Abstract
There is a high co-occurrence of risky substance use among adults with traumatic brain injury (TBI), although it is unknown if the neurologic sequelae of TBI can promote this behavior. We propose that to conclude that TBI can cause risky substance use, it must be determined that TBI precedes risky substance use, that confounders with the potential to increase the likelihood of both TBI and risky substance use must be ruled out, and that there must be a plausible mechanism of action. In this review, we address these factors by providing an overview of key clinical and preclinical studies and list plausible mechanisms by which TBI could increase risky substance use. Human and animal studies have identified an association between TBI and risky substance use, although the strength of this association varies. Factors that may limit detection of this relationship include differential variability due to substance, sex, age of injury, and confounders that may influence the likelihood of both TBI and risky substance use. We propose possible mechanisms by which TBI could increase substance use that include damage-associated neuroplasticity, chronic changes in neuroimmune signaling, and TBI-associated alterations in brain networks.
Given the high co-occurrence of risky substance use and/or substance use disorders (SUDs) among adults with traumatic brain injury (TBI) (1–3), investigators have asked whether TBI causes SUD and/or SUD causes TBI. Several reviews have concluded that at-risk substance use is more likely to cause TBI than TBI is to cause SUD (4–6), while others have concluded that there is insufficient evidence to make definitive conclusions about the directionality of causal influences (1,2). There is little doubt that engaging in risky substance use can cause TBI. Intoxication, whether by alcohol, cannabis, or other drugs, increases the likelihood of injury, which can include a TBI (7). At least 2 studies have found that among those treated for an injury in an emergency department, the greater the alcohol intoxication, the more likely the injury included a TBI (8,9).
The more nuanced question is whether TBI causes risky substance use. Some authors have cited the tendency for TBI cohorts to reduce use immediately after injury, especially if the injury is associated with use (10–12), as evidence that TBI does not cause risky substance use. However, several studies have shown that most people who used alcohol before TBI eventually return to pre-injury patterns of use unless a medical condition precludes use (11–13). The logic of this argument is further undermined by what Corrigan et al. have called the chicken or the egg problem (14). The difficulty in examining a causal relationship between TBI and SUD is highly affected by the injury used to anchor the question. Previous investigators who concluded that SUD causes TBI but TBI does not cause SUD anchored their analyses in studies that used samples of patients in adult trauma or acute rehabilitation units. A large percentage of participants in these samples had histories of at-risk substance use or a diagnosable SUD that preceded their injury. However, these studies did not determine whether the TBI that led to their inclusion in the cohort was their first TBI, which ignores whether one or more TBIs earlier in life may have influenced their development of risky use behaviors. Several studies support the conclusion that childhood TBI could lead to the development of adolescent or adult at-risk substance use (15–19); thus, the conclusion that causality is only in the direction of substance use causing TBI appears flawed.
In this review, we will explore this more nuanced direction of causality—can TBI cause the development of at-risk substance use and/or SUD? To allow a focused consideration of this question, we will not address whether a TBI can make preexisting substance use worse. To confidently conclude that TBI is the cause for the development of at-risk substance use and/or SUD would require establishment of several relationships among these conditions. First, most obviously, the TBI would need to precede the development of the problematic substance use. Second, the relationship between TBI and problematic substance use could not be due to a confounder that precedes both and causes each. Confounders that have been hypothesized include childhood exposures (e.g., parental attributes, parenting, adverse childhood experiences, socioeconomic status, community risk factors) and a behavioral phenotype for risk taking (e.g., a personality trait that could lead to both TBI and problematic substance use). Finally, and perhaps most challenging, to establish causality, there would need to be a mechanism of action that provides a plausible explanation for how TBI could do so. In the following sections, we will explore first human, then preclinical evidence for the conditions required to conclude causality (summarized in Tables 1 and 2, respectively). Evidence from these two sources of findings is uneven, and surprisingly, despite the greater control afforded via preclinical studies, unequivocal evidence to inform this question is not easily derived.
Table 1.
Human Studies of the Association Between TBI and Risky Substance Use
| Study | Sample and Population | Method | Risky Alcohol Use or Alcohol Use Disorder | Use of Other Substances |
|---|---|---|---|---|
| Corrigan et al. (20) | N = 4464 adults 1–20 years after acute rehabilitation for TBI and enrolled in TBI model systems | Prospective cohort (retrospective self-report of TBI; past-month [preindex injury] or past-year [postindex injury] use) | Those with history of TBI vs. those without more likely to engage in risky drinking before index injury (42.4% vs. 31.3%) and after index injury (22.8% vs. 13.5%) | Those with history of TBI vs. those without more likely to use illicit drugs before index injury (27.9% vs. 20.3%) and after index injury (17.8% vs. 8.1%) |
| Dams-O’Connor et al. (21) | N = 586 adults treated for TBI and enrolled in the TRACK-TBI study | Prospective cohort (retrospective self-report of TBI; past-month use) | Those with history of TBI vs. those without more likely to engage in risky drinking before index injury (56.6% vs. 37.1%) | Those with history of TBI vs. those without more likely to use illicit drugs before index injury (35.3% vs. 14.9%) |
| Adams et al. (22) | N = 4645 army soldiers following combat deployment who participated in Army STARRS study | Prospective cohort (retrospective self-report of TBI; past-month drinking) Adjusted for predeployment drinking, predeployment psychiatric diagnosis, combat/deployment stress severity, personal life stress during deployment, PTSD symptoms during deployment, and sociodemographic and military service characteristics |
Three months after deployment, lifetime TBI, but not deployment-acquired TBI, associated with increased binge (AOR = 1.39, 95% CI: 1.20–1.60) and heavy drinking (AOR = 1.28, 95% CI: 1.09–1.49). Having both predeployment lifetime history and a deployment-acquired TBI increased heavy drinking 6 months later (AOR = 1.42, 95% CI: 1.03–1.95) | Not studied |
| Silver et al. (23) | N = 5034 adults from greater metropolitan New Haven, Connecticut | Population survey (retrospective self-report of lifetime TBI; use disorders from standardized interview) Adjusted for sociodemographics and health-related quality of life variables |
Alcohol abuse or dependence (AOR = 2.2, 95% CI: 1.7–2.8) | Drug abuse or dependence (AOR = 1.8, 95% CI: 1.2–2.5) |
| Anstey et al. (24) | N = 7485 adults from 2 southeast Australian metropolitan areas | Population survey (retrospective self-report of lifetime TBI; past-month drinking) Adjusted for age group, gender, financial problems, physical health, and psychoticism |
No difference for TBI vs. no TBI except young adult females (p = .045) | Not studied |
| Ilie et al. (25) | N = 1988 adults in the province of Ontario, Canada | Population survey (retrospective self-report of lifetime TBI; past-month substance use) Adjusted for age, sex, marital status, family income, and education |
