Abstract
Purpose:
Programmed cell death protein 1(PD-1)/programmed death-ligand 1 (PD-L1) interaction suppresses local T cell responses and promotes peripheral tolerance. In the present study, we focus on PD-1/PD-L1 co-location as a surrogate for this interaction and assess its association with immunotherapy outcomes in patients with non-small cell lung cancer (NSCLC).
Experimental Design:
Pre-treatment biopsies from a retrospective cohort of 154 immunotherapy-treated patients with advanced NSCLC were analysed. Expression of PD-1 and PD-L1 was assessed by multiplexed quantitative immunofluorescence (QIF) and PD-1 expression in the same pixels as PD-L1 (called a co-location score) was measured using an algorithm to define overlapping expression areas. Co-location scores were correlated with immunotherapy outcomes and PD-L1 tumor proportion score.
Results:
PD-1/PD-L1 co-location score was associated with best overall response (p=0.0012), progression free survival (p=0.0341) and overall survival after immunotherapy (p=0.0249). The association was driven by patients receiving immune checkpoint inhibitors in the second or subsequent line of treatment. PD-L1 TPS by IHC was also correlated with best overall response and progression-free survival. PD-L1 measured within the tumor compartment by QIF did not show any significant association with either best overall response or overall survival. Finally, co-location score was not associated with PD-L1 expression by either method.
Conclusions:
Based on our findings, co-location score shows promise as a biomarker associated with outcome after immunotherapy. With further validation, it could have value as a predictive biomarker for the selection of NSCLC patients receiving treatment with immune checkpoint inhibitors.
Keywords: PD-1, PD-L1, immunotherapy, NSCLC, co-location, quantitative immunofluorescence, immunohistochemistry (IHC)
Introduction
Programmed cell death protein 1(PD-1)/programmed death-ligand 1 (PD-L1) axis inhibitors have become the new standard in the treatment of locally advanced or metastatic non-small cell lung cancer (NSCLC). Pembrolizumab has received FDA approval as single agent in the first line of treatment of NSCLC patients with locally advanced or metastatic disease and PD-L1 Tumor Proportion Score (TPS) ≥50%, assessed by the 22C3 (DAKO) assay companion diagnostic test. Additionally, regimens combining immunotherapy (ITx) with chemotherapy have been approved for all patients, irrespective of PD-L1 expression (1, 2). Nivolumab monotherapy, administered after progression to platinum-based chemotherapy regardless of PD-L1 expression, has also exhibited good results with objective response rates (ORR) up to 20% (3, 4). Pembrolizumab has also been approved as a treatment option for patients whose tumors present with high microsatellite instability (MSI-H) or high tumor mutational burden (TMB-H), who have progressed to platinum-based chemotherapy (5, 6). Thus, PD-L1 expression by IHC is the ultimate companion diagnostic test to guide therapeutic decisions in everyday practice. Nonetheless, PD-L1 protein heterogeneity, limited response rates even among high PD-L1 expressors as well as documentation of response among patients with low PD-L1 expression, constitute PD-L1 assessment by IHC an imperfect biomarker (7).
Several studies have addressed the issue of PD-1/PD-L1 spatial relationship and have correlated PD-1/PD-L1 proximity with ITx response. Using serial sections stained for target markers followed by cell quantification by spatial analysis algorithm, Giraldo et al showed that proximity of PD1+ to PD-L1+ cells was associated with response to ITx in Merkel cell carcinoma (8). Also, the PD-1/PD-L1 interaction was defined using multiplexed quantitative immunofluorescence (QIF) by the Automated Quantitative Analysis (AQUA™). Co-location scores were associated with ITx outcomes in patients with advanced melanoma (9). Tumeh et al also showed the association of PD-1/PD-L1 proximity with response to ITx in patients with advanced melanoma; following multiplexed immunofluorescence for PD-1 and PD-L1, proximity was quantified as the number of overlapping positive disks among 10,000 randomly selected disks per tumor region and subsequently correlated with response to ITx in patients with melanoma (10). Most recently, Sánchez-Magraner et al showed that PD-1/PD-L1 interaction, evaluated by Forster resonance energy transfer (FRET), was associated with overall survival in patients with melanoma and NSCLC that received immune checkpoints inhibitors (ICIs) (11).
In this study, we assessed the co-location of PD-1 and its ligand PD-L1 on a retrospective cohort of ITx-treated NSCLC patients, where we define co-location as expression in the same pixels (about 0.5 um resolution). We hypothesized that anti-PD1/PD-L1 agents would preferentially exert their effect on tumors with close cell-to-cell proximity, as reflected by the PD-1/PD-L1 co-location score, rather than high PD-L1 expression alone.
