Abstract
Ammonia is one of the most abundant and simple nitrogen sources with decent stability and reactivity. Direct insertion of ammonia into a carbon skeleton is an ideal approach to building valuable N-heterocycles for extensive applications with unprecedented atom and step economy. Here, we show an electrochemical dehydrogenative method in which ammonia is inserted directly into alkenes to build aromatic N-heterocycles in a single step without the use of any external oxidant. This new approach achieves 98–99.2% atom economy with hydrogen as the only byproduct. Quinoline and pyridine with diverse substitutions are readily available. In this work, electrochemistry was used to drive a 4-electron oxidation reaction that is hard to access by other protocols, providing a parallel pathway to nitrene chemistry. In a tandem transformation that included three distinct electrochemical processes, the insertion of ammonia further showcased the tremendous potential to manipulate heterocycles derived from Hantzsch ester to diazine via pyridine and pyrrole.
Subject terms: Synthetic chemistry methodology, Reaction mechanisms
Aromatic heterocycles containing nitrogen are ubiquitous in biologically relevant small molecules. Here the authors show an unorthodox methodology for their synthesis, by inserting the nitrogen atom into a carbon ring, with ammonia in electrochemical conditions.
Introduction
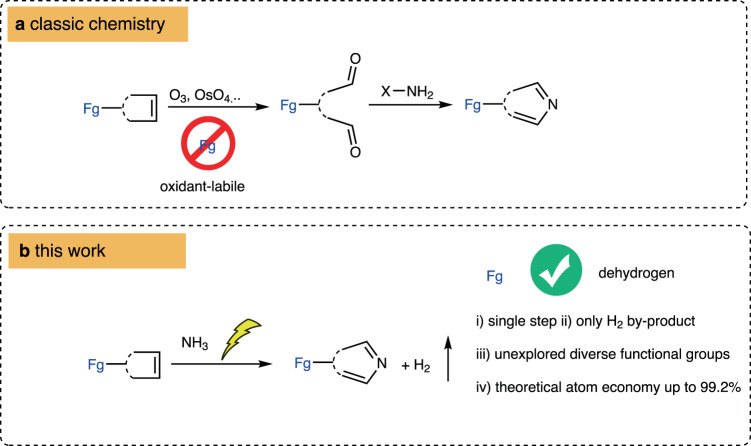
Aromatic N-heterocycles are common moieties in natural products1, and they have been extensively applied to pharmaceutical design2. The classic syntheses of aromatic N-heterocycles are frequently conducted with condensation reactions using pre-oxidized materials. For example, in early efforts to synthesize isoquinoline from indene, oxidation cleavage of alkenes using ozone3,4 or OsO45 was necessary, resulting in a limited scope of functionality (Fig. 1a)6. However, the insertion of heteroatoms into the carbon skeleton exhibits multiple advantages, including atom/step economy, oxidation-labile functional group (Fg) tolerance, and unexplored selectivity (Fig. 1b). However, it is difficult to find a method as efficient as oxidation for cleaving C–C bonds, especially the bonds of alkenes and alkynes. Such an intrinsic obstacle makes the direct insertion of heteroatoms into alkenes to build heterocycles an elusive goal. To regulate the oxidation state of organic molecules, the dehydrogenation reaction is a complementary route to the oxygenation reaction7–21. Dehydrogenative cross-coupling exhibits a unique ability to achieve oxidative bond formation in the absence of external oxidants22–26. In particular, the electrochemical protocol demonstrates its intrinsic potential to drive dehydrogenative cross-coupling reactions with cathodic hydrogen evolution27–30. As one of the most important C-heteroatom connections, considerable efforts have been made to construct C-N bonds, including electrochemical protocols31–33. In recent years, the electrochemical dehydrogenative intermolecular construction of C-N bonds has witnessed tremendous progress, for example, in reactions of aromatic C-H amination34–43, benzylic C-H amination44–46, alkyne amination47, alkene azidation48, alkane amination49, aromatic C-X/N-H cross coupling50–53, alkene aziridination49,54–59, and other innovations60. Despite these achievements, electricity has not fulfilled the task of sewing NH3 and alkene to aromatic N-heterocycles.
Fig. 1. Comparison of protocols to construct aromatic N-heterocycles from alkenes.
a Classic chemistry, oxidation/condensation. b This work, dehydrogenative insertion of NH3. Fg = functional groups.
In this work, we report the insertion of ammonia into carbon skeletons to build diverse aromatic N-heterocycles via a multiple electron transfer pathway involving hydrogen evolution with up to 99.2% theoretical atom economy.
