Abstract
Background:
Lead exposure is associated with behavioral problems in children, but the age(s) of greatest susceptibility to low-level lead exposure is unknown.
Objective:
We evaluated the association of repeated blood lead concentrations with parent-reported behaviors to identify periods of heightened susceptibility during infancy and childhood (HOME Study; Cincinnati, Ohio; 2003–2006; n=244).
Methods:
We quantified lead in whole blood samples (ages 1-,2-,3-,4-,5-, and 8-years) and assessed behavior using the Behavioral Assessment System for Children-2 (BASC-2; ages 2-,3-,4-,5-, and 8-years). We used multiple informant models and modified Poisson regression to estimate covariate-adjusted associations of ln-transformed blood lead concentrations with continuous BASC-2 T-scores and the relative risk of behavior scores classified as at-risk or clinically significant, respectively.
Results:
We observed trends indicating that higher blood lead concentrations at all ages were adversely associated with scores on behavioral scales. On the Externalizing Problems and Adaptive Skills scales, these associations were strongest for blood lead concentrations at age 8-years (β=3.1-point; 95%CI=0.7, 5.4 and β=−2.2-point; 95%CI= −4.9, 0.5, respectively) compared with other ages. Overall, higher blood lead concentrations were associated with elevated risk of behavior scores classified as at-risk or clinically significant on the Adaptive Skills, Behavioral Symptom Index, and Externalizing Problems scales.
Keywords: lead, children’s health, behavior, periods of heightened susceptibility
INTRODUCTION
Lead is a recognized neurotoxicant with no safe level of exposure (1). Studies have reported adverse associations of higher blood lead concentrations with cognition, cardiovascular health, and kidney function in adulthood (2–5). Primary prevention is the preferred solution to eliminate the burden of lead-related neurobehavioral consequences including diminished intellectual and academic abilities in childhood, as well as hyperactivity and attention problems — the primary features of attention-deficit-hyperactivity (ADHD) disorder (1,2).
Current practices to protect children from lead toxicity focus on blood lead screening, surveillance, and secondary prevention during the first two years of life (6). Furthermore, the Centers for Disease Control and Prevention (CDC) emphasizes a higher risk for children less than six years old (7). The biological processes underlying rapid neurodevelopment during this period may result in heightened susceptibility to lead toxicity (8). Current efforts have also targeted this life stage because of the recognition that developmentally appropriate behaviors can increase lead exposure from lead-based paint, lead-contaminated water, house dust, and soil. These practices, however, are primarily informed by studies of children with higher blood lead concentrations than contemporary children (9–12). Thus, it is unclear whether heightened susceptibility to lead exposure extends beyond early childhood into later childhood, particularly in populations with low-level exposure from a variety of sources that would require more extensive primary prevention interventions.
The objective of this study was to evaluate the association of blood lead concentrations collected during the first 8-years of life with longitudinal measures of behaviors in a contemporary cohort and identify periods of heightened susceptibility to low-level lead exposure.
SUBJECTS AND METHODS
Health Outcomes and Measures of the Environment (HOME) Study Recruitment
Between 2003 and 2006, HOME Study research staff recruited pregnant women living in the Cincinnati, Ohio metropolitan area from nine local prenatal clinics (13). Eligibility criteria included: English fluency; 18 years of age or older; 16 ± 3 weeks gestation; plan to continue prenatal care and deliver at collaborating clinics and hospitals; residence in a home built in or prior to 1978 which was not a mobile or trailer home; plan to live in the greater Cincinnati metropolitan area for at least one year; HIV-negative; not taking medications for seizures or thyroid disorders; not diagnosed with diabetes, bipolar disorder, schizophrenia, or cancer. We targeted women living in homes built before 1978, the year residential lead paints were banned in the United States, and we oversampled women self-identifying as non-Hispanic Black because of their children’s increased risk of lead exposure. A residential lead hazard intervention study was conducted as part of the HOME Study (14). For participants randomly assigned to receive the residential lead hazard intervention, we implemented a series of measures to remove or reduce lead hazards in paint, dust, water, and bare soil in and around the homes of pregnant women prior to delivery of the infant. We maintained the interventions for the first three years of childhood. If a participant moved prior to age 21 months, we attempted to implement the intervention in the new residence. For the control group, we installed injury prevention devices, including stair gates, cabinet locks, and smoke detectors, as well as made residential modifications to prevent injury (14,15).
Of the 355 women who participated in the intervention, eligible participants for this analysis included singletons born without a congenital abnormality to women who identified as non-Hispanic Black or non-Hispanic White and completed at least one study visit after baseline (n=271). We included participants who had the outcome measures or whole blood samples collected during at least one study visit and complete covariate information (n=244). The institutional review boards (IRB) of Cincinnati Children’s Hospital Medical Center (CCHMC) and the participating prenatal clinics and delivery hospitals approved the HOME Study. Brown University deferred to the CCHMC IRB as the IRB of record. Women provided written informed consent for themselves and their children after research assistants explained study protocols during face-to-face visits.
Blood Lead Concentrations
We collected whole blood samples from children at ages 1-, 2-, 3-, 4-, 5-, and 8-years using collection materials that were prescreened for lead contamination. We stored blood samples at −80°C until shipment to the CDC laboratory for analysis. Blood lead concentrations were quantified using inductively coupled plasma mass spectrometry (Limit of Detection=0.07 μg/dL for samples quantified at age 8 years and 0.25 μg/dL for samples from earlier ages)(16). All batches included reagent blanks and quality control samples (coefficient of variation, <3.5%).
Behavioral Assessment System for Children-2
At ages 2-, 3-, 4-, 5-, and 8-years, caregivers rated their children’s behavior using the Behavioral Assessment System for Children-2 (BASC-2), a valid and reliable caregiver-reported measure of child behavior (17). We analyzed the four composite scales - Adaptive Skills, Behavioral Symptoms Index (BSI), Externalizing Problems, and Internalizing Problems. The Adaptive Skills composite score reflects adaptability, social skills, as well as leadership, and the BSI score is a rating of the child’s overall level of problem behaviors. The Externalizing Problems score assesses disruptive behaviors such as aggression, conduct problems and hyperactivity, while the Internalizing Problems score reflects inwardly directed behaviors, such as anxiety, depression, and somatization.
We used U.S. population-based normative referent data to calculate combined sex normalized T-scores (mean: 50, standard deviation [SD]: 10) for each scale. Lower scores on the Adaptive Skills scale indicate less adaptive behaviors and higher scores on the BSI, Externalizing Problems, and Internalizing Problems scales indicate more problem behaviors. We further explored relations of blood lead concentrations with the Externalizing Problems subscales related to: Aggression, Hyperactivity, and Conduct Problems (age 8-years only) because lead has previously and consistently been associated with these ADHD-related behavioral features (18,19). We assessed all composite scores and subscales as continuous measures, and also dichotomized BASC-2 scores using thresholds recommended by the test developer to identify at-risk or clinically significant behavior problems (Adaptive Skills score ≤ 40 on original scale and BASC-2 scores ≥ 60 for other scales).
Participant Characteristics
At baseline (around 20-weeks’ gestation), trained research staff administered standardized interviews to collect information on maternal race and ethnicity, household income, maternal education, and residential ownership status (own – yes/no). We extracted the construction year for the residence from housing assessors’ databases. We assessed maternal IQ using the Wechsler Abbreviated Scale of Intelligence- WASI (20) and, at the one-year study visits, we assessed the children’s caregiving environment using the Home Observation for Measurement of the Environment (HOME) Inventory Score (21). To estimate gestational and childhood tobacco smoke exposure, we averaged maternal cotinine concentrations in serum samples collected at 16- and 26-weeks’ gestation, as well as children’s cotinine concentration quantified in serum samples collected at ages 1-, 2-, 3- and 4-years (22). At each study visit, we administered the Beck Depression Inventory-II to the mother to assess maternal depressive symptoms (23).
Statistical Analysis
We characterized the distributions of blood lead concentrations at each visit. We calculated the intraclass correlation coefficient (ICC) for blood lead concentrations at ages 1-, 2-, 3-, 4-, 5-, and 8-years to assess the reproducibility within children over time. Based on the distribution of the blood lead concentrations, we used the natural logarithm (ln) to transform blood lead concentration to reduce the influence of outliers. We also examined the distribution of the BASC-2 scores over time and calculated the ICC for composite and subscale scores.
