Abstract
Huntington’s disease (HD) mouse models suggest that cardiovascular exercise may enhance neuroplasticity and delay disease signs, however, the effects of exercise on neuroplasticity in people with HD are unknown. Using a repeated-measures experimental design, we compared the effects of a single bout of high-intensity exercise, moderate-intensity exercise, or rest, on motor cortex synaptic plasticity in 14 HD CAG-expanded participants (9 premanifest and 5 early manifest) and 20 CAG-healthy control participants, using transcranial magnetic stimulation. Measures of cortico-motor excitability, short-interval intracortical inhibition and intracortical facilitation were obtained before and after a 20-min bout of either high-intensity interval exercise, moderate-intensity continuous exercise, or rest, and again after intermittent theta burst stimulation (iTBS). HD participants showed less inhibition at baseline compared to controls. Whereas the control group showed increased excitability and facilitation following high-intensity exercise and iTBS, the HD group showed no differences in neuroplasticity responses following either exercise intensity or rest, with follow-up Bayesian analyses providing consistent evidence that these effects were absent in the HD group. These findings indicate that exercise-induced synaptic plasticity mechanisms in response to acute exercise may be attenuated in HD, and demonstrate the need for future research to further investigate exercise and plasticity mechanisms in people with HD.
Subject terms: Neuroscience, Neurology
Introduction
Huntington’s disease (HD) is an autosomal dominant neurodegenerative disease resulting in motor dysfunction, cognitive impairment and neuropsychiatric symptoms1. Onset is typically in midlife, although the onset and course of HD is variable, which is proposed to be due to genetic and environmental factors, including lifestyle2. Exercise has emerged as a promising lifestyle candidate to modify disease onset and progression2. Research using HD mouse-models demonstrates that cardiovascular exercise alters biomarkers of neuroplasticity, such as brain derived neurotrophic factor (BDNF)3,4, and delays signs of disease5–8. Additionally, one retrospective study of people with HD found that a lifestyle comprising less time sitting was associated with later disease onset9. Recently, a number of exercise interventions have been trialed in HD with mixed results10–13, although one study provided preliminary evidence that a 9 month multidisciplinary intervention that included exercise increased grey matter volume and improved learning and memory13. One of the key obstacles to designing effective exercise interventions in HD is the lack of clarity regarding the neurophysiological mechanisms that underlie the effect of exercise on the brain in people with the HD CAG-expansion.
A strong candidate mechanism is that each bout of exercise opens a window wherein the capacity for synaptic plasticity is enhanced14. Synaptic plasticity refers to the biological process that modifies the strength of communication between neurons15. In healthy adults, an acute bout of cardiovascular exercise can transiently increase synaptic plasticity in the motor cortex16. For example, 20–30 min of moderate-intensity continuous cycling reduces inhibition17,18, increases facilitation19, and amplifies the response to a plasticity induction protocol20. Additionally, high-intensity interval training (HIIT), where short intervals of high-intensity exercise are interspersed with periods of less intense exercise, elevates BDNF levels21, which may further benefit synaptic plasticity21–23. Recently, we reported the first direct comparison of high-intensity interval exercise and moderate-intensity continuous exercise on synaptic plasticity in motor cortex. We induced a long-term potentiation (LTP) ‘like’ effect by using intermittent theta burst stimulation (iTBS), which comprises brief, high-frequency subthreshold trains of cortical magnetic stimulation pulses in the gamma frequency band (50 Hz), superimposed upon a theta rhythm (5 Hz)24,25. In a sample of 20 healthy adults, we showed that high-intensity interval exercise combined with iTBS enhanced synaptic plasticity, compared to rest, with moderate-intensity continuous exercise showing an intermediate response26. Whether high-intensity exercise is optimal to enhance synaptic plasticity in people living with neurodegenerative disease is currently unknown.
To date, no study has investigated the acute effects of exercise on motor cortex plasticity in HD. This is important because there is evidence that early disruption to cortico-striatal circuits reduces inhibition in the motor cortex27, and previous studies have shown that people with the CAG-expansion for HD have attenuated plasticity responses to several non-invasive brain stimulation protocols28–31. The absence of the normal neuroplasticity response may be attributable to altered or dysregulated dopamine signaling, as well as reduced production of BDNF in HD. Specifically, the indirect (D2) dopaminergic pathway within the striatum is affected early in HD. The indirect pathway connects to the motor cortex via the nigrostriatal pathway32, and is important for motor cortex plasticity27. Additionally, mutant huntingtin has been found to decrease the level of BDNF and its receptor tropomyosin-related kinase B (TrkB) in human and mouse brains, and reduced release of BDNF has been observed in cortical neurons of an HD mouse-model33. Given its known effects on dopamine and BDNF, exercise could present a possible early intervention strategy for people with the HD CAG-expansion to counteract these neurophysiological changes and boost neuroplasticity, optimize cognitive reserve, and delay symptom onset or slow progression34. The aim of the current study was therefore to investigate whether a single bout of either HIIT or moderate-intensity continuous exercise enhances the response to iTBS in people with premanifest and early manifest HD, in comparison to an HD-CAG healthy control group. Using our established transcranial magnetic stimulation (TMS) methodology26, we measured how exercise intensity alters inhibition and excitation in the motor cortex, and synaptic plasticity as induced by iTBS. We hypothesized that similar to healthy controls, HD gene-expanded participants would show the largest enhancement of synaptic plasticity following high-intensity, rather than moderate-intensity exercise (compared to rest). An alternative hypothesis was that baseline alterations to motor cortex neurophysiology (e.g., reduced inhibition and increased facilitation) would attenuate this effect.
Methods
Participants
The sample comprised 20 CAG healthy control participants (Control Group), and 14 HD CAG gene-expanded participants (HD Group; 9 premanifest, 5 early manifest) recruited from the ENRU-Stout HD database held at Monash University, and the Statewide Progressive Neurological Disease Service at Calvary Health Care Bethlehem in Melbourne, Australia. Participants were aged between 21 and 70 years, right-handed, and were screened for contraindications to TMS35 and exercise (e.g., cardiovascular disease, uncontrolled hypertension)36. Exclusion criteria were a history of any psychiatric or neurological illness (except HD for the HD group), seizure, any serious medical conditions, or current pregnancy.
For the HD Group, participants were required to have a genetically-confirmed expansion of the HD CAG repeat sequence (≥ 39 CAG repeats), and no more than mild functional impairment, defined as a Total Functional Capacity (TFC) score of ≥ 1137 on the Unified Huntington’s Disease Rating Scale38 (UHDRS). Participants were classified as premanifest if they had never received a clinical diagnosis of HD, and as early HD if they had received a clinical diagnosis of HD. Disease Burden Score (calculated as age × [CAG-35.5]) ranged from 91 to 44139, equating to estimated years to onset ranging from 0 to 43 years. For those who also participated in the longitudinal, observational Enroll-HD study (n = 13), UHDRS Total Motor Score was obtained from their most recent annual visit. Handedness was self-reported using the Edinburgh Handedness Inventory40, anxiety and depression symptoms were measured using the Hospital Anxiety and Depression Scale41,42, and current physical activity levels were assessed using the International Physical Activity Questionnaire (IPAQ)43. Groups did not differ in age, gender distribution, years of education, self-reported anxiety or depression, or current levels of physical activity (see Table 1). Of the HD participants, three reported mild anxiety symptoms, one reported moderate anxiety symptoms, and two reported mild depression symptoms. Of the control participants, three reported mild anxiety symptoms, one reported moderate anxiety symptoms, and one reported mild depression symptoms. These rates were consistent with normative data for the HADS for the general adult population44. The study was approved by the Human Research Ethics Committees of Monash University (Project Reference: 2019-7055-37585) and Calvary Health Care Bethlehem (Project Reference: 16081804) in Melbourne, Australia, and all participants gave written informed consent. All procedures were performed in accordance with the World Medical Association Declaration of Helsinki.