Results
Optimization of insertion of ammonia into indene
In the initial attempt, 1a was adopted as the substrate with the conjugated tetrasubstituted alkene as the target bond to insert a nitrogen atom with ammonia (Table 1) at 0 °C. The first observed insertion product 2a had a 16% 1H NMR yield when graphite felt (GF) was used as the electrodes and methanol was used as the solvent (entry 1). Pt (entry 2) and Ag (entry 3) were applied as cathodes to enhance the evolution of hydrogen, and the corresponding yields were improved to 30 and 52%, respectively. The decrease (entry 4) and increase (entry 5) in cell voltage slightly decreased the yield. Lowering the reaction temperature to −10 °C brought only trace conversion (entry 6). However, the reaction at room temperature could give the desired isoquinoline in comparable yield (entry 7). The application of DCM as a co-solvent could improve the solubility of the substrate and lead to a 65% isolated yield (entry 8). When iPrOH was the solvent with dichloromethane (DCM) as the co-solvent, the conversion of alkene was not observed due to the low conductivity (entry 9). Instead of an ammonia atmosphere, a solution of ammonia at a concentration of 0.28 mol/L was adequate to give a comparable yield (entry 10). The screening of other supporting electrolytes showed that LiCl resulted in inferior yields (entry 11), and LiBF4 gave acceptable results (entry 12). A reaction using Pb(OAc)4 as the terminal oxidant61 with ammonia did not give conversion of the substrate (entry 13, see Supplementary Tables 1 and 2 for more details).
Table 1.
Initial model reaction of ammonia insertion and optimization.
 | ||||
|---|---|---|---|---|
| Entry | Solvent | Electrodes | Electrolyte | Yield (%)g |
| 1a | CH3OH | GF+/GF− | Mg(ClO4)2 | 16 |
| 2a | CH3OH | GF+/Pt− | Mg(ClO4)2 | 30 |
| 3a | CH3OH | GF+/Ag− | Mg(ClO4)2 | 52 |
| 4b | CH3OH | GF+/Ag− | Mg(ClO4)2 | 50 |
| 5c | CH3OH | GF+/Ag− | Mg(ClO4)2 | 47 |
| 6d | CH3OH | GF+/Ag− | Mg(ClO4)2 | Trace |
| 7e | CH3OH | GF+/Ag− | Mg(ClO4)2 | 55 |
| 8f | CH3OH/DCM | GF+/Ag− | Mg(ClO4)2 | 68(65h) |
| 9i | iPrOH/DCM | GF+/Ag− | Mg(ClO4)2 | N. R. |
| 10j | CH3OH/DCM | GF+/Ag− | Mg(ClO4)2 | 64 |
| 11f | CH3OH/DCM | GF+/Ag− | LiCl | 30 |
| 12f | CH3OH/DCM | GF+/Ag− | LiBF4 | 67 |
| 13 | NH3/Pb(OAc)4 | N.R. | ||
a Reaction conditions: 1a (0.1 mmol), NH3 (balloon, ca. 1 atm), graphite felt (GF) anode, Ag cathode, supporting electrolyte (0.1 mmol), MeOH (5.0 mL), 0 °C, 4 V cell voltage, 3 h.
b 3.5 V cell voltage.
c 5 V cell voltage.
d −10 °C.
e rt instead of 0 °C.
f MeOH (4.0 mL) and DCM (dichloromethane, 1.0 mL), rt, 3 h.
g 1H NMR yields of product 2a.
h Isolated yields of product 2a.
i iPrOH (4.0 mL) and dichloromethane, DCM (1.0 mL), rt, 3 h.
j NH3 (0.28 mol/L).
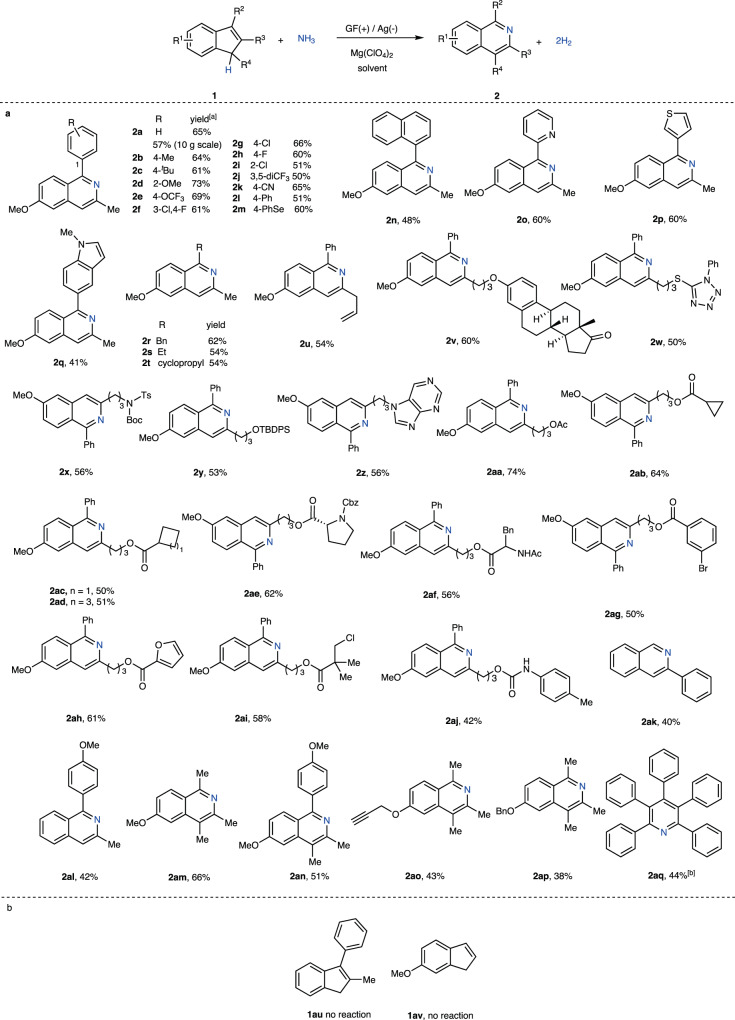
Substrate scope of reaction of insertion of ammonia
With the optimized conditions (Table 1, entry 8), we explored the scope of the other cyclic alkenes (Fig. 2). Products 2a–2 m with different aryl groups at the R2 position were obtained in moderate to good yields. In a 10-gram scale reaction, product 2a was isolated in a similar yield (57%) to that shown in Table 1 (65%, entry 6). Next, naphthalenyl 2n and biheterocycles 2o–2q were prepared with the same protocol. The electron-deficient pyridine 2o and electron-rich thiophene 2p and indole 2q were all