We used a multiple informant approach with generalized estimating equations (GEE) to jointly evaluate the relations of ln-transformed blood lead concentrations at ages 1-, 2-, 3-, 4-, 5-, and 8-years with repeated continuous BASC-2 scores at ages 2-, 3-, 4-, 5-, and 8-years. This model assumes that the predefined exposure periods have the same timing for all participants, the exposure effect is homogenous within each exposure period, and there is at least one exposure sample per participant (24). Furthermore, the model assumes that data are missing completely at random. In these models, we assessed the relation of blood lead concentrations at each timepoint with the BASC-2 scores reported at both the concurrent and subsequent timepoints. For example, when estimating the relation of blood lead concentrations quantified at age 3-years with BASC, we included BASC-2 scores at ages 3-, 4-, 5-, and 8-years. Using this approach, we also assessed if the relation of blood lead concentrations with BASC-2 scores varied at one age, relative to other ages, using a lead by exposure period product term (24). We considered a heterogeneity product term p-value < 0.20 to be suggestive of potential differences in these associations by exposure period. We used this approach, instead of separate regression models for each exposure timepoint, to statistically compare the associations between blood lead and behavior across timepoints.
Next, we used modified Poisson regression to examine relations of repeated ln-transformed blood lead concentrations at ages 2-, 3-, 4-, 5-, and 8-years with the relative risk of having BASC-2 scores classified as at-risk or clinically significant at ages 2-, 3-, 4-, 5-, and 8-years. The resulting beta coefficients from this analysis represent the cross-sectional and longitudinal association of repeated blood lead concentrations and repeated behavior measures.
We selected covariates a priori based on the literature and adjusted all models for the child’s age (months) at BASC-2 assessment, child sex (categorical), household income (continuous), residential intervention group (categorical), maternal race/ethnicity (categorical), maternal depressive symptoms at time of evaluation (continuous), and child’s average serum cotinine concentration (continuous) (25,26).
In secondary analyses, we evaluated if the periods of heightened susceptibility to lead varied by sex. First, we used the multiple informant model and assessed the heterogeneity p-value for a sex by blood lead by exposure period product term; we considered p-values < 0.20 to be suggestive of potential sex differences in the periods of heightened susceptibility to lead toxicity. This analysis specifically evaluates if the pattern of associations between repeated blood lead concentrations and BASC-2 scores varies by child sex. When we identified sex differences, we further examined these in adjusted sex-stratified models. We evaluated heterogeneity in the association between blood lead concentrations at each exposure period and BASC-2 scores using the lead by exposure period product term p-value within each sex strata.
We evaluated sex differences in the associations between repeated blood lead concentrations and repeated BASC-2 scores by adding a sex by blood lead product term to fully-adjusted modified Poisson regression models. In these models, we considered product term p-values < 0.05 as evidence of potential sex-differences in the association between blood lead and risk of having BASC-2 scores classified as at-risk or clinically significant.
Sensitivity Analyses
We conducted a series of sensitivity analyses to evaluate the robustness of our results to several assumptions. First, we adjusted our main models for maternal average serum cotinine instead of average serum cotinine during childhood. We also adjusted for both prenatal and childhood cotinine in the models. Prior research suggests that prenatal tobacco smoke exposure could be related to childhood behavior (18). Second, to evaluate the robustness of models, we also adjusted models for maternal IQ scores, HOME Inventory Scores, and maternal education. Third, we assessed results from the multiple informant analyses in the injury prevention and lead hazard intervention groups separately because the lead hazard intervention had previously been associated with lower blood lead concentrations and subtle improvements in children’s neurobehavioral outcomes (14). We also evaluated our results after removing the residential intervention variable from adjusted models. Finally, we conducted additional analyses using only the 8-year BASC outcomes in the multiple informant models. While using repeated BASC-2 measures reduces within-person variation in our outcome, it is possible that variation in the exposure-outcome association across exposure timepoints could be due to heightened susceptibility to lead or latency in the manifestation of behavioral outcomes. Because we previously observed that BASC-2 scores had fair to good reproducibility over childhood (27), we hypothesized that the results would not meaningfully differ when we only used the 8-year BASC scores.
RESULTS
Relations of Blood Lead and BASC-2 Scores with Participant Characteristics
The distributions of blood lead concentrations were similar at ages 1 and 2 years (Figure 1; median blood lead concentration at age 1-year = 1.5 μg/dL; 25th, 75th= 1.0, 2.3). Blood lead concentrations tended to gradually decline for subsequent visits, with the lowest concentrations occurring at age 8-years (median blood lead concentration at 8-years= 0.5 μg/dL; 25th, 75th= 0.4, 0.9). Repeated blood lead concentrations had good reproducibility (ICC= 0.59). Children of mothers who self-identified as non-Hispanic Black, had a high school diploma or less, and a household income less than $45,000 tended to have higher blood lead concentrations compared with other groups (Table 1). On average, children who lived in older rental housing units had higher blood lead concentrations compared with those living in a newer and caregiver-owned residence.
Figure 1. Distribution of children’s blood lead concentrations by study visit at ages 1, 2, 3, 4, 5, and 8 years (The HOME Study; Cincinnati, OH).
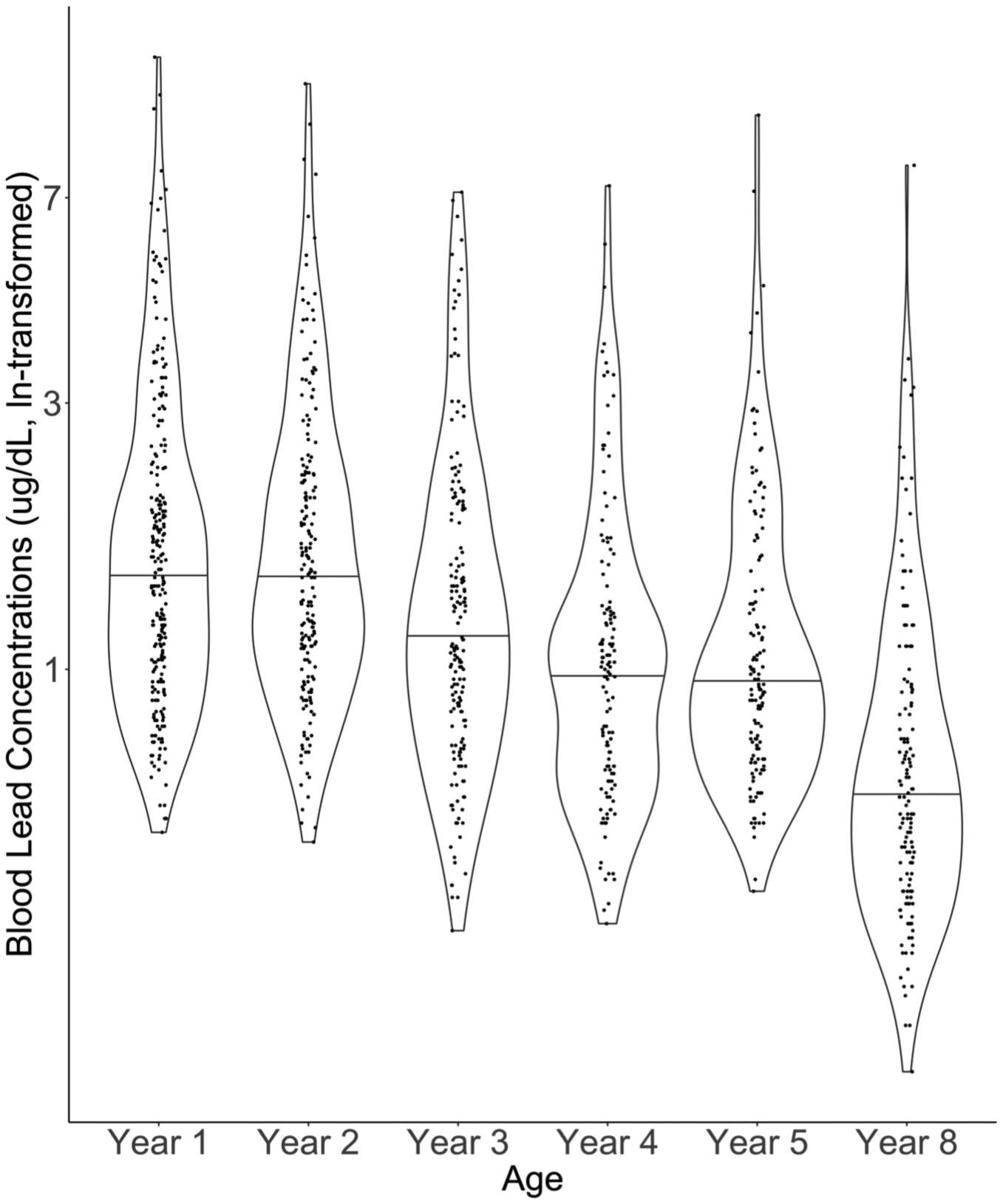
Solid line indicates median of each distribution. Jittered dots are individual observations. Smoothed lines are density functions. Labels on the y-axis have been back-transformed. Observations: Year 1= 229; Year 2 = 203; Year 3= 182; Year 4= 133; Year 5 = 140; Year 8= 147.