Table 1.
Participant characteristics.
| HD gene-expanded | Healthy controls | Test statistic | p value | |
|---|---|---|---|---|
| N | 14 | 20 | ||
| Age (years) | 39.71 (13.73), 26–70 | 35.15 (13.25), 21–64 | T32 = − .97 | .34 |
| Women | 10 (71%) | 12 (60%) | χ2 1 = .47 | .49 |
| Years of education | 15.86 (2.31) | 16.85 (3.05) | T32 = 1.03 | .31 |
| CAG repeat length | 41.71 (1.82), 39–46 | – | ||
| Disease-burden score | 246 (104), 91–441 | – | ||
| UHDRS-TMS (n = 13) | 4.92 (8.98), 0–25 | – | ||
| UHDRS-TFC | 12.46 (0.87), 11–13 | – | ||
| HADS anxiety | 5.57 (3.39), 0–11 | 4.90 (3.34), 0–13 | T32 = − .57 | .57 |
| HADS depression | 3.42 (3.18), 0–10 | 2.65 (2.30), 0–8 | T32 = − .83 | .41 |
| Handedness, EHI | + 79.62 (15.13) | + 80.20 (13.27) | T31 = .15 | .88 |
| BMI | 24.52 (2.41) | 23.73 (4.10) | T32 = − .64 | .52 |
| IPAQ | 6949 (4916) | 4681 (2287) | T15.42 = − 1.56 | .14 |
| Resting heart rate, BPM | 63 (7.38) | 68 (12.51) | T31.28 = 1.43 | .16 |
| Rest threshold (%MSO) | 61 (10.96) | 66 (10.26) | F1,32 = 1.65 | .21 |
| Active threshold (%MSO) | 49 (8.23) | 52 (9.28) | F1,32 = 1.28 | .26 |
| Test stimulus (%MSO) | 78 (15.55) | 79 (12.40) | F1,32 = .03 | .86 |
| Conditioning stimulus (%MSO) | 41 (7.65) | 41 (8.18) | F1,32 = .02 | .90 |
| iTBS stimulus (%MSO) | 29 (5.09) | 31 (4.97) | F1,32 = .69 | .41 |
| Baseline 1 mV (NC) | .95 (.25) | 1.07 (.28) | F1,32 = 1.70 | .20 |
| Baseline SICI (C/NC ratio) | .61 (.24) | .50 (.07) | F1,32 = 5.11 | .03 |
| Baseline ICF (C/NC ratio) | 1.19 (.33) | 1.18 (.40) | F1,32 = .005 | .94 |
Data are mean (SD), range or number (%).
Significant values are in bold.
UHDRS-TMS total motor score: possible scores range from 0 to 124, UHDRS-TFC total functional capacity: possible scores range from 0 to 13, UHDRS-TMS total motor score: possible scores range from 0 to 124, HADS hospital anxiety and depression scale, EHI Edinburgh handedness inventory, [range: − 100 (left-handed) to + 100 (right-handed)], BMI body mass index, IPAQ International physical activity questionnaire (higher scores indicate higher levels of physical activity), BPM beats per minute, RMT resting motor threshold, MSO maximum stimulator output, AMT active motor threshold with monophasic stimulation, TS test stimulus, CS conditioning stimulus, iTBS intermittent theta burst stimulation, NC non-conditioned, C conditioned, SICI short interval intracortical inhibition, ICF intracortical facilitation.
Study design and methods
Each participant (HD, controls) completed three sessions separated by at least 72 h, with session order counterbalanced within each group, in a pseudorandom manner, to ensure there were no systematic differences in session order between groups. Participants were instructed to refrain from moderate and vigorous physical activity for 24 h prior to each session.
Session protocol
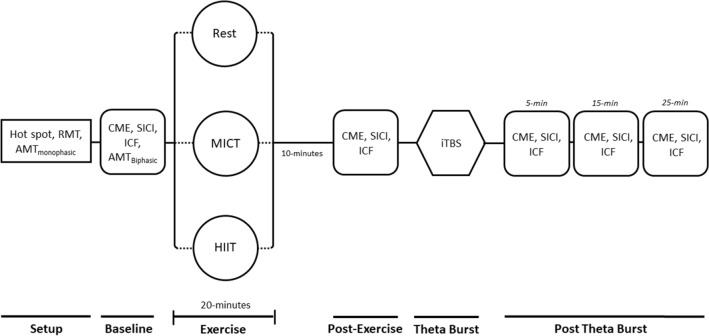
Participants were fitted with a chest-strap heart-rate monitor (Polar H7, Polar Electro), then seated for the Baseline TMS assessment. Next, they completed 20 min of either high-intensity interval stationary cycling, moderate-intensity continuous stationary cycling, or rest, followed by a 10-min cool down, the post-exercise TMS assessment, iTBS, then Post-iTBS TMS assessments at 5, 15 and 25 min (see Fig. 1).
Figure 1.
Sequence of events for each session. RMT resting motor threshold, AMT active motor threshold, CME cortico-motor excitability, SICI short-interval intracortical inhibition, ICF intracortical facilitation, HIIT high-intensity interval training, MICT moderate-intensity continuous training, iTBS intermittent theta burst stimulation. Participants completed one 20-min bout of exercise, or equivalent period of rest, per session.
TMS
We recorded several common single- and paired-pulse TMS measures, including corticomotor excitability (CME), short-interval intracortical inhibition (SICI), and intracortical facilitation (ICF)26,45. SICI is a measure of gamma-amino butyric acid (GABA) receptor A inhibition, whereas ICF has been linked to both the excitatory neurotransmitter glutamate and catecholamine function46. Obtaining both SICI and ICF enables assessment of the excitation-inhibition balance26,47.
