (47%) | <0.0001 |
| Length of hospital stay, days | 14.0 (10.0-19.0) | 13.0 (10.0-18.0) | 29.0 (18.0-52.0) | <0.0001 |
| ICU admission | 75 (4%) | 50 (3%) | 25 (24%) | <0.0001 |
| Length of ICU stay, days | 14.0 (7.0-27.0) | 10.0 (5.0-21.0) | 21.0 (13.0-40.0) | 0.0082 |
| Time from discharge to follow-up, days | 308.0 (191.0-322.0) | 308.0 (185.0-322.0) | 297.0 (268.0-318.0) | 0.15 |
| Time from symptom onset to follow-up, days | 342.0 (223.0-358.0) | 341.0 (220.0-357.0) | 351.0 (326.0-367.0) | 0.0004 |
Data are n (%), n/N(%), or median (IQR). The different denominators used indicate number of participants with data for corresponding variables.
Other types of antibiotics include peptide, lincomycin, thiazolidone, glycopeptide, nitroimidazole, and cyclic lipopeptide.
AKI=acute kidney injury. HFNC=high-flow nasal cannula for oxygen therapy. NIV=non-invasive ventilation. IMV=invasive mechanical ventilation. ECMO=extracorporeal membrane oxygenation. ICU=intensive care unit.
The characteristics of study participants according to percentage of eGFR decreased from acute phase to follow-up were shown in Table S2. Those with higher percentage of eGFR decreased were more likely to have comorbidity including hypertension (p<0.0001), diabetes (p<0.0001), coronary heart disease (p=0.0010), and CKD (p=0.0005), more severely ill (p<0.0001), more likely to receive corticosteroids (p=0.011) and intravenous immunoglobulin (p=0.018), and also more likely to admit to ICU (p<0.0001).
Laboratory findings of study participants at follow-up
Table 2 shows the laboratory findings of study participants at follow-up according to AKI status. Participants with AKI had higher levels of leukocyte count (p=0.0009), higher serum creatinine (p=0.0012) and percentage of eGFR decreased from acute phase to follow-up (p<0.0001), and lower level of eGFR (p=0.0020) compared with those without AKI. The proportions of study participants with eGFR less than 60 mL/min per 1.73 m2 (p<0.0020) and proteinuria (p=0.0017) were both higher among participants who developed AKI. Although the difference of glycated hemoglobin A1C (HbA1C) was statistically significant (p=0.0007) but likely not clinically significant given small absolute differences.
Table 2.
Laboratory findings of study participants at follow-up according to acute kidney injury status at acute phase.
| Characteristics | Total (n=1734) | No AKI (n=1629) | AKI (n=105) | p value |
|---|---|---|---|---|
| Leukocyte count, × 109 per L | 6.1 (5.1-7.3) | 6.1 (5.1-7.2) | 6.7 (5.7-7.8) | 0.0009 |
| Lymphocyte count, × 109 per L | 1.9 (1.6-2.4) | 1.9 (1.6-2.4) | 2.0 (1.5-2.6) | 0.31 |
| Haemoglobin, g/dL | 142.5 (133.0-154.0) | 143.0 (133.0-154.0) | 141.0 (134.0-151.5) | 0.52 |
| Platelet count, × 109 per L | 217.0 (184.0-254.0) | 217.0 (184.0-254.0) | 225.5 (187.5-260.5) | 0.44 |
