Abstract
Background:
Average lifetime risk for heart failure (HF) is high, but differs significantly across and within sex-race groups. No models for estimating long-term risk for HF exist, which would allow for earlier identification and interventions in high-risk subsets. The authors aim to derive 30-year HF risk equations.
Methods:
Adults between the ages of 20 to 59 years and free of cardiovascular disease at baseline from 5 population-based cohorts were included. Among 24,838 participants (55% women, 25% Black based on self-report), follow-up consisted of 599,551 person-years. Sex- and race-specific 30-year HF risk equations were derived and validated accounting for competing risk of non-HF death. HF was based on a clinical diagnosis. Model discrimation and calibration were assessed using 10-fold cross-validation. Finally, the model was applied to varying risk factor patterns for systematic examination.
Results:
The rate of incident HF was 4.0 per 1000 person-years. Harrell’s c statistics were 0.82 (0.80–0.83) and 0.84 (0.82–0.85) in White and Black men, and 0.84 (0.82–0.85) and 0.85 (0.83–0.87) in White and Black women, respectively. Hosmer-Lemeshow calibration was acceptable, with χ2 <30 in all subgroups. Risk estimation varied across sex-race groups: for example, in an average 40-year-old non-smoker with an untreated systolic blood pressure of 140 mm Hg and body mass index of 30 kg/m2, risk was estimated to be 22.8% in a Black man, 13.7% in a White man, 13.0% in a Black woman, and 12.1% in a White woman.
Conclusions:
Sex- and race-specific equations for prediction of long-term risk of HF demonstrated high discrimination and adequate calibration.
Subject Terms: Epidemiology, Heart Failure, Primary Prevention, Risk Factors
Graphical Abstract
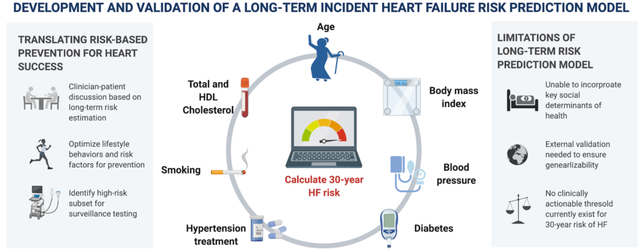
This study demonstrates that long-term risk for incident heart failure varies significantly among young and middle-aged adults and can be reliably estimated using clinical risk factor levels that are readily available in the primary care setting. We derived and validated a novel risk tool that can guide discussions between patients and clinicians about strategies to lower risk of heart failure across the life course through a combination of lifestyle and pharamaceutical approaches. Furthermore, emerging therapies such as sodium glucose co-transporter 2 inhibitors may be considered earlier in individuals at high long-term risk to prevent heart failure. Our findings translate the epidemiologic evidence about lifetime risk of heart failure into a practical risk tool that can be applied in the clinical setting.
INTRODUCTION
Heart failure (HF) is an important and growing contributor to loss of total and healthy life years.1–3 In the US alone, it accounts for over 1.2 million hospitalizations and contributes to over 300,000 deaths annually.4 Age-adjusted mortality rates for HF are increasing, particularly among young adults <65 years.5 Without effective risk-based prevention strategies for HF that can be implemented in younger adulthood, prevalence of HF is expected to exceed 8 million by 2030.6 The high and growing prevalence of HF is associated with a significant burden on the US healthcare system, with direct annual costs of $11 billion in 2014, and projected direct annual costs of $53 billion dollars by 2030 if current trends continue.2, 7 Significant and pervasive race-based disparities persist with higher rates of incident HF in Black compared with White men and women that are due, in large part, to differences in risk factor levels, healthcare access, and upstream social determinants of health.8
Recent guidelines by American College of Cardiology/American Heart Association (ACC/AHA) prioritize identifying individuals at increased risk of developing HF so they can be undergo more detailed surveillance (e.g., biomarker testing, echocardiography) and be targeted for aggressive preventive interventions, such as intensive blood pressure (BP) lowering or emerging therapies for HF prevention (e.g., sodium glucose co-transporter 2 inhibitors [SGLT2i]).9, 10 Cumulative exposures to known modifiable risk factors are largely responsible for HF risk, and validated short-term risk estimation models, such as our recently published Pooled Cohort Equations to Prevent Heart Failure (PCP-HF), can enhance identification of individuals at high short-term (10-year) risk for incident HF.11 However, long-term risk can not be readily extrapolated from short-term (10-year) risks. Further, some individuals at low 10-year risk of HF may be at high long-term risk for HF.12 Therefore, understanding both short- and long-term risks for HF may be especially relevant among younger adults who, because of their age, have a low absolute 10-year risk. Some of these adults likely have a high lifetime risk and will experience greater relative benefits from interventions earlier in the life course.13
Average lifetime risk estimates of HF vary significantly between 20 to 46% in the population.14, 15 However, to date, no models for individualizing long-term risk estimates of HF (which must account for competing risks) exist. Therefore, we sought to derive risk models to estimate individualized long-term risk of incident HF and determine the impact of varying risk factor levels and combinations.
METHODS
Data Availability.
All data and materials utilized in this manuscript are made publicly available by the National Heart, Lung, and Blood Institute Biologic Specimen and Data Repository Information Coordinating Center (BioLINCC).
Study Sample.
For the derivation sample, we included pooled individual-level data from 5 large, racially and geographically diverse, National Heart Lung and Blood Institute (NHLBI)-sponsored cohort studies with long-term follow-up: the Atherosclerosis Risk in Communities (ARIC) Study, Coronary Artery Risk Development in Young Adults (CARDIA) Study, Framingham Heart Study (FHS), Framingham Offspring Cohort (FOF), and Multi-Ethnic Study of Atherosclerosis (MESA).16 The study designs of each cohort have been previously described.16–21 All cohorts represent community-based or population-based samples with direct measurement of risk factors and surveillance and adjudication for incident HF. In each cohort, race was based on self-report and is included with the acknowledgement that race is as a social construct. Participants who self-reported race other than Black or White were excluded due to small sample sizes.
Patient and Public Involvement.
Development and validation of the risk prediction equations did not directly involve patients, as pre-existing databases were utilized that did not require additional patient involvement or consent. Dissemination of results to study participants directly is not feasible but our published results will be available to any relevant study participant in addition to the greater public.
Model Inputs: Exposure and Outcome Ascertainment.
