Abstract
Alpha-fetoprotein (AFP) is an oncofetal glycoprotein that has been used as a tumor marker for hepatocellular carcinoma (HCC) in combination with ultrasound and other imaging modalities. Its utility is limited because of both low sensitivity and specificity, and discrepancies among the different methods of measurements. Moreover, its accuracy varies according to patient characteristics and the AFP cut-off values used. Combination of AFP with novel biomarkers such as AFP-L3, Golgi specific membrane protein (GP73) and des-gamma-carboxyprothrombin significantly improved its accuracy in detecting HCC. Increased AFP level could also signify severity of hepatic destruction and subsequent regeneration and is commonly observed in patients with acute and chronic liver conditions and cirrhosis. Hereditary and other non-hepatic disorders can also cause AFP elevation.
Keywords: Alpha-fetoprotein, Hepatocellular carcinoma, Alpha-fetoprotein-L3, Cirrhosis, Tumor markers, Hereditary persistence of alpha-fetoprotein
Core Tip: Alpha-fetoprotein has been used commonly as a biomarker for hepatocellular carcinoma (HCC) surveillance. Its sensitivity and specificity can be affected by the assay methods, patient characteristics and severity of the underlying liver conditions. Combination with other novel markers has shown promising results. Algorithms integrating these serum markers with noninvasive diagnostic imaging modalities are essential for the accurate and timely diagnosis of HCC.
INTRODUCTION
During fetal and neonatal life, alpha-fetoprotein (AFP) is produced by yolk sac, fetal liver and gastrointestinal tract. Only a trace amount of AFP can be measured in adults due to its rapid decline in adults[1-4]. The use of AFP as a tumor marker for hepatocellular carcinoma (HCC) was first proposed in 1960s. The utility of AFP as a surveillance and diagnostic test has been criticized due to both low sensitivity and specificity. AFP, however, continues to play a significant role for HCC surveillance in combination with ultrasound (US) and other imaging modalities[5-8]. This review focused on both the clinical roles and limitations of AFP. In addition, the patterns of AFP elevation in various non-HCC etiologies were discussed.
BIOLOGY OF AFP
AFP is an oncofetal glycoprotein consisting of a single polypeptide chain of approximately 600 amino acids and nearly 4% carbohydrate. With molecular weight of 67500 dalton, it is a negatively charged protein with an isoelectric point of pH 4.57 and displays several charge isomers by extended agarose gel electrophoresis[1,2]. During the first trimester, AFP is mainly produced by yolk sac. After the atresia of yolk sac by 11-12 wk, fetal liver becomes the predominant source of AFP. A trace amount is also produced by the gastrointestinal tract of the fetus[1]. AFP in fetal serum can be detected as early as 29 d after conception and reaches its peak value of 3.0 × 106 ng/mL by 14th week of gestation. It declines to 2.0 × 105–3.0 × 105 ng/mL by week 32 and further decreases to 20-120 ng/mL at term[3]. By six months of life, serum AFP level is approximately 30 ng/mL. Thereafter, it is detectable between 3 and 15 ng/mL in adulthood[3,4].
AFP belongs to the family of serum albumin and the genes of which are present on the chromosome 4. It shares structure and physiochemical properties with its family members namely albumin, vitamin D-binding protein and afamin[9]. As both albumin and AFP show significant amino acid homologies, AFP is suggested to be an embryonic analogue of albumin[10]. Furthermore, because of its similarity to albumin, it has been postulated that AFP could be a carrier protein. In addition, its role as an immune-regulator and as a carrier protein for bilirubin has also been suggested[11].
AFP, being a principal serum binding protein in the fetus, plays a vital role in carrying various ligands such as fatty acids, hormones, minerals and bilirubin[12]. Though it performs many physiological functions during fetal development, elevated levels in adults are frequently observed in liver carcinogenesis and various disease processes[13-15]. Many biologic studies support the pro-oncogenic as well as anti-apoptotic effects of AFP. AFP can stimulate cell proliferation, cell motility and invasive properties of HCC cell lines and there is evidence that apoptosis of cancer cells could be achieved by blocking AFP[16,17]. On the other hand, a number of cancerous and non- cancerous causes involving both the liver and other organs can also lead to AFP elevation. AFP, therefore, is a non-specific marker of HCC[7] (Table 1).
Table 1.
|
Hepatic
|
Non-hepatic
|
| Neoplastic | Neoplastic |
| Hepatocellular carcinoma | Germ cell tumors (testicular and ovarian malignancies) |
| Intrahepatic cholangiocarcinoma | Gastric cancer |
| Non-neoplastic | Non-neoplastic |
| Liver cirrhosis | Normal pregnancy/infancy |
| Fulminant acute hepatitis | Colitis |
| Acute and chronic viral hepatitis | Fetal disorders (Gastroschisis, Neural tube defect) |
| Chronic liver diseases | Ataxia telangiectasia |
| Biliary obstruction (Intrahepatic and extrahepatic causes) | Hereditary tyrosinemia type 1 |
| Drug induced hepatitis | Hereditary AFP persistence |
| Alcohol liver disease | Beckwith-Wiedemann syndrome |
| Non-alcoholic liver disease | Systemic lupus erythematosus |
| Neonatal hepatitis | Hirschsprung’s disease |
| Massive hepatic necrosis | |
| Wilson disease | |
| Hemochromatosis |
AFP: Alpha-fetoprotein.
HOW IS AFP MEASURED?
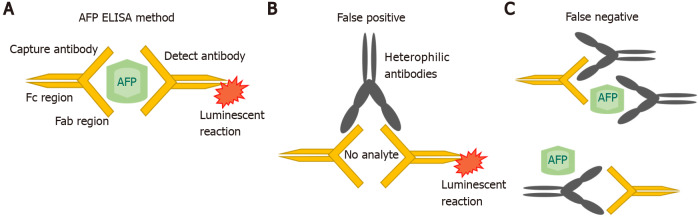
AFP was originally measured by immunoelectophoresis, but this method was not very sensitive. In the 1970s and 1980s, new techniques such as radioimmunoassay and enzyme immunoassays were used. Subsequently, a quantitative automated chemiluminescent enzyme immunoassay was developed, which replaced and refined the previous clinical assays[18,19]. In this method, the serum sample is placed on a magnetic plate which is already bound by an anti-AFP antibody. A second chemiluminescent detection antibody, added to the same magnetic plate, then binds to all the AFP that is present in excess. All the unbound detection antibody is washed off and an organic substrate called as developer is added which emits light and become luminescent (Figure 1A). A chemiluminometer is used to detect the antibody, and quantification of the results is done against the known AFP standards. However, measurement interference may occur. In a single step method of AFP detection, sometimes interfering antibodies would bind to both the capture and detect antibodies leading to a false positive result (Figure 1B). Conversely, the interfering antibodies may blind to the reagents and inhibiting the proper interaction of AFP with the specific anti-AFP antibodies[20] (Figure 1C).
Figure 1.
ELISA measures. A: ELISA measures alpha-fetoprotein (AFP) by detecting reactivity with two anti-AFP antibodies; B: Interfering antibodies that bind directly to the capture and detect reagents without the target AFP (no analytes). This binding would emit light and become luminescent causing false positive results and high values; C: Interfering antibodies can inhibit reactivity of the ELISA by binding to the reagents and prevent the detection of AFP by the anti-AFP antibodies; that leads to a false negative result. AFP: Alpha-fetoprotein.
AFP as a diagnostic marker for HCC
The association between AFP and HCC is well recognized; however, the sensitivity and specificity of the AFP assay varies according to patient characteristics, design of the study, and the AFP cut-off values used[21,22] (Table 2). A systematic review of studies using AFP at a threshold value of 20 ng/mL in patients with cirrhosis, the sensitivity and specificity of detecting HCC were 41%-65% and 80%-94%, respectively[22]. Lowering the cut-off can increase the sensitivity of AFP but at the cost of higher false positives. By increasing the AFP value from 20 to 50 ng/mL, the specificity increased to 96% with a positive predictive value of 75% but sensitivity was reduced to 47%[23]. AFP values also vary according to the tumor size. The correlation of AFP to the size of the tumor was evaluated by Saffroy et al[24]. The bigger tumors generally had higher AFP values. The sensitivity of AFP, therefore, decreased from 52% for HCC > 3 cm to 25% for those < 3 cm in diameter[24].
