Abstract
Background:
Patients with head and neck cancer (HNC) treated with radiation therapy (RT) are at risk for jaw osteoradionecrosis (ORN) which is largely characterized by presence of exposed necrotic bone. This report describes the incidence and clinical course of and risk factors for exposed intraoral bone in the multicenter Observational Study of Dental Outcomes in Head and Neck Cancer Patients (OraRad) cohort.
Methods:
Participants were evaluated before RT and at 6, 12, 18 and 24 months after RT. Exposed bone was characterized by location, sequestrum formation, and other associated features. Radiation dose to the affected area was determined and history of treatment for exposed bone was recorded.
Results:
The study enrolled 572 participants; 35 (6.1%) were diagnosed with incident exposed bone at 6 (47% of reports), 12 (24%), 18 (20%), and 24 (8%) months, with 60% being sequestrum and 7 cases (20%) persisting for >6 months. The average maximum RT dose to the affected area of exposed bone was 5456 cGy (SD 1768); the most frequent associated primary RT sites were the oropharynx (42.9%) and oral cavity (31.4%); 76% of episodes occurred in the mandible. ORN diagnosis was confirmed in 18 participants, an incidence rate of 3.1% (18/572). Risk factors included pre-RT extractions (p=0.008), higher RT dose (p=0.039) and tobacco use (p=0.048).
Conclusions:
The two-year incidence of exposed bone in the OraRad cohort was 6.1%; the incidence of confirmed ORN was 3.1%. Exposed bone after RT for HNC is relatively uncommon and in most cases a short-term, not recurring or persistent complication.
Keywords: Head and neck cancer, radiation therapy, osteonecrosis, osteoradionecrosis, jaw osteonecrosis
Precis for use in the Table of Contents
The two-year incidence of exposed bone in the OraRad cohort was 6.1% and the incidence of confirmed cases of jaw osteoradionecrosis was 3.3%. Exposed bone after radiation therapy for head and neck cancer is relatively uncommon and in most cases a short-term, not recurring or persistent complication.
Introduction
Head and neck cancer (HNC) is the sixth most common malignancy in the United States, affecting over 50,000 individuals and accounting for over 10,000 deaths annually(1, 2). Primary or adjuvant radiation therapy (RT), frequently delivered with concurrent chemotherapy, is a mainstay of HNC management protocols(3). Despite advances in RT treatment planning and delivery, acute and late toxicities are common and associated with significant morbidity(4). The oral cavity is at particularly high risk for complications including mucositis, salivary gland dysfunction, rampant dental decay, taste changes, trismus, and osteoradionecrosis (ORN), which negatively affect oral-health-related quality of life both during and after treatment(5, 6).
Jaw ORN is an infrequent but potentially debilitating condition characterized by exposed necrotic bone, secondary infection, tooth loss, pain, disability, and in advanced cases, orocutaneous fistula formation and risk of pathologic mandibular fracture(7). Despite being well-characterized clinically, limited data is available on the natural course and risk factors associated with development of ORN(8). The Observational Study of Dental Outcomes in Head and Neck Cancer Patients (OraRad, NCT02057510) prospectively followed patients with HNC from before RT initiation through two years after therapy initiation(9). This report characterizes the incidence, clinical course, and risk factors for development of exposed intraoral bone after completing RT in the multicenter OraRad cohort.
Methods
Study cohort
This prospective cohort study enrolled 572 adult men and women at six enrollment centers throughout the eastern United States (Atrium Health’s Carolinas Medical Center, University of Connecticut, University of North Carolina, University of Pennsylvania, New York University, and Brigham and Women’s Hospital) with a diagnosis of head and neck squamous cell carcinoma, salivary gland cancer, non-squamous cell carcinoma, and non-salivary gland malignancies of the head and neck region(9). Participants were enrolled from February 2014 through November 2017. Participants enrolled in OraRad received primary or adjuvant external beam RT (at least 45 Gy to one intraoral site) with or without concomitant chemotherapy as clinically indicated(9). Participants were required to have at least one non-hopeless tooth (“hopeless” defined as non-restorable/non-salvageable and indicated for extraction) remaining or expected to remain in the mouth after completing any required pre-RT dental extractions. The study was approved by the Institutional Review Board of each center and written informed consent was obtained from every participant before participation in this observational study.
Clinical Assessments
Study participants were evaluated after any initial surgery and before beginning RT (baseline, V0), then examined at 6-month intervals for 24 months after the start of RT (V06, V12, V18, V24), with each visit consisting of self-reported assessments and a comprehensive oral and dental examination performed by a calibrated examiner(9). Medical records were reviewed throughout the study period to record cancer diagnosis, cancer therapies, pre- and post-RT dental treatments, and other related clinical details. Panoramic radiographs were obtained at baseline. Teeth were deemed “hopeless” if present at the time of examination but otherwise deemed non-restorable (e.g., extensive caries, severe mobility) and indicated for extraction.
Exposed bone was defined as an area of visibly exposed bone with loss of overlying mucosal soft tissue(9). When detected, the exposed bone’s location was characterized using the tooth numbers of the teeth nearest the exposed bone. When an area of exposed bone was observed over multiple visits, it was considered a single site of exposed bone even if there were slight changes in its location or dimensions. Exposed bone was considered to develop “spontaneously” in locations where there was no dental extraction or exfoliation or hopeless teeth at that site or at an adjacent tooth site, either at an earlier study visit (including pre-RT extractions) or at the same study visit. Other clinical features described included the presence of sequestrum formation (mobile fragment of exposed bone), orocutaneous fistula, associated tooth mobility in the field of exposed bone, swelling, induration and evidence of pathological fracture(9).
After an exposed bone report, additional RT data was obtained from medical records to determine the radiation dose to the affected area, including mean and maximum dose to right and left mandible, parotid gland, and maxilla, as well as maximum dose to the tooth/teeth of the affected area. Participants with a diagnosis of exposed bone were referred for evaluation and management if the diagnosis was not already established. Management provided was per institutional/practitioner standard of care and was not protocol-directed. Diagnosis and staging of ORN were performed according to the American Association of Oral and Maxillofacial Surgeons’ (AAOMS) classification system for medication-related jaw osteonecrosis (MRONJ) given the lack of a universally accepted classification system for ORN(10). Any history of treatment for exposed bone was recorded.