compatible. Next, the reactions employing substrates bearing alkyl R2 groups gave the products 2r–2t. In the case of 2t, the radical clock cyclopropyl group was intact during the transformation. Next, the other R3 side chains were evaluated using a series of functional groups. Allyl 2 u, estrone-derived ether 2 v, mercaptophenyltetrazole-derived thioether 2w, amide protected with tbutoxycarbonyl (Boc) and p-tolyl sulfonyl (Ts) 2x, silyl ether 2 y, and purine 2z were all robust under these reaction conditions, and the corresponding products were achieved in 50–60% yield. In addition, substrates 1, which incorporated different ester groups, were synthesized as tested under the standard conditions, and the desired products 2aa–2ai were obtained in up to 74% yield, revealing further tolerance towards Ar-Br 2ag, furan 2ah, and R-Cl 2ai groups. A carbamate 2aj was achieved in moderate 42% yield. Next, the indenes 1 with varied substitution patterns was converted to isoquinolines 2ak–2ap. In these cases, the yields were lower partially because of the unidentified side reactions involving MeOH as a nucleophile. We further explored the substrate scope beyond the indene. Tetraphenyl cyclopentadiene was converted to the corresponding tetraphenyl pyridine 2aq in a single step in 44% yield. Functional groups, such as alkenes, alkynes, selenides, thioethers, thiophenes62,63, and indole64, which react with ozone readily, were all tolerated during the transformations. In the example of 2 v, a theoretical atom economy of 99.2% was realized. However, there was a limit on the pattern of substitution. For example, substrates 1au and 1av showed that the electron-donating group on the indene backbone and aryl ring on the alkene were important because they were capable of stabilizing the cationic and radical intermediates.
Fig. 2. The insertion of ammonia into various alkenes.
a Reaction conditions: graphite felt (GF) anode and Ag cathode, 1 (0.1 mmol), NH3 (balloon, ca. 1 atm), Mg(ClO4)2 (0.1 mmol), MeOH/DCM = 4 mL/1 mL, or 3 mL/2 mL, 3.5–4.5 V cell potential, rt, 3–4 h. [a] isolated yields are reported for all cases. [b] reaction conditions: GF anode and Ag cathode, 1aq (0.05 mmol), Mg(ClO4)2 (0.1 mmol), MeOH/toluene = 3 mL/2 mL, 5.5 V cell potential, rt, 3 h. b Substrates did not react.
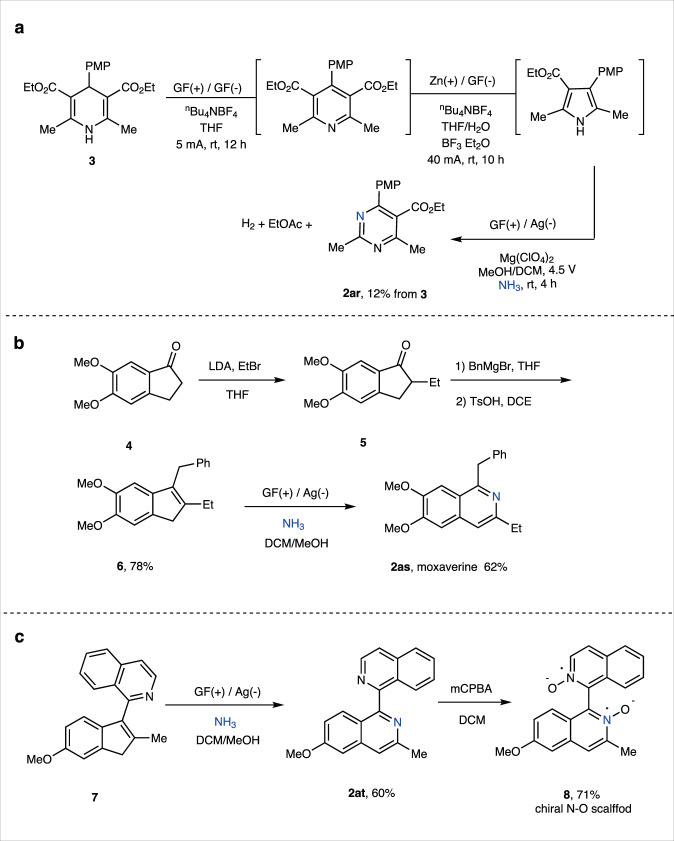
Tandem reaction involving the insertion of ammonia
Subsequently, we explored the insertion of ammonia into alkenes in a three-step tandem electrochemical reaction (Fig. 3a). By employing Hantzsch ester 3 as the starting material, tandem electrochemical dehydrogenative oxidation/reductive ring contraction/NH3 insertion gave 1,3-diazine 2ar without isolation of intermediates. The overall side products for this transformation were ethyl acetate65 and hydrogen, and the obtained tetra-substituted 1,3-diazine was not accessible by any other methods (see Supplementary Figs. 36 and 37 for more details). With this method, the commercial pharmaceutical compound moxaverine 2as was synthesized in four steps from indanone 4 with ammonia insertion as the key step in 62% yield (Fig. 3b). Next, Compound 7 was converted to unsymmetric bi-quinoline 2at, which was further oxidized to N-O oxide with 3-chloroperoxybenzoic acid (mCPBA). The achieved bi-N-O oxide 8 was an axial chiral scaffold (Fig. 3c, see Supplementary Fig. 38 for more details)66.