Table 2.
|
AFP cut-off value
|
Sensitivity
|
Specificity
|
| 20 | Approximately 60% | 90% |
| 50 | 47% | 96% |
| 100 | 31.2% | 98.8% |
| 400 | 17% | 99.4% |
AFP: Alpha-fetoprotein.
For AFP-producing HCC, AFP plays an important role in initiating monitoring and terminating HCC therapy. A complete response of the treatment can be expected if preceding levels of AFP decrease to normal on follow ups. AFP, however, cannot be applied to monitor treatment response if its levels were not elevated prior to treatment[25-27]. Approximately one-third of the patients with HCC have no AFP elevation[28]. Nomura et al[29] characterized those HCCs with low or no elevation of AFP. These patients generally have favorable prognosis with low probability of HCC recurrence and improved survival as compared to those with elevated AFP levels[29,30]. In a large retrospective study including 1800 patients with HCC, 42% had AFP < 20 ng/mL whereas 16% had AFP levels between 20 and 100 ng/mL. Thus, a total of 58% had AFP < 100 ng/mL. The authors observed that 67% of patients with HCC smaller than 5 cm had AFP ≤ 100 ng/mL vs only 49% of the patients with HCC larger than 5 cm had AFP ≤ 100 ng/mL. In addition, HCCs without AFP elevation (≤ 100 ng/mL) were less likely to develop portal vein thrombosis[31].
AFP-L3 and other HCC biomarkers
Based on the binding capacity of AFP to lens culinaris agglutinin (LCA), three separated bands of AFP glycoforms can be identified with the Western blotting. These are AFP-L1 (non-binding), AFP-L2 (intermediate binding) and AFP-L3 (LCA-reactive). AFP-L1 generally correlates with hepatic inflammation in chronic hepatitis while AFP-L3 is more specific for HCC. AFP-L2 is derived from yolk sac and is detectable in the maternal serum during pregnancy[32] (Table 3).
Table 3.
Overview of the diagnostic parameters for hepatocellular carcinoma
|
Diagnostic parameter (cut off)
|
Remark
|
AUROC
|
| AFP (> 20 ng/mL) | Sensitivity: 40%–65% | 0.54–0.80 |
| Specificity: 76%–96% | ||
| AFP-L3 (> 15%) | Sensitivity: 45%–90% | 0.74–0.84 |
| Specificity: 95% | ||
| AFP-L3 + AFP-P4 + P5 (> 15% for both biomarkers) | Sensitivity: 55.3%–61% | 0.76 |
| Specificity: 82.3%–93.9% | ||
| AFP, AFP-L3 and DCP (3-25 ng/mL, 1%-10%, 0.48 ng/mL or 40 mAu/mL) | Sensitivity: 81%–93% | 0.88–0.93 |
| Specificity: 69%-87% | ||
| AFP and GP73 | Sensitivity: 75%-91% | 0.91–0.95 |
| Specificity: 81%-97% |
AFP: Alpha-fetoprotein; DCP: Des-gamma-carboxyprothrombin.
AFP-L3 is calculated as a fraction of AFP-L3 to total AFP. The cut-off values most commonly used for a positive AFP-L3 test for patients with HCC is 10% though levels above 15% are more specific[33]. It is secreted in the initial tumor stages and thus can be used as an early HCC diagnostic marker[32,34]. AFP-L3 level can also distinguish the histological differentiation of tumors[25]. In patients with total AFP level ≤ 200 ng/mL, AFP-L3 specificity may reach 100% for HCC if it increases more than 35% of the total AFP[35]. In a large multicenter prospective study of hepatitis C virus related HCC, the specificity of AFP-L3 was observed to be 92% but its sensitivity was fairly low at 37%, irrespective of the tumor stage[36]. On the contrary, a number of recent studies reported a much higher sensitivity of AFP-L3 for diagnosing HCC[37-39]. Ibrahim et al[39] evaluated the diagnostic efficacy of AFP-L3 among 20 healthy individuals, 40 patients of chronic active hepatitis and 40 HCC patients with underlying chronic hepatitis B (CHB) or C. Among the HCC patients, 30 (75%) had tumor size > 5 cm. At a cut-off point of > 12.3 ng/mL, AFP-L3 was 100% accurate in diagnosing the HCC[39]. In a study by Ibrahim et al[39], 20 healthy individuals, 20 chronic liver disease patients and 40 patients HCV-induced HCC were included in the analysis. A large proportion (65%) of the HCC patients had advanced stage of HCC. By using a cut-off value of 23 ng/mL, AFP-L3 had a sensitivity of 97.5% and specificity of 100% in predicting HCC at[37]. Cerban et al[38] investigated the diagnostic performance of AFP, AFP-L3, PIVKA-II and GPC-3 for HCC in a cohort including 52 cirrhotic and 101 HCC patients. AFP-L3 at a cut-off point of > 13.5 ng/mL was found to have a higher sensitivity of 84.7%, compared to other biomarkers[38]. The sensitivity of AFP-L3 for HCC detection can be affected by its cut-off values, as well as the tumor size and stage[40]. Data suggested that AFP-L3 could potentially be a valuable prognostic tool. The persistent elevation of AFP-L3 after HCC therapy was found to be associated with shorter survival[25].
Recently, a highly-sensitive AFP-L3 or hs-AFP-L3 was developed. Toyoda el al. determined the performance of hs-AFP-L3 among patients with 44 patients with chronic hepatitis or cirrhosis, and 54 patients with HCC. The sensitivity and specificity of hs-AFP-L3 were 84.9% and 88.6%, respectively[41].
Since both total AFP and AFP-L3 have limitations for the detection of HCC, studies have been conducted to combine AFP and AFP-L3 with other novel immunoassays to improve their performance. Des-gamma-carboxyprothrombin (DCP), also known as PIVKA-II (Protein Induced by vitamin K absence or antagonist-II), is a promising HCC biomarker. PIVKA-II is an abnormal product of liver carboxylation that reflects oncogenesis and HCC progression and is undetectable in healthy patients[25,42].
A systematic review noted that AFP, AFP-L3 combined with DCP enhanced their individual HCC predictive values with pooled sensitivity and specificity of 88% and 79%, respectively[43,44]. Since 2002, Japan has included these 3 biomarkers in their HCC surveillance programs and they noted improved rates of early HCC identification and prognosis[43]. When applying the GALAD score (age, sex, AFP, AFP-L3 and DCP), the HCC diagnostic accuracy was even higher [area under curve (AUC) 0.976][45,46]. A recent meta-analysis reported that AFP combined with GP73 (a Golgi specific membrane protein found in epithelial cells of bile ducts) has favorable sensitivity (84%) and specificity (92%) in diagnosing HCC with an AUROC value of 0.93[47].
Lately, a new HCC diagnostic marker, the long noncoding RNAs (lncRNAs), was developed[48]. LncRNAs are transcribed by RNA polymerase II and play a role in the regulation of transcription and translation of proteins. Small extracellular vesicles (EV) transfer proteins, DNA, and RNA between tumor and nontumor cells. The dysregulated lncRNAs including EV-derived lncRNAs contribute to HCC progression and metastasis. EV-derived lncRNAs are, therefore, potential novel serum biomarkers. Kim et al[48] evaluated LINC00853, an EV-derived lncRNA in 90 patients with HCC and 92 without HCC in a cohort study. The AUROC of LINC00853 was 0.935 for identifying all-stage HCC and 0.969 for detecting early-stage HCC. These results were significantly better compared to AFP[48]. In another study by Yu et al[49] reported that the combination of AFP with 2-lncRNA had a higher discriminative power than that of AFP alone in the diagnosis of HCC[49].
AFP ELEVATION IN LIVER DISEASES AND CIRRHOSIS
Rise in AFP has been recorded in different chronic liver diseases without HCC and other malignancies, hence, the results of AFP levels should be interpreted with caution[50]. AFP is known to have a role in liver regeneration, inflammation and liver fibrosis. Less pronounced AFP elevation > 10 ng/mL has been reported in 15%-58% of chronic hepatitis and 11%-47% of cirrhosis[51]. The usual cut off for differentiating benign vs malignant liver conditions was at 500 ng/mL but can be variable[52]. Studies have reported a progressive rise in serum AFP levels with increased histological severity from inflammation to cirrhosis to HCC[53].