Statistical Considerations and Analysis
The OraRad Study’s sample size was chosen to give sufficient power for estimating the fraction of participants with tooth loss by the two-year followup. In particular, it was not powered for analyses related to exposed bone or ORN. Nonetheless, it provided enough power to detect several risk factors for exposed bone even when cases with sequestrum were excluded.
Comparisons between participants who had vs. did not have any reports of exposed bone, or between participants who had spontaneous vs. non-spontaneous exposed bone, used Fisher’s exact test for categorical measures (e.g., sex, primary RT site) and two-sample t-tests for measures on continuous scales (e.g., age, RT dose to primary site). We checked the t-tests using Wilcoxon’s rank-sum test for measures with skewed distributions, e.g., RT dose to primary site, which gave substantively identical results and are thus not reported. Associations of potential risk factors with having vs. not having any reports of exposed bone were estimated and tested using logistic regression; P-values are from the likelihood-ratio test, confidence intervals are Wald intervals. Time to the first study visit with an exposed-bone report was analyzed using the Kaplan-Meier method with confidence intervals by the likelihood method; this was done first counting as events all persons who had exposed bone reports and separately counting as events only those persons who were later confirmed to have ORN. All analyses used JMP Pro (v. 14.0.0, SAS Institute, Cary NC).
Results
Patient Demographics
The study enrolled 572 participants (76.9% male) with an average age of 58.3 years (SD 11.1; Table 1). The most frequent primary RT sites included the oropharynx (49%), oral cavity (15%), salivary gland (10%), and larynx/hypopharynx (7.5%), with an average received RT dose of 66 Gy (Table 1). Before the baseline visit, 182 participants (31.8%) had one or more teeth extracted (880 total teeth extracted); 24 other participants (4.2%) had teeth that were deemed hopeless but had no teeth extracted. During followup, there were 15 tooth loss events at V06 (6 extractions or exfoliations, 9 newly “hopeless”), 99 at V12 (53 extractions/exfoliations, 46 newly “hopeless”), 66 at V18 (31 extractions/exfoliations, 35 newly “hopeless”), and 82 at V24 (42 extractions/exfoliations, 40 newly “hopeless”). The V24 visit was attended by 83% of participants who remained alive and had not withdrawn consent (421/505); of those not attending V24, 62 (11% of 572) were deceased and 5 (1% of 572) had withdrawn consent.
Table 1.
Participant characteristics.
| Characteristic | All Participants | No Report of Exposed Bone | Any Report of Exposed Bone | P-value |
|---|---|---|---|---|
| n | 572 | 537 | 35 | |
| Age (SD) | 58.3 (11.1) | 58.3 (11.2) | 57.7 (8.9) | 0.74 |
| Male | 440 (76.9%) | 411 (76.4%) | 29 (82.9%) | 0.53 |
| Race* | 0.086 | |||
| White | 474 (84.9%) | 446 (85.0%) | 28 (84.9%) | |
| African American | 45 (8.1%) | 40 (7.6%) | 5 (15.2%) | |
| Other | 39 (7.0%) | 39 (7.4%) | 0 (0%) | |
| Hispanic | 29 (5.1%) | 27 (5.0%) | 2 (5.7%) | 0.70 |
| Primary RT site¶ | 0.087 | |||
| Oropharynx | 262 (49.2%) | 247 (49.6%) | 15 (42.9%) | |
| Oral Cavity | 82 (15.4%) | 71 (14.3%) | 11 (31.4%) | |
| Salivary Gland | 54 (10.1%) | 53 (10.6%) | 1 (2.9%) | |
| Larynx/Hypopharynx | 40 (7.5%) | 37 (7.4%) | 3 (8.6%) | |
| Other | 95 (17.8%) | 90 (18.0%) | 5 (14.3%) | |
| Definitive RT¶¶ | 219 (41.1%) | 204 (40.6%) | 15 (46.9%) | 0.58 |
| RT type | 0.76 | |||
| IMRT | 538 (94.1%) | 504 (93.9%) | 34 (97.1%) | |
| Proton | 31 (5.4%) | 30 (5.6%) | 1 (2.9%) | |
| Other or missing | 3 (0.5%) | 3 (0.6%) | 0 (0.0%) | |
| Total RT dose in cGy to primary site (SD)** | 6573 (663) | 6560 (674) | 6773 (399) | 0.065 |
| Received chemotherapy (chemoRT) | 364 (63.6%) | 340 (63.3%) | 24 (68.6%) | 0.59 |
| BL status*** | 0.008 | |||
| Extraction | 182 (31.8%) | 163 (30.4%) | 19 (54.3%) | |
| Hopeless | 24 (4.2%) | 22 (4.1%) | 2 (5.7%) | |
| No extraction or hopeless | 366 (64.0%) | 352 (65.5%) | 14 (40.0%) | |
| BL n teeth (SD)**** | 22.9 (5.8) | 23.0 (5.7) | 21.3 (6.5) | 0.090 |
| Dental Insurance | 366 (64.0%) | 345 (64.2%) | 21 (60.0%) | 0.59 |
| Checkup last year | 414 (72.4%) | 393 (73.2%) | 21 (60.0%) | 0.12 |
| Education > High Schoolß | 412 (72.3%) | 390 (72.9%) | 22 (62.9%) | 0.24 |
| Brush/floss§ | 242 (42.3%) | 226 (42.1%) | 16 (45.7%) | 0.73 |
| Fluoride§ | 156 (27.4%) | 150 (28.0%) | 6 (17.7%) | 0.24 |
| WMA PD (SD)§§ | 2.35 (0.52) | 2.34 (0.51) | 2.51 (0.53) | 0.091 |
| % sites PD≥5 (SD) | 3.0 (6.0) | 2.9 (5.8) | 5.0 (8.5) | 0.055 |
| % sites PD≥4 (SD) | 11.1 (13.0) | 10.9 (12.8) | 14.2 (14.8) | 0.18 |
| WMA CAL (SD) | 1.92 (0.99) | 1.90 (0.99) | 2.20 (0.97) | 0.11 |
| % sites CAL≥3 (SD) | 27.8 (25.2) | 27.5 (25.2) | 33.3 (26.3) | 0.22 |
| % sites CAL≥2 (SD) | 53.1 (27.1) | 52.6 (27.2) | 61.1 (23.0) | 0.097 |
n = 558, 525 for “none”, 33 for “any”; 14 answered “Don’t know/decline to state”. “Other" includes people who selected more than one race.
n = 533, n = 498 for “none”, 35 for “any”; the other 39 had “Other” or “Don’t know”.
n = 534 for definitive vs. adjuvant post-surgical
n = 571, n = 536 for “none”, 35 for “any”; the other 1 had missing data.