| Serum albumin, g/L | 45.8 (44.1-47.6) | 45.8 (44.1-47.6) | 45.8 (44.3-48.0) | 0.78 |
| Alanine aminotransferase, U/L | 20.0 (14.0-29.0) | 20.0 (14.0-29.0) | 21.0 (14.0-29.0) | 0.50 |
| Aspartate aminotransferase, U/L | 26.0 (22.0-31.0) | 26.0 (22.0-31.0) | 25.0 (21.0-32.0) | 0.66 |
| HbA1C, % | 5.4 (5.1-5.8) | 5.4 (5.1-5.8) | 5.7 (5.3-6.1) | 0.0007 |
| Serum creatinine, μmol/L | 70.1 (59.0-82.7) | 69.7 (59.0-82.2) | 78.0 (61.6-95.1) | 0.0012 |
| Percentage of eGFR decreased, % | 7.2 (2.2-14.3) | 6.7 (2.0-13.3) | 19.8 (11.5-26.5) | <0.0001 |
| eGFR, mL/min per 1.73 m2 | 98.5 (86.0-108.6) | 98.7 (86.6-109.0) | 93.7 (74.3-106.2) | 0.0020 |
| Classification of eGFR | 0.0002 | |||
| ≥90 mL/min per 1.73 m2 | 1161/1707 (68%) | 1103/1605 (69%) | 58/102 (57%) | |
| 60-89.9 mL/min per 1.73 m2 | 476/1707 (28%) | 446/1605 (28%) | 30/102 (29%) | |
| 30-59.9 mL/min per 1.73 m2 | 60/1707 (4%) | 50/1605 (3%) | 10/102 (10%) | |
| 15-29.9 mL/min per 1.73 m2 | 7/1707 (0%) | 5/1605 (0%) | 2/102 (2%) | |
| <15 mL/min per 1.73 m2 | 3/1707 (0%) | 1/1605 (0%) | 2/102 (2%) | |
| Proteinuria | 0.0017 | |||
| Negative | 1161/1259 (92%) | 1095/1177 (93%) | 66/82 (80%) | |
| 1+ | 54/1259 (4%) | 46/1177 (4%) | 8/82 (10%) | |
| ≥2+ | 44/1259 (3%) | 36/1177 (3%) | 8/82 (10%) | |
| Hematuresis | 0.32 | |||
| Negative | 1017/1259 (81%) | 946/1177 (80%) | 71/82 (87%) | |
| 1+ | 157/1259 (12%) | 151/1177 (13%) | 6/82 (7%) | |
| ≥2+ | 85/1259 (7%) | 80/1177 (7%) | 5/82 (6%) |
Data are n (%), n/N(%), or median (IQR). The differing denominators used indicate number of participants with data for corresponding variables.
AKI=acute kidney injury. eGFR=estimated glomerular filtration rate. HbA1C= glycated hemoglobin A1C.
Kidney function status and its change
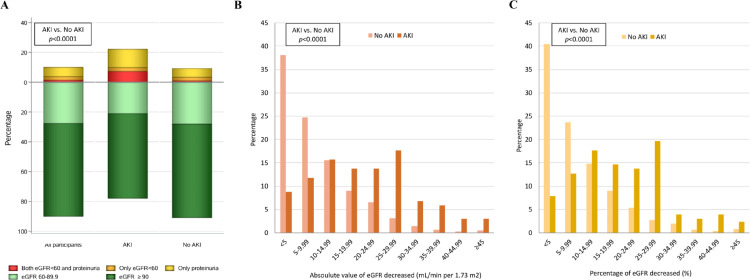
Figure 2 shows the kidney function status at follow-up and its change from acute phase to follow-up by AKI status. The kidney function status was different between those with and without AKI (p<0.0001, Figure 2A). The proportions of study participants with both eGFR<60 mL/min per 1.73 m2 and proteinuria, only eGFR<60 mL/min per 1.73 m2, only proteinuria, eGFR 60-89.9 mL/min per 1.73 m2, and eGFR ≥90 mL/min per 1.73 m2 were 7.4%, 2.5%, 12.4%, 21.0%, and 56.8% among those who developed AKI, while the corresponding proportions were 1.1%, 2.3%, 5.9%, 28.0%, and 62.7% among those without AKI, respectively. Furthermore, both the absolute level and percentage of eGFR decreased from acute phase to follow-up were higher in AKI group compared with no AKI group (both p<0.0001, Figure 2B-C). The proportions of participants with a 25% or greater drop in eGFR was 32.8% among those who developed AKI, but the proportion was only 6.5% among those without AKI.
Figure 2.
Distributions of kidney function status at follow-up (A) and absolute value (B) or percentage (C) of eGFR decreased from acute phase to follow-up.