ACUTE HEPATITIS
The degree of AFP elevation in acute hepatitis is related to the severity of hepatic destruction[50,54]. The levels range from 10 ng/mL-1000 ng/mL with occasional higher value from 3000 to 7190 ng/mL has been reported. Among children with acute hepatitis B, AFP was detected within 1 wk of the onset of clinical hepatitis and returned to normal by the time of recovery with loss of HBsAg[55]. The possible mechanisms of AFP elevation include acute phase reaction to the liver injury, hepatocyte regeneration, or viral control or mediated AFP synthesis[56]. AFP elevation after significant alanine aminotransferase (ALT) elevation is most likely due to liver regeneration and there is generally a latent period of 5-16 d[57]. AFP generally peaks at a time when liver destruction is subsiding and hepatic remodeling begins[57]. Hence, the highest level of AFP in acute hepatitis generally occurs during the recovery phase of illness and is an indicator of the liver regenerative process[58].
CHB
Elevated AFP levels are frequently observed during the course of CHB without HCC. The commonest cause of AFP elevation was the exacerbation of the underlying liver disease with or without changes in the status of hepatitis B virus (HBV) replication[41,52]. High AFP levels associated with hepatitis flare were also found to be predictive of HBeAg to anti-HBe seroconversion[59,60]. AFP levels usually decrease within 12 mo on antiviral therapy[61]. Kim et al[61] reported that AFP normalization (< 20 ng/mL) was achieved in 89.5% of treated patients but remained abnormal in 40.6% of antiviral treatment naïve patients[61].
Persistent elevation of AFP > 100 ng/mL without a parallel increase in ALT level was reported to be a predictor of HCC with a sensitivity of 98.7% and specificity of 66.7%[59]. Yuan et al[62] conducted a retrospective study of 302 treatment naive CHB patients with AFP positive status. After antiviral therapy, they found that there was a 6.35-fold increased HCC risk among those with persistently elevated AFP compared to those with normalized AFP levels[62].
In a metanalysis, Peng et al[63] concluded that among HBV–related HCCs with low AFP levels, circulating miRNAs could be potential valuable biomarkers. For patients with AFP levels < 400 ng/mL, miR-125b and miR-205 demonstrated a sensitivity of 90% while combination of miR-15b and miR-130b had > 90% sensitivity and specificity. For AFP < 20 ng/mL, miR-26a, 27a, 7b and combination of miR-122 and miR-7b exhibited a sensitivity of 80% while combination of mir-29a, 29c, 133a, 143, 145, 192 and 505 produced a specificity of > 80%. Additionally, the combination of miR-15b and miR-130b showed high diagnostic accuracy with sensitivity and specificity exceeding 90%. For differentiating HCC patients with low AFP levels (< 20 ng/mL) from non-HCC patients, the overall sensitivity and specificity of miRNAs were 0.85 and 0.74, respectively with AUC of 0.88 (95%CI: 0.85–0.90). For patients with AFP < 400ng/mL, the overall sensitivity and specificity were 0.84 and 0.76 with AUC of 0.88 (95%CI: 0.84–0.90). Hence, miRNAs are attractive HCC markers to detect HBV-associated HCC with low AFP levels[63].
Dickkopf WNT signaling pathway inhibitor 1 (DKK-1) is a glycoprotein that is expressed in various malignancies. Shen et al[64] conducted a study on 424 HCC, 98 CHB, 96 cirrhosis and 213 healthy controls and measured their DKK-1 Levels. The diagnostic cut-off was established at 2.153 ng/mL. DKK-1 proved useful in the detection of AFP-negative HCC with 70.4% sensitivity and 90.0% specificity. These values were validated in another cohort. When combined with AFP, DKK-1 increased detection rate of early-stage HCC with 73.1% sensitivity and 90.0% specificity. Additionally, elevated DKK-1 Levels can help differentiate HCC from other liver diseases such as CHB and cirrhosis with 69.1% sensitivity and 84.7% specificity[64,65].
Hepatitis B flare is defined as an abrupt elevation of serum ALT to 5 times the ULN or greater than 3-fold increase from baseline[66]. During hepatitis flare, peak AFP level was usually observed 1-2 wk after peak ALT rise. Significant AFP elevation to > 2500 ng/mL had been documented with hepatitis flare. The normalization of AFP might take up to 3-12 mo[66].
The presentation varies from asymptomatic to overt hepatitis with decompensation and liver failure. AFP elevation was noted in 25%-30% of the patients with hepatitis flares. A study reported the annual incidence of hepatitis flares to be 27% in HBeAg-positive patients and 10% in HBeAg-negative patients with a mean follow-up period of 2 years. Chang et al[67] reported that patients with AFP > 100 ng/mL during flare cleared HBeAg at a rate of 31% in 3 mo, 62% in 12 mo and 72% in 18 mo. In contrast, the corresponding HBeAg clearance rates for those with AFP < 100 ng/mL were only 4%, 15% and 19% in 3, 12 and 18 mo respectively[67]. Patients with bridging hepatic necrosis (BHN) had increased activation of the AFP-producing oval cell. BHN was noted in 80% of the cases with AFP levels > 100 mg/dL[53]. BHN, AFP > 100 ng/mL and decreasing HBV DNA titers were identified as good prognostic indicators of an effective immune control with HBV DNA suppression and/or HBeAg clearance.
Severe and repeated hepatitis flares could also lead to development of cirrhosis or hepatic decompensation. Patients with severe hepatitis flare with BHN or AFP > 100 ng/mL but fail to suppress HBV DNA should be treated promptly with antiviral therapy.
CHRONIC HEPATITIS C
AFP elevation is more greatly associated with HCV-associated HCC than HBV-associated HCC[64]. The incidence of elevated AFP in chronic hepatitis C (CHC) patients range from 10%-43%[61]. Elevated AFP > 20 ng/dL in CHC is associated with female gender, black race, increased age, genotype 1b, low albumin level, elevated aspartate aminotransferase (AST), elevated AST/ALT ratio, low platelet, prolonged PT, and increased ferritin levels[68,69]. Yang et al[70] evaluated 279 CHC patients and found no correlation between AFP and the levels of HCV RNA[70]. In the HALT C (Hepatitis C Antiviral Long-term Treatment) study, Di Bisceglie et al[71] observed AFP > 20 ng/mL in 16.6% of patients without HCC; it was associated with cirrhosis, female and Black patients[71]. Black patients with CHC tend to have higher AFP elevation compared to other racial groups[71]. Generally, HCV-related HCC does not have significant AFP elevation. Thus, AFP has especially low sensitivity to identify HCC in Black populations[71].
Fouad et al[72] reported that sustained virological response with direct-acting antiviral agents (DAA) therapy was associated with a significant reduction in serum AFP and might be used as a predictor of treatment response[72]. In another study, 60% of the DAA treated patients normalized AFP compared to only 23% without therapy[61].
Therapeutic phlebotomy has also been reported to reduce AFP levels in CHC[44]. It has been postulated that iron mediated oxidative stress is associated with hepatic injury; iron depletion decreases the oxidative stress and indirectly lowers the AFP level[73].
NONALCOHOLIC FATTY LIVER DISEASE
Nonalcoholic fatty liver disease (NAFLD) has become the most common liver disease in the Western world with about 10%-30% progress to cirrhosis[74]. It is estimated that 6 million people in the United States have NASH with 1%-2% incidence of HCC annually. Published reports estimated that over 25% of NASH-related HCC occurred in patients without cirrhosis. With the high prevalence of NAFLD, risk stratification is essential to implement HCC surveillance programs[75]. Currently, no surveillance of NALFD patients for HCC is recommended by AASLD[76]. However, it has been suggested that NAFLD patients with cirrhosis or possible fibrosis or diabetes mellitus should have 6 monthly surveillances via ultrasound (US) and tumor markers (AFP, AFP-L3 and DCP). Abdominal fat can be a hindrance, hence, patients with obesity can be alternatively surveilled using CT-Scan or magnetic resonance imaging[77].
