Abstract
Oral administration of a water-soluble iodine contrast agent (gastrografin) was reported to assist in the appropriate contouring of the small intestine on computed tomography (CT)-based radiotherapy (RT) planning. The efficacy and optimal dose of gastrografin in CT-based image-guided brachytherapy (IGBT) for cervical cancer remain unknown. This study aimed to investigate the efficacy of pretreatment oral administration of gastrografin at a small dose of 50 ml in CT-based IGBT for cervical cancer. A total of 422 sessions in 137 patients who underwent CT-based IGBT with 50 ml of oral gastrografin (concentration, 3% or 4%) were analyzed. Preparation of gastrografin was judged as effective when the small intestine was contrast-enhanced at the area where the small intestine was in contact with the uterus/adnexa. About 287 out of 422 sessions (68%) were judged as effective with gastrografin preparation. The 135 ineffective sessions were considered as follows: (i) the contrast enhancement of the small intestine was not confirmed (n = 36), (ii) the small intestine was not in contact with the uterus/adnexa despite the confirmation of the contrast enhancement of the small intestine (n = 34), and (iii) gastrografin was absent in the small intestine at the area in contact with the uterus/adnexa, even when gastrografin was observed in the small intestine at the area not in contact with the uterus/adnexa (n = 65). In conclusion, pretreatment oral administration of a small dose gastrografin achieved moderate efficacy for accurate contouring of the small intestine close to the uterus/adnexa in CT-based IGBT for cervical cancer.
Keywords: cervical cancer, image-guided brachytherapy (IGBT), gastrografin, computed tomography (CT)
INTRODUCTION
Cervical cancer is a common global malignancy in women. In 2018, approximately 570 000 women worldwide were newly diagnosed with cervical cancer and 310 000 died from the disease [1]. Radical radiotherapy (RT) for cervical cancer consists of external beam radiotherapy (EBRT) and intracavitary brachytherapy (ICBT) [2]. In recent years, 3-dimensional image-guided ICBT (3D-IGBT) has rapidly replaced conventional 2D-ICBT [3–8]. The 3D-IGBT improves oncologic outcomes and reduces adverse events compared to 2D-ICBT by achieving more optimal dose distribution with the sufficient dose for the tumor while minimizing the dose to surrounding normal organs [9, 10].
Magnetic resonance imaging (MRI) provides better tissue contrast and is recommended for 3D-IGBT [11, 12], but computed tomography (CT) based 3D-IGBT is also widely used [3]. It has been reported that CT-based 3D-IGBT can achieve favorable local control and reduced adverse events [13–15]. In CT-based 3D-IGBT, delineating the small intestine and uterus/adnexa where they are in contact is usually difficult. However, the accurate contouring of the small intestine is important to avoid unintentional high-dose exposure to the small intestine and to reduce the risk of late adverse events such as enteritis or worse, perforation. The accurate identification of the small intestine would also contribute to accurate contouring of the High-Risk Clinical Target Volume by facilitating definition of the boundary between the uterus and the small intestine.
Oral administration of contrast agent facilitates contouring of small intestine in CT-based 3D-IGBT. In a case report, pretreatment oral administration of gastrografin was reported to be useful and cost-effective in CT-based 3D-IGBT [16]. Whereas, transvenous contrast administration was not practical in CT-based 3D-IGBT because it is costly, requires manpower, and increases adverse effects [17–20]. Gastrografin (generic name diatrizoate) is a water-soluble iodine contrast agent indicated for gastrointestinal imaging and contains 370 mg/ml of iodine.
There are very few reports on the use of gastrografin for radiation treatment planning on CT images in the pelvic region [16, 21]. Therefore, the efficacy, safety and appropriate dose of gastrografin in CT-based 3D-IGBT are still unknown. In particular, the appropriate dose of gastrografin needs to be further investigated. This is because there are only reports of 250–500 ml of gastrografin being administered orally for RT treatment planning CT scans. However, 250 ml or more of gastrografin is sometimes too much to drink, especially for patients with nausea or diarrhea as adverse effects of RT and/or chemotherapy or in elderly patients.
At our institution, CT-based 3D-IGBT was performed after a dose of 50 ml of oral gastrografin administration. The study aimed to evaluate the safety and efficacy of oral gastrografin administration, especially at low doses (50 ml).