The TMS protocol used in this study has been described elsewhere26. Briefly, participants were seated with both hands resting on a pillow on their laps. Surface electromyography (EMG) was recorded from first dorsal interosseous (FDI) of the right hand in a belly-tendon montage. Monophasic TMS pulses were applied to left primary motor cortex using a 70 mm diameter figure-of-eight coil connected to a MagVenture MagPro X100 stimulator (MagVenture Ltd.). At the beginning of each session we determined resting motor threshold, active motor threshold (i.e., the lowest stimulation intensity required to elicit MEPs of ≥ 200 μV during a tonic contraction of the FDI in at least 5 out of 10 consecutive trials48), and the test stimulus intensity required to produce a stable MEP of ~ 1 mV. Paired-pulse TMS was used to assess SICI and ICF, with the sub-threshold conditioning stimulus preceding the supra-threshold ~ 1 mV test stimulus by 2 ms and 12 ms, respectively49. The conditioning stimulus intensity was titrated to produce 50% inhibition of the non-conditioned (NC) MEP then held constant for all SICI and ICF measures. To ensure valid comparison of paired-pulse measures across different time-points, where necessary we adjusted the test stimulus, so that test MEP sizes were matched to within 30% of the comparison time-point (i.e. Post-Exercise was matched to Baseline, Post-iTBS was matched to Post-Exercise)22,26. For all single- and paired-pulse measurements, 16 stimuli were delivered at 5 s intervals.
iTBS was applied to the same motor cortex site, using the same TMS coil and stimulator, following standard procedure24. The iTBS intensity was set at 80% of biphasic active threshold, and three high frequency (50 Hz) biphasic pulses were delivered every 200 ms for 2 s, repeated every 10 s for 20 repetitions.
Exercise
Participants completed the exercise sessions on a stationary cycle ergometer (Wattbike). To avoid stimulation of the FDI muscle during exercise sessions but not rest sessions, which could affect subsequent MEP size, we asked participants to refrain from gripping the handlebar of the bike during exercise. Heart rate was continuously monitored, and the intensity of each exercise session was tailored to individual participants based on their estimated heart rate reserve (HRR), which is an indication of the functional range of the heart during exercise. Resting heart rate was measured while participants were seated and HRR was computed by subtracting resting heart rate from age-predicted maximum heart rate (220—current age).
The high-intensity exercise protocol comprised alternating 3 min bouts of cycling at 50% HRR, with 2 min bouts of cycling at up to 90% HRR, for a total duration of 20 min26. The moderate-intensity exercise protocol comprised 20 min cycling at 50% HRR. For the rest session, participants sat quietly for 20 min. Participants self-reported their perceived exertion (RPE) using Borg’s scale (ranging from 6, no exertion at all, to 20, maximal exertion)50. Participants completed a 2-min warm-up and 2-min cool-down and the beginning and end of each exercise session, followed by a 10-min quiet rest period before the TMS protocol resumed.
With respect to the effectiveness of our methodology in manipulating exercise intensity methodology, we validated the high-intensity and moderate-intensity protocols by characterizing the effects of these sessions on percentage of HRR reached during each session, as well as power:weight ratio, cadence, and RPE, in each group (see Table 2). During the final epoch of the high-intensity exercise session, the HD and control groups reached a maximum of 93% and 91% HRR, respectively. In contrast, for the moderate intensity protocol, on average the HD and control groups exercised at 52% and 50% of HRR. During the high-intensity session, there were no differences between the groups on power, power:weight ratio, cadence, HRR, or RPE for any of the epochs (all ps > 0.08). During the moderate-intensity session, there were no differences between groups for power, power:weight ratio, cadence or HRR (all ps > 0.14;); however, the control group reported a higher perceived exertion than the HD group on average during the moderate intensity session t28.7 = 2.99, p = 0.006).
Table 2.
Effects of high-intensity interval training and moderate-intensity continuous training on exercise performance.
| Session | Power (Watts) | Power:Weight (Watts:kg) | Cadence (RPM) | %HRR | RPE | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Controls | HD | Controls | HD | Controls | HD | Controls | HD | Controls | HD | |
| HIIT | ||||||||||
| 0–3 min | 68 (17.20) | 68 (24.45) | 1.00 (0.24) | 1.05 (0.31) | 76 (7.93) | 73 (11.92) | 43% (0.05) | 45% (0.07) | 10 (1.47) | 10 (1.73) |
| 4–5 min | 106 (34.69) | 105 (31.64) | 1.56 (0.45) | 1.61 (0.33) | 90 (8.96) | 88 (13.34) | 62% (0.07) | 64% (0.12) | 13 (1.43) | 13 (2.05) |
| 6–8 min | 71 (17.88) | 75 (30.04) | 1.05 (0.26) | 1.16 (0.39) | 77 (8.05) | 75 (12.62) | 52% (0.05) | 52% (0.06) | 11 (1.30) | 11 (1.36) |
| 9–10 min | 111 (32.37) | 114 (36.82) | 1.63 (0.44) | 1.73 (0.41) | 95 (7.88) | 90 (15.47) | 73% (0.04) | 74% (0.11) | 13 (1.66) | 13 (2.01) |
| 11–13 min | 73 (18.62) | 76 (30.07) | 1.07 (0.25) | 1.17 (0.37) | 78 (8.26) | 75 (12.78) | 57% (0.04) | 58% (0.07) | 11 (1.34) | 11 (1.05) |
| 14–15 min | 122 (32.18) | 126 (47.61) | 1.80 (0.47) | 1.90 (0.54) | 101 (17.87) | 96 (14.92) | 82% (0.04) | 80% (0.10) | 14 (1.63) | 15 (1.51) |
| 16–18 min | 72 (17.83) | 73 (29.05) | 1.06 (0.24) | 1.11 (0.37) | 77 (9.86) | 75 (13.50) | 62% (0.06) | 59% (0.07) | 12 (1.60) | 12 (1.48) |
| 19–20 min | 156 (61.45) | 165 (59.30) | 2.30 (0.86) | 2.51 (0.63) | 105 (12.41) | 108 (15.87) | 91% (0.06) | 93% (0.15) | 18 (1.32) | 17 (1.63) |
| MICT | ||||||||||
| 0–20 min | 75 (19.14) | 76 (27.39) | 1.09 (0.23) | 1.14 (0.37) | 79 (9.78) | 77 (10.87) | 50% (0.05) | 52% (0.03) | 12 (1.18) | 11 (0.54) |
Data are mean (SD).
HIIT high-intensity interval training, MICT moderate-intensity continuous training, RPM revolutions per minute, %HRR percentage of heart rate reserve, RPE rating of perceived exertion.
Data analysis
EMG recordings were inspected offline, and trials discarded if contaminated with muscle activity in the 100 ms before the TMS pulse, or if root mean square EMG was ≥ 10 μV, because muscle activity at the time of the TMS pulse is known to influence MEP amplitudes. For CME, MEP amplitudes were normalized to the mean MEP at the post-exercise time-point (i.e., a value of 1 was assigned to the post-exercise time-point and all other values were expressed relative to this value24). For paired-pulse measures, the conditioned (C) MEP was expressed as a ratio of the non-conditioned (NC) MEP (C/NC). To reduce the influence of outliers, we used an a priori trimmed mean procedure, in which the highest and lowest values of each set of 16 MEPs were discarded51. Any univariate outliers identified (z ± 3.29; four in the HD group and four in the Control group) were adjusted to a unit higher than the next most extreme score in their respective condition. Due to technical constraints, two values were not obtained at Baseline for a control participant in the Rest condition, and two values were not obtained at the Post-iTBS 15-min time-point for an HD participant in the Rest condition. These values were imputed using mean value replacement. Prior to analysis, a visual inspection of histograms showed positive skew for SICI and ICF variables. To satisfy the normality assumption for statistical analyses, we transformed these variables using a natural logarithm, however, untransformed data are presented in Figures.
