Abstract
N6-methyladenosine (m6A) is the most common epigenetic modification of eukaryotic RNA, which can participate in the growth and development of the body and a variety of physiological and disease processes by affecting the splicing, processing, localization, transport, translation, and degradation of RNA. Increasing evidence shows that non-coding RNAs, particularly microRNA, long non-coding RNA, and circular RNA, can also regulate the RNA m6A modification process by affecting the expression of m6A-related enzymes. The interaction between m6A modification and non-coding RNAs provides a new perspective for the exploration of the potential mechanism of tumor genesis and development. In this review, we summarize the potential mechanisms and effects of m6A and non-coding RNAs in gastrointestinal tract cancers.
Keywords: m6A modification, non-coding RNAs (ncRNAs), gastrointestinal tract cancers, colorectal cancer, long non-coding RNA (lncRNA)
Background
Tumors of the digestive system are the most common malignant tumors, mainly including colorectal cancer, gastric cancer, liver cancer, pancreatic cancer, esophageal cancer, and gallbladder cancer. Since the early symptoms of gastrointestinal tract cancers are not obvious, it is often easier to be ignored. Patients are often treated in a late-stage, which leads to difficult treatment and poor prognosis. An in-depth study of the molecular mechanism of the occurrence and development of gastrointestinal tract cancers is helpful to find targets for early diagnosis and treatment, so as to improve the level of comprehensive diagnosis and treatment of gastrointestinal tract cancers.
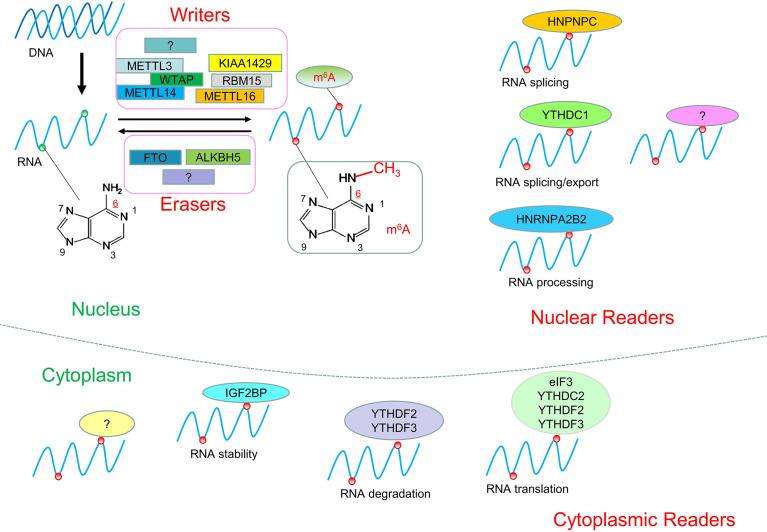
Epigenetics is a branch of genetics that studies the heritable changes in gene expression without changes in the nucleotide sequence of genes. It mainly includes DNA/RNA methylation, histone modification, chromatin remodeling, and non-coding RNAs regulation. Known eukaryotic RNA has more than 100 kinds of modifications, among which the common ones include: N1-methyladenosine (m1A), m6A, and 5-methylcytosine (m5C), of which RNA m6A is the most common eukaryotic RNA Epigenetic modification, which is involved in many key processes of mammalian growth and development and disease (1–4). N6-methyladenosine (m6A) refers to the methylation of the nitrogen atom at the sixth position of the RNA molecule adenine. It is known that m6A modification is the most common in mRNAs, and can also appear in tRNAs, rRNAs, and non-coding RNAs. It regulates gene expression by influencing various metabolic pathways such as RNA processing, transport, translation, and degradation, thus participating in various physiological and pathological processes (1, 5). It is known that m6A is involved in the growth and development of the body (6), learning and memory (7), immune response (8), and disease occurrence (9). The level of RNA methylation in the body is regulated by three enzymes (Writers, Erasers, Readers) in a dynamic equilibrium state ( Figure 1 ). When the balance is broken, it will cause disease. Recently, the study of epigenetic modification, especially RNA m6A, in prognosis prediction and treatment of tumors has been increasing. In lung and bladder cancer, for example, high expression of the m6A-related enzyme METTL3 indicates a worse prognosis (10, 11). Meclofenamic acid (MA) and its ethyl ester derivative MA2 can selectively inhibit the demethylation of m6A-related enzyme FTO and have been used in the treatment of glioma (12, 13).
Figure 1.
RNA m6A modification. RNA m6A modification is a dynamic and reversible process. RNA could be methylated by Writers and demethylated by Erasers. RNA modified by m6A can be recognized by Readers to regulate RNA processing, localization, stability, translation, and degradation.
In recent years, research on RNA m6A modification has become more and more popular, but the main focus is on m6A modification of mRNAs, and less attention has been paid to m6A modification of non-coding RNAs. Recently, a growing body of evidence suggests that RNA m6A modification and non-coding RNAs play an important role in the occurrence and development of tumors, which opens a new door for the diagnosis and treatment of malignant tumors. In this review, we mainly elaborated on the role of m6A and non-coding RNAs interaction in gastrointestinal tract cancers.
Research Progress of RNA m6A Modification
Interest in RNA m6A modification has been ongoing since its discovery in the 1970s (14, 15). The discovery of FTO in 2011 revealed that m6A modification is a dynamic and reversible process (16). In 2012, the discovery of m6A modification detection methods m6A-seq and MeRIP-seq brought about a great turning point in the study of RNA m6A modification (17, 18). It has been found that m6A modification sites are often located in the consensus sequence RRACH (R = G or A and H = A, C, or U), which tends to be found in 3′untranslated regions (3′UTRs) and stop codons (17, 18). The level of m6A modification in the human body is in a dynamic equilibrium state, and its abnormally high or low level will lead to the occurrence of disease. For example, the level of m6A modification is increased in hepatocellular carcinoma and decreased in cervical carcinoma (19, 20). An increasing amount of evidence suggests that abnormal m6A modifications are associated with many types of cancer, including lung, breast, cervical, bladder, glioma, and others (10, 21–24). The dynamic reversible process of m6A was mainly related to the regulation of three types of enzymes: Writers, Erasers, and Readers ( Table 1 and Figure 1 ).
Table 1.
Related enzymes and functions of RNA m6A.
| Type | Enzyme | Function | References |
|---|---|---|---|
| Writers | METTL3 | catalytic core, catalyzes m6A modification of RNA in vivo and in vitro | (25) |
| METTL14 | RNA binding platform that specifically recognizes binding target RNA: Stabilize the METTL3 structure | (25) | |
| WTAP | promotes recognition of m6A modification sites and activates the methyltransferase complex | (26) | |
| KIAA1429 | aggregates the methyltransferase complex at a specific site | (27) | |
| ZC3H13 | promotes localization of the m6A complex into the nucleus | (28) | |
| METTL16 | catalyzes m6A methylation of RNA | (29) | |
| RBM15/15B | helps to gather the enzyme complex to the target site | (30) | |
| ZCCHC4 | is involved in methylation of human 28S rRNA. | (31) | |
| Erasers | FTO | RNA demethylase | (32) |
| ALKBH5 | RNA demethylase | (32) | |
| Readers | YTHDC1 | promotes the transport and splicing of RNA | (33) |
| YTHDC2 | promotes RNA translation | (34) | |
| YTHDF1 | promotes RNA translation | (35) | |
| YTHDF2 | promotes RNA degradation | (36) | |
| YTHDF3 | interaction with YTHDF1 enhances its ability to promote mRNA translation or interaction with YTHDF2 enhances its ability to promote mRNA degradation | (37) | |
| HNRNPC | regulates the selective splicing and structural changes of RNA | (38) | |
| HNRNPA2B1 | regulates the selective splicing and structural changes of RNA | (38) | |
| eIF3 | promotes RNA translation | (35) | |
| IGF2BP1/2/3 | increases RNA stability and translation | (39) |
Writers are methyltransferases, which use S-adenosylmethionine (SAM) as a methyl donor to mediate the process of methylation modification of RNA ( Table 1 ). Writers mainly exist in the form of complexes to play their catalytic role (40). The currently known methyltransferase complex components mainly include METTL3 (methyltransferase-like protein 3), METTL14 (methyltransferase-like protein 14), WTAP (Wilms tumor suppressor-1-associated protein). Among them, METTL3 is the catalytic core, and METTL14 is the RNA binding platform (25); WTAP itself does not have methylation activity. It co-localizes in nuclear speckles with the METTL3-METTL14 heterodimer in the nucleus, which helps the methylase complex quickly recognize the modification site of m6A and activate the METTL3-METTL14 complex (26). Writers also include KIAA1429, ZC3H13 (Zinc finger CCCH-type containing 13), RBM15/15B(RNA binding motif protein 15/15B), ZCCHC4 (zinc finger CCHC-type containing 4), and METTL16 (27, 29, 30). KIAA1429(also known as VIRMA, Vir like m6A methyltransferase associated) recruits the METTL3-METTL14-WTAP complex to specific sites (41). ZC3H13 promotes the localization of the m6A complex into the nucleus (28). ZC3H13 plays an important role in the progression of colorectal, breast, and kidney cancers (42–44). RBM15/15B contributes to the aggregation of the transferase complex to the target (30), which is involved in the progression of hematopoietic diseases, HCC, and laryngeal cancer (45–47). In addition, a new methyltransferase, ZCCHC4, has been discovered in recent years. ZCCHC4 is mainly involved in the methylation of human 28S rRNA and is highly expressed in HCC (31).
Erasers are demethylases that mediate the process of demethylation modification of RNA ( Table 1 ). Erasers mainly include FTO (fat mass and obesity associated protein) and ALKBH5 (ALKB homolog 5). Both belong to the AlkB family and rely on ferric divalent ions and α-ketoglutaric acid for their demethylation function (32). Studies have shown that FTO is related to the occurrence and development of multiple malignant tumors such as breast cancer (48), glioblastoma (49), and acute myeloid leukemia (50).