Not studied | Uses marijuana (AOR = 2.80, 95% CI: 1.79–4.39) Uses nonprescription opioids (AOR = 2.90, 95% CI: 1.50–5.59) |
| Ilie et al. (26) | N = 6074 adults in the province of Ontario, Canada | Population survey (retrospective self-report of lifetime TBI; past-month harmful or hazardous drinking) | Of persons with history of TBI, 21.4% reported harmful or hazardous drinking vs. 13.2% of no TBI respondents | Not studied |
| Whiteneck et al. (27) | N = 2701 noninstitutionalized adults residing in the state of Colorado | Population survey (retrospective self-report of lifetime TBI history; past-month drinking) Adjusted for place of medical care, age, sex, and race |
At-risk alcohol use no more prevalent for persons with history of TBI than general population | Not studied |
| Bogner et al. (28) | N = 6996 noninstitutionalized adults residing in the state of Ohio | Population survey Behavioral Risk Factors Surveillance System (retrospective self-report of lifetime TBI; past-month alcohol use) Adjusted for age, sex, and race/ethnicity |
Binge drinking (AOR = 1.5, 95% CI: 1.1–2.0) Heavy drinking (AOR = 1.7, 95% CI: 1.1–2.6) Those with first TBI before age 15 years: no difference in binge or heavy drinking |
Not studied |
| Corrigan et al. (29) | N = 2935 noninstitutionalized adults with history of TBI with loss of consciousness residing in the state of Ohio | Population survey Behavioral Risk Factors Surveillance System (retrospective self-report of lifetime TBI; past-month alcohol use) Adjusted for age, sex, and race/ethnicity |
Those with first TBI before age 20 more likely to binge drink in adulthood (28.5% vs. 20.4%, p = .003) Those with first mild TBI before age 20 more likely to binge drink in adulthood (31.9% vs. 19.3%, p < .001) |
Not studied |
| Waltzman et al. (30) | N = 3605 noninstitutionalized adults with history of TBI with loss of consciousness residing in the state of North Carolina | Population survey Behavioral Risk Factors Surveillance System (retrospective self-report of lifetime TBI; past-month alcohol use) Adjusted for sex, age, veteran status, marital status, educational attainment, employment status, and annual income |
Those with history of TBI more likely to binge drink (AOR = 1.7, 95% CI: 1.2–2.4) Those with first TBI before age 18: no difference in binge drinking |
Not studied |
| McKinlay et al. (138) | N = 1265 children born in 1977 in Christchurch, New Zealand | Birth cohort followed to age 25 (TBI from medical record abstraction of prospectively collected medical records; standardized structured interview to determine substance use disorder) Adjusted for sociodemographic factors, early behavioral problems, and parental substance abuse |
Alcohol dependence n.s. for each group of age at first injury (0–5, 6–15, 16–21 years old) | Drug dependence for first injury 0–5 years old: AOR = 2.85, 95% CI: 1.11–7.32 Drug dependence for first injury 6–15 years old: AOR n.s. Drug dependence for first injury 16–21 years old: AOR = 2.55, 95% CI: 1.07–6.12 |
| Timonen et al. (33) | N = 10,934 children born in 1966 in Northern Finland | Birth cohort followed to age 31 (medically diagnosed TBI; heavy drinking defined by diagnosis of alcohol abuse or dependence [DSM-III criteria] or having at least 2 registered drunk driving offenses) Adjusted for mother’s marital status and father’s social class at time of birth and urban vs. rural residence at approximately age 14 |
Heavy alcohol use no different for childhood TBI vs. no childhood TBI Among heavy drinkers, children with first TBI before age 12 began heavy drinking 6 years before those with first TBI at 12–15 years old |
Not studied |
| Kennedy et al. (34) | N = 11,412 children born 1991–1992 in South West region of England (n = 800 with mild TBI; n = 2305 with orthopedic injury; n = 8307 uninjured children) | Birth cohort followed to age 17 (medically diagnosed injuries through age 16; self-reported recent use) Adjusted for prebirth sociodemographic factors, family environment and parenting style, and history of criminal activity |
Alcohol use disorder vs. orthopedic injured: AOR = 1.69, 95% CI: 1.17–2.45 Alcohol use disorder vs. general population: AOR = 1.31, 95% CI: 0.94–1.82 |
Cannabis misuse vs. orthopedic injured (n.s.) Nicotine dependence vs. orthopedic injured (n.s.) |
AOR, adjusted odds ratio; CI, confidence interval; n.s., nonsignificant; PTSD, posttraumatic stress disorder; STARRS, Study to Assess Risk and Resilience in Servicemembers; TBI, traumatic brain injury; TRACK-TBI, Transforming Research and Clinical Knowledge in Traumatic Brain Injury.
Table 2.
Summary of Preclinical Studies of TBI and Drug Reward, Reinforcement, and Seeking
| TBI Model | Single/Repeated Injury | Drug of Abuse | Reward/Reinforcement Model | Species/Strain | Sex | Age at Time of Injury | TBI→Testing Delay | Effect of TBI | Reference |
|---|---|---|---|---|---|---|---|---|---|
| TBI→Drug | |||||||||
| CCI (closed head) | Single | Alcohol | Two-bottle choice (drinking in the dark) | Mouse/C57BL/6 | M | 6–7 wk | 4–10 days | Reduced intake | (37) |
| Weight drop | Single | Alcohol | Two-bottle choice | Mouse/Swiss Webster (selectively bred for stress analgesia) | NS | 8 wk | 7–19 days | Reduced intake (only in LA mice) | (36) |
| 62–72 days | No effect | ||||||||
| Blast | Single | Alcohol | Two-bottle choice Two-bottle choice (alcohol deprivation and quinine adulteration tests) |
Rat/Sprague Dawley | M | 8 wk | 1–7 wk | No effect | (38) |
| 8 wk | Increased 1-hour intake in upper 50% after median split | ||||||||
| 10–24 wk | No effect | ||||||||
| CCI (closed head) | Single | Alcohol | Two-bottle choice | Mouse/Swiss Webster | M | 21 days | 10–22 days | Increased intake | (40) |
| CCI (closed head) | Single | Alcohol | Two-bottle choice | Mouse/Swiss Webster | M | 21 days | 8–9 wk | No effect | (41) |
| F | 21 days | 8–9 wk | Increased intake | ||||||
| M | 60 days | 3–4 wk | No effect | ||||||
| F | 60 days | 3–4 wk | No effect | ||||||
| CCI (closed head) | Single | Alcohol | CPP | Mouse/Swiss Webster | M | 21 days | 8–9 wk | No effect | (41) |
| F | 21 days | 8–9 wk | Increased CPP | ||||||
| Blast | Single Repeated (3×) | Alcohol | Two-bottle choice | Mouse/C57BL/6 | M | 12–16 wk | 4–8 wk | No effect Decreased 24-hour intake Increased 2-hour proportional intake |
(39) |
| CCI (open head) | Single | Cocaine | CPP | Mouse/C57BL/6 | M | 6 wk | 2–3 wk | Increased CPP | (42,43) |
| CCI (open head) | Single | Cocaine | CPP | Mouse/C57BL/6 | M | 6 wk | 2–3 wk | Increased CPP | (44) |
| 8 wk | 2–3 wk | No effect | |||||||
| CCI (open head) | Single | Cocaine | CPP | Mouse/C57BL/6 | F (E/P)a F (M/D)b |
6 wk | 2–3 wk | No effect Increased CPP |
(44) |
| CCI (open head) | Single | Cocaine | SA | Rat/Long-Evans | M | 12 wk | 2–4 wk | Increased SA | (46) |
| Blast | Single | Cocaine | SA | Rat/Sprague Dawley | M | 8 wk | 4–8 wk | No effect on SA | (47) |
| 10–11 wk | No effect on extinction | ||||||||
| 12–14 wk | No effect on cue- or drug-primed reinstatement | ||||||||
| Blast | Repeated (3×) | Oxycodone | SA | Rat/Sprague Dawley | M | 8 wk | 4–8 wk | No effect on FR-1 SA | (48) |
| 5–7 wk | Reduced FR-2 SA; no effect on FR-4 SA | ||||||||
| 7–9 wk | Increased drug seeking in FR-4 extinction sessions | ||||||||
| 10–23 wk | No effect on FR-1 extinction or cue reinstatement of drug seeking | ||||||||
| Blast | Repeated (3×) | Oxycodone | SA | Rat/Sprague Dawley | M | 8 wk | 6 wk | Increased drug seeking | (123) |
| Drug→TBI→Drug | |||||||||
| LFP | Single | Alcohol | Operant alcohol SA | Rat/Wistar | F | 16 wk | 2–10 days | No effect | (128) |
| LFP | Single | Alcohol | Operant alcohol SA | Rat/Wistar | M | 16 wk | 9 days | Increased breakpoint for alcohol | (129) |
| LFP | Single | Alcohol | Operant alcohol SA | Rat/Wistar | M | 16 wk | 2–15 days | Increased alcohol SA: preinjury intake positively correlated with postinjury increase in intake | (130) |
CCI, controlled cortical impact; CPP, conditioned place preference; F, female; FR, fixed ratio; LA, low analgesia; LFP, lateral fluid percussion; M, male; NS, not specified; SA, self-administration; TBI, traumatic brain injury.