Materials and Methods
Cohort characteristics
Retrospectively collected, pre-treatment, formalin-fixed paraffin-embedded tumor specimens represented in tissue microarray (TMA) format were analyzed. The study cohort consisted of 154 NSCLC patients treated with ITx at Yale New Haven Hospital from 2011 to 2020, represented in two TMAs, YTMA 404 and YTMA 471, constructed at separate timepoints. For each TMA different master blocks were created using non-adjacent tumor cores of 0.6mm diameter from each patient biopsy. 5um-thick cuts from 2 independent blocks for each TMA, each containing a different core from the same patient tumor, were used for staining. Clinical annotations were retrieved from clinical records and pathology reports. PD-L1 TPS were retrieved from pathology reports evaluated with an FDA-approved assay and scored by trained pathologists on matched biopsy samples with those included in the TMAs. Response to ITx was assessed using RECIST v1.1, as complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD). Data were available for best overall response (BOR), progression free survival (PFS) and overall survival (OS). Durable clinical benefit was determined for patients presenting CR, PR or SD for over 6 months; long-term benefit was determined for patients that did not relapse within 12 months post treatment initiation. Written informed consent or waiver of consent was provided by all the patients. The study was approved by the Yale Human Investigation Committee protocol #9505008219 and conducted in accordance with the Declaration of Helsinki. Supplemental Table 1 summarizes the baseline characteristics of the patients included in our study cohort. Cased dichotomized by clinical benefit and long-term benefit and their matching PD-1/PD-L1 co-location scores are included in Supplemental Table 2.
Multiplexed Immunofluorescence Staining
As previously described (12), TMA slides were deparaffinized and subjected to antigen retrieval with 1 mM ethylenediaminetetraacetic acid buffer (pH 8) at 97oC for 20 minutes in a pressure heating container (PT module, Lab Vision). Next, slides were incubated with a solution of 0.3% hydrogen peroxide in methanol for 30 minutes to inactivate endogenous peroxidase, followed by another 30 minutes of incubation with 0.3% bovine serum albumin with 0.05% tween-20 blocking solution. Subsequently, a sequential multiplexed immunofluorescence staining was performed with primary antibodies to detect epithelial tumor cells (pan-cytokeratin, polyclonal, Agilent), PD-1 (clone NAT105, Abcam) and PD-L1 (clone E1L3N, Cell Signaling Technology, RRID:AB_2687655) on the same TMA section (13). Isotype-specific horseradish peroxidase (HRP)-conjugated secondary antibodies and tyramide-based amplification systems were used for signal detection. 4’,6-diamino-2-phenylindole (DAPI) was used to highlight all nuclei. Control slides from index arrays created for this specific purpose were included in each staining experiment to ensure reproducibility.
Fluorescence Image Acquisition and PD1/L1 Co-location
Signal quantification for PD-1 and PD-L1 was determined by the AQUA™ method of QIF on fluorescence images acquired using a PM-2000 system (Navigate Biopharma, Carlsbad, CA, USA), as previously described. Targets were measured within three different compartments: the tumor mask, created by binarizing and then dilating the cytokeratin (CK) signal; the DAPI mask, created by binarizing and then dilating the DAPI signal; and the stromal mask, created by excluding the tumor mask from the DAPI mask, QIF scores were generated by dividing the summed pixel intensities for the marker of interest by the area of the compartment in which it was measured and then normalized to the exposure time and bit depth at which the images were captured. Cases with staining artifacts or less than 2% compartment area were systematically excluded after visual inspection.
PD-1/PD-L1 co-location score was calculated using an algorithm based on previous studies (9). In brief, DAPI signal was dilated to the size of a cell radius, PD-L1 signal was used to create a PD-L1+ cell mask overlapping with all cells and PD-1 signal was used to create a PD-1+ cell mask overlapping with CK negative cells. Our goal was to capture the signal of PD-L1+ cells among both tumor and immune cells, hence the overlapping of PD-L1+ mask with all cells (DAPI). Accordingly, since PD-1+ cells are found exclusively among immune cells, PD-1+ signal overlapping with non-malignant cell (CK negative) area was used to create the PD-1+ mask.PD-L1+ mask was dilated to obtain a mask that also includes the cells in proximity with PD-L1+. This close-proximity mask was then combined with the PD-1+ cell mask to acquire the co-location area of PD-L1+ and PD-1+ cells which were hypothesized to demonstrate interaction at a resolution of about 0.5 um (see Fig. 1). For our experiments, staining, target and co-location score measurements were performed in two independent TMA blocks, with each block containing one nonadjacent tumor core per patient to achieve more accurate tissue representation; QIF scores and co-location scores were then averaged for each case.