Had an extraction at BL vs. had no extractions but had a tooth declared hopeless vs. had no teeth extracted or declared hopeless.
n = 571, n = 536 for “none”, 35 for “any”; the other 1 had missing data.
n = 570, n = 535 for “none”, 35 for “any”; 2 declined to state.
Brush/floss: Reported brushing at least 2x daily and flossing at least daily; Fluoride: reporting using prescription fluoride at least daily. Fluoride compliance n = 569, n = 535 for “none”, 34 for “any”.
WMA = “whole mouth average”; n = 533 for BL periodontal data; n = 503 for “none”, 30 for “any”. The other 39 did not have periodontal measures taken, almost all because they would have required antibiotic prophylaxis.
RT = radiation therapy; IMRT = intensity modulated radiation therapy; BL = baseline.
Exposed Bone Characteristics
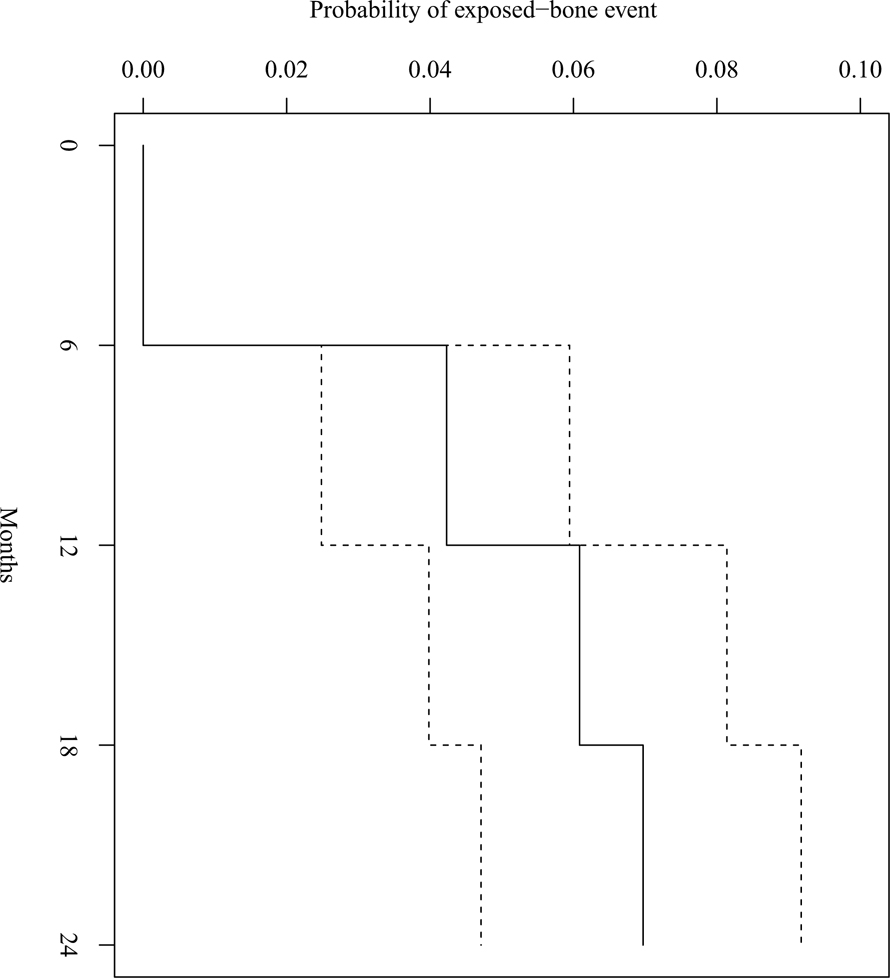
At baseline examination four participants had exposed bone; none of these areas persisted or recurred at subsequent visits. Three of these four participants had undergone pre-RT dental extractions at the affected sites, while the fourth had tooth #30 extracted pre-RT and exposed bone near tooth #32 at the baseline examination. Incident exposed bone after baseline was diagnosed in 35 participants (6.1%). These 35 participants had a total of 49 exposed bone reports, with 23 reports of exposed bone at V06 (47% of reports), 12 at V12 (24%), 10 at V18 (20%), and 4 at V24 (8%). Time-to-event analysis is shown in Figure 1; the majority of exposed bone cases were detected at V06 with no new reports of exposed bone at V24, and the same is true for the subset of exposed bone cases that were later confirmed to be ORN. Several participants had multiple reports at a given site, described in detail below; two participants had exposed bone diagnosed at two distinct locations. Of the 35 participants, 11 had only spontaneous exposed-bone reports, while 24 had only non-spontaneous reports; of the two participants with exposed bone at two distinct locations, one person had spontaneous exposed bone at both locations and the other person had non-spontaneous exposed bone at both locations. Exposed bone was more frequently reported in the mandible (76% of episodes) than the maxilla, with no statistically significant difference with respect to laterality. The average maximum RT dose to the affected area of exposed bone was 5456 cGy (SD 1768), and the most frequent primary RT sites associated with exposed bone were the oropharynx (42.9%) and oral cavity (31.4%; Table 1). Average maximum RT dose did not differ significantly between locations having spontaneous (5663 cGy, SD 1416) and non-spontaneous (5344 cGy, SD 1951) exposed bone (P = 0.61). Sequestrum formation was present in more than half of persons with exposed bone (21/35, 60%).
Figure 1.
Kaplan-Meier estimates of the fraction of the cohort having an exposed bone event by 6, 12, 18, and 24 months. There were no first exposed bone reports at 24 months. The dashed lines represent 95% confidence intervals.