MATERIAL & METHODS
Patients
A total of 159 patients underwent CT-based 3D-IGBT between January 2017 and December 2019 at the University of the Ryukyus hospital as a part of definitive RT for pathologically proven uterine cervical cancer. Of those, 137 received 50 ml (concentration, 3% or 4%) of oral gastrografin in preparation for CT-based 3D-IGBT. Gastrografin was administered orally two hours before the estimated start of ICBT applicator placement. Gastrografin administration was avoided if the patient was allergic to iodine contrast media, had a history of asthma, or if the patient refused to take the medication. A total of 422 CT-based 3D-IGBT sessions for 137 patients were included in the study. Cases of postoperative irradiation were not included in the study.
Table 1 shows the patients’ characteristics. Height, weight and body mass index (BMI) were checked in order to analyze whether the differences in body size affected the efficacy of oral gastrografin.
Table 1.
Patient characteristics (N = 137)
| Characteristics | n |
|---|---|
| Age, years Median (range) |
58 (31–90) |
| Height, cm Median (range) |
159 (130–173) |
| Weight, kg Median (range) |
47 (29–95) |
| BMI, kg/m2 Median (range) |
18.6 (12.8–36.5) |
| History of pelvic surgery Yes No |
22a 115 |
| FIGO Stage (2008) | |
| I (IA, IB) II (IIA, IIB) III (IIIA, IIIB) IV (IVA, IVBb) |
47 (1, 46) 46 (8, 38) 38 (3, 35) 6 (5, 1b) |
| Pathology | |
| SCC AC, ASC Others |
125 10 2 |
| Treatment RT CCRT |
41 96 |
| IGBT frequency 1 2 3 4 |
1 3 111 22 |
Abbreviations: BMI = Body mass index; FIGO = International Federation of Gynecology and Obstetrics; SCC = Squamous cell carcinoma; AC = Adenocarcinoma; ASC = Adenosquamous carcinoma; RT = Radiotherapy; CCRT = Concurrent chemoradiotherapy.
aTwenty-two cases was as follows: excision of appendicitis (n = 8), Caesarean section (n = 8), ovarian/fallopian tube surgery (n = 4) and resection of colorectal cancer (n = 3), including duplicates.
bOne patient with cervical cancer at stage IVB had inguinal lymph node metastasis and underwent curative RT.
Gastrografin was administered to patients with or without suspected diarrhea. To determine whether diarrhea affects the effect of oral gastrografin, the frequency of defecation before and after the administration of gastrografin was recorded. The frequency of defecation before and after administration of gastrografin used for analysis was determined to be the larger one or two days before and after administration, respectively. Eighty-four patients (61.3%) were prescribed loperamide hydrochloride as anti-diarrheal medication. Additionally, almost all patients were prescribed antiemetics, such as metoclopramide. Patients with inadequate oral intake were administered an infusion; there were no patients with clinically suspected dehydration.
This retrospective study was approved by the Institutional Review Board of our institution, and the requirement for written informed consent was waived.
Radiotherapy and chemotherapy
Forty-one patients underwent RT alone, whereas 96 patients underwent concurrent chemoradiotherapy. RT alone was indicated for patients with the International Federation of Gynecology and Oncology (FIGO) 2008 stage IB1/IIA1 cervical cancer with a tumor diameter of <25 mm, or when chemotherapy could not be administered due to old age (over 70 years) or poor general condition.
The standard dose for EBRT was 50 Gy in 25 fractions. After 40 Gy, central shielding was inserted. A 6 Gy in three fractions of boost irradiation to the parametrium of the uterus or lymph node metastases was performed at the discretion of the radiation oncologist. The EBRT was delivered 5 days a week. A 3D-conformal 4-field box technique with 10 MV photons (Clinac iX, Varian Medical Systems, California, USA) was adopted in all cases.
The fields of EBRT were the whole pelvis in 103 patients (75.2%), small pelvis in 14 patients (10.2%) and extended fields including paraaortic lymph nodes in 20 patients (14.6%). Small pelvic field RT was mainly indicated for patients over 75 years old. Extended fields RT was indicated for patients with the paraaortic or common iliac lymph node metastasis.