The protocols used to measure and define demographic and clinical phenotypes (predictor variables) and ascertainment of outcomes (HF events and non-HF related deaths) for the included model derivation cohorts (ARIC, CARDIA, FHS, FOF, MESA) have been described previously and were generally similar across the included cohorts.16–21 Height, weight, BP, and fasting glucose and cholesterol were measured directly in all participants at in-person examinations; smoking status was self-reported. All included cohorts had near complete (>99%) ascertainment of vital status during follow-up. Incident HF events were adjudicated with the use of strategies selected by each cohort’s investigator group with available clinical records and pre-specified criteria (detailed descriptions are provided in the Expanded Methods in the Online Supplement). We defined non-HF related death (the competing risk in our analyses) as death due to other causes in participants without prior HF. Black and White participants from the pooled cohorts were included in the analysis if they were between the ages of 20 to 59 years old, and free from cardiovascular disease at baseline (coronary heart disease, stroke, and HF). Participants were excluded if they had incomplete baseline measurement of risk factors. The study protocol was approved by the Institutional Review Board at Northwestern University.
Long-term Risk Prediction Modeling.
Long-term risk estimation conveys the cumulative risk of developing a disease during the remainder of an individual’s life. Competing risk analyses are recommended in analyses such as these where a significant proportion of participants may not experience the outcome of interest (such as HF), because they have already experienced a “competing event” such as death from non-HF related causes. Failure to account for non-HF related death as a competing risk tends to lead to overestimation of HF risk, especially among older populations.15 Therefore, we used cause-specific hazard modeling to account for competing risks and we developed sex- and race-specific long-term models to estimate 30-year risk of HF with death from non-HF causes accounted for as a competing risk. We used the same predictor variables as the published 10-year PCP-HF model, which included age, systolic BP, body mass index (BMI), total cholesterol, high density lipoprotein cholesterol, and smoking, diabetes, and hypertension treatment status across sex-race groups.11 These covariates are established risk factors for HF.
To predict cumulative incidence rates for HF events and non-HF death, two Cox regression models for cause-specific risk of HF events and non-HF death were fitted to the data. The baseline cumulative hazard function and regression coefficients were estimated, which were then used to estimate the long-term or 30-year HF risk using a previously published formula to derive the competing cumulative incidence function (detailed descriptions of analytic approach in Online Supplement).22, 23 Specifically, this formula multiplies the hazard contribution for HF at a given age by the probability of being alive and free of HF at that age, and then sums these estimates across the age range of interest. It can be used to calculate the cumulative incidence function for an individual participant based on age at baseline and long-term follow-up, and can also incorporate the patient’s covariate values to estimate individual cumulative incidence values. We used the cut point of 30-years of follow-up in our definition of long-term risk estimation as this is consistent with the available data. We compared the estimates derived from the age-specific ten-year sub-datasets (age 20–29, 30–39, 40–49, 50–59 years) with the overall sample (age 20–59 years), and there was no significant difference for the estimated probability values and their pattern with varying risk profile for long-term HF risk. Finally, we combined all participants and fitted continuous age as a predictor. The analysis was employed separately for each sex and race group based on self-report, given known differences in both HF risk and competing risks of death across sex-race groups.14 However, it must be acknowledged that race was incorporated as a social construct to develop unique models based on participant self-report for each sex-race group and race does not reflect a predictor or risk factor. Model performance was assessed using C-statistic and Hosmer-Lemeshow calibration statistics. Based on prior publications, we defined the C-statistic a priori as less than 0.70, 0.70 to 0.80, and greater than 0.80 as inadequate, acceptable, and excellent discrimination levels, respectively. Model calibration was evaluated by the Greenwood-Nam-D’Agostino (GND) approach with adequate calibration defined a priori as χ2<20.24
Systematic Examination: Variation of Single and Multiple Risk Factors.
In order to systematically examine the chracteristics of the 30-year HF risk estimation models and examine the distribution of long-term risk estimates across sex-race groups, we applied the newly derived 30-year models across varying risk factor profiles.25, 26 Specifically, we generated 30-year risk estimates for a hypothetical White man, Black man, White woman, and Black woman from 20 to 59 years of age. This approach does not examine risk for a specific person or specific populations; rather, it varies risk factor levels individually and in pre-specified combinations in a systematic manner to examine the range of outputs for the 30-year HF risk prediction tool. We used age-adjusted national mean values for risk factor levels derived from published analyses for US adults aged 20–59 years from the National Health and Nutrition Examination Survey (NHANES) cycles 2013–2016 (Online Table I).25 For treated and untreated systolic BP (SBP) and BMI, we used age-specific values to reflect changes in these risk factor values with normative aging and among those who were and were not treated with antihypertensive medications (See Online Supplement for detailed analyses).
All statistical analyses were performed with the use of SAS statistical software version 9.4 (SAS institute) and R Studio version 3.1.2. Two-sided values of P < 0.05 were considered statistically significant.
RESULTS
Characteristics of the Derivation Sample.
Baseline characteristics and total counts of incident HF among White and Black adults who formed the study sample are shown in Table 1. We identified 24,838 participants aged 20–59 years at baseline for the derivation cohort, of whom 55% were women and 25% were Black based on self-report; mean age of the overall sample was 43±12 years. Prevalence of risk factors varied across sex-race groups with lower prevalence of diabetes in White men and women compared with Black men and women. Overall, risk factor levels are representative of the general US population. Baseline demographics and mean risk factor values by cohort are shown in Online Table II. Over a combined follow-up of 599,551 person-years, rate of incident HF was 4.0 per 1000 person-years (Online Table III). Significant sex-race differences existed with highest rates of incident HF in Black men (5.1 per 1,000 person-years) and lowest rates in White women (3.5 per 1,000 person-years).
Table 1.
Baseline Characteristics among Black and White Men and Women in the Derivation Sample from 5 Population-Based Cohorts*
| Black | White | |||
|---|---|---|---|---|
| Men N=2626 |
Women N=3659 |
Men N=8647 |
Women N=9906 |
|
| Mean age, years (SD) | 39.9 (14.0) | 40.8 (13.6) | 44.5 (11.6) | 44.5 (11.5) |
| Diabetes, n (%) | 255 (9.7) | 357 (9.8) | 462 (5.3) | 377 (3.8) |
| Current smoking, n (%) | 941 (36) | 1001 (27) | 2800 (32) | 3123 (32) |
| Mean systolic blood pressure, mm Hg (SD) | 123 (18) | 119 (20) | 122 (16) | 116 (18) |
| Hypertension treatment, n (%) | 515 (20) | 970 (27) | 963 (11) | 1216 (12) |
| Mean total cholesterol, mg/dL (SD) | 193 (42) | 196 (43) | 204 (41) | 206 (43) |
| Mean HDL cholesterol, mg/dL (SD) | 51 (16) | 56 (16) | 45 (13) | 56 (16) |
| Mean BMI, kg/m 2 | 26.8 (5.1) | 29.1 (7.1) | 26.7 (4.0) | 25.5 (5.2) |
| Incident heart failure, n (%) | 292 (11.1) | 376 (10.3) | 861 (10.0) | 855 (8.6) |
Individual-level participant data pooled across 5 cohorts, which included ARIC, CARDIA, FHS, FOF, and MESA.
Model Development & Performance.