Table 1.
Participant characteristics, BASC-2 Composite Scale Scores, and blood lead concentrations (first observation only).
| Characteristic | Total | Adaptive Skills | Behavioral Symptom Index | Externalizing Problems | Internalizing Problems | Blood Lead (μg/dL) |
|---|---|---|---|---|---|---|
| n (%) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Median (25th, 75th) | |
| Total | 244 (100) | 43 (7.8) | 50 (7.3) | 48 (7.8) | 46 (8.4) | 1.5 (1.0, 2.3) |
| Child Sex | ||||||
| Male | 112 (46) | 42 (7.3) | 51 (7.0) | 49 (7.1) | 45 (6.7) | 1.4 (1.0, 2.3) |
| Female | 132 (54) | 45 (8.0) | 50 (7.4) | 47 (8.2) | 46 (9.5) | 1.5 (1.0, 2.5) |
| Maternal Race/Ethnicity | ||||||
| non-Hispanic White | 189 (77) | 43 (6.9) | 50 (6.5) | 48 (7.2) | 45 (6.8) | 1.3 (0.9, 1.9) |
| non-Hispanic Black | 55 (23) | 45 (10) | 51 (9.6) | 49 (9.5) | 48 (12) | 2.6 (1.7, 3.6) |
| Intervention | ||||||
| Injury | 125 (51) | 44 (8.1) | 51 (7.7) | 49 (8.3) | 47 (9.3) | 1.6 (1.0, 2.5) |
| Lead | 119 (49) | 43 (7.5) | 50 (6.7) | 47 (7.2) | 45 (7.3) | 1.4 (1.0, 2.0) |
| Maternal Education | ||||||
| High School or Less | 37 (15) | 44 (8.8) | 53 (9.7) | 51 (10) | 50 (14) | 3.1 (2.0, 3.8) |
| Some College | 59 (24) | 43 (8.8) | 49 (7.1) | 47 (8.1) | 44 (7.2) | 1.7 (1.1, 2.4) |
| College Graduate | 148 (61) | 43 (7.2) | 50 (6.6) | 47 (6.7) | 46 (6.6) | 1.2 (0.9, 1.8) |
| Household Income | ||||||
| < $45,000 | 70 (28) | 45 (9.3) | 52 (8.8) | 50 (9.5) | 48 (11) | 2.4 (1.6, 3.6) |
| $45,000–75,000 | 92 (38) | 42 (7.9) | 50 (6.4) | 46 (6.3) | 44 (6.7) | 1.3 (0.9, 1.9) |
| >$75,000 | 82 (34) | 43 (6.2) | 50 (6.6) | 48 (7.4) | 46 (6.4) | 1.2 (0.9, 1.9) |
| Average Maternal Serum Cotinine (ng/mL) | ||||||
| <0.015 | 100 (41) | 42 (6.9) | 50 (6.9) | 48 (7.0) | 46 (6.6) | 1.3 (0.9, 1.9) |
| 0.015 to 3.0 | 122 (50) | 44 (8.5) | 50 (7.7) | 48 (8.3) | 46 (9.6) | 1.5 (1.0, 2.7) |
| >3.0 | 22 (9) | 42 (7.5) | 51 (6.9) | 50 (8.5) | 45 (8.1) | 3.2 (1.2, 3.8) |
| Average Child’ Serum Cotinine (ng/mL) | ||||||
| <0.02 | 61 (25) | 43 (6.3) | 50 (6.9) | 48 (7.2) | 45 (7.0) | 1.2 (0.9, 1.7) |
| 0.02 to 0.22 | 123 (50) | 43 (7.5) | 49 (6.3) | 47 (6.8) | 45 (6.7) | 1.4 (1.0, 2.0) |
| >0.22 | 60 (25) | 44 (9.7) | 52 (9.0) | 51 (9.4) | 49 (12) | 2.8 (1.4, 3.6) |
| Year Residence was Builta | ||||||
| =<1924 | 76 (31) | 43 (7.5) | 51 (8.3) | 48 (8.9) | 48 (10) | 2.2 (1.5, 3.4) |
| >1924 to 1955 | 97 (40) | 44 (8.2) | 50 (6.7) | 47 (7.3) | 46 (7.3) | 1.3 (1.0, 1.9) |
| >1955 – 1978 | 70 (29) | 43 (7.6) | 50 (6.8) | 48 (7.3) | 44 (7.2) | 1.2 (0.9, 1.9) |
| Residential Ownershipa | ||||||
| No | 61 (25) | 45 (9.0) | 51 (8.9) | 49 (9.3) | 48 (12) | 2.6 (1.5, 3.7) |
| Yes | 182 (75) | 43 (7.4) | 50 (6.7) | 47 (7.1) | 45 (6.5) | 1.3 (0.9, 1.9) |
Missing participant data (n=1)
BASC-2 scores had fair to good reproducibility over childhood (ICC ranging from 0.48 to 0.59; Table S1). Compared with males, females tended to have scores indicative of fewer behavioral problems on most scales. BASC-2 scale scores did not strongly vary by other demographic characteristics. The characteristics of participants who completed the study visit at age 8-years were similar to participant characteristics of the full sample at baseline (Table S2). However, a higher proportion of participants at baseline had a mother who was a college graduate compared to the characteristics of participants who were included from the 8-year study visit.
Differences in the Relations of Blood Lead Concentrations Across Ages with Childhood Behavior
In general, we found trends suggesting positive associations between blood lead concentrations during all ages and higher scores on the BSI and Externalizing Problems scales (Figure 2 and Table S3). We found modest evidence that the associations between blood lead concentrations and the Externalizing Problems scores varied by exposure period (heterogeneity p-value=0.20). Specifically, blood lead concentrations at 8-years (β=3.1-point per unit increase; 95% CI=0.7, 5.4) was more strongly associated with higher Externalizing Problems scores compared to other exposure periods. We also found slight evidence of an adverse association between higher blood lead concentrations and Adaptive Skills scores that was stronger at age 8-years (β= −2.2-point per unit increase; 95% CI= −4.9, 0.5) years compared with other exposure periods (heterogeneity p-value=0.22). In contrast, we did not find evidence to suggest that associations of blood lead concentrations with BSI and Internalizing Problems scores varied by exposure period (heterogeneity p-values= 0.45 and 0.89, respectively).
Figure 2. Adjusted difference in repeated BASC-2 scores at ages 2–8 years per unit increase in ln-transformed blood lead concentration at ages 1, 2, 3, 4, 5, and 8 years.