An association between AFP elevation and NAFLD was first established by Babalı et al[78] in 2009. This study demonstrated a significant increase in AFP levels in patients suffering from NAFLD (4.09 ± 1.68) in comparison to the control group (2.95 ± 0.41) with P value < 0.05.Additionally, three subgroups of NAFLD were created based on the liver ultrasonography findings with presence of hepatorenal contrast or bright liver corresponding to grade 1, both hepatorenal contrast and bright liver signifying grade 2, and both pre-mentioned findings with bright liver being severe representing grade 3. The results showed a significant increase in AFP in grade 3 NAFLD (5.43 ± 1.51) in comparison to grade 2 (3.97 ± 1.45) and grade 1 (2.92 ± 1.06) with a P value of 0.001. Triglyceride, cholesterol, low density lipoprotein, high-density lipoprotein, glucose and ALT levels were not significantly different in these groups[78].
Best et al[79] published a case control study on German centers in 2020 describing the relationship between HCC and NASH using AFP, AFP-L3, DCP and the GALAD score for assessment. 125 NASH patients with HCC and 231 NASH control patients without HCC were taken. The GALAD score recognized patients with HCC at any stage with an AUC of 0.96 which was superior to AUC of AFP (AUC 0.88), AFP-L3 (AUC 0.86) and DCP (AUC 0.87) levels with P < 0.001 at a cut off about -0.63. The sensitivity was 84.8% and specificity was 95.2% at this cut off. They also conducted Japanese cohort study on 392 patients with NAFLD out of which 28 patients developed HCC. The mean GALAD score in these 28 patients predicted occurrence of HCC 1.5 years before its diagnosis. GALAD score was considerably higher in these patients compared to NASH patients who did not develop HCC[79].
EARLY VS ADVANCED LIVER CIRRHOSIS WITH DECOMPENSATION
Arrieta et al[80] reported that AFP ≥ 200 and 400 ng/mL had a sensitivity of 36.3% and 20.2% in determining HCC with 100% specificity. Improved method of analysis using progressive elevation of AFP ≥ 7 ng/mL/mo among cirrhotic patients increased the sensitivity to 71.4% with 100% specificity. This method was successful even when AFP values were below 200 ng/mL[80].
AFP can be elevated in patients with CHC or cirrhosis without any evidence of HCC[81]. A persistent AFP value of 17.8 ng/mL was noted to have a 98.6% specificity, 35% sensitivity and a positive predictive value of 97.7% in identifying patients with cirrhosis[82]. Sudden fluctuations in AFP levels in patients with cirrhosis can be indicative of hepatitis flare, deterioration of liver disease and development of HCC[83]. AFP has been applied routinely for HCC surveillance in cirrhotic patients. Harada et al[83] showed that 40% of cirrhotic patients had AFP levels > 20 mg/dL[83]. Manuc et al[84] reported in a study of 2068 CHC patients with cirrhosis, 30.1% without HCC had AFP > 15 ng/mL due to advanced age, severe liver injury with raised AST and ALT levels and low platelets[84].
In patients with cirrhosis, both the sensitivity and specificity of AFP for detection of early-stage HCC diagnosis can be enhanced by combining it with US. In a meta-analysis, the pooled sensitivity of AFP and US for any stage and early-stage HCC were 95% (83%-100%) and 60% (45%–74%) respectively, compared to only 72% (56%–86%) and 40% (22%–58%) for US only[85]. Surveillance with AFP and ultrasonography in cirrhotic patients having an annual risk of HCC > 0.4% is cost-effective[86].
There are many risk scores that have been developed to predict HCC development among patients with chronic hepatitis and cirrhosis. However, many of them have not been standardized or carefully validated in different patient populations. Ideally, HCC prediction models should be developed for treated and untreated patients taking into account the patient ethnicity, significant comorbid conditions and ideally the molecular biomarkers[87].
PERSISTENT AFP ELEVATION IN THE ABSENCE OF LIVER DISEASE
AFP is an important screening test for HCC and is also used in the diagnostic evaluation of other hepatic and non-hepatic conditions. Persistent AFP elevation has also been reported in patients without malignant or nonmalignant conditions. Hereditary persistence of AFP (HPAFP), a benign autosomal dominant disorder with no apparent disease or abnormality, should be considered as one of the differential diagnoses in patients with unclear etiology of persistent AFP elevation[88]. HPAFP is a rare condition with only 20 reported cases in the literature[89]. In contrast to malignant tumors with AFP usually > 500 ng/mL, the AFP concentration in HPAFP is mostly below 200 ng/mL, but levels up to 1500 ng/mL have been reported in some cases[88,90,91]. The molecular mechanism of HPAFP can differ in unrelated families. Specific heterozygous point mutations are frequently found in the promoter region of the AFP gene related to hepatocyte nuclear factor 1 (HNF1) binding sites[92,93]. These mutations usually result in an increased affinity for HNF1 and subsequently lead to increased AFP promoter activity and AFP gene transcription[94]. Two-point mutations have been identified; the upstream substitution of cytosine (C) with adenosine (A) at amino acid position of 55 (-55 C > A) in the proximal HNF-1 binding site and upstream mutation caused by the substitution of Guanine (G) by Adenosine (A) at position 119 (-119 G > A) in the distal HNF-1 binding site[95,96]. The first case of HPAFP was reported in 1984 by Greenberg et al[91] in a Scottish family where a 38-year-old woman was noted to have persistently elevated AFP during post-partum[91]. The AFP concentration of her amniotic fluid was normal and HPAFP was later confirmed by evaluating serum AFP levels and molecular testing in family members. In another report of 20 HPAFP cases, 2 patients underwent unnecessary surgery and 3 had unnecessary chemotherapy due to their persistently elevated AFP[96]. Though rare, hereditary causes should be considered in patients with unexplained and persistent AFP elevation. In a recent study by Jeon et al[89] on 4 Korean patients with persistently elevated AFP levels from 12.1 to 186.1 ng/mL for > 1 year, 1 patient was found to have a hereditary cause by pedigree analysis even though the typical mutation of the AFP gene in the promoter region was absent. This case elucidated the heterogeneous nature of persistent AFP elevation and HPAFP is not always the result of mutation in the AFP transcription regulatory regions[89].
CONCLUSION
AFP has been used for HCC surveillance widely in clinical practice. It, however, has limited sensitivity and specificity for HCC detection, and a proportion of patients with advanced HCC do not have AFP secretion. Moreover, patients with chronic liver diseases, especially those with cirrhosis are commonly identified with persistent AFP elevation without radiological evidence of HCC. For all these reasons, AFP is not recommended to be used as a sole marker alone for HCC surveillance. The diagnostic potential of AFP for early diagnosis of HCC can be enhanced by combining it with other novel diagnostic markers such as AFP-L3, PIVKA-II, and GP73. Although many have already endorsed diagnostic value, large number of multicenter studies encompassing larger cohorts and long-term assessment are required to confirm clinical utility. Additionally, algorithms integrating these serum markers with noninvasive diagnostic imaging modalities are needed to be developed for the early and accurate diagnosis of HCC.
Footnotes
Conflict-of-interest statement: There is no conflict of interest.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: October 4, 2021
First decision: October 20, 2021
Article in press: December 31, 2021
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: El-Sokkary AM, Song L S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
Figure
Figure 1 ELISA measures. A: ELISA measures alpha-fetoprotein (AFP) by detecting reactivity with two anti-AFP antibodies; B: Interfering antibodies that bind directly to the capture and detect reagents without the target AFP (no analytes). This binding would emit light and become luminescent causing false positive results and high values; C: Interfering antibodies can inhibit reactivity of the ELISA by binding to the reagents and prevent the detection of AFP by the anti-AFP antibodies; that leads to a false negative result. AFP: Alpha-fetoprotein.
Contributor Information
Hira Hanif, Liver Center, Department of Medicine, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA 02215, United States.
Mukarram Jamat Ali, Liver Center, Department of Medicine, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA 02215, United States.
Ammu T Susheela, Internal Medicine, Loyola MacNeal Hospital, Berwyn, PA 60402, United States.
Iman Waheed Khan, Liver Center, Department of Medicine, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA 02215, United States.
Maria Alejandra Luna-Cuadros, Liver Center, Department of Medicine, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA 02215, United States.