Phase at time of injury; E/P, estrus/proestrus.
Phase at time of injury; M/P, metestrus/diestrus.
HUMAN STUDIES CONTRIBUTING TO CAUSALITY
Human studies that allow scrutiny of a causal relationship between TBI and problematic substance use have been limited to examining the temporal onset and presence of confounders. These studies have used three methods: 1) eliciting lifetime exposure in TBI cohorts; 2) population surveys examining the association of the two conditions; and 3) birth cohorts examining both onset and association. TBI cohorts in which lifetime exposure was studied have included the TBI model systems (20), Transforming Research and Clinical Knowledge in Traumatic Brain Injury (TRACK-TBI) (21), and Army Study to Assess Risk and Resilience in Servicemembers (STARRS) 22). Lifetime TBI identification has been done with standardized methods of self-report, and substance use is typically self-reported past-month behavior. Population surveys have used a variety of methods for detecting both TBI and substance use, with the former varying from single-item elicitation [i.e., New Haven, Connecticut (23); Southeast Australia (24); Ontario, Canada (25,26)] to protocols based on standardized methods of retrospective self-report [i.e., Colorado (27), Ohio (28,29), North Carolina (30)]. Only a small number of birth cohort studies have allowed examination of the onset and development of TBI and at-risk substance use, including those conducted in Christchurch, New Zealand (25); Northern Finland (31–33); and Avon, United Kingdom (34). TBI identification is typically based on medical records; substance use has been both medically documented or self-reported recent behavior. Each of these methodologies has strengths and weaknesses for examining the temporal onset and presence of confounders, as shown in Box S1.
Temporal Onset
Several studies support a relationship between childhood TBI and later adolescent or adult at-risk substance use (20–22). Studies in TBI cohorts that capture lifetime history of TBI have found that earlier life injuries are more common among those with alcohol use problems, although temporal ordering is not possible. Studies in 2 U.S. states did not find that children injured earlier in life (before age 15 and before age 18 years) than persons injured after those ages were not more likely to engage in risky alcohol use as adults (28,30). In contrast, Corrigan et al. found that adults who had experienced a mild TBI with loss of consciousness before age 20 were more likely to engage in binge drinking than those who had a first mild TBI with loss of consciousness at an older age (29). This risk was largely due to a first TBI being incurred both at 10 to 14 and 15 to 19 years. These two groups were equally likely to engage in adult binge drinking. Indeed, had either 15 or 18 years been used as the cut point for age at first TBI, as was done in the two other state population studies, the difference would not have been significant. While any adolescent onset of TBI seems to increase the likelihood of problem alcohol use in adulthood, unfortunately, it is not definitive that TBI preceded the problematic use.
The Christchurch birth cohort study found that children hospitalized with a mild TBI before the age of 6 were more likely to develop alcohol problems in adolescence than children with no TBI or those with a mild TBI that did not require hospitalization (35). The birth cohort study in Avon found that TBIs occurring before age 17 were associated with problem alcohol consumption at age 17 (34). The Northern Finland birth cohort study did not find a difference in heavy drinking for those with or without childhood TBI; however, those children who incurred a TBI before age 12 initiated heavy drinking 6 years earlier than those with a first TBI at 12 to 15 years old (33). Note that the Christchurch results would appear to un-equivocally support that TBI preceded problematic substance use, but the Avon cohort is more ambiguous due to the possibility that alcohol consumption could begin in early adolescence.
Potential Confounders
Studies using the Northern Finland birth cohort reported multiple risk factors associated with incurring a TBI, including that if parents misused alcohol, there was a twofold greater chance of childhood TBI (31), and that a first TBI after age 12 that occurred while drinking alcohol resulted in a fourfold greater risk of repeat TBI by age 34 (32). Findings noted above for the Christchurch and Avon birth cohort studies were significant after controlling for multiple demographic, parental, and developmental characteristics (34,35). The Avon birth cohort studies also compared those with a TBI to an orthopedic injury control group intended to represent a behavioral phenotype for risk taking. While the association with problem alcohol use was significantly higher in the orthopedic injury group than the uninjured comparison group, the TBI group was still significantly greater than the orthopedic control group. Their findings were specific to alcohol because the TBI and orthopedic injury groups did not differ significantly in their likelihood of smoking either cannabis or nicotine.
These few studies may lend support for the effect of TBI not being due to influences such as parental attributes, parenting, or socioeconomic status. Influences from adverse childhood experiences or community characteristics have not been explored. The single study that investigated a risk-taking behavioral phenotype still found an additional effect of TBI on drinking at age 17 (34). This finding is perhaps strengthened by risk taking having equivalent risk for the likelihood of cannabis and tobacco use. Still, it is a single study.
In summary, from human studies, a significant association is frequently found between TBI and at-risk substance use and/or SUD. These studies have been largely limited to alcohol. The strength of association observed may be small, thus contributing to variation in findings. The ability to detect the relationship may be masked by variations in manifestation of the influence of TBI, for instance, age at injury or context of the injury (e.g., whether it occurs during a period of stress). The utility of the human literature for establishing a causal relationship between TBI and risky substance use is specifically limited by uncertainty about temporal onset during adolescence. Without specificity about the age at injury and the age at initiation and/or risky use of substances, temporality will be difficult to ascertain. Finally, the study of confounders is limited by the ability to operationalize constructs such as adverse childhood experiences or behavioral phenotypes in population-based cohorts. However, studies that have controlled for characteristics of parents, parenting, and the home environment seem to consistently suggest that these factors are unlikely sources of confound.
PRECLINICAL STUDIES CONTRIBUTING TO CAUSALITY
Temporal Onset
An advantage of preclinical research is that temporal ordering of TBI and exposure to drugs of abuse is controlled. To date, most preclinical research has focused on modeling the question of whether TBI can increase risky drug use (see Box S2 for commonly used models of drug reward/reinforcement; see Table 2 for a summary of experimental findings).