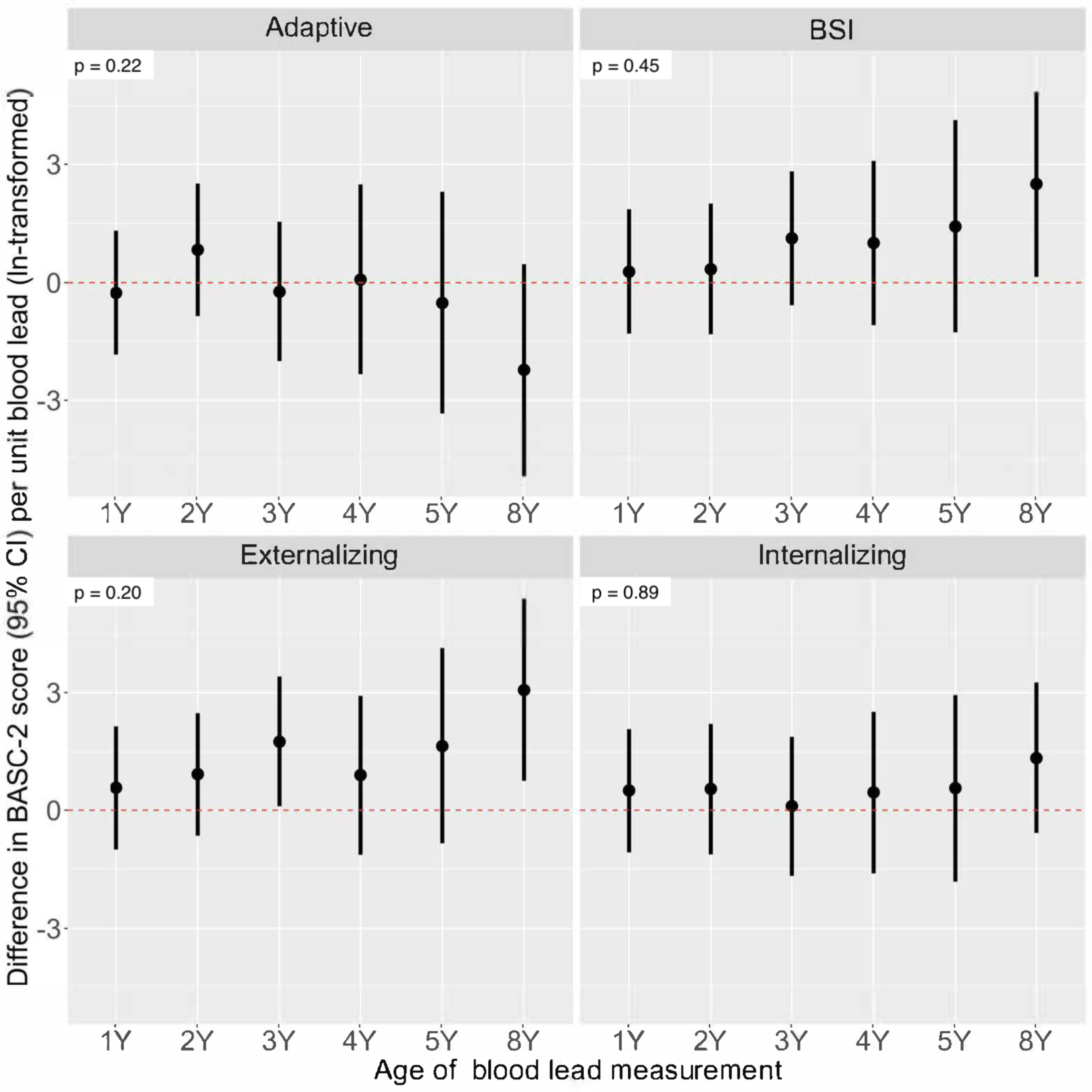
Model adjusted for child’s age, sex, intervention group, household income, maternal race, maternal depressive symptoms, child’s serum cotinine. Total participants = 244 (BASC observations: 2Y = 222; 3Y = 210; 4Y = 151; 5Y = 164; 8Y = 171). Heterogeneity p-values included to indicate difference in lead-BASC associations by child’s age at blood lead measurement.
When we further examined the association between blood lead concentrations and Externalizing Problems subscales we found evidence suggesting that higher blood lead concentrations across all ages were associated with more aggressive and hyperactive behaviors, as well as conduct problems. We did not find strong evidence to suggest that these relations varied by exposure period for the Aggression and Conduct subscales (Figure 3 and Table S4). We did, however, find some evidence suggesting that the association between blood lead concentrations and Hyperactivity scores varied by exposure period (heterogeneity p-value=0.25). Similar to patterns observed for the Externalizing Problems scale, blood lead concentrations at age 8-years (β=3.2-point per unit increase; 95% CI=0.6, 5.8) was more strongly associated with higher Hyperactivity scores compared with other exposure periods.
Figure 3. Adjusted difference in repeated BASC-2 Externalizing Subscale scores at ages 2–8 years per unit increase in ln-transformed blood lead concentration at ages 1, 2, 3, 4, 5, and 8 years.
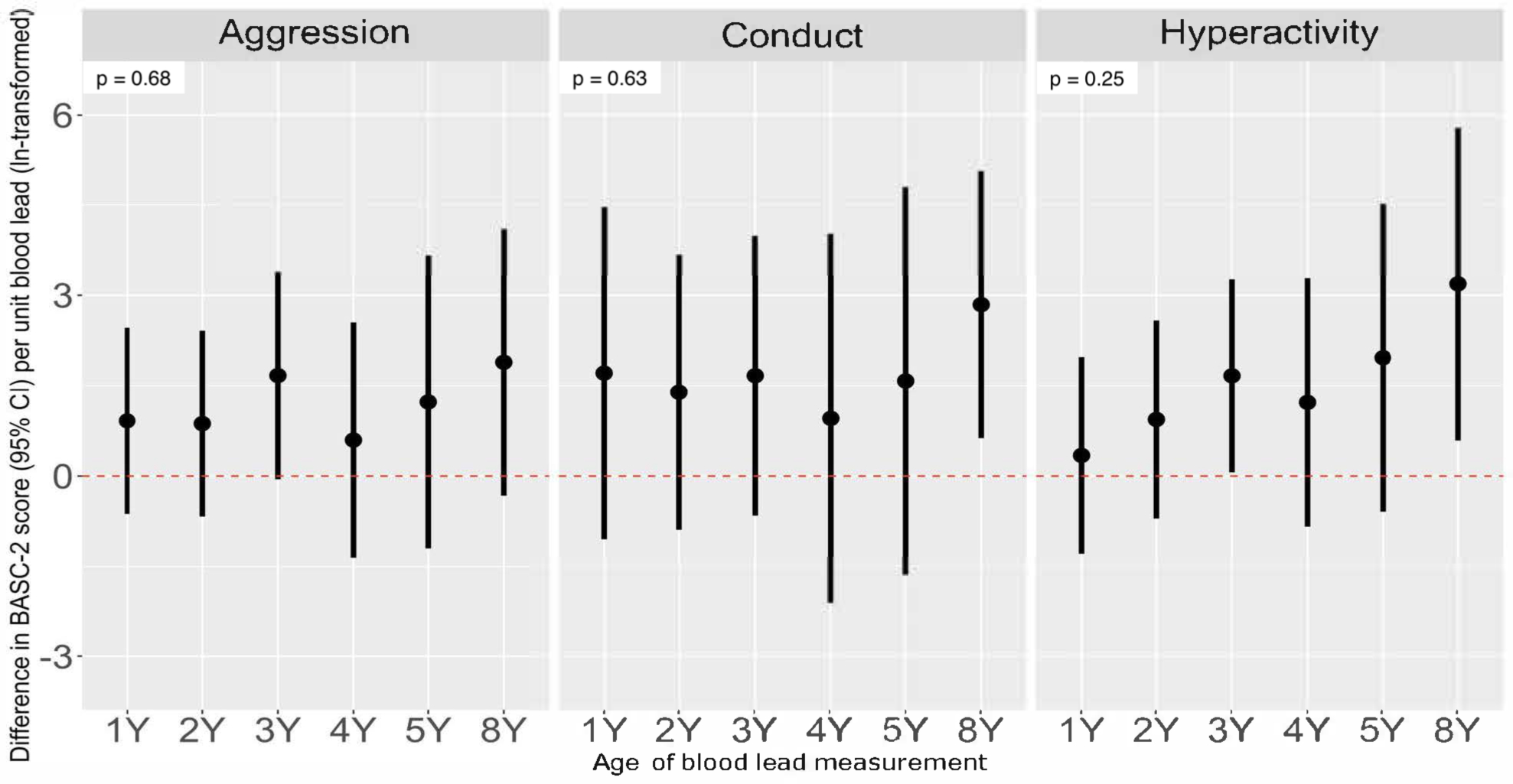
Model adjusted for child’s age, sex, intervention group, household income, maternal race, maternal depressive symptoms, child’s serum cotinine. Total participants for Aggression and Hyperactivity Subscales = 244 (BASC observations: 2Y = 222; 3Y = 210; 4Y = 151; 5Y = 164; 8Y = 171). Conduct models include blood lead concentrations at age 1, 2, 3, 4, 5 and 8 years and BASC-2 scores at age 8 years only (participants n=147). Heterogeneity p-values included to indicate difference in lead-BASC associations by child’s age at blood lead measurement.