Association of AKI with kidney function status and its change
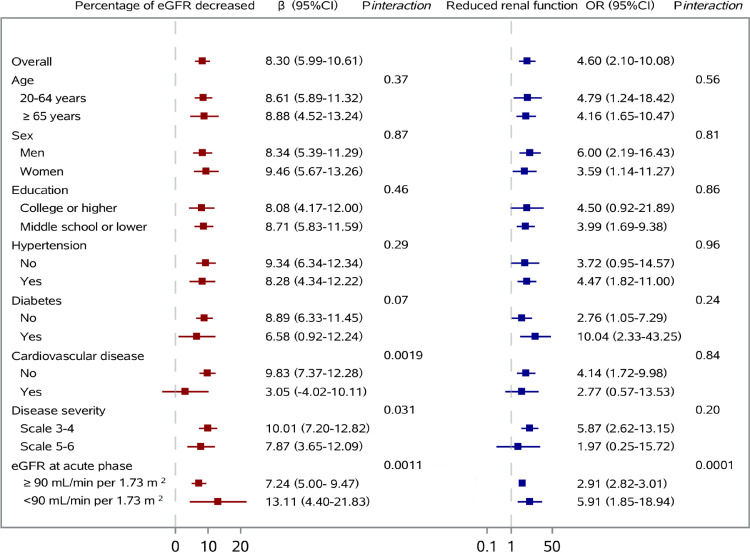
The associations of AKI with percentage of eGFR decreased from acute phase to follow-up and reduced renal function at follow-up are shown in Figure 3, and association with proteinuria at follow-up is shown in Figure S1. After multivariable adjustment for age, sex, cigarette smoking, education, disease severity, comorbidity including hypertension, diabetes, CKD, cardiovascular disease, and rheumatoid arthritis, and duration from symptom onset to follow-up, the percentage of eGFR decreased from acute phase to follow-up was 8.30% (95% CI, 5.99-10.61) higher for AKI participants than those without development of AKI at acute phase. Participants with AKI had an odds ratio (OR) of 4.60 (95% CI, 2.10-10.08) for reduced renal function and an OR of 2.52 (95% CI, 1.25-5.10) for proteinuria at follow-up.
Figure 3.
Association of acute kidney injury at acute phase with percentage of eGFR decreased from acute phase to follow-up and reduced renal function at follow-up.
The interaction of AKI with categorization of highest eGFR level at acute phase was statistically significant for the association of AKI with all three outcomes (pinteraction =0.0011, 0.0001, 0.0046 for percentage of eGFR decreased, reduced renal function, and proteinuria, respectively). The lower eGFR level at acute phase (<90 mL/min per 1.73 m2) strengthened the association between AKI and three outcomes. History of cardiovascular disease and more severe disease status during hospitalization weakened the association of AKI with percentage of eGFR decreased. For interaction of diabetes history with AKI, the effect of AKI on reduced renal function was numerically higher among participants with diabetes than those without diabetes although the interaction term was not statistically significant. The other variables showed no evidence of modifying the association of AKI with outcomes.
The sensitivity analysis after excluding participant with self-reported history of CKD before SARS-CoV-2 infection did not substantially change the associations of AKI with three outcomes (Table S3). Specifically, the association magnitude of AKI with percentage of eGFR decreased from acute phase to follow-up tended to be slightly smaller. The sensitivity analysis with further adjustment for antibiotics use at acute phase showed similar results with that before adjustment.