Sex- and race-specific equations for 30-year risk of HF included age, SBP, BMI, total cholesterol, high density lipoprotein cholesterol, and smoking, diabetes, and hypertension treatment status (Online Table IV). Similar coefficients were calculated for White adults when FHS and FOS were excluded (Online Table V). Discrimination of the 30-year risk model for HF, adjusted for competing risk of non-HF death, was excellent in all groups, with Harrell’s c statistics (95% confidence interval) of 0.82 (0.80, 0.83) and 0.84 (0.82, 0.85) in White and Black men, and 0.84 (0.82, 0.85) and 0.85 (0.83, 0.87) in White and Black women, respectively. Hosmer-Lemeshow calibration statistic χ2 was <30 in all subgroups with a statistically significant p-value in White women only (Figure 1). The R Function of the model for use is available in the Online Supplement. The risk factor levels must be in the following clinical range for continuous inputs based on the available range in the derivation sample (SBP [80 to 200 mm Hg]; TC [100 to 300 mg/dL]; HDL-C [30 to 100 mg/dL]). This is consistent with previously published risk prediction equations like the 2013 ACC/AHA Pooled Cohort Equations for ten-year risk prediction of ASCVD.
Figure 1. (A-D). Calibration Plots of 30-year risk model of heart failure adjusted for competing risk of non-HF death.
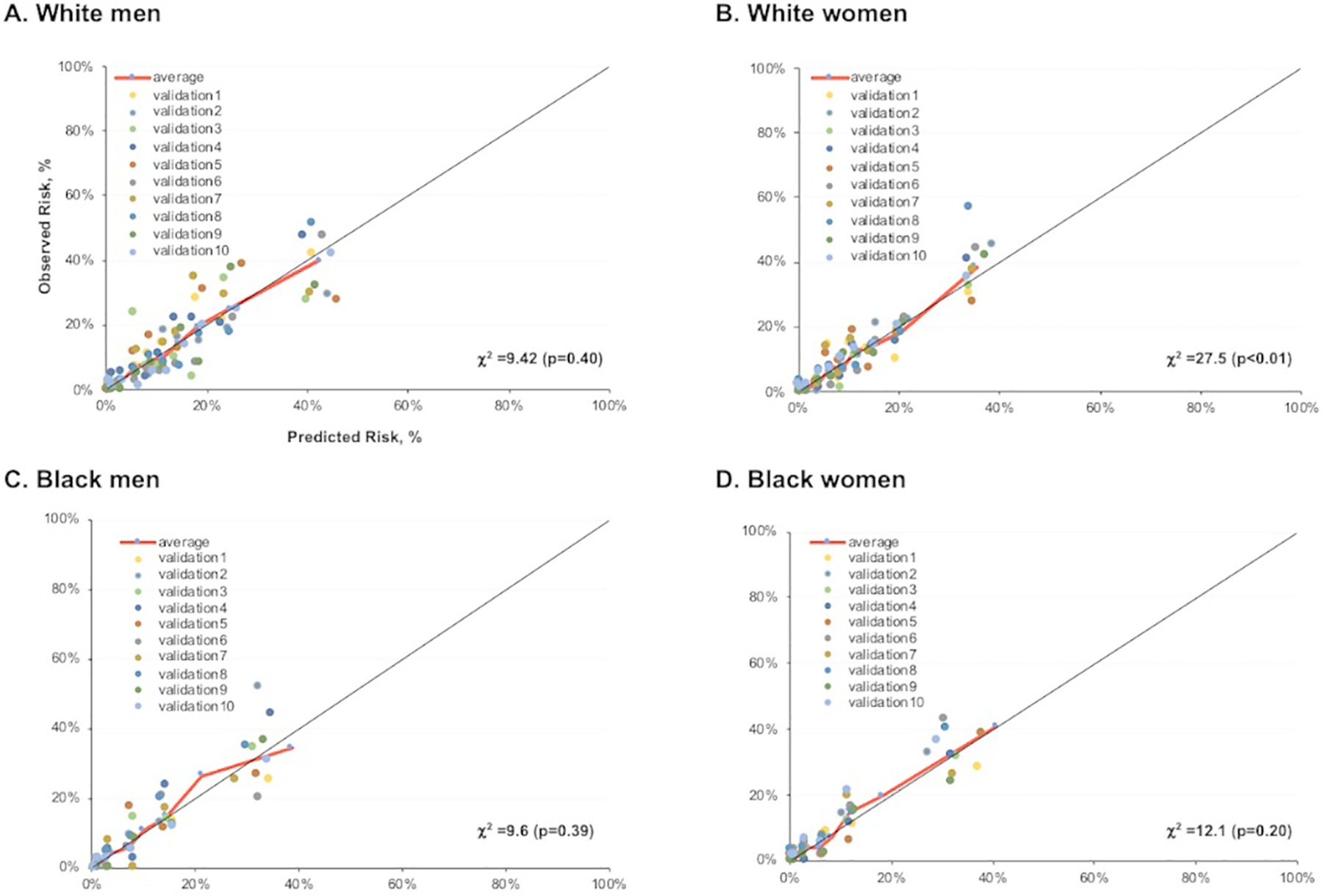
Observed compared with predicted 30-year risk of heart failure (HF) among White men (A), Black men (B), White women (C), and Black women (D) adjusted for competing risk of non-HF death using a 10-fold cross validation approach. Each data point represents one of the ten-fold cross validation runs with the average represented by the red line. Risk factor values are restricted to the clinical range in the derivation sample, including systolic blood pressure 80 to 200 mm Hg, total cholesterol 100 to 300 mg/dL, high density lipoprotein cholesterol 30 to 100 mg/dL.
Distribution of 30-year Predicted HF Risk.
When all other risk factors were held constant at the age-adjusted national mean levels (based on NHANES 2013–2016) for each sex-race group, 30-year predicted HF risk was substantially higher with older age (Figure 2). Estimated 30-year HF risks for a Black and White man and woman across individual levels of SBP and BMI are shown in Figure 3 and Online Figures I–IV; for each analysis, all other risk factors are held constant at age-adjusted mean levels with normative values of non-smoking and non-DM status. The absolute risks and slopes of association across risk factor values were more prominent at older ages, and generally highest for Black men.
Figure 2. (A-D) and Central Illustration. 30-year risk of heart failure across sex-race and age groups adjusted for competing risk of non-cardiovascular death.