The standard dose for high-dose-rate IGBT was 18 Gy in three fractions. The regimen of brachytherapy was adjusted in the range of 6 Gy in one fraction to 24 Gy in four fractions, taking into account the therapeutic effect and the irradiated dose of organs at risk. The IGBT dose was prescribed at Point A with standard loading of the source dwell positions and weighting according to the Manchester System, or graphically arranged taking into account target and risk organs doses. A standard applicator set of tandem and ovoid was usually used (metallic applicator, n = 218; plastic applicator, n = 184), and tandem and a vaginal cylinder were used for patients with vaginal invasion (n = 9). When the tumor could not be covered by the intracavitary approach only, hybrid irradiation with intratissue irradiation and intracavitary irradiation was performed (n = 11). All treatment planning was performed with the CT-based 3D-IGBT technique using Oncentra Brachy (Elekta, Stockholm, Sweden). IGBT was administered once a week with a microSelectron HDR brachytherapy afterloader (Elekta).
The standard chemotherapy regimen was weekly cisplatin 40 mg/m2 administration concurrently with RT. When the common iliac lymph node or paraaortic lymph node metastasis was positive, two courses of TP regimen (paclitaxel, 175 mg/m2; cisplatin, 50 mg/m2) were performed every three weeks as neoadjuvant chemotherapy prior to RT, and TP was administered every three weeks concurrently with RT. The chemotherapy regimens were standardized as described above, but could be adjusted at the discretion of the gynecologic oncologist according to the patient or tumor status.
Planning-CT images
Planning-CT images were acquired after ICBT applicator insertion for every IGBT session with LightSpeed VCT (GE Healthcare, Chicago, USA) or discovery RT (GE Healthcare), with a slice thickness of 2.5 mm. The scan area was generally set to include the cranial border at the level of the L5 vertebrae and the caudal border of the perineum. To decrease IGBT doses delivered to the small bowel, the bladder was filled with saline (approximately 100–150 ml).
Evaluation
Effective was defined as the presence of gastrografin in the small intestine in the area adjoining the uterus/adnexa on an IGBT-planning CT scan. If the small intestine was not in contact with the uterus/adnexa, or if the small intestine was in contact with the uterus/adnexa but no contrast enhancement was seen in the area contacting with the uterus/adnexa, it was judged as ineffective.
Additionally, whether gastrografin had reached the cecum was investigated, because if the gastrografin was given too early, it would pass through the small intestine and not work effectively. To assess the reaching of gastrografin to the distal end of the small intestine, the cecum, which is easier to identify than the ileal end, was used instead. Besides, the reach of gastrografin into the sigmoid colon and rectum was also confirmed.
The contours of the organs were determined in consultation with a minimum of two radiation oncologists. The decisions of effective or ineffective were made in consultation with Maemoto H and Ogura T.
To investigate whether the positional relation between the uterus/adnexa and small intestine during each IGBT session could be predicted in advance, MRIs that had been obtained usually within the two weeks before the first IGBT were checked.
Statistical analysis
The student’s t-test and one-way analysis of variance test were used for the analysis of the continuous variables. The Chi-squared test for discrete variables was used to compare proportions. P-values <0.05 were decided to be statistically significant. All statistical analyses were performed using JMP Pro software (version 15.0; SAS Institute, Cary, NC, USA).
RESULTS
Safety
Of the 137 patients included in this study, two or three patients suffered from the adverse effects of gastrografin. One patient developed nausea after the first administration of gastrografin and did not continue. Another patient who had been receiving treatment for asthma for a long time experience an asthma attack several days after the second administration of gastrografin and thus did not continue. Gastrografin was avoided in patients with a history of asthma in the study. However, it was prescribed for this patient because she had not experienced any asthma attacks for a long time. Additionally, one patient took gastrografin only at the first IGBT and did not take it again. The medical records of this patient were insufficient and the details could not have been found. In summary, 134 out of 137 patients (97.8%) took 50 ml of gastrografin without any problems.
The frequency of defecation for each of the two days before and after oral gastrografin administration was available for reference in 291 of the 422 sessions. The median frequency of defecation before and after administration of gastrografin was two (range, 0–10 times) and two (range, 0–16), respectively. The frequency of defecation increased by more than three times after the administration of gastrografin in 18 out of 291 sessions (6.2%). The frequency of defecation before gastrografin was five or more times in the 41 sessions. Of the 41 sessions, only one session had an increase in three or more defecations after the administration of gastrografin.