Muzammil Muhammad Khan, Liver Center, Department of Medicine, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA 02215, United States.
Daryl Tan-Yeung Lau, Liver Center, Department of Medicine, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA 02215, United States. dlau@bidmc.harvard.edu.
References
- 1.Gitlin D, Perricelli A, Gitlin GM. Synthesis of -fetoprotein by liver, yolk sac, and gastrointestinal tract of the human conceptus. Cancer Res. 1972;32:979–982. [PubMed] [Google Scholar]
- 2.Yachnin S. The clinical significance of human alpha-fetoprotein. Ann Clin Lab Sci. 1978;8:84–90. [PubMed] [Google Scholar]
- 3.Masseyeff R, Gilli J, Krebs B, Calluaud A, Bonet C. Evolution of alpha-fetoprotein serum levels throughout life in humans and rats, and during pregnancy in the rat. Ann N Y Acad Sci. 1975;259:17–28. doi: 10.1111/j.1749-6632.1975.tb25398.x. [DOI] [PubMed] [Google Scholar]
- 4.Ball D, Rose E, Alpert E. Alpha-fetoprotein levels in normal adults. Am J Med Sci. 1992;303:157–159. doi: 10.1097/00000441-199203000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Thompson Coon J, Rogers G, Hewson P, Wright D, Anderson R, Cramp M, Jackson S, Ryder S, Price A, Stein K. Surveillance of cirrhosis for hepatocellular carcinoma: systematic review and economic analysis. Health Technol Assess. 2007;11:1–206. doi: 10.3310/hta11340. [DOI] [PubMed] [Google Scholar]
- 6.Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723–750. doi: 10.1002/hep.29913. [DOI] [PubMed] [Google Scholar]
- 7.Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, Tateishi R, Han KH, Chawla YK, Shiina S, Jafri W, Payawal DA, Ohki T, Ogasawara S, Chen PJ, Lesmana CRA, Lesmana LA, Gani RA, Obi S, Dokmeci AK, Sarin SK. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11:317–370. doi: 10.1007/s12072-017-9799-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim DY, Han KH. Epidemiology and surveillance of hepatocellular carcinoma. Liver Cancer. 2012;1:2–14. doi: 10.1159/000339016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Terentiev AA, Moldogazieva NT. Alpha-fetoprotein: a renaissance. Tumour Biol. 2013;34:2075–2091. doi: 10.1007/s13277-013-0904-y. [DOI] [PubMed] [Google Scholar]
- 10.Ruoslahti E, Terry WD. alpha foetoprotein and serum albumin show sequence homology. Nature. 1976;260:804–805. doi: 10.1038/260804a0. [DOI] [PubMed] [Google Scholar]
- 11.Crandall BF. Alpha-fetoprotein: a review. Crit Rev Clin Lab Sci. 1981;15:127–185. doi: 10.3109/10408368109105870. [DOI] [PubMed] [Google Scholar]
- 12.Murray MJ, Nicholson JC. α-Fetoprotein. Arch Dis Child Educ Pract Ed. 2011;96:141–147. doi: 10.1136/adc.2011.213181. [DOI] [PubMed] [Google Scholar]
- 13.Bloomer JR, Waldmann TA, McIntire KR, Klatskin G. alpha-fetoprotein in noneoplastic hepatic disorders. JAMA. 1975;233:38–41. doi: 10.1001/jama.233.1.38. [DOI] [PubMed] [Google Scholar]
- 14.Shen WF, Zhong W, Xu F, Kan T, Geng L, Xie F, Sui CJ, Yang JM. Clinicopathological and prognostic analysis of 429 patients with intrahepatic cholangiocarcinoma. World J Gastroenterol. 2009;15:5976–5982. doi: 10.3748/wjg.15.5976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schneider DT, Calaminus G, Göbel U. Diagnostic value of alpha 1-fetoprotein and beta-human chorionic gonadotropin in infancy and childhood. Pediatr Hematol Oncol. 2001;18:11–26. doi: 10.1080/088800101750059828. [DOI] [PubMed] [Google Scholar]
- 16.Sauzay C, Petit A, Bourgeois AM, Barbare JC, Chauffert B, Galmiche A, Houessinon A. Alpha-foetoprotein (AFP): A multi-purpose marker in hepatocellular carcinoma. Clin Chim Acta. 2016;463:39–44. doi: 10.1016/j.cca.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 17.Wang X, Wang Q. Alpha-Fetoprotein and Hepatocellular Carcinoma Immunity. Can J Gastroenterol Hepatol. 2018;2018:9049252. doi: 10.1155/2018/9049252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Law SW, Dugaiczyk A. Homology between the primary structure of alpha-fetoprotein, deduced from a complete cDNA sequence, and serum albumin. Nature. 1981;291:201–205. doi: 10.1038/291201a0. [DOI] [PubMed] [Google Scholar]
- 19.Nishizono I, Iida S, Suzuki N, Kawada H, Murakami H, Ashihara Y, Okada M. Rapid and sensitive chemiluminescent enzyme immunoassay for measuring tumor markers. Clin Chem. 1991;37:1639–1644. [PubMed] [Google Scholar]
- 20.Shah PA, Onken A, Ishtiaq R, Maqsood MH, Patel SS, Campoverde Reyes KJ, Herskovits AZ, Lau DTY. A challenging case of alpha-fetoprotein-result discrepancies in a patient with chronic hepatitis B. Gastroenterol Rep (Oxf) 2020;8:484–486. doi: 10.1093/gastro/goaa052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abelev GI, Perova SD, Khramkova NI, Postnikova ZA, Irlin IS. Production of embryonal alpha-globulin by transplantable mouse hepatomas. Transplantation. 1963;1:174–180. doi: 10.1097/00007890-196301020-00004. [DOI] [PubMed] [Google Scholar]
- 22.Gupta S, Bent S, Kohlwes J. Test characteristics of alpha-fetoprotein for detecting hepatocellular carcinoma in patients with hepatitis C. A systematic review and critical analysis. Ann Intern Med. 2003;139:46–50. doi: 10.7326/0003-4819-139-1-200307010-00012. [DOI] [PubMed] [Google Scholar]
- 23.Gambarin-Gelwan M, Wolf DC, Shapiro R, Schwartz ME, Min AD. Sensitivity of commonly available screening tests in detecting hepatocellular carcinoma in cirrhotic patients undergoing liver transplantation. Am J Gastroenterol. 2000;95:1535–1538. doi: 10.1111/j.1572-0241.2000.02091.x. [DOI] [PubMed] [Google Scholar]
- 24.Saffroy R, Pham P, Reffas M, Takka M, Lemoine A, Debuire B. New perspectives and strategy research biomarkers for hepatocellular carcinoma. Clin Chem Lab Med. 2007;45:1169–1179. doi: 10.1515/CCLM.2007.262. [DOI] [PubMed] [Google Scholar]
- 25.Bertino G, Ardiri A, Malaguarnera M, Malaguarnera G, Bertino N, Calvagno GS. Hepatocellualar carcinoma serum markers. Semin Oncol. 2012;39:410–433. doi: 10.1053/j.seminoncol.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 26.Luo P, Wu S, Yu Y, Ming X, Li S, Zuo X, Tu J. Current Status and Perspective Biomarkers in AFP Negative HCC: Towards Screening for and Diagnosing Hepatocellular Carcinoma at an Earlier Stage. Pathol Oncol Res. 2020;26:599–603. doi: 10.1007/s12253-019-00585-5. [DOI] [PubMed] [Google Scholar]
- 27.Sonbol MB, Riaz IB, Naqvi SAA, Almquist DR, Mina S, Almasri J, Shah S, Almader-Douglas D, Uson Junior PLS, Mahipal A, Ma WW, Jin Z, Mody K, Starr J, Borad MJ, Ahn DH, Murad MH, Bekaii-Saab T. Systemic Therapy and Sequencing Options in Advanced Hepatocellular Carcinoma: A Systematic Review and Network Meta-analysis. JAMA Oncol. 2020;6:e204930. doi: 10.1001/jamaoncol.2020.4930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Breborowicz J. Microheterogeneity of human alphafetoprotein. Tumour Biol. 1988;9:3–14. doi: 10.1159/000217540. [DOI] [PubMed] [Google Scholar]