Blood Lead Concentrations and Relative Risk of BASC-2 Scores Classified as At-risk or Clinically Significant
Adjusted for covariates, higher repeated blood lead concentrations were associated with higher risk of having scores classified as at-risk or clinically significant on the Adaptive Skills, BSI, and Externalizing Problems scales (Table 2). Furthermore, higher blood lead concentrations were associated with higher risk of having at-risk or clinically significant Hyperactivity, Aggression, and Conduct Problems scores.
Table 2.
Adjusted relative risk of BASC-2 scores classified as at-risk or clinically significant at ages 2–8 years per ln-unit increase in blood lead concentrations.
| BASC-2 Scale | Observationsa / Total | Adjusted RR (95% CI) |
|---|---|---|
| Adaptive Skills | 151/790 | 1.31 (0.98, 1.80) |
| Behavioral Symptom Index (BSI) | 86/790 | 1.60 (1.03, 2.48) |
| Internalizing | 73/790 | 0.96 (0.53, 1.72) |
| Externalizing | 94/790 | 1.56 (0.95, 2.56) |
| Hyperactivity | 114/790 | 1.48 (1.08, 2.02) |
| Aggression | 80/790 | 1.72 (1.19, 2.51) |
| Conduct | 17/147 | 1.73 (0.97, 3.07) |
The number of observations that met the threshold for being considered at- risk for clinically significant problematic behaviors. Total refers to the total number of observations.
Adjusted model includes child’s age, sex, intervention group, household income, maternal race, maternal depressive symptoms, child’s serum cotinine.
Adaptive Skills, BSI, Internalizing, and Externalizing Composite, as well as Hyperactivity and Aggression Subscale models include time-varying blood lead concentrations (ages 2–8 years) and time-varying BASC-2 score category (participants n=232).
Conduct models include blood lead concentrations at age 8 years and BASC-2 scores at age 8 years only (participants n=147).
At-risk for clinically significant problematic behaviors indicated by Adaptive Skills scores ≤ 40, or BSI, Internalizing, as well as Externalizing composite and subscale scores ≥ 60.
Secondary Stratified Analyses
Our results suggested that the pattern in the association between blood lead concentrations at each exposure period and Adaptive Skills scores varied by sex (sex by blood lead by exposure period heterogeneity p-value=0.02). For females, the association between blood lead concentrations and Adaptive Skills scores varied by exposure period (blood lead by exposure period heterogeneity p-value=0.15); higher blood lead concentrations at age 8-years, compared with other exposure periods, were associated with lower Adaptive Skills scores (β=4.8-point per unit decrease; 95% CI=0.6, 5.8). For males, we found slight evidence suggesting the association between blood lead concentrations and Adaptive Skills scores may vary by exposure period (blood lead by exposure period heterogeneity p-value=0.25). However, the confidence intervals for the effect estimates at all exposure periods included the null. Patterns of associations between blood lead concentrations across exposure periods and the other BASC-2 scales were similar among females and males (Figure 4 and Figure S1). Overall, we did not find evidence to suggest that associations between higher repeated blood lead concentrations and the risk of having BASC-2 scores classified as at-risk or clinically significant varied by sex (Table S5, sex by blood lead p-values=0.19–0.94).
Figure 4. Adjusted difference in repeated BASC-2 composite scores at ages 2–8 years per unit increase in ln-transformed blood lead concentration at ages 1, 2, 3, 4, 5, and 8 years by sex.
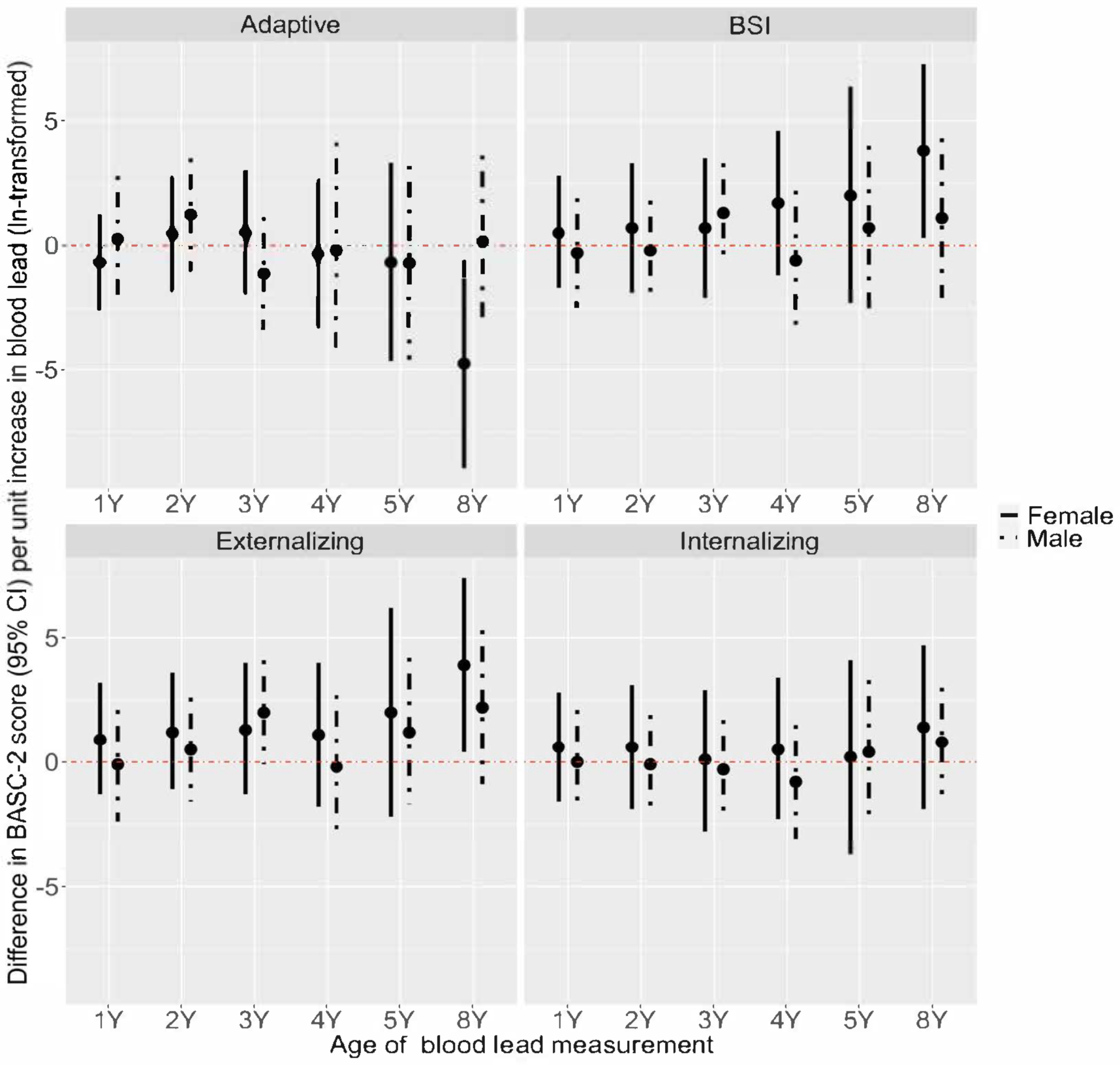
Model adjusted for child’s age, household income, maternal race, maternal depressive symptoms, child’s serum cotinine, intervention group. Total participants = 244 (BASC observations: 2Y = 222; 3Y = 210; 4Y = 151; 5Y = 164; 8Y = 171). Female (n=132) Heterogeneity p-value: Adaptive = 0.15; BSI = 0.39; Externalizing = 0.42; Internalizing = 0.95; Male (n=112) Heterogeneity p-value: Adaptive = 0.25; BSI = 0.21; Externalizing = 0.18; Internalizing = 0.84.