Readers are m6A binding proteins, which can specifically recognize RNA m6A modification site information to perform different biological functions ( Table 1 ). Readers mainly include YTHDF1-3 and YTHDC1-2 containing the YTH domain, HNRNPC, and HNRNPA2B1 from the heterogeneous nuclear ribonucleoprotein (HNRNP) family, as well as eIF3 (eukaryotic translation initiation factor), IGF2BP1/2/3 (insulin-like growth factor 2 mRNA-binding protein 1/2/3) (39). The YTH domain at the carboxyl terminal of YTHDF1-3 protein can bind to RNA, and the P/Q/N rich region at the amino terminal of YTHDF1-3 protein can bind to the m6A site of mRNA (35, 51). YTHDC1 promotes the transport and splicing of m6A-modified RNA (33); YTHDC2 can promote RNA translation (34); YTHDF1 interacts with translation initiation factor eIF3 to stimulate translation of the corresponding RNA (35); YTHDF2 promotes RNA degradation (36); YTHDF3 has a bidirectional regulation effect. When it binds to YTHDF1, it can enhance the ability of YTHDF1 to promote RNA translation, and when it binds to YTHDF2, it can also enhance the ability of YTHDF2 to promote RNA degradation (37); HNRNPC and HNRNPA2B1 regulate the selective splicing and structural changes of RNA (38).
Non-Coding RNAs
Non-coding RNAs (ncRNAs) are non-protein-coding RNAs, which mainly include micro RNAs (miRNAs), circular RNAs (circRNAs), and long noncoding RNAs (lncRNAs). Non-coding RNA can participate in the protein expression process in a variety of ways.
MiRNAs are endogenous non-coding RNA molecules of approximately 20-22 nucleotides in length. Firstly, the miRNA gene is transcribed into the precursor miRNA (pre-miRNA). Subsequently, in the cytoplasm, the pre-miRNA is cleaved by the Dicer enzyme into mature miRNA (52). Functionally, miRNAs bind to the 3 ‘untranslated region (UTR) of the target gene, resulting in mRNA degradation of the target gene or translation inhibition at the post-transcriptional level of the target gene (53);
CircRNAs are formed by reverse splicing of precursor mRNA(pre-mRNA) (54). There are three main cyclization mechanisms of circRNAs: Intron reverse complementary sequence driven cyclization; RNA binding proteins drive cyclization; Lasso drive cyclization (55–57). Functionally, circRNAs mainly achieve their epigenetic regulation through the following pathways: circRNAs as ceRNA sponges miRNAs to block or reduce the inhibition of miRNAs on target genes; circRNAs can regulate transcription and splicing of target genes. In addition, circRNAs can also be directly involved in protein-protein interactions. (57–60);
LncRNAs are non-coding RNAs larger than 200 nucleotides in length. The synthesis process of lncRNAs is similar to mRNA, with 5 ‘-terminated 7-methylguanosine cap and 3’ -terminated polyadenylate tail. But unlike the mRNA that encodes the protein, lncRNAs don’t have an open reading frame (ORF) (61, 62). LncRNAs can interact with different molecules to exert epigenetic regulation: LncRNAs can regulate gene transcription by reshaping chromatin or directly contacting RNA polymerase and transcription factors; LncRNAs can bind to mRNA and affect its processing and translation; LncRNAs bind to proteins to regulate protein activity; Recent studies have found that lncRNAs can also serve as ceRNA as miRNA sponges. (63–67).
m6A and ncRNAs
In recent years, many studies have proved that not only m6A modification exists in mRNAs, but many ncRNAs are also regulated by m6A modification (68–70). The m6A modification of ncRNAs can not only regulate its processing, splicing, and expression but also affect its positioning and stability. For example, in pancreatic cancer, IGF2BP2 as Reader recognizes m6A-modified lncRNA DANCR, which promotes the progression of pancreatic cancer by improving the stability of DANCR (71). Similarly, ncRNAs can also regulate the process of RNA m6A modification by affecting the expression of m6A-related enzymes. For example, in hepatoblastoma (HB), microRNA miR-186 negatively regulates the expression of METTL3, thereby inhibiting the proliferation and invasion of HB cells (72).
The role of m6A Interaction With ncRNAs in Gastrointestinal Tract Cancers
Currently, an increasing number of studies have proved that m6A and ncRNAs are associated with the occurrence and development of a variety of tumors. RNA m6A modification and ncRNAs can affect the expression of downstream oncogenes or tumor suppressor genes through a variety of pathways, thus influencing tumor progression ( Table 2 ).
Table 2.
Interaction between m6A and ncRNAs in gastrointestinal tract cancers.
| Cancer | Enzyme | Type | Enzyme expression | ncRNA | ncRNA expression | Mechanism | Biological function | References |
|---|---|---|---|---|---|---|---|---|
| Colorectal cancer | METTL3 | Writer | Up | A subset of miRNAs (miR-483, miR-676, and miR-877) | Up | METTL3 promotes the post-transcriptional processing of subsets of miRNAs | promotes tumor growth | (73) |
| METTL3 | Writer | Up | lncRNA RP11 | Up | METTL3 promotes the expression of lncRNA RP11 | promotes CRC cells migration, invasion, and EMT | (70) | |
| METTL3 | Writer | Up | microRNA miR-1246 | Up | METTL3 promotes miR-1246 maturation | promotes the migration and invasion of CRC cells | (69) | |
| METTL3 | Writer | Up | lncRNA LINC01605 | Up | LINC01605 promotes m6A modification of SPTBN2 mRNA by METTL3 | promotes the proliferation, migration and invasion of CRC cells | (74) | |
| METTL3 | Writer | Up | lncRNA LBX2-AS1 | Up | METTL3 promotes lncRNA LBX2-AS1 expression | promotes the proliferation, migration, invasion and 5-FU resistance of CRC cells | (75) | |
| METTL14 | Writer | Down | lncRNA XIST | Up | METTL14 promotes the degradation of lncRNA XIST | inhibits proliferation and invasion of CRC cells | (76) | |
| METTL14 | Writer | Down | microRNA miR-375 | Down | METTL14 promotes miR-375 processing | inhibits the migration and invasion of CRC cells | (77) | |
| ALKBH5 | Eraser | Up | lncRNA NEAT1 | Up | ALKBH5 promotes the expression of lncRNA NEAT1 | promotes the proliferation and migration of colon cancer cells | (78) | |
| YTHDC1 | Reader | – | circNSUN2 | Up | YTHDC1 promotes the export of circNSUN2 from the nucleus to the cytoplasm | promotes liver metastasis of CRC | (68) | |
| YTHDF3 | Reader | Up | lncRNA GAS5 | Down | lncRNA GAS5 inhibits the transcriptional expression of YTHDF3 | promotes the proliferation and invasion of CRC cells | (79) | |
| IGF2BP2 | Reader | Up | lncRNA LINRIS | Up | LINRIS blocks IGF2BP2 degradation | promotes MYC-mediated glycolysis and CRC cells proliferation | (80) | |
| IGF2BP2 | Reader | – | lncRNA LINC00460 | Up | The formation of LINC00460/DHX9/IGF2BP2 complex enhances the stability of HMGA1 mRNA | promotes the proliferation, migration and invasion of CRC cells | (81) | |
| Gastric cancer | METTL3 | Writer | – | lncRNA LINC00470 | Up | LINC00470 promotes the interaction between METTL3 and PTEN mRNA, thereby promoting the degradation of PTEN mRNA and decreasing its expression | promotes the proliferation, migration and invasion of gastric cancer cells | (82) |
| METTL3 | Writer | – | lncRNA ARHGAP5-AS1 | Up | lncRNA ARHGAP5-AS1 promotes ARHGAP5 transcription and improves the stability of ARHGAP5 mRNA by recruiting METTL3 to modify ARHGAP5 mRNA with m6A | promotes chemotherapy resistance in gastric cancer | (83) | |
| METTL3 | Writer | Up | miR-4429 | Down | miR-4429 targets METTL3 and prevents its stabilization of oncogene SEC62 mRNA | inhibits GC proliferation and promote apoptosis | (84) | |
| METTL3 | Writer | Up | lncRNA BLACAT2 | Up | BLACAT2 promotes METTL3 expression | promotes the progression of gastric cancer | (85) | |
| KIAA1429 | Writer | Up | lncRNA LINC00958 | Up | KIAA1429 up-regulates the expression of LINC00958 | promotes aerobic glycolysis of GC cells | (86) | |
| ALKBH5 | Eraser | Up | lncRNA NEAT1 | Up | ALKBH5 promotes the expression of lncRNA NEAT1 | promotes the invasion and metastasis of gastric cancer | (87) | |
| Liver cancer | METTL3 | Writer | Up | lncRNA LINC00958 | Up | METTL3 upregulates the expression of LINC00958 by stabilizing the RNA transcription of LINC00958 | promotes proliferation, migration, invasion and lipogenesis of HCC cells | (88) |
| METTL3 | Writer | Up | circRNA-SORE | Up | METTL3 increases the stability of circRNA-SORE RNA | induces sorafenib resistance in HCC | (89) | |
