Abstract
There is an urgent need to understand the intracellular mechanisms by which synthetic opioids, such as fentanyl, depress breathing. We used L-NAME (NG-nitro-L-arginine methyl ester), a nitric oxide synthase (NOS) inhibitor, to provide evidence for a role of nitric oxide (NO) and nitrosyl factors, including S-nitrosothiols, in fentanyl-induced suppression of breathing in rats. We measured breathing parameters using unrestrained plethysmography to record the changes produced by bolus administration of fentanyl (25 μg/kg, IV) in male Sprague Dawley rats that were pretreated with vehicle (saline), L-NAME (50 μmol/kg, IV) or the inactive D-isomer, D-NAME (50 μmol/kg, IV), 15 min previously. L-NAME produced a series of ventilatory changes that included (i) sustained elevations in breathing frequency, due to the reductions in the durations of inspiration and expiration, (ii) sustained elevations in minute ventilation, accompanied by minimal changes in tidal volume, and (iii) increases in inspiratory drive and expiratory drive, and peak inspiratory flow and peak expiratory flow. Subsequent administration of fentanyl in rats pretreated with vehicle produced negative effects on breathing, including decreases in frequency, tidal volume and therefore minute ventilation. Fentanyl elicited markedly different responses in rats that were pretreated with L-NAME, and conclusively, the negative effects of fentanyl were augmented by the NOS inhibitor. D-NAME did not alter ventilatory parameters or modulate the effects of fentanyl on breathing. Our study fully characterized the effects of L-NAME on ventilation in rats and is the first to suggest a potential role of nitrosyl factors in the ventilatory responses to fentanyl. Our data shows that nitrosyl factors reduce the expression of fentanyl-induced changes in ventilation.
Keywords: Fentanyl, ventilatory parameters, nitric oxide synthase, S-nitrosothiols, rats
Graphical Abstract
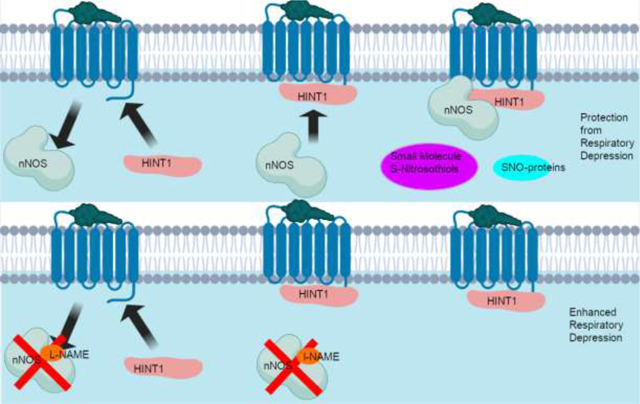
Panel A: Fentanyl-activated μ-ORs recruit histidine triad nucleotide-binding protein 1 (HINT1) while simultaneously activating neuronal NOS (nNOS). HINT1 then recruits nNOS to the μ-OR-HINT1 complex (Sánchez-Blázquez et al., 2010; Rodríguez-Muñoz et al., 2011). nNOS produces small molecule Snitrosothiols and S-nitrosylated proteins which serve to protect against fentanyl-induced respiratory depression. Panel B: Fentanyl-activated μ-ORs recruit HINT1 but nNOS is inhibited by L-NAME and nNOS cannot produce small molecule S-nitrosothiols and S-nitrosylated proteins. Blockade of nNOS has a number of effects including preventing the formation of the μ-OR-NMDA (N-methyl-D-aspartate) receptor super complex, which is a possible mechanism for the potentiation of fentanyl-induced respiratory depression in the absence of NOS activity (Rodríguez-Muñoz and Garzón, 2013; Shah et al., 2019).
1. Introduction
Fentanyl is a potent opioid receptor (OR) agonist that is widely prescribed for pain relief (Muijsers Wagstaff, 2001; Nelson and Schwaner, 2009). The misuse of fentanyl causes several adverse consequences, including fatal depression of breathing (Nelson and Schwaner, 2009; Baby et al., 2021a). Fentanyl is usually considered to be selective for μ-ORs (Trescot et al., 2008; Hajiha et al., 2009) and indeed has high affinity/efficacy at these ORs (Raynor et al., 1994; Huang et al., 2001). However, fentanyl also has biologically relevant affinities and intrinsic activities for δ- and κ-ORs (Yeadon and Kitchen, 1990; Zhu et al., 1996; Butelman et al., 2002; Gharagozlou et al., 2006).
The potential sites involved in the suppression of breathing and antinociception/analgesia effects produced by fentanyl drugs are a constant area of study (Mayer et al., 1989; Sarton et al., 1999; Moshourab and Stein, 2012). Henderson et al (2014) found that pretreatment with the peripherally restricted μ-OR antagonist, naloxone methiodide, diminished, but did not abolish the actions of fentanyl on ventilatory parameters and gas-exchange in the lungs of rats. As such, the actions of fentanyl most likely involve peripheral (e.g., carotid bodies), central structures free of an effective blood-brain barrier (e.g., area postrema), and brain structures within the barrier such as the nucleus tractus solitarius (NTS), Kölliker-Fuse nucleus and pre-Bötzinger complex (Mayer et al., 1989; Campbell et al., 1995; Boom et al., 2012; Henderson et al., 2014; Varga et al., 2020).
Nitric oxide (NO), and a multitude of nitrosyl factors, including S-nitrosothiols (SNOs), circulating in the blood (Doctor et al., 2005; Allen et al., 2009; Gaston et al., 2014; 2020), the carotid bodies (Wang et al., 1995; Moya et al., 2012; Prabhakar and Semenza, 2012; Gaston et al., 2020) and brain nuclei, such as the NTS (Ogawa et al., 1995; Vitagliano et al., 1996; Lipton et al., 2001; Granjeiro and Machado, 2009), ventral and dorsal respiratory groups, nucleus retrofacialis of the pre-Bötzinger complex (Li et al., 2003), nucleus raphe magnus (Nucci et al., 2004), rostral ventrolateral medulla (de Paula and Branco, 2003), medial pontine reticular formation (Leonard and Lydic, 1997), locus coeruleus (Fabris et al., 1999, 2000), and pontine respiratory group (Ling et al., 1992), play key roles in the regulation of breathing. Moreover, systemic injections of NO synthesis inhibitors affect breathing and the ventilatory responses to hypoxia (Haxhiu et al., 1995; Gozal et al., 1996a,b, 1997; Reeves et al., 2008). The involvement of nitrosyl factors with regards to the effects of fentanyl, including analgesia, have received some attention (Kissin et al., 2000; Maegawa and Tonussi, 2003; Célérier et al., 2006; Erkant et al., 2006; Lu et al., 2014; Gupta et al., 2015). However, there is nothing present in the literature as to whether nitrosyl factors participate in the deleterious actions of fentanyl on breathing. The aim of the present investigation was to evaluate how pretreatment with the NOS inhibitor, NG-nitro-L-arginine methyl ester (L-NAME) (Rees et al., 1990; Whalen et al., 2000; Mendoza et al., 2014) modulates the deleterious changes in breathing induced by fentanyl in unanesthetized male rats.
2. Methods
2.1. Rats and surgeries
All of the experiments were performed in strict agreement with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80–23) revised in 1996. The protocols were approved by the Animal Care and Use Committees of Case Western Reserve University and the University of Virginia. Adult male Sprague Dawley rats were purchased from Harlan industries (Madison, WI, USA). The rats were anesthetized (2% isoflurane) and a catheter was placed in the jugular vein and exteriorized to the back of the neck. All incisions were closed, and the rats were given 7 days for recovery. The jugular vein catheters were flushed with phosphate-buffered saline (0.1 M, pH 7.4) 3–4h before commencing the studies. The experiments were done in a quiet room with relative humidity of 51 ± 2% temperature of 21.2 ± 0.2 °C.
2.2. Ventilatory parameters
Ventilatory parameters (Table 1) were continuously monitored in unrestrained rats using whole body plethysmography (PLY3223; Data Sciences International, St. Paul, MN) as detailed previously (Young et al., 2013; Jenkins et al., 2021; a,b et al., 2018, 2021; Gaston et al., 2020, 2021). The in-built software program – Fine Pointe, BUXCO – provided constant corrections of the digitized values for alterations in the humidity and temperature of the chambers. The fluctuating changes in chamber pressure due to the respiratory waveforms were converted to volumes via algorithms developed by Epstein and associates (Epstein and Epstein, 1978; Epstein et al., 1980). The directly recorded and derived parameters detailed in Table 1 were chosen to provide the first set of analyses detailing how blockade of NOS affects respiratory timing and ventilatory mechanics in unanesthetized rats, and to define the processes by which inhibiting NOS modulates fentanyl-induced changes in ventilatory parameters, including percentage (%) of non-eupneic breaths per epoch (i.e., non-eupneic breathing index, NEBI). Since there were no between group differences in body weights (Table 2), the ventilatory (i.e., flow-related) data were not corrected for body weight. The nonsignificant, small changes in body temperature (Table 5) meant that the above algorithms provided accurate conversions of the box flow signals (Mortola and Frappell, 1998; Henderson et al., 2014).
Table 1.
Description of ventilatory parameters
| Parameter | Abbreviation | Units | Definition |
|---|---|---|---|
|
| |||
| A. Directly recorded parameters | |||
| Frequency of breaths | Freq | breaths/min | Rate of breathing |
| Inspiratory Time | Ti | sec | Duration of inspiration |
| Expiratory Time | Te | sec | Duration of expiration |
| End Inspiratory Pause | EIP | msec | Pause between end of inspiration start of expiration |
| End Expiratory Pause | EEP | msec | Pause between end of expiration and start of inspiration |
| Relaxation time | RT | sec | Decay of expiration to 36% maximum |
| Tidal Volume | TV | ml | Volume of inspired air per breath |
| Minute Ventilation | MV = freq × TV | ml/min | Total volume of air inspired per min |
| Peak Inspiratory Flow | PIF | ml/sec | Maximum inspiratory flow |
| Peak Expiratory Flow | PEF | ml/sec | Maximum expiratory flow |
| Expiratory flow at 50% | EF50 | ml/sec | Expiratory flow at 50% expired TV |
| Non-eupneic breathing index | NEBI | % | % of non-eupneic breaths per epoch |
|
| |||
| B. Derived parameters | |||
| Inspiratory Drive | TV/Ti | ml/sec | Central urge to inhale |
| Expiratory Drive | TV/Te | ml/sec | Central drive to exhale |
| NEBI/Frequency | NEBI/Freq | %/(b/min) | Balanced rejection index |
| Apneic Pause | AP = (Te/RT)-1 | No units | Elongated expiration |
Table 2.