Efficacy
Out of the 422 IGBT sessions for 137 patients, 287 (68%) were judged to be effective (Fig. 1). In other words, contrast enhancement of the small bowel in contact with the uterus/adnexa (Fig. 2A). The 135 ineffective sessions included: (i) the contrast enhancement of the small intestine within the CT-scanned field was not confirmed (n = 36), (ii) the small intestine was not in contact with the uterus/adnexa, although contrast enhancement of the small intestine was confirmed (n = 34) (Fig. 2B), and (iii) gastrografin was absent in the small intestine in the area in contact with the uterus/adnexa, even when gastrografin was observed in the small intestine in the area where it was not in contact with the uterus/adnexa (n = 65) (Fig. 2C).
Fig. 1.
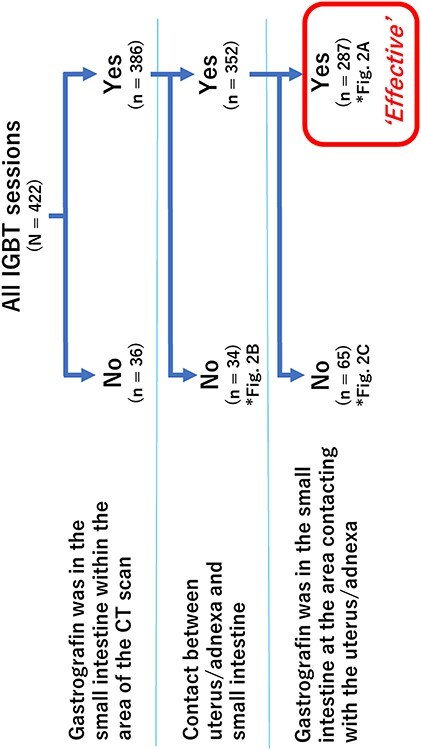
Number of sessions in the gastrografin-effective or ineffective groups.
Fig. 2.
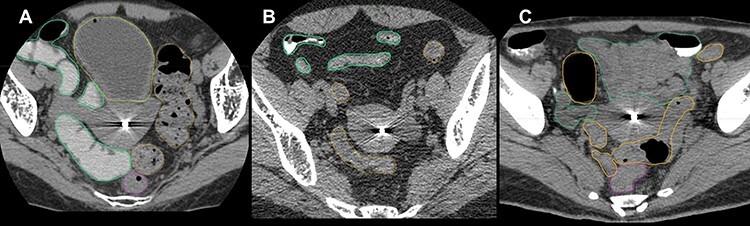
Examples of effective or ineffective cases. The green, yellow, orange and magenta lines indicate the small intestine, bladder, sigmoid colon and rectum, respectively. The tandem applicator inserted in the endometrial cavity is displayed as high density. (A) An effective case of oral gastrografin. (B) An ineffective case of oral gastrografin because the uterus/adnexa was not in contact with any part of the small intestine. (C) An ineffective case of oral gastrografin because gastrografin was absent in the small intestine at the area in contact with the uterus/adnexa, although gastrografin was observed in the small intestine at the area where it was not in contact with the uterus or adnexa.
Sixty-one out of the 137 (44.5%) patients were judged to have had effective IGBT for all sessions, 59 (43.1%) patients had both effective and ineffective sessions and 17 (12.4%) patients had ineffective IGBT in all sessions. The mean BMI of the 61 patients for whom gastrografin was effective in all their IGBT sessions was significantly lower than that of the remaining 76 patients (Fig. 3).
Fig. 3.
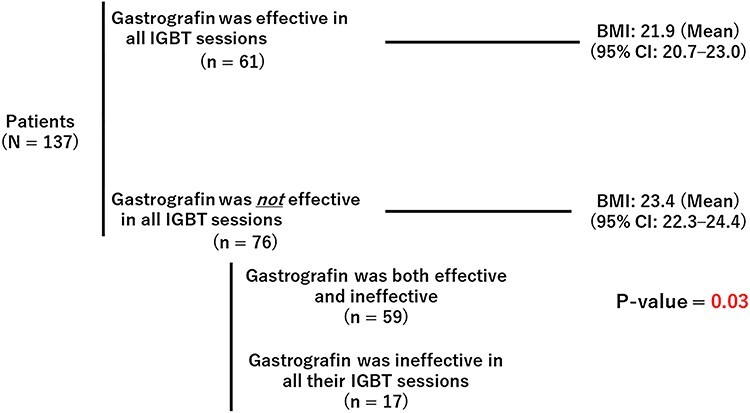
Mean BMI of the patients for whom gastrografin was effective in all IGBT sessions and the others. IGBT = image-guided brachytherapy; CI = confidence interval.