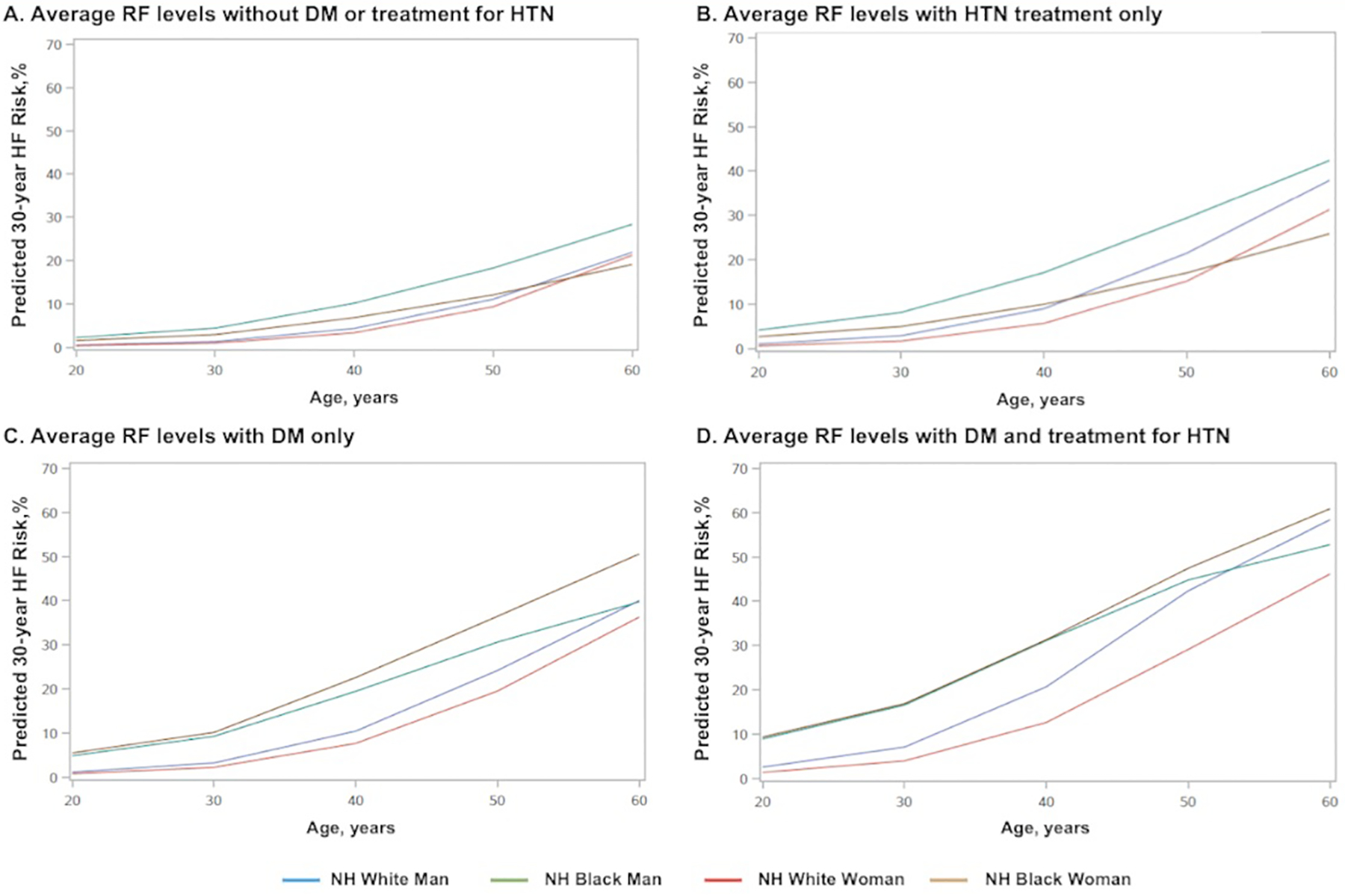
30-year predicted risk for heart failure (HF) for a hypothetical White or Black man or woman at interval selected ages, with risk factors held constant at approximate age-adjusted national means (among nonsmokers). (A) 30-year HF risk estimates for those without DM and not taking antihypertensive medications (B) 30-year HF risk estimates for those without DM and taking antihypertensive medications. (C) 30-year HF risk estimates for those with DM but not taking antihypertensive medications. (D) 30-year HF risk estimates for those with DM and taking antihypertensive medications. BP= blood pressure; DM= diabetes mellitus; HTN=hypertension; RF=risk factor
Figure 3. (A-H). 30-year predicted heart failure risk across different age groups with varying risk factor levels of systolic blood pressure (untreated) and body mass index adjusted for competing risk of non-HF death.
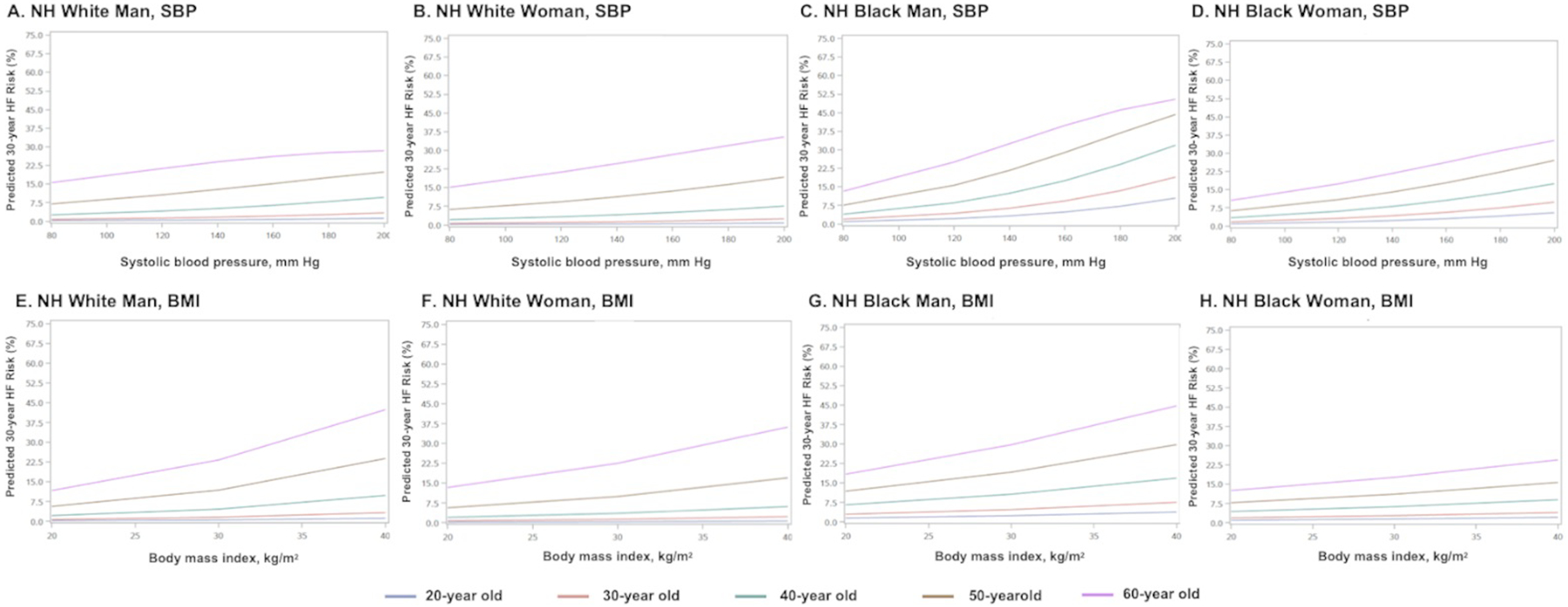
Ten-year predicted risks for heart failure by varying levels of single risk factors in a hypothetical White man at selected ages, with other risk factors held constant at approximate age-adjusted national means (including nonsmoking). SBP= systolic blood pressure; BMI=body mass index; NH=non-Hispanic
Thirty-year predicted HF risk with simultaneous variation of multiple risk factors is demonstrated for a hypothetical 40-year-old of each sex and race group in Figure 4 and Online Figures V–VIII. In all sex and race groups with TC and HDL-C at age-adjusted national means, the lowest 30-year predicted HF risk was observed in a hypothetical individual with the following optimal risk factor levels entered: non-smoker, non-DM with untreated SBP of 120mmHg and BMI of 20 kg/m2. The highest observed 10-year predicted HF risk (with TC and HDL-C at sex-race specific age-adjusted means) was in a hypothetical individual with treated SBP of 160 mm Hg and BMI of 35 kg/m2. Thirty-year predicted HF risk in a hypothetical 40-year-old varied from 5.4% to 63.7% in a White man, 10.2% to 63.6% in a Black man, 5.7% to 45.6% in a White woman, and 7.0% to 70.0% in a Black woman.