Description of rats used in whole body plethysmography and baseline ventilatory parameters
| Parameter | Vehicle | L-NAME | D-NAME |
|---|---|---|---|
|
| |||
| Number | 6 | 6 | 6 |
| Age, days | 80 ± 2 | 81 ± 2 | 80 ± 2 |
| Body weight, grams | 328 ± 2 | 330 ± 2 | 331 ± 3 |
| Frequency, breaths/min | 99 ± 4 | 106 ± 4 | 103 ± 4 |
| Tidal Volume (TV), ml | 2.469 ± 0.045 | 2.323 ± 0.035 | 2.380 ± 0.051 |
| Minute Ventilation, ml/min | 245 ± 12 | 247 ± 9 | 243 ± 15 |
| Inspiratory time (Ti), sec | 0.203 ± 0.013 | 0.191 ± 0.011 | 0.197 ± 0.014 |
| Expiratory time (Te), sec | 0.375 ± 0.019 | 0.350 ± 0.025 | 0.368 ± 0.035 |
| End inspiratory pause, msec | 6.80 ± 0.23 | 7.18 ± 0.37 | 6.93 ± 0.33 |
| End expiratory pause, msec | 45.4 ± 7.7 | 47.5 ± 7.6 | 47.0 ± 6.5 |
| Peak inspiratory flow, ml/sec | 20.5 ± 1.2 | 21.5 ± 1.7 | 20.7 ± 1.9 |
| Peak expiratory flow, ml/sec | 16.2 ± 0.5 | 16.5 ± 1.6 | 17.0 ± 1.8 |
| EF50, ml/sec | 0.43 ± 0.06 | 0.46 ± 0.05 | 0.44 ± 0.07 |
| Relaxation time (RT), sec | 0.224 ± 0.010 | 0.206 ± 0.011 | 0.213 ± 0.022 |
| Apneic pause, (Te/RT)-1 | 0.78 ± 0.13 | 0.73 ± 0.07 | 0.76 ± 0.09 |
| TV/Ti, ml/sec | 12.5 ± 1.0 | 12.4 ± 0.7 | 12.1 ± 1.2 |
| TV/Te, ml/sec | 6.8 ± 0.4 | 6.9 ± 0.6 | 6.7 ± 0.8 |
| NEBI, % | 5.5 ± 0.5 | 5.6 ± 0.7 | 5.4 ± 0.6 |
| NEBI/frequency, %/(breaths/min) | 5.4 ± 0.5 | 5.3 ± 0.7 | 5.5 ± 0.8 |
NEBI, non-eupneic breathing index. The data are presented as mean ± SEM. There were 6 rats in each of the vehicle-treated and L-NAME-treated groups, and 6 rats in the D-NAME-treated group. There were no between group differences for any parameter (P > 0.05, for all comparisons).
Table 5.
Changes in body temperatures during various time points of the experimental protocol
| Stage | Time (min) | Vehicle | L-NAME |
|---|---|---|---|
|
| |||
| Pre | −15 | 37.5 ± 0.1 | 37.4 ± 0.1 |
| 0 | 37.4 ± 0.1 | 37.4 ± 0.1 | |
| Average | 37.5 ± 0.1 | 37.4 ± 0.1 | |
| Post-vehicle or L-NAME | +5 | 37.6 ± 0.1 | 37.5 ± 0.1 |
| +10 | 37.5 ± 0.1 | 37.5 ± 0.1 | |
| +15 | 37.4 ± 0.1 | 37.3 ± 0.1 | |
| Post-fentanyl | +5 | 37.5 ± 0.1 | 37.4 ± 0.1 |
| +10 | 37.6 ± 0.1 | 37.4 ± 0.1 | |
| +15 | 37.5 ± 0.1 | 37.3 ± 0.1 | |
| +30 | 37.7 ± 0.1 | 37.6 ± 0.2 | |
| +45 | 37.7 ± 0.2 | 37.6 ± 0.2 | |
| +60 | 37.9 ± 0.2 | 37.8 ± 0.1 | |
|
| |||
| Arithmetic changes from the average pre value | |||
|
| |||
| Post-vehicle or L-NAME | +5 | +0.1 ± 0.1 | +0.0 ± 0.1 |
| +10 | 0.0 ± 0.1 | +0.0 ± 0.1 | |
| +15 | 0.0 ± 0.1 | −0.0 ± 0.1 | |
| Post-fentanyl | +5 | 0.0 ± 0.1 | +0.0 ± 0.1 |
| +10 | +0.1 ± 0.1 | +0.0 ± 0.1 | |
| +15 | +0.0 ± 0.1 | −0.0 ± 0.1 | |
| +30 | +0.2 ± 0.1 | +0.2 ± 0.1 | |
| +45 | +0.3 ± 0.2 | +0.2 ± 0.1 | |
| +60 | +0.4 ± 0.1* | +0.4 ± 0.1* | |
The data are presented as mean ± SEM. There were 5 rats in each group.
P < 0.05, significant change from pre-value (Pre). There were no between group differences at any time point (P > 0.05, for all comparisons).
2.3. Protocols for plethysmography studies
The rats used in this study (Table 2) were placed in whole body plethysmography chambers for 60–75 min to settle before beginning the study protocol. Data were recorded continuously (i.e., every breath was accounted for) during the entire experiment, including the acclimatization and study phases. One group of rats (n=6) was injected with vehicle (saline, 100 μL/100 gram, IV). Another group (n=6) was injected with L-NAME (50 μmol/kg, IV). Another group (n=6) was injected with D-NAME (50 μmol/kg, IV). Following 15 min, all the rats were injected with fentanyl (25 μg/kg, IV) and breathing was continuously monitored for an additional 60 min.
2.4. Protocols for body temperature studies
Male Sprague Dawley rats were placed in open top plastic containers and given 60 min to settle. The body temperature of the rats was recorded by placing a thermistor probe 5–6 cm into the rectum (Henderson et al., 2014). The probe cable was attached to a telethermometer (Yellow Springs Instruments, South Burlington, Vermont) that was attached to the tail of the rat by tape. Temperature was recorded at regular intervals throughout the experiment (Table 5). One group of rats (n=5; 315 ± 2 grams) was injected with vehicle (saline, 100 μL/100 g body weight, IV) and after 15 min was injected with fentanyl (25 μg/kg, IV). Another group (n=5; 317 ± 3 grams) was injected with L-NAME (50 μmol/kg, IV) and then after 15 min was injected with fentanyl (25 μg/kg, IV).
2.5. Drugs
Fentanyl citrate (50 μg/ml) was provided by the Animal Resource Centers of Case Western Reserve University and the University of Virginia. L- and D-NAME were obtained from Sigma Aldrich (St. Louis, MO, USA).
2.6. Statistics
Directly recorded data points (binned into 15 sec epochs), parameters derived from directly recorded parameters, and the calculated response areas (i.e., the cumulative percent changes from pre-values) were analyzed. To accurately determine baseline values for each parameter, the pre-drug 1 min bins (i.e., pre-values) taken for analyses did not include occasional marked deviations from baseline that were caused by abrupt movements, such as grooming or scratching. All data are shown as mean ± SEM and were analyzed by one-way or two-way analysis of variance (ANOVA) (Winer, 1971) followed by Student’s modified t-test with Bonferroni corrections for multiple comparisons between means using the error mean square terms from each ANOVA (Wallenstein et al., 1980; Ludbrook, 1998; McHugh, 2011). A value of P < 0.05 was taken to denote the initial level of statistical significance that was modified according to the number of comparisons between means as described in detail by Wallenstein et al (1980). A full description of the above statistical approaches we used is provided in Getsy et al (2021).
3. Results
3.1. Baseline ventilatory parameters
The numbers, ages and body weights of the three groups of rats that were used in the whole body plethysmography studies are presented in Table 2. The ages and body weights of the three groups were equivalent to one another (P > 0.05, for all comparisons). The average resting (i.e., pre-drug) ventilatory parameter values for the three groups are also presented in Table 2. As can be seen, there were no differences between the groups for any parameter (P > 0.05, for all comparisons).