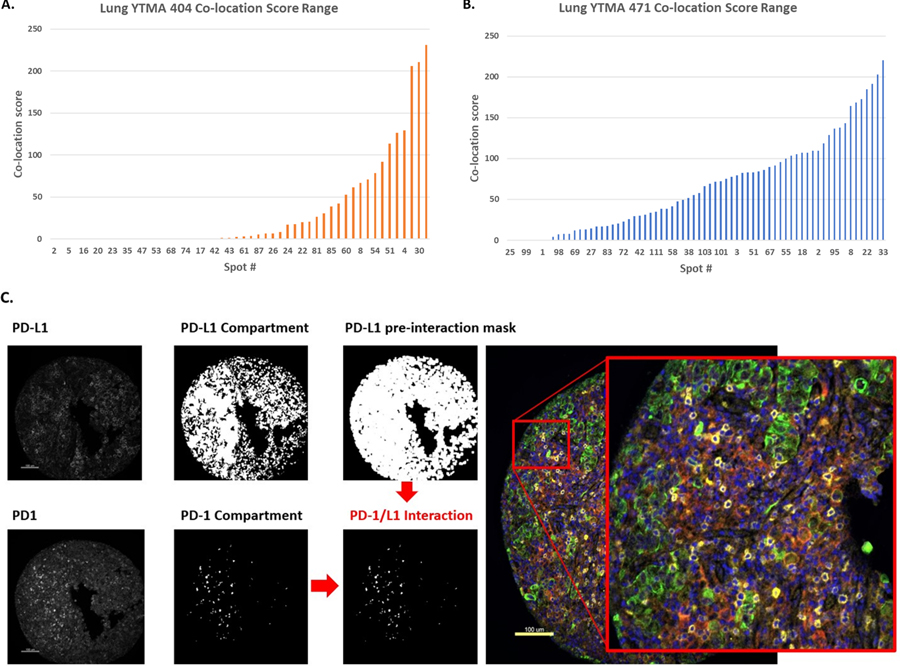
Figure 1.
PD-1/L1 co-location score calculation by QIF.
Statistical analysis
Spearman’s Rank Correlation Coefficient (R) was used to examine the linear association between co-location scores and PD-L1 QIF scores. Comparison of non-continuous variables was performed using the Mann-Whitney U test for two groups and the Kruskal-Wallis H test for three or more groups. Survival curves for OS and PFS were constructed using the Kaplan–Meier estimator and statistical significance was determined using the log-rank test. Multivariate analysis was performed using the Cox proportional hazards model. Cases were stratified into high and low groups by using the median as a cutoff for co-location scores. PD-L1 QIF scores were divided in low for the lowest 75% and high for the highest 25% of detected expression, according to known percentages for low versus high PD-L1 expressors in NSCLC (14). For PD-L1 expression by TPS, groups were stratified by cutoffs of 0%, 1–49% and ≥50%. All hypothesis testing was performed at a two-sided significance level of α equal to 0.05. Statistical analyses were performed using GraphPad Prism v9.0.2 software (GraphPad Software, La Jolla, CA, USA, RRID:SCR_002798) and R. studio 1.4.1106 (Inc., Boston, MA).
Data availability
All data generated and analyzed in this study are housed in Yale AQUAmine interface and are available upon request from the corresponding author Dr D. Rimm.
Results
Co-location score is associated with ITx outcomes in NSCLC
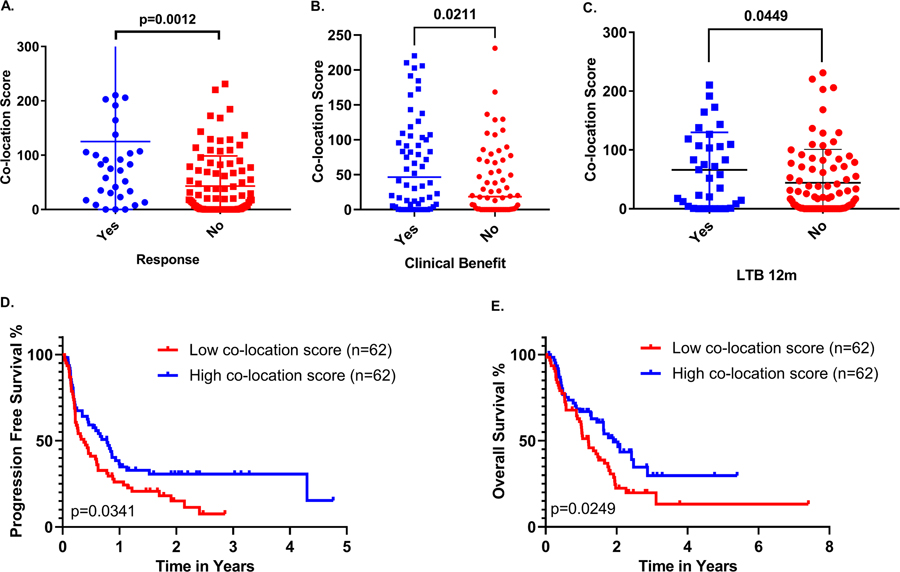
QIF scores, for PD-1 and PD-L1, and co-location scores were evaluable in 124 patients. Co-location scores ranged from 0 to 231 (Fig. 2A, B). Using the median cutoff point, cases were dichotomized in high (n=62) and low (n=62) according to PD-1/PD-L1 co-location score. A representative case with high PD-1/PD-L1 co-location score can be seen in Fig. 2C. To evaluate the association of co-location score with response to ITx, cases were stratified by BOR in responders (CR, PR; n=30) and non-responders (SD, PD; n=91). Responders had significantly higher co-location scores than non-responders (p=0.0012) (Fig. 3A). Durable clinical benefit was correlated