Figure 4. (A-D): 30-year predicted heart failure risk in a hypothetical 40-year old adult varying multiple risk factors and adjusted for competing risk of non-HF related death.
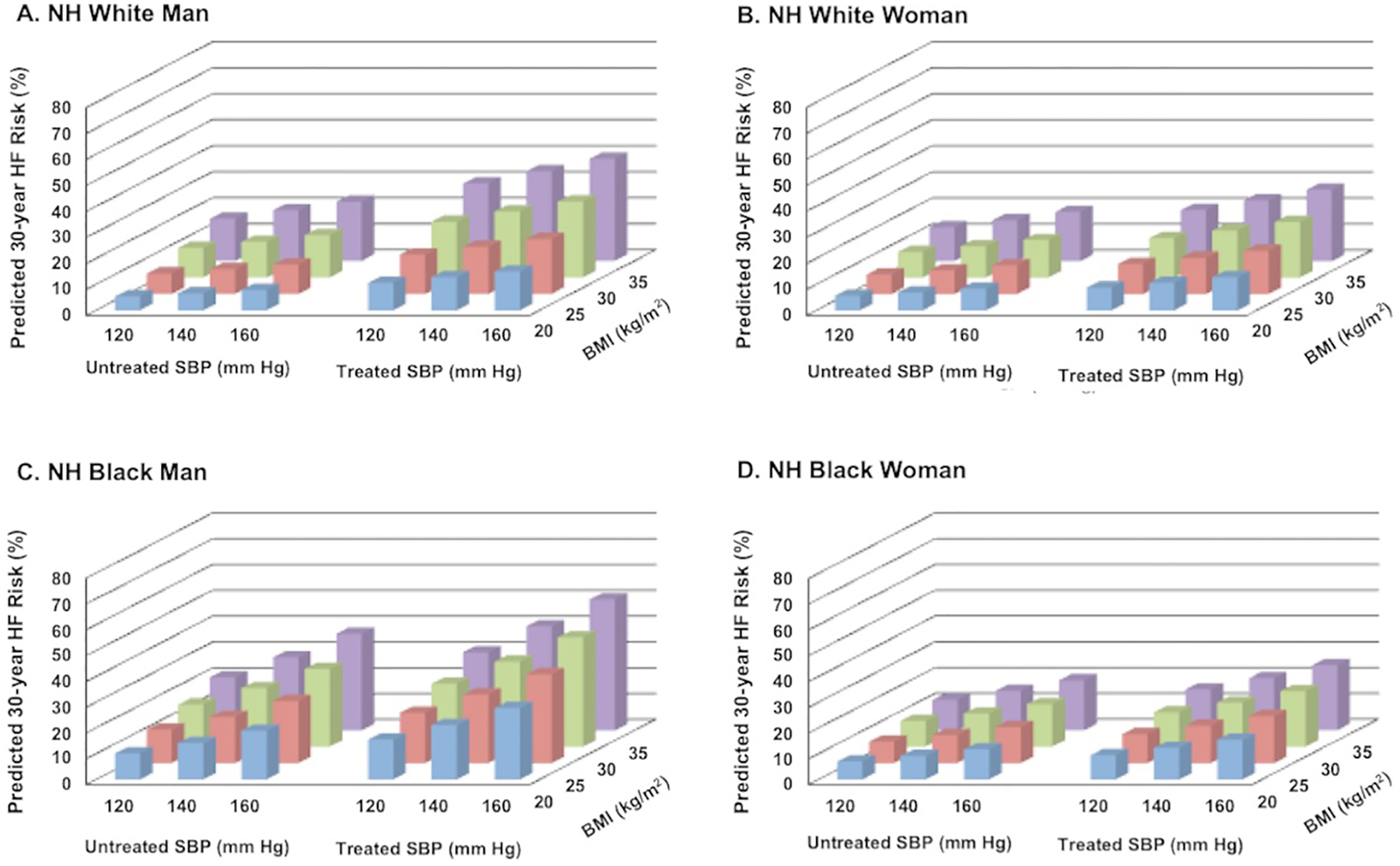
Ten-year predicted risks for heart failure by varying levels of multiple risk factors in a hypothetical White man (A) and Black man (B) at 60 years of age. BMI= body mass index; BP= blood pressure; DM= diabetes mellitus; SBP = systolic blood pressure
DISCUSSION
Summary of Principal Findings.
In this study, we developed novel sex- and race-specific 30-year risk prediction models for incident HF using individual-level data from 5 population-based cohorts. The present study integrates clinical variables routinely available in the primary care setting into a robust tool to provide a summary estimate of an individual’s likelihood of experiencing symptomatic HF (ACC/AHA Stage C/D) over a 30-year period. Importantly, the current model does not use biomarkers or variables from imaging (e.g., electrocardiogram, echocardiography) that are not standard of practice in asymptomatic adults for screening, which supports the potential for widespread and cost-effective implementation of this tool. The 30-year HF risk models were derived from a sample of participants without prevalent CVD from US-based population-based cohorts and demonstrate excellent discrimination and good calibration in all sex-race groups. We also systematically examined the intrinsic properties of the 30-year derived HF risk prediction tool under various risk factor combinations that can inform clinician-patient discussions regarding potential clinical interventions to reduce risk for HF (e.g., intensive BP lowering).
Findings in Context of Prior Studies on Lifetime Risk of HF.