The current study included patients who received 50 ml of gastrografin at a concentration of 3% or 4%. There was no significant difference in the percentage of these two concentrations between the gastrografin-effective and ineffective groups. The history of pelvic surgery or concurrent chemotherapy had no significant effect on the effectiveness of gastrografin. There was also no significant difference in height, weight, or the rate of loperamide hydrochloride prescription between the gastrografin-effective and ineffective groups.
A total of 281 out of 422 sessions had a record of defecation frequency around the IGBT. The median frequency of defecation before administration of gastrografin was two (range, 0–10). There was no significant difference in the mean number of defecations before the administration of gastrografin between the effective sessions (2.3 times, 95% confidence interval [CI] 2.0–2.6 times) and ineffective sessions (2.1 times, 95% CI 1.7–2.5 times).
Contact of small intestine with uterus or adnexa
As shown in Fig. 2B, the small intestine was not in contact with the uterus/adnexa in 34 sessions. Of the 137 patients, the small intestine was in contact with the uterus/adnexa at every IGBT session in 116 patients. Thirteen patients had both contact and no contact sessions, and eight patients had no contact between the uterus/adnexa and small intestine at any IGBT session. Additionally, the relationship between BMI and the contact between the uterus/adnexa and the small intestine was investigated, as it was expected that the small intestine would be more likely to come in contact with the uterus in slim patients because of the smaller space in the pelvis. The mean BMI of the 116 patients whose small intestine and uterus/adnexa were in contact at all their IGBT sessions was significantly lower than the mean BMI of the 21 patients who were not (Fig. 4).
Fig. 4.
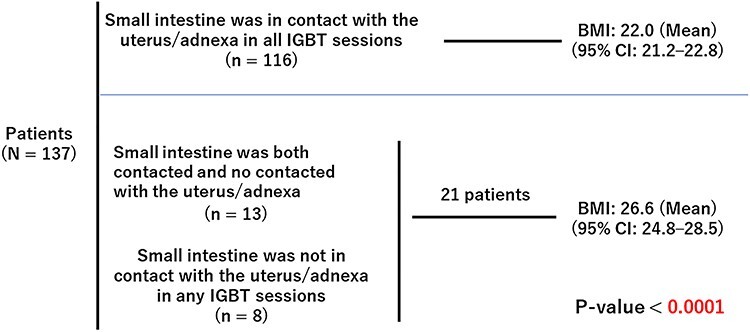
Relationship between BMI and contact of the small intestine with the uterus/adnexa.
There were 117 of the 137 patients who had contact between the small intestine and the uterus/adnexa on MR images during treatment taken within two weeks prior to the first IGBT, and 16 of the 137 patients did not (Fig. 5). The chi-square analysis showed that the percentage of patients who had contact between the small intestine and the uterus/adnexa at every IGBT session was significantly higher in the patients who had contact between the small intestine and uterus/adnexa on MR images taken during the treatment (p < 0.0001). Four out of 137 patients were excluded from this analysis because their MR images during treatment were not confirmed.
Fig. 5.
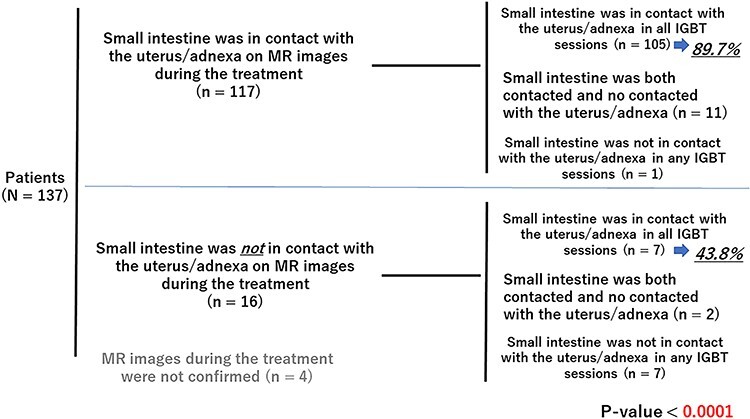
Contact of the small intestine with the uterus/adnexa on MR images before IGBT and the contact in IGBT sessions.