with higher co-location scores (p=0.0211) (Fig. 3B). Long-term benefit at 12 months was also correlated with higher co-location scores (p=0.0449) (Fig. 3C). High co-location scores were significantly associated with longer OS (p=0.0249, HR=0.60; 95% CI=0.39–0.93) and PFS (p=0.0341, HR=0.64; 95% CI=0.43–0.97) (Fig. 3D–E) and significance was maintained for both OS and PFS in multivariate analysis with respect to age, gender, smoking status and histological subtype (Supp. Fig. 1A–B).
Figure 2.
A,B. Dynamic range of co-location score for all NSCLC cases (YTMA 404 & YTMA 471). C. Representative TMA core (YTMA 471_spot 95) with high co-location score.
Figure 3.
PD-1/L1 co-location is associated with A. response, B. clinical benefit and C. long-term clinical benefit (>12 months) in ITx treated NSCLC patients. D-E. High co-location score is significantly associated with longer OS (p=0.0246) and PFS (p=0.0341).
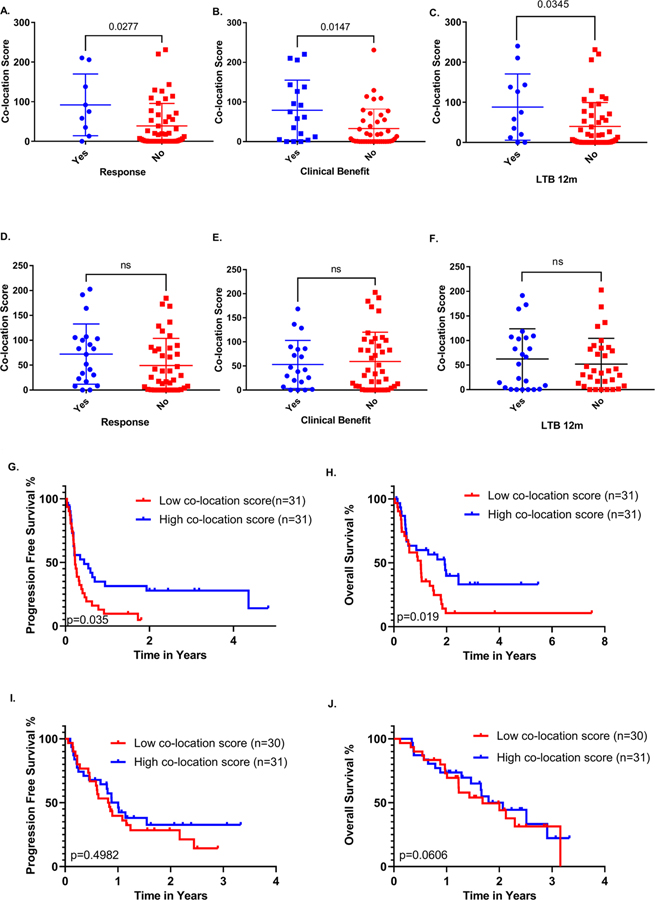
Next, we investigated the effect of PD-1/PD-L1 co-location score with respect to line of treatment. In our study cohort, 69 patients (44.8%) received ITx as first line treatment and 85 patients (55.2%) received ITx in the second or subsequent line of treatment. For the purpose of the analysis, cases were stratified in two groups: first line of treatment and second or subsequent line of treatment. Interestingly, co-location scores correlated with durable clinical benefit in the second or subsequent line of treatment (p=0.0147) (Fig. 4A–C), whereas no similar effect was observed when ITx was administered in the first line of treatment (Fig. 4D–F). Though this may represent an artifact of small sample size, it may be a meaningful biological finding. Additionally, ITx administered in the second or subsequent line of treatment led to significantly longer PFS (p=0.035, HR=0.51; 95% CI=0.29–0.89 and OS (p=0.019, HR=0,52; 95% CI=0.29–0.95) among patients with high PD-1/L1 co-location scores (Fig. 4G–H) an effect that also remained significant in a multivariate model (Supp. Fig. 1C–D); PD-1/PD-L1 co-location scores did not show any clear or consistent association with either PFS or OS for patients that received ITx as first-line treatment (Fig. 4I–J).