Our findings build upon and expand prior reports estimating average levels of lifetime risk for HF in the general population. While other studies have reported overall lifetime risk estimates for risk strata (e.g. BMI categories) at specific ages, ours is the first to model individual-level 30-year risk of incident HF based on objective clinical data inputting continuous risk factor levels across a broad range of ages from participants enrolled in community-based cohorts representative of the general US population.14, 15, 27–29 We also confirm previously demonstrated associations of risk factors with short-term risk of HF and extend these with assessment of long-term risk and adjustment for competing risk of non-HF death that did not differ between cohorts.11
Race-Based Disparities in Short-Term and Long-Term Risk of HF.
Significant health disparities in HF risk factors and incidence of HF exist across sex and race groups that have been previously demonstrated in the cohorts included here. In the Coronary Artery Risk Development in Young Adults Study, premature development of incident HF occurred far more commonly among Black than White adults with 26 of 27 CARDIA participants who developed HF before 50 years of age being Black.8 These racial disparities are, in part, attributable to differences in risk factor burden as well as upstream social determinants of health (e.g., access to health care). Specifically, differences in HF risk may be related to differences in prevalence of HF risk factors of obesity, diabetes, and hypertension. The root cause of disparities in HF and HF risk factors are well-documented in upstream social determinants of health that contribute to both development of risk factors and risk of HF and include structural and systemic barriers, such as anti-Black racism, residential segregation, and exposure to adverse life events and stressors disproportionately borne by Black adults in the US.30 Among Black adults in the CARDIA cohort, participants who moved to a more segregated neighborhood experienced a greater increase in BP over 25 years of follow-up, a key risk factor for HF. This may also contribute to distinct mechanisms of HF that are observed by race, with preceding history of MI noted less commonly among Black adults with HF.31 Specifically, Black adults may be exposed to higher cumulative levels of key risk factors such as obesity and hypertension across the life course, which may also lead to earlier onset of HF in addition to greater risk of HF. By deriving the long-term risk prediction models in each self-described race-sex group, we identified unique coefficients that would be applicable to similar groups.
Urgent Need for Prevention Efforts in HF Earlier in the Life Course.
Age-adjusted mortality rates due to HF are increasing with greatest relative increases among younger adults before the age of 65 years.5, 32 Ample evidence supports successful prevention or postponement of HF development through modification or prevention of risk factors with the greatest population attributable fraction for hypertension associated with HF.33, 34 Therefore, focused efforts on prevention of HF are of paramount importance and have been highlighted in the research priorities of the National Heart, Lung, and Blood Institute as an overarching strategic goal.35 Given the rising prevalence and significant morbidity and mortality associated with HF, the ACC/AHA and ESC have emphasized recognition of individuals at high risk for HF to target with primary prevention strategies.9, 10 While several short-term risk models for HF exist, risk estimates are driven, in large part, by age and therefore, lack sensitivity to identify risk at younger ages. Individual assessment of long-term risk for HF, which has been lacking to date, will provide another much-needed tool in our armamentarium.
In adults identified to be at high 30-year risk of HF, comprehensive prevention strategies that target both lifestyle modifications and risk factor control (e.g. maintenance of a healthy body weight, adequate moderate-vigorous physical activity, smoking cessation, BP and glucose management) while addressing and accounting for adverse social factors that enhance risk for HF are needed for public health translatability of a risk-based prevention paradigm in HF.36 The importance of aggressive BP lowering to reduce risk of HF was demonstrated in the Systolic Blood Pressure Reduction Intervention Trial. Similar to the implementation of ASCVD risk estimation to guide SBP targets described in the 2017 ACC/AHA HTN guidelines with a goal SBP<130/80mm Hg for those at a 10-year ASCVD risk of ≥7.5%, an elevated HF risk may be helpful to inform clinician-patient risk discussions regarding aggressive BP management for HF prevention.37 However, national data suggests that BP control has worsened in the last decade and race-based disparities in BP control are pervasive. Disruptive strategies that focus on community-engaged approaches, such as barbershops, should be considered to ensure optimal implementation of evidence-based guidelines for risk factor modification.
In addition to targeted risk factor modification, the 30-year HF risk estimates also provide a framework that may be useful to test the efficacy of specific emerging therapies for treatment of risk factors, such as diabetes, among individuals at high risk for HF. For example, sodium-glucose cotransporter 2 inhibitors (SGLT2i) are an appealing therapeutic approach among those at high risk given robust data demonstrating improvement in cardiac mechanics in preclinical and clinical studies in those with and without diabetes.38–40 However, it is of paramount importance to ensure that clinical practice and research equitably promote prevention of HF in all adults.
Study Limitations.
There are several limitations to this study. First, the current model is not able to discriminate between HF with reduced or preserved ejection fraction and the pre-specified definitions to adjudicate incident HF events varied somewhat between cohorts. However, refinement of risk specifying each HF subtype may not be clinically meaningful as interventions targeting primordial and primary prevention strategies focus on shared antecedent risk factors (e.g., intensive BP lowering, choice of SGLT2i for DM management) regardless of HF subtype. Additionally, prognosis is equally poor with each subtype of HF.41 As a result, a composite HF risk model will allow broader upstream clinical utility and generalizability.42, 43 Second, while our ten-fold cross validation is a robust method for model derivation, external validation is still needed in follow-up studies. Third, our study relied on self-report of race to stratify participants for model development. We acknowledge that race is a social construct and we were not able to incorporate social determinants of health in the risk model. However, adverse social factors are key contributors to risk of HF that need to be addressed and future models should incorporate the explicit social determinants of health that race is reflecting. Socioeconomic variables were not consistently ascertained in each of the cohorts and therefore have considerable challenges in harmonization for model development and translatability in clinical settings where these factors are not assessed. Our analysis does capture proximate key clinical risk factors which are on the causal pathway through which social determinants are likely largely operating. Fourth, the individual cohorts represent specific geographic regions, particularly among the Black adults included in the study. These regional differences may confound race-based differences and may limit the generalizability of the risk model. Fifth, our study was limited to examination of White and Black adults based on the limited sample size of other race and ethnic groups and further research is needed to evaluate the accuracy of the predicted 30-year HF risk in other race and ethnic groups as we may under- or over-estimate true HF risk in selected subgroups. Sixth, the present observational study design cannot predict the effect of specific antihypertensive or antidiabetic therapies on reducing risk for incident HF. Nevertheless, our estimates of risk for HF based on these clinical covariates may help guide clinicians and patients in decision-making regarding prevention strategies and/or intensification of therapies in individuals at higher risk for HF (e.g. aggressive BP lowering). Seventh, the risk models are based on historical data to allow sufficient follow-up for long-term risk prediction over which time secular trends in risk factor levels and treatment approaches have changed. However, prior studies have demonstrated that birth cohort effects do not change the relationship between risk factors and cardiovascular disease and calculated coefficients did not change meaningfully when we excluded FHS and FOS for White adults.44 Lastly, the cohorts included here overlap with those utilized in ten-year risk prediction (PCP-HF).