Association of AKI stage with kidney function status and its change
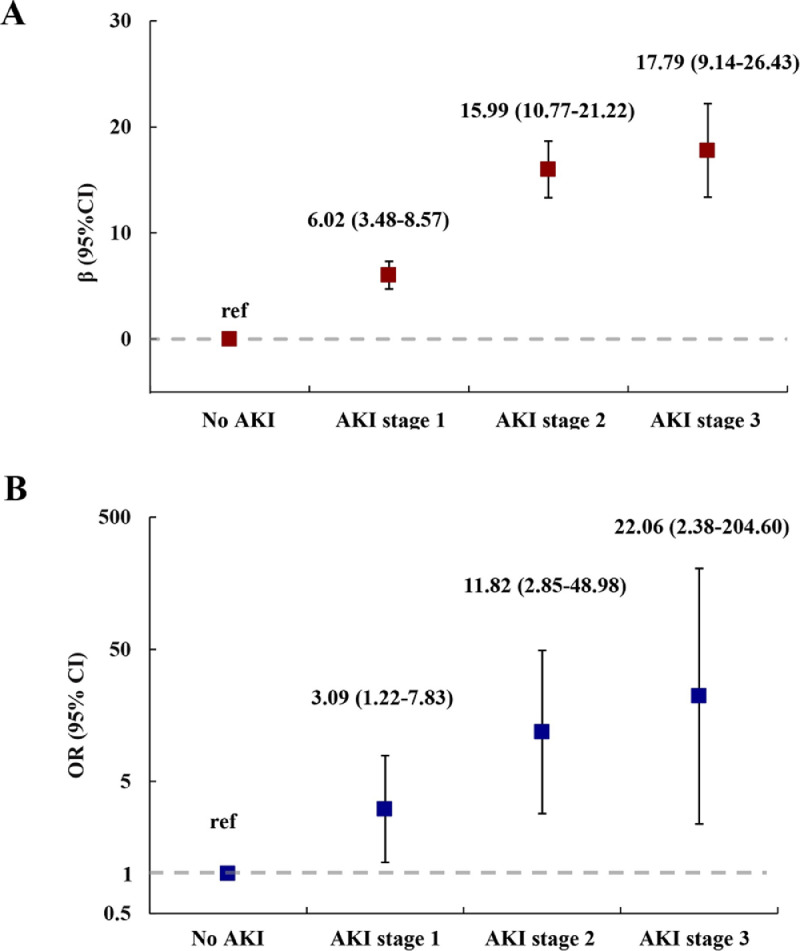
The associations of AKI stage with percentage of eGFR decreased from acute phase to follow-up and reduced renal function at follow-up are shown in Figure 4, and association with proteinuria at follow-up is shown in Figure S2. After multivariable adjustment, a graded increase in percentage of eGFR decreased and risk of reduced renal function and proteinuria was found with higher AKI stage. The percentages of eGFR decreased for participants with development of AKI stage 1, stage 2, and stage 3 were 6.02% (95% CI, 3.48-8.57), 15.99% (95% CI, 10.77-21.22), and 17.79% (95% CI, 9.14-26.43) higher compared with those without AKI, respectively. The ORs of reduced renal function for those with stage 1, stage 2, and stage 3 were 3.09 (95% CI, 1.22-7.83), 11.82 (95% CI, 2.85-48.89), and 22.06 (95% CI, 2.38-204.60), respectively. The ORs of proteinuria for stage 1, stage 2, and stage 3 were 2.11 (95% CI, 0.97-4.61), 2.98 (95% CI, 0.68-13.02), and 14.44 (95% CI, 2.07-100.50), respectively. The sensitivity analyses after excluding participant with self-reported history of CKD before SARS-CoV-2 infection showed similar results, except that the percentage of eGFR decreased from acute phase to follow-up was similar between AKI stage 2 and stage 3 (Table S3). The sensitivity analysis with further adjustment for antibiotics use at acute phase also showed similar results with that before adjustment.
Figure 4.
Percentage of eGFR decreased from acute phase to follow-up (A) and risk of reduced renal function at follow-up (B) by acute kidney injury stage.
Risk factors associated with AKI
After multivariable adjustment, age, comorbidity including hypertension and diabetes, disease severity characterized by highest seven-category scale during hospitalization, and use of corticosteroids or antibiotics during hospitalization were shown to be associated with development of AKI during hospitalization (Table S4). The OR of AKI per 10-year increase of age was 1.19 (95% CI, 1.02-1.40). The ORs were 2.58 (95% CI, 1.62-4.13) and 2.24 (95% CI, 1.29-3.89) for participants with history of hypertension and participants with history of diabetes before SARS-CoV-2 infection compared with those without, respectively. Compared with participants with scale 3, the ORs of AKI were 2.30 (95% CI, 1.12-4.72) and 2.30 (95% CI, 1.12-4.72) among participants with scale 4 and scale 5-6, respectively. Participant who received corticosteroids had an OR of 2.09 (95% CI, 1.24-3.54) for AKI compared with those who did not, while the OR was 6.24 (95% CI, 1.49-26.15) for those who received antibiotics compared with those who did not.