Figure 4.
A-C. Association of PD-1/L1 co-location score with response, clinical benefit and long term benefit (>12 months) for >1st line ITx treatment. D-F. 1st line ITx was not associated with response, clinical benefit or long term benefit. G-J. Survival curves depicting the correlation of higher co-location scores with improved OS and PFS for >1st line ITx but not for 1st line ITx in our NSCLC cohort.
PD-L1 TPS association with ITx outcomes and co-location score
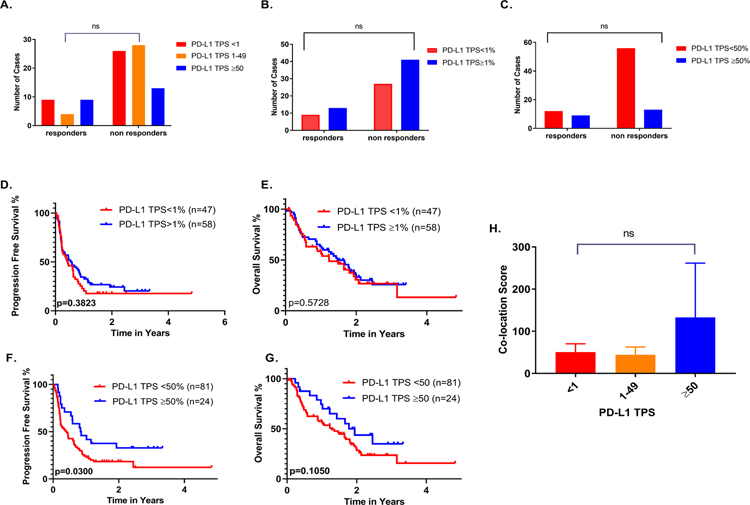
To rule out the possibility that co-location score association with ITx outcomes is a surrogate for PD-L1 expression, we performed response and survival analyses in the study cohort with available PD-L1 TPS data, per FDA guidelines for PD-L1 assessment in NSCLC. In this cohort, PD-L1 expression by IHC was not correlated with response to ITx when cases were stratified to negative (TPS=0%), low (TPS=1–49%) and high (TPS≥50%) expressors as both reponders and non-responders were identified in each group (Fig. 5A–C). PD-L1 TPS also failed to correlate with OS using either the 1% or 50% cutoff points. However, high PD-L1 expression (TPS≥50%) was significantly associated with longer PFS (p=0.0300, HR=0.47;95% CI=0.29–0.89). This suggests that this cohort is roughly similar to clinical trials data where PD-L1 is a predictive biomarker, but is not always significant. (Fig. 5D–G).
Figure 5.
PD-L1 TPS association with response and survival by various cutpoints in whole pt cohort A-G.PD-L1 TPS stratified by various cutpoints did not correlate with co-location score H.
When lines of treatment were examined separately, in contrast to PD-1/PD-L1 co-location score, PD-L1 TPS did not demonstrate any significant association with BOR, PFS or OS neither in the first nor in the second or subsequent lines of treatment (Supp.Fig. 2A–N). Most importantly, using various cutoffs, PD-L1 TPS did not correlate with PD-1/PD-L1 co-location score indicating that co-location score may add to the predictive value of PD-L1 assessment by IHC (Fig. 5H).