Fig. 3. The application of ammonia insertion protocol.
a Three-step electrochemical synthesis of diazine 2ar from Hantzsch ester 3. b Synthesis of choroidal blood regulator moxaverine 2as. c Synthesis of 6-methoxy-3-methyl-[1,1’-biisoquinoline] 2,2’-dioxide. GF, graphite felt; mCPBA; LDA, lithium diisopropylamide; 3-chloroperoxybenzoic acid, PMP, 4-MeOPh.
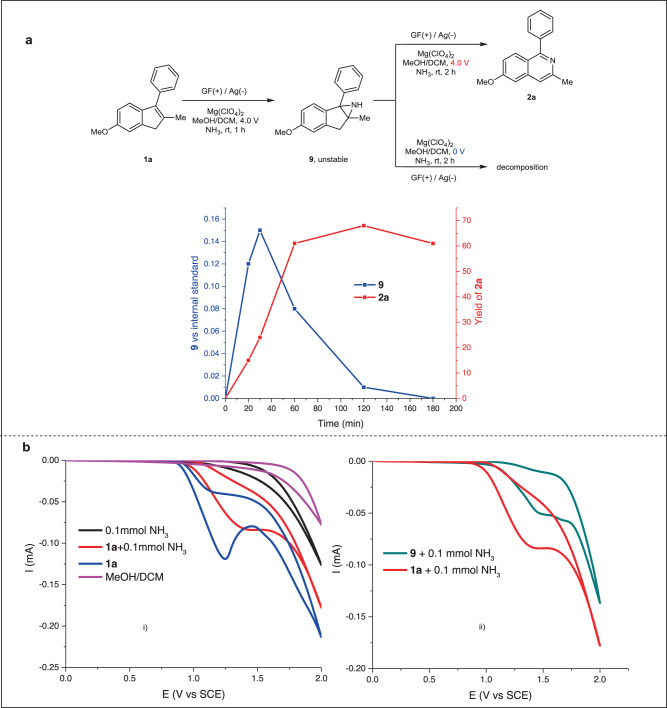
Mechanistic studies
Next, experiments under controlled conditions were conducted to gain some information about the reaction pathway (Fig. 4). At first, the reaction of 1a and ammonia was interrupted at an early stage before the full consumption of the starting alkene. An unstable intermediate aziridine 9 was detected, isolated, and characterized. Next, we subjected Compound 9 to the same electrochemical conditions, and the final isoquinoline 2a was produced as the major product. However, when Compound 9 was subjected to the same chemical environment without electricity, decomposition to an unknown complex mixture was observed, and target Compound 2a was not detected at all (see Supplementary Figs. 7–9 for more details). In the kinetic study, the concentration of intermediate 9 reached a maximum at 30 min and then decreased to full consumption until 180 min. In comparison, product 2a was generated continuously for 120 min, and then the yield dropped slightly (Fig. 4a, see Supplementary Figs. 10 and 11 for more details). Next, cyclic voltammetry (CV) was performed on the species in the reaction (Fig. 4b). The results revealed that 1a more readily lost electrons than ammonia when it was the sole solute in MeOH (Fig. 4b-i, blue curve). When 1a and ammonia were exposed to anodic oxidation together, the onset potential (1.0 V vs. saturated calomel electrode SCE) of the reactants was similar to that of 1a, but the peak potential shifted to 1.5 V vs. SCE, suggesting that ammonia affected the electrode environment during electron transfer from 1a to the anode (Fig. 4b–i, red curve). Furthermore, it was found that the oxidation of 1a proceeded slightly more readily than the oxidation of the intermediate (9) (Fig. 4b-ii, see Supplementary Figs. 21–26 for details).
Fig. 4. Experiments investigating the reagents and intermediates.
a Investigation and tracking of intermediates 9 under different conditions. b Cyclic voltammetry experiments of reactants and 9. GF, graphite felt; DCM, dichloromethane.