| METTL3 | Writer | Up | microRNA miR-186 | Down | miR-186 negatively regulates METTL3 expression | inhibits the proliferation and invasion of HB cells | (72) | |
| METTL3 | Writer | Up | circ-ARL3 | Up | METTL3 promotes reverse splicing and formation of circ-ARL3 | promotes the proliferation and invasion of HBV+ HCC cells | (90) | |
| METTL3 | Writer | Up | lncRNA ILF3-AS1 | Up | ILF3-AS1 increases the methylation level of ILF3 by recruiting METTL3, thereby inhibiting degradation of ILF3 | promotes the proliferation, migration and invasion of HCC cells. | (91) | |
| METTL3 | Writer | Up | lncRNA NIFK−AS1 | Up | METTL3 promotes lncRNA NIFK AS1 expression | promotes the growth and invasion of HCC cells and the resistance to sorafenib | (92) | |
| METTL3 | Writer | Up | LncRNA MEG3 | Down | METTL3 inhibits MEG3 expression | promotes the growth and invasion of HCC cells | (93) | |
| KIAA1429 | Writer | Up | lncRNA GATA3-AS | – | lncRNA GATA3-AS targeting promotes m6A modification of GATA3 pre-mRNA by KIAA1429, resulting in decreased stability of GATA3 pre-mRNA and decreases expression of GATA3 | promotes the invasion and migration of HCC cells | (94) | |
| KIAA1429 | Writer | Up | circDLC1 | Down | KIAA1429 inhibits the expression of circDLC1 | promotes the proliferation and movement of HCC cells | (95) | |
| WTAP | Writer | Up | miR-139-5p | Down | miR-139-5p inhibits the expression of WTAP | Inhibits the EMT process of HCC | (96) | |
| YTHDF1 | Reader | Up | lncRNA TPTEP1 | – | YTHDF1-mediated increases GNAS translation inhibited the interaction between lncRNA TPTEP1 and STAT3 | promotes LPS-induced growth and invasion of HCC cells | (97) | |
| Pancreatic cancer | METTL3 | Writer | Up | lncRNA LINC00857 | Up | METTL3 enhances the stability of LINC00857 RNA, resulting in up-regulation of its expression | promotes the proliferation of pancreatic cancer cells and inhibit apoptosis | (98, 99) |
| METTL3 | Writer | Up | miR-25-3p | Up | METTL3 promotes the maturation of miR-25-3p | promotes the initiation and progress of PDAC | (100) | |
| ALKBH5 | Eraser | Down | LncRNA KCNK15-AS1 | Down | ALKBH5 promotes the expression of lncRNA KCNK15-AS1 | inhibits the migration and invasion of pancreatic cancer cells | (101) | |
| IGF2BP2 | Reader | Up | LncRNA DANCR | Up | IGF2BP2 improves the stability of lncRNA DANCR | promotes pancreatic cancer cell proliferation, stem cell-like properties, and tumorigenesis | (71) | |
| HNRNPC | Reader | Up | has-miR-183-3p | – | rs7495 in HNRNPC 3’UTR disrupts the binding site of has-miR-183-3p, thereby increasing the expression of HNRNPC | promotes the proliferation of PDAC cells | (102) | |
| Carcinoma of esophagus | WTAP | Reader | Up | lncRNA EMS | Up | LncRNA EMS promotes WTAP expression | promotes cisplatin resistance of esophageal carcinoma cells. | (103) |
| ALKBH5 | Eraser | Up | lncRNA LINC00278 | Down | ALKBH5 inhibits LINC00278 encoded micropeptide YY1BM | promotes the progress of ESCC | (104) | |
| Gallbladder cancer | METTL3 | Writer | – | miR-2b-3p | Up | METTL3 promotes the maturation of miR-92b-3p | promotes the progression of gallbladder cancer | (105) |
Colorectal Cancer
Colorectal cancer (CRC) is one of the most common cancers of the digestive system, with the third highest incidence (10.0%) in the world, behind breast cancer (11.7%) and lung cancer (11.4%), and the second highest mortality (9.4%) in the world, only after lung cancer (18%) (106). Although traditional surgery combined with radiotherapy and chemotherapy has greatly improved the poor prognosis of colorectal cancer in recent years, the 5-year survival rate of colorectal cancer is still only 63.5%, and postoperative recurrence and liver metastasis of colorectal cancer are the main reasons for poor prognosis of patients with colorectal cancer (107, 108). Therefore, the exploration of the pathogenesis of colorectal cancer has a very important strategic significance. Through a comprehensive analysis of lncRNAs m6A modification in CRC, it was found that the methylation level of lncRNAs in CRC tissues was significantly higher than that in adjacent normal tissues (109); METTL14 promotes the m6A modification of the carcinogen lncRNA XIST, which makes XIST degrade in a YTHDF2-mediated manner, thereby inhibiting the proliferation and invasion of CRC cells (76); Similarly, METTL14 also acts on the tumor suppressor microRNA miR-375 and promotes the processing and maturation of miR-375. Finally, METTL14 inhibits the growth of CRC cells through the miR-375/YAP1 pathway and inhibits the migration and invasion of CRC cells through the miR-375/SP1 pathway (77); Epithelial-mesenchymal transition (EMT) is the first and most important step in cancer cell metastasis (110). METTL3-mediated m6A modification up-regulates the expression of lncRNA RP11. RP11 can trigger the migration, invasion, and EMT of colorectal cancer cells by promoting the post-translational up-regulation of the EMT transcription factor (EMT-TF) Zeb1 (70); The RNA-binding protein RALY (also known as hnRNPCL2) enhances the m6A modification of a subset of miRNAs (miR-483, miR-676, and miR-877) through METTL3, which promotes the post-transcriptional processing of a subset of miRNAs. These miRNAs systematically down-regulate the expression of metabolism-related genes (ATP5I, ATP5G1, ATP5G3, and CYC1), thereby reprogramming mitochondrial metabolism in colorectal cancer cells. Knockout of RALY gene can inhibit the growth and progression of colorectal tumors (73); METTL3 also up-regulates the expression of microRNA miR-1246 by promoting the maturation of miR-1246. miR-1246 negatively regulates the expression of the tumor suppressor gene SPRED2, thereby inactivating the Raf/MEK/ERK pathway and promoting the migration and invasion of CRC cells (69); LINC01605 is significantly overexpressed in CRC, and it could bind to METTL3 to promote m6A modification of SPTBN2 mRNA by METTL3, thus enhancing the translation of SPTBN2 mRNA. Overexpression of SPTBN2 leads to proliferation, migration, and invasion of CRC cells (74). In colorectal cancer, METTL3 induces m6A methylation of lncRNA LBX2-AS1 mRNA, thereby improving its mRNA stability and ultimately promoting its expression. LncRNA LBX2-AS1 is associated with proliferation, migration, invasion, and 5-FU resistance of colorectal cancer (75). In colon cancer, ALKBH5 can up-regulate the expression of lncRNA NEAT1 through demethylation modification, thereby promoting tumor progression. Moreover, ALKBH5 or NEAT1 gene knockout can partially inhibit the malignant behavior of colon cancer (78); CircNSUN2 is often upregulated in patients with liver metastasis (LM) from colorectal cancer and is exported from nucleus to cytoplasm in an m6A-dependent manner through binding to YTHDC1. CircNSUN2 in cytoplasm interacts with IGF2BP2 to form circNSUN2/IGF2BP2/HMGA2 RNA-protein ternary complex, thus enhancing the stability of HMGA2 (high mobility group AT-hook 2) mRNA. This ultimately leads to increased expression of HMGA2, which promotes liver metastasis from colorectal cancer (68); In CRC, lncRNA GAS5 directly binds to YAP and promotes YAP phosphorylation and ubiquitin-mediated degradation. This reduces YAP-mediated transcription of YTHDF3, thereby inhibiting CRC cell proliferation and invasion. On the contrary, YTHDF3 reversibly and selectively binds to the m6A modified GAS5 to trigger the decay of GAS5, thus promoting the progress of CRC and forming a negative feedback loop (79); In colorectal cancer, lncRNA LINRIS (Long Intergene Non-Coding RNA For IGF2BP2 Stability) is highly expressed. LINRIS blocks the degradation of Reader IGF2BP2 through the ubiquitination-autophagy pathway, thereby maintaining the stability of MYC mRNA (MYC mRNA is a typical target of IGF2BP2 and one of the core regulators of glycolysis) and promotes MYC-mediated Glycolysis and proliferation of colorectal cancer cells. This confirms the potential of LINRIS-IGF2BP2-MYC axis for colorectal cancer targeted therapy (80); LncRNA LINC00460 interacts with IGF2BP2 and DHX9 to form the LINC00460/DHX9/IGF2BP2 complex. This complex may increase the stability of HMGA1 mRNA by recognizing the m6A modification site of HMGA1 (high-mobility group at-hook 1), thereby enhancing the expression of HMGA1, and ultimately promoting the proliferation, migration, and invasion process of colorectal cancer cells (81).