3.2. Ventilatory parameters
3.2.1. Frequency of breathing, tidal volume, and minute ventilation
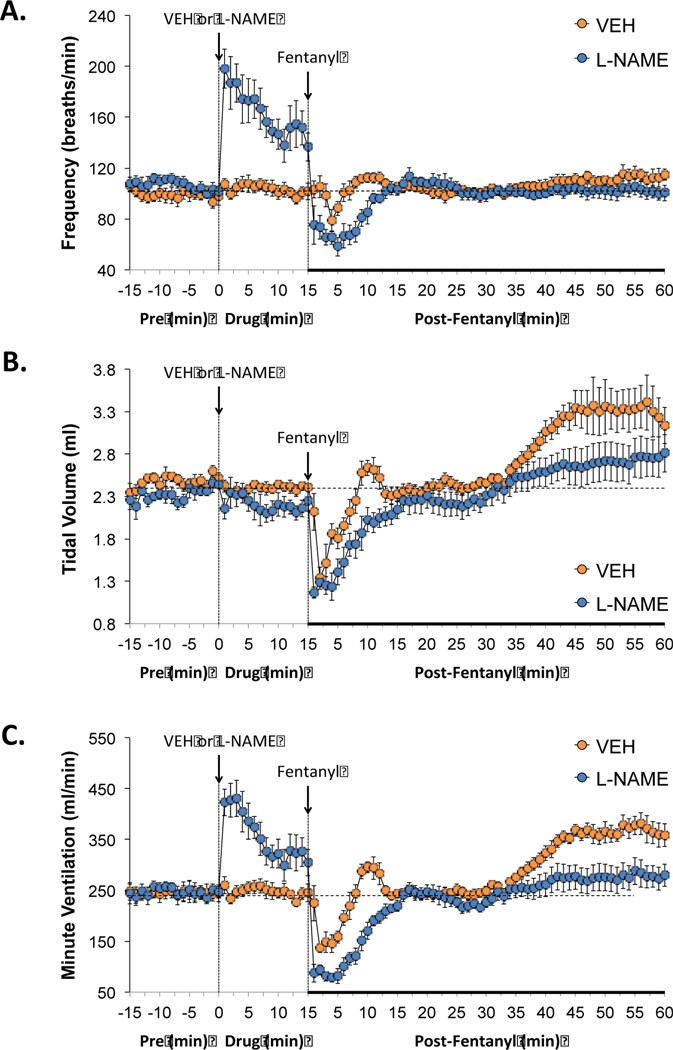
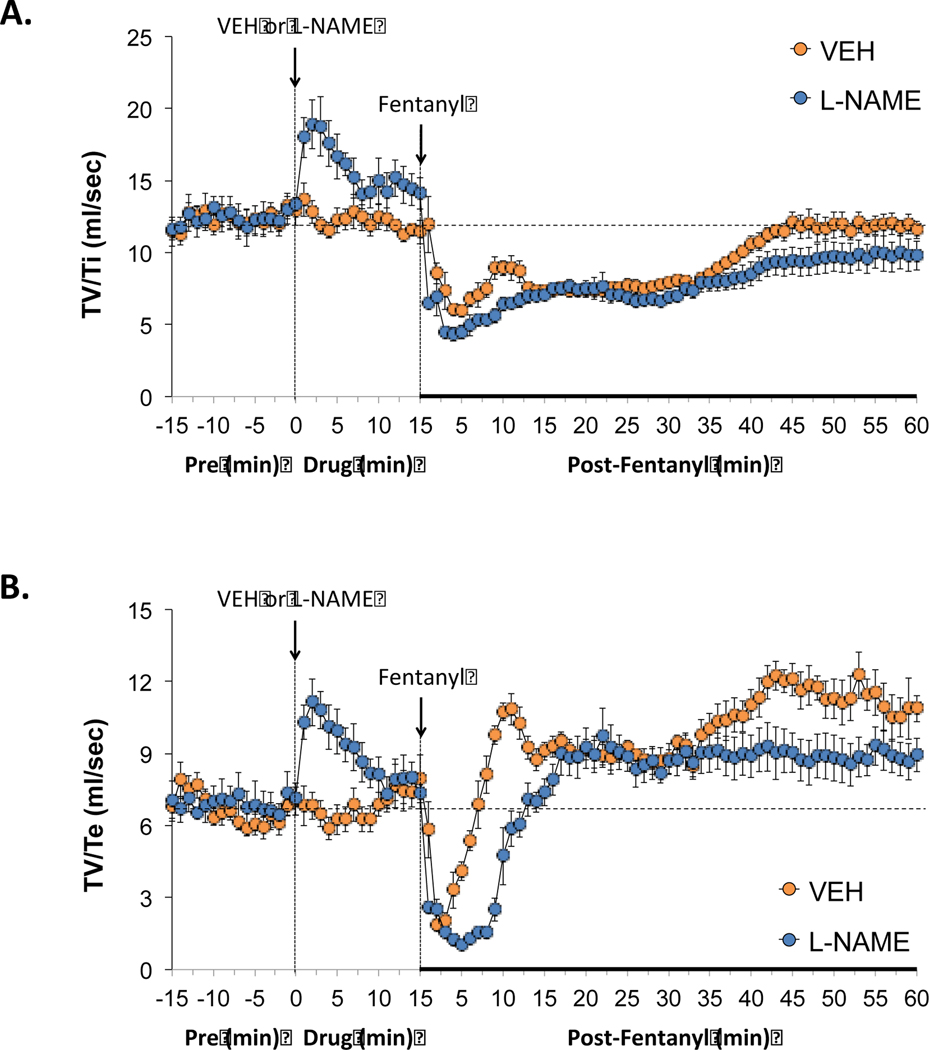
The administration of vehicle produced minor changes in ventilation (Figures 1–7). As can be seen in Figure 1 (panel A), the administration L-NAME (50 μmol/kg, IV) elicited an immediate increase in breathing frequency that remained in effect at 15 min post-injection (i.e., the moment that fentanyl was administered. L-NAME had minimal effects on tidal volume (panel B), thus the sustained increase in minute ventilation (i.e., frequency × tidal volume, panel C) was due entirely to the increase in breathing frequency. Administration of fentanyl (25 μg/kg, IV) to rats pretreated with vehicle produced a relatively transient fall in breathing frequency and tidal volume, and therefore minute ventilation, which lasted approximately 5–10 min and then returned close to baseline. Tidal volume began to increase from baseline about 35 min post-injection of fentanyl in the vehicle group and was still evident at 60 min. Administration of fentanyl into the L-NAME-treated rats elicited substantially greater transient decreases in breathing frequency, tidal volume, and minute ventilation compared to rats pretreated with vehicle. In addition, the post-fentanyl increases in tidal volume that were seen in vehicle-treated rats were not seen in the L-NAME-treated rats.
Figure 1.
Effects of NOS inhibition on fentanyl-induced changes in frequency of breathing, tidal volume and minute ventilation. L-NAME elicits pronounced effects of frequency of breathing and therefore minute ventilation and the negative effects of fentanyl on all three parameters were exaggerated in the presence of L-NAME. Panels: Frequency of breathing (panel A), tidal volume (panel B) and minute ventilation (panel C) prior to (pre) and following the administration of vehicle or L-NAME (50 μmol/kg, IV) and then fentanyl (25 μg/kg, IV) in unanesthetized adult Sprague Dawley rats. There were 6 rats in each group. The data are presented as mean ± SEM.
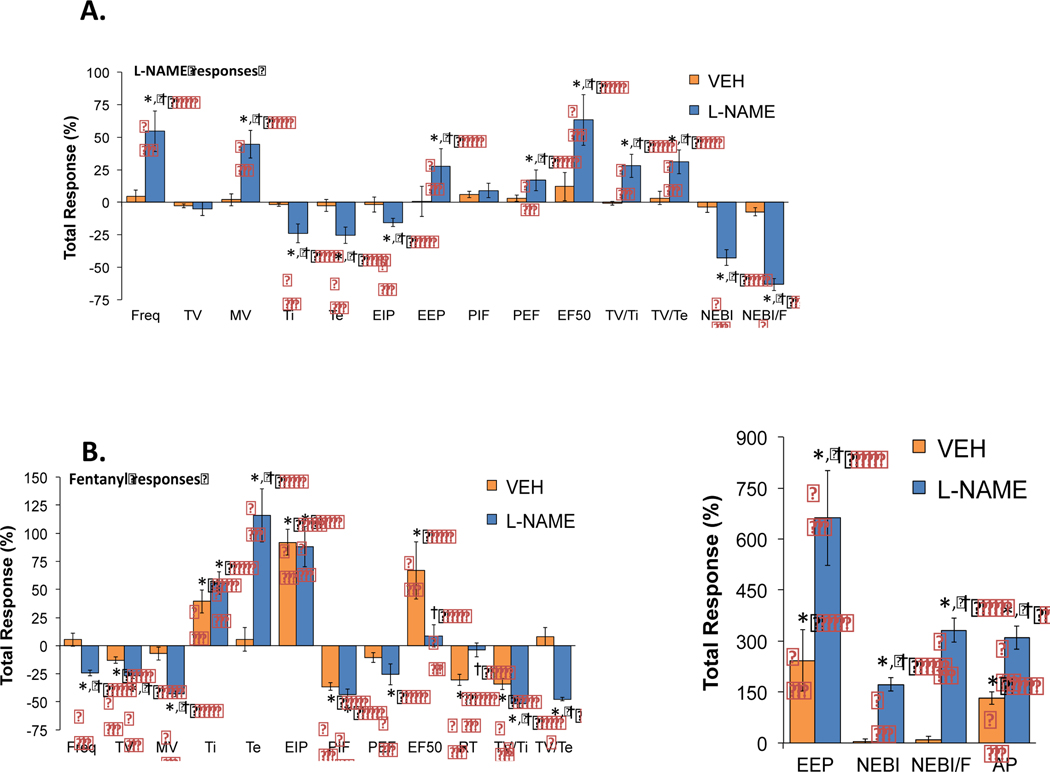
Figure 7.
Effects of NOS inhibition on the total fentanyl-induced changes in ventilatory parameters. Inhibition of NOS markedly alters the negative effects of fentanyl on many individual ventilatory parameters. Panels: The Total ventilatory responses elicited by vehicle or L-NAME (50 μmol/kg, IV) (panel A) or by fentanyl (25 μg/kg, IV) in the vehicle- or L-NAME-treated rats (panels B). There were 6 unanesthetized adult Sprague Dawley rats in each group. The data are presented as mean ± SEM. *P < 0.05, significant response. † P < 0.05, L-NAME-treated versus vehicle-treated.
3.2.2. Inspiratory and expiratory times
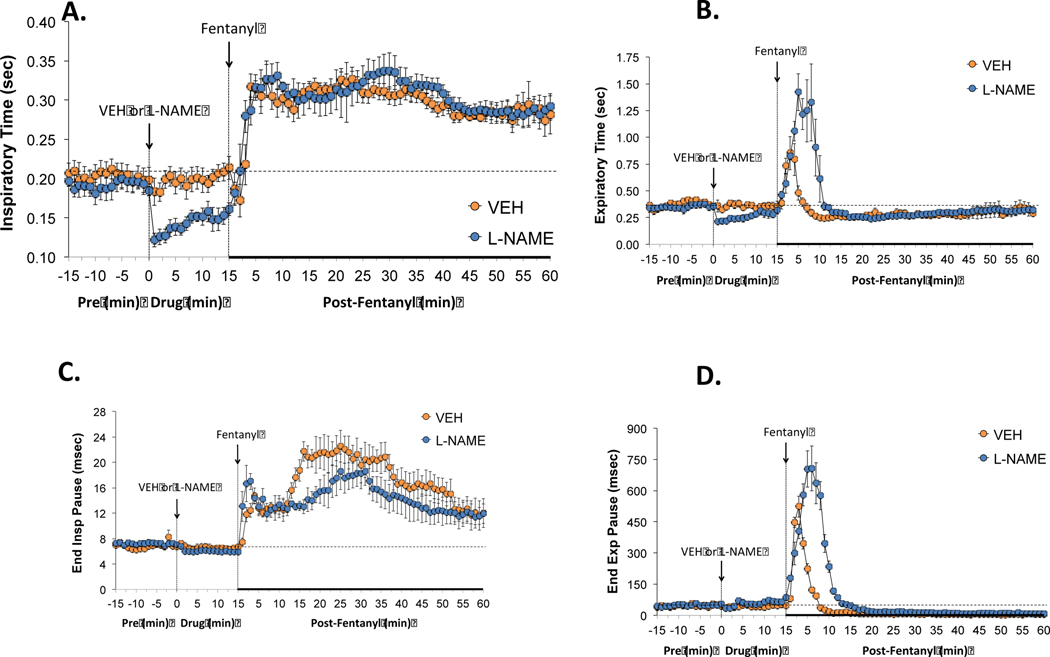
As can be seen in Figure 2, the administration of L-NAME (50 μmol/kg, IV) produced immediate decreases in inspiratory time (i.e., inspiratory duration) (panel A) and expiratory time (i.e., expiratory duration) (panel B) that were consistent with the increases in breathing frequency (Figure 1). Subsequent administration of fentanyl (25 μg/kg, IV) in rats that received L-NAME elicited rapid and sustained increases in inspiratory time that were similar in the rats that received vehicle (panel A). In panel B, the subsequent administration of fentanyl elevated expiratory time in both rats treated with vehicle or L-NAME, however, the elevation in expiratory time was greater and longer lasting in the rats that had received L-NAME.
Figure 2.
Effects of NOS inhibition on fentanyl-induced changes in inspiratory/expiratory timing. L-NAME reduced both inspiratory and expiratory times but did not affect end inspiratory or end expiratory pauses. The negative effects of fentanyl on inspiratory time and end inspiratory pause were not affected by L-NAME whereas the negative effects of fentanyl of expiratory time and end expiratory pause are markedly augmented in the presence of the NOS inhibitor. Panels: Inspiratory time (panel A), expiratory time (panel B), end inspiratory pause (panel C) and end expiratory pause (panel D) prior to (pre) and following the administration of vehicle or L-NAME (50 μmol/kg, IV) and then fentanyl (25 μg/kg, IV) in unanesthetized adult Sprague Dawley rats. There were 6 rats in each group. The data are presented as mean ± SEM.