Early studies on the effects of TBI on subsequent drug use focused on alcohol. In mice, experimental TBI led to reduced alcohol intake within the first 2 weeks following injury (36,37). In rats, blast TBI resulted in similar alcohol intake during a 7-week course of two-bottle choice but divergent responses on a final 1-hour session (38). Similar results were found in mice after repeated blast TBI: the proportion of daily alcohol consumed in the first 2 hours was higher in injured mice (39). Weil and colleagues demonstrated that adolescent mice had elevated alcohol drinking 1 week after TBI (40). A subsequent study found that females but not males had higher alcohol consumption and conditioned place preference (CPP) (41).
Adolescent, but not adult, TBI resulted in elevated cocaine CPP in male mice (42–44), but female mice showed differential effects based on estrus status. Mice in met- or diestrus at the time of injury had significantly elevated cocaine CPP, while those in proestrus or estrus had no change (45). Moderate to severe frontal TBI increased cocaine self-administration (46), while mild TBI found no difference in self-administration, extinction, or cue- or cocaine-primed induced reinstatement (47). Repeated blast resulted in similar levels of oxycodone self-administration, but subsequent drug seeking was elevated in the injured group (48). Thus, there are several examples of increased susceptibility for substance use in rodent models, but outcomes differ based on factors such as injury mechanism and severity, age at the time of injury, the drug studied, and time after injury.
Potential Confounders
Although preclinical studies can be designed to reduce the impact of confounding variables, these may not always be considered. Unknown individual differences that are present prior to experiment onset could independently contribute to both TBI recovery and addiction-related outcomes. Many studies use outbred rats [e.g., (46,48)] or mice [e.g., (40)], and this genetic variability may explain individual differences in drug reward/reinforcement following TBI (38,46). Similarly, individual differences in traits such as impulsivity exist in experimental animals and, similar to humans, can explain differences in addiction-related behaviors (49–51). Consideration of these differences and testing models of human confounders [e.g., models of early-life adversity (52,53)] will be important to better understand human variability in the relationship between TBI and risky substance use.
Plausible Mechanisms of Action
TBI induces myriad neurochemical changes, the nature of which is affected by factors including injury mechanism and severity, genetics, age, and sex. This section will focus on biological effects of TBI that are linked with neuroplasticity and describe plausible mechanisms by which these effects could increase addiction liability (summarized in Table 3). The reader is referred to (54–57) for more comprehensive reviews on the neurochemical sequelae of TBI. For simplicity, we will discuss these mechanisms in 2 broad timeframes: acute (within the first few days of injury) and chronic (after the first few days). The acute stage will be discussed in terms of the initial effects of injury that can set the stage for enduring neuroplasticity relevant to addiction liability, and the chronic stage will present evidence that persistent sequelae could influence physiological responses to drugs of abuse and addiction liability.
Table 3.
Biological Effects of TBI That Are Linked With Neuroplasticity and Plausible Mechanisms by Which These Effects Could Increase Risky Substance Use
| Time | Biological Processes | Downstream Effects/Processes | Link to Neuroplasticity | Plausible Link to Risky Substance Use | Clinical Evidence for Mechanistic Link | References |
|---|---|---|---|---|---|---|
| Immediate | Mechanical deformation/mechanoporation–induced ion flux: K+ efflux, Na+ and Ca2+ influx | Membrane depolarization Excitotoxicity |
Increased intrinsic excitability of neurons | PFC: increase or decrease in intrinsic excitability during cocaine or remifentanil abstinence (sex and treatment regimen dependent) NAc: decrease in intrinsic excitability during cocaine abstinence VTA: increased intrinsic excitability of dopamine neurons during cocaine abstinence |
(54,65,139,140) | |
| Minutes/Hours | Necrosis | Release of DAMPs/engagement of TLR2, 3, 4, 7, 9; engagement of purinergic receptors, NLRs, RLRs, ALRs Energy crisis |
DAMP-associated TLR4 signaling: increased insertion of CP-AMPARs, increased NMDA-mediated Ca2+ flux; decrease in dopamine cell firing rate DAMP-associated TLR3, 7 signaling: growth cone collapse, inhibition of neurite outgrowth, neurodegeneration ATP-associated P2X signaling: release of glutamate from astrocytes NLR signaling: see Downstream Effects/processes/inflammasome activation TLR4 signaling: increased NF-κB → modulation of LTD; antiapoptotic gene regulation TLR4 signaling: increased IL-6 → Short IL-6 exposure: increased intrinsic excitability Long IL-6 exposure: reduced depolarization-induced Na+ and Ca2+ currents, downregulation of presynaptic mGluR2/3 |
NAc: increase in CP-AMPARs during cocaine abstinence; reduction of NAc CP-AMPARs reduces drug seeking VTA: increase in CP-AMPARs after nicotine, cocaine, amphetamine, morphine, heroin, ethanol, and cannabis Intra-VTA LPS (TLR4 agonist) sufficient to induce reinstatement of cocaine seeking TLR4 agonist sufficient to increase alcohol consumption TLR4 antagonist suppressed incubation of heroin craving Reduction of mGluR2/3 function in mesocorticolimbic brain regions is also observed following exposure to nicotine, cocaine, or alcohol Treatment with mGluR2/3 agonists or positive modulators can reduce the reinforcing effects and seeking of drugs |
Lower volumes of gray matter in substance use disorder: largest effects in putamen, thalamus, insula, and anterior cingulate cortex Polymorphisms of human NF-κB1 associated with chronic alcohol use Elevated NF-κB in brains of chronic alcohol users |
(64–66,72–76,92,110,119,141–147) |
| Axonal injury (shearing, calpain-associated proteolysis of cytoskeletal proteins) | Impaired synaptic transmission, necrosis/apoptosis | See Downstream Effects/processes/necrosis/apoptosis | Impaired prefrontal connectivity in TBI and risky substance use | Hypofrontality observed in substance use disorder with many drugs of abuse Axonal injury in polydrug abuse |
(148–154) | |
| Biphasic: Minutes/Hours, Then Days and Beyond | Increased BBB permeability | Extravasation of peripheral immune cells and immunogens Activation of the complement system |
See Biological Processes/gliosis Complement signaling: synapse loss, neuroprotection against excitotoxicity, increased neurogenesis |
Increased alcohol intake following BBB disruption | Reduced BBB integrity in postmortem tissue from risky alcohol use | (36,155–159) |
| Generation of reactive oxygen species | Inflammasome activation → generation of IL-1β, IL-18; induction of pyroptosis Inhibition of astrocytic transport of glutamate Decreased NMDA receptor–mediated Ca2+ flux Elevated NF-κB |
IL-1β: reduction of Nav, L- and N-type Cav, and Kv channel activity; increased NMDA-mediated Ca2+ flux; increased or decreased GABAA-mediated Cl− current (cell-type specific); increased Ca2+-dependent glutamate release, decreased Ca2+-dependent GABA release; inhibition of LTP Pyroptosis: see Biological Processes/apoptosis Elevated extracellular glutamate→promotion of excitotoxicity NF-κB: modulation of LTD; antiapoptotic gene regulation; increase in expression of mu, delta opioid receptors and NK1Rs, increase in expression of β-endorphin and enkephalin propeptides, reduction of dynorphin expression |