Gastric Cancer
The incidence of gastric cancer (GC) ranks fifth in the world, and it is also the fourth leading cause of cancer-related deaths in the world (106). The 5-year survival rate of patients with gastric cancer metastasis is less than 5% (111). Studies have found that in gastric cancer, the expression of METTL3 is elevated. METTL3 modifies the oncogene SEC62 mRNA by m6A, which promotes the stabilizing effect of IGF2BP1 on SEC62 mRNA and increases the expression of SEC62. Interestingly, miR-4429 inhibited the expression of METTL3, resulting in down-regulation of SEC62 expression, thereby preventing the progression of gastric cancer (84); LncRNA LINC00470 is significantly up-regulated in gastric cancer tissues and cell lines. LINC00470 enhances the modification of tumor suppressor PTEN mRNA by binding with METTL3. The up-regulation of m6A modification level of PTEN mRNA promotes the degradation of PTEN mRNA and decreases its expression, and finally promotes the proliferation, migration, and invasion of gastric cancer cells (82); In gastric cancer, the autophagy degradation of lncRNA ARHGAP5-AS1 is impaired, leading to its upregulation in chemotherapy resistant cancer cells. LncRNA ARHGAP5 -AS1 promotes the transcription of ARHGAP5 by interacting with the ARHGAP5 promoter and enhances the stability of ARHGAP5 mRNA in the cytoplasm by recruiting METTL3 to conduct m6A modification on ARHGAP5 mRNA. Finally, the expression of ARHGAP5 is up-regulated, which promotes the chemotherapy resistance of gastric cancer (83); LncRNA BLACAT2 is significantly upregulated in GC. LncRNA BLACAT2 can sponge miR-193b-5p, thereby blocking the inhibitory effect of miR-193b-5p on METTL3 and promoting the progression of GC (85). KIAA1429 up-regulated the expression of lncRNA LINC00958 in GC cells in an m6A dependent manner. LINC00958 promotes aerobic glycolysis of GC cells by enhancing the stability of GLUT1 mRNA (86); ALKBH5 promotes the high expression of lncRNA NEAT1 in gastric cancer cells and tissues through demethylation. NEAT1 can act as a scaffold to bind to EZH2 (a subunit of the multicomb-inhibiting complex), promote the expression of downstream genes of EZH2, and eventually lead to the invasion and metastasis of gastric cancer (87); MicroRNA miR-660, a tumor suppressor, is significantly reduced in gastric cancer tissues and cell lines. MiR-660 inhibits the expression of oncogene E2F3 (E2F transcription factor 3) by directly binding to E2F3 3’-UTR, and ultimately inhibits the proliferation of gastric cancer cells, in which m6A modification is a necessary condition for the interaction between miR-660 and E2F3 (112); In poorly differentiated gastric adenocarcinoma (PDGA), most of the differentiated expressed circRNAs (DECs) are modified by m6A, and the variation trend of m6A modification is basically consistent with the expression level of circRNAs. This suggests that the m6A modification of DECs may play a potential role in the progression of gastric cancer (113);
Liver Cancer
Liver cancer is the third leading cause of death from tumors worldwide, causing more than 830,000 deaths each year (106). The high recurrence rate and high metastasis rate of liver cancer lead to poor prognosis, with a 5-year survival rate of less than 20%. Its symptoms are hidden, 80% of liver cancer patients are often found in the middle and late stage (114, 115). Hepatitis B virus (HBV) infection is an important cause of liver cancer. However, the pathogenesis of liver cancer is not fully understood, and the study of the molecular mechanism of liver cancer will be helpful to the development of new targeted drugs. Primary liver cancer includes hepatocellular carcinoma (HCC), intrahepatic cholangiocarcinoma (ICC), and HCC-ICC mixed type. The most common one is HCC, which accounts for about 90%. In HCC, METTL3 regulates the m6A modification level of lncRNA LINC00958, thereby increasing the stability of LINC00958 and upregulating its expression. LINC00958 promotes the proliferation, migration, invasion, and adipogenesis of HCC cells through miR-3619-5p/HDGF axis (88); In hepatoblastoma (HB), the expression of the tumor suppressor microRNA miR-186 is decreased. MiR-186 targets and negatively regulates METTL3 expression and inhibits the activation of Wnt/β-catenin signaling pathway, thereby inhibiting the proliferation and invasion of HB cells (72); In HBV-associated HCC, HBx protein encoded by HBV x gene upregulates METTL3 expression. This increases the m6A modification level of circ-ARL3. The combination of YTHDC1 and circ-ARL3 modified by m6A is conducive to reverse splicing and formation of circ-ARL3. Circ-ARL3 sponges miR-1305 and antagonizes the inhibition of miR-1305 on a group of target oncogenes, thereby promoting the progression of HBV+ HCC (90); LncRNA ILF3-AS1 expression is increased in HCC tissues. ILF3-AS1 increases the methylation level of ILF3 by recruiting METTL3, thereby inhibiting degradation of ILF3 and ultimately promoting the proliferation, migration, and invasion of HCC cells (91). In HCC, METTL3 enhanced the stability of lncRNA NIFK-AS1 mRNA by increasing the methylation modification level of NIFK−AS1, resulting in increased expression of NIFK-AS1. NIFK-AS1 can promote the growth and invasion of HCC cells and the resistance to sorafenib (92). LncRNA MEG3 is underexpressed in HCC tissues and cells. MEG3 can regulate the expression of BTG2 by sponging miR-544b, thus exerting its anticancer effect. Further studies found that METTL3 could inhibit MEG3 expression (93). It was found that the expression of KIAA1429, a key component of the m6A methyltransferase complex, is significantly up-regulated in HCC tissues. KIAA1429 regulates the expression of the tumor suppressor circDLC1, which is negatively correlated with the expression of circDLC1 in HCC tissues. CircDLC1 can bind to the RNA-binding protein Hur and reduce the interaction between Hur and MMP1 mRNA, thereby inhibiting the expression of MMP1 and ultimately inhibiting the progression of HCC (95); LncRNA GATA3-AS, transcribed from the antisense chain of GATA3 gene, specifically promotes the m6A modification of the tumor suppressor GATA3 precursor mRNA (pre-mRNA) by methyltransferase KIAA1429, which reduces the stability of GATA3 pre-mRNA. The decreased expression of GATA3 promoted the malignant phenotype of HCC cells (94); Additionally, miR-139-5p inhibits the EMT process of HCC by negatively regulating the expression of WTAP (96); Recent studies have shown that HCC is often associated with chronic inflammation. Lipopolysaccharide (LPS) stimulation promotes YTHDF1-mediated G-protein alpha-subunit (GNAS) translation in HCC cells by increasing the m6A modification of GNAS mRNA. The high expression of GNAS promotes the activation of STAT3 in LPS-induced HCC cells by inhibiting the interaction between the lncRNA TPTEP1 and STAT3, and ultimately led to the growth and invasion of LPS-induced HCC cells (97); The expression of circRNA circ_KIAA1429 is up-regulated in HCC. It can maintain the expression of ZEB1 through m6A-YTHDF3-ZEB1 mechanism, thereby promoting the migration, invasion, and occurrence of EMT in HCC cells (116); In sorafenib resistant HCC cells, the increased level of m6A modification of circRNA-SORE improves the stability of its RNA, thereby upregulating the level of circRNA-SORE. CircRNA-SORE acts as a miRNA sponge to isolate miR-103a-2-5p and miR-660-3p, and thus competitively activates the Wnt/β-catenin pathway, ultimately inducing sorafenib resistance in HCC (89);
Pancreatic Cancer
Pancreatic cancer (PC) mainly originates from pancreatic ductal epithelial cells and follicular cells. The 5-year relative survival rate of pancreatic cancer is only 9%, which is one of the worst prognostic malignancies (117). A study in European countries predicts that pancreatic cancer will surpass breast cancer by 2025 and become the third leading cause of cancer death (106). In pancreatic cancer, m6A modification is highly enriched in LINC00857, which enhances its RNA stability and leads to up-regulation of its expression. LINC00857 acts as a ceRNA sponge to bind and inhibit miR-150-5p, resulting in enhanced expression of E2F3 (the target of miR-150-5p), thereby promoting PC cell proliferation and inhibiting apoptosis (98, 99); Cigarette smoke can induce the upregulation of METTL3 expression and the maturation of miR-25-3p by m6A modification. MiR-25-3p targets to inhibit the expression of tumor suppressor PHLPP2, thereby activating AKT-p70S6K oncogene signal and promoting the start and development of pancreatic ductal adenocarcinoma (PDAC) (100); ALKBH5 is down-regulated in pancreatic cancer tissues. It can demethylate lncRNA KCNK15-AS1, thereby promoting the expression of KCNK15-AS1. KCNK15-AS1 and ALKBH5 can inhibit EMT, thereby inhibiting the migration and invasion of pancreatic cancer cells (101); In pancreatic cancer, IGF2BP2 as a reader recognizes m6A-modified lncRNA DANCR, thereby improving the stability of DANCR, and ultimately promoting tumorigenesis, cell proliferation and stem cell-like properties of pancreatic cancer (71); The study found that rs7495 (SNP) in the 3’UTR of HNRNPC may destroy its binding site with has-miR-183-3p, thereby increasing the expression of HNRNPC and promoting the proliferation of PDAC cells (102);
Carcinoma of Esophagus
Esophageal carcinoma is mainly divided into squamous cell carcinoma and adenocarcinoma, the most common clinical symptoms of progressive dysphagia. Esophageal cancer is the fourth leading cause of cancer death in China (118). Hypoxia leads to increased expression of lncRNA EMS in esophageal carcinoma. Overexpressed lncRNA EMS targets miR-758-3p, thereby increasing the expression of WTAP and ultimately promoting cisplatin resistance in esophageal cancer cells (103); Y-linked lncRNA LINC00278 encodes a micropeptide named YY1BM, which inhibits the expression of eEF2K by blocking the interaction between YY1(Yin Yang 1) and androgen receptor (AR). This led to the apoptosis of the cells of the Esophageal squamous cell carcinoma (ESCC). Smoking increases the expression of ALKBH5 and decreases the m6A modification level of LINC00278, thus inhibiting the translation of YY1BM and promoting the development of ESCC (104).
Gallbladder Cancer
Gallbladder cancer (GBC) is the most common malignant tumor of the biliary tract. Although it is relatively rare, its median survival rate is only about 6 months (119). Deoxycholic acid (DCA) is down-regulated in GBC. DCA inhibits the maturation of miR-92b-3p by promoting the disintegration of METTL3 in the METTL3-METTL14-WTAP complex, thereby reducing the m6A modification level of pri-miR-92b in GBC cells. The reduction of miR-2B-3p leads to increased expression of the new miR-92B-3p target ——PTEN (phosphatase and tensin homolog) mRNA, thereby inhibiting the oncogenic PI3K/AKT signaling pathway in gallbladder carcinoma. This suggests that DCA acts as a tumor suppressor in GBC, and DCA therapy may provide a new therapeutic strategy for GBC (105).
Conclusions and Perspectives
At present, there are more and more studies on RNA m6A modification, but the main focus is on the methylation of mRNAs, and less attention is paid to the methylation modification of ncRNAs. NcRNAs mainly include miRNAs, circRNAs, and lncRNAs, which can participate in protein expression through a variety of pathways. The interaction between m6A modification and ncRNA is related to the occurrence and progression of a variety of cancers. Therefore, the study of the interaction mechanism between m6A and ncRNA is conducive to the further development of related drugs for the treatment of tumors. METTL3 has been proved to be highly expressed in a variety of tumors and play a role in tumor progression, which provides ideas for the clinical application of METTL3 inhibitors. At present, METTL3 inhibitors have been identified as a promising anticancer therapy strategy in AML (acute myelogenous leukemia) (120, 121)
In this paper, the mechanism of the interaction between m6A and ncRNAs in gastrointestinal tract cancers was discussed in detail ( Table 2 ), suggesting that: (1) m6A modification can affect the metabolism of ncRNAs, and similarly, ncRNAs can also affect the process of m6A modification, thereby regulating the occurrence and progression of tumors. For example, in colorectal cancer, METTL14 inhibits the proliferation and invasion of CRC cells by promoting the m6A modification of lncRNA XIST (76); lncRNA GAS5 inhibits the proliferation and invasion of CRC cells by reducing the transcription of YTHDF3 (79). (2) An m6A-related enzyme can influence tumor progression by regulating the metabolism of different ncRNAs. For example, METTL14 inhibits the proliferation and invasion of CRC cells by promoting the degradation of lncRNA XIST and the processing and maturation of microRNA miR-375 (76, 77); (3) The same molecule can play the opposite roles in different tumors. For example, METTL3 promotes the progression of CRC but inhibits the progression of gastric cancer (69, 70, 84). These findings reveal that the occurrence of tumors is co-regulated by multiple molecules and multiple pathways, and suggest that targeted therapies targeting multiple molecules and multiple pathways can be carried out simultaneously in the treatment of tumors.