Of the 35 participants with exposed bone, seven (20%) presented with persistent exposed bone, defined as exposed bone at a given site at more than one study visit (Table 2). Of these seven cases, five were located in the posterior mandible and two in the posterior maxilla. Four were spontaneous, one was in the site of a pre-RT extraction, and two occurred in sites of post-RT extractions. Of these seven participants, five continued to have exposed bone at V24 (or at last visit if before V24). Osteoradionecrosis (ORN) diagnosis was confirmed in six of these seven cases; the remaining case had insufficient available documentation to determine whether ORN was diagnosed. The remaining 28 participants were observed to have exposed bone at one visit only; 21 of the 28 were available for follow-up examination at subsequent study visits during which no exposed bone was recorded. Of these 28 participants, 14 were specifically noted to have “sequestrum”.
Table 2.
Exposed bone that persisted for more than one study visit.
| Participant | Location | Previous Extraction at Same or Neighboring Site | Sequestrum | Comments |
|---|---|---|---|---|
| A | 29–31 | None | Yes | One site with variable size/extent during V06, V18; attended V12 with no report. |
| B | 17–18 | None | No/Yes | Exposed bone V06 at 17, V12 at 18; sequestrum at V12 at 18 |
| C | 28–32 | None | No | One site with variable size/extent, reported all 4 visits |
| D | 1 | Same | No | Extracted 1 at BL, exposed bone at 1 V18, V24 (not V06, V12) |
| E | 16 | Neighbor | No | BL: 12, 13 hopeless, 14, 15 extracted; exposed bone 16 V12, V18, V24 (not V06) |
| F | 18–19 | None | Yes | Exposed bone V12 at 18 and 19, V18 at 18; missed V24. No extractions at baseline or during followup. |
| G | 27–31 | Same* | Yes | V06: 30 hopeless & exposed bone reported; V12: 30 extracted, exposed bone 28–31; exposed bone 27–29 V18, V24 |
Five of these seven individuals attended all visits (V06, V12, V18, V24). Person B attended V06 and V12 and then died before V18, while Person F attended V06, V12, and V18 but missed V24.
Person G had exposed bone at tooth #30 at V06 and tooth #30 was declared hopeless at V06. It was then extracted at V12; exposed bone was reported at this location at V12.
Imaging studies were available for 12 participants with exposed bone, which variably demonstrated sequestrum formation (17%) and both mottled (25%) and osteolytic (33%) bone changes. Management of exposed bone included chlorhexidine rinses (41%), surgical sequestrectomy (41%), surgical debridement (31%), antibiotics (31%), pentoxifylline and vitamin E (16%), analgesics (13%), hyperbaric oxygen therapy (9%), and surgical resection (3%).
Exposed Bone and History of Dental Extractions
Of the 35 participants with a report of exposed bone, 11 participants (31%) had spontaneous exposed bone (i.e., they had no teeth extracted, exfoliated, or declared hopeless at the site of exposed bone or at adjacent tooth sites, before or at the study visit at which their exposed bone was reported). Of the 11 participants with spontaneous exposed bone, one person had exposed bone at two distinct locations. Of these 12 instances of spontaneous exposed bone, 11 occurred in the molar regions and 10 in the mandible; four cases persisted over multiple visits.
Of the remaining 24 participants with non-spontaneous exposed bone, one had exposed bone at two distinct locations. Of these 25 locations, 24 included the molars or premolars, of which two were large enough also to include the canines, while one location was exclusively anterior. Seven locations were in the maxilla and 18 locations were in the mandible. Of the 25 locations, 19 had a previous or concurrently-reported extraction, exfoliation, or a hopeless tooth at the same site, while 6 had one at an adjacent site.
Confirmed Cases of Osteoradionecrosis
Osteoradionecrosis (ORN) diagnosis was confirmed in 18 participants, for an overall confirmed ORN incidence rate of 3.1% (18/572). Of the 35 participants with observed exposed bone, four did not attend the subsequent referral visit and of those who did, five had no chart record confirming or disconfirming ORN. Thus the overall incidence of ORN could be higher than 3.1%. Of those with confirmed ORN, five were Stage 1 and 13 were Stage 2 at their worst, with none diagnosed as Stage 3. Of the five whose worst stage was Stage 1, two remained Stage 1 and three were fully healed at the last assessment recorded in their chart at the time of chart review. Of the 13 whose worst stage was Stage 2, three remained Stage 2, one was Stage 1, and nine were fully healed at the last assessment. No confirmed ORN cases worsened in stage.
All of the participants with confirmed ORN received treatment for the diagnosis. Treatments included chlorhexidine rinses (n=10; 56%), antibiotics (n=8; 44%), surgical debridement (n=8; 44%), sequestrectomy (n=7; 39%), pentoxifylline and vitamin E (n=5; 28%), analgesics (n=4; 22%), hyperbaric oxygen therapy (n=3; 17%), and surgical resection (n=1; 6%). Of the 18 participants with ORN, 12 (67%) experienced complete resolution/healing. There were no statistically significant associations between the various treatments provided and resolution outcome (data not shown). One participant received HBO before dental extractions (15 dives); two participants underwent HBO after RT (18 dives for the participant who received HBO before extractions; 40 dives for the other participant).
Of the 35 participants with exposed bone, eight were confirmed as not having a diagnosis of ORN; all experienced complete resolution/healing.
Tooth Loss after Exposed Bone Observation
Five participants were observed with exposed bone (three at V06 and two at V12) who later had teeth extracted, exfoliated, or deemed hopeless at or adjacent to a site where exposed bone was reported; four of these exposed-bone sites were in the posterior mandible. All five of these participants also had teeth extracted, exfoliated, or deemed hopeless before or concurrent with their report of exposed bone, i.e., their exposed bone was not spontaneous.