IL-1β antagonist in VTA reduced cocaine seeking in rats TLR4 antagonist in VTA reduced cocaine-primed drug seeking in rats Intra-NAc NF-κB inhibitor reduced morphine and cocaine CPP NF-κB inhibitor reduced naloxone-precipitated morphine withdrawal severity |
Peripheral IL-1β positively correlated with duration of methadone maintenance in former heroin users Peripheral IL-1β elevated in abstinent severe cocaine users with comorbid psychiatric diagnosis Polymorphisms of human NF-κB1 associated with chronic alcohol use Elevated NF-κB in brains of chronic alcohol users |
(69,101,110,142,160–165) | |
| Days–Weeks | Gliosis | Potentiation of drug-associated dopamine increase Shift to IDO metabolism Cytokine disruption of tetrahydrobiopterin (BH4) Cytokine increase in levels and activity of SERT Cytokine reduction in DAT Cytokine-induced release of astrocytic glutamate and reduced astrocytic glutamate uptake Cytokine-induced increase in cell surface AMPA and NMDA receptors Cytokine-induced decrease in cell surface GABAA receptors Cytokine-induced increase in levels and activity of acetylcholinesterase and decrease in acetylcholine release Thrombospondin-mediated increase in axonal sprouting Astroglial scar formation |
IDO-biased metabolism reduces serotonin production and produces neuroactive metabolites kynurenic acid (antagonist of AMPA, kainite, and NDMA glutamate receptors) and quinolinic acid (agonist of NMDA receptors) BH4 disruption reduces production of dopamine, norepinephrine, and epinephrine Cytokine-associated changes in glutamate and GABA receptor function produce a net increase in synaptic strength Astroglial scar formation associated with cognitive/motor deficits TNF-α: Involved in synaptic scaling: increases mEPSC frequency and amplitude. Pathological concentrations of TNF-α inhibit LTP Induction of glutamate release from astrocytes and microglia, which acts on extrasynaptic GluN2B containing NMDA receptors BDNF reduction of inhibitory transmission via decrease of Cl− transport |
Pharmacological shift of IDO metabolism from quinolinic acid to kynurenic acid pathway abolished the alcohol intake in 2 preclinical models and reduced cue-induced cocaine and alcohol seeking Reduced tonic dopamine and norepinephrine reduces motivational arousal Reduced phasic dopamine in striatum associated with increased cocaine self-administration, reversed by L-Dopa G-CSF increases cocaine self-administration and increases evoked dopamine release TBI-primed immune response may prime immune response to drugs of abuse Dexamethasone reduced TBI-associated increase in cocaine CPP Minocycline reduced TBI-associated increase in alcohol drinking |
Lower kynurenic acid in patients with alcohol use disorder Reduced DAT in brain injury and methamphetamine abuse Upregulation of immune-related genes is a consistent finding in postmortem tissue from chronic alcohol users Stress and cue-triggered drug craving elevated plasma TNF-α in cocaine-dependent individuals Microgliosis elevated in orbital frontal cortex, striatum, and midbrain of methamphetamine users, inversely proportional to abstinence duration |
(40,55,69,84,91,92,159,166–181) |
| Weeks | Demyelination | Impaired action potential propagation | See Biological Processes/axonal injury | See Biological Processes/axonal injury | (54) | |
| Months | Network-level changes | Decreased communication between the default mode network and salience network Widespread elevations in functional connectivity |
(56) | |||
| Neurodegenerative processes | Phospho-tau accumulation β-amyloid accumulation |
See Downstream Effects/processes/inflammasome activation | Elevated phospho-tau in rat striatum following heroin self-administration | Elevated phospho-tau in postmortem tissue from heroin abusers Elevated phospho-tau in postmortem tissue from individuals with high levels of prescription opioid use Elevated β-amyloid in polydrug users |
(154,182–186) |
ALR, AIM2-like receptor; ATP, adenosine triphosphate; BBB, blood-brain barrier; BDNF, brain-derived neurotrophic factor; CP-AMPAR, Ca2+-permeable AMPA receptor; CPP, conditioned place preference; DAMP, damage-associated molecular pattern; DAT, dopamine transporter; GABA, gamma-aminobutyric acid; G-CSF, granulocyte colony-stimulating factor; IL, interleukin; LPS, lipopolysaccharide; LTD, long-term depression; LTP, long-term potentiation; mEPSC, miniature excitatory postsynaptic current; mGluR, metabotropic glutamate receptor; NAc, nucleus accumbens; NF-κB, nuclear factor-κB; NK1R, neurokinin 1 receptor; NLR, NOD-like receptor; PFC, prefrontal cortex; RLR, rig-1 like receptor; SERT, serotonin transporter; TBI, traumatic brain injury; TLR, toll-like receptor; TNF-α, tumor necrosis factor α; VTA, ventral tegmental area.
Acute Effects: Damage-Associated Molecular Pattern Mediated Increases in Ca2+-Permeable AMPA Receptors.
Primary mechanisms of TBI involve impact-associated forces, which stretch tissue and shear axons (54). At impact, mechanical deformation and/or mechanoporation leads to ion flux that ultimately leads to profound membrane depolarization of neurons (54,58,59). This depolarization promotes neuronal excitotoxicity and necrosis, which releases damage-associated molecular patterns (DAMPs) (59–61). DAMPs promote activation of astrocytes and microglia (collectively referred to as gliosis), resulting in release of cytokines and chemokines (54,61). DAMP-associated TLR4 (toll-like receptor 4) signaling triggers robust synaptic plasticity, increasing synaptic levels of Ca2+-permeable AMPA receptors (CP-AMPARs) and increasing Ca2+ conductance through NMDA receptors (62). Both experimental TBI and injury to cultured neurons were found to elevate synaptic CP-AMPARs (63,64). In rodents, drugs of abuse also increase synaptic CP-AMPARs in areas of the brain involved in drug seeking, including the ventral tegmental area (VTA), nucleus accumbens, and prefrontal cortex (PFC), and these effects often persist for weeks or months (65,66). Elevated CP-AMPAR expression is observed in the VTA after exposure to morphine, cocaine, ethanol, and cannabis (65). CP-AMPARs are also increased in the nucleus accumbens after cocaine withdrawal, and reversal of this phenomenon is sufficient to reduce drug seeking (67,68). Thus, the DAMP→TLR4→CP-AMPAR cascade is one plausible mechanism by which TBI could promote subsequent substance use.
Acute Effects: Cytokine Regulation of Neuronal Transmission.
Glial and peripheral cytokines can influence synaptic plasticity and have been linked to addiction liability. Interleukin 1β (IL-1β) modulates neuronal ion flux via several mechanisms (69,70), and chronic IL-6 downregulates metabotropic glutamate 2/3 receptors (mGluR2/3) (71). Reduction of mGluR2/3 function in mesocorticolimbic brain regions is also observed following exposure to nicotine, cocaine, or alcohol, and treatment with mGluR2/3 agonists or positive modulators reduces the reinforcing effects and seeking of drugs (72–76). Tumor necrosis factor α strongly increases the balance of excitatory/inhibitory transmission by increasing cell surface CP-AMPARs and internalizing GABAA (gamma-aminobutyric acid A) receptors (77). Although cytokine responses occur acutely following injury, there is extensive evidence for prolonged elevations in excitatory neurotransmission following immune challenge, including susceptibility to seizures (78–80). Persistent increases in neuronal excitability and seizure susceptibility are also common following TBI (81,82). Thus, IL-6–associated reduction of mGluR2/3 and tumor necrosis factor α–associated elevation of CP-AMPARs are plausible mechanisms by which TBI-associated cytokine signaling could promote subsequent substance use.
Chronic Effects.