Of course, in addition to the common miRNAs, circRNAs, and lncRNAs, ncRNAs also include snRNAs, snoRNAs, piRNAs, etc (122). The effect of the interaction between m6A and these ncRNAs on tumor development is still worthy of further study.
Author Contributions
YF provided direction and guidance throughout the preparation of this manuscript. LY wrote and edited the manuscript. RH, LH, J-BH, S-YX, and ZD collected and prepared the related papers. X-YW and C-FM reviewed and made significant revisions to the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by Jiangsu Innovative Team Leading Talent Fund (CXTDC2016006, QNRC2016446), Jiangsu 333 Talent Fund (BRA2020016),Jiangsu Provincial Key Research and Development Special Fund (BE2015666), Jiangsu Six High Peak Talent Fund (WSW-205, WSW236), Zhenjiang Key Research and Development Fund (SH2021038), Suqian Science and Technology Support Project Fund (K201907).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
- ALKBH5
ALKB homolog 5
- AR
androgen receptor
- CRC
Colorectal cancer
- CircRNAs
circular RNAs
- DECs
differentiated expressed circRNAs
- DCA
Deoxycholic acid
- eIF3
eukaryotic translation initiation factor
- EMT
Epithelial-mesenchymal transition
- E2F3
E2F transcription factor 3
- EMT-TF
EMT transcription factor
- ESCC
Esophageal squamous cell carcinoma
- FTO
fat mass and obesity associated protein
- GC
Gastric cancer
- GNAS
G-protein alpha-subunit
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HMGA1
high-mobility group at-hook 1
- HMGA2
high mobility group AT-hook 2
- HB
hepatoblastoma
- HNRNP
heterogeneous nuclear ribonucleoprotein
- ICC
intrahepatic cholangiocarcinoma
- IGF2BP1/2/3
insulin-like growth factor 2 mRNA-binding protein 1/2/3
- LINRIS
Long Intergene Non-Coding RNA For IGF2BP2 Stability
- LM
liver metastasis
- lncRNAs
long noncoding RNAs
- LPS
Lipopolysaccharide
- m1A
N1-methyladenosine
- m6A
N6-methyladenosine
- m5C
5-methylcytosine
- MA
Meclofenamic acid
- miRNAs
micro RNAs
- METTL3
methyltransferase-like protein 3
- METTL14
methyltransferase-like protein 14
- ncRNAs
non-coding RNAs
- PTEN
phosphatase and tensin homolog
- PDGA
poorly differentiated gastric adenocarcinoma
- pre-mRNA
precursor mRNA
- PC
Pancreatic cancer
- PDAC
pancreatic ductal adenocarcinoma
- SAM
S-adenosylmethionine
- VIRMA
Vir like m6A methyltransferase associated
- WTAP
Wilms tumor suppressor-1-associated protein
- YY1
Yin Yang 1
- 3’-UTRs
3’untranslated regions
References
- 1. Liu J, Jia G. Methylation Modifications in Eukaryotic Messenger RNA. J Genet Genomics (2014) 41(1):21–33. doi: 10.1016/j.jgg.2013.10.002 [DOI] [PubMed] [Google Scholar]
- 2. Dominissini D, Nachtergaele S, Moshitch-Moshkovitz S, Peer E, Kol N, Ben-Haim MS, et al. The Dynamic N(1)-Methyladenosine Methylome in Eukaryotic Messenger RNA. Nature (2016) 530(7591):441–6. doi: 10.1038/nature16998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. You C, Dai X, Wang Y. Position-Dependent Effects of Regioisomeric Methylated Adenine and Guanine Ribonucleosides on Translation. Nucleic Acids Res (2017) 45(15):9059–67. doi: 10.1093/nar/gkx515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen J, Zhang YC, Huang C, Shen H, Sun B, Cheng X, et al. M(6)A Regulates Neurogenesis and Neuronal Development by Modulating Histone Methyltransferase Ezh2. Genomics Proteomics Bioinf (2019) 17(2):154–68. doi: 10.1016/j.gpb.2018.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boccaletto P, Machnicka MA, Purta E, Piatkowski P, Baginski B, Wirecki TK, et al. MODOMICS: A Database of RNA Modification Pathways. 2017 Update. Nucleic Acids Res (2018) 46(D1):D303–7. doi: 10.1093/nar/gkx1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Edens B, Vissers C, Su J, Arumugam S, Xu Z, Shi H, et al. FMRP Modulates Neural Differentiation Through mA-Dependent mRNA Nuclear Export. Cell Rep (2019) 28(4):845–854.e845. doi: 10.1016/j.celrep.2019.06.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shi H, Zhang X, Weng Y, Lu Z, Liu Y, Lu Z, et al. mA Facilitates Hippocampus-Dependent Learning and Memory Through YTHDF1. Nature (2018) 563(7730):249–53. doi: 10.1038/s41586-018-0666-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li H, Tong J, Zhu S, Batista P, Duffy E, Zhao J, et al. mA mRNA Methylation Controls T Cell Homeostasis by Targeting the IL-7/STAT5/SOCS Pathways. Nature (2017) 548(7667):338–42. doi: 10.1038/nature23450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Han M, Liu Z, Xu Y, Liu X, Wang D, Li F, et al. Abnormality of M6a mRNA Methylation Is Involved in Alzheimer’s Disease. Front Neurosci (2020) 14:98. doi: 10.3389/fnins.2020.00098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Han J, Wang JZ, Yang X, Yu H, Zhou R, Lu HC, et al. METTL3 Promote Tumor Proliferation of Bladder Cancer by Accelerating Pri-Mir221/222 Maturation in M6a-Dependent Manner. Mol Cancer (2019) 18(1):110. doi: 10.1186/s12943-019-1036-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jin D, Guo J, Wu Y, Du J, Yang L, Wang X, et al. M(6)A mRNA Methylation Initiated by METTL3 Directly Promotes YAP Translation and Increases YAP Activity by Regulating the MALAT1-miR-1914-3p-YAP Axis to Induce NSCLC Drug Resistance and Metastasis. J Hematol Oncol (2019) 12(1):135. doi: 10.1186/s13045-019-0830-6 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12. Huang Y, Yan J, Li Q, Li J, Gong S, Zhou H, et al. Meclofenamic Acid Selectively Inhibits FTO Demethylation of M6a Over ALKBH5. Nucleic Acids Res (2015) 43(1):373–84. doi: 10.1093/nar/gku1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang S, Zhao BS, Zhou A, Lin K, Zheng S, Lu Z, et al. M(6)A Demethylase ALKBH5 Maintains Tumorigenicity of Glioblastoma Stem-Like Cells by Sustaining FOXM1 Expression and Cell Proliferation Program. Cancer Cell (2017) 31 591-606(4):e596. doi: 10.1016/j.ccell.2017.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Desrosiers R, Friderici K, Rottman F. Identification of Methylated Nucleosides in Messenger RNA From Novikoff Hepatoma Cells. Proc Natl Acad Sci USA (1974) 71(10):3971–5. doi: 10.1073/pnas.71.10.3971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Perry RP, Kelley DE. Existence of Methylated Messenger RNA in Mouse L Cells. Cell (1974) 1(1):37–42. doi: 10.1016/0092-8674(74)90153-6 [DOI] [Google Scholar]
- 16. Jia G, Fu Y, Zhao X, Dai Q, Zheng G, Yang Y, et al. N6-Methyladenosine in Nuclear RNA is a Major Substrate of the Obesity-Associated FTO. Nat Chem Biol (2011) 7(12):885–7. doi: 10.1038/nchembio.687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dominissini D, Moshitch-Moshkovitz S, Schwartz S, Salmon-Divon M, Ungar L, Osenberg S, et al. Topology of the Human and Mouse M6a RNA Methylomes Revealed by M6a-Seq. Nature (2012) 485(7397):201–6. doi: 10.1038/nature11112 [DOI] [PubMed] [Google Scholar]
- 18. Meyer KD, Saletore Y, Zumbo P, Elemento O, Mason CE, Jaffrey SR. Comprehensive Analysis of mRNA Methylation Reveals Enrichment in 3’ UTRs and Near Stop Codons. Cell (2012) 149(7):1635–46. doi: 10.1016/j.cell.2012.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang X, Li Z, Kong B, Song C, Cong J, Hou J, et al. Reduced mA mRNA Methylation is Correlated With the Progression of Human Cervical Cancer. Oncotarget (2017) 8(58):98918–30. doi: 10.18632/oncotarget.22041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen M, Wei L, Law CT, Tsang FH, Shen J, Cheng CL, et al. RNA N6-Methyladenosine Methyltransferase-Like 3 Promotes Liver Cancer Progression Through YTHDF2-Dependent Posttranscriptional Silencing of SOCS2. Hepatology (2018) 67(6):2254–70. doi: 10.1002/hep.29683 [DOI] [PubMed] [Google Scholar]
- 21. Wang H, Deng Q, Lv Z, Ling Y, Hou X, Chen Z, et al. N6-Methyladenosine Induced miR-143-3p Promotes the Brain Metastasis of Lung Cancer via Regulation of VASH1. Mol Cancer (2019) 18(1):181. doi: 10.1186/s12943-019-1108-x [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22. Xiao L, Li X, Mu Z, Zhou J, Zhou P, Xie C, et al. FTO Inhibition Enhances the Antitumor Effect of Temozolomide by Targeting MYC-miR-155/23a Cluster-MXI1 Feedback Circuit in Glioma. Cancer Res (2020) 80(18):3945–58. doi: 10.1158/0008-5472.CAN-20-0132 [DOI] [PubMed] [Google Scholar]