Frequentist analyses were conducted using the Statistical Package for the Social Sciences (Version 25; Chicago, IL, USA), with alpha set to 0.05. To determine differences between groups in the absence of exercise, we compared the HD and control groups on TMS measures at baseline, by undertaking two-way mixed model analysis of variance (ANOVA), with Exercise Session as the within-subjects variable and Group as the between-subjects variable. Separate ANOVAs were conducted for each of the TMS measures of interest (Rest threshold, Active threshold, CME, SICI, ICF). Where appropriate, Greenhouse–Geisser epsilon was used to correct for violations of sphericity. To examine differences in synaptic plasticity following exercise across groups, we used three-way mixed model ANOVAs, where Exercise Session and Time were within-subjects factors, and Group was the between subjects factor. Separate ANOVAs were conducted for the TMS measures of CME, SICI and ICF. Significant main effects and interactions were examined further using one-way ANOVAs. We also performed planned group comparisons of change in these TMS measures across each session using t tests. To assess magnitude of effects, we calculated partial eta-squared (ηp2)52.
Given our small sample size, and to more directly compare the effect of exercise condition on change in neuroplasticity measures (CME, SICI, ICF) post iTBS within each group, Bayesian analysis is also reported. Bayesian analyses are helpful in the context of non-significant results, as they provide quantification of evidence in favor of the null hypothesis, and therefore indicate whether the observed data truly provide evidence of absence, or simply indicate an absence of evidence53,54. In order to assess the strength of the evidence for the presence or absence of an effect of exercise on the neuroplasticity measures (CME, SICI and ICF) within each group separately, one-way repeated measures Bayesian ANOVAs were run for each group, where Exercise Session was the within-subjects factor, using JASP software55. Specifically, we calculated Bayes Factors corresponding to evidence in favor of H1 relative to the H0 (BF10), using default priors for ANOVA56. By convention, only Bayes factor values below 0.33 or above 3 are considered noteworthy, with a BF10 value ≥ 3 indicating that the alternative hypothesis is ≥ 3 times more likely than the null hypothesis, and conversely a BF10 value ≤ 0.33 indicating the null hypothesis is ≥ 3 times more likely than the alternative hypothesis, i.e., at least moderate evidence in favor of the alternative, or null hypothesis, respectively57,58. Values between 0.33 and 3 are considered inconclusive and only anecdotal evidence in favor of either hypothesis. ANOVA results with a Bayes Factors ≥ 3 were followed up with post hoc comparisons based on the default Bayesian t test with a Cauchy prior.
Results
The results from the healthy control group have been reported before26, and are included here only to provide a comparison with the HD group.
Baseline motor cortex neurophysiology
The HD and control groups were similar in terms of baseline resting and active motor cortex thresholds, and facilitation (ICF), with no significant main effects of Group or Session, and no interaction effects (Table 1 displays main effects of Group results for these variables). HD participants showed significantly less inhibition (SICI) at baseline than controls across all sessions, represented by a significant main effect of Group (F1,32 = 5.11, p = 0.03, ηp2 = 0.14).
Cortico-motor excitability
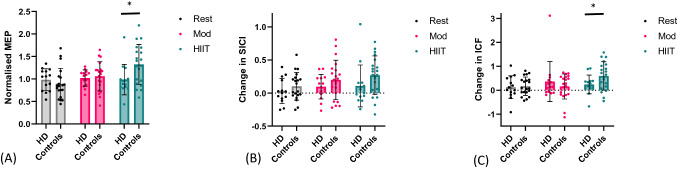
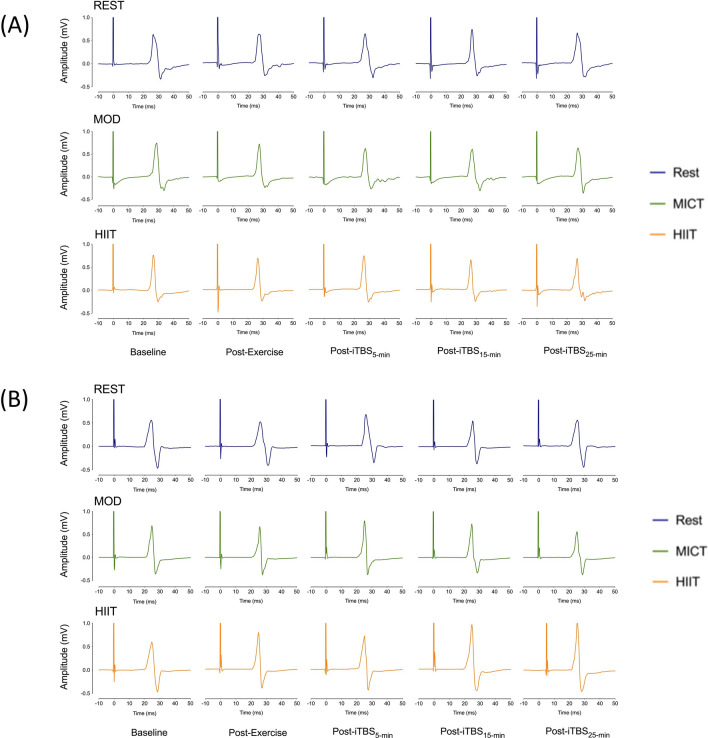
We examined the effect of exercise on CME by normalizing the average post-iTBS MEP to the post-exercise time-point. Two-way ANOVA revealed a significant Group x Session interaction, (F2,64 = 3.72, p = 0.03, ηp2 = 0.10), where the Control group showed significantly larger MEPs following iTBS in the high-intensity exercise condition than the HD group (t (32) = 2.39, p = 0.02), but there were no significant differences in MEPs between groups in either the Moderate intensity (t (32) = 0.41, p = 0.70) or Rest condition (t (32) = − 0.83, p = 0.42) (Fig. 2a). For the HD group, the Bayesian one-way repeated measures ANOVA provided moderate evidence in favor of the null hypothesis, that is, that neither high- nor moderate-intensity exercise boosted CME post-iTBS (B10 = 0.18; null hypothesis approximately 5.4 times more likely than the alterative hypothesis). In contrast, the control group showed very strong evidence of a significant difference between sessions, (BF10 = 36.23), with post-hoc comparisons revealing strong evidence that high intensity exercise increased CME post-iTBS compared to rest (BF10 = 20.15). In contrast, there was no convincing evidence that high-intensity exercise enhanced CME compared to moderate-intensity exercise (BF10 = 1.35), nor that moderate-intensity exercise increased CME in comparison to rest (BF10 = 0.68). Figure 3 shows examples of raw, single MEPs at each time-point within each condition from a representative HD participant and control participant, which demonstrate these patterns in CME seen in each group.
Figure 2.
Effect of high-intensity interval training (HIIT), moderate-intensity continuous training (MICT) and rest on TMS measures for each group. Data represent the mean (standard deviation), with individual participant values also shown. ITBS intermittent theta burst stimulation. (A) Cortico-motor excitability (CME), average post-iTBS MEP, normalised from post-exercise time-point. (B) Short-interval intracortical inhibition (SICI). Change from pre-exercise baseline to average post-iTBS time-points. (C) Intracortical facilitation (ICF). Change from pre-exercise baseline to average post-iTBS time-points. *p < .05.
Figure 3.