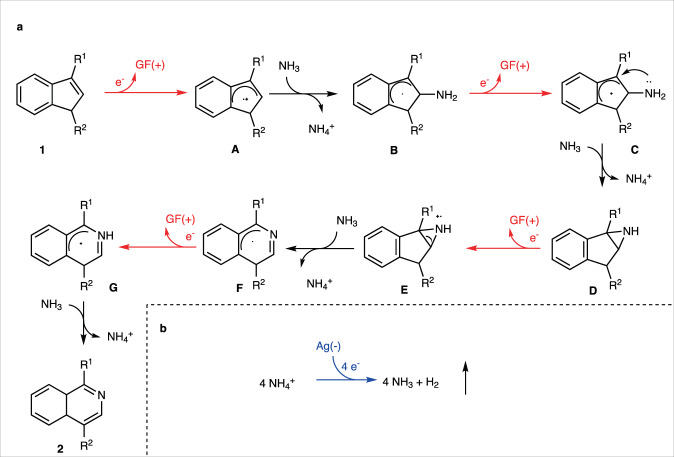
A plausible reaction mechanism
With these observations, we suggest a plausible reaction pathway, shown in Fig. 5. First, the reaction starts with anodic oxidation of alkene 1 and the subsequent trapping of cationic radical A with ammonia (for more details of the radical kinetics, see Supplementary Figs. 15–19). The second oxidation converts the consequent neutral radical B to cation C, which undergoes annulation to aziridine D. A third electron transfer oxidation of nitrogen during the conversion of D to E triggers deprotonation/rearrangement, yielding dihydroisoquinoline radical F. The fourth electron transfer and deprotonation results in the final product 2. Finally, the evolution of 2 equivalents of hydrogen molecules at the cathode accomplishes the whole electron cycle.
Fig. 5. A plausible reaction pathway involving 4e oxidation.
a Anodic reactions converting substrate 1 to product 2, GF( + ) is graphite felt anode. b Cathodic hydrogen evolution.
Discussion
In summary, we developed a direct insertion of ammonia into cyclic alkenes to synthesize aromatic N-heterocycles. The reaction utilizes electrochemical hydrogen evolution instead of oxygenation to drive the reaction. The reaction proceeds via electrochemical aziridination and ring rearrangement with up to 99.2% theoretical atom economy. By avoiding the usage of external oxidants, various functional groups labile towards oxidation were compatible in this transformation. Divergent N-heterocycles are available with this oxidant-free pathway.
Methods
General procedure for the synthesis of 2
A 10 mL three-necked heart-shaped flask was charged with the substrate alkene (0.1 mmol), Mg(ClO4)2 (22.3 mg, 0.1 mmol) and a magnetic stir bar. The flask was equipped with a rubber stopper, graphite felt (2 cm × 1 cm x 0.5 cm) as the anode and Ag plate (2 cm × 1 cm) as the cathode. The flask was evacuated and backfilled with ammonia gas three times, and then an ammonia gas balloon was connected to this flask via a needle. Next, anhydrous solvent (5 mL) was added via syringe. Electrolysis with constant cell potential was carried out at room temperature. The reaction was monitored with TLC and GC–MS, and when it was complete, the mixture was concentrated under reduced pressure. The residue was purified by chromatography on silica gel to afford the desired product (see Supplementary Fig. 1 for more details).
Gram-scale reaction to prepare 2a: A 400 mL rectangular flask was charged with substrate 1a (10.0 g, 42 mmol), LiBF4 (0.60 g, 6 mmol) and a magnetic stir bar. The flask was equipped with two pieces of graphite felt (8.5 cm × 6.5 cm × 0.5 cm, 6.5 cm × 6.5 cm × 0.5 cm) as the anode (2 pieces) and one piece of silver flake (6.5 cm × 6.5 cm × 0.5 cm) as the cathode. Two electrodes were separated and fixed with a 1.0 cm stick. The graphite felt anode was attached to a platinum wire, and cathode was attached to a silver wire (see Supplementary Figs. 2–6 for details). The flask was evacuated once and backfilled with gaseous NH3, and 200 mL of anhydrous MeOH and 100 mL of anhydrous DCM were added via syringe. Electrolysis under a controlled cell potential (8 V due to the extended distance between anode and cathode) was carried out in a water bath at room temperature. After 14 h, the mixture was concentrated under reduced pressure. The residue was purified by chromatography on silica gel to afford desired product 2a (6.0 g, 57%).
Supplementary information
Acknowledgements
This work was supported by the National Science Foundation of China (nos. 22071105, 22031008, Xu Cheng) and the Qinglan Project of Jiangsu Education Department (Xu Cheng).
Author contributions
S.L. carried out the work on optimization of the reaction conditions, exploration of the substrate scope, elucidation of the mechanism and wrote the supplementary information. X.C. conceived and supervised the project and wrote the manuscript.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Data availability
The authors declare that all other data supporting the findings of this study are available within the article and Supplementary Information files and are also available from the corresponding author upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-022-28099-w.
References
- 1.Joule, J. A. In Adv. Heterocycl. Chem. 119 (eds Eric F. V. Scriven & Christopher A. Ramsden) 81–106 (Academic Press, 2016).
- 2.Vitaku E, Smith DT, Njardarson JT. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among U.S. FDA approved pharmaceuticals. J. Med. Chem. 2014;57:10257–10274. doi: 10.1021/jm501100b. [DOI] [PubMed] [Google Scholar]
- 3.Fremery MI, Fields EK. Amozonolysis of cycloolefins. J. Org. Chem. 1964;29:2240–2243. [Google Scholar]
- 4.Miller RB, Frincke JM. Synthesis of isoquinolines from indenes. J. Org. Chem. 1980;45:5312–5315. [Google Scholar]
- 5.Dime DS, McLean S. Synthesis of isoquinolines from indenes. J. Org. Chem. 1981;46:4999–5000. [Google Scholar]
- 6.Quin, L. D. & Tyrell, J. A. Fundamentals of Heterocyclic Chemistry: Importance in Nature and in the Synthesis of Pharmaceuticals. 344 (John Wiley & Sons, Inc, 2010).