Sensitivity Analyses
In sensitivity analyses, our main results did not meaningfully change when we included maternal cotinine, instead of childhood cotinine, in models or adjusted for both maternal and childhood cotinine (Table S6 and S7). Our results also did not meaningfully change when we adjusted for HOME Inventory scores, maternal IQ, or maternal education (Table S8 and S9). Furthermore, the results did not meaningful change when we stratified by residential intervention group (Figure S2) or when we removed residential intervention as a covariate in the fully-adjusted models (Table S10).
When we used only 8-year BASC-2 scores in multiple informant models, the magnitude of the associations at each timepoint did not meaningfully differ. However, for the Externalizing Problems scale, the associations were more homogenous for blood lead measures between ages 1- and 5-years than our main results (Table S10). We also observed more heterogeneity in the associations of blood lead concentrations with Adaptive Skills scores (heterogeneity p-value=0.05). Higher blood lead concentrations at 8-years (β= −2.2; 95% CI= −4.8, 0.5) were more strongly associated with poorer adaptive skills at age 8-years.
DISCUSSION
Our results indicated that higher blood lead concentrations during the first 8-years of life were associated with elevated risk of several ADHD-related behaviors, specifically aggression, conduct problems, and hyperactivity. We observed trends indicating that higher blood lead concentrations between ages 1- and 8-years were associated with these behaviors; however, we found consistent evidence indicating that higher blood lead concentrations at age 8-years was more strongly associated with these behaviors than at other ages. We found similar results when we restricted our analysis to 8-year BASC-2 scores, the timepoint that is potentially most relevant to long-term behavioral outcomes. In secondary analyses, we found evidence of adverse associations between higher blood lead concentrations at age 8-years and adaptive skills among females, but not males. Overall, our results suggest that the relatively low blood lead levels quantified among contemporary pediatric populations may increase risk for the behavioral features of ADHD.
Our results are consistent with decades of literature indicating that lead exposure at higher levels relative to our contemporary population is associated with cognitive impairments and higher risk of ADHD-related behavioral problems (1,18,28–31). Lead exposure may be directly related to ADHD-related behavior problems during childhood, but it may also indirectly contribute to these behaviors by impairing cognition (32). Froehlich et al (2009) previously estimated that approximately 1 in 5 cases of ADHD among US children ages 8 to 15 years may be attributable to lead exposure.
The removal of lead from gasoline and residential paint has resulted in dramatic declines in blood lead levels for the general US population since earlier studies of lead toxicity. Still, the geometric mean blood lead level during our study period was 1.3 μg/dL (2007–2010) and 0.6 μg/dL (2011–2016) among US children ages 1–5 and 6–11 years, respectively (33). Moreover, structural inequalities disproportionately expose Black Americans and low-income communities to higher levels of lead (33–35). In our study, the geometric mean blood lead concentrations among children ages 1–5 years was 1.3 μg/dL, suggesting that blood lead concentrations, at levels below the CDC reference value of 5 μg/dL, are associated with ADHD-related behaviors. Our results confirm again that there is no safe level of lead exposure, and indicate that susceptibility to lead toxicity extends into middle childhood.
Earlier investigations of lead neurotoxicity suggested that peak blood lead concentrations typically occur around age two years and these concentrations are most strongly associated with childhood cognitive deficits (9,36,37). However, as prospective cohort studies extended later into childhood, growing evidence indicates that blood lead concentrations among school-aged children may be more strongly associated with cognition, as well as associated with gray matter volume in adulthood (31,38–44). We are not aware of any prior studies that have directly compared relations of sequential blood lead concentrations over early life developmental periods with childhood behavior. Prior research conducted by Horton et al used dentine biomarkers to identify periods of heightened susceptibility to lead exposure between 3-months gestation and 1-year postnatally. Results from this analysis identified heightened susceptibility to lead beginning postnatally after 8 months when higher dentine lead concentrations were associated with externalizing behaviors, especially hyperactivity, assessed using the BASC-2 at ages 8–11 years (45).
Overall, the magnitude of differences in age-specific associations was small relative to overall effect sizes and we did not find strong evidence that the association between blood lead concentrations and behavioral profiles varied by exposure timing. We did, however, find consistent evidence indicating that higher blood lead concentrations at age 8-years were associated with more ADHD behavioral features. It is possible that susceptibility to lead toxicity varies during childhood due to time-specific developmental processes. For example, mid-childhood and adolescence could be a period of heightened susceptibility to lead exposure due to structural changes that occur in the frontal cortex and other areas of the brain that control dimensions of attention, self-regulation, and social cognition (46–48). However, it is also plausible that blood lead concentrations at these ages provide more stable estimates of lead body burden, and thus, could be more predictive of lead-related neurobehavioral outcomes (41). Blood lead concentrations primarily reflect recent exposure over the previous months in adults, but blood lead concentrations are also influenced by the release of accumulated lead stored in bones (2). Recent evidence suggests the half-life of blood lead varies by age, with a blood lead half-life equal to approximately 6.9 and 19.3 days for children between the ages of 1 to 3 and from 3 to 14, respectively (49). Furthermore, the relation between lead intake and blood lead concentration differs with age due to variation in gastrointestinal absorption (2,50). Thus, it is plausible that blood lead screening conducted during the first several years of life fails to identify some school-aged children who have higher blood lead concentrations in mid-childhood and are at-risk for lead-related behavioral problems later in life.
Guided by the early lead toxicity studies among toddlers, blood lead screening and prevention programs have heavily focused on the first two years of life (6). The CDC Childhood Lead Poisoning Prevention Program also depicts children less than six years as having heightened susceptibility to lead (7). Our analysis leverages novel statistical methods and repeated blood lead measures to directly compare relations of behavioral profiles with blood lead concentrations during multiple developmental periods (24). We did not find strong evidence to suggest that the first two to six years of life represents a unique period of heightened susceptibility to lead toxicity among children with low-level exposure. Instead, our results suggest that lead exposure at any time during childhood may negatively impact behavior. Because school-aged children spend more time outside of the home and have different time-activity patterns compared to toddlers, it is plausible that the primary exposure sources contributing to blood lead concentrations vary across these developmental periods. Future research could examine if primary prevention, as well as secondary interventions for school-aged children diagnosed with conduct or externalizing behavioral problems can help reduce the burden of lead-related cognitive and behavioral impairments. Our results also highlight the need to identify and reduce additional sources of lead exposure among infants and school-aged children.
Our results indicating that blood lead at age 8 years was associated with poorer adaptive skills among females, but not males, is consistent with some prior epidemiologic and animal studies (12,51). For example, in studies of the Port Pirie cohort, investigators reported a pattern across studies suggesting that females may be more at-risk for lead-related effects on cognition and mental health outcomes in childhood, adolescence, and adulthood (12). Yet, results from studies have also reported no sex-differences or have suggested that males may be more susceptible than females to lead-related cognitive and neurodevelopmental effects (for example: (18,52–55). Our results suggest that inconsistencies in sex-specific associations reported across studies may be due to differences in the timing of the exposure and blood lead concentrations, but this area warrants further investigation in larger studies. During mid-childhood and adolescence, periods of heightened susceptibility to lead exposure may be sex-specific due to differences in the timing and underlying biology of structural changes in regions of the brain controlling self-regulation and social cognition (46,56,57).
Our analysis included a relatively small number of participants with blood lead concentrations above the CDC reference values and consisted primarily of non-Hispanic White participants. Therefore, while we were able to quantify the relations of low blood lead levels with behavioral profiles, the results may not be generalizable to more diverse populations with higher levels of exposure or additional risk factors for ADHD-related behaviors.
Our study had some limitations. First, other socioeconomic and behavioral factors that we did not consider in adjusted models could contribute to residual confounding. However, we report consistent trends in associations between blood lead concentrations and BASC-2 scores across several sensitivity analyses with different covariates. Second, it is possible that data used in the multiple informant models are not missing completely at random, which could bias our results. Furthermore, there is slight variation in exact age of blood lead measurement at each study visit, which may lead to misclassification of the exposure timing that could bias our results. This variation in exposure timing was smaller at younger ages (e.g., at 2-year visit age range≅ 0.6 years) than the 8-year visit (e.g., age range≅ 2.5 years). However, we adjusted all models for exact age (months) of BASC-2 assessment. Third, our sample size may have limited our ability to detect associations, especially in sex stratified analyses of periods of heightened susceptibility to lead toxicity.