Risk Factors
Several patient characteristics were associated with having at least one report of exposed bone (Table 3). Those with pre-RT extractions prior to baseline were more likely to have exposed bone during follow-up (10.4% vs. 3.8% with no pre-RT extractions prior to baseline, P = 0.008), with the chance of exposed bone increasing with the number of pre-RT extractions (odds ratio for any vs. no exposed bone 1.11 per extracted tooth, 95% CI 1.04 to 1.18, P = 0.01). Those with pre-RT extractions prior to baseline or during follow-up were even more likely to have an exposed-bone report (12.2% vs. 2.8% with no extractions, P < 0.0001). Those with a higher dose to their primary RT site were more likely to have exposed bone (odds ratio per 1000 cGy higher dose 1.97, 95% CI 1.03 to 4.12, P = 0.039). Current tobacco users (28 of 570 who reported on their tobacco use) were at much higher risk of exposed bone compared to former and never users, with 17.9% reporting exposed bone compared to 5.8% and 5.2% respectively. Risk of exposed bone varied depending on primary RT site, though not reaching statistical significance (P = 0.087), with oral cavity being the primary RT site having most frequent exposed bone (13.4%) and salivary gland the least (1.9%). Patients receiving concurrent chemotherapy (chemoradiation therapy) had frequency of exposed bone that was only slightly, and not significantly, higher (6.6% vs. 5.3%, P = 0.59).
Table 3.
Tests of association of potential predictors with exposed bone outcomes.
| P-value | |||
|---|---|---|---|
| Potential Predictor | Any exposed bone vs. none | Exposed bone, no sequestrum | Description of the association Odds ratios (ORs) are for exposed bone vs. no exposed bone |
| Pre-RT extractions or hopeless teeth at BL* | 0.008 | 0.043 | Any vs. none: Extraction 10.4%, hopeless 8.3%, neither 3.8% No sequestrum: Extraction 5.0%, hopeless 0%, neither 1.4% |
| Any extractions or hopeless teeth by V24** | <0.0001 | 0.0013 | Any vs. none: Lost 12.2%, hopeless 0%, neither 2.8%. No sequestrum: Lost 5.6%, hopeless 0%, neither 0.6%. |
| N teeth extracted pre-RT prior to BL | 0.006 | 0.060 | Any vs. none: OR per tooth 1.11 (95% CI 1.04 to 1.18) No sequestrum: OR per tooth 1.11 (95% CI 1.01 to 1.21) |
| N teeth present at BL | 0.11 | 0.49 | Any vs. none: OR per additional tooth present 0.96 (95% CI 0.91 to 1.01) No sequestrum: OR per additional tooth present 0.97 (95% CI 0.89 to 1.05) |
| Total RT dose to primary site*** | 0.039 | 0.31 | Any vs. none: OR per 1000 cGy higher dose 1.97 (95% CI 1.03 to 4.12). No sequestrum: OR per 1000 cGy higher dose 1.64 (95% CI 0.59 to 4.58). |
| Primary RT site¶ | 0.087 | 0.70 | Any vs. none: oral cavity 13.4%, larynx/hypopharynx 7.5%, oropharynx 5.7%, other 5.3%, salivary gland 1.9%. No sequestrum: oral cavity 3.7%, larynx/hypopharynx 0%, oropharynx 2.3%, other 4.2%, salivary gland 1.9%. |
| Definitive vs. Post-operative RT | 0.58 | 1.00 | Any vs. none: definitive 6.9%, post-operative 5.4% No sequestrum: definitive 2.3%, post-operative 2.5% |
| RT type (IMRT vs. proton) | 0.71 | 1.00 | Any vs. none: IMRT 6.3%, proton 3.2% No sequestrum: IMRT 2.6%, proton 0% |
| Follow-up fluoride§ | 0.85 | 0.76 | Any vs. none: compliant 5.8%; non-compliant 6.9% No sequestrum: compliant 2.0%; non-compliant 2.8% |
| Follow-up brush/floss§ | 1.00 | 0.24 | Any vs. none: compliant 6.3%; non-compliant 6.7% No sequestrum: compliant 1.2%; non-compliant 3.2% |
| Tobacco, ever v. never v. current | 0.048 | 0.0047 | Any vs. none: Never 5.2%, Former 5.8%, Current 17.9% No sequestrum: Never 2.0%, Former 1.7%, Current 14.3% |
| Chemotherapy (Y/N) | 0.59 | 0.78 | Any vs. none: chemotherapy 6.6%; none 5.3% No sequestrum: chemotherapy 2.8%; none 1.9% |
| Anti-resorptive (Y/N) | 0.31 | 0.37 | Any vs. none: anti-resorptive 11.1%; none 6.0% No sequestrum: anti-resorptive 5.6%; none 2.4% |
| Enrollment site (clinic) | 0.0024 | 0.018 | Any vs. none: CMC 13.5%, UConn 11.3%, BW 5.7%, UNC 3.2%, UPenn 2.7%, NYU 1.3%. No sequestrum: CMC 5.8%, UConn 7.6%, BW 1.3%, UNC 0%, UPenn 0.7%, NYU 1.3%. |
| Whole mouth average (WMA) PD§§ | 0.10 | 0.13 | Any vs. none: OR for +0.5 mm WMA PD 1.33 (95% CI 0.95 to 1.85)† No sequestrum: OR for +0.5 mm WMA PD 1.45 (95% CI 0.91 to 2.34)† |
| % sites PD≥4 | 0.21 | 0.16 | Any vs. none: OR for +13 percentage points 1.23 (95% CI 0.91 to 1.68)† No sequestrum: OR for +13 percentage points 1.37 (95% CI 0.92 to 2.06)† |
| % sites PD≥5 | 0.10 | 0.086 | Any vs. none: OR for +6 percentage points 1.26 (95% CI 0.98 to 1.62)† No sequestrum: OR for +6 percentage points 1.37 (95% CI 1.01 to 1.85)† |
| Whole mouth average (WMA) CAL | 0.13 | 0.30 | Any vs. none: OR for +1mm WMA CAL 1.29 (95% CI 0.94 to 1.78)† No sequestrum: OR for +1mm WMA CAL 1.30 (95% CI 0.82 to 2.05)† |
| % sites CAL≥2 | 0.098 | 0.40 | Any vs. none: OR for +27 percentage points 1.36 (95% CI 0.94 to 1.98)† No sequestrum: OR for +27 percentage points 1.26 (95% CI 0.73 to 2.20)† |
| % sites CAL≥3 | 0.24 | 0.53 | Any vs. none: OR for +25 percentage points 1.23 (95% CI 0.88 to 1.72)† No sequestrum: OR for +25 percentage points 1.18 (95% CI 0.71 to 1.95† |
Had an extraction at BL vs. had no extractions but had a tooth declared hopeless vs. had no teeth extracted or declared hopeless.