Examples of raw single motor-evoked potentials (MEPs) from one HD participant (A) and one control participant (B) during rest, moderate-intensity continuous training (MICT) and high-intensity interval training (HIIT). These MEPs were selected to illustrate the changes in cortico-motor excitability from baseline to post intermittent theta burst stimulation (iTBS) in the HIIT condition for control participants, but not HD participants.
Short-interval intracortical inhibition
A 3 Session × 5 Time × 2 Group mixed model ANOVA revealed a significant reduction in inhibition following high- and moderate-intensity exercise and iTBS in the control group but not the HD group, reflected by a significant Session × Group interaction effect (F2,64 = 3.45, p = 0.04, ηp2 = 0.10), and a significant main effect of Time (F4,128 = 3.35, p = 0.01, ηp2 = 0.10). There were no main effects of Session, or Group, or interaction effects between Session × Time, Time × Group, or Session × Time × Group. The change in SICI from the Pre-Exercise time-point to the average of the post-iTBS time-points for each group is shown in Fig. 2b. There were no significant differences between groups for any of the conditions (Rest: (t (32) = 1.05, p = 0.30); Moderate-intensity condition: (t (32) = 1.1, p = 0.27); High-intensity condition: (t (32) = 1.5, p = 0.14)). For the HD group, Bayesian one-way repeated-measures ANOVA provided moderate evidence in favor of the null hypothesis, that is, that neither high- nor moderate-intensity exercise reduced SICI post iTBS (BF10 = 0.27). For the control group, the evidence that the change in SICI differed between sessions was inconclusive (BF10 = 1.20).
Intracortical facilitation
A 3 Session × 5 Time × 2 Group mixed model ANOVA revealed a significant increase in facilitation following high-intensity exercise and iTBS in the control group but not the HD group, reflected by a significant Session x Group interaction effect (F2,64 = 3.93, p = 0.03, ηp2 = 0.11), and a significant main effect of Time (F4,128 = 5.83, p < 0.001, ηp2 = 0.15). There were no main effects of Session, or Group, or interaction effects between Session × Time, Time × Group, or Session × Time × Group. The change in ICF from the Pre-Exercise time-point to the average of the post-iTBS time-points for each group is shown in Fig. 2c. There was significantly more facilitation following high-intensity exercise and iTBS in the control group in comparison to the HD group, (t (32) = 2.13, p = 0.04), but there were no between group differences in the moderate exercise (t (32) = − 0.89, p = 0.38) or rest (t (32) = 0.09, p = 0.93) conditions. For the HD group, Bayesian one-way ANOVA again revealed moderate evidence in favor of the null hypothesis, indicating neither the high- nor moderate-intensity exercise had any effect on ICF post iTBS (BF10 = 0.27). In contrast, for the control group, there was strong evidence in favor of the alternative hypothesis, that ICF differed across groups (BF10 = 14.39), with moderate evidence that high-intensity exercise induced a larger increase in ICF following iTBS than rest (BF10 = 6.20), but no conclusive evidence that high-intensity exercise induced a larger increase in ICF following iTBS than moderate-intensity exercise (BF10 = 2.38). In comparison, there was moderate evidence that moderate-intensity exercise had no effect on ICF, in comparison to rest (BF10 = 0.24).
Relationships between demographic and clinical characteristics and TMS outcomes
We investigated relationships between age, self-reported physical activity levels, mood symptoms, and delta change values (Post-iTBS—Baseline) in the TMS measures (CME, SICI, or ICF), using Pearson correlations (Spearman correlations for non-normally distributed variables) and a more conservative significance level of p < 0.01 due to multiple comparisons. There were no significant relationships between these variables in either group across any of the three conditions. There were also no significant relationships between DBS and any of the TMS measures in the HD group.
Discussion
Contrary to our first hypothesis, the premanifest and early HD participants did not show enhancement of synaptic plasticity following high-intensity interval exercise, in comparison to either moderate intensity exercise or rest. Specifically, the HD group showed no increases in cortico-motor excitability, glutamatergic facilitation, or decreases in GABAAergic inhibition following either high- or moderate-intensity exercise, a finding which was further supported by the follow-up Bayesian analyses. The HD group showed lower inhibition at baseline which may have attenuated the effect of exercise on plasticity, consistent with our alternative hypothesis. Taken together, the current findings indicate that HD may be associated with an abnormal, attenuated plasticity response to an acute bout of cardiorespiratory exercise in premanifest and early HD, which has implications for the design of exercise interventions in this population.
Several possible mechanisms may account for the absence of exercise induced plasticity in the HD group. The first is that the HD group showed low baseline levels of GABAAergic intracortical inhibition compared to controls, which has been observed in several previous studies29,30,59. In healthy adults, exercise transiently reduces GABAAergic intracortical inhibition17,18. In HD, due to homeostatic mechanisms, the already reduced SICI in HD at baseline may have precluded further reductions in response to exercise60. However, baseline levels of corticomotor excitability and intracortical facilitation were not different than controls. Given the HD group also did not show the expected plasticity response for these variables, homeostatic mechanisms are unlikely to wholly account for the lack of responsivity of neuroplasticity to exercise. Instead, this attenuated synaptic plasticity response might also be attributable to altered dopamine signaling, or reduced production of BDNF, both of which have previously been demonstrated in HD61,62. In healthy adults, acute exercise triggers the release of lactate, dopamine, and the synthesis and release of BDNF63, which are associated with decreased cortical inhibition and corresponding increase in neuroplasticity63,64. Future research should investigate the effects of exercise on dopamine and BDNF release in people with the HD CAG-expansion, to better understand these potential underlying mechanisms.
An additional explanation for the lack of plasticity response in the HD group, is that exercise applied prior to the application of iTBS may have triggered a homeostatic inhibitory response65. However, we note that the healthy control group did not show evidence of this kind of homeostatic suppression of plasticity, and generally, exercise by itself does not induce changes in plasticity, but rather is thought to create an environment conducive for plasticity16. Further, recent research investigating homeostatic metaplasticity in HD mouse models reported disruption of these processes in the mouse brains66, although this has not been directly studied in humans. In the current study, the break between the cessation of exercise and the application of iTBS was approximately 15 min (10-min cool down followed by TMS measures). A previous study67 found that two trains of iTBS separated by 5 min resulted in an inhibitory effect of CME, whereas a 15 min interval resulted in a facilitatory effect on CME. The authors suggested that the 5-min interval may have triggered homeostatic plasticity, whereas the longer interval may have induced a more stable LTP-like effect. Therefore, even if exercise did induce LTP-like plasticity, the timings used in the current study mean that homeostatic plasticity is unlikely to account for the attenuated plasticity effect in seen in the HD group.
These findings have important implications for studies of exercise in HD, as attenuated brain responses to exercise may contribute to the mixed outcomes reported for exercise interventions in HD to date. Although some non-randomized controlled trials of exercise interventions have reported promising results, a recent meta-analysis of motor and cognitive effects from randomized controlled trials indicated no significant effects from the interventions on either the primary outcome (UHDRS motor score) or secondary outcomes (cognitive, health status or physical)11. The current findings indicate that more research is urgently needed to understand under what circumstances exercise may elicit an optimal neurophysiological response in HD (e.g., by investigating response to different types of exercise, or if there are effects over the longer-term following multiple exercise sessions), to inform the future direction of exercise intervention research in this population.