Discussion
In this ambidirectional cohort study conducted among COVID-19 patients discharged from hospital, we found AKI developed during hospitalization was associated with higher percentage of eGFR decreased from acute phase to one year after symptom onset, and also increased risk of reduced renal function and proteinuria. Furthermore, the graded increase in percentage of eGFR decreased and risk of reduced renal function and proteinuria was observed with higher AKI stage. To our knowledge, this cohort study is the largest one with the longest follow-up duration to explore the effect of AKI and AKI stage on longitudinal change and post-acute status of kidney function among COVID-19 patients discharged from hospital. The findings indicated the importance of long-term kidney disease management among COVID-19 survivors.
The occurrence of AKI has been widely reported among COVID-19 patients. Dissecting the risk factors of COVID-19 induced AKI and its pathogenesis can help clinicians to recognize patients with higher risk of AKI earlier. In our study, the results about factors including age, comorbidity including hypertension and diabetes, and disease severity found to be associated with AKI were consistent with previous studies.6,25, 26, 27, 28 The pathophysiology of COVID-19 induced AKI is multifactorial. Direct kidney tissue damage caused by SARS-CoV-2 is the most comprehensively studied mechanism of AKI.29, 30, 31 The other plausible mechanisms of AKI include microvascular dysfunction secondary to endothelial damage, and the potential role that cytokine storm plays in the immunopathology of AKI.31,32
The burden of AKI especially severe AKI and requirement for renal replacement therapy were higher compared with those without COVID-19, resulting in special concern for AKI occurred among COVID-19 patients.7 However, data about post-acute kidney function status and evidence for the impact of AKI on longitudinal change of kidney function were very limited.13,16 AKI and CKD are closely interconnected with each other and AKI has been recognized as a gateway to progression for CKD.33, 34, 35 The long-term decline in kidney function after AKI has been described in people with specific clinical features such as those who discharged after ICU admission or those who underwent coronary angiography.36,37 Recognizing the effect of COVID-19 associated AKI on longitudinal change of kidney function and post-acute kidney function can inform clinicians to develop more precise and intense follow-up strategies for kidney disease management among people recovering from COVID-19 according to their AKI status at acute phase.
To elucidate effect of AKI on long-term kidney outcomes among COVID-19 patients, making comparison between those who developed and did not develop AKI at acute phase is important. One previous study including only 60 participants who were COVID-19 patients admitted to ICU showed that AKI lasting for more than 7 days was associated with higher risk of CKD.14 Our study showed consistent result among more generalized COVID-19 survivors. We also found the graded relationship between AKI stage and longitudinal change of kidney function, and the effect modification of eGFR level at acute phase. These findings indicated earlier and more intense kidney function follow-up should be performed among those with more severe AKI and lower eGFR level during hospitalization. However, how long the impact of AKI on longitudinal change of kidney status among COVID-19 survivors would last and whether the effect would change as patients with AKI gradually recovered need to better explored in cohort study with longer follow-up duration. In addition, attention should also be paid to population who were recovering from COVID-19 but who did not develop AKI at acute phase. Immunologic aberrations and inflammatory damage in response to the acute infection was indicated as one of the possible mechanisms of post-acute COVID-19.3,29,38 The possibility that SARS-CoV-2 did not cause AKI at acute phase but still resulted in faster eGFR decline compared with general population who were not infected with SARS-CoV-2 cannot be excluded.
Several limitations of this study should be addressed. Firstly, this is a study with enrollment of COVID-19 patients who discharged alive at the early stage of pandemic, with proportion of AKI as 6.1%, which may limit the generalizability of study findings. For the definition of AKI, the lowest creatinine level during hospitalization was used as baseline creatinine level which has been described in other studies.23 Secondly, urine dipstick test has been used to measure proteinuria due to the lack of spot urine albumin-to-creatinine ratio and protein-to-creatinine ratio. Thirdly, participants with CKD before COVID-19 may have been included in the assessment as the kidney function status of study participants on admission was not systematically evaluated due to the emergency state of hospital at early stage of pandemic. However, the sensitivity analyses after excluding self-reported history of CKD before SARS-CoV-2 infection did not substantially change the associations of AKI with outcomes. Fourthly, 21.8% of eligible participants were not included in final analysis due to loss of follow-up or missing information for key variable. Fortunately, no statistically significant difference was observed for majority of baseline characteristics between participants included and not included in final analysis. Lastly, information bias resulted from recalling information such as smoking and self-reported comorbidity cannot be excluded, even though misclassification of these variables was more likely to be non-differential in our cohort study.