3.2.3. End inspiratory and expiratory pauses
As also seen in Figure 2, administration of L-NAME (50 μmol/kg, IV) produced minor changes in end inspiratory pause (EIP) (panel C) and end expiratory pause (EEP) (panel D). Subsequent administration of fentanyl (25 μg/kg, IV) in the rats that had received L-NAME, produced prompt and sustained elevations in EIP that were somewhat similar to the rats that had received vehicle (panel C). The subsequent administration of fentanyl elevated EEP in rats that had received vehicle or L-NAME. Nonetheless, the increase in EEP was greater and longer lasting in the rats that had received L-NAME.
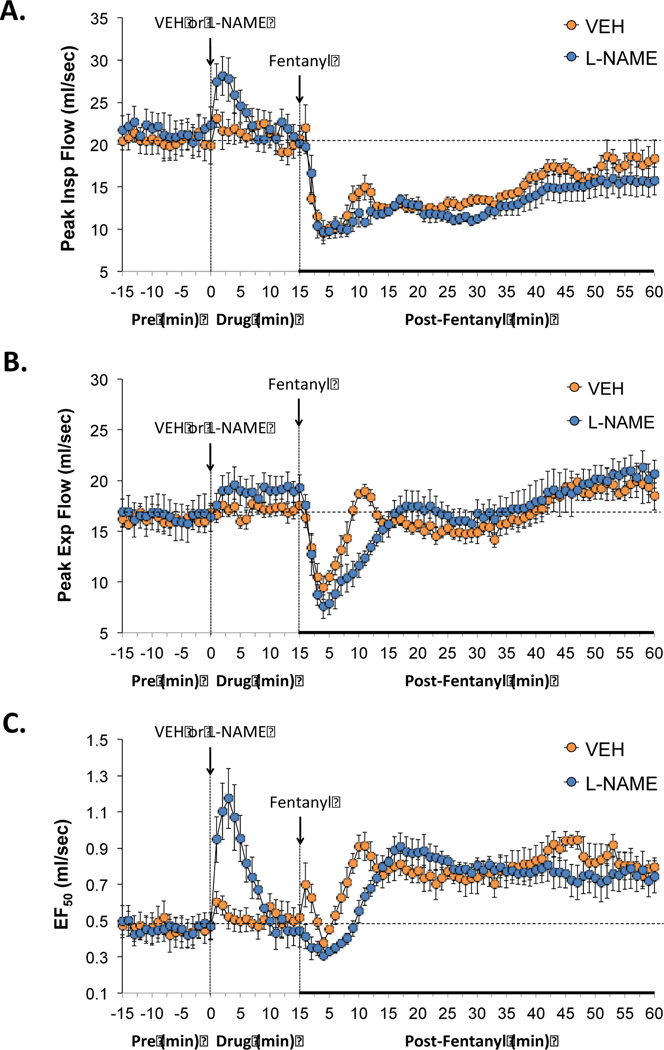
3.2.4. Peak inspiratory and expiratory flows and EF50
As seen in Figure 3, the injection of L-NAME (50 μmol/kg, IV) elicited a transient elevation in peak inspiratory flow (PIF) (panel A) and EF50 (panel C), and a smaller sustained elevation in peak expiratory flow (PEF) (panel B). The administration of fentanyl (25 μg/kg, IV) elicited rapid and sustained decreases in PIF that were similar in the rats that received either vehicle or L-NAME (panel A). Administration of fentanyl also produced a decrease in PEF in the rats that received vehicle or L-NAME (panel B), however, this response lasted longer in rats treated with L-NAME (panel B). Additional, administration of fentanyl (25 μg/kg, IV) produced sustained elevations in EF50 that were comparable in rats that had received vehicle or L-NAME, however this sustained increase in EF50 was not immediate and seen approximately 7 min post fentanyl injection.
Figure 3.
Effects of NOS inhibition on fentanyl-induced changes in inspiratory and expiratory flows. L-NAME increased peak inspiratory flows and EF50 while minimally affecting peak expiratory flow. The negative effects of fentanyl on peak expiratory flow and EF50 were augmented in the presence of L-NAME. Panels: Peak inspiratory flow (panel A), peak expiratory flow (panel B) and EF50 (panel C) prior to (pre) and following the administration of vehicle or L-NAME (50 μmol/kg, IV) and then fentanyl (25 μg/kg, IV) in unanesthetized adult Sprague Dawley rats. There were 6 rats in each group. The data are presented as mean ± SEM.
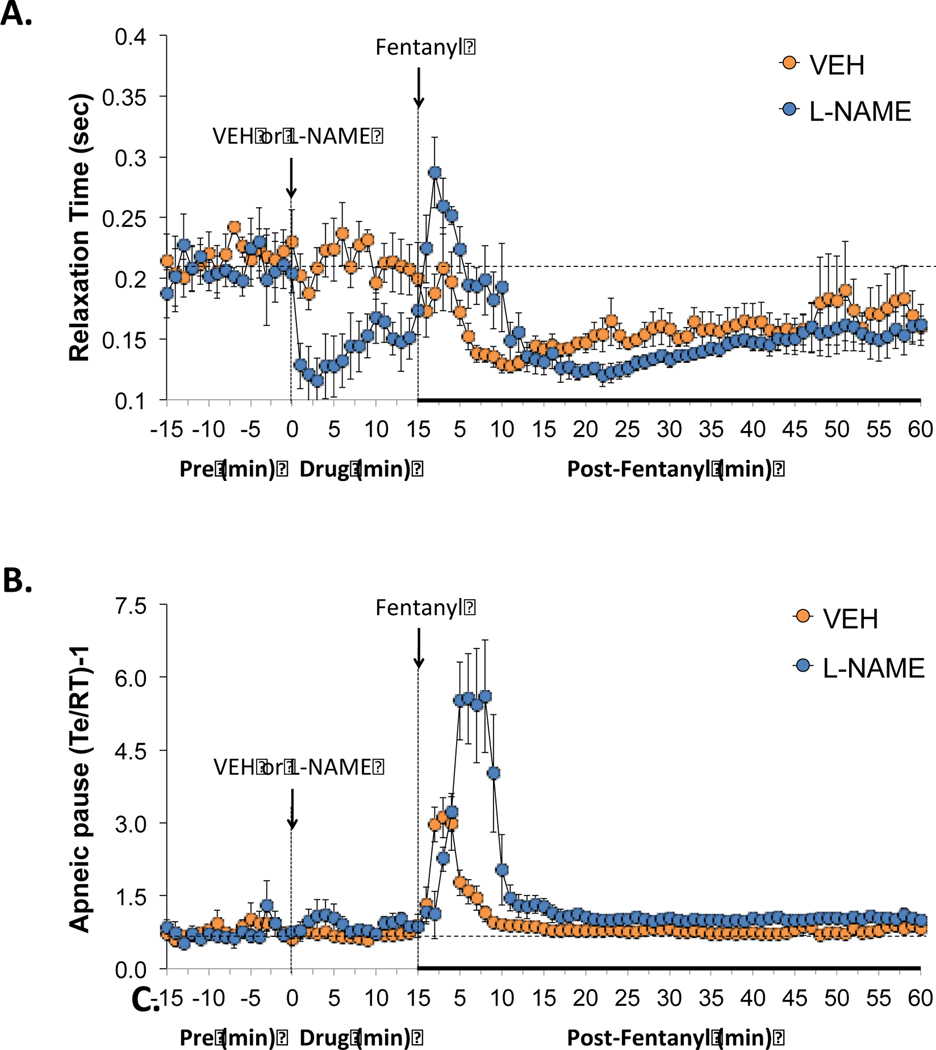
3.2.5. Relaxation time and apneic pause
As seen in Figure 4, administration of L-NAME (50 μmol/kg, IV) produced a pronounced decrease in relaxation time (panel A), but did not affect apneic pause [AP = (RT/Te)-1] (panel B). The administration of fentanyl (25 μg/kg, IV) produced transient increases followed by sustained decreases in relaxation time of similar pattern in rats that received vehicle or L-NAME (panel A). The administration of fentanyl (25 μg/kg, IV) elicited a prompt increase in apneic pause in rats that had either received vehicle or L-NAME, with the response being of substantially greater magnitude in the rats that had received L-NAME.
Figure 4.
Effects of NOS inhibition on fentanyl-induced changes in relative expiratory timing. L-NAME decreased relaxation time but minimally affected apneic pause. The negative effects of fentanyl on apneic pause were markedly augmented in the presence of L-NAME. Panels: Relaxation time (panel A) and apneic pause (expiratory time/relaxation time)-1), (Te/RT)-1) (panel B) prior to (pre) and following the administration of vehicle or L-NAME (50 μmol/kg, IV) and then fentanyl (25 μg/kg, IV) in unanesthetized adult Sprague Dawley rats. There were 6 rats in each group. The data are presented as mean ± SEM.
3.2.6. Inspiratory and expiratory drives
As shown in Figure 5, administration of L-NAME (50 μmol/kg, IV) elicited substantial increases in inspiratory drive (TV/Ti) (panel A) and expiratory drive (TV/Te) (panel B) that were not seen in vehicle-treated rats. Administration of fentanyl (25 μg/kg, IV) caused rapid long-lasting decreases in inspiratory drive that were similar in rats that had received vehicle or L-NAME (panel A). The administration of fentanyl elicited a prompt reduction in expiratory drive in rats that had received vehicle or L-NAME. Nevertheless, the transient decrease in expiratory drive was of greater duration in rats that had received L-NAME (panel B).
Figure 5.
Effects of NOS inhibition on fentanyl-induced changes in inspiratory drive and expiratory drive. L-NAME enhanced inspiratory drive and expiratory drive but enhanced the negative effects of fentanyl on expiratory drive. Panels: Tidal volume/inspiratory time (TV/Ti) (panel A) and tidal volume/expiratory time (TV/Te) (panel B) prior to (pre) and following the administration of vehicle or L-NAME (50 μmol/kg, IV) and then fentanyl (25 μg/kg, IV) in unanesthetized adult Sprague Dawley rats. There were 6 rats in each group. The data are presented as mean ± SEM.