Postmortem and neuroimaging studies demonstrate gliosis that persists months or years after injury (55,57), and elevated levels of IL-1β, major histocompatibility complex class II, and IL-6 have been reported several months after experimental TBI (55,57). Drug use has been linked to chronic gliosis. Alcohol (83), cocaine (84,85), methamphetamine (86), and opioids (87,88) engage innate immune signaling, and alcohol and methamphetamine can also induce gliosis via other mechanisms, such as the generation of reactive oxygen species (89,90). Methamphetamine users had elevated binding of the microglial activation marker (R)-[11C]-PK11195, which was inversely proportional to abstinence duration (91). Similarly, upregulation of immune-related genes is a consistent finding in postmortem tissue from chronic alcohol users (92), and gliosis is prominent in animal studies of alcohol and opioid self-administration (92–94).
Chronic Effects: Microglial Priming—Implications for Drug Exposure, Craving, and Relapse.
TBI can lead to microglial priming, where the cells are hyperresponsive to subsequent immune challenge, even at distal time points (95–97). It is plausible that TBI-associated priming could also affect immune responses to drugs of abuse. Morphine and other opioids prolong recovery from nerve injury (98,99), and this has been proposed to be due to injury-induced priming of spinal microglia (100). Neuroimmune activation has previously been proposed to create the biology of addiction (101). In the context of TBI, injury-associated priming may contribute to addiction liability by altering biological responses to substances and triggers of drug craving: re-exposure to the drug, stress, and drug-associated cues (102–105).
Prenatal immune activation increased drug-primed reinstatement to methamphetamine seeking and CPP for amphetamine (106,107), and adult immune activation increased alcohol drinking (108,109). These data suggest that immune activation primed the response to these substances of abuse in a manner consistent with greater risk of risky substance use. In a model of comorbid TBI and cocaine use, cocaine intake was positively correlated with neuroinflammatory markers in the frontal cortex (46). Drug exposure itself can also prime neuroimmune responses. A history of cocaine primes the cocaine-induced increase in IL-1β, nuclear factor-κB, and CD11b messenger RNA in the VTA, and blockade of IL-1 receptors in the VTA suppresses cocaine-primed reinstatement of drug seeking (110). Similarly, rats exposed to morphine in adolescence had an exaggerated immune response to morphine-elevated morphine CPP in adulthood (111). The glial modulator ibudilast given during adolescent morphine exposure blocked the later increase in morphine CPP, suggesting that gliosis is a critical mediator of the effect (111). Supporting the notion that a TBI-primed response to drug can influence subsequent drug seeking, the steroidal anti-inflammatory drug dexamethasone reduced the TBI-associated elevation in cocaine CPP without affecting CPP in noninjured animals (43). Similarly, the glial modulator minocycline reduced TBI-associated increase in voluntary alcohol consumption but had no effect in uninjured animals (40). Complementing these studies that demonstrate the necessity of neuroimmune signaling for drug seeking, intra-VTA injection of the TLR4 agonist lipopolysaccharide was found to be sufficient to reinstate cocaine seeking (110).
Stress is the most-reported trigger of drug craving for several drugs of abuse (112). Stress triggers a neuroimmune response and has been proposed to act as a potential trigger for microglial priming (113,114). In mice, early-life stress increased central immune responses to cocaine (115), suggesting that stress-associated microglial priming is relevant to the immunologic response to drugs of abuse. Preclinical studies support the notion that stress-induced immune responses are important in drug seeking and craving: ibudilast blocked stress-induced reinstatement of methamphetamine seeking (116).
Exposure to drug cues (e.g., places and things associated with substance use) is also a powerful trigger of drug craving (117), and there is evidence of conditioned immunologic effects of drug-associated cues. Exposure to cocaine-associated cues elevated plasma tumor necrosis factor α (118), and heroin seeking evoked by exposure to the drug environment was suppressed by the non-opioid TLR4 antagonist (+)-naltrexone (119).
Chronic Effects: Changes in Function of Brain Networks.
Preclinical studies have identified TBI-associated increased network excitability in the cortex that emerges over time, and increased excitatory and decreased inhibitory synaptic inputs have been identified as putative mechanisms of increased excitability of pyramidal neurons (120–122). A rodent model of comorbid TBI and oxycodone abuse identified interactive effects of TBI and drug exposure on increasing widespread connectivity (123), a phenomenon associated with worse TBI outcomes (124,125) and abstinence from prior heroin use (126). The PFC is a region that is highly vulnerable to injury in TBI (127), and the same study found that structural and functional outcomes in the PFC correlated with drug seeking (123).
DISCUSSION
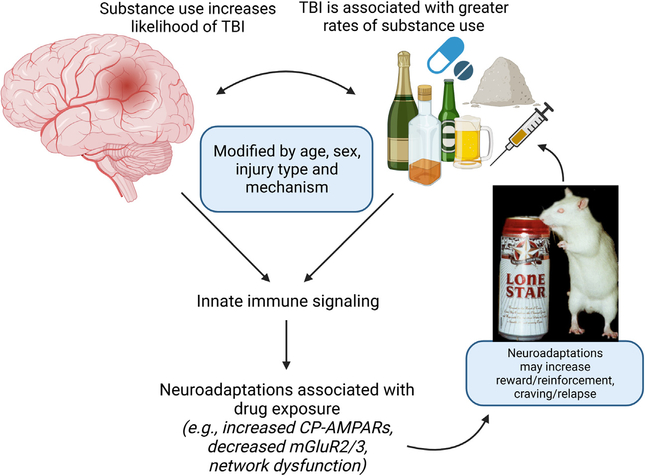
The relationship between TBI and risky substance use is difficult to study, and there is evidence for each to increase the incidence of the other (Figure 1). We have summarized human and animal studies that reflect on the question, does TBI cause risky substance use or SUD? However, there is not sufficient evidence to definitively conclude that TBI can cause such use. In general, preclinical studies outnumber human investigations. Both human and animal studies have identified an association between TBI and risky substance use, although the strength of this association varies. This variability may imply a weak signal or may be due to methodological limitations.
Figure 1.
Theoretical framework of the relationship between TBI and substance use. CP-AMPARs, Ca2+-permeable AMPA receptors; mGluR2/3, metabotropic glutamate 2/3 receptors; TBI, traumatic brain injury.
A weak signal also may be due to specific characteristics that modify the presence or strength of relationship. For instance, the relationship between TBI and substance use may vary by the substance studied. Human and animal studies have been almost exclusively about alcohol, although recent studies include other substances. Particularly pertinent may be the sex of the organism, as well as the developmental stage when injury and/or substances are introduced. There are both human and animal studies that suggest that TBI in adolescence shows a greater association to substance use proclivity. It should be noted that prior substance use itself may alter the ability of TBI to increase subsequent risky drug use. A plethora of human studies indicate that pre-injury misuse of substances increases the likelihood of substance misuse after. Similarly, male (but not female) rats with a history of alcohol drinking were found to increase intake following injury (128–130).
Ruling out confounding effects has been more difficult. Human studies controlling for parental and household factors suggest that these factors may not be a source of confound. A human population study using uninjured sibling control subjects to specifically address risky substance use would be a useful addition to these studies. It has been more difficult to rule out personality traits that may be sources of confound. Risk taking, conduct disorders, and childhood traumatization have all been posited. While animal studies could be designed to test such factors, we are not aware of any that have examined these factors in relationship to the effects of TBI on drug reward or seeking.