- 29.Nomura F, Ohnishi K, Tanabe Y. Clinical features and prognosis of hepatocellular carcinoma with reference to serum alpha-fetoprotein levels. Analysis of 606 patients. Cancer. 1989;64:1700–1707. doi: 10.1002/1097-0142(19891015)64:8<1700::aid-cncr2820640824>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 30.Agopian VG, Harlander-Locke MP, Markovic D, Zarrinpar A, Kaldas FM, Cheng EY, Yersiz H, Farmer DG, Hiatt JR, Busuttil RW. Evaluation of Patients With Hepatocellular Carcinomas That Do Not Produce α-Fetoprotein. JAMA Surg. 2017;152:55–64. doi: 10.1001/jamasurg.2016.3310. [DOI] [PubMed] [Google Scholar]
- 31.Carr BI, Akkiz H, Üsküdar O, Yalçın K, Guerra V, Kuran S, Karaoğullarından Ü, Altıntaş E, Özakyol A, Tokmak S, Ballı T, Yücesoy M, Bahçeci Hİ, Ülkü A, Akçam T, Polat KY, Ekinci N, Şimşek H, Örmeci N, Sonsuz A, Demir M, Kılıç M, Uygun A, Demir A, Delik A, Arslan B, Doran F, Yilmaz S, Tokat Y. HCC with low- and normal-serum alpha-fetoprotein levels. Clin Pract (Lond) 2018;15:453–464. doi: 10.4172/clinical-practice.1000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taketa K, Sekiya C, Namiki M, Akamatsu K, Ohta Y, Endo Y, Kosaka K. Lectin-reactive profiles of alpha-fetoprotein characterizing hepatocellular carcinoma and related conditions. Gastroenterology. 1990;99:508–518. doi: 10.1016/0016-5085(90)91034-4. [DOI] [PubMed] [Google Scholar]
- 33.Zhou H, Wang H, Zhou D, Wang Q, Zou S, Tu Q, Wu M, Hu H. Hepatitis B virus-associated intrahepatic cholangiocarcinoma and hepatocellular carcinoma may hold common disease process for carcinogenesis. Eur J Cancer. 2010;46:1056–1061. doi: 10.1016/j.ejca.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 34.AlSalloom AA. An update of biochemical markers of hepatocellular carcinoma. Int J Health Sci (Qassim) 2016;10:121–136. [PMC free article] [PubMed] [Google Scholar]
- 35.Leerapun A, Suravarapu SV, Bida JP, Clark RJ, Sanders EL, Mettler TA, Stadheim LM, Aderca I, Moser CD, Nagorney DM, LaRusso NF, de Groen PC, Menon KV, Lazaridis KN, Gores GJ, Charlton MR, Roberts RO, Therneau TM, Katzmann JA, Roberts LR. The utility of Lens culinaris agglutinin-reactive alpha-fetoprotein in the diagnosis of hepatocellular carcinoma: evaluation in a United States referral population. Clin Gastroenterol Hepatol. 2007;5:394–402; quiz 267. doi: 10.1016/j.cgh.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sterling RK, Jeffers L, Gordon F, Venook AP, Reddy KR, Satomura S, Kanke F, Schwartz ME, Sherman M. Utility of Lens culinaris agglutinin-reactive fraction of alpha-fetoprotein and des-gamma-carboxy prothrombin, alone or in combination, as biomarkers for hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2009;7:104–113. doi: 10.1016/j.cgh.2008.08.041. [DOI] [PubMed] [Google Scholar]
- 37.Ibrahim AA, Allah RM, El Hammady AM, Sarhan RS, Khalil MA, Rahman MM. Diagnostic accuracy of lectin-reactive α-fetoprotein (AFP-L3) in the diagnosis of hepatitis C virus-related hepatocellular carcinoma. Benha Medical J. 2018;35:312. [Google Scholar]
- 38.Cerban R, Ester C, Iacob S, Ghioca M, Paslaru L, Dumitru R, Grasu M, Constantin G, Popescu I, Gheorghe L. Alpha-fetoprotein, alpha-fetoprotein-L3, protein induced by vitamin K absence, glypican 3 and its combinations for diagnosis of hepatocellular carcinoma. Surg Gastroenterol. 2019;24:37–44. [Google Scholar]
- 39.Ibrahim HM, Elghannam MZ, Elkhawaga OAY, El-Sokkary AMA. Evaluation of serum alpha fetoprotein-L3 as an accuracy novel biomarker for the early diagnosis of hepatocellular carcinoma in Egyptian patients. Saudi J Biol Sci. 2021;28:5760–5764. doi: 10.1016/j.sjbs.2021.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oka H, Saito A, Ito K, Kumada T, Satomura S, Kasugai H, Osaki Y, Seki T, Kudo M, Tanaka M Collaborative Hepato-Oncology Study Group of Japan. Multicenter prospective analysis of newly diagnosed hepatocellular carcinoma with respect to the percentage of Lens culinaris agglutinin-reactive alpha-fetoprotein. J Gastroenterol Hepatol. 2001;16:1378–1383. doi: 10.1046/j.1440-1746.2001.02643.x. [DOI] [PubMed] [Google Scholar]
- 41.Toyoda H, Kumada T, Tada T, Sone Y, Kaneoka Y, Maeda A. Tumor Markers for Hepatocellular Carcinoma: Simple and Significant Predictors of Outcome in Patients with HCC. Liver Cancer. 2015;4:126–136. doi: 10.1159/000367735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu R, Tan Z, Xiang X, Dan Y, Deng G. Effectiveness of PIVKA-II in the detection of hepatocellular carcinoma based on real-world clinical data. BMC Cancer. 2017;17:608. doi: 10.1186/s12885-017-3609-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang X, Zhang Y, Yang N, He H, Tao X, Kou C, Jiang J. Evaluation of the Combined Application of AFP, AFP-L3%, and DCP for Hepatocellular Carcinoma Diagnosis: A Meta-analysis. Biomed Res Int. 2020;2020:5087643. doi: 10.1155/2020/5087643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lim TS, Kim DY, Han KH, Kim HS, Shin SH, Jung KS, Kim BK, Kim SU, Park JY, Ahn SH. Combined use of AFP, PIVKA-II, and AFP-L3 as tumor markers enhances diagnostic accuracy for hepatocellular carcinoma in cirrhotic patients. Scand J Gastroenterol. 2016;51:344–353. doi: 10.3109/00365521.2015.1082190. [DOI] [PubMed] [Google Scholar]
- 45.Caviglia GP, Abate ML, Petrini E, Gaia S, Rizzetto M, Smedile A. Highly sensitive alpha-fetoprotein, Lens culinaris agglutinin-reactive fraction of alpha-fetoprotein and des-gamma-carboxyprothrombin for hepatocellular carcinoma detection. Hepatol Res. 2016;46:E130–E135. doi: 10.1111/hepr.12544. [DOI] [PubMed] [Google Scholar]
- 46.Zhou L, Liu J, Luo F. Serum tumor markers for detection of hepatocellular carcinoma. World J Gastroenterol. 2006;12:1175–1181. doi: 10.3748/wjg.v12.i8.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fang YS, Wu Q, Zhao HC, Zhou Y, Ye L, Liu SS, Li XX, Du WD. Do combined assays of serum AFP, AFP-L3, DCP, GP73, and DKK-1 efficiently improve the clinical values of biomarkers in decision-making for hepatocellular carcinoma? Expert Rev Gastroenterol Hepatol. 2021;15:1065–1076. doi: 10.1080/17474124.2021.1900731. [DOI] [PubMed] [Google Scholar]