The interval from the administration of gastrografin to CT scan
The median interval from oral gastrografin administration to treatment planning CT scan was 154 min (range, 40–294 min). In the gastrografin-effective 285 sessions, the mean interval from the administration of gastrografin to CT scan was 157 min (95% CI: 152–162 min), while that in the ineffective 126 sessions was 148 min (95% CI: 141–156 min). The interval from administration to CT tended to be longer in the effective group, but the difference was small and not significant (p = 0.07). Eleven sessions were excluded from this analysis because the time of gastrografin administration was not recorded.
Gastrografin’s reach to the cecum and its effectiveness or ineffectiveness
Contrast enhancement of the cecum was investigated in 404 sessions (Fig. 6). The effective rate was significantly higher when gastrografin was identified in the cecum than when it was not (p < 0.0001). Next, the relationship between the interval from the administration of gastrografin to planning CT scans and the presence or absence of evident gastrografin in the cecum was investigated. The mean interval from administration to treatment planning CT scan in the sessions with confirmed gastrografin in the cecum was significantly longer than the mean interval in the remaining sessions without evident gastrografin in the cecum (p < 0.0001).
Fig. 6.
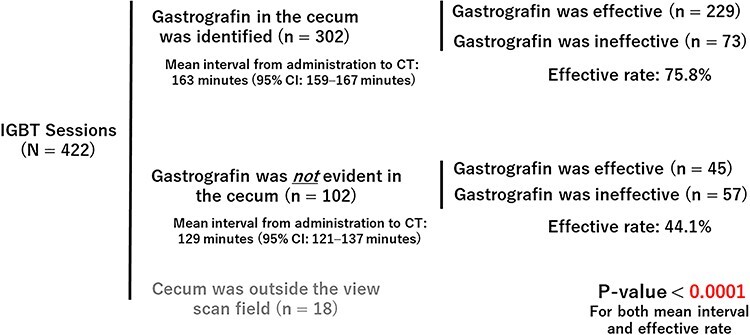
Gastrografin’s reach to the cecum and effective rate.
Gastrografin in the sigmoid colon and rectum
Gastrografin was observed in the sigmoid colon or rectum in 16 IGBT sessions in 11 patients. The mean interval from the administration of gastrografin to treatment planning CT scan in the 16 sessions was 148 min (95% CI: 127–169 min). This was not significantly different from the mean of the other sessions (155 min, 95% CI: 151–159 min). The mean number of the frequency of defecation on the day before or two days before administration of gastrografin was 2.2 (95% CI: 1.1–3.2) in the 16 sessions which were the same as the mean of 2.3 (95% CI:2.0–2.5) in the other sessions.
DISCUSSION
This is the largest study to investigate the safety and efficacy of oral gastrografin in CT-based 3D-IGBT for cervical cancer. In addition, this is also the first study to evaluate the effectiveness of a small dose of 50 ml of oral gastrografin in CT-based 3D-IGBT for cervical cancer. This study revealed that even a small dose of 50 ml of oral gastrografin helped in the contouring of the small bowel in CT-based 3D-IGBT in quite a few cases.
There have been few case series reports that 250–500 ml of oral gastrografin was effective in planning EBRT or IGBT [16, 21]. Patients with nausea or diarrhea during RT and/or chemotherapy for uterine cervical cancer, especially the elderly, may find oral doses of 250–500 ml burdensome and difficult. Therefore, the results of this study are significant and showed that even 50 ml of oral gastrografin would assist radiation oncologists in contouring the small intestine in CT-based 3D-IGBT.
In this study, it was found that oral gastrografin is more likely to work effectively when the small intestine and uterus/adnexa are in contact in MR images before IGBT, and in slim patients. Although the data is not presented, the result of analysis with CT images taken before the start of EBRT, including treatment planning CT, was almost the same as the result of analysis with MRI. Gastrografin was more effective in slim patients, probably because the pelvic cavity is narrower in slim patients, so the small intestine and uterus/adnexa are more likely to be in contact.
Regarding the timing of oral administration, there had been some concern that if the gastrografin was given too early, it would pass through the small intestine and would not work effectively. However, the interval from administration to CT scan tended to be longer in the effective group, although the difference was small and not significant. Moreover, it was found that the effective rate was significantly higher in cases where gastrografin had reached the cecum.