Finally, there are several plausible mechanisms of action for TBI to cause a predisposition for risky substance use. Acute and chronic effects of TBI can result in increased CP-AMPARs and decreased mGluR2/3 expression, hallmarks of prior drug exposure. Emerging evidence suggests that microglial priming is a strong candidate for TBI to alter responses to substances in a way that promotes future use. Although research on the immunologic interactions between TBI and drug use is still in its infancy, the therapeutic potential of neuroimmune modulation for the treatment of risky substance use (independent of brain injury) is under investigation (131,132). Another plausible mechanism is by altering the function of brain networks, especially those involving the PFC. The PFC is particularly vulnerable to TBI, and clinical and preclinical studies identify it as a key node in drug craving and seeking (133–135).
The question of whether TBI can cause risky substance use is important for human health: knowledge of prior TBI may be used to guide personalized substance use treatment (136), and there is evidence that prior TBI may change the therapeutic approach for SUD treatment in individuals with comorbid TBI [e.g., dexamethasone reduced cocaine CPP only in TBI animals (43)]. To the extent that childhood TBI may predispose to adult risky substance use, secondary prevention to reduce the likelihood of that outcome could become a target for future research, not unlike adverse childhood experience (137). Future research would benefit from population studies specifically designed to address this question, as well as preclinical studies to test potential therapeutics for substance use in animals with and without brain injuries.
Supplementary Material
ACKNOWLEDGMENTS AND DISCLOSURES
This work was supported by the United States Department of Veteran Affairs (Grant No. RX002931 [to CMO]); National Institutes of Health (Grant No. DA042792 [to CMO]); and the National Institute on Disability, Independent Living, and Rehabilitation Research (Grant No. 90DP0040 [to JDC]).
National Institute on Disability, Independent Living, and Rehabilitation Research is a Center within the Administration for Community Living (ACL), Department of Health and Human Services. The contents of this publication do not necessarily represent the policy of the United States Department of Veteran Affairs; National Institute on Disability, Independent Living, and Rehabilitation Research; Administration for Community Living; National Institutes of Health; or Department of Health and Human Services, and you should not assume endorsement by the Federal Government.
We thank Cole Vonder Haar, Ph.D., and Antje Kroner-Milsch, M.D., Ph.D., for thoughtful comments on prior versions of this manuscript.
Figure 1 was created with BioRender.com.
Footnotes
The authors report no biomedical financial interests or potential conflicts of interest.
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.biopsych.2021.07.013.
Contributor Information
Christopher M. Olsen, Department of Pharmacology and Toxicology, Neuroscience Research Center, Department of Neurosurgery, Medical College of Wisconsin, Milwaukee, Wisconsin
John D. Corrigan, Department of Physical Medicine & Rehabilitation, Wexner Medical Center, The Ohio State University, Columbus, Ohio
REFERENCES
- 1.Bjork JM, Grant SJ (2009): Does traumatic brain injury increase risk for substance abuse? J Neurotrauma 26:1077–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Graham DP, Cardon AL (2008): An update on substance use and treatment following traumatic brain injury. Ann N Y Acad Sci 1141:148–162. [DOI] [PubMed] [Google Scholar]
- 3.Parry-Jones BL, Vaughan FL, Miles Cox W (2006): Traumatic brain injury and substance misuse: A systematic review of prevalence and outcomes research (1994–2004). Neuropsychol Rehabil 16:537–560. [DOI] [PubMed] [Google Scholar]
- 4.Rogers JM, Read CA (2007): Psychiatric comorbidity following traumatic brain injury. Brain Inj 21:1321–1333. [DOI] [PubMed] [Google Scholar]
- 5.Institute of Medicine (2009): Gulf War and Health: Volume 7: Long-Term Consequences of Traumatic Brain Injury. Washington, DC: The National Academies Press. [PubMed] [Google Scholar]
- 6.Ponsford J, Alway Y, Gould KR (2018): Epidemiology and natural history of psychiatric disorders after TBI. J Neuropsychiatry Clin Neurosci 30:262–270. [DOI] [PubMed] [Google Scholar]
- 7.Macdonald S, Anglin-Bodrug K, Mann RE, Erickson P, Hathaway A, Chipman M, Rylett M (2003): Injury risk associated with cannabis and cocaine use. Drug Alcohol Depend 72:99–115. [DOI] [PubMed] [Google Scholar]
- 8.Chen CM, Yi HY, Yoon YH, Dong C (2012): Alcohol use at time of injury and survival following traumatic brain injury: Results from the National Trauma Data Bank. J Stud Alcohol Drugs 73:531–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Savola O, Niemelä O, Hillbom M (2005): Alcohol intake and the pattern of trauma in young adults and working aged people admitted after trauma. Alcohol 40:269–273. [DOI] [PubMed] [Google Scholar]
- 10.Kreutzer JS, Wltol AD, Sander AM, Cifn DX, Martvitz JH, Delmonico R (1996): A prospective longitudinal multicenter analysis of alcohol use patterns among persons with traumatic brain injury. J Head Trauma Rehabil 11:58–69. [Google Scholar]
- 11.Adams RS, Ketchum JM, Nakase-Richardson R, Katz DI, Corrigan JD (2021): Prevalence of drinking within low-risk guidelines during the first 2 years after inpatient rehabilitation for moderate or severe traumatic brain injury. Am J Phys Med Rehabil 100:815–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corrigan JD, Cuthbert JP, Harrison-Felix C, Whiteneck GG, Bell JM, Miller AC, et al. (2014): US population estimates of health and social outcomes 5 years after rehabilitation for traumatic brain injury. J Head Trauma Rehabil 29:E1–E9. [DOI] [PubMed] [Google Scholar]
- 13.Awan N, DiSanto D, Juengst SB, Kumar RG, Bertisch H, Niemeier J, et al. (2020): Interrelationships between post-TBI employment and substance abuse: A cross-lagged structural equation modeling analysis. Arch Phys Med Rehabil 101:797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corrigan J, Adams R, Dams-O’Conner K (2021): At-risk substance use and substance use disorders. In: Zasler ND, Katz DI, Zafonte RD, editors. Brain Injury Medicine: Principles and Practice, 3rd ed. New York: Springer Publishing Company, LLC, 1241–1251. [Google Scholar]
- 15.Cannella LA, McGary H, Ramirez SH (2019): Brain interrupted: Early life traumatic brain injury and addiction vulnerability. Exp Neurol 317:191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corrigan JD, Bogner J, Holloman C (2012): Lifetime history of traumatic brain injury among persons with substance use disorders. Brain Inj 26:139–150. [DOI] [PubMed] [Google Scholar]
- 17.McKinlay A, Grace RC, Horwood LJ, Fergusson DM, Ridder EM, MacFarlane MR (2008): Prevalence of traumatic brain injury among children, adolescents and young adults: Prospective evidence from a birth cohort. Brain Inj 22:175–181. [DOI] [PubMed] [Google Scholar]
- 18.Weil ZM, Karelina K, Corrigan JD (2019): Does pediatric traumatic brain injury cause adult alcohol misuse: Combining preclinical and epidemiological approaches. Exp Neurol 317:284–290. [DOI] [PubMed] [Google Scholar]