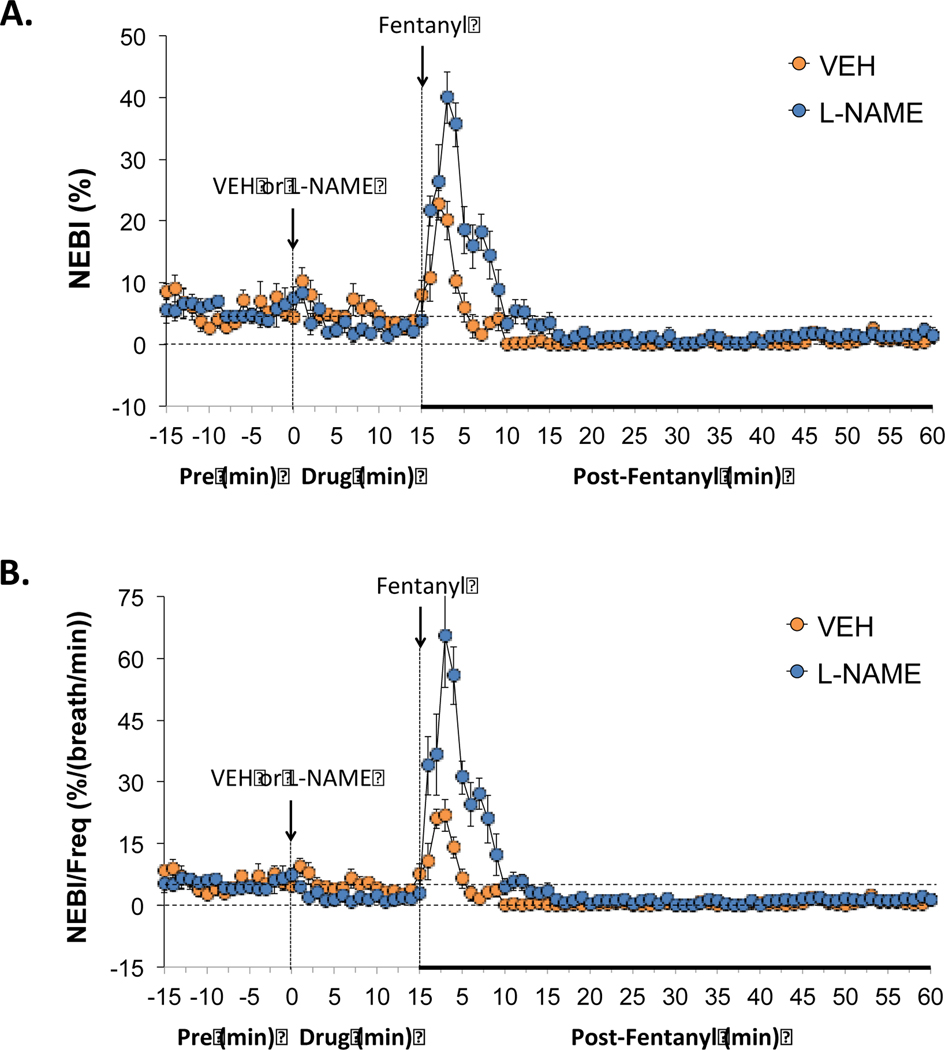
3.2.7. Non-eupneic breathing indices
As seen in Figure 6, administration of L-NAME (50 μmol/kg, IV) produced small, non-significant reductions in the non-eupneic breathing index (NEBI) (panel A), as well as the corrected rejection index (NEBI/Freq) (panel B). The administration of fentanyl (25 μg/kg, IV) produced a transient (4–5 min) increase in both parameters in rats that had received vehicle or L-NAME (panels A and B). The magnitudes and durations of these fentanyl-induced increases in NEBI and NEBI/Freq were much greater in magnitude in the rats that had received L-NAME (panels A and B).
Figure 6.
Effects of NOS inhibition on fentanyl-induced changes in non-eupneic breathing indices. L-NAME only minimally affected the non-eupneic breathing index (NEBI) or NEBI corrected for the frequency of breathing but augmented the negative effects of fentanyl on non-eupneic breathing. Panels: NEBI) (panel A) and NEBI/frequency of breathing (NEBI/Freq) (panel B) prior to (pre) and following the administration of vehicle or L-NAME (50 μmol/kg, IV) and then fentanyl (25 μg/kg, IV) in unanesthetized adult Sprague Dawley rats. There were 6 rats in each group. The data are presented as mean ± SEM.
3.3. Peak responses
The peak alterations in ventilatory parameters produced by fentanyl (25 μg/kg, IV) in rats that received vehicle, L-NAME (50 μmol/kg, IV) or D-NAME (50 μmol/kg, IV) are shown in Table 3. The peak responses elicited by fentanyl in these three groups of rats were generally equivalent to one another, except for the following parameters: (1) the decrease in minute ventilation was greater in rats that had received L-NAME than in those that received vehicle, and (2) the increases in (a) expiratory time, (b) end expiratory pause, (c) apneic pause, (d) NEBI and (e) NEBI/Freq were greater in rats that had received L-NAME than in those that received vehicle. The peak responses elicited by fentanyl in the D-NAME-treated rats were equivalent to those in vehicle-treated rats.
Table 3.
Peak changes in ventilatory parameters elicited by fentanyl in the three treatment groups
| Parameter | Vehicle | L-NAME | D-NAME |
|---|---|---|---|
|
| |||
| Frequency, breaths/min | −25 ± 7* | −39 ± 5* | −24 ± 4* |
| Tidal Volume (TV), ml | −46 ± 5* | −47 ± 7* | −45 ± 4* |
| Minute Ventilation, ml/min | −44 ± 4* | −68 ± 3*,† | −43 ± 5* |
| Inspiratory time (Ti), sec | +54 ± 14* | +74 ± 9* | +53 ± 8* |
| Expiratory time (Te), sec | +131 ± 21* | +312 ± 46*,† | +133 ± 17* |
| End inspiratory pause, msec | +112 ± 13* | +139 ± 12* | +107 ± 15* |
| End expiratory pause, msec | +1102 ± 136* | +1702 ± 138*,† | +1092 ± 109* |
| Peak inspiratory flow, ml/sec | −51 ± 4* | −55 ± 6* | −52 ± 5* |
| Peak expiratory flow, ml/sec | −41 ± 6* | −51 ± 6* | −43 ± 5* |
| EF50, ml/sec | +118 ± 12* | +105 ± 16* | +113 ± 13* |
| Relaxation time (RT), sec | −36 ± 6* | −36 ± 3* | −33 ± 4* |
| Apneic pause, (Te/RT)-1 | +365 ± 64* | +752 ± 149*,† | +357 ± 44* |
| TV/Ti, ml/sec | −51 ± 4* | −58 ± 4* | −53 ± 7* |
| TV/Te, ml/sec | −72 ± 6* | −85 ± 5* | −78 ± 9* |
| NEBI, % | +345 ± 23 | +724 ± 83*,† | +319 ± 27* |
| NEBI/Freq, %/(breaths/min) | +352 ± 62* | +1296 ± 62*,† | +355 ± 51* |
NEBI, non-eupneic breathing index. The data are presented as mean ± SEM. There were 6 rats in each of the vehicle-treated, and L-NAME- and D-NAME-treated groups.
P < 0.05, significant change from pre-value.
P < 0.05, L-NAME-treated versus vehicle-treated.
3.4. Total ventilatory responses
3.4.1. L-NAME
The total responses (i.e., the sum of response values over a 15 min period, presented as %change from baseline (i.e., pre) values) of each ventilatory parameter elicited by vehicle or L-NAME (50 μmol/kg, IV) are shown in panel A of Figure 7. Administration of vehicle caused minor alterations in all parameters, none of which were significant. L-NAME elicited substantial elevations in frequency of breathing (Freq), minute ventilation (MV), end expiratory pause (EEP), peak inspiratory flow (PIF), EF50, apneic pauses (AP), and inspiratory (TV/Ti) and expiratory (TV/Te) drives. Additionally, L-NAME produced substantial reductions in inspiratory time (Ti) and expiratory time (Te), end inspiratory pause (EIP), relaxation time (RT), non-eupneic breathing index (NEBI), and corrected rejection index (NEBI/F).
3.4.2. Fentanyl
The total responses (i.e., the sum of the response values presented as %change from baseline (i.e, pre) values) recorded in the first 15 min after injection of fentanyl (25 μg/kg, IV) in rats that had received vehicle-or L-NAME- (50 μmol/kg, IV) are shown in panel B of Figure 7. In rats that had received vehicle, fentanyl produced a reduction in tidal volume (TV), peak inspiratory flow (PIF), relaxation time (RT), and inspiratory (TV/Ti) and expiratory (TV/Te) drives. Additionally, fentanyl induced increases in inspiratory time (TI), end inspiratory pause (EIP), EF50, end expiratory pause (EEP) and the number of apneic pauses (AP). In rats that had received L-NAME, fentanyl elicited (i) greater decreases in breathing frequency (Freq), tidal volume (TV), minute ventilation (MV), and inspiratory (TV/Ti) and expiratory (TV/Te) drives than in vehicle-treated rats, (ii) similar changes in inspiratory time (Ti), end inspiratory pause (EIP), and peak inspiratory (PIF) and expiratory (PEF) flows compared to vehicle-treated rats, (iii) a diminished increase in EF50 compared to vehicle-treated rats, and (iv) enhanced increases in expiratory time (Te), end expiratory pause (EEP), NEBI, corrected rejection index (NEBI/F) and apneic pauses (AP) compared to rats that had received vehicle.
3.5. Effects of D-NAME
As shown in Table 4, D-NAME (50 μmol/kg, IV) did not affect resting breathing frequency, tidal volume or minute ventilation (data presented as total responses recorded during the 15 min after injection) or modify the total negative responses of fentanyl on these parameters.
Table 4.
Total responses elicited by D-NAME and fentanyl in D-NAME-treated rats
| D-NAME responses | Fentanyl responses | |||
|---|---|---|---|---|
|
|
|
|||
| Parameter | Vehicle | D-NAME | Vehicle | D-NAME |
|
|
|
|
||
| ΔFrequency, % | +4.7 ± 4.6 | +4.6 ± 3.9 | +5.2 ± 5.7 | +4.9 ± 3.3 |
| ΔTidal Volume, % | −2.9 ± 1.5 | −2.7 ± 1.8 | −12.9 ± 3.0* | −14.3 ± 2.1* |
| ΔMinute Ventilation, % | +1.9 ± 4.8 | +2.0 ± 4.1 | −7.1 ± 5.7 | −8.8 ± 5.2 |
The data are presented as mean ± SEM. There were 6 rats in the vehicle-treated group and 6 rats in the D-NAME-treated group.
P < 0.05, significant change from pre-value. There were no between group differences for any parameter (P > 0.05, for all comparisons).
3.6. Body temperature study
The alterations in body temperatures in response to the injection of fentanyl (25 μg/kg, IV) in the vehicle- or L-NAME- (50 μmol/kg, IV) treated rats are shown in Table 5. Administration of fentanyl elicited minimal changes in body temperature in the rats that had received vehicle, although the increase in body temperature compared to Pre at the 60 min time point reached statistical significance in vehicle-treated rats. The administration of L-NAME did not elicit changes body temperature, and the minor effects elicited by fentanyl were equivalent to those recorded in the rats that had received vehicle (P > 0.05, for all comparisons).