Since gastrografin is also useful in distinguishing between the small intestine and sigmoid colon, the most desirable situation is one in which the gastrografin has reached the small intestine around the uterus but not the sigmoid colon. In the current study, gastrografin was seen in the sigmoid colon or rectum in only 3.8% of cases, so the timing of oral administration in this study was appropriate. This result was consistent with another report, which mentioned that gastrografin reached the small intestine but not the sigmoid colon at 2–4 h after oral administration [16].
Although patients with enteritis are generally considered to require caution when taking gastrografin [22], there were few gastrointestinal side effects associated with oral gastrografin in the current study. This indicates that gastrografin could be administered safely in patients undergoing RT for cervical cancer. One of the reasons for the low frequency of side effects in this study may be that many patients were prescribed anti-diarrheal and antiemetic drugs as supportive care for RT and/or chemotherapy. There had been concerns that radiation enteritis affects the effective rate. However, there was no clear association between gastrografin effectiveness and the frequency of defecation or anti-diarrheal medication.
The main limitation of this study is that it was a single-center retrospective study. In some cases, the time of gastrografin administration or the frequency of defecation were not recorded. The number of IGBTs administered to patients varied from one to four. However, since the number of eligible cases was reasonably large in this study, it is unlikely that the above limitations could limit the results. Some patients took a 3% concentration of gastrografin, while others took a 4% concentration. However, there was no significant difference in the percentage of these two concentrations between the gastrografin-effective and ineffective groups. There was a wide range of intervals from the administration of gastrografin to IGBT planning CT scan due to the retrospective nature of this study. Nonetheless, the time is different in actual clinical practice. Hence, the results of this study may be more in line with the actual clinical situation. How to classify the gastrografin-effective and ineffective groups in this study may have been controversial. For example, it might have been suggested that cases in which the uterus/adnexa and small intestine were not in contact should be excluded from the analysis. However, the criteria used in this study were considered reasonable in assessing the proportion of cases in which gastrografin was useful among the total number of cases with oral gastrografin administration. The efficacy rate for another criterion can be calculated from the number of patients in each group (Fig. 1). For instance, if all the cases in which gastrografin was observed in the small intestine were judged as effective, the efficacy rate would be 91.5% (386 of 422). Whether gastrografin administration actually leads to a reduction in small bowel adverse events was not assessed in the study, although the authors believe that administration of gastrografin allows easier small bowel contouring at a low cost. The accurate identification of the small intestine with gastrografin not only has the potential to reduce the adverse events in the small intestine, but can also contribute to accurate contouring of the High-Risk Clinical Target Volume clarifying the boundary between the uterus and the small intestine, especially in patients with poor adipose tissue around the uterus as is often the cases in Asians.
A dose of 50 ml of oral gastrografin was effective in 68% of all the sessions, and this result was not fully satisfactory. Increasing the dose of gastrografin may increase the efficacy rate. Since gastrografin was confirmed to be safe in this study, it would be better to increase the dose of gastrografin in patients without difficulty in taking large amounts of gastrografin.
In conclusion, pretreatment oral administration of a small dose gastrografin achieved moderate efficacy for accurate contouring of small bowel close to the uterus/adnexa in CT-based IGBT for cervical cancer. It was suggested that oral gastrografin may be more likely to work effectively in slim patients. Gastrografin may be safely used during RT and/or chemotherapy for cervical cancer, although care must be taken in case of a history of asthma or iodine allergy.
CONFLICT OF INTEREST
The authors have no competing financial interests.