Our study also had several strengths. We used repeated measures of blood lead concentrations along with the repeated measures of the validated and reliable BASC-2 instrument across infancy and childhood. Additionally, the HOME Study collected other information about behavioral and mental health data that we considered as covariates in our analyses.
In conclusion, our results suggest that contemporary levels of lead exposure during childhood are associated with ADHD-related behaviors, specifically hyperactivity, aggression, and conduct problems. Understanding how blood lead concentrations across childhood relate to behavior problems can guide public health policy focused on reducing lead-related behavioral effects for all children.
Supplementary Material
Significance:
Contemporary levels of lead exposure during the first 8-years of life were associated with ADHD-related behaviors, specifically aggression, hyperactivity, and conduct problems.
Impact Statement:
Our results highlight the importance of primary lead prevention across childhood.
ACKNOWLEDGEMENTS:
The authors would like to acknowledge David E. Jacobs, Sherry Dixon, Jonathan Wilson, and Kofi Berko for their contribution to this research. The authors and publisher are solely responsible for the accuracy of the statements and interpretations contained in this publication. Such interpretations do not necessarily reflect the views of the US Government.
FUNDING:
The HOME Study and this research was supported by the National Institutes of Environmental Health Sciences grants P01ES011261, R01ES014575, and R01ES020349. Additional support for this research was received through a grant (MDLTS0008-18) from the United States Department of Housing and Urban Development.
Footnotes
ETHICAL APPROVAL: The institutional review boards (IRB) of Cincinnati Children’s Hospital Medical Center (CCHMC) and the participating prenatal clinics and delivery hospitals approved the HOME Study. Brown University deferred to the CCHMC IRB as the IRB of record. Women provided written informed consent for themselves and their children after research assistants explained study protocols during face-to-face visits.
COMPETING INTERESTS: JMB’s institution was financially compensated for his services as an expert witness for plaintiffs in litigation related to PFAS-contaminated drinking water; these funds were not paid to JMB directly. BPL has served as an expert witness for plaintiffs in litigation related to lead poisoning prevention cases, but he received no personal payments for his services. His institution was financially compensated for some of those cases. The other authors declare they have no actual or potential competing financial interests.
DATA AVAILABILITY:
Data are available upon reasonable request. The HOME Study Principal Investigators welcome new collaborations with other investigators and have actively engaged in collaborative data sharing projects. Interested investigators should contact Drs Joseph M. Braun (joseph_braun_1@brown.edu) and Kimberly Yolton (kimberly.yolton@cchmc.org) to obtain additional information about The HOME Study, discuss collaborative opportunities, and request a project proposal form. The HOME Study Protocol Review Committee reviews proposed research projects to ensure that they do not overlap with extant projects and are an efficient use of scarce resources (eg, biospecimens).
References
- 1.American Academy of Pediatrics, Council on Environmental Health. Prevention of Childhood Lead Toxicity. Pediatrics. 2016;138(1):e20161493. [DOI] [PubMed] [Google Scholar]
- 2.Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Lead. Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service; 2020. Accessed September 29, 2020 at www.atsdr.cdc.gov/ToxProfiles/tp13.pdf [Google Scholar]
- 3.Lanphear BP, Rauch S, Auinger P, Allen RW, Hornung RW. Low-level lead exposure and mortality in US adults: a population-based cohort study. Lancet Public Health. 2018;3(4):e177–84. [DOI] [PubMed] [Google Scholar]
- 4.National Toxicology Program. NTP Monograph on Health Effects of Low-Level Lead. Office of Health Assessment and Translation Division of the National Toxicology Program, National Institute of Environmental Health Sciences, National Institutes of Health, US Dept of Health and Human Services; 2012. Accessed January 22, 2021 at https://ntp.niehs.nih.gov/whatwestudy/assessments/noncancer/completed/lead/index.html
- 5.Reuben A, Caspi A, Belsky DW, Broadbent J, Harrington H, Sugden K, et al. Association of childhood blood lead levels with cognitive function and socioeconomic status at age 38 years and with IQ change and socioeconomic mobility between childhood and adulthood. JAMA. 2017. Mar 28;317(12):1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ettinger AS, Leonard ML, Mason J. CDC’s lead poisoning prevention program: A long-standing responsibility and commitment to protect children from lead exposure. J Public Health Manag Pract. 2019;25 Suppl 1, Lead Poisoning Prevention:S5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Childhood Lead Poisoning Prevention. Populations at Higher Risk. 2020. Available from: https://www.cdc.gov/nceh/lead/prevention/populations.htm
- 8.Bellinger DC, Matthews-Bellinger JA, Kordas K. A developmental perspective on early-life exposure to neurotoxicants. Environ Int. 2016;94:103–12. [DOI] [PubMed] [Google Scholar]
- 9.Bellinger DC, Stiles KM, Needleman HL. Low-level lead exposure, intelligence and academic achievement: A long-term follow-up study. Pediatrics. 1992;90(6). [PubMed] [Google Scholar]
- 10.Dietrich KN, Succop PA, Berger OG, Hammond PB, Bornschein RL. Lead exposure and the cognitive development of urban preschool children: The Cincinnati Lead Study Cohort at age 4 years. Neurotoxicol Teratol. 1991;13:203–11. [DOI] [PubMed] [Google Scholar]
- 11.Raymond J, Brown M. Childhood blood lead levels in children aged <5 years — United States, 2009–2014. MMWR Surveil Summ. 2017;66(No.SS-3):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Searle AK, Baghurst PA, van Hooff M, Sawyer MG, Sim MR, Galletly C, et al. Tracing the long-term legacy of childhood lead exposure: A review of three decades of the Port Pirie Cohort study. NeuroToxicology. 2014;43:46–56. [DOI] [PubMed] [Google Scholar]
- 13.Braun JM, Kalloo G, Chen A, Dietrich KN, Liddy-Hicks S, Morgan S, et al. Cohort Profile: The Health Outcomes and Measures of the Environment (HOME) study. Int J Epidemiol. 2016. Mar 22;dyw006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Braun JM, Hornung R, Chen A, Dietrich KN, Jacobs DE, Jones R, et al. Effect of residential lead-hazard interventions on childhood blood Lead concentrations and neurobehavioral outcomes: A randomized clinical trial. JAMA Pediatr. 2018;172(10):934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phelan KJ, Khoury J, Xu Y, Liddy S, Hornung R, Lanphear BP. A randomized controlled trial of home injury hazard reduction: The HOME Injury Study. Arch Pediatr Adolesc Med. 2011;165(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones DR, Jarrett JM, Tevis DS, Franklin M, Mullinix NJ, Wallon KL, et al. Analysis of whole human blood for Pb, Cd, Hg, Se, and Mn by ICP-DRC-MS for biomonitoring and acute exposures. Talanta. 2017;162:114–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reynolds C, Kamphaus R. Behavior Assessment System for Children Manual. 2nd ed. Bloomington, MN: Pearson; 2004. [Google Scholar]
- 18.Braun JM, Kahn RS, Froehlich T, Auinger P, Lanphear BP. Exposures to environmental toxicants and Attention Deficit Hyperactivity Disorder in U.S. Children. Environ Health Perspect. 2006;114(12):1904–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marcus DK, Fulton JJ, Clarke EJ. Lead and conduct problems: A meta-analysis. J Clin Child Adolesc Psychol. 2010;39(2):234–41. [DOI] [PubMed] [Google Scholar]
- 20.The Psychological Corporation. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Psychological Corp; 1999. [Google Scholar]
- 21.Caldwell BM, Bradley R. Home Observation for Measurement of the Environment. Little Rock, AR: University of Arkansas; 1984. [Google Scholar]
- 22.Bernert JT, McGuffey JE, Morrison MA, Pirkle JL. Comparison of serum and salivary cotinine measurements by a sensitive high-performance liquid chromatography-tandem mass spectrometry method as an indicator of exposure to tobacco smoke among smokers and nonsmokers. J Anal Toxicol. 2000;24(5):333–9. [DOI] [PubMed] [Google Scholar]