- 48.Kim SS, Baek GO, Ahn HR, Sung S, Seo CW, Cho HJ, Nam SW, Cheong JY, Eun JW. Serum small extracellular vesicle-derived LINC00853 as a novel diagnostic marker for early hepatocellular carcinoma. Mol Oncol. 2020;14:2646–2659. doi: 10.1002/1878-0261.12745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu J, Han J, Zhang J, Li G, Liu H, Cui X, Xu Y, Li T, Liu J, Wang C. The long noncoding RNAs PVT1 and uc002mbe.2 in sera provide a new supplementary method for hepatocellular carcinoma diagnosis. Medicine (Baltimore) 2016;95:e4436. doi: 10.1097/MD.0000000000004436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wong RJ, Ahmed A, Gish RG. Elevated alpha-fetoprotein: differential diagnosis - hepatocellular carcinoma and other disorders. Clin Liver Dis. 2015;19:309–323. doi: 10.1016/j.cld.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 51.Taketa K. Alpha-fetoprotein: reevaluation in hepatology. Hepatology. 1990;12:1420–1432. doi: 10.1002/hep.1840120625. [DOI] [PubMed] [Google Scholar]
- 52.Lok AS, Lai CL. alpha-Fetoprotein monitoring in Chinese patients with chronic hepatitis B virus infection: role in the early detection of hepatocellular carcinoma. Hepatology. 1989;9:110–115. doi: 10.1002/hep.1840090119. [DOI] [PubMed] [Google Scholar]
- 53.Liu YR, Lin BB, Zeng DW, Zhu YY, Chen J, Zheng Q, Dong J, Jiang JJ. Alpha-fetoprotein level as a biomarker of liver fibrosis status: a cross-sectional study of 619 consecutive patients with chronic hepatitis B. BMC Gastroenterol. 2014;14:145. doi: 10.1186/1471-230X-14-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liaw YF, Tai DI, Chen TJ, Chu CM, Huang MJ. Alpha-fetoprotein changes in the course of chronic hepatitis: relation to bridging hepatic necrosis and hepatocellular carcinoma. Liver. 1986;6:133–137. doi: 10.1111/j.1600-0676.1986.tb00279.x. [DOI] [PubMed] [Google Scholar]
- 55.Pongpipat D, Suvatte V, Tuchinda M, Assateerawatt A. Occurrence of alpha 1-fetoprotein in acute viral hepatitis in children. Am J Dis Child. 1979;133:551–552. doi: 10.1001/archpedi.1979.02130050095020. [DOI] [PubMed] [Google Scholar]
- 56.Kew MC, Purves LR, Bersohn I. Serum alpha-fetoprotein levels in acute viral hepatitis. Gut. 1973;14:939–942. doi: 10.1136/gut.14.12.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Silver HK, Deneault J, Gold P, Thompson WG, Shuster J, Freedman SO. The detection of alpha 1-fetoprotein in patients with viral hepatitis. Cancer Res. 1974;34:244–247. [PubMed] [Google Scholar]
- 58.Seo SI, Kim SS, Choi BY, Lee SH, Kim SJ, Park HW, Kim HS, Shin WG, Kim KH, Lee JH, Kim HY, Jang MK. Clinical significance of elevated serum alpha-fetoprotein (AFP) level in acute viral hepatitis A (AHA) Hepatogastroenterology. 2013;60:1592–1596. [PubMed] [Google Scholar]
- 59.Song G, Rao H, Feng B, Wei L. Prediction of spontaneous HBeAg seroconversion in HBeAg-positive chronic hepatitis B patients during the immune clearance phase. J Med Virol. 2014;86:1838–1844. doi: 10.1002/jmv.24032. [DOI] [PubMed] [Google Scholar]
- 60.Liaw YF, Chu CM, Huang MJ, Sheen IS, Yang CY, Lin DY. Determinants for hepatitis B e antigen clearance in chronic type B hepatitis. Liver. 1984;4:301–306. doi: 10.1111/j.1600-0676.1984.tb00942.x. [DOI] [PubMed] [Google Scholar]
- 61.Kim CY, Kim BR, Lee SS, Jeon DH, Lee CM, Kim WS, Cho HC, Kim JJ, Lee JM, Kim HJ, Ha CY, Kim TH, Jung WT, Lee OJ. Clinical features of hepatitis B and C virus infections, with high α-fetoprotein levels but not hepatocellular carcinoma. Medicine (Baltimore) 2017;96:e5844. doi: 10.1097/MD.0000000000005844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yuan G, Zhou Y, Liu J, Hu C, Huang H, Ren Y, Yu W, Guo Y, Zhang YY. AFP specificity for HCC surveillance is increased by mitigating liver injury among treated chronic hepatitis B patients with elevated AFP. Int J Clin Exp Pathol. 2019;12:1315–1323. [PMC free article] [PubMed] [Google Scholar]
- 63.Peng C, Li Z, Xie Z, Wang Z, Ye Y, Li B, Li W. The role of circulating microRNAs for the diagnosis of hepatitis B virus-associated hepatocellular carcinoma with low alpha-fetoprotein level: a systematic review and meta-analysis. BMC Gastroenterol. 2020;20:249. doi: 10.1186/s12876-020-01345-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shen Q, Fan J, Yang XR, Tan Y, Zhao W, Xu Y, Wang N, Niu Y, Wu Z, Zhou J, Qiu SJ, Shi YH, Yu B, Tang N, Chu W, Wang M, Wu J, Zhang Z, Yang S, Gu J, Wang H, Qin W. Serum DKK1 as a protein biomarker for the diagnosis of hepatocellular carcinoma: a large-scale, multicentre study. Lancet Oncol. 2012;13:817–826. doi: 10.1016/S1470-2045(12)70233-4. [DOI] [PubMed] [Google Scholar]
- 65.Inoue T, Tanaka Y. Novel biomarkers for the management of chronic hepatitis B. Clin Mol Hepatol. 2020;26:261–279. doi: 10.3350/cmh.2020.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ekpanyapong S, Reddy KR. Hepatitis B Virus Reactivation: What Is the Issue, and How Should It Be Managed? Clin Liver Dis. 2020;24:317–333. doi: 10.1016/j.cld.2020.04.002. [DOI] [PubMed] [Google Scholar]
- 67.Chang ML, Liaw YF. Hepatitis B flares in chronic hepatitis B: pathogenesis, natural course, and management. J Hepatol. 2014;61:1407–1417. doi: 10.1016/j.jhep.2014.08.033. [DOI] [PubMed] [Google Scholar]
- 68.Chu CW, Hwang SJ, Luo JC, Lai CR, Tsay SH, Li CP, Wu JC, Chang FY, Lee SD. Clinical, virologic, and pathologic significance of elevated serum alpha-fetoprotein levels in patients with chronic hepatitis C. J Clin Gastroenterol. 2001;32:240–244. doi: 10.1097/00004836-200103000-00014. [DOI] [PubMed] [Google Scholar]
- 69.Chen TM, Huang PT, Tsai MH, Lin LF, Liu CC, Ho KS, Siauw CP, Chao PL, Tung JN. Predictors of alpha-fetoprotein elevation in patients with chronic hepatitis C, but not hepatocellular carcinoma, and its normalization after pegylated interferon alfa 2a-ribavirin combination therapy. J Gastroenterol Hepatol. 2007;22:669–675. doi: 10.1111/j.1440-1746.2007.04898.x. [DOI] [PubMed] [Google Scholar]
- 70.Yang N, Li Z, Yan M, Xiao W, Zhang W, Long Y, Cheng Y, Ming K, Xu B. Evaluation of Serum Alpha-Fetoprotein Level in Chronic Hepatitis C Patients. Clin Lab. 2019;65 doi: 10.7754/Clin.Lab.2018.180607. [DOI] [PubMed] [Google Scholar]
- 71.Di Bisceglie AM, Sterling RK, Chung RT, Everhart JE, Dienstag JL, Bonkovsky HL, Wright EC, Everson GT, Lindsay KL, Lok AS, Lee WM, Morgan TR, Ghany MG, Gretch DR HALT-C Trial Group. Serum alpha-fetoprotein levels in patients with advanced hepatitis C: results from the HALT-C Trial. J Hepatol. 2005;43:434–441. doi: 10.1016/j.jhep.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 72.Fouad R, Elsharkawy A, Abdel Alem S, El Kassas M, Alboraie M, Sweedy A, Afify S, Abdellatif Z, Khairy M, Esmat G. Clinical impact of serum α-fetoprotein and its relation on changes in liver fibrosis in hepatitis C virus patients receiving direct-acting antivirals. Eur J Gastroenterol Hepatol. 2019;31:1129–1134. doi: 10.1097/MEG.0000000000001400. [DOI] [PubMed] [Google Scholar]