4. Discussion
The principal finding of this study was that the ventilatory depressant effects of fentanyl were substantially modified in the presence of the NO synthase inhibitor, L-NAME, but not in the presence of D-NAME (consistent with evidence that the D-isomer is inactive). There is substantial evidence revealing how systemic administration of NO synthesis inhibitors, such as L-NAME, affect breathing frequency, tidal volume and minute ventilation in unanesthetized rats (Haxhiu et al., 1995; Gozal et al., 1996a,b, 1997; Reeves et al., 2008). In general, L-NAME elicits robust sustained increases in frequency of breathing that are associated with minimal at first, but then gradually occurring and relatively minor decreases in tidal volume as time progresses. Thus, the observed increases in minute ventilation last for about 15 minutes. Our study extends these findings by showing that intravenous injection of L-NAME elicited numerous robust changes in ventilatory parameters in unanesthetized Sprague Dawley rats that were not elicited by D-NAME. In brief, L-NAME elicited (i) a sustained elevation in breathing frequency via reductions in both inspiratory and expiratory durations, (ii) minimal changes (slight decreases) in tidal volume, (iii) a sustained elevation of minute ventilation, (iv) sustained increases in peak inspiratory and expiratory flows accompanied by a robust, relatively transient increase in EF50, (v) sustained decreases in relaxation time, but no change in apneic pause, (vi) sustained elevations in both inspiratory drive and expiratory drive, and (vii) sustained decreases in NEBI (non-eupneic breathing index) per se and NEBI corrected for breathing frequency (NEBI/Freq). Currently, we do not know whether these effects on ventilatory parameters elicited by L-NAME involve inhibition of neuronal (type 1) and/or endothelial (type 3) isoforms of NO synthase since L-NAME blocks both (Rees et al., 1990; Gozal et al., 1996a,b). However, there is literature showing that L-NAME exerts its effects on ventilation by inhibiting NO synthase in the carotid bodies (Wang et al., 1995; Gozal et al., 1996a; Reeves, et al., 2008; Moya et al., 2012) and brainstem nuclei that regulate breathing (Ogawa et al., 1995; Fabris et al., 1999, 2000; de Paula and Branco, 2003; Nucci et al., 2004; Granjeiro and Machado, 2009).
The ventilatory responses elicited by fentanyl in the unanesthetized male Sprague Dawley rats are consistent with previous reports in such rats (Henderson et al., 2014; Baby et al., 2021a; Jenkins et al., 2021). Briefly stated, fentanyl elicited (i) a relatively minor and transient decrease in frequency of breathing that was associated with a substantial transient decrease in tidal volume and therefore minute ventilation, (ii) sustained elevations in inspiratory time and end inspiratory pause accompanied by robust, but more transient elevations in expiratory time and end expiratory pause, (iii) pronounced decreases in peak inspiratory and expiratory flows along with initial minor, but eventual robust, increases in EF50, (iv) a substantial decrease in relaxation time associated with a pronounced, but transient increase in apneic pause, (v) robust and long-lasting decreases in inspiratory and expiratory drives, and (vi) a brief increase in non-eupneic breathing index (NEBI). These responses elicited by fentanyl may involve stimulation of ORs on neurons in the brainstem and spinal cord that are involved in ventilatory control (Laferriere et al., 1999; Wang et al., 2002; Haji et al., 2003; Lonergan et al., 2003a; Lonergan et al., 2003b), as well as structures in the periphery including the carotid bodies, pulmonary arteries, and neural and muscular elements of the chest-wall and diaphragm (Schurig et al., 1978; Shook et al., 1990; Hakim et al., 1992; Haji et al., 2000; Dahan et al., 2010).
The ability of L-NAME to markedly alter an array of ventilatory responses that were elicited by fentanyl strongly indicates that NO and/or nitrosyl factors, such as S-nitrosothiols, actively participate in the expression of the effects of the synthetic opioid. In particular, the ability of fentanyl to decrease breathing frequency, tidal volume and minute ventilation were substantially exaggerated in rats pretreated with L-NAME. Thus, it is evident that the loss of newly generated nitrosyl factors augment the negative effects of fentanyl on breathing. In other words, it appears that the active production of nitrosyl factors within the carotid bodies and/or central brainstem nuclei normally counteract the processes by which fentanyl exerts its negative effects on ventilation.
The enhanced fentanyl-induced depression of breathing frequency in the L-NAME-treated rats was accompanied by greater increases in expiratory time and end expiratory pause compared to vehicle-treated rats. However the sustained fentanyl-induced increases in inspiratory time and end inspiratory pause were equivalent in rats that received vehicle or L-NAME. As such, it appears that the nitrosyl factors play a greater role in combating the expiratory phase rather than the inspiratory phase of breathing. This concept is further supported by data showing that the negative actions of fentanyl on expiratory drive, apneic pause [(expiratory time/relaxation time)-1], peak expiratory flow, and the early stages of depression of EF50 were exaggerated in rats that were pretreated with L-NAME in comparison to those that received vehicle, whereas the effects on inspiratory drive and peak inspiratory flow were not. Another key finding was that L-NAME enhanced the increase in non-eupneic breathing episodes (NEBI) that were elicited by fentanyl. Comparably previous studies provide evidence that fentanyl destabilizes breathing in rats most likely by enhancing the occurrence of apneas (Henderson et al., 2014; Baby et al., 2021a; Jenkins et al., 2021), and that this negative effect is normally counteracted by endogenous nitrosyl factors.
Finally, our data support the findings of Carnio et al (1999) and Benamar et al (2001) that administration of a 50 μmol/kg dose of L-NAME elicited negligible effects on body temperature in Sprague Dawley rats. Also similar to previous evidence, the administration of a 25 μg/kg dose of fentanyl elicited a minor elevation in body temperature in rats treated with vehicle (Geller et al., 1983; Savić Vujović et al., 2013), which was only evident at 60 min post-injection. This increase in body temperature elicited by fentanyl in rats treated with L-NAME was very similar to those in the rats that were pretreatment with vehicle. These results agree with evidence that L-NAME does not affect the hyperthermia elicited by a 4 mg/kg dose of morphine although it should be noted that L-NAME did blunt the pronounced hyperthermia elicited by a 15 mg/kg dose of morphine (Benamar et al., 2001). Nevertheless, the small increases in body temperature elicited by fentanyl in the rats that were pr etreated with vehicle or L-NAME (both approximately 0.4°C) is unlikely to have influenced the changes in ventilatory parameters observed in these rats.
5. Conclusion
The finding that the deleterious actions of fentanyl on breathing were augmented by the NOS inhibitor, L-NAME, raises several interesting possibilities about the potential roles of NO and nitrosyl factors, such as S-nitrosothiols, in the actions of this potent synthetic opioid. It has been well documented that NO exerts inhibitory actions in carotid bodies (Lahiri et al., 2006; Prabhakar and Semenza, 2012). Therefore, the ability of L-NAME to augment the negative effects of fentanyl on breathing could involve the inhibition of NO generation via blockade of NO synthase in structures, such as the carotid bodies, and in particular, carotid sinus chemoafferent nerve terminals whose cell bodies reside in the petrosal ganglia, and autonomic nerve terminals that emanate from the superior cervical ganglia (Höhler et al., 1994; Atanasova et al., 2016). Moreover, L-NAME may also inhibit the de novo synthesis and actions of S-nitrosothiols, which normally exert positive effects on carotid body activation in rats, that under normal circumstances, induce carotid sinus chemoafferent nerve-dependent increases in minute ventilation (Gaston et al., 2020). Furthermore, it has been reported that microinjection of NO (Ogawa et al., 1995) and S-nitrosothiols, such as S-nitroso-L-cysteine (Lipton et al., 2001) in the nucleus tractus solitarius (NTS), a key brainstem structure involved in ventilatory signaling (Ogawa et al., 1995), exert positive effects on ventilation.
The intravenous injection of 50 μmol/kg dose of L-NAME would be expected to elicit a sustained hypertension in these unanesthetized rats (Whalen et al., 2006; Davisson et al., 2014). In contrast, administration of a 25 μg/kg dose fentanyl would be expected to produce a transient decrease in arterial blood pressure in these rats (Gautret et al., 1985; Yadav et al., 2018; Haouzi et al., 2020). The possibility that these cardiovascular effects of fentanyl are modified by L-NAME has yet to be determined. This study will soon be followed by reports on how intravenous infusion of the endogenous S-nitrosothiol, S-nitroso-L-cysteine (Myers et al., 1990; Gaston et al., 2020), markedly blunts the deleterious actions of fentanyl and morphine on ventilatory parameters, arterial blood gases - pH, pCO2, pO2 and sO2, and Alveolar-arterial gradient (i.e., an indicator of gas exchange within lung alveoli) in unanesthetized and anesthetized rats. The finding that L-NAME modulates the negative effects of fentanyl on ventilatory parameters in unanesthetized rats raises numerous questions about the role that nitrosyl factors may have in (1) the expression of the pharmacological effects of fentanyl and other opioids, and (2) the actions of drugs being developed to selectively block the effects of opioids on breathing. We have recently reported that L-glutathione ethyl ester (Jenkins et al., 2021), Dcystine diethyl ester and D-cystine dimethyl ester (Gaston et al., 2021) and the superoxide/free radical scavenger, tempol (Baby et al., 2021a,b) markedly attenuate the negative effects of systemically-injected opioids (morphine, fentanyl) on breathing in unanesthetized rats while sparing both the analgesic and sedative actions of the opioids. We are evaluating the concepts that (1) L-glutathione ethyl ester and the reduced forms of the cystine thiolesters (D-cysteine ethyl ester, D-cysteine methyl ester) exert their effects against opioids by becoming S-nitrosothiols (S-nitroso-D-cysteine ethyl ester, S-nitroso-D-cysteine methyl ester), which act via numerous signaling pathways (see Gaston et al., 2020), and (2) tempol may exert its affects, at least in part by preventing the breakdown of endogenous S-nitrosothiols generated by opioids, thereby enhancing the signaling mechanisms by which S-nitrosothiols exert their effects.
With respect to potential mechanisms by which inhibition of NOS potentiates the negative effects of fentanyl on breathing, we have provided a graphical abstract that lays out a tentative proposal. As is depicted in Panel A, fentanyl-activated μ-ORs recruit histidine triad nucleotide-binding protein 1 (HINT1) while simultaneously activating the neuronal form of NOS (nNOS). HINT1 then recruits nNOS to the μ-OR-HINT1 complex (Sánchez-Blázquez et al., 2010; Rodríguez-Muñoz et al., 2011). A key process is that nNOS produces small molecule S-nitrosothiols and S-nitrosylated proteins which protect against fentanyl-induced respiratory depression. As depicted in Panel B, fentanyl-activated μ-ORs recruit HINT1 but when nNOS is inhibited by L-NAME, nNOS cannot then produce small molecule S-nitrosothiols and S-nitrosylated proteins. Such blockade of nNOS has a number of effects including preventing the formation of the μ-OR-NMDA (N-methyl-D-aspartate) receptor super complex, which is a possible mechanism for potentiation of fentanyl-induced respiratory depression in the absence of NOS activity (Rodríguez-Muñoz and Garzón, 2013; Shah et al., 2019).