- 23.Beck A, Steer R, Brown G. Beck Depression Inventory - II. San Antonio, TX: The Psychological Corporation; 1996. [Google Scholar]
- 24.Sánchez BN, Hu H, Litman HJ, Téllez-Rojo MM. Statistical methods to study timing of vulnerability with sparsely sampled data on environmental toxicants. Environ Health Perspect. 2011;119(3):409–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hernán MA. Causal knowledge as a prerequisite for confounding evaluation: An application to birth defects epidemiology. Am J Epidemiol. 2002;155(2):176–84. [DOI] [PubMed] [Google Scholar]
- 26.Hernán MA, Hernández-Díaz S, Robins JM. A structural approach to selection bias. Epidemiology. 2004;15(5):615–25. [DOI] [PubMed] [Google Scholar]
- 27.Braun JM, Yolton K, Stacy SL, Erar B, Papandonatos GD, Bellinger DC, et al. Prenatal environmental chemical exposures and longitudinal patterns of child neurobehavior. NeuroToxicology. 2017;62:192–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Braun JM, Froehlich TE, Daniels JL, Dietrich KN, Hornung R, Auinger P, et al. Association of environmental toxicants and conduct disorder in U.S. children: NHANES 2001–2004. Environ Health Perspect. 2008;116(7):956–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hong S-B, Im M-H, Kim J-W, Park E-J, Shin M-S, Kim B-N, et al. Environmental lead exposure and attention deficit/hyperactivity disorder symptom domains in a community sample of South Korean school-age children. Environ Health Perspect. 2015;123(3):271–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lanphear BP, Dietrich K, Auinger P, Cox C. Cognitive deficits associated with blood lead concentrations <10 pg/dL in US children and adolescents. Public Health Rep. 2000;1:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lanphear BP, Hornung R, Khoury J, Yolton K, Baghurst P, Bellinger DC, et al. Low-level environmental lead exposure and children’s intellectual function: An international pooled analysis. Environ Health Perspect. 2005;113(7):894–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen A, Cai B, Dietrich KN, Radcliffe J, Rogan WJ. Lead exposure, IQ, and behavior in urban 5- to 7-year-olds: Does lead affect behavior only by lowering IQ? Pediatrics. 2007;119(3):e650–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Egan KB, Cornwell CR, Courtney JG, Ettinger AS. Blood lead levels in U.S. children ages 1–11 years, 1976–2016. Environ Health Perspect. 2021;129(3):EHP7932, 037003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muller C, Sampson RJ, Winter AS. Environmental inequality: The social causes and consequences of lead exposure. Annu Rev Sociol. 2018;44(1):263–82. [Google Scholar]
- 35.Whitehead LS, Buchanan SD. Childhood lead poisoning: A perpetual environmental justice issue? J Public Health Manag Pract. 2019;25:S115–20. [DOI] [PubMed] [Google Scholar]
- 36.Pocock SJ, Smith M, Baghurst P. Environmental lead and children’s intelligence: A systematic review of the epidemiological evidence. BMJ. 1994;309:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwartz J Low-level lead exposure and children’s IQ: A meta-analysis and search for a threshold. Env Res. 1994;65:42–55. [DOI] [PubMed] [Google Scholar]
- 38.Baghurst PA, McMichael AJ, Wigg NR, Vimpani GV, Robertson EF, Roberts RJ, et al. Environmental exposure to lead and children’s intelligence at the age of seven years: The Port Pirie Cohort Study. N Engl J Med. 1992;327(18):1279–84. [DOI] [PubMed] [Google Scholar]
- 39.Brubaker CJ, Dietrich KN, Lanphear BP, Cecil KM. The influence of age of lead exposure on adult gray matter volume. NeuroToxicology. 2010;31(3):259–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen A, Dietrich KN, Ware JH, Radcliffe J, Rogan WJ. IQ and blood lead from 2 to 7 years of age: Are the effects in older children the residual of high blood lead concentrations in 2-year-olds? Environ Health Perspect. 2005;113(5):597–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hornung RW, Lanphear BP, Dietrich KN. Age of greatest susceptibility to childhood lead exposure: A new statistical approach. Environ Health Perspect. 2009;117(8):1309–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marshall AT, Betts S, Kan EC, McConnell R, Lanphear BP, Sowell ER. Association of lead-exposure risk and family income with childhood brain outcomes. Nat Med. 2020;26(1):91–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mazumdar M, Bellinger DC, Gregas M, Abanilla K, Bacic J, Needleman HL. Low-level environmental lead exposure in childhood and adult intellectual function: a follow-up study. Environ Health;2011;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reuben A, Elliott ML, Abraham WC, Broadbent J, Houts RM, Ireland D, et al. Association of childhood lead exposure with MRI measurements of structural brain integrity in midlife. JAMA;2020;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Horton MK, Hsu L, Claus Henn B, Margolis A, Austin C, Svensson K, et al. Dentine biomarkers of prenatal and early childhood exposure to manganese, zinc and lead and childhood behavior. Environ Int;121:148–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mills KL, Lalonde F, Clasen LS, Giedd JN, Blakemore S-J. Developmental changes in the structure of the social brain in late childhood and adolescence. Soc Cogn Affect Neurosci. 2014;9(1):123–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Keshavan MS, Giedd J, Lau JYF, Lewis DA, Paus T. Changes in the adolescent brain and the pathophysiology of psychotic disorders. Lancet Psychiatry. 2014;1(7):549–58. [DOI] [PubMed] [Google Scholar]
- 48.Baron Nelson M, O’Neil SH, Wisnowski JL, Hart D, Sawardekar S, Rauh V, et al. Maturation of brain microstructure and metabolism associates with increased capacity for self-regulation during the transition from childhood to adolescence. J Neurosci. 2019;39(42):8362–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Specht AJ, Weisskopf M, Nie LH. Childhood lead biokinetics and associations with age among a group of lead-poisoned children in China. J Expo Sci Environ Epidemiol. 2019;29(3):416–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mushak P Gastro-intestinal absorption of lead in children and adults: Overview of biological and biophysico-chemical aspects. Chem Speciat Bioavailab. 1991;3(3–4):87–104. [Google Scholar]
- 51.Anderson DW, Mettil W, Schneider JS. Effects of low level lead exposure on associative learning and memory in the rat: Influences of sex and developmental timing of exposure. Toxicol Lett. 2016;246:57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dietrich KM, Krafft KM, Bornschein RL, Hammond PB, Berger O, Succop PA, et al. Low-level fetal lead exposure effect on neurobehavioral development in early infancy. Pediatrics. 1987;80(5):721–30. [PubMed] [Google Scholar]
- 53.Pocock SJ, Ashby D, Smith MA. Lead exposure and children’s intellectual performance. Int J Epidemiol. 1987;16(1):57–67. [DOI] [PubMed] [Google Scholar]
- 54.Ris MD, Dietrich KN, Succop PA, Berger OG, Bornschein RL. Early exposure to lead and neuropsychological outcome in adolescence. J Int Neuropsychol Soc. 2004;10(2):261–70. [DOI] [PubMed] [Google Scholar]
- 55.Singh G, Singh V, Sobolewski M, Cory-Slechta DA, Schneider JS. Sex-dependent effects of developmental lead exposure on the brain. Front Genet. 2018;9:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chung YS, Calhoun V, Stevens MC. Adolescent sex differences in cortico-subcortical functional connectivity during response inhibition. Cogn Affect Behav Neurosci. 2020;20(1):1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lenroot RK, Giedd JN. Sex differences in the adolescent brain. Brain Cogn. 2010;72(1):46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request. The HOME Study Principal Investigators welcome new collaborations with other investigators and have actively engaged in collaborative data sharing projects. Interested investigators should contact Drs Joseph M. Braun (joseph_braun_1@brown.edu) and Kimberly Yolton (kimberly.yolton@cchmc.org) to obtain additional information about The HOME Study, discuss collaborative opportunities, and request a project proposal form. The HOME Study Protocol Review Committee reviews proposed research projects to ensure that they do not overlap with extant projects and are an efficient use of scarce resources (eg, biospecimens).