Some teeth exited the mouth (lost) vs. no teeth exited the mouth but had a tooth declared hopeless vs. no teeth lost or declared hopeless.
n = 571, n = 536 for “none”, 35 for “any”; the other 1 had missing data.
n = 533, n = 498 for “none”, 35 for “any”; the other 39 had “Other” or “Donť know”.
Brush/floss: Reported brushing at least 2x daily and flossing at least daily for all followup visits attended; Fluoride: reporting using prescription fluoride at least daily for all followup visits attended.
WMA = “whole mouth average”; n = 533 for BL periodontal data, n = 503 for “none”, 30 for “any”.
Odds ratios (ORs) are for an approximately 1 standard deviation increase in the predictor measure. Standard deviations are: WMA PD 0.52 mm; % sites PD≥4 13.0 percentage points; % sites PD≥5 6.0 percentage points; WMA CAL 0.99 mm; % sites CAL≥3 27.1 percentage points; % sites CAL ≥3 25.2 percentage points.
RT = radiation therapy; IMRT = intensity modulated radiation therapy; BL = baseline.
Discussion
OraRad represents the first multicenter study, which also has the largest cohort prospectively followed, to describe this important but relatively rare oral complication of head and neck RT. This prospective multicenter study of HNC patients treated with RT found a 6.1% incidence of exposed bone by two-years. More than half of all exposed bone observations were made by the 1-year followup, and one-fifth of persons with exposed bone had reports persisting over more than one 6-month interval between visits. Risk factors for developing exposed bone included pre-RT extractions or teeth deemed “hopeless” either at baseline or during the study period, the location of and RT dose to the primary tumor site, and current tobacco use.
We applied the AAOMS MRONJ definition and classification system to analyze the incidence and severity of ORN in our cohort(11). While various definitions and classification systems for ORN exist, it was felt that the AAOMS system, which is widely recognized in the context of MRONJ (which clinically follows the same principles as ORN with respect to diagnosis, severity and management), provided the most clinically meaningful assessment instrument(12, 13). While 6.1% of participants had exposed bone observations, the incidence of ORN was 3.1%. However, given that four participants with exposed bone did not attend a referral visit for further evaluation and possible ORN diagnosis, and five participants did not have sufficient information from chart review to confirm or disconfirm ORN diagnosis, it is possible that this figure underestimates the true incidence of ORN (which, if the 18 confirmed cases and the 9 unknown cases are counted as having ORN, would reach 4.7%). Tsai et al. reported a retrospective study of 402 patients with oropharyngeal cancer with 7.5% incidence of ORN developing at a median of 8 months after RT(14). Other reports have described onset of ORN occuring even up to 5 years after radiation(13, 15).
Several retrospective studies have explored the increased incidence of ORN as a result of post-RT extractions. Dental extractions after radiation, in particular with a radiation dose greater than 60Gy, increase the risk of developing ORN(16–19). Kuhnt et al. reported an ORN incidence of 6.6%, with increased risk associated with both post-RT dental extractions as well as the primary RT site(20). Nabil et al. reported ORN incidence of 7% in an analysis of pooled reports of ORN following dental extractions post-RT in 828 patients(16). Both higher radiation dose (>66Gy) and location of the radiation were associated with development of ORN after dental extractions(18, 21, 22). Epstein et al. reported a cohort of 146 patients, of whom 92 had teeth removed pre-RT, 12 during RT and 42 post-RT. This patient cohort had 8 cases of ORN, of whom 5 patients had pre-RT extractions and 3 had post-RT extractions (23). Epstein et al. reported a cohort of patients in which every instance of ORN was preceded by teeth being extracted or declared hopeless (12). Others have found that the risk of developing exposed bone and ORN increases when a tooth is extracted post-RT(17). Ruther et al. describes a cohort of 830 patients with 68 (8.2%) developing ORN; of these, 34 (50%) were associated with a tooth extraction(18).
In the present study, while the majority of exposed bone reports followed dental extractions and were therefore considered “non-spontaneous”, 11 participants had observations of spontaneously occuring exposed bone. Most of these reports occurred in the posterior mandible, similar to the location for lingual mandibular sequestration and ulceration, a condition occurring in otherwise healthy individuals without a history of RT and with no known injury or trauma to the affected area(24). Periodontal disease has also been identified as a potential risk factor for development of jaw osteonecrosis(25). Koga et al. reported a retrospective analysis of 17 patients with ORN of whom 5 (29.4%) were described as spontaneous (not related to surgery or dental extractions)(26). Beumer et al. reported 83 cases of ORN with 19 episodes described as occuring spontaneously, but related to dental disease and the field of radiation(19). Thorn et al. described spontaneous development of ORN in 23 of 80 patients (29%) with some cases related to presence of periodontal disease(27). In our cohort, spontaneous exposed bone reports were over-represented (compared with non-spontaneous reports) among sites with persistent exposed bone, underscoring the recognition that dental extraction is not the only factor involved. Not surprisingly, MRONJ may also present spontaneously(10).
As in previous studies, in the OraRad cohort dental extractions post-RT and higher RT dose to the primary site were both identified as risk factors for development of exposed bone(18). Receiving a radiation dose greater than or equal to 65Gy significantly increases the risk of ORN(17, 21, 28). Tsai et al. discussed the significant difference in incidence of ORN between treatment with 50 Gy and 60Gy (P = 0.02) (14). Horiot et al. explored the effect of topical fluoride on dental caries and development of ORN, and while the caries rate was reduced with topical fluoride use, ORN was not(29). In the current cohort, current tobacco use was associated with an increased risk of observed exposed bone. Raguse et al. reported 42% of the ORN cases involved tobacco use after RT but did not find a significant association with development of ORN(30).