- 73.Noritake H, Kobayashi Y, Ooba Y, Kitsugi K, Shimoyama S, Yamazaki S, Chida T, Watanabe S, Kawata K, Suda T. Improved Serum Alpha-Fetoprotein Levels after Iron Reduction Therapy in HCV Patients. ISRN Hepatol. 2014;2014:875140. doi: 10.1155/2014/875140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pennisi G, Celsa C, Giammanco A, Spatola F, Petta S. The Burden of Hepatocellular Carcinoma in Non-Alcoholic Fatty Liver Disease: Screening Issue and Future Perspectives. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20225613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Singal AG, Lampertico P, Nahon P. Epidemiology and surveillance for hepatocellular carcinoma: New trends. J Hepatol. 2020;72:250–261. doi: 10.1016/j.jhep.2019.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lim J, Singal AG. Surveillance and Diagnosis of Hepatocellular Carcinoma. Clin Liver Dis (Hoboken) 2019;13:2–5. doi: 10.1002/cld.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sumida Y, Yoneda M, Seko Y, Ishiba H, Hara T, Toyoda H, Yasuda S, Kumada T, Hayashi H, Kobayashi T, Imajo K, Tada T, Kawaguchi T, Eguchi Y, Oeda S, Takahashi H, Tomita E, Okanoue T, Nakajima A Japan Study Group Of Nafld Jsg-Nafld. Surveillance of Hepatocellular Carcinoma in Nonalcoholic Fatty Liver Disease. Diagnostics (Basel) 2020;10 doi: 10.3390/diagnostics10080579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Babalı A, Cakal E, Purnak T, Bıyıkoğlu I, Cakal B, Yüksel O, Köklü S. Serum α-fetoprotein levels in liver steatosis. Hepatol Int. 2009;3:551–555. doi: 10.1007/s12072-009-9156-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Best J, Bechmann LP, Sowa JP, Sydor S, Dechêne A, Pflanz K, Bedreli S, Schotten C, Geier A, Berg T, Fischer J, Vogel A, Bantel H, Weinmann A, Schattenberg JM, Huber Y, Wege H, von Felden J, Schulze K, Bettinger D, Thimme R, Sinner F, Schütte K, Weiss KH, Toyoda H, Yasuda S, Kumada T, Berhane S, Wichert M, Heider D, Gerken G, Johnson P, Canbay A. GALAD Score Detects Early Hepatocellular Carcinoma in an International Cohort of Patients With Nonalcoholic Steatohepatitis. Clin Gastroenterol Hepatol. 2020;18:728–735.e4. doi: 10.1016/j.cgh.2019.11.012. [DOI] [PubMed] [Google Scholar]
- 80.Arrieta O, Cacho B, Morales-Espinosa D, Ruelas-Villavicencio A, Flores-Estrada D, Hernández-Pedro N. The progressive elevation of alpha fetoprotein for the diagnosis of hepatocellular carcinoma in patients with liver cirrhosis. BMC Cancer. 2007;7:28. doi: 10.1186/1471-2407-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kobeisy MA, Morsy KH, Galal M, Sayed SK, Ashmawy MM, Mohammad FM. Clinical significance of elevated alpha-foetoprotein (AFP) in patients with chronic hepatitis C without hepatocellular carcinoma in upper EGYPT. Arab J Gastroenterol. 2012;13:49–53. doi: 10.1016/j.ajg.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 82.Bayati N, Silverman AL, Gordon SC. Serum alpha-fetoprotein levels and liver histology in patients with chronic hepatitis C. Am J Gastroenterol. 1998;93:2452–2456. doi: 10.1111/j.1572-0241.1998.00703.x. [DOI] [PubMed] [Google Scholar]
- 83.Harada T, Shigeta K, Noda K, Fukumoto Y, Nishimura H, Mizuta M, Takemoto T. Clinical implications of alpha-fetoprotein in liver cirrhosis: five-year follow-up study. Hepatogastroenterology. 1980;27:169–175. [PubMed] [Google Scholar]
- 84.Manuc D, Preda CM, Sandra I, Baicus C, Cerban R, Constantinescu I, Olteanu AO, Ciora CA, Manuc T, Chiriac DE, Chifulescu AE, Diculescu M, Tieranu C, Negreanu L, Oprea-Calin G, Manuc M. Signification of Serum Alpha-Fetoprotein Levels in Cases of Compensated Cirrhosis and Hepatitis C Virus without Hepatocellular Carcinoma. J Med Life. 2020;13:68–74. doi: 10.25122/jml-2019-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tzartzeva K, Obi J, Rich NE, Parikh ND, Marrero JA, Yopp A, Waljee AK, Singal AG. Surveillance Imaging and Alpha Fetoprotein for Early Detection of Hepatocellular Carcinoma in Patients With Cirrhosis: A Meta-analysis. Gastroenterology. 2018;154:1706–1718.e1. doi: 10.1053/j.gastro.2018.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Parikh ND, Singal AG, Hutton DW, Tapper EB. Cost-Effectiveness of Hepatocellular Carcinoma Surveillance: An Assessment of Benefits and Harms. Am J Gastroenterol. 2020;115:1642–1649. doi: 10.14309/ajg.0000000000000715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kubota N, Fujiwara N, Hoshida Y. Clinical and Molecular Prediction of Hepatocellular Carcinoma Risk. J Clin Med. 2020;9 doi: 10.3390/jcm9123843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li X, Alexander S. Hereditary persistence of alpha-fetoprotein. Pediatr Blood Cancer. 2009;52:403–405. doi: 10.1002/pbc.21816. [DOI] [PubMed] [Google Scholar]
- 89.Jeon Y, Choi YS, Jang ES, Kim JW, Jeong SH. Persistent α-Fetoprotein Elevation in Healthy Adults and Mutational Analysis of α-Fetoprotein Promoter, Enhancer, and Silencer Regions. Gut Liver. 2017;11:136–141. doi: 10.5009/gnl16069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schefer H, Mattmann S, Joss RA. Hereditary persistence of alpha-fetoprotein. Case report and review of the literature. Ann Oncol. 1998;9:667–672. doi: 10.1023/a:1008243311122. [DOI] [PubMed] [Google Scholar]
- 91.Greenberg F, Rose E, Alpert E. Hereditary persistence of alpha-fetoprotein. Gastroenterology. 1990;98:1083–1085. doi: 10.1016/0016-5085(90)90037-2. [DOI] [PubMed] [Google Scholar]
- 92.Alj Y, Milgrom E, Guiochon-Mantel A. Rapid determination of alpha-fetoprotein gene promoter mutations in hereditary persistence of alpha-fetoprotein. Clin Chem. 2004;50:1706–1707. doi: 10.1373/clinchem.2004.034157. [DOI] [PubMed] [Google Scholar]
- 93.Blesa JR, Vidal J, Giner-Durán R, Lacalle ML, Catalán I, Hernández-Yago J. Detection of hereditary persistence of alpha-fetoprotein by conformation-sensitive gel electrophoresis analysis. Clin Chem. 2003;49:2102–2103. doi: 10.1373/clinchem.2003.024232. [DOI] [PubMed] [Google Scholar]
- 94.Bonfig W, Hempel M, Teichert-von Lüttichau I, Liptay S, Burdach S. Avoiding harmful procedures in patients with elevated α-fetoprotein concentrations: hereditary persistence of α-fetoprotein is an important and benign differential diagnosis! J Pediatr Hematol Oncol. 2012;34:e301–e303. doi: 10.1097/MPH.0b013e3182441256. [DOI] [PubMed] [Google Scholar]
- 95.McVey JH, Michaelides K, Hansen LP, Ferguson-Smith M, Tilghman S, Krumlauf R, Tuddenham EG. A G-->A substitution in an HNF I binding site in the human alpha-fetoprotein gene is associated with hereditary persistence of alpha-fetoprotein (HPAFP) Hum Mol Genet. 1993;2:379–384. doi: 10.1093/hmg/2.4.379. [DOI] [PubMed] [Google Scholar]
- 96.Houwert AC, Giltay JC, Lentjes EG, Lock MT. Hereditary persistence of alpha-fetoprotein (HPAF P): review of the literature. Neth J Med. 2010;68:354–358. [PubMed] [Google Scholar]