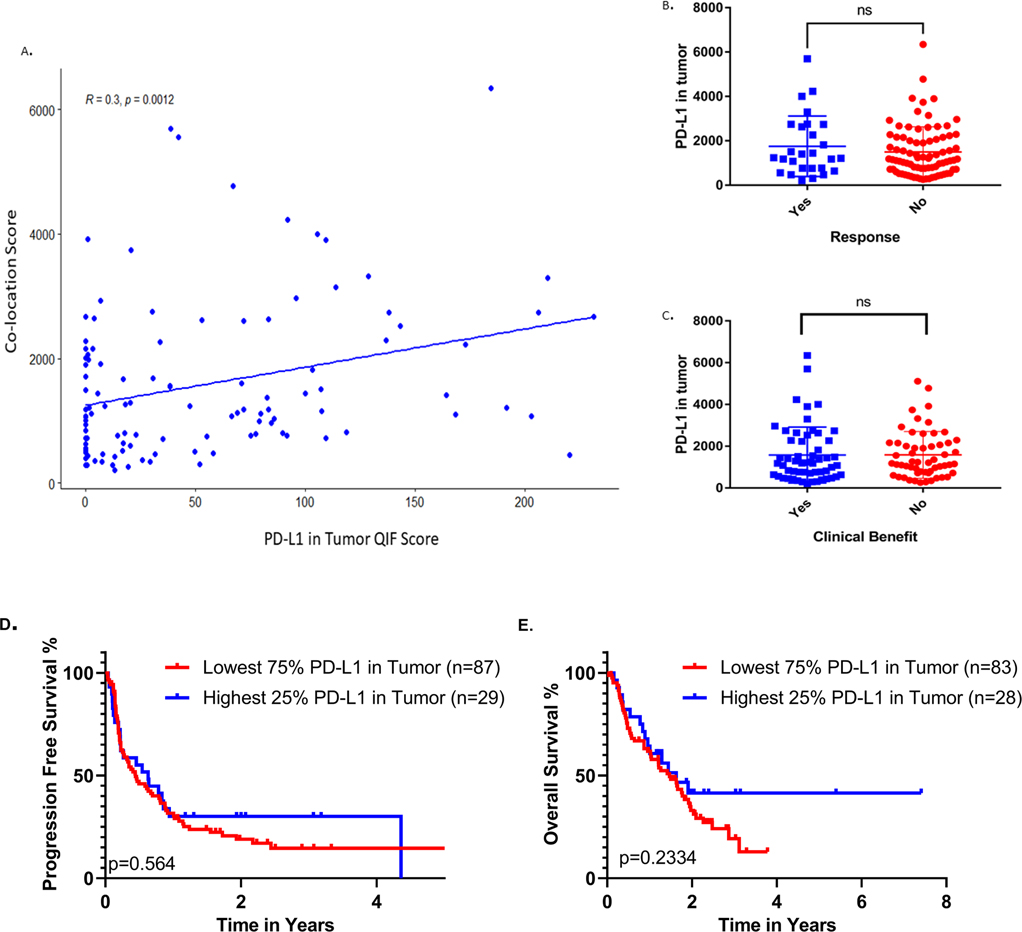
To further exclude the possibility of our observations being the result of high PD-L1 expression regardless of PD-1/PD-L1 proximity, as reflected by co-location score, we measured PD-L1 expression within the tumor compartment by QIF. PD-L1 QIF scores showed moderate correlation with co-location scores (R=0.3) quantified in the same TMA spots (Fig. 6A). Also, QIF scores of PD-L1 in tumor were not associated with BOR or durable clinical benefit after treatment with ICIs (Fig. 6B–C); PD-L1 in tumor by QIF showed no association with PFS or OS neither when all lines of treatment were analyzed together (Fig. 6D–E), nor when first and second or subsequent lines of treatment were examined separately (Supp.Fig. 3A–D). Thus, our observations show that the association between co-location score and outcome are unlikely to be influenced by PD-L1 expression scores.
Figure 6.
A. Correlation of PD-L1 in tumor QIFscores with PD-1/L1 co-location scores. B-C. PD-L1 expression in tumor by QIF is not associated with response or clinical benefit. PD-L1 in tumor by QIF does not correlate with OS or PFS D-E.
Discussion
For many years, PD-L1 has been the only target for IHC companion diagnostic tests, in spite of the fact that the principle behind PD-1 axis drugs is to prevent the interaction between PD-1 and PD-L1. Here we seek to use evidence of the interaction as a companion diagnostic test. Our study indicates that PD-1/PD-L1 co-location, defined by QIF, depicts the interaction of PD-1 with its ligand PD-L1 and could directly probe the target for activity of currently approved ICIs for NSCLC. In addition, the lack of correlation of co-location score with PD-L1 expression shows that co-location score is a discrete biomarker potentially sensing the actual target of the ITx drugs. PD-1/L1 co-location score was correlated with favorable response and prolonged survival for immunotherapy-treated patients with advanced or metastatic NSCLC offering a novel strategy for prediction of ITx outcomes. Under physiologic conditions, PD-1 is predominantly expressed by activated T cells after TCR engagement as well as by NK cells, myeloid cells and antigen presenting cells (APCs) to prevent cell over-activation in acute inflammatory states and protect against autoimmunity. These actions are mediated by several downstream pathways such as PI3K-AKT, RAS and ERK, which are activated after PD-1 engagement with one of its ligands, PD-L1 or PD-L2 and result in reduced cell proliferation and activity and ultimately lead to cell exhaustion (15). Accordingly, while PD-L1 is also encountered on the surface of many immune cell subtypes including T cells, B-cells and APCs it is also expressed by cancer cells (16). In malignancy, this previously described physiologically protective PD-1/L1 interaction acts in favor of tumor immune evasion with catastrophic repercussions for the host (17). The ability of tumor cells to use this mechanism to their advantage presented a target for the development of anti-PD1/L1 ICIs aiming to block PD-1/L1 interaction and reinstate anti-cancer immunity (18). Consequently, as ITx effectiveness depends on a pre-existing interaction rather than the presence of a single target, it can be argued that PD-L1 expression on its own provides only partial understanding of the interplay between tumor and immune cells within the complex tumor microenvironment (TME).