- 7.Ito Y, Hirao T, Saegusa T. Synthesis of.alpha.,.beta.-unsaturated carbonyl compounds by palladium(II)-catalyzed dehydrosilylation of silyl enol ethers. J. Org. Chem. 1978;43:1011–1013. [Google Scholar]
- 8.Nicolaou KC, Zhong YL, Baran PS. A new method for the one-step synthesis of α,β-unsaturated carbonyl systems from saturated alcohols and carbonyl compounds. J. Am. Chem. Soc. 2000;122:7596–7597. [Google Scholar]
- 9.Diao T, Stahl SS. Synthesis of cyclic enones via direct palladium-catalyzed aerobic dehydrogenation of ketones. J. Am. Chem. Soc. 2011;133:14566–14569. doi: 10.1021/ja206575j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Izawa Y, Zheng C, Stahl SS. Aerobic oxidative Heck/dehydrogenation reactions of cyclohexenones: efficient access to meta-substituted phenols. Angew. Chem. Int. Ed. 2013;52:3672–3675. doi: 10.1002/anie.201209457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jin X, Koizumi Y, Yamaguchi K, Nozaki K, Mizuno N. Selective synthesis of primary anilines from cyclohexanone oximes by the concerted catalysis of a Mg–Al layered double hydroxide supported Pd catalyst. J. Am. Chem. Soc. 2017;139:13821–13829. doi: 10.1021/jacs.7b07347. [DOI] [PubMed] [Google Scholar]
- 12.Wang M-M, Ning X-S, Qu J-P, Kang Y-B. Dehydrogenative synthesis of linear α,β-unsaturated aldehydes with oxygen at room temperature enabled by tBuONO. ACS Catal. 2017;7:4000–4003. [Google Scholar]
- 13.Chen M, Rago AJ, Dong G. Platinum-catalyzed desaturation of lactams, ketones, and lactones. Angew. Chem. Int. Ed. 2018;57:16205–16209. doi: 10.1002/anie.201811197. [DOI] [PubMed] [Google Scholar]
- 14.Chuentragool P, Parasram M, Shi Y, Gevorgyan V. General, mild, and selective method for desaturation of aliphatic amines. J. Am. Chem. Soc. 2018;140:2465–2468. doi: 10.1021/jacs.8b00488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Z, He Z, Zhang L, Huang Y. Iridium-catalyzed aerobic α,β-dehydrogenation of γ,δ-unsaturated amides and acids: activation of both α- and β-C–H bonds through an allyl–iridium intermediate. J. Am. Chem. Soc. 2018;140:735–740. doi: 10.1021/jacs.7b11351. [DOI] [PubMed] [Google Scholar]
- 16.Hu R, Chen F-J, Zhang X, Zhang M, Su W. Copper-catalyzed dehydrogenative γ-C(sp3)-H amination of saturated ketones for synthesis of polysubstituted anilines. Nat. Commun. 2019;10:3681. doi: 10.1038/s41467-019-11624-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teskey CJ, Adler P, Gonçalves CR, Maulide N. Chemoselective α,β-dehydrogenation of saturated amides. Angew. Chem. Int. Ed. 2019;58:447–451. doi: 10.1002/anie.201808794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen M, Dong G. Platinum-catalyzed α,β-desaturation of cyclic ketones through direct metal–enolate formation. Angew. Chem. Int. Ed. 2021;60:7956–7961. doi: 10.1002/anie.202013628. [DOI] [PubMed] [Google Scholar]
- 19.Gnaim S, et al. Electrochemically driven desaturation of carbonyl compounds. Nat. Chem. 2021;13:367–372. doi: 10.1038/s41557-021-00640-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu R, Tao Y, Zhang X, Su W. 1,2-Aryl migration induced by amide C−N bond-formation: reaction of alkyl aryl ketones with primary amines towards α,α-diaryl β,γ-unsaturated γ-lactams. Angew. Chem. Int. Ed. 2021;60:8425–8430. doi: 10.1002/anie.202014900. [DOI] [PubMed] [Google Scholar]
- 21.Huang D, Newhouse TR. Dehydrogenative Pd and Ni catalysis for total synthesis. Acc. Chem. Res. 2021;54:1118–1130. doi: 10.1021/acs.accounts.0c00787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li C-J. Cross-dehydrogenative coupling (CDC): exploring C−C bond formations beyond functional group transformations. Acc. Chem. Res. 2009;42:335–344. doi: 10.1021/ar800164n. [DOI] [PubMed] [Google Scholar]
- 23.Scheuermann CJ. Beyond traditional cross couplings: the scope of the cross dehydrogenative coupling reaction. Chem. Asian J. 2010;5:436–451. doi: 10.1002/asia.200900487. [DOI] [PubMed] [Google Scholar]
- 24.Yeung CS, Dong VM. Catalytic dehydrogenative cross-coupling: forming carbon−carbon bonds by oxidizing two carbon−hydrogen bonds. Chem. Rev. 2011;111:1215–1292. doi: 10.1021/cr100280d. [DOI] [PubMed] [Google Scholar]
- 25.Liu C, et al. Oxidative coupling between two hydrocarbons: an update of recent C–H functionalizations. Chem. Rev. 2015;115:12138–12204. doi: 10.1021/cr500431s. [DOI] [PubMed] [Google Scholar]