This analysis of exposed bone within the OraRad cohort has some limitations. Despite calibration of examiners, it is uncertain how consistently an observation of bone “sequestrum” was described. A bone sequestrum would be anticipated to be more likely to resolve, especially if removed as part of a procedure, compared to exposed bone that is non-mobile and cannot be removed. While exposed bone was observed during study examinations, diagnosis of ORN was determined by chart review of medical and/or dental records and ensuring that specific criteria were met. As prior studies of ORN have used various definitions and criteria, direct comparisons may not be entirely accurate. In the present study, some cases had limited available follow-up data, so the prevalence of ORN might have been underreported, although it is unlikely to have been higher than the two-year reported incidence of observed exposed bone (6.1%). Follow-up was limited to two years, so the incidence and course of exposed bone that develops or persists past this period is unknown. Similarly, some participants were lost to follow-up, making it impossible to determine the actual course of their condition.
The study also had several strengths, including its multicenter prospective design and in-depth data on pre-RT dental status and treatment provided. Given that all subjects were evaluated according to the same schedule and in a calibrated manner, no obvious bias should have affected the rate of exposed bone observations. Further, once exposed bone was observed, the participant’s medical and dental records were carefully reviewed to determine whether ORN diagnostic criteria were fulfilled.
In summary, the two-year incidence of exposed bone in the OraRad cohort was 6.1%, and the incidence of confirmed ORN was 3.1%. Most exposed bone reports were at 6 months after RT, with the mandible affected most frequently. Risk factors included pre- and post-RT dental extractions and higher RT dose, and the oral cavity was the highest risk primary cancer site. Approximately one-third of exposed bone reports were spontaneous, with most occurring in the posterior mandible. Based on these findings, we conclude that development of exposed bone after RT for HNC is relatively uncommon and in most cases is a short-term but not recurring or persistent complication.
Acknowledgements
The authors gratefully acknowledge the contributions of the study participants, study staff at each clinical site, and Helen Voelker and Leslie Long-Simpson of the Data and Coordinating Center at the University of Minnesota.
This study was funded by grant number U01DE022939 from the National Institute for Dental and Craniofacial Research (NIDCR), National Institutes of Health (NIH), USA.
Footnotes
The authors declare no relevant conflicts of interest.
Contributor Information
Nathaniel S. Treister, Division of Oral Medicine and Dentistry, Brigham and Women’s Hospital, 1620 Tremont Street, 3rd Floor, Boston, MA 02120, USA. Department of Oral Medicine, Infection and Immunity, Harvard School of Dental Medicine, 188 Longwood Avenue, Boston, MA 02115, USA.
Michael T. Brennan, Department of Oral Medicine, Atrium Health’s Carolinas Medical Center, 1000 Blythe Blvd., Charlotte, NC 28203, USA..
Thomas P. Sollecito, Department of Oral Medicine, University of Pennsylvania School of Dental Medicine, 240 South 40th Street, Philadelphia, PA 19104, USA. Division of Oral Medicine, University of Pennsylvania Health System, 3400 Civic Center Blvd, Philadelphia, PA 19104, USA.
Brian L. Schmidt, Department of Oral & Maxillofacial Surgery and Bluestone Center for Clinical Research, New York University College of Dentistry, 421 First Avenue, New York, New York 10010, USA..
Lauren L. Patton, Division of Craniofacial and Surgical Care, Adams School of Dentistry, University of North Carolina, CB 7450, Chapel Hill, NC, USA..
Rebecca Mitchell, Division of Biostatistics, School of Public Health, University of Minnesota, 2221 University Ave SE Suite 200, Minneapolis, MN 55414, USA..
Robert I. Haddad, Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA, USA..
Roy B. Tishler, Department of Radiation Oncology, Dana-Farber Cancer Institute/Brigham & Women’s Hospital, 450 Brookline Ave, Boston, MA 02215, United States..
Ryann Shadick, Division of Oral Medicine and Dentistry, Brigham and Women’s Hospital, 1620 Tremont Street, 3rd Floor, Boston, MA 02120, USA..
James S. Hodges, Division of Biostatistics, School of Public Health, University of Minnesota, 2221 University Ave SE Suite 200, Minneapolis, MN 55414, USA..
Rajesh V. Lalla, Section of Oral Medicine, MC3912, University of Connecticut Health, 263 Farmington Avenue, Farmington, CT 06030-3912, USA..
References
- 1.American Cancer Society. American Cancer Society: Cancer Facts and Statistics [Available from: https://cancerstatisticscenter.cancer.org/?_ga=2.214893202.42047244.1569264094-1510505257.1568219754#!/cancer-site/Oral%20cavity%20and%20pharynx.
- 2.Lo Nigro C, Denaro N, Merlotti A, Merlano M. Head and neck cancer: improving outcomes with a multidisciplinary approach. Cancer Manag Res. 2017;9:363–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marur S, Forastiere AA. Head and Neck Squamous Cell Carcinoma: Update on Epidemiology, Diagnosis, and Treatment. Mayo Clin Proc. 2016;91(3):386–96. [DOI] [PubMed] [Google Scholar]
- 4.National Cancer Institute. Head and Neck Cancers 2017. [Available from: https://www.cancer.gov/types/head-and-neck/head-neck-fact-sheet#how-are-head-and-neck-cancers-treated.