Based on our findings, PD-1/PD-L1 co-location score also correlates with improved ITx outcomes for higher than first-line treatment cases. While ITx has shown superior efficacy in the management of NSCLC as second or subsequent line of treatment compared to chemotherapy regimens (19), response rate is limited to 14–20% and 40% of patients experience disease progression (2, 3, 20, 21). PD-L1 has shown no predictive value in this setting and as a result, in the absence of an approved companion diagnostic test ITx has been universally administered at the cost of mediocre response rates and patient exclusion from alternative treatment options in clinical trials. Nowadays, all patients with advanced NSCLC are eligible to receive ITx as first-line of treatment, either as monotherapy for high PD-L1 expressors or in combination with chemotherapy. This, given our study’s findings, sets a limit to the potential clinical applicability of PD-1/PD-L1 co-location score. Nonetheless, our results reflect an important biological process within the TME, which dictates the necessity for receptor/ligand proximity, rather than high PD-L1 expression, for ITx effectiveness to be achieved in heavily pre-treated patients, and thus provide a strong incentive for further investigation.
This study has a number of limitations. First, the experiments were conducted on a retrospectively collected cohort with heterogeneous treatment and limited size due to the treatment patterns at our institution. In addition, treatment heterogeneity is seen in the various PD-1/L1 inhibitors that were used, either administered as monotherapy or, in some cases, in combination with other agents. Another limitation is that work was done using a tissue microarray strategy, thus limiting the tissue sample size. However, an argument can be made that if the finding is seen in a TMA spot, it would generalize to a conventional tissue section. Furthermore, biopsies of primary tumors were used in a substantial proportion of patients which may not be representative of PD-L1 expression status in metastatic tissue. Another limitation is that we did not include a cohort of non-IO treated pts. Therefore, the utility of co-location score as a predictive rather than prognostic biomarker cannot be demonstrated since it is no longer ethically possible to have a placebo arm and evaluate statistical interaction. Finally, further validation of our findings on tissue biopsy sections in additional cohorts is necessary before drawing definite conclusions on co-location’s score accuracy.
In summary, we showed that PD-1/L1 co-location score is associated with improved ITx outcomes in NSCLC and can be specific to higher lines of treatment regardless of PD-L1 expression.
Supplementary Material
Translational relevance.
Immunotherapy (ITx) for advanced non-small-cell lung cancer (NSCLC) is based on the inhibition of programmed death 1 (PD-1) interaction with its ligand, programmed death-ligand 1 (PD-L1) by targeted monoclonal antibodies. Tumor cell PD-L1 expression measured by immunohistochemistry is the most broadly used predictive biomarker for clinical decision-making regarding ITx. However, a considerable number of low PD-L1 expressors have been found to derive benefit from ITx suggesting that better biomarkers are needed. Previous studies have illustrated significant associations of PD-1/L1 interaction with ITx outcomes for a range of malignancies. In the present study, using quantitative immunofluorescence, we demonstrated that co-location of PD-1 and PD-L1 in the tumor microenvironment can accurately depict their interaction and has the potential to more accurately identify candidates for treatment with immune checkpoint inhibitors in NSCLC, compared with conventional PD-L1 expression assays.
Acknowledgments
Dr N. Gavrielatou is supported by a scholarship from the Hellenic Society of Medical Oncologists (HESMO). Drs. D. Rimm and K. Schalper are supported by the Yale SPORE in Lung Cancer
Footnotes
Disclosure of Potential Conflict of Interest
David L. Rimm has served as an advisor for Astra Zeneca, Agendia, Amgen, BMS, Cell Signaling Technology, Cepheid, Danaher, Daiichi Sankyo, Genoptix/Novartis, GSK, Konica Minolta, Merck, NanoString, PAIGE.AI, Perkin Elmer, Roche, Sanofi, Ventana and Ultivue. Amgen, Cepheid, NavigateBP, NextCure, and Konica Minolta fund research in David L. Rimm’s lab. Kurt A. Schalper received research funding from Genoptix/Navigate (Novartis), Tesaro/GSK, Moderna Therapeutics, Takeda, Surface Oncology, Pierre-Fabre Research Institute, Merck, Bristol-Myers Squibb, AstraZeneca, Ribon Theraputics and Eli Lilly. He has received honoraria for consultant/advisory roles from Moderna Therapeutics, Shattuck Labs, Pierre-Fabre, AstraZeneca, EMD Serono, Ono Pharmaceuticals, Clinica Alemana de Santiago, Agenus and Takeda. Other authors have no potential conflicts of interest.
