Key Teaching Points.
-
•
This case demonstrated an unmasked type 1 Brugada electrocardiogram (ECG) pattern not associated with a body temperature rise several days after a COVID-19 vaccination. This finding suggested a possible risk of a COVID-19 vaccine in Brugada syndrome (BrS) patients, especially those without implantable cardioverter-defibrillator therapy or without an ECG diagnosis of BrS.
-
•
This case suggested the necessity of ECG screening before the initial COVID-19 vaccination to find any latent BrS and to reduce the risk of sudden cardiac death, even in those without any symptoms of faintness or syncope or a familial history of sudden cardiac death.
-
•
A careful in-hospital monitoring would be recommended regardless of an elevated fever until the resolution of the ECG, especially in patients with a remarkable ECG change to a type 1 Brugada ECG pattern after the vaccination.
Introduction
Brugada syndrome (BrS) patients are regarded as a high-risk population under the COVID-19 pandemic because infection-induced fevers may unmask a type 1 Brugada electrocardiogram (ECG) pattern potentially leading to lethal ventricular arrhythmias.1 Vaccinations seem to be crucial in BrS patients; however, careful observation and antipyretic drugs are necessary for vaccine-induced fevers.2 Among the enormous number of COVID-19 vaccinations around the world, although the incidence is rare, death events, including sudden cardiac death, after a vaccination have been reported.3 The causal relationship between the death and vaccination has remained unclear in all cases.
We present a case with an unmasked type 1 Brugada ECG pattern without a fever after a COVID-19 vaccination. This patient had previously been pointed out to have a non–type 1 Brugada ECG pattern but had no symptoms. He experienced faintness and general fatigue for several days after the initial vaccination, which resolved spontaneously.
Case report
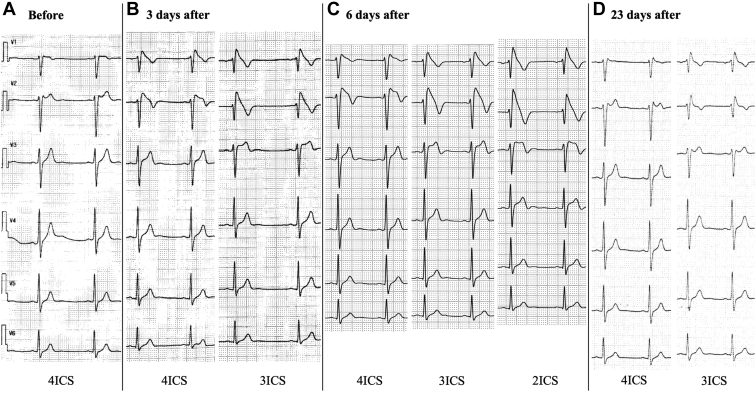
The patient in this case was a 32-year-old man. It had been pointed out that he had a type 2 Brugada ECG pattern from the age of 30 years during a regular medical checkup (Figure 1A). He had no spontaneous type 1 Brugada ECG pattern, history of faintness or syncope, or familial history of sudden cardiac death.
Figure 1.
Chest-lead electrocardiograms (ECGs) before and after the vaccination. A: Before the vaccination. The ECG shows a non–type 1 Brugada ECG pattern at the fourth intercostal space (ICS) 2 years before the vaccination. B: Three days after the vaccination. The ECG shows a type 1 Brugada ECG pattern at the third and fourth ICS. C: Six days after the vaccination. The ECG shows a type 1 Brugada ECG pattern at the second, third, and fourth ICS. D: Twenty-three days after the vaccination. The ECG shows a non–type 1 Brugada ECG pattern at the third and fourth ICS. ECG, electrocardiogram; ICS, intercostal space.
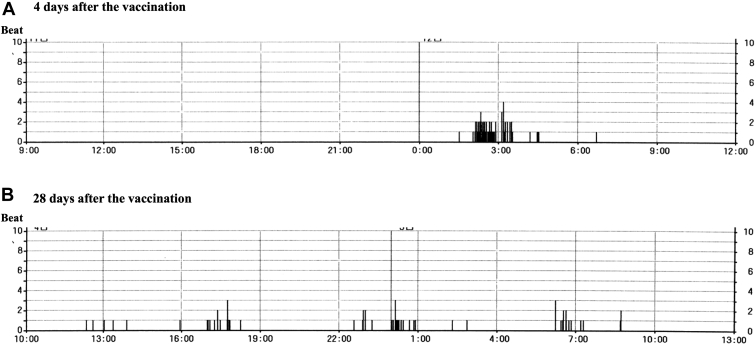
He received a first dose of a BNT162b2 COVID-19 vaccine (Pfizer-BioNTech) in October 2021. He experienced faintness and general fatigue 3 days after the vaccination and visited a cardiovascular clinic (his father was the director). His physical findings, including blood pressure, heart rate, and body temperature, were all within normal range. The 12-lead ECG exhibited a type 1 Brugada ECG in the right-sided precordial leads at the third and fourth intercostal spaces (Figure 1B). The transthoracic echocardiography showed no structural abnormal findings. The 24-hour Holter ECG monitoring performed on the same day showed a gradual decrease in the heart rate after meals, sinus bradycardia around 50 beats/min, and frequent premature ventricular extrasystoles (PVCs) while sleeping (Figure 2A). The laboratory data, including the white blood cell count and serum high-sensitive C-type reactive protein level, were within normal range. The high-sensitive cardiac troponin I was also negative.