Our findings contrast with reports of exercise effects on the brain in HD mouse models. For example, wheel running in R6/1 transgenic HD mice increases BDNF gene expression3,4 and delays onset of motor signs7, whereas treadmill exercise in CAG140 knock-in HD mice restores dopamine D2 receptor expression6. The exercise interventions in these studies were long-term, however, rather than a single bout of exercise as implemented in the current study. In HD, longer-term exercise may be needed to have potent beneficial effects on brain chemistry, such as BDNF and dopamine levels.
Our study included people with the HD CAG expansion in very early stage, as much as 43 years before predicted onset, but also participants who had already been diagnosed. Manifest HD participants may have had too much neurodegeneration to respond optimally to exercise, although we did not find support for this possibility, in that disease burden score in our HD sample was not associated with the size of plasticity response following exercise. Given our small sample size, however, replication with a larger sample would be needed before any conclusions could be drawn regarding whether neuroplasticity alterations in HD track with disease progression. An additional consideration was the age of the HD participants, as some participants were older (3 participants were aged over 50 years), and in healthy populations, older participants have shown attenuated neuroplasticity response to TMS protocols68. In the current study, however, the control group did not show an attenuated plasticity response, despite some control participants also being older (4 participants aged over 50 years), making age a less likely explanation for the lower plasticity response seen in the HD group. Further, age was not significantly associated with the measures of plasticity in either group. In HD, there is a relationship between CAG expansion, age and the timing and severity of disease onset, whereby people with larger CAG expansions tend to have a younger and more severe onset of symptoms in HD1,39. Therefore, future research should investigate the relationships between age, disease severity and neuroplasticity response in larger HD samples.
Unlike the control group, who showed an increased facilitatory response to iTBS following high-intensity exercise, HD participants did not show any change to iTBS response following either exercise intensity. Only one previous study has utilized a theta-burst paradigm within a HD population. Orth et al.28 investigated responses to continuous TBS (cTBS) in a mixed premanifest and manifest HD group and found that cTBS had no effect on inhibition in HD, whereas cTBS resulted in significantly increased inhibition in the control group. Our current study extends this to show that in a premanifest and early HD sample, there was no detectable effect of iTBS on excitability, inhibition, or facilitation, either alone or primed with moderate- or high-intensity exercise. This suggests that these neuroplasticity mechanisms are affected early on in the disease course of HD, which has important implications for the consideration of non-invasive brain stimulation interventions in HD. Future research could also investigate whether exercise paired with a non-invasive brain stimulation protocol that suppresses excitability, such as continuous TBS69, might be more effective in altering plasticity in HD, given the lower baseline SICI seen in this population.
The current study has several limitations. We did not include an objective measure of cardiorespiratory fitness (e.g., VO2max), and therefore we could not examine any relationships between physical fitness and synaptic plasticity. However, groups did not differ on self-reported physical activity levels, resting heart rate, or objective measures of exercise performance, suggesting our groups were reasonably well matched. We also did not assess the potential role of BDNF genotype as a mediator of the plasticity response within the HD group, due to sample size limitations. Our group and others have previously found BDNF genotype to mediate the plasticity response to iTBS in healthy adults26,70. Further, our small sample size, particularly in the HD group, means that we may have been underpowered to detect an effect of exercise on neuroplasticity in the HD sample. Our follow up Bayesian analyses, however, provide moderate evidence that the results likely reflect a true absence of effect of exercise.
Conclusions
In conclusion, the current study is the first to investigate the acute effects of exercise and iTBS on measures of motor cortex plasticity in premanifest and early HD. In contrast to the control group, we found no effect of either high- or moderate-intensity exercise and iTBS on the individual plasticity measures in the HD participants. These findings call into question the assumption of the benefits of exercise to the brain in HD, and demonstrate the need for future research to better understand exercise and plasticity mechanisms in people with the HD gene-expansion. Research is needed to investigate whether optimal neuroplasticity responses are elicited following longer cardiovascular exercise intervention protocols, such as those over days or weeks, as well as the effect of alternative exercise and non-invasive brain stimulation protocols. Answering these questions will be essential for creating physical activity guidelines and designing exercise interventions in HD that are most likely to have significant clinical benefit.
Acknowledgements
This study was funded by a Huntington’s Disease Society of America Human Biology Project Grant. Sophie Andrews received salary support from the Huntington’s Disease Society of America. We thank the participants for their time and effort which made this study possible. We also thank Jaeger Wongtrakun, Joshua Hendrikse, Melissa Pelly and Claire Cadwallader for their assistance with data collection on this project.
Author contributions
S.C.A., J.P.C. and J.C.S. conceptualised and designed the study. S.C.A. and D.C. acquired the data. S.C.A. ran the analysis, interpreted the data, and drafted the manuscript. All authors reviewed and edited the manuscript.
Competing interests
Prof. Stout has served on Scientific Advisory Boards (Teva-Australia, Spark Therapeutics), and has performed consulting services for the CHDI Foundation, uniQure NV, and Triplet Therapeutics. She is a director with financial responsibilities for Zindametrix Pty Ltd, which has held contracts in the past 12 months with Vaccinex, uniQure NV, Triplet Therapeutics, and Voyager Therapeutics. Dr. Andrews, Mr. Curtin and Dr. Coxon declare no potential conflict of interest.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors jointly supervised this work: James P. Coxon and Julie C. Stout.