- 23. Zhang Y, Wang D, Wu D, Zhang D, Sun M. Long Noncoding RNA KCNMB2-AS1 Stabilized by N(6)-Methyladenosine Modification Promotes Cervical Cancer Growth Through Acting as a Competing Endogenous RNA. Cell Transplant (2020) 29:963689720964382. doi: 10.1177/0963689720964382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rong D, Dong Q, Qu H, Deng X, Gao F, Li Q, et al. M(6)A-Induced LINC00958 Promotes Breast Cancer Tumorigenesis via the miR-378a-3p/YY1 Axis. Cell Death Discovery (2021) 7(1):27. doi: 10.1038/s41420-020-00382-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang X, Feng J, Xue Y, Guan Z, Zhang D, Liu Z, et al. Structural Basis of N-6-Adenosine Methylation by the METTL3-METTL14 Complex. Nature (2016) 534(7608):575–8. doi: 10.1038/nature18298 [DOI] [PubMed] [Google Scholar]
- 26. Liu J, Yue Y, Han D, Wang X, Fu Y, Zhang L, et al. A METTL3-METTL14 Complex Mediates Mammalian Nuclear RNA N6-Adenosine Methylation. Nat Chem Biol (2014) 10(2):93–5. doi: 10.1038/nchembio.1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schwartz S, Mumbach MR, Jovanovic M, Wang T, Maciag K, Bushkin GG, et al. Perturbation of M6a Writers Reveals Two Distinct Classes of mRNA Methylation at Internal and 5’ Sites. Cell Rep (2014) 8(1):284–96. doi: 10.1016/j.celrep.2014.05.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wen J, Lv R, Ma H, Shen H, He C, Wang J, et al. Zc3h13 Regulates Nuclear RNA M(6)A Methylation and Mouse Embryonic Stem Cell Self-Renewal. Mol Cell (2018) 69(6):1028–38.e1026. doi: 10.1016/j.molcel.2018.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pendleton KE, Chen B, Liu K, Hunter OV, Xie Y, Tu BP, et al. The U6 snRNA M(6)A Methyltransferase METTL16 Regulates SAM Synthetase Intron Retention. Cell (2017) 169(5):824–35:e814. doi: 10.1016/j.cell.2017.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Patil DP, Chen CK, Pickering BF, Chow A, Jackson C, Guttman M, et al. M(6)A RNA Methylation Promotes XIST-Mediated Transcriptional Repression. Nature (2016) 537(7620):369–73. doi: 10.1038/nature19342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ma H, Wang X, Cai J, Dai Q, Natchiar SK, Lv R, et al. N6-Methyladenosine Methyltransferase ZCCHC4 Mediates Ribosomal RNA Methylation. Nat Chem Biol (2018) 15(1):88–94. doi: 10.1038/s41589-018-0184-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gerken T, Girard CA, Tung YC, Webby CJ, Saudek V, Hewitson KS, et al. The Obesity-Associated FTO Gene Encodes a 2-Oxoglutarate-Dependent Nucleic Acid Demethylase. Science (2007) 318(5855):1469–72. doi: 10.1126/science.1151710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xiao W, Adhikari S, Dahal U, Chen YS, Hao YJ, Sun BF, et al. Nuclear M(6)A Reader YTHDC1 Regulates mRNA Splicing. Mol Cell (2016) 61(4):507–19. doi: 10.1016/j.molcel.2016.01.012 [DOI] [PubMed] [Google Scholar]
- 34. Wojtas MN, Pandey RR, Mendel M, Homolka D, Sachidanandam R, Pillai RS. Regulation of M(6)A Transcripts by the 3’–>5’ RNA Helicase YTHDC2 Is Essential for a Successful Meiotic Program in the Mammalian Germline. Mol Cell (2017) 68(2):374–87.e312. doi: 10.1016/j.molcel.2017.09.021 [DOI] [PubMed] [Google Scholar]
- 35. Wang X, Zhao BS, Roundtree IA, Lu Z, Han D, Ma H, et al. N(6)-Methyladenosine Modulates Messenger RNA Translation Efficiency. Cell (2015) 161(6):1388–99. doi: 10.1016/j.cell.2015.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li Z, Qian P, Shao W, Shi H, He XC, Gogol M, et al. Suppression of M(6)A Reader Ythdf2 Promotes Hematopoietic Stem Cell Expansion. Cell Res (2018) 28(9):904–17. doi: 10.1038/s41422-018-0072-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shi H, Wang X, Lu Z, Zhao BS, Ma H, Hsu PJ, et al. YTHDF3 Facilitates Translation and Decay of N(6)-Methyladenosine-Modified RNA. Cell Res (2017) 27(3):315–28. doi: 10.1038/cr.2017.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Liu N, Dai Q, Zheng G, He C, Parisien M, Pan T. N(6)-Methyladenosine-Dependent RNA Structural Switches Regulate RNA-Protein Interactions. Nature (2015) 518(7540):560–4. doi: 10.1038/nature14234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shi H, Wei J, He C. Where, When, and How: Context-Dependent Functions of RNA Methylation Writers, Readers, and Erasers. Mol Cell (2019) 74(4):640–50. doi: 10.1016/j.molcel.2019.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bokar JA, Rath-Shambaugh ME, Ludwiczak R, Narayan P, Rottman F. Characterization and Partial Purification of mRNA N6-Adenosine Methyltransferase From HeLa Cell Nuclei. Internal mRNA Methylation Requires a Multisubunit Complex. J Biol Chem (1994) 269(26):17697–704. doi: 10.1016/s0021-9258(17)32497-3 [DOI] [PubMed] [Google Scholar]
- 41. Yue Y, Liu J, Cui X, Cao J, Luo G, Zhang Z, et al. VIRMA Mediates Preferential M(6)A mRNA Methylation in 3’UTR and Near Stop Codon and Associates With Alternative Polyadenylation. Cell Discovery (2018) 4:10. doi: 10.1038/s41421-018-0019-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhu D, Zhou J, Zhao J, Jiang G, Zhang X, Zhang Y, et al. ZC3H13 Suppresses Colorectal Cancer Proliferation and Invasion via Inactivating Ras-ERK Signaling. J Cell Physiol (2019) 234(6):8899–907. doi: 10.1002/jcp.27551 [DOI] [PubMed] [Google Scholar]
- 43. Gong PJ, Shao YC, Yang Y, Song WJ, He X, Zeng YF, et al. Analysis of N6-Methyladenosine Methyltransferase Reveals METTL14 and ZC3H13 as Tumor Suppressor Genes in Breast Cancer. Front Oncol (2020) 10:578963. doi: 10.3389/fonc.2020.578963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Guo T, Duan H, Chen J, Liu J, Othmane B, Hu J, et al. N6-Methyladenosine Writer Gene ZC3H13 Predicts Immune Phenotype and Therapeutic Opportunities in Kidney Renal Clear Cell Carcinoma. Front Oncol (2021) 11:718644. doi: 10.3389/fonc.2021.718644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Raffel G, Mercher T, Shigematsu H, Williams I, Cullen D, Akashi K, et al. Ott1(Rbm15) has Pleiotropic Roles in Hematopoietic Development. Proc Natl Acad Sci U S A (2007) 104(14):6001–6. doi: 10.1073/pnas.0609041104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cai X, Chen Y, Man D, Yang B, Feng X, Zhang D, et al. RBM15 Promotes Hepatocellular Carcinoma Progression by Regulating N6-Methyladenosine Modification of YES1 mRNA in an IGF2BP1-Dependent Manner. Cell Death Discovery (2021) 7(1):315. doi: 10.1038/s41420-021-00703-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang X, Tian L, Li Y, Wang J, Yan B, Yang L, et al. RBM15 Facilitates Laryngeal Squamous Cell Carcinoma Progression by Regulating TMBIM6 Stability Through IGF2BP3 Dependent. J Exp Clin Cancer Res (2021) 40(1):80. doi: 10.1186/s13046-021-01871-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang Y, Cheng Z, Xu J, Lai M, Liu L, Zuo M, et al. (2021). Fat Mass and Obesity-Associated Protein (FTO) Mediates Signal Transducer and Activator of Transcription 3 (STAT3)-Drived Resistance of Breast Cancer to Doxorubicin. Bioengineered 12(1):1874-89. doi: 10.1080/21655979.2021.1924544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Huff S, Tiwari SK, Gonzalez GM, Wang Y, Rana TM. M(6)A-RNA Demethylase FTO Inhibitors Impair Self-Renewal in Glioblastoma Stem Cells. ACS Chem Biol (2021) 16(2):324–33. doi: 10.1021/acschembio.0c00841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Li Z, Weng H, Su R, Weng X, Zuo Z, Li C, et al. FTO Plays an Oncogenic Role in Acute Myeloid Leukemia as a N(6)-Methyladenosine RNA Demethylase. Cancer Cell (2017) 31(1):127–41. doi: 10.1016/j.ccell.2016.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Li A, Chen YS, Ping XL, Yang X, Xiao W, Yang Y, et al. Cytoplasmic M(6)A Reader YTHDF3 Promotes mRNA Translation. Cell Res (2017) 27(3):444–7. doi: 10.1038/cr.2017.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ha M, Kim VN. Regulation of microRNA Biogenesis. Nat Rev Mol Cell Biol (2014) 15(8):509–24. doi: 10.1038/nrm3838 [DOI] [PubMed] [Google Scholar]
- 53. Michlewski G, Caceres JF. Post-Transcriptional Control of miRNA Biogenesis. RNA (2019) 25(1):1–16. doi: 10.1261/rna.068692.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Salzman J, Gawad C, Wang PL, Lacayo N, Brown PO. Circular RNAs are the Predominant Transcript Isoform From Hundreds of Human Genes in Diverse Cell Types. PloS One (2012) 7(2):e30733. doi: 10.1371/journal.pone.0030733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jeck WR, Sorrentino JA, Wang K, Slevin MK, Burd CE, Liu J, et al. Circular RNAs are Abundant, Conserved, and Associated With ALU Repeats. RNA (2013) 19(2):141–57. doi: 10.1261/rna.035667.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Conn SJ, Pillman KA, Toubia J, Conn VM, Salmanidis M, Phillips CA, et al. The RNA Binding Protein Quaking Regulates Formation of circRNAs. Cell (2015) 160(6):1125–34. doi: 10.1016/j.cell.2015.02.014 [DOI] [PubMed] [Google Scholar]