Funding
This work was supported by NIH grants 1P01HL101871, 1R61HL154136–01 and U01DA051373.
Footnotes
Availability of data
The datasets generated by this investigation are available freely when requested by contacting the author for correspondence.
Declaration of competing interest
The authors declare that they have no competing financial interests or personal relationships that would have influenced the studies that are described in this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen BW, Stamler JS, Piantadosi CA, 2009. Hemoglobin, nitric oxide and molecular mechanisms of hypoxic vasodilation. Trends Mol. Med. 10, 452–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atanasova DY, Dimitrov ND, Lazarov NE, 2016. Expression of nitric oxide-containing structures in the rat carotid body. Acta Histochem. 118, 770–775. [DOI] [PubMed] [Google Scholar]
- Baby SM, Gruber RB, Young AP, MacFarlane PM, Teppema LJ, Lewis SJ, 2018. Bilateral carotid sinus nerve transection exacerbates morphine-induced respiratory depression. Eur. J. Pharmacol. 834, 17–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baby S, Gruber R, Discala J, Puskovic V, Jose N, Cheng F, Jenkins M, Seckler J, Lewis S, 2021a. Systemic Administration of Tempol Attenuates the Cardiorespiratory Depressant Effects of Fentanyl. Front. Pharmacol. 12, 690407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baby SM, Discala JF, Gruber R, Getsy PM, Cheng F, Damron DS, Lewis SJ, 2021b. Tempol Reverses the Negative Effects of Morphine on Arterial Blood-Gas Chemistry and Tissue Oxygen Saturation in Freely-Moving Rats. Front Pharmacol. 12, 749084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benamar K, Xin L, Geller EB, Adler MW, 2001. Effect of central and peripheral administration of a nitric oxide synthase inhibitor on morphine hyperthermia in rats. Brain Res. 894, 266–273. [DOI] [PubMed] [Google Scholar]
- Boom M, Niesters M, Sarton E, Aarts L, Smith TW, Dahan A, 2012. Non-analgesic effects of opioids: opioid-induced respiratory depression. Curr Pharmaceutical Design 18, 5994–6004. [DOI] [PubMed] [Google Scholar]
- Butelman ER, Ball JW, Kreek MJ, 2002. Comparison of the discriminative and neuroendocrine effects of centrally penetrating kappa-opioid agonists in rhesus monkeys. Psychopharmacology (Berl) 164, 115–120. [DOI] [PubMed] [Google Scholar]
- Campbell C, Weinger MB, Quinn M, 1995. Alterations in diaphragm EMG activity during opiate-induced respiratory depression. Respir. Physiol. 100, 107–117. [DOI] [PubMed] [Google Scholar]
- Carnio EC, Almeida MC, Fabris G, Branco LG, 1999. Role of nitric oxide in 2-deoxy-D-glucose-induced hypothermia in rats. Neuroreport 10, 3101–3104. [DOI] [PubMed] [Google Scholar]
- Célérier E, González JR, Maldonado R, Cabañero D, Puig MM, 2006. Opioid-induced hyperalgesia in a murine model of postoperative pain: role of nitric oxide generated from the inducible nitric oxide synthase. Anesthesiology 104, 546–555. [DOI] [PubMed] [Google Scholar]
- Davisson RL, Bates JN, Johnson AK, Lewis SJ, 2014. Effects of intracerebroventricular injections of 5-HT on systemic vascular resistances of conscious rats. Microvasc. Res. 95, 116–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Paula PM, Branco LG, 2003. Nitric oxide in the rostral ventrolateral medulla modulates hyperpnea but not anapyrexia induced by hypoxia. Brain Res. 977, 231–238. [DOI] [PubMed] [Google Scholar]
- Doctor A, Platt R, Sheram ML, Eischeid A, McMahon T, Maxey T, Doherty J, Axelrod M, Kline J, Gurka M, Gow A, Gaston B, 2005. Hemoglobin conformation couples erythrocyte S-nitrosothiol content to O2 gradients. Proc. Natl. Acad. Sci. USA 102, 5709–5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erkent U, Iskit AB, Onur R, Ilhan M, 2006. The effect of nitric oxide on fentanyl and haloperidol-induced catalepsy in mice. Eur. J. Anaesthesiol. 7, 580–585. [DOI] [PubMed] [Google Scholar]
- Epstein MA, Epstein RA, 1978. A theoretical analysis of the barometric method for measurement of tidal volume. Respir. Physiol. 32, 105–120. [DOI] [PubMed] [Google Scholar]
- Epstein RA, Epstein MA, Haddad GG, Mellins RB, 1980. Practical implementation of the barometric method for measurement of tidal volume. J. Appl. Physiol. 49, 1107–1115 (1980). [DOI] [PubMed] [Google Scholar]
- Fabris G, Anselmo-Franci JA, Branco LG, 1999. Role of nitric oxide in hypoxia-induced hyperventilation and hypothermia: participation of the locus coeruleus. Braz. J. Med. Biol. Res. 32, 1389–1398. [DOI] [PubMed] [Google Scholar]
- Fabris G, Steiner AA, nselmo-Franci JA, Branco LG, 2000. Role of nitric oxide in rat locus coeruleus in hypoxia-induced hyperventilation and hypothermia. Neuroreport 11, 2991–2995. [DOI] [PubMed] [Google Scholar]
- Gaston B, May WJ, Sullivan S, Yemen S, Marozkina NV, Palmer LA, Bates JN, Lewis SJ, 2014. Essential role of hemoglobin beta-93-cysteine in posthypoxia facilitation of breathing in conscious mice. J. Appl. Physiol. (1985) 116, 1290–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaston B, Smith L, Bosch J, Seckler J, Kunze D, Kiselar J, Marozkina N, Hodges CA, Wintrobe P, McGee K, Morozkina TS, Burton ST, Lewis T, Strassmaier T, Getsy P, Bates JN, Lewis SJ, 2020. Voltage-gated potassium channel proteins and stereoselective S-nitroso-l-cysteine signaling. JCI Insights 5, e134174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaston B, Baby SM, May WJ, Young AP, Grossfield A, Bates JN, Seckler JM, Wilson CG, Lewis SJ, 2021. D-Cystine di(m)ethyl ester reverses the deleterious effects of morphine on ventilation and arterial blood gas chemistry while promoting antinociception. Sci. Rep. 11, 10038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautret B, Schmitt HJ, 1985. Multiple sites for the cardiovascular actions of fentanyl in rats. Cardiovasc. Pharmacol. 7, 649–652. [DOI] [PubMed] [Google Scholar]
- Getsy PM, Sundararajan S, May WJ, von Schill GC, McLaughlin DK, Palmer LA, Lewis SJ, 2021. Short-term facilitation of breathing upon cessation of hypoxic challenge is impaired in male but not female endothelial NOS knock-out mice. Sci. Rep. 11, 18346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharagozlou P, Hashemi E, DeLorey TM, Clark JD, Lameh J, 2006. Pharmacological profiles of opioid ligands at kappa opioid receptors. BMC Pharmacol. 6, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozal D, Gozal E, Gozal YM, Torres JE, 1996a. Nitric oxide synthase isoforms and peripheral chemoreceptor stimulation in conscious rats. Neuroreport 7, 1145–1148. [DOI] [PubMed] [Google Scholar]
- Gozal D, Torres JE, Gozal YM, Littwin SM, 1996b. Effect of nitric oxide synthase inhibition on cardiorespiratory responses in the conscious rat. J. Appl. Physiol. 81, 2068–2077. [DOI] [PubMed] [Google Scholar]
- Gozal D, Gozal E, Torres JE, Gozal YM, Nuckton TJ, Hornby PJ, 1997. Nitric oxide modulates ventilatory responses to hypoxia in the developing rat. Am. J. Respir. Crit. Care Med. 155, 1755–1762. [DOI] [PubMed] [Google Scholar]
- Granjeiro EM, Machado BH, 2009. NO in the caudal NTS modulates the increase in respiratory frequency in response to chemoreflex activation in awake rats. Respir. Physiol. Neurobiol. 166, 32–40. [DOI] [PubMed] [Google Scholar]
- Gupta M, Poonawala T, Farooqui M, Ericson ME, Gupta K, 2015. Topical fentanyl stimulates healing of ischemic wounds in diabetic rats. J. Diabetes 7, 573–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haouzi P, Guck D, McCann M, Sternick M, Sonobe T, Tubbs N, 2020. Severe Hypoxemia Prevents Spontaneous and Naloxone-induced Breathing Recovery after Fentanyl Overdose in Awake and Sedated Rats. Anesthesiology 132, 1138–1150. [DOI] [PubMed] [Google Scholar]
- Haxhiu MA, Chang CH, Dreshaj IA, Erokwu B, Prabhakar NR, Cherniack NS, 1995. Nitric oxide and ventilatory response to hypoxia. Respir. Physiol. 101, 257–266. [DOI] [PubMed] [Google Scholar]
- Hajiha M, DuBord MA, Liu H, Horner RL, 2009. Opioid receptor mechanisms at the hypoglossal motor pool and effects on tongue muscle activity in vivo. J. Physiol. 587, 2677–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson F, May WJ, Gruber RB, Discala JF, Puskovic V, Young AP, Baby SM, Lewis SJ, 2014. Role of central and peripheral opiate receptors in the effects of fentanyl on analgesia, ventilation and arterial blood-gas chemistry in conscious rats. Respir. Physiol. Neurobiol. 191, 95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höhler B, Mayer B, Kummer W, 1994. Nitric oxide synthase in the rat carotid body and carotid sinus. Cell Tissue Res. 276, 559–564. [DOI] [PubMed] [Google Scholar]
- Huang P, Kehner GB, Cowan A, Liu-Chen LY, 2001. Comparison of pharmacological activities of buprenorphine and norbuprenorphine: norbuprenorphine is a potent opioid agonist. J. Pharmacol. Exper. Ther. 297, 688–695. [PubMed] [Google Scholar]
- Jenkins MW, Khalid F, Baby SM, May WJ, Young AP, Bates JN, Cheng F, Seckler JM, Lewis SJ, 2021. Glutathione ethyl ester reverses the deleterious effects of fentanyl on ventilation and arterial blood-gas chemistry while prolonging fentanyl-induced analgesia. Sci. Rep. 11, 6985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissin I, Bright CA, Bradley EL Jr., 2000. Acute tolerance to continuously infused alfentanil: the role of cholecystokinin and N-methyl-D-aspartate-nitric oxide systems. Anesth. Analg. 91, 110–116. [DOI] [PubMed] [Google Scholar]
- Lahiri S, Roy A, Baby SM, Hoshi T, Semenza GL, Prabhakar NR, 2006. Oxygen sensing in the body. Prog. Biophys. Mol. Biol. 91, 249–286. [DOI] [PubMed] [Google Scholar]