Conclusion.
In summary, we present an analysis from 24,838 men and women from 5 population-based cohorts describing the development of a new model to estimate long-term risk of HF. We further performed a 10-fold cross validation, which demonstrated excellent model discrimination and good calibration. Our approach may help refine and further personalize risk stratification of HF on a population-level and may facilitate risk communication on an individual level beginning earlier in the life course. Lastly, the present study examining the new model outputs for various risk factor combinations provides clinical context to guide physician-patient risk-based decision.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What Is Known?
In the past decade, hospitalizations and mortality due to heart failure have increased significantly, particularly among young to middle-aged adults.
Concurrently, prevalence of risk factors (e.g., obesity and diabetes) have increased and risk factor control (e.g., hypertension) has declined in the United States with significant race-related disparities that are due to upstream social determinants of health
Short-term risk prediction models for heart failure that exist under-estimate risk as they are highly dependent on age and can not be extrapolated to long-term risk for young to middle-aged adults.
What New Information Does This Article Contribute?
This study reports the derivation and validation of the first set of long-term risk prediction models over a 30-year period for adults aged 20 to 59 years that integrates clinically relevant continuous risk factor levels that are readily available in the primary care setting
Risk estimates varied significantly across risk factor levels within each sex and race group and can inform clinician-patient discussions to optimize lifestyle and risk factor management (e.g., intensive blood pressure lowering) for risk-based prevention of heart failure beginning earlier in the life course
ACKNOWLEDGEMENTS
We thank the investigators of all the cohort studies included in this analysis for their hard work and dedication in collecting the underlying data, and especially the study participants, whose time and commitment have transformed our understanding of health and disease.
SOURCES OF FUNDING
Research reported in this publication was supported, in part, by the National Institutes of Health’s National Center for Advancing Translational Sciences (KL2TR001424), the NHLBI (R01HL159250, and U01HL160279), and the American Heart Association (#19TPA34890060) to SSK as well as a grant from the National Institutes of Health/National Heart, Lung, and Blood Institute to Dr. Lloyd-Jones (R21 HL085375). Funders were not directly involved in the study design, data collection, analysis and interpretation, or drafting of this report. We confirm the independence of researchers from funders and that all authors had full access to all of the data in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis.
Nonstandard Abbreviations and Acronyms:
- HF
heart failure
- SGLT2i
sodium glucose co-transporter 2 inhibitor
- PCP-HF
Pooled Cohort Equations to Prevent Heart Failure
- SBP
systolic blood pressure
- BMI
body mass index
Footnotes
DISCLOSURE
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD, Ferguson JF, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Lutsey PL, Mackey JS, Matchar DB, Matsushita K, Mussolino ME, Nasir K, O’Flaherty M, Palaniappan LP, Pandey A, Pandey DK, Reeves MJ, Ritchey MD, Rodriguez CJ, Roth GA, Rosamond WD, Sampson UKA, Satou GM, Shah SH, Spartano NL, Tirschwell DL, Tsao CW, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P, American Heart Association Council on E, Prevention Statistics C and Stroke Statistics S. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation. 2018;137:e67–e492. [DOI] [PubMed] [Google Scholar]
- 2.Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, Ikonomidis JS, Khavjou O, Konstam M and Maddox T. Forecasting the impact of heart failure in the United States. Circ Heart Fail. 2013;6:606–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Jordan LC, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, O’Flaherty M, Pandey A, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Spartano NL, Stokes A, Tirschwell DL, Tsao CW, Turakhia MP, VanWagner LB, Wilkins JT, Wong SS and Virani SS. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation. 2019;139:e56–e528. [DOI] [PubMed] [Google Scholar]
- 4.Jackson SL, Tong X, King RJ, Loustalot F, Hong Y and Ritchey MD. National Burden of Heart Failure Events in the United States, 2006 to 2014. Circ Heart Fail. 2018;11:e004873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glynn P, Lloyd-Jones DM, Feinstein MJ, Carnethon M and Khan SS. Disparities in Cardiovascular Mortality Related to Heart Failure in the United States. J Am Coll Cardiol. 2019;73:2354–2355. [DOI] [PubMed] [Google Scholar]
- 6.Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, Ikonomidis JS, Khavjou O, Konstam MA, Maddox TM, Nichol G, Pham M, Pina IL, Trogdon JG, American Heart Association Advocacy Coordinating C, Council on Arteriosclerosis T, Vascular B, Council on Cardiovascular R, Intervention, Council on Clinical C, Council on E, Prevention and Stroke C. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6:606–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jackson SL, Tong X, King RJ, Loustalot F, Hong Y and Ritchey MD. National Burden of Heart Failure Events in the United States, 2006 to 2014. Circulation: Heart Failure. 2018;11:e004873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bibbins-Domingo K, Pletcher MJ, Lin F, Vittinghoff E, Gardin JM, Arynchyn A, Lewis CE, Williams OD and Hulley SB. Racial differences in incident heart failure among young adults. N Engl J Med. 2009;360:1179–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr., Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW and Westlake C. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017;136:e137–e161. [DOI] [PubMed] [Google Scholar]
- 10.Coats AJS, Pieske B, Linde C, Jankowska EA, Ruschitzka F, Rutten FH, Rosano GMC, Bueno H, Riley JP, Cleland JGF, Parissis JT, González-Juanatey JR, Ruilope LM, Jessup M, van der Meer P, Nihoyannopoulos P, Anker SD, Harjola V-P, Falk V, Voors AA, Ponikowski P and Group ESD. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. European Heart Journal. 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 11.Khan SS, Ning H, Shah SJ, Yancy CW, Carnethon M, Berry JD, Mentz RJ, O’Brien E, Correa A, Suthahar N, de Boer RA, Wilkins JT and Lloyd-Jones DM. 10-Year Risk Equations for Incident Heart Failure in the General Population. J Am Coll Cardiol. 2019;73:2388–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marma AK, Berry JD, Ning H, Persell SD and Lloyd-Jones DM. Distribution of 10-year and lifetime predicted risks for cardiovascular disease in US adults: findings from the National Health and Nutrition Examination Survey 2003 to 2006. Circ Cardiovasc Qual Outcomes. 2010;3:8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thanassoulis G, Sniderman AD and Pencina MJ. A Long-term Benefit Approach vs Standard Risk-Based Approaches for Statin Eligibility in Primary Prevention. JAMA Cardiol. 2018;3:1090–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huffman MD, Berry JD, Ning H, Dyer AR, Garside DB, Cai X, Daviglus ML and Lloyd-Jones DM. Lifetime risk for heart failure among white and black Americans: cardiovascular lifetime risk pooling project. J Am Coll Cardiol. 2013;61:1510–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lloyd-Jones DM, Larson MG, Leip EP, Beiser A, D’Agostino RB, Kannel WB, Murabito JM, Vasan RS, Benjamin EJ, Levy D and Framingham Heart S. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation. 2002;106:3068–72. [DOI] [PubMed] [Google Scholar]