- 57. Li Z, Huang C, Bao C, Chen L, Lin M, Wang X, et al. Exon-Intron Circular RNAs Regulate Transcription in the Nucleus. Nat Struct Mol Biol (2015) 22(3):256–64. doi: 10.1038/nsmb.2959 [DOI] [PubMed] [Google Scholar]
- 58. Lasda E, Parker R. Circular RNAs: Diversity of Form and Function. RNA (2014) 20(12):1829–42. doi: 10.1261/rna.047126.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Conn VM, Hugouvieux V, Nayak A, Conos SA, Capovilla G, Cildir G, et al. A circRNA From SEPALLATA3 Regulates Splicing of its Cognate mRNA Through R-Loop Formation. Nat Plants (2017) 3:17053. doi: 10.1038/nplants.2017.53 [DOI] [PubMed] [Google Scholar]
- 60. Pamudurti NR, Bartok O, Jens M, Ashwal-Fluss R, Stottmeister C, Ruhe L, et al. Translation of CircRNAs. Mol Cell (2017) 66 9-21(1):e27. doi: 10.1016/j.molcel.2017.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kapranov P, Cheng J, Dike S, Nix DA, Duttagupta R, Willingham AT, et al. RNA Maps Reveal New RNA Classes and a Possible Function for Pervasive Transcription. Science (2007) 316(5830):1484–8. doi: 10.1126/science.1138341 [DOI] [PubMed] [Google Scholar]
- 62. Ulitsky I, Bartel DP. lincRNAs: Genomics, Evolution, and Mechanisms. Cell (2013) 154(1):26–46. doi: 10.1016/j.cell.2013.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Geisler S, Coller J. RNA in Unexpected Places: Long non-Coding RNA Functions in Diverse Cellular Contexts. Nat Rev Mol Cell Biol (2013) 14(11):699–712. doi: 10.1038/nrm3679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wang X, Sun W, Shen W, Xia M, Chen C, Xiang D, et al. Long non-Coding RNA DILC Regulates Liver Cancer Stem Cells via IL-6/STAT3 Axis. J Hepatol (2016) 64(6):1283–94. doi: 10.1016/j.jhep.2016.01.019 [DOI] [PubMed] [Google Scholar]
- 65. Lan Y, Xiao X, He Z, Luo Y, Wu C, Li L, et al. Long Noncoding RNA OCC-1 Suppresses Cell Growth Through Destabilizing HuR Protein in Colorectal Cancer. Nucleic Acids Res (2018) 46(11):5809–21. doi: 10.1093/nar/gky214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Xiao G, Yao J, Kong D, Ye C, Chen R, Li L, et al. The Long Noncoding RNA TTTY15, Which Is Located on the Y Chromosome, Promotes Prostate Cancer Progression by Sponging Let-7. Eur Urol (2019) 76(3):315–26. doi: 10.1016/j.eururo.2018.11.012 [DOI] [PubMed] [Google Scholar]
- 67. Zhuo W, Liu Y, Li S, Guo D, Sun Q, Jin J, et al. Long Noncoding RNA GMAN, Up-Regulated in Gastric Cancer Tissues, Is Associated With Metastasis in Patients and Promotes Translation of Ephrin A1 by Competitively Binding GMAN-AS. Gastroenterology (2019) 156(3):676–691 e611. doi: 10.1053/j.gastro.2018.10.054 [DOI] [PubMed] [Google Scholar]
- 68. Chen RX, Chen X, Xia LP, Zhang JX, Pan ZZ, Ma XD, et al. N(6)-Methyladenosine Modification of Circnsun2 Facilitates Cytoplasmic Export and Stabilizes HMGA2 to Promote Colorectal Liver Metastasis. Nat Commun (2019) 10(1):4695. doi: 10.1038/s41467-019-12651-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Peng W, Li J, Chen R, Gu Q, Yang P, Qian W, et al. Upregulated METTL3 Promotes Metastasis of Colorectal Cancer via miR-1246/SPRED2/MAPK Signaling Pathway. J Exp Clin Cancer Res (2019) 38(1):393. doi: 10.1186/s13046-019-1408-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wu Y, Yang X, Chen Z, Tian L, Jiang G, Chen F, et al. M(6)A-Induced lncRNA RP11 Triggers the Dissemination of Colorectal Cancer Cells via Upregulation of Zeb1. Mol Cancer (2019) 18(1):87. doi: 10.1186/s12943-019-1014-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hu X, Peng WX, Zhou H, Jiang J, Zhou X, Huang D, et al. IGF2BP2 Regulates DANCR by Serving as an N6-Methyladenosine Reader. Cell Death Differ (2020) 27(6):1782–94. doi: 10.1038/s41418-019-0461-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Cui X, Wang Z, Li J, Zhu J, Ren Z, Zhang D, et al. Cross Talk Between RNA N6-Methyladenosine Methyltransferase-Like 3 and miR-186 Regulates Hepatoblastoma Progression Through Wnt/beta-Catenin Signalling Pathway. Cell Prolif (2020) 53(3):e12768. doi: 10.1111/cpr.12768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sun L, Wan A, Zhou Z, Chen D, Liang H, Liu C, et al. RNA-Binding Protein RALY Reprogrammes Mitochondrial Metabolism via Mediating miRNA Processing in Colorectal Cancer. Gut (2020) 70(9):1698–712. doi: 10.1136/gutjnl-2020-320652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Yue M, Liu T, Yan G, Luo X, Wang L. LINC01605, Regulated by the EP300-SMYD2 Complex, Potentiates the Binding Between METTL3 and SPTBN2 in Colorectal Cancer. Cancer Cell Int (2021) 21(1):504. doi: 10.1186/s12935-021-02180-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ma YN, Hong YG, Yu GY, Jiang SY, Zhao BL, Guo A, et al. LncRNA LBX2-AS1 Promotes Colorectal Cancer Progression and 5-Fluorouracil Resistance. Cancer Cell Int (2021) 21(1):501. doi: 10.1186/s12935-021-02209-y [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 76. Yang X, Zhang S, He C, Xue P, Zhang L, He Z, et al. METTL14 Suppresses Proliferation and Metastasis of Colorectal Cancer by Down-Regulating Oncogenic Long non-Coding RNA XIST. Mol Cancer (2020) 19(1):46. doi: 10.1186/s12943-020-1146-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Chen X, Xu M, Xu X, Zeng K, Liu X, Sun L, et al. METTL14 Suppresses CRC Progression via Regulating N6-Methyladenosine-Dependent Primary miR-375 Processing. Mol Ther (2020) 28(2):599–612. doi: 10.1016/j.ymthe.2019.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 78. Guo T, Liu D, Peng S, Xu AM. ALKBH5 Promotes Colon Cancer Progression by Decreasing Methylation of the lncRNA Neat1. Am J Transl Res (2020) 12(8):4542–9. [PMC free article] [PubMed] [Google Scholar]
- 79. Ni W, Yao S, Zhou Y, Liu Y, Huang P, Zhou A, et al. Long Noncoding RNA GAS5 Inhibits Progression of Colorectal Cancer by Interacting With and Triggering YAP Phosphorylation and Degradation and is Negatively Regulated by the M(6)A Reader YTHDF3. Mol Cancer (2019) 18(1):143. doi: 10.1186/s12943-019-1079-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wang Y, Lu JH, Wu QN, Jin Y, Wang DS, Chen YX, et al. LncRNA LINRIS Stabilizes IGF2BP2 and Promotes the Aerobic Glycolysis in Colorectal Cancer. Mol Cancer (2019) 18(1):174. doi: 10.1186/s12943-019-1105-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Hou P, Meng S, Li M, Lin T, Chu S, Li Z, et al. LINC00460/DHX9/IGF2BP2 Complex Promotes Colorectal Cancer Proliferation and Metastasis by Mediating HMGA1 mRNA Stability Depending on M6a Modification. J Exp Clin Cancer Res (2021) 40(1):52. doi: 10.1186/s13046-021-01857-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Yan J, Huang X, Zhang X, Chen Z, Ye C, Xiang W, et al. LncRNA LINC00470 Promotes the Degradation of PTEN mRNA to Facilitate Malignant Behavior in Gastric Cancer Cells. Biochem Biophys Res Commun (2020) 521(4):887–93. doi: 10.1016/j.bbrc.2019.11.016 [DOI] [PubMed] [Google Scholar]
- 83. Zhu L, Zhu Y, Han S, Chen M, Song P, Dai D, et al. Impaired Autophagic Degradation of lncRNA ARHGAP5-AS1 Promotes Chemoresistance in Gastric Cancer. Cell Death Dis (2019) 10(6):383. doi: 10.1038/s41419-019-1585-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. He H, Wu W, Sun Z, Chai L. MiR-4429 Prevented Gastric Cancer Progression Through Targeting METTL3 to Inhibit M(6)A-Caused Stabilization of SEC62. Biochem Biophys Res Commun (2019) 517(4):581–7. doi: 10.1016/j.bbrc.2019.07.058 [DOI] [PubMed] [Google Scholar]
- 85. Hu H, Kong Q, Huang XX, Zhang HR, Hu KF, Jing Y, et al. Longnon-Coding RNA BLACAT2 Promotes Gastric Cancer Progression via the miR-193b-5p/METTL3 Pathway. J Cancer (2021) 12(11):3209–21. doi: 10.7150/jca.50403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Yang D, Chang S, Li F, Ma M, Yang J, Lv X, et al. M(6) A Transferase KIAA1429-Stabilized LINC00958 Accelerates Gastric Cancer Aerobic Glycolysis Through Targeting GLUT1. IUBMB Life (2021) 73(11):1325–33. doi: 10.1002/iub.2545 [DOI] [PubMed] [Google Scholar]
- 87. Zhang J, Guo S, Piao HY, Wang Y, Wu Y, Meng XY, et al. ALKBH5 Promotes Invasion and Metastasis of Gastric Cancer by Decreasing Methylation of the lncRNA Neat1. J Physiol Biochem (2019) 75(3):379–89. doi: 10.1007/s13105-019-00690-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Zuo X, Chen Z, Gao W, Zhang Y, Wang J, Wang J, et al. M6A-Mediated Upregulation of LINC00958 Increases Lipogenesis and Acts as a Nanotherapeutic Target in Hepatocellular Carcinoma. J Hematol Oncol (2020) 13(1):5. doi: 10.1186/s13045-019-0839-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Xu J, Wan Z, Tang M, Lin Z, Jiang S, Ji L, et al. N(6)-Methyladenosine-Modified CircRNA-SORE Sustains Sorafenib Resistance in Hepatocellular Carcinoma by Regulating Beta-Catenin Signaling. Mol Cancer (2020) 19(1):163. doi: 10.1186/s12943-020-01281-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Rao X, Lai L, Li X, Wang L, Li A, Yang Q. N(6) -Methyladenosine Modification of Circular RNA Circ-ARL3 Facilitates Hepatitis B Virus-Associated Hepatocellular Carcinoma via Sponging miR-1305. IUBMB Life (2021) 73(2):408–17. doi: 10.1002/iub.2438 [DOI] [PubMed] [Google Scholar]