- 26.Chen B, Wu L-Z, Tung C-H. Photocatalytic activation of less reactive bonds and their functionalization via hydrogen-evolution cross-couplings. Acc. Chem. Res. 2018;51:2512–2523. doi: 10.1021/acs.accounts.8b00267. [DOI] [PubMed] [Google Scholar]
- 27.Tang S, Liu Y, Lei A. Electrochemical oxidative cross-coupling with hydrogen evolution: a green and sustainable way for bond formation. Chem. 2018;4:27–45. [Google Scholar]
- 28.Yuan Y, Lei A. Electrochemical oxidative cross-coupling with hydrogen evolution reactions. Acc. Chem. Res. 2019;52:3309–3324. doi: 10.1021/acs.accounts.9b00512. [DOI] [PubMed] [Google Scholar]
- 29.Röckl JL, Pollok D, Franke R, Waldvogel SR. A decade of electrochemical dehydrogenative C,C-coupling of aryls. Acc. Chem. Res. 2020;53:45–61. doi: 10.1021/acs.accounts.9b00511. [DOI] [PubMed] [Google Scholar]
- 30.Wang P, Gao XL, Huang PF, Lei AW. Recent advances in electrochemical oxidative cross-coupling of alkenes with H2 evolution. Chemcatchem. 2020;12:27–40. [Google Scholar]
- 31.Jiang Y, Xu K, Zeng C. Use of electrochemistry in the synthesis of heterocyclic structures. Chem. Rev. 2018;118:4485–4540. doi: 10.1021/acs.chemrev.7b00271. [DOI] [PubMed] [Google Scholar]
- 32.Kärkäs MD. Electrochemical strategies for C–H functionalization and C–N bond formation. Chem. Soc. Rev. 2018;47:5786–5865. doi: 10.1039/c7cs00619e. [DOI] [PubMed] [Google Scholar]
- 33.Xiong P, Xu H-C. Chemistry with electrochemically generated N-centered radicals. Acc. Chem. Res. 2019;52:3339–3350. doi: 10.1021/acs.accounts.9b00472. [DOI] [PubMed] [Google Scholar]
- 34.Qiu Y, Struwe J, Meyer TH, Oliveira JCA, Ackermann L. Catalyst- and reagent-free electrochemical azole C−H amination. Chem. Eur. J. 2018;24:12784–12789. doi: 10.1002/chem.201802832. [DOI] [PubMed] [Google Scholar]
- 35.Yang Q-L, et al. Copper-catalyzed electrochemical C–H amination of arenes with secondary amines. J. Am. Chem. Soc. 2018;140:11487–11494. doi: 10.1021/jacs.8b07380. [DOI] [PubMed] [Google Scholar]
- 36.Sauermann N, Mei R, Ackermann L. Electrochemical C−H amination by cobalt catalysis in a renewable solvent. Angew. Chem. Int. Ed. 2018;57:5090–5094. doi: 10.1002/anie.201802206. [DOI] [PubMed] [Google Scholar]
- 37.Morofuji T, Shimizu A, Yoshida J-I. Electrochemical C–H amination: synthesis of aromatic primary amines via N-arylpyridinium ions. J. Am. Chem. Soc. 2013;135:5000–5003. doi: 10.1021/ja402083e. [DOI] [PubMed] [Google Scholar]
- 38.Hu X, Zhang G, Nie L, Kong T, Lei A. Electrochemical oxidation induced intermolecular aromatic C-H imidation. Nat. Commun. 2019;10:5467. doi: 10.1038/s41467-019-13524-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu K, et al. Electrooxidative para-selective C–H/N–H cross-coupling with hydrogen evolution to synthesize triarylamine derivatives. Nat. Commun. 2019;10:639. doi: 10.1038/s41467-019-08414-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morofuji T, Shimizu A, Yoshida J-I. Heterocyclization approach for electrooxidative coupling of functional primary alkylamines with aromatics. J. Am. Chem. Soc. 2015;137:9816–9819. doi: 10.1021/jacs.5b06526. [DOI] [PubMed] [Google Scholar]
- 41.Wesenberg LJ, et al. Metal-free twofold electrochemical C−H amination of activated arenes: application to medicinally relevant precursor synthesis. Chem. Eur. J. 2020;26:17574–17580. doi: 10.1002/chem.202003852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang L, et al. Photoelectrocatalytic arene C–H amination. Nat. Catal. 2019;2:366–373. doi: 10.1038/s41929-019-0231-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang J-H, et al. Regioselective ortho amination of an aromatic C–H bond by trifluoroacetic acid via electrochemistry. Org. Lett. 2019;21:5581–5585. doi: 10.1021/acs.orglett.9b01910. [DOI] [PubMed] [Google Scholar]
- 44.Niu L, et al. Manganese-catalyzed oxidative azidation of C(sp3)–H bonds under electrophotocatalytic conditions. J. Am. Chem. Soc. 2020;142:17693–17702. doi: 10.1021/jacs.0c08437. [DOI] [PubMed] [Google Scholar]
- 45.Hou Z-W, et al. Site-selective electrochemical benzylic C−H amination. Angew. Chem. Int. Ed. 2021;60:2943–2947. doi: 10.1002/anie.202013478. [DOI] [PubMed] [Google Scholar]