FUNDING
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1. Arbyn M, Weiderpass E, Bruni L et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health 2020;8:e191–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Monk BJ, Tewari KS, Koh WJ. Multimodality therapy for locally advanced cervical carcinoma: state of the art and future directions. J Clin Oncol 2007;25:2952–65. [DOI] [PubMed] [Google Scholar]
- 3. Toita T, Ohno T, Ikushima H et al. National survey of intracavitary brachytherapy for intact uterine cervical cancer in Japan. J Radiat Res 2018;59:469–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Viswanathan AN, Erickson BA. Three-dimensional imaging in gynecologic brachytherapy: a survey of the American brachytherapy society. Int J Radiat Oncol Biol Phys 2010;76:104–9. [DOI] [PubMed] [Google Scholar]
- 5. Grover S, Harkenrider MM, Cho LP et al. Image guided cervical brachytherapy: 2014 survey of the American brachytherapy society. Int J Radiat Oncol Biol Phys 2016;94:598–604. [DOI] [PubMed] [Google Scholar]
- 6. Pavamani S, D’Souza DP, Portelance L et al. Image-guided brachytherapy for cervical cancer: a Canadian brachytherapy group survey. Brachytherapy 2011;10:345–51. [DOI] [PubMed] [Google Scholar]
- 7. Taggar AS, Phan T, Traptow L et al. Cervical cancer brachytherapy in Canada: a focus on interstitial brachytherapy utilization. Brachytherapy 2017;16:161–6. [DOI] [PubMed] [Google Scholar]
- 8. de Boer P, Jürgenliemk-Schulz IM, Westerveld H et al. Patterns of care survey: radiotherapy for women with locally advanced cervical cancer. Radiother Oncol 2017;123:306–11. [DOI] [PubMed] [Google Scholar]
- 9. Rijkmans EC, Nout RA, Rutten IH et al. Improved survival of patients with cervical cancer treated with image-guided brachytherapy compared with conventional brachytherapy. Gynecol Oncol 2014;135:231–8. [DOI] [PubMed] [Google Scholar]
- 10. Ohno T, Noda SE, Okonogi N et al. In-room computed tomography–based brachytherapy for uterine cervical cancer: results of a 5-year retrospective study. J Radiat Res 2017;58:543–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pötter R, Haie-Meder C, Van Limbergen E et al. Recommendations from gynaecological (GYN) GEC ESTRO working group (II): concepts and terms in 3D image-based treatment planning in cervix cancer brachytherapy-3D dose volume parameters and aspects of 3D image-based anatomy, radiation physics, radiobiology. Radiother Oncol 2006;78:67–77. [DOI] [PubMed] [Google Scholar]
- 12. Harkenrider MM, Alite F, Silva SR et al. Image-based brachytherapy for the treatment of cervical cancer. Int J Radiat Oncol Biol Phys 2015;92:921–34. [DOI] [PubMed] [Google Scholar]
- 13. Zolciak-Siwinska A, Gruszczynska E, Bijok M et al. Computed tomography-planned high-dose-rate brachytherapy for treating uterine cervical cancer. Int J Radiat Oncol Biol Phys 2016;96:87–92. [DOI] [PubMed] [Google Scholar]
- 14. Okazaki S, Murata K, Noda SE et al. Dose-volume parameters and local tumor control in cervical cancer treated with central-shielding external-beam radiotherapy and CT-based image-guided brachytherapy. J Radiat Res 2019;60:490–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kusada T, Toita T, Ariga T et al. Computed tomography–based image-guided brachytherapy for cervical cancer: correlations between dose–volume parameters and clinical outcomes. J Radiat Res 2018;59:67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Irie D, Murata K, Kaminuma T et al. Oral Gastrografin facilitates delineation of intestinal tracts in CT-based brachytherapy for uterine cervical cancer. Cureus 2020;12:e8367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Katayama H, Yamaguchi K, Kozuka T et al. Adverse reactions to ionic and nonionic contrast media: a report from the Japanese committee on the safety of contrast media. Radiology 1990;175:621–8. [DOI] [PubMed] [Google Scholar]
- 18. Cochran ST, Bomyea K, Sayre JW. Trends in adverse events after IV administration of contrast media. AJR Am J Roentgenol 2001;176:1385–8. [DOI] [PubMed] [Google Scholar]
- 19. Mortelé KJ, Oliva MR, Ondategui S et al. Universal use of nonionic iodinated contrast medium for CT: evaluation of safety in a large urban teaching hospital. AJR Am J Roentgenol 2005;184:31–4. [DOI] [PubMed] [Google Scholar]
- 20. Wang CL, Cohan RH, Ellis JH et al. Frequency, outcome, and appropriateness of treatment of nonionic iodinated contrast media reactions. AJR Am J Roentgenol 2008;191:409–15. [DOI] [PubMed] [Google Scholar]
- 21. Deodato F, Macchia G, Grimaldi L et al. Stereotactic radiotherapy in recurrent gynecological cancer: a case series. Oncol Rep 2009;22:415–9. [PubMed] [Google Scholar]
- 22. Drug information . Gastrografin® Oral/enema. https://pins.japic.or.jp/pdf/newPINS/00051515.pdf; (1 June 2021, date last accessed) (in Japanese).