- 16.Wilkins JT, Karmali KN, Huffman MD, Allen NB, Ning H, Berry JD, Garside DB, Dyer A and Lloyd-Jones DM. Data Resource Profile: The Cardiovascular Disease Lifetime Risk Pooling Project. Int J Epidemiol. 2015;44:1557–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 18.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR Jr., Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M and Tracy RP. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–81. [DOI] [PubMed] [Google Scholar]
- 19.Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR Jr., Liu K and Savage PJ. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41:1105–16. [DOI] [PubMed] [Google Scholar]
- 20.Kannel WB, Feinleib M, McNamara PM, Garrison RJ and Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979;110:281–90. [DOI] [PubMed] [Google Scholar]
- 21.Dawber TR, Kannel WB and Lyell LP. An approach to longitudinal studies in a community: the Framingham Study. Ann N Y Acad Sci. 1963;107:539–56. [DOI] [PubMed] [Google Scholar]
- 22.Kalbfleisch JPR. The staitistical analysis of failure time data. Hoboken. 2002. [Google Scholar]
- 23.Hippisley-Cox J, Coupland C, Robson J and Brindle P. Derivation, validation, and evaluation of a new QRISK model to estimate lifetime risk of cardiovascular disease: cohort study using QResearch database. BMJ. 2010;341:c6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lloyd-Jones DM. Cardiovascular risk prediction: basic concepts, current status, and future directions. Circulation. 2010;121:1768–77. [DOI] [PubMed] [Google Scholar]
- 25.Karmali KN, Goff DC, Ning H and Lloyd-Jones DM. A systematic examination of the 2013 ACC/AHA pooled cohort risk assessment tool for atherosclerotic cardiovascular disease. Journal of the American College of Cardiology. 2014;64:959–968. [DOI] [PubMed] [Google Scholar]
- 26.Marma AK and Lloyd-Jones DM. Systematic examination of the updated Framingham heart study general cardiovascular risk profile. Circulation. 2009;120:384–390. [DOI] [PubMed] [Google Scholar]
- 27.Ahmad FS, Ning H, Rich JD, Yancy CW, Lloyd-Jones DM and Wilkins JT. Hypertension, Obesity, Diabetes, and Heart Failure-Free Survival: The Cardiovascular Disease Lifetime Risk Pooling Project. JACC Heart Fail. 2016;4:911–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khan SS, Ning H, Wilkins JT, Allen N, Carnethon M, Berry JD, Sweis RN and Lloyd-Jones DM. Association of Body Mass Index With Lifetime Risk of Cardiovascular Disease and Compression of Morbidity. JAMA Cardiol. 2018;3:280–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pandey A, Omar W, Ayers C, LaMonte M, Klein L, Allen NB, Kuller LH, Greenland P, Eaton CB, Gottdiener JS, Lloyd-Jones DM and Berry JD. Sex and Race Differences in Lifetime Risk of Heart Failure With Preserved Ejection Fraction and Heart Failure With Reduced Ejection Fraction. Circulation. 2018;137:1814–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalogeropoulos A, Georgiopoulou V, Kritchevsky SB, Psaty BM, Smith NL, Newman AB, Rodondi N, Satterfield S, Bauer DC, Bibbins-Domingo K, Smith AL, Wilson PW, Vasan RS, Harris TB and Butler J. Epidemiology of incident heart failure in a contemporary elderly cohort: the health, aging, and body composition study. Arch Intern Med. 2009;169:708–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bahrami H, Kronmal R, Bluemke DA, Olson J, Shea S, Liu K, Burke GL and Lima JAC. Differences in the incidence of congestive heart failure by ethnicity: the multi-ethnic study of atherosclerosis. Archives of internal medicine. 2008;168:2138–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sidney S, Quesenberry CP Jr., Jaffe MG, Sorel M, Go AS and Rana JS. Heterogeneity in national U.S. mortality trends within heart disease subgroups, 2000–2015. BMC cardiovascular disorders. 2017;17:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Butler J Primary prevention of heart failure. ISRN Cardiol. 2012;2012:982417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD, American Heart Association Strategic Planning Task F and Statistics C. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. [DOI] [PubMed] [Google Scholar]
- 35.Lauer MS, Kiley JP, Mockrin SC, Mensah GA, Hoots WK, Patel Y, Cook NL, Patterson AP and Gibbons GH. National Heart, Lung, and Blood Institute (NHLBI) strategic visioning: setting an agenda together for the NHLBI of 2025. J Am Coll Cardiol. 2015;65:1130–3. [DOI] [PubMed] [Google Scholar]
- 36.Folsom AR, Shah AM, Lutsey PL, Roetker NS, Alonso A, Avery CL, Miedema MD, Konety S, Chang PP and Solomon SD. American Heart Association’s Life’s Simple 7: Avoiding Heart Failure and Preserving Cardiac Structure and Function. Am J Med. 2015;128:970–6 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA, Williamson JD and Wright JT. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults. A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. 2018;71:e127–e248. [DOI] [PubMed] [Google Scholar]
- 38.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE and Woerle HJ. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. New England Journal of Medicine. 2015;373:2117–2128. [DOI] [PubMed] [Google Scholar]
- 39.Patorno E, Pawar A, Franklin JM, Najafzadeh M, Deruaz-Luyet A, Brodovicz KG, Sambevski S, Bessette LG, Santiago Ortiz AJ, Kulldorff M and Schneeweiss S. Empagliflozin and the Risk of Heart Failure Hospitalization in Routine Clinical Care: A First Analysis from the Empagliflozin Comparative Effectiveness and Safety (EMPRISE) Study. Circulation. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF and Murphy SA. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. New England Journal of Medicine. 2019;380:347–357. [DOI] [PubMed] [Google Scholar]
- 41.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL and Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–9. [DOI] [PubMed] [Google Scholar]
- 42.Tsao CW, Lyass A, Enserro D, Larson MG, Ho JE, Kizer JR, Gottdiener JS, Psaty BM and Vasan RS. Temporal Trends in the Incidence of and Mortality Associated With Heart Failure With Preserved and Reduced Ejection Fraction. JACC Heart Fail. 2018;6:678–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davies MJ, D’Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, Rossing P, Tsapas A, Wexler DJ and Buse JB. Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41:2669–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berry JD, Dyer A, Cai X, Garside DB, Ning H, Thomas A, Greenland P, Van Horn L, Tracy RP and Lloyd-Jones DM. Lifetime risks of cardiovascular disease. N Engl J Med. 2012;366:321–9. 10.1056/NEJMoa1012848. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data and materials utilized in this manuscript are made publicly available by the National Heart, Lung, and Blood Institute Biologic Specimen and Data Repository Information Coordinating Center (BioLINCC).