- 46.Shen T, Lambert TH. C–H amination via electrophotocatalytic ritter-type reaction. J. Am. Chem. Soc. 2021;143:8597–8602. doi: 10.1021/jacs.1c03718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kong W-J, Shen Z, Finger LH, Ackermann L. Electrochemical access to aza-polycyclic aromatic hydrocarbons: rhoda-electrocatalyzed domino alkyne annulations. Angew. Chem. Int. Ed. 2020;59:5551–5556. doi: 10.1002/anie.201914775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fu N, Sauer GS, Saha A, Loo A, Lin S. Metal-catalyzed electrochemical diazidation of alkenes. Science. 2017;357:575–579. doi: 10.1126/science.aan6206. [DOI] [PubMed] [Google Scholar]
- 49.Shen T, Lambert TH. Electrophotocatalytic diamination of vicinal C–H bonds. Science. 2021;371:620–626. doi: 10.1126/science.abf2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li C, et al. Electrochemically enabled, nickel-catalyzed amination. Angew. Chem. Int. Ed. 2017;56:13088–13093. doi: 10.1002/anie.201707906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Adeli Y, et al. Electrochemically oxidative C–C bond cleavage of alkylarenes for anilines synthesis. ACS Catal. 2019;9:2063–2067. [Google Scholar]
- 52.Kawamata Y, et al. Electrochemically driven, Ni-catalyzed aryl amination: scope, mechanism, and applications. J. Am. Chem. Soc. 2019;141:6392–6402. doi: 10.1021/jacs.9b01886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Walker BR, Manabe S, Brusoe AT, Sevov CS. Mediator-enabled electrocatalysis with ligandless copper for anaerobic Chan–Lam coupling reactions. J. Am. Chem. Soc. 2021;143:6257–6265. doi: 10.1021/jacs.1c02103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Siu T, Yudin AK. Practical olefin aziridination with a broad substrate scope. J. Am. Chem. Soc. 2002;124:530–531. doi: 10.1021/ja0172215. [DOI] [PubMed] [Google Scholar]
- 55.Chen J, et al. Electrocatalytic aziridination of alkenes mediated by n-Bu4NI: a radical pathway. Org. Lett. 2015;17:986–989. doi: 10.1021/acs.orglett.5b00083. [DOI] [PubMed] [Google Scholar]
- 56.Li J, et al. Electrochemical aziridination by alkene activation using a sulfamate as the nitrogen source. Angew. Chem. Int. Ed. 2018;57:5695–5698. doi: 10.1002/anie.201801106. [DOI] [PubMed] [Google Scholar]
- 57.Ošeka M, et al. Electrochemical aziridination of Internal alkenes with primary amines. Chem. 2021;7:255–266. [Google Scholar]
- 58.Liu, S. et al. Electrochemical aziridination of tetrasubstituted alkenes with ammonia. CCS Chem. 10.31635/ccschem.021.202100826 (2021).
- 59.Holst DE, Wang DJ, Kim MJ, Guzei IA, Wickens ZK. Aziridines synthesis by coupling amines and alkenes via an electro-generated dication. Nature. 2021;596:74–79. doi: 10.1038/s41586-021-03717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim JE, Choi S, Balamurugan M, Jang JH, Nam KT. Electrochemical C-N bond formation for sustainable amine synthesis. Trends Chem. 2020;2:1004–1019. [Google Scholar]
- 61.Narasimhan K, Kumar PR. Addition of phthalimido nitrene to substituted cyclopentadienes. Heterocycles. 1984;22:1369–1375. [Google Scholar]
- 62.Bailey PS, Hwang HH. Ozonation of phenyl-substituted thiophenes. J. Org. Chem. 1985;50:1778–1779. [Google Scholar]
- 63.Andreev PY. Reaction of ozone with five-membered hetarenes in a liquid phase. Russ. J. Appl. Chem. 2012;85:1395–1398. [Google Scholar]
- 64.Lee S-G, WooLim C, EuiSong C, HanPark D. Formation of stable solvent-trapped carbonyl oxides from the ozonolysis of indole derivatives. Synth. Commun. 1996;26:4623–4631. [Google Scholar]
- 65.Liu X, Liu C, Cheng X. Ring-contraction of hantzsch esters and their derivatives to pyrroles via electrochemical extrusion of ethyl acetate out of aromatic rings. Green Chem. 2021;23:3468–3473. [Google Scholar]
- 66.Reep C, Morgante P, Peverati R, Takenaka N. Axial-chiral biisoquinoline N,N′-dioxides bearing polar aromatic C-H bonds as catalysts in Sakurai-Hosomi-Denmark allylation. Org. Lett. 2018;20:5757–5761. doi: 10.1021/acs.orglett.8b02457. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that all other data supporting the findings of this study are available within the article and Supplementary Information files and are also available from the corresponding author upon request.