- 91. Bo C, Li N, He L, Zhang S, An Y. Long non-Coding RNA ILF3-AS1 Facilitates Hepatocellular Carcinoma Progression by Stabilizing ILF3 mRNA in an mA-Dependent Manner. Hum Cell (2021) 34(6):1843–54. doi: 10.1007/s13577-021-00608-x [DOI] [PubMed] [Google Scholar]
- 92. Chen YT, Xiang D, Zhao XY, Chu XY. Upregulation of lncRNA NIFK-AS1 in Hepatocellular Carcinoma by M(6)A Methylation Promotes Disease Progression and Sorafenib Resistance. Hum Cell (2021) 34(6):1800–11. doi: 10.1007/s13577-021-00587-z [DOI] [PubMed] [Google Scholar]
- 93. Wu J, Pang R, Li M, Chen B, Huang J, Zhu Y. M6a-Induced LncRNA MEG3 Suppresses the Proliferation, Migration and Invasion of Hepatocellular Carcinoma Cell Through miR-544b/BTG2 Signaling. Onco Targets Ther (2021) 14:3745–55. doi: 10.2147/OTT.S289198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Lan T, Li H, Zhang D, Xu L, Liu H, Hao X, et al. KIAA1429 Contributes to Liver Cancer Progression Through N6-Methyladenosine-Dependent Post-Transcriptional Modification of GATA3. Mol Cancer (2019) 18(1):186. doi: 10.1186/s12943-019-1106-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Liu H, Lan T, Li H, Xu L, Chen X, Liao H, et al. Circular RNA Circdlc1 Inhibits MMP1-Mediated Liver Cancer Progression via Interaction With HuR. Theranostics (2021) 11(3):1396–411. doi: 10.7150/thno.53227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Liu W, Gao X, Chen X, Zhao N, Sun Y, Zou Y, et al. miR-139-5p Loss-Mediated WTAP Activation Contributes to Hepatocellular Carcinoma Progression by Promoting the Epithelial to Mesenchymal Transition. Front Oncol (2021) 11:611544. doi: 10.3389/fonc.2021.611544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Ding H, Zhang X, Su Y, Jia C, Dai C. GNAS Promotes Inflammation-Related Hepatocellular Carcinoma Progression by Promoting STAT3 Activation. Cell Mol Biol Lett (2020) 25:8. doi: 10.1186/s11658-020-00204-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Xia T, Wu X, Cao M, Zhang P, Shi G, Zhang J, et al. The RNA M6a Methyltransferase METTL3 Promotes Pancreatic Cancer Cell Proliferation and Invasion. Pathol Res Pract (2019) 215(11):152666. doi: 10.1016/j.prp.2019.152666 [DOI] [PubMed] [Google Scholar]
- 99. Meng X, Deng Y, He S, Niu L, Zhu H. M(6)A-Mediated Upregulation of LINC00857 Promotes Pancreatic Cancer Tumorigenesis by Regulating the miR-150-5p/E2F3 Axis. Front Oncol (2021) 11:629947. doi: 10.3389/fonc.2021.629947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Zhang J, Bai R, Li M, Ye H, Wu C, Wang C, et al. Excessive miR-25-3p Maturation via N(6)-Methyladenosine Stimulated by Cigarette Smoke Promotes Pancreatic Cancer Progression. Nat Commun (2019) 10(1):1858. doi: 10.1038/s41467-019-09712-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. He Y, Hu H, Wang Y, Yuan H, Lu Z, Wu P, et al. ALKBH5 Inhibits Pancreatic Cancer Motility by Decreasing Long Non-Coding RNA KCNK15-AS1 Methylation. Cell Physiol Biochem (2018) 48(2):838–46. doi: 10.1159/000491915 [DOI] [PubMed] [Google Scholar]
- 102. Ying P, Li Y, Yang N, Wang X, Wang H, He H, et al. Identification of Genetic Variants in M(6)A Modification Genes Associated With Pancreatic Cancer Risk in the Chinese Population. Arch Toxicol (2021) 95(3):1117–28. doi: 10.1007/s00204-021-02978-5 [DOI] [PubMed] [Google Scholar]
- 103. Zhu Z, Pang Y, Jin G, Zhang H, Wang W, Liu J, et al. Hypoxia Induces Chemoresistance of Esophageal Cancer Cells to Cisplatin Through Regulating the lncRNA-EMS/miR-758-3p/WTAP Axis. Aging (Albany NY) (2021) 13(13):17155–76. doi: 10.18632/aging.203062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Wu S, Zhang L, Deng J, Guo B, Li F, Wang Y, et al. A Novel Micropeptide Encoded by Y-Linked LINC00278 Links Cigarette Smoking and AR Signaling in Male Esophageal Squamous Cell Carcinoma. Cancer Res (2020) 80(13):2790–803. doi: 10.1158/0008-5472.CAN-19-3440 [DOI] [PubMed] [Google Scholar]
- 105. Lin R, Zhan M, Yang L, Wang H, Shen H, Huang S, et al. Deoxycholic Acid Modulates the Progression of Gallbladder Cancer Through N(6)-Methyladenosine-Dependent microRNA Maturation. Oncogene (2020) 39(26):4983–5000. doi: 10.1038/s41388-020-1349-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 107. Roth AD, Tejpar S, Delorenzi M, Yan P, Fiocca R, Klingbiel D, et al. Prognostic Role of KRAS and BRAF in Stage II and III Resected Colon Cancer: Results of the Translational Study on the PETACC-3, EORTC 40993, SAKK 60-00 Trial. J Clin Oncol (2010) 28(3):466–74. doi: 10.1200/JCO.2009.23.3452 [DOI] [PubMed] [Google Scholar]
- 108. Aguiar Junior S, Oliveira MM, Silva D, Mello CAL, Calsavara VF, Curado MP. Survival of Patients With Colorectal Cancer in a Cancer Center. Arq Gastroenterol (2020) 57(2):172–7. doi: 10.1590/S0004-2803.202000000-32 [DOI] [PubMed] [Google Scholar]
- 109. Zuo L, Su H, Zhang Q, Wu W, Zeng Y, Li X, et al. Comprehensive Analysis of lncRNAs N-Methyladenosine Modification in Colorectal Cancer. Aging (Albany NY) (2021) 13(3):4182–98. doi: 10.18632/aging.202383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. De Craene B, Berx G. Regulatory Networks Defining EMT During Cancer Initiation and Progression. Nat Rev Cancer (2013) 13(2):97–110. doi: 10.1038/nrc3447 [DOI] [PubMed] [Google Scholar]
- 111. Thrumurthy SG, Chaudry MA, Chau I, Allum W. Does Surgery Have a Role in Managing Incurable Gastric Cancer? Nat Rev Clin Oncol (2015) 12(11):676–82. doi: 10.1038/nrclinonc.2015.132 [DOI] [PubMed] [Google Scholar]
- 112. He X, Shu Y. RNA N6-Methyladenosine Modification Participates in miR-660/E2F3 Axis-Mediated Inhibition of Cell Proliferation in Gastric Cancer. Pathol Res Pract (2019) 215(6):152393. doi: 10.1016/j.prp.2019.03.021 [DOI] [PubMed] [Google Scholar]
- 113. Zhang C, Wang J, Geng X, Tu J, Gao H, Li L, et al. Circular RNA Expression Profile and M6a Modification Analysis in Poorly Differentiated Adenocarcinoma of the Stomach. Epigenomics (2020) 12(12):1027–40. doi: 10.2217/epi-2019-0153 [DOI] [PubMed] [Google Scholar]
- 114. Finn R, Zhu A, Farah W, Almasri J, Zaiem F, Prokop L, et al. Therapies for Advanced Stage Hepatocellular Carcinoma With Macrovascular Invasion or Metastatic Disease: A Systematic Review and Meta-Analysis. Hepatology (2018) 67(1):422–35. doi: 10.1002/hep.29486 [DOI] [PubMed] [Google Scholar]
- 115. Hlady R, Robertson KJH. A Three-Pronged Epigenetic Approach to the Treatment of Hepatocellular Carcinoma. Hepatology (2018) 68(4):1226–8. doi: 10.1002/hep.30133 [DOI] [PubMed] [Google Scholar]
- 116. Wang M, Yang Y, Yang J, Yang J, Han S. Circ_KIAA1429 Accelerates Hepatocellular Carcinoma Advancement Through the Mechanism of M(6)A-YTHDF3-Zeb1. Life Sci (2020) 257:118082. doi: 10.1016/j.lfs.2020.118082 [DOI] [PubMed] [Google Scholar]
- 117. Siegel RL, Miller KD, Jemal A. Cancer Statistics 2020. CA Cancer J Clin (2020) 70(1):7–30. doi: 10.3322/caac.21590 [DOI] [PubMed] [Google Scholar]
- 118. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer Statistics in Chin. CA Cancer J Clin (2016) 66(2):115–32. doi: 10.3322/caac.21338 [DOI] [PubMed] [Google Scholar]
- 119. Jing C, Wang Z, Fu X. Effect of Diabetes Mellitus on Survival in Patients With Gallbladder Cancer: A Systematic Review and Meta-Analysis. BMC Cancer (2020) 20(1):689. doi: 10.1186/s12885-020-07139-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Yankova E, Blackaby W, Albertella M, Rak J, De Braekeleer E, Tsagkogeorga G, et al. Small-Molecule Inhibition of METTL3 as a Strategy Against Myeloid Leukaemia. Nature (2021) 593(7860): 597–601. doi: 10.1038/s41586-021-03536-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Li J, Gregory R. Mining for METTL3 Inhibitors to Suppress Cancer. Nat Struct Mol Biol (2021) 28(6):460–2. doi: 10.1038/s41594-021-00606-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Linder B, Grozhik A, Olarerin-George A, Meydan C, Mason C, Jaffrey S. Single-Nucleotide-Resolution Mapping of M6a and m6Am Throughout the Transcriptome. Nat Methods (2015) 12(8):767–72. doi: 10.1038/nmeth.3453 [DOI] [PMC free article] [PubMed] [Google Scholar]