References
- 1.Walker FO. Huntington's disease. Lancet. 2007;369:218–228. doi: 10.1016/S0140-6736(07)60111-1. [DOI] [PubMed] [Google Scholar]
- 2.Mo C, Hannan AJ, Renoir T. Environmental factors as modulators of neurodegeneration: Insights from gene-environment interactions in Huntington's disease. Neurosci. Biobehav. Rev. 2015;52:178–192. doi: 10.1016/j.neubiorev.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Zajac MS, et al. Wheel running and environmental enrichment differentially modify exon-specific BDNF expression in the hippocampus of wild-type and pre-motor symptomatic male and female Huntington's disease mice. Hippocampus. 2010;20:621–636. doi: 10.1002/hipo.20658. [DOI] [PubMed] [Google Scholar]
- 4.Pang TYC, Stam NC, Nithianantharajah J, Howard ML, Hannan AJ. Differential effects of voluntary physical exercise on behavioral and brain-derived neurotrophic factor expression deficits in Huntington's disease transgenic mice. Neuroscience. 2006;141:569–584. doi: 10.1016/j.neuroscience.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 5.Caldwell CC, Petzinger GM, Jakowec MW, Cadenas E. Treadmill exercise rescues mitochondrial function and motor behavior in the CAG140 knock-in mouse model of Huntington's disease. Chem. Biol. Interact. 2020;315:108907. doi: 10.1016/j.cbi.2019.108907. [DOI] [PubMed] [Google Scholar]
- 6.Stefanko DP, Shah VD, Yamasaki WK, Petzinger GM, Jakowec MW. Treadmill exercise delays the onset of non-motor behaviors and striatal pathology in the CAG140 knock-in mouse model of Huntington's disease. Neurobiol. Dis. 2017;105:15–32. doi: 10.1016/j.nbd.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 7.van Dellen A, Cordery PM, Spires TL, Blakemore C, Hannan AJ. Wheel running from a juvenile age delays onset of specific motor deficits but does not alter protein aggregate density in a mouse model of Huntington's disease. BMC Neurosci. 2008;9:34. doi: 10.1186/1471-2202-9-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harrison DJ, et al. Exercise attenuates neuropathology and has greater benefit on cognitive than motor deficits in the R6/1 Huntington's disease mouse model. Exp. Neurol. 2013;248:457–469. doi: 10.1016/j.expneurol.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 9.Trembath MK, et al. A retrospective study of the impact of lifestyle on age at onset of Huntington disease. Mov. Disord. 2010;25:1444–1450. doi: 10.1002/mds.23108. [DOI] [PubMed] [Google Scholar]
- 10.Fritz NE, et al. Physical therapy and exercise interventions in Huntington's disease: A mixed methods systematic review. J. Huntingtons Dis. 2017;6:217–235. doi: 10.3233/JHD-170260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Playle R, Dimitropoulou P, Kelson M, Quinn L, Busse M. Exercise interventions in Huntington's disease: An individual patient data meta-analysis. Mov. Disord. Clin. Pract. 2019;6:567–575. doi: 10.1002/mdc3.12809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cruickshank TM, et al. Effects of multidisciplinary therapy on physical function in Huntington's disease. Acta Neurol. Scand. 2018;138:500–507. doi: 10.1111/ane.13002. [DOI] [PubMed] [Google Scholar]
- 13.Cruickshank TM, et al. The effect of multidisciplinary rehabilitation on brain structure and cognition in Huntington's disease: An exploratory study. Brain Behav. 2015;5:e00312. doi: 10.1002/brb3.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hendrikse J, Kandola A, Coxon J, Rogasch N, Yucel M. Combining aerobic exercise and repetitive transcranial magnetic stimulation to improve brain function in health and disease. Neurosci. Biobehav. Rev. 2017;83:11–20. doi: 10.1016/j.neubiorev.2017.09.023. [DOI] [PubMed] [Google Scholar]
- 15.Magee JC, Grienberger C. Synaptic plasticity forms and functions. Annu. Rev. Neurosci. 2020;43:95–117. doi: 10.1146/annurev-neuro-090919-022842. [DOI] [PubMed] [Google Scholar]
- 16.Mellow ML, Goldsworthy MR, Coussens S, Smith AE. Acute aerobic exercise and neuroplasticity of the motor cortex: A systematic review. J. Sci. Med. Sport. 2020;23:408–414. doi: 10.1016/j.jsams.2019.10.015. [DOI] [PubMed] [Google Scholar]
- 17.Mooney RA, et al. Acute aerobic exercise modulates primary motor cortex inhibition. Exp. Brain Res. 2016;234:3669–3676. doi: 10.1007/s00221-016-4767-5. [DOI] [PubMed] [Google Scholar]
- 18.Smith AE, Goldsworthy MR, Garside T, Wood FM, Ridding MC. The influence of a single bout of aerobic exercise on short-interval intracortical excitability. Exp. Brain Res. 2014;232:1875–1882. doi: 10.1007/s00221-014-3879-z. [DOI] [PubMed] [Google Scholar]
- 19.Singh AM, Duncan RE, Neva JL, Staines WR. Aerobic exercise modulates intracortical inhibition and facilitation in a nonexercised upper limb muscle. BMC Sports Sci. Med. Rehabil. 2014;6:23. doi: 10.1186/2052-1847-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh AM, Neva JL, Staines WR. Acute exercise enhances the response to paired associative stimulation-induced plasticity in the primary motor cortex. Exp. Brain Res. 2014;232:3675–3685. doi: 10.1007/s00221-014-4049-z. [DOI] [PubMed] [Google Scholar]
- 21.Saucedo Marquez CM, Vanaudenaerde B, Troosters T, Wenderoth N. High-intensity interval training evokes larger serum BDNF levels compared with intense continuous exercise. J. Appl. Physiol. (1985) 2015;119:1363–1373. doi: 10.1152/japplphysiol.00126.2015. [DOI] [PubMed] [Google Scholar]
- 22.Stavrinos EL, Coxon JP. High-intensity interval exercise promotes motor cortex disinhibition and early motor skill consolidation. J. Cogn. Neurosci. 2017;29:593–604. doi: 10.1162/jocn_a_01078. [DOI] [PubMed] [Google Scholar]
- 23.Mang CS, Snow NJ, Campbell KL, Ross CJ, Boyd LA. A single bout of high-intensity aerobic exercise facilitates response to paired associative stimulation and promotes sequence-specific implicit motor learning. J. Appl. Physiol. 2014;1985(117):1325–1336. doi: 10.1152/japplphysiol.00498.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 25.Suppa A, et al. Ten years of theta burst stimulation in humans: established knowledge, unknowns and prospects. Brain Stimul. 2016;9:323–335. doi: 10.1016/j.brs.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 26.Andrews SC, et al. Intensity matters: High-intensity interval exercise enhances motor cortex plasticity more than moderate exercise. Cereb. Cortex. 2020;30:101–112. doi: 10.1093/cercor/bhz075. [DOI] [PubMed] [Google Scholar]
- 27.McColgan P, Joubert J, Tabrizi SJ, Rees G. The human motor cortex microcircuit: Insights for neurodegenerative disease. Nat. Rev. Neurosci. 2020;21(8):401–415. doi: 10.1038/s41583-020-0315-1. [DOI] [PubMed] [Google Scholar]
- 28.Orth M, et al. Abnormal motor cortex plasticity in premanifest and very early manifest Huntington disease. J. Neurol. Neurosurg. Psychiatry. 2010;81:267–270. doi: 10.1136/jnnp.2009.171926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Philpott AL, et al. Cortical inhibitory deficits in premanifest and early Huntington's disease. Behav. Brain Res. 2016;296:311–317. doi: 10.1016/j.bbr.2015.09.030. [DOI] [PubMed] [Google Scholar]
- 30.Schippling S, et al. Abnormal motor cortex excitability in preclinical and very early Huntington's disease. Biol. Psychiatry. 2009;65:959–965. doi: 10.1016/j.biopsych.2008.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crupi D, et al. Cortical and brainstem LTP-like plasticity in Huntington's disease. Brain Res. Bull. 2008;75:107–114. doi: 10.1016/j.brainresbull.2007.07.029. [DOI] [PubMed] [Google Scholar]
- 32.Blumenstock S, Dudanova I. Cortical and striatal circuits in Huntington's disease. Front. Neurosci. 2020;14:82. doi: 10.3389/fnins.2020.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.He YY, Zhang XY, Yung WH, Zhu JN, Wang JJ. Role of BDNF in central motor structures and motor diseases. Mol. Neurobiol. 2013;48:783–793. doi: 10.1007/s12035-013-8466-y. [DOI] [PubMed] [Google Scholar]
- 34.Hannan AJ. Environmental enrichment and brain repair: Harnessing the therapeutic effects of cognitive stimulation and physical activity to enhance experience-dependent plasticity. Neuropathol. Appl. Neurobiol. 2014;40:13–25. doi: 10.1111/nan.12102. [DOI] [PubMed] [Google Scholar]
- 35.Rossi S, Hallett M, Rossini PM, Pascual-Leone A, Safety of, T.M.S.C.G Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin. Neurophysiol. 2009;120:2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sports Medicine Australia. Sports Medicine Australia Pre-exercise Screening System. (Government of Australia, Canberra, ACT, 2011).