- 5.Parliament M, Alidrisi M, Munroe M, Wolfaardt J, Scrimger R, Thompson H, et al. Implications of radiation dosimetry of the mandible in patients with carcinomas of the oral cavity and nasopharynx treated with intensity modulated radiation therapy. Int J Oral Maxillofac Surg. 2005;34(2):114–21. [DOI] [PubMed] [Google Scholar]
- 6.Rogers SN, D’Souza JJ, Lowe D, Kanatas A. Longitudinal evaluation of health-related quality of life after osteoradionecrosis of the mandible. Br J Oral Maxillofac Surg. 2015;53(9):854–7. [DOI] [PubMed] [Google Scholar]
- 7.Owosho AA, Tsai CJ, Lee RS, Freymiller H, Kadempour A, Varthis S, et al. The prevalence and risk factors associated with osteoradionecrosis of the jaw in oral and oropharyngeal cancer patients treated with intensity-modulated radiation therapy (IMRT): The Memorial Sloan Kettering Cancer Center experience. Oral Oncol. 2017;64:44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chronopoulos A, Zarra T, Ehrenfeld M, Otto S. Osteoradionecrosis of the jaws: definition, epidemiology, staging and clinical and radiological findings. A concise review. Int Dent J. 2018;68(1):22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lalla RV, Long-Simpson L, Hodges JS, Treister N, Sollecito T, Schmidt B, et al. Clinical registry of dental outcomes in head and neck cancer patients (OraRad): rationale, methods, and recruitment considerations. BMC Oral Health. 2017;17(1):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruggiero SL. Diagnosis and Staging of Medication-Related Osteonecrosis of the Jaw. Oral Maxillofac Surg Clin North Am. 2015;27(4):479–87. [DOI] [PubMed] [Google Scholar]
- 11.Ruggiero SL, Dodson TB, Fantasia J, Goodday R, Aghaloo T, Mehrotra B, et al. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw−−2014 update. J Oral Maxillofac Surg. 2014;72(10):1938–56. [DOI] [PubMed] [Google Scholar]
- 12.Epstein JB, Wong FL, Stevenson-Moore P. Osteoradionecrosis: clinical experience and a proposal for classification. J Oral Maxillofac Surg. 1987;45(2):104–10. [DOI] [PubMed] [Google Scholar]
- 13.He Y, Liu Z, Tian Z, Dai T, Qiu W, Zhang Z. Retrospective analysis of osteoradionecrosis of the mandible: proposing a novel clinical classification and staging system. Int J Oral Maxillofac Surg. 2015;44(12):1547–57. [DOI] [PubMed] [Google Scholar]
- 14.Tsai CJ, Hofstede TM, Sturgis EM, Garden AS, Lindberg ME, Wei Q, et al. Osteoradionecrosis and radiation dose to the mandible in patients with oropharyngeal cancer. Int J Radiat Oncol Biol Phys. 2013;85(2):415–20. [DOI] [PubMed] [Google Scholar]
- 15.Monnier Y, Broome M, Betz M, Bouferrache K, Ozsahin M, Jaques B. Mandibular osteoradionecrosis in squamous cell carcinoma of the oral cavity and oropharynx: incidence and risk factors. Otolaryngol Head Neck Surg. 2011;144(5):726–32. [DOI] [PubMed] [Google Scholar]
- 16.Nabil S, Samman N. Incidence and prevention of osteoradionecrosis after dental extraction in irradiated patients: a systematic review. Int J Oral Maxillofac Surg. 2011;40(3):229–43. [DOI] [PubMed] [Google Scholar]
- 17.Beumer J, Harrison R, Sanders B, Kurrasch M. Postradiation dental extractions: a review of the literature and a report of 72 episodes. Head Neck Surg. 1983;6(1):581–6. [DOI] [PubMed] [Google Scholar]
- 18.Reuther T, Schuster T, Mende U, Kübler A. Osteoradionecrosis of the jaws as a side effect of radiotherapy of head and neck tumour patients--a report of a thirty year retrospective review. Int J Oral Maxillofac Surg. 2003;32(3):289–95. [DOI] [PubMed] [Google Scholar]
- 19.Beumer J, Harrison R, Sanders B, Kurrasch M. Osteoradionecrosis: predisposing factors and outcomes of therapy. Head Neck Surg. 1984;6(4):819–27. [DOI] [PubMed] [Google Scholar]
- 20.Potential risk factors for jaw osteoradionecrosis after radiotherapy for head and neck cancer [Internet]. 2016. [DOI] [PMC free article] [PubMed]
- 21.Goldwaser BR, Chuang SK, Kaban LB, August M. Risk factor assessment for the development of osteoradionecrosis. J Oral Maxillofac Surg. 2007;65(11):2311–6. [DOI] [PubMed] [Google Scholar]
- 22.Epstein JvdM, McKenzie Erik, Wong Michael, Lepawsky Frances, Michael Stevenson-Moore Peter. Postradiation osteonecrosis of the mandible: A long-term follow-up study. 1997;83(6):657–62. [DOI] [PubMed] [Google Scholar]
- 23.Epstein JB, Rea G, Wong FL, Spinelli J, Stevenson-Moore P. Osteonecrosis: study of the relationship of dental extractions in patients receiving radiotherapy. Head Neck Surg. 1987;10(1):48–54. [DOI] [PubMed] [Google Scholar]
- 24.Peters E, Lovas GL, Wysocki GP. Lingual mandibular sequestration and ulceration. Oral Surg Oral Med Oral Pathol. 1993;75(6):739–43. [DOI] [PubMed] [Google Scholar]
- 25.Lorenzo-Pouso AI, Pérez-Sayáns M, Chamorro-Petronacci C, Gándara-Vila P, López-Jornet P, Carballo J, et al. Association between periodontitis and medication-related osteonecrosis of the jaw: A systematic review and meta-analysis. J Oral Pathol Med. 2020;49(3):190–200. [DOI] [PubMed] [Google Scholar]
- 26.Koga DH, Salvajoli JV, Kowalski LP, Nishimoto IN, Alves FA. Dental extractions related to head and neck radiotherapy: ten-year experience of a single institution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105(5):e1–6. [DOI] [PubMed] [Google Scholar]
- 27.Thorn JJ, Hansen HS, Specht L, Bastholt L. Osteoradionecrosis of the jaws: clinical characteristics and relation to the field of irradiation. J Oral Maxillofac Surg. 2000;58(10):1088–93; discussion 93–5. [DOI] [PubMed] [Google Scholar]
- 28.Chen JA, Wang CC, Wong YK, Wang CP, Jiang RS, Lin JC, et al. Osteoradionecrosis of mandible bone in patients with oral cancer--associated factors and treatment outcomes. Head Neck. 2016;38(5):762–8. [DOI] [PubMed] [Google Scholar]
- 29.Horiot JC, Schraub S, Bone MC, Bain Y, Ramadier J, Chaplain G, et al. Dental preservation in patients irradiated for head and neck tumours: A 10-year experience with topical fluoride and a randomized trial between two fluoridation methods. Radiother Oncol. 1983;1(1):77–82. [DOI] [PubMed] [Google Scholar]
- 30.Raguse JD, Hossamo J, Tinhofer I, Hoffmeister B, Budach V, Jamil B, et al. Patient and treatment-related risk factors for osteoradionecrosis of the jaw in patients with head and neck cancer. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;121(3):215–21.e1. [DOI] [PubMed] [Google Scholar]