In conclusion, the cohort study with one-year follow-up duration after symptom onset among people recovering from COVID-19 lend credence to the association of AKI with longitudinal decline and post-acute status of kidney function. This study also provided evidence for the graded relationship between AKI stage and kidney function outcomes. The findings were important for development of more precise and intense follow-up strategies for future kidney disease management among people recovering from COVID-19. Furthermore, studies with longer follow-up duration for COVID-19 should be conducted to understand long-term impact of AKI on longitudinal change of kidney function more comprehensively because whether the effect size of AKI on kidney function would be different beyond one year after symptom onset needs to be further evaluated.
Declaration of interests
We declare no competing interests.
Acknowledgments
Contributors
BC had the idea for and designed the study and all authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. XG and BC drafted the paper. XG did the analysis and all authors critically revised the manuscript for important intellectual content and agreed to submit the final version for publication. XG, LH, DC, YeW, and BC completed the follow-up work, and collected and verified the data. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Acknowledgements
This work was supported by Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (CIFMS 2020-I2M-CoV19-005, 2018-I2M-1-003, and 2020-I2M-2-013); the National Natural Science Foundation of China (82041011); the National Key Research and Development Program of China (2018YFC1200102); and Major Projects of National Science and Technology on New Drug Creation and Development of Pulmonary Tuberculosis (2020ZX09201001). This work was also supported by the China Evergrande Group, Jack Ma Foundation, Sino Biopharmaceutical Limited, Ping An Insurance (Group), and New Sunshine Charity Foundation. We acknowledged all subjects who participated in this study and their families. We would like to thank all staff of this follow-up study team.
Data sharing
Restrictions apply to the availability of these data and so are not publicly available. However, data are available from the authors upon reasonable request and with the permission of the institution.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ebiom.2022.103817.
Supplementary materials
Reference
- 1.World Health Organization . 2021. WHO Coronavirus Disease (COVID-19) dashboard.https://covid19.who.int/table Accessed 17 December 2021. [PubMed] [Google Scholar]
- 2.Gupta A, Madhavan MV, Sehgal K, et al. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26(7):1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27(4):601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng Y, Luo R, Wang K, et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97(5):829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirsch JS, Ng JH, Ross DW, et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98(1):209–218. doi: 10.1016/j.kint.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fisher M, Neugarten J, Bellin E, et al. AKI in Hospitalized Patients with and without COVID-19: A Comparison Study. J Am Soc Nephrol. 2020;31(9):2145–2157. doi: 10.1681/ASN.2020040509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan L, Chaudhary K, Saha A, et al. AKI in Hospitalized Patients with COVID-19. J Am Soc Nephrol. 2021;32(1):151–160. doi: 10.1681/ASN.2020050615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ng JH, Hirsch JS, Hazzan A, et al. Outcomes Among Patients Hospitalized With COVID-19 and Acute Kidney Injury. Am J Kidney Dis. 2021;77(2):204–215. doi: 10.1053/j.ajkd.2020.09.002. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perico L, Benigni A, Remuzzi G. Should COVID-19 Concern Nephrologists? Why and to What Extent? The Emerging Impasse of Angiotensin Blockade. Nephron. 2020;144(5):213–221. doi: 10.1159/000507305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Remuzzi G, Kolhe NV, Fluck RJ, Selby NM, Taal MW. Acute kidney injury associated with COVID-19: A retrospective cohort study. PLoS Med. 2020;17(10) doi: 10.1371/journal.pmed.1003406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moledina DG, Simonov M, Yamamoto Y, et al. The Association of COVID-19 With Acute Kidney Injury Independent of Severity of Illness: A Multicenter Cohort Study. Am J Kidney Dis. 2021;77(4):490–499. doi: 10.1053/j.ajkd.2020.12.007. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nugent J, Aklilu A, Yamamoto Y, et al. Assessment of Acute Kidney Injury and Longitudinal Kidney Function After Hospital Discharge Among Patients With and Without COVID-19. JAMA Netw Open. 2021;4(3) doi: 10.1001/jamanetworkopen.2021.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hultstrom M, Lipcsey M, Wallin E, Larsson IM, Larsson A, Frithiof R. Severe acute kidney injury associated with progression of chronic kidney disease after critical COVID-19. Crit Care. 2021;25(1):37. doi: 10.1186/s13054-021-03461-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bowe B, Xie Y, Xu E, Al-Aly Z. Kidney Outcomes in Long COVID. J Am Soc Nephrol. 2021;32(11):2851–2862. doi: 10.1681/ASN.2021060734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang L, Yao Q, Gu X, et al. 1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study. Lancet. 2021;398(10302):747–758. doi: 10.1016/S0140-6736(21)01755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest. 1988;93(3):580–586. doi: 10.1378/chest.93.3.580. [DOI] [PubMed] [Google Scholar]
- 19.Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L) Qual Life Res. 2011;20(10):1727–1736. doi: 10.1007/s11136-011-9903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33(5):337–343. doi: 10.3109/07853890109002087. [DOI] [PubMed] [Google Scholar]
- 21.Xie W, Wu Y, Wang W, et al. A longitudinal study of carotid plaque and risk of ischemic cardiovascular disease in the Chinese population. J Am Soc Echocardiogr. 2011;24(7):729–737. doi: 10.1016/j.echo.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 22.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2:1-138.