- 37.Shoulson I, Fahn S. Huntington disease: Clinical care and evaluation. Neurology. 1979;29:1–3. doi: 10.1212/wnl.29.1.1. [DOI] [PubMed] [Google Scholar]
- 38.Huntington Study Group Unified Huntington's disease rating scale: Reliability and consistency. Mov. Disord. 1996;11:136–142. doi: 10.1002/mds.870110204. [DOI] [PubMed] [Google Scholar]
- 39.Penney JB, Jr, Vonsattel JP, MacDonald ME, Gusella JF, Myers RH. CAG repeat number governs the development rate of pathology in Huntington's disease. Ann. Neurol. 1997;41:689–692. doi: 10.1002/ana.410410521. [DOI] [PubMed] [Google Scholar]
- 40.Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 41.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 42.De Souza J, Jones LA, Rickards H. Validation of self-report depression rating scales in Huntington's disease. Mov. Disord. 2010;25:91–96. doi: 10.1002/mds.22837. [DOI] [PubMed] [Google Scholar]
- 43.Craig CL, et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003;35:1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 44.Breeman S, Cotton S, Fielding S, Jones GT. Normative data for the hospital anxiety and depression scale. Qual. Life Res. 2015;24:391–398. doi: 10.1007/s11136-014-0763-z. [DOI] [PubMed] [Google Scholar]
- 45.Philpott AL, Fitzgerald PB, Cummins TD, Georgiou-Karistianis N. Transcranial magnetic stimulation as a tool for understanding neurophysiology in Huntington's disease: A review. Neurosci. Biobehav. Rev. 2013;37:1420–1433. doi: 10.1016/j.neubiorev.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 46.Ziemann U, et al. TMS and drugs revisited 2014. Clin. Neurophysiol. 2015;126:1847–1868. doi: 10.1016/j.clinph.2014.08.028. [DOI] [PubMed] [Google Scholar]
- 47.Carcea I, Froemke RC. Cortical plasticity, excitatory-inhibitory balance, and sensory perception. Prog. Brain Res. 2013;207:65–90. doi: 10.1016/B978-0-444-63327-9.00003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rossini PM, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: Basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr. Clin. Neurophysiol. 1994;91:79–92. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 49.Cash, R.F.H. & Ziemann, U. Paired-pulse interactions. in The Oxford Handbook of Transcranial Stimulation, Second Edition (eds. Wassermann, E.M., et al.) (2021).
- 50.Borg G. Perceived exertion as an indicator of somatic stress. Scand. J. Rehabil. Med. 1970;2:92–98. [PubMed] [Google Scholar]
- 51.Coxon JP, Stinear CM, Byblow WD. Intracortical inhibition during volitional inhibition of prepared action. J. Neurophysiol. 2006;95:3371–3383. doi: 10.1152/jn.01334.2005. [DOI] [PubMed] [Google Scholar]
- 52.Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Front. Psychol. 2013;4:863. doi: 10.3389/fpsyg.2013.00863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wagenmakers EJ, et al. Bayesian inference for psychology. Part I: Theoretical advantages and practical ramifications. Psychon. B Rev. 2018;25:35–57. doi: 10.3758/s13423-017-1343-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dienes Z. Using Bayes to get the most out of non-significant results. Front. Psychol. 2014;5:781. doi: 10.3389/fpsyg.2014.00781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.JASP Team. JASP. (2020).
- 56.Rouder JN, Morey R, Speckman PL, Province JM. Default Bayes factors for ANOVA designs. J. Math. Psychol. 2012;56:356–374. [Google Scholar]
- 57.Jeffreys H. Theory of Probability. Oxford University Press; 1961. [Google Scholar]
- 58.Lee MD, Wagenmakers EJ. Bayesian Cognitive Modeling: A Practical Course. Cambridge University Press; 2014. [Google Scholar]
- 59.Abbruzzese G, et al. Intracortical inhibition and facilitation are abnormal in Huntington's disease: A paired magnetic stimulation study. Neurosci. Lett. 1997;228:87–90. doi: 10.1016/s0304-3940(97)00363-7. [DOI] [PubMed] [Google Scholar]
- 60.Muller-Dahlhaus F, Ziemann U. Metaplasticity in human cortex. Neuroscientist. 2015;21:185–202. doi: 10.1177/1073858414526645. [DOI] [PubMed] [Google Scholar]
- 61.Zuccato C, Cattaneo E. Role of brain-derived neurotrophic factor in Huntington's disease. Prog. Neurobiol. 2007;81:294–330. doi: 10.1016/j.pneurobio.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 62.Cepeda C, Murphy KP, Parent M, Levine MS. The role of dopamine in Huntington's disease. Prog. Brain Res. 2014;211:235–254. doi: 10.1016/B978-0-444-63425-2.00010-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McMorris, T., Turner, A., Hale, B. & Sproule, J. Beyond the Catecholamines Hypothesis for an Acute Exercise–Cognition Interaction: A Neurochemical Perspective. in Exercise-Cognition Interaction: Neuroscience Perspectives (ed. McMorris, T.) 65–103 (Academic Press, Elsevier, 2016).
- 64.Gundersen V, Storm-Mathisen J, Bergersen LH. Neuroglial transmission. Physiol. Rev. 2015;95:695–726. doi: 10.1152/physrev.00024.2014. [DOI] [PubMed] [Google Scholar]
- 65.Murakami T, Muller-Dahlhaus F, Lu MK, Ziemann U. Homeostatic metaplasticity of corticospinal excitatory and intracortical inhibitory neural circuits in human motor cortex. J. Physiol. 2012;590:5765–5781. doi: 10.1113/jphysiol.2012.238519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Smith-Dijak AI, et al. Impairment and restoration of homeostatic plasticity in cultured cortical neurons from a mouse model of Huntington disease. Front. Cell. Neurosci. 2019;13:209. doi: 10.3389/fncel.2019.00209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tse NY, et al. The effect of stimulation interval on plasticity following repeated blocks of intermittent theta burst stimulation. Sci. Rep. 2018;8:8526. doi: 10.1038/s41598-018-26791-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bhandari A, et al. A meta-analysis of the effects of aging on motor cortex neurophysiology assessed by transcranial magnetic stimulation. Clin. Neurophysiol. 2016;127:2834–2845. doi: 10.1016/j.clinph.2016.05.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McDonnell MN, Buckley JD, Opie GM, Ridding MC, Semmler JG. A single bout of aerobic exercise promotes motor cortical neuroplasticity. J. Appl. Physiol. 2013;1985(114):1174–1182. doi: 10.1152/japplphysiol.01378.2012. [DOI] [PubMed] [Google Scholar]
- 70.Antal A, et al. Brain-derived neurotrophic factor (BDNF) gene polymorphisms shape cortical plasticity in humans. Brain Stimul. 2010;3:230–237. doi: 10.1016/j.brs.2009.12.003. [DOI] [PubMed] [Google Scholar]