Figure 2.
Premature ventricular extrasystoles (PVCs) on the 24-hour Holter electrocardiogram (ECG). A: Four days after the vaccination. The PVC trend shows that PVCs occur frequently only during sleep. B: Twenty-eight days after the vaccination. The PVC trend shows that PVCs occurred infrequently during periods other than sleep.
The diagnosis made was a suspected COVID-19 vaccine–induced type 1 Brugada ECG pattern with ventricular arrhythmias because the changes in the conditions inducing the type 1 Brugada ECG pattern, such as his lifestyle (including bathing and eating habits leading to gastric filling) and his medications, before and after the vaccination were not determined. His condition was carefully observed in the cardiovascular clinic (his home) while monitoring his ECG continuously and preparing an automatic external defibrillator. We basically checked the 12-lead ECGs in the late afternoon and/or night. In addition, we checked the ECG when his symptoms occurred. The ECG changed to a typical type 1 Brugada ECG pattern 6 days after the vaccination (Figure 1C). The ECG findings fluctuated from day to day and his symptoms recurred repeatedly after meals and during bathing until the 22nd day after the vaccination. No fever was observed during the course. An electrophysiological study was not performed because he did not wish to have an invasive examination. The ECG findings resolved spontaneously to a non–type 1 Brugada ECG pattern along with his symptom improvement (Figure 1D). Holter ECG monitoring performed 28 days after the vaccination showed infrequent PVCs during his sleep (Figure 2B). The type 1 Brugada ECG pattern no longer presented more than 1 month after the resolution of the type 1 ECG.
Discussion
This case demonstrated an unmasked type 1 Brugada ECG pattern not associated with a body temperature rise several days after a COVID-19 vaccination. This finding suggested a possible risk of a COVID-19 vaccine in BrS patients, especially those without implantable cardioverter-defibrillator therapy or without an ECG diagnosis of BrS. This case suggested lethal ventricular arrhythmias as one of the causes of sudden cardiac death after a COVID-19 vaccination.
The possible diagnosis in this case may have included acute coronary syndrome, pericarditis, and myocarditis. However, those diseases were unlikely because there was no typical chest pain or elevation of the inflammation markers and myocardial injury. In addition, no abnormal findings, including a left ventricular wall motion abnormality or pericardial effusion, were detected by echocardiography. The underling mechanism of the ECG changes after the vaccination was unclear, and a fever-induced change was excluded. Whether there were any changes in the other conditions inducing the type 1 Brugada ECG pattern before and after the vaccination was not determined. The vaccine-induced activation of the parasympathetic nervous system, which might be supported by new-onset symptoms and bradycardia, may be one possible mechanism.
This case suggested the necessity of ECG screening before the initial COVID-19 vaccination to find any latent BrS and to reduce the risk of sudden cardiac death, even in those without any symptoms of faintness or syncope or a familial history of sudden cardiac death. A careful in-hospital monitoring would be recommended regardless of an elevated fever until the resolution of the ECG, especially in patients with a remarkable ECG change to a type 1 Brugada ECG pattern after the vaccination.
One of the limitations of this case report was the uncertainty of the previous appearance of the type 1 Brugada ECG pattern before the vaccination. Although we evaluated only the 12-lead ECGs obtained during the medical checkups and did not perform any further examination, including a 24-hour 12-lead ECG, before the vaccination, he had received multiple ECG evaluations during his medical checkups since he was 15 years old. A type 1 Brugada ECG pattern on the 12-lead ECGs had not been observed for 17 years. Further, the type 1 Brugada ECG pattern no longer presented after the spontaneous resolution of the type 1 ECG pattern. Another limitation was the uncertainty of the risk of other types of COVID-19 vaccines. A recent study reported that the incidence of cardiac arrhythmias and death after BNT162b2 vaccinations was the lowest among all COVID-19 vaccines.4
Conclusion
A COVID-19 vaccine may unmask a type 1 Brugada ECG pattern several days after a vaccination without a fever.
Acknowledgments
The authors thank Ken Okumura for proofreading the article and John Martin for providing linguistic assistance.
Footnotes
Funding Support: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Disclosures: All authors have no conflict to disclose.
References
- 1.Wu C.I., Postema P.G., Arbelo E., et al. SARS-CoV-2, COVID-19, and inherited arrhythmia syndromes. Heart Rhythm. 2020;17:1456–1462. doi: 10.1016/j.hrthm.2020.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caturano A., Pafundi P.C., Sasso F.C., Dendramis G., Brugada P., Russo V. Brugada syndrome and COVID-19 vaccines. Europace. 2021;23:1871–1872. doi: 10.1093/europace/euab211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu S., Huang R., Sy L.S., et al. COVID-19 vaccination and non-COVID-19 mortality risk - seven integrated health care organizations, United States, December 14, 2020-July 31, 2021. MMWR Morb Mortal Wkly Rpt. 2021;70:1520–1524. doi: 10.15585/mmwr.mm7043e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cari L., Alhosseini M.N., Fiore P., et al. Cardiovascular, neurological, and pulmonary events following vaccination with the BNT162b2, ChAdOx1 nCoV-19, and Ad26.COV2.S vaccines: an analysis of European data. J Autoimmun. 2021;125 doi: 10.1016/j.jaut.2021.102742. 102742. [DOI] [PMC free article] [PubMed] [Google Scholar]