References
- 1.Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med. 2018;378:2078–92. [DOI] [PubMed] [Google Scholar]
- 2.Reck M, Mok TSK, Nishio M, Jotte RM, Cappuzzo F, Orlandi F, et al. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir Med. 2019;7:387–401. [DOI] [PubMed] [Google Scholar]
- 3.Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373:123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373:1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marcus L, Lemery SJ, Keegan P, Pazdur R. FDA Approval Summary: Pembrolizumab for the Treatment of Microsatellite Instability-High Solid Tumors. Clin Cancer Res. 2019;25:3753–8. [DOI] [PubMed] [Google Scholar]
- 6.Marabelle A, Fakih M, Lopez J, Shah M, Shapira-Frommer R, Nakagawa K, et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020;21:1353–65. [DOI] [PubMed] [Google Scholar]
- 7.Doroshow DB, Bhalla S, Beasley MB, Sholl LM, Kerr KM, Gnjatic S, et al. PD-L1 as a biomarker of response to immune-checkpoint inhibitors. Nat Rev Clin Oncol. 2021. [DOI] [PubMed] [Google Scholar]
- 8.Giraldo NA, Nguyen P, Engle EL, Kaunitz GJ, Cottrell TR, Berry S, et al. Multidimensional, quantitative assessment of PD-1/PD-L1 expression in patients with Merkel cell carcinoma and association with response to pembrolizumab. J Immunother Cancer. 2018;6:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson DB, Bordeaux J, Kim JY, Vaupel C, Rimm DL, Ho TH, et al. Quantitative Spatial Profiling of PD-1/PD-L1 Interaction and HLA-DR/IDO-1 Predicts Improved Outcomes of Anti-PD-1 Therapies in Metastatic Melanoma. Clin Cancer Res. 2018;24:5250–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanchez-Magraner L, Miles J, Baker CL, Applebee CJ, Lee DJ, Elsheikh S, et al. High PD-1/PD-L1 Checkpoint Interaction Infers Tumor Selection and Therapeutic Sensitivity to Anti-PD-1/PD-L1 Treatment. Cancer Res. 2020;80:4244–57. [DOI] [PubMed] [Google Scholar]
- 12.Zugazagoitia J, Liu Y, Toki M, McGuire J, Ahmed FS, Henick BS, et al. Quantitative Assessment of CMTM6 in the Tumor Microenvironment and Association with Response to PD-1 Pathway Blockade in Advanced-Stage Non–Small Cell Lung Cancer. Journal of Thoracic Oncology. 2019;14:2084–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MacNeil T, Vathiotis IA, Martinez-Morilla S, Yaghoobi V, Zugazagoitia J, Liu Y, et al. Antibody validation for protein expression on tissue slides: a protocol for immunohistochemistry. Biotechniques. 2020;69:460–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu H, Boyle TA, Zhou C, Rimm DL, Hirsch FR. PD-L1 Expression in Lung Cancer. J Thorac Oncol. 2016;11:964–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharpe AH, Pauken KE. The diverse functions of the PD1 inhibitory pathway. Nat Rev Immunol. 2018;18:153–67. [DOI] [PubMed] [Google Scholar]
- 16.Chen S, Crabill GA, Pritchard TS, McMiller TL, Wei P, Pardoll DM, et al. Mechanisms regulating PD-L1 expression on tumor and immune cells. J Immunother Cancer. 2019;7:305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun C, Mezzadra R, Schumacher TN. Regulation and Function of the PD-L1 Checkpoint. Immunity. 2018;48:434–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci Transl Med. 2016;8:328rv4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schulz C, Gandara D, Berardo CG, Rosenthal R, Foo J, Morel C, et al. Comparative Efficacy of Second- and Subsequent-line Treatments for Metastatic NSCLC: A Fractional Polynomials Network Meta-analysis of Cancer Immunotherapies. Clin Lung Cancer. 2019;20:451–60 e5. [DOI] [PubMed] [Google Scholar]
- 20.Carbone DP, Reck M, Paz-Ares L, Creelan B, Horn L, Steins M, et al. First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer. N Engl J Med. 2017;376:2415–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, Han JY, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540–50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated and analyzed in this study are housed in Yale AQUAmine interface and are available upon request from the corresponding author Dr D. Rimm.