- 23.Siew ED, Matheny ME. Choice of Reference Serum Creatinine in Defining Acute Kidney Injury. Nephron. 2015;131(2):107–112. doi: 10.1159/000439144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stevens LA, Claybon MA, Schmid CH, et al. Evaluation of the Chronic Kidney Disease Epidemiology Collaboration equation for estimating the glomerular filtration rate in multiple ethnicities. Kidney Int. 2011;79(5):555–562. doi: 10.1038/ki.2010.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin L, Wang X, Ren J, et al. Risk factors and prognosis for COVID-19-induced acute kidney injury: a meta-analysis. BMJ Open. 2020;10(11) doi: 10.1136/bmjopen-2020-042573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gabarre P, Dumas G, Dupont T, Darmon M, Azoulay E, Zafrani L. Acute kidney injury in critically ill patients with COVID-19. Intensive Care Med. 2020;46(7):1339–1348. doi: 10.1007/s00134-020-06153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bowe B, Cai M, Xie Y, Gibson AK, Maddukuri G, Al-Aly Z. Acute Kidney Injury in a National Cohort of Hospitalized US Veterans with COVID-19. Clin J Am Soc Nephrol. 2020;16(1):14–25. doi: 10.2215/CJN.09610620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.See YP, Young BE, Ang LW, et al. Risk Factors for Development of Acute Kidney Injury in COVID-19 Patients: A Retrospective Observational Cohort Study. Nephron. 2021;145(3):256–264. doi: 10.1159/000514064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Puelles VG, Lütgehetmann M, Lindenmeyer MT, et al. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med. 2020;383(6):590–592. doi: 10.1056/NEJMc2011400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Su H, Yang M, Wan C, et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98(1):219–227. doi: 10.1016/j.kint.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li H, Liu L, Zhang D, Xu J. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet. 2020;395:1517–1520. doi: 10.1016/S0140-6736(20)30920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chawla LS, Eggers PW, Star RA, Kimmel PL. Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med. 2014;371(1):58–66. doi: 10.1056/NEJMra1214243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heung M, Chawla LS. Acute kidney injury: gateway to chronic kidney disease. Nephron Clin Pract. 2014;127(1-4):30–34. doi: 10.1159/000363675. [DOI] [PubMed] [Google Scholar]
- 35.Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2012;81(5):442–448. doi: 10.1038/ki.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haines RW, Powell-Tuck J, Leonard H, Crichton S, Ostermann M. Long-term kidney function of patients discharged from hospital after an intensive care admission: observational cohort study. Sci Rep. 2021;11(1):9928. doi: 10.1038/s41598-021-89454-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.James MT, Ghali WA, Tonelli M, et al. Acute kidney injury following coronary angiography is associated with a long-term decline in kidney function. Kidney Int. 2010;78(8):803–809. doi: 10.1038/ki.2010.258. [DOI] [PubMed] [Google Scholar]
- 38.Naicker S, Yang CW, Hwang SJ, Liu BC, Chen JH, Jha V. The Novel Coronavirus 2019 epidemic and kidneys. Kidney Int. 2020;97(5):824–828. doi: 10.1016/j.kint.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.