- Leonard TO, Lydic R, 1997. Pontine nitric oxide modulates acetylcholine release, rapid eye movement sleep generation, and respiratory rate. J. Neurosci. 17, 774–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ZQ, Wu ZH, Shi Y, Wang NQ, 2003. Nitric oxide is involved in the modulation of central respiratory rhythm. Sheng Li Xue Bao 55, 560–564. [PubMed] [Google Scholar]
- Ling L, Karius DR, Fiscus RR, Speck DF, 1992. Endogenous nitric oxide required for an integrative respiratory function in the cat brain. J. Neurophysiol. 68, 1910–1912. [DOI] [PubMed] [Google Scholar]
- Lipton AJ, Johnson MA, Macdonald T, Lieberman MW, Gozal D, Gaston B, 2001. S-nitrosothiols signal the ventilatory response to hypoxia. Nature 413, 171–174. [DOI] [PubMed] [Google Scholar]
- Lu Y, Hu J, Zhang Y, Dong C, 2014. Spinal neuronal NOS activation mediates intrathecal fentanyl preconditioning induced remote cardioprotection in rats. Internat. Immunopharmacol. 19, 127–131. [DOI] [PubMed] [Google Scholar]
- Ludbrook J, 1998. Multiple comparison procedures updated. Clin. Exp. Pharmacol. Physiol. 25, 1032–1037. [DOI] [PubMed] [Google Scholar]
- Ma S, Abboud FM, Felder RB, 1995. Effects of l-arginine-derived nitric oxidesynthesis on neuronal activity in nucleus tractus solitarius. Am. J. Physiol. 268, R487–R491. [DOI] [PubMed] [Google Scholar]
- Maegawa FA, Tonussi CR, 2003. The L-arginine/nitric oxide/cyclic-GMP pathway apparently mediates the peripheral antihyperalgesic action of fentanyl in rats. Braz. J. Med. Biol. Res. 36, 1701–1707. [DOI] [PubMed] [Google Scholar]
- Mayer N, Zimpfer M, Raberger G, Beck A, 1989. Fentanyl inhibits the canine carotid chemoreceptor reflex. Anesth. Analg. 69, 756–762. [PubMed] [Google Scholar]
- McHugh ML, 2011. Multiple comparison analysis testing in ANOVA. Biochem. Med. (Zagreb) 21, 203–209. [DOI] [PubMed] [Google Scholar]
- Mendoza JP, Passafaro RJ, Baby SM, Young AP, Bates JN, Gaston B, Lewis SJ, 2014. Role of nitric oxide-containing factors in the ventilatory and cardiovascular responses elicited by hypoxic challenge in isoflurane-anesthetized rats. J. Appl. Physiol. (1985) 116, 1371–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortola JP, Frappell PB, 1998. On the barometric method for measurements of ventilation, and its use in small animals. Can. J. Physiol. Pharmacol. 76, 937–944. [DOI] [PubMed] [Google Scholar]
- Moshourab R, Stein C, 2012. Fentanyl decreases discharges of C and A nociceptors to suprathreshold mechanical stimulation in chronic inflammation. J. Neurophysiol. 108, 2827–2836. [DOI] [PubMed] [Google Scholar]
- Moya EA, Alcayaga J, Iturriaga R, 2012. NO modulation of carotid body chemoreception in health and disease. Respir. Physiol. Neurobiol. 184, 158–164. [DOI] [PubMed] [Google Scholar]
- Muijsers RB, Wagstaff AJ, 2001. Transdermal fentanyl: an updated review of its pharmacological properties and therapeutic efficacy in chronic cancer pain control. Drugs 61, 2289–2307. [DOI] [PubMed] [Google Scholar]
- Myers PR, Minor RL Jr., Guerra R Jr., Bates JN, Harrison DG, 1990. Vasorelaxant properties of the endothelium-derived relaxing factor more closely resemble S-nitrosocysteine than nitric oxide. Nature 345, 161–163. [DOI] [PubMed] [Google Scholar]
- Nelson L, Schwaner R, 2009. Transdermal fentanyl: pharmacology and toxicology. J. Med. Toxicol. 5, 230–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nucci TB, Branco LG, Gargaglioni LH, 2004. Nitric oxide pathway in the nucleus raphe magnus modulates hypoxic ventilatory response but not anapyrexia in rats. Brain Res. 1017, 39–45. [DOI] [PubMed] [Google Scholar]
- Ogawa H, Mizusawa A, Kikuchi Y, Hida W, Miki H, Shirato K, 1995. Nitric oxide as a retrograde messenger in the nucleus tractus solitarii of rats during hypoxia. J. Physiol. 486, 495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelligrino DA, Laurito CE, VadeBoncouer TR, 1996. Nitric oxide synthase inhibition modulates the ventilatory depressant and antinociceptive actions of fourth ventricular infusions of morphine in the awake dog. Anesthesiology 85, 1367–1377. [DOI] [PubMed] [Google Scholar]
- Prabhakar NR, Semenza GL, 2012. Gaseous messengers in oxygen sensing. J. Mol. Med. (Berl). 90, 265–272. [DOI] [PubMed] [Google Scholar]
- Raynor K, Kong H, Chen Y, Yasuda K, Yu L, Bell GI, Reisine T, 1994. Pharmacological characterization of the cloned kappa-, delta-, and mu-opioid receptors. Mol. Pharmacol. 45, 330–334. [PubMed] [Google Scholar]
- Rees DD, Palmer RM, Schulz R, Hodson HF, Moncada S, 1990. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Brit. J. Pharmacol. 101, 746–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves SR, Simakajornboon N, Gozal D, 2008. The role of nitric oxide in the neural control of breathing. Respir. Physiol. Neurobiol. 164, 143–150. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Muñoz M, de la Torre-Madrid E, Sánchez-Blázquez P, Garzón J, 2011. NO-released zinc supports the simultaneous binding of Raf-1 and PKCγ cysteine-rich domains to HINT1 protein at the mu-opioid receptor. Antioxid. Redox Signal. 14, 2413–2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Muñoz M, Garzón J, 2013. Nitric oxide and zinc-mediated protein assemblies involved in mu opioid receptor signaling. Mol. Neurobiol. 48, 769–782. [DOI] [PubMed] [Google Scholar]
- Sánchez-Blázquez P, Rodríguez-Muñoz M, Garzón J, 2010. Mu-opioid receptors transiently activate the Akt-nNOS pathway to produce sustained potentiation of PKC-mediated NMDAR-CaMKII signaling. PLoS One, e11278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarton E, Teppema L, Dahan A, 1999. Sex differences in morphine-induced ventilatory depression reside within the peripheral chemoreflex loop. Anesthesiology 90, 1329–1338. [DOI] [PubMed] [Google Scholar]
- Savić Vujović KR, Vučković S, Srebro D, Ivanović M, Došen-Mićović L, Vučetić Č, Džoljić E, Prostran MA, 2013. comparison of the antinociceptive and temperature responses to morphine and fentanyl derivatives in rats. Arch. Pharm. Res. 36, 501–508. [DOI] [PubMed] [Google Scholar]
- Shah RM, Peterson C, Strom A, Dillenburg M, Finzel B, Kitto KF, Fairbanks C, Wilcox G, Wagner CR., 2019. Inhibition of HINT1 Modulates Spinal Nociception and NMDA Evoked Behavior in Mice. ACS Chem. Neurosci. 10, 4385–4393. [DOI] [PubMed] [Google Scholar]
- Tagawa T, Imaizumi T, Harada S, Endo T, Shiramoto M, Hirooka Y, Takeshita A, 1994. Nitric oxide influences neuronal activity in the nucleus tractus solitarius of rat brainstem slices. Circ. Res. 75, 70–76. [DOI] [PubMed] [Google Scholar]
- Torres JE, Kreisman NR, Gozal D, 1997. Nitric oxide modulates in vitro intrinsic optical signal and neural activity in the nucleus tractus solitarius of the rat. Neurosci. Lett. 232, 175–178. [DOI] [PubMed] [Google Scholar]
- Travagli RA, Gillis RA, 1994. Nitric oxide-mediated excitatory effect on neuronsof dorsal motor nucleus of vagus. Am. J. Physiol. 266, G154–G160. [DOI] [PubMed] [Google Scholar]
- Trescot AM, Datta S, Lee M, Hansen H, 2008. Opioid pharmacology. Pain Physician 11(2 Suppl) S133–S153. [PubMed] [Google Scholar]
- Varga AG, Reid BT, Kieffer BL, Levitt ES, 2020. Differential impact of two critical respiratory centres in opioid-induced respiratory depression in awake mice. J. Physiol. 598, 189–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitagliano S, Berrino L, D’Amico M, Maione S, De Novellis V, Rossi F, 1996. Involvement of nitric oxide in cardiorespiratory regulation in the nucleus tractus solitarii. Neuropharmacology 35, 625–631. [DOI] [PubMed] [Google Scholar]
- Wallenstein S, Zucker CL, Fleiss JC, 1980. Some statistical methods useful in circulation research. Circ. Res. 47, 1–9. [DOI] [PubMed] [Google Scholar]
- Wang ZZ, Dinger BG, Stensaas LJ, Fidone SJ, 1995. The role of nitric oxide in carotid chemoreception. Biol. Signals 4, 109–116. [DOI] [PubMed] [Google Scholar]
- Whalen EJ, Johnson AK, Lewis SJ, 2000. Beta-adrenoceptor dysfunction after inhibition of NO synthesis. Hypertension 36, 376–382. [DOI] [PubMed] [Google Scholar]
- Whalen EJ, Bates JN, Johnson AK, Lewis SJ, 2006. Downregulation of propranolol-sensitive beta-adrenoceptor signaling after inhibition of nitric oxide synthesis. Br. J. Pharmacol. 147, 755–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer BJ, 1971. Statistical Principles of Experimental Design, McGraw-Hill, New York, NY, pp. 752–809. [Google Scholar]
- Yadav SK, Kumar D, Kumar P, Gupta PK, Bhattacharya R, 2018. Biochemical, Oxidative, and Physiological Changes Caused by Acute Exposure of Fentanyl and Its 3 Analogs in Rodents. Int. J. Toxicol. 37, 28–37. [DOI] [PubMed] [Google Scholar]
- Yeadon M, Kitchen I, 1990. Multiple opioid receptors mediate the respiratory depressant effects of fentanyl-like drugs in the rat. Gen. Pharmacol. 21, 655–664. [DOI] [PubMed] [Google Scholar]
- Young AP, Gruber RB, Discala JF, May WJ, McLaughlin D, Palmer LA, Lewis SJ, 2013. Co-activation of μ- and δ-opioid receptors elicits tolerance to morphine-induced ventilatory depression via generation of peroxynitrite. Respir. Physiol. Neurobiol. 186, 255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Xue JC, Law PY, Claude PA, Luo LY, Yin J, Chen C, Liu-Chen LY, 1996. The region in the mu opioid receptor conferring selectivity for sufentanil over the delta receptor is different from that over the kappa receptor. FEBS Letters, 384, 198–202. [DOI] [PubMed] [Google Scholar]