- 19.Winqvist S, Jokelainen J, Luukinen H, Hillbom M (2006): Adolescents’ drinking habits predict later occurrence of traumatic brain injury: 35-year follow-up of the northern Finland 1966 birth cohort. J Adolesc Health 39:275.e1–275.e7. [DOI] [PubMed] [Google Scholar]
- 20.Corrigan JD, Bogner J, Mellick D, Bushnik T, Dams-O’Connor K, Hammond FM, et al. (2013): Prior history of traumatic brain injury among persons in the Traumatic Brain Injury Model Systems National Database. Arch Phys Med Rehabil 94:1940–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dams-O’Connor K, Spielman L, Singh A, Gordon WA, Lingsma HF, Maas AI, et al. (2013): The impact of previous traumatic brain injury on health and functioning: A TRACK-TBI study. J Neurotrauma 30:2014–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adams RS, Campbell-Sills L, Stein MB, Sun X, Larson MJ, Kessler RC, et al. (2020): The association of lifetime and deployment-acquired traumatic brain injury with postdeployment binge and heavy drinking. J Head Trauma Rehabil 35:27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silver JM, Kramer R, Greenwald S, Weissman M (2001): The association between head injuries and psychiatric disorders: Findings from the New Haven NIMH Epidemiologic Catchment Area Study. Brain Inj 15:935–945. [DOI] [PubMed] [Google Scholar]
- 24.Anstey KJ, Butterworth P, Jorm AF, Christensen H, Rodgers B, Windsor TD (2004): A population survey found an association between self-reports of traumatic brain injury and increased psychiatric symptoms. J Clin Epidemiol 57:1202–1209. [DOI] [PubMed] [Google Scholar]
- 25.Ilie G, Adlaf EM, Mann RE, Ialomiteanu A, Hamilton H, Rehm J, et al. (2015): Associations between a history of traumatic brain injuries and current cigarette smoking, substance use, and elevated psychological distress in a population sample of Canadian adults. J Neurotrauma 32:1130–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ilie G, Wickens CM, Ialomiteanu A, Adlaf EM, Asbridge M, Hamilton H, et al. (2019): Traumatic brain injury and hazardous/harmful drinking: Concurrent and single associations with poor mental health and roadway aggression. Psychiatry Res 272:458–466. [DOI] [PubMed] [Google Scholar]
- 27.Whiteneck GG, Cuthbert JP, Corrigan JD, Bogner JA (2016): Prevalence of self-reported lifetime history of traumatic brain injury and associated disability: A statewide population-based survey. J Head Trauma Rehabil 31:E55–E62. [DOI] [PubMed] [Google Scholar]
- 28.Bogner J, Corrigan JD, Yi H, Singichetti B, Manchester K, Huang L, Yang J (2020): Lifetime history of traumatic brain injury and behavioral health problems in a population-based sample. J Head Trauma Rehabil 35:E43–E50. [DOI] [PubMed] [Google Scholar]
- 29.Corrigan JD, Hagemeyer AN, Weil ZM, Sullivan L, Shi J, Bogner J, Yang J (2020): Is pediatric traumatic brain injury associated with adult alcohol misuse? J Neurotrauma 37:1637–1644. [DOI] [PubMed] [Google Scholar]
- 30.Waltzman D, Daugherty J, Sarmiento K, Proescholdbell S (2021): Lifetime history of traumatic brain injury with loss of consciousness and the likelihood for lifetime depression and risk behaviors: 2017 BRFSS North Carolina. J Head Trauma Rehabil 36:E40–E49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Winqvist S, Jokelainen J, Luukinen H, Hillbom M (2007): Parental alcohol misuse is a powerful predictor for the risk of traumatic brain injury in childhood. Brain Inj 21:1079–1085. [DOI] [PubMed] [Google Scholar]
- 32.Winqvist S, Luukinen H, Jokelainen J, Lehtilahti M, Näyhä S, Hillbom M (2008): Recurrent traumatic brain injury is predicted by the index injury occurring under the influence of alcohol. Brain Inj 22:780–785. [DOI] [PubMed] [Google Scholar]
- 33.Timonen M, Miettunen J, Hakko H, Zitting P, Veijola J, von Wendt L, Räsänen P (2002): The association of preceding traumatic brain injury with mental disorders, alcoholism and criminality: The Northern Finland 1966 Birth Cohort Study. Psychiatry Res 113:217–226. [DOI] [PubMed] [Google Scholar]
- 34.Kennedy E, Heron J, Munafò M (2017): Substance use, criminal behaviour and psychiatric symptoms following childhood traumatic brain injury: Findings from the ALSPAC cohort. Eur Child Adolesc Psychiatry 26:1197–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McKinlay A, Grace R, Horwood J, Fergusson D, MacFarlane M (2009): Adolescent psychiatric symptoms following preschool childhood mild traumatic brain injury: Evidence from a birth cohort. J Head Trauma Rehabil 24:221–227. [DOI] [PubMed] [Google Scholar]
- 36.Poznanski P, Lesniak A, Korostynski M, Sacharczuk M (2020): Ethanol consumption following mild traumatic brain injury is related to blood-brain barrier permeability. Addict Biol 25:e12683. [DOI] [PubMed] [Google Scholar]
- 37.Lowing JL, Susick LL, Caruso JP, Provenzano AM, Raghupathi R, Conti AC (2014): Experimental traumatic brain injury alters ethanol consumption and sensitivity. J Neurotrauma 31:1700–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lim YW, Meyer NP, Shah AS, Budde MD, Stemper BD, Olsen CM (2015): Voluntary alcohol intake following blast exposure in a rat model of mild traumatic brain injury. PLoS One 10:e0125130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schindler AG, Baskin B, Juarez B, Janet Lee S, Hendrickson R, Pagulayan K, et al. (2021): Repetitive blast mild traumatic brain injury increases ethanol sensitivity in male mice and risky drinking behavior in male combat veterans. Alcohol Clin Exp Res 45:1051–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karelina K, Nicholson S, Weil ZM (2018): Minocycline blocks traumatic brain injury-induced alcohol consumption and nucleus accumbens inflammation in adolescent male mice. Brain Behav Immun 69:532–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weil ZM, Karelina K, Gaier KR, Corrigan TE, Corrigan JD (2016): Juvenile traumatic brain injury increases alcohol consumption and reward in female mice. J Neurotrauma 33:895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Merkel SF, Razmpour R, Lutton EM, Tallarida CS, Heldt NA, Cannella LA, et al. (2017): Adolescent traumatic brain injury induces chronic mesolimbic neuroinflammation with concurrent enhancement in the rewarding effects of cocaine in mice during adulthood. J Neurotrauma 34:165–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Merkel SF, Andrews AM, Lutton EM, Razmpour R, Cannella LA, Ramirez SH (2017): Dexamethasone attenuates the enhanced rewarding effects of cocaine following experimental traumatic brain injury. Cell Transplant 26:1178–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cannella LA, Andrews AM, Tran F, Razmpour R, McGary H, Collie C, et al. (2020): Experimental traumatic brain injury during adolescence enhances cocaine rewarding efficacy and dysregulates dopamine and neuroimmune systems in brain reward substrates. J Neurotrauma 37:27–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cannella LA, Andrews AM, Razmpour R, McGary H, Corbett CB, Kahn J, Ramirez SH (2020): Reward and immune responses in adolescent females following experimental traumatic brain injury. Behav Brain Res 379:112333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vonder Haar C, Ferland JN, Kaur S, Riparip LK, Rosi S, Winstanley CA (2019): Cocaine self-administration is increased after frontal traumatic brain injury and associated with neuroinflammation. Eur J Neurosci 50:2134–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muelbl MJ, Slaker ML, Shah AS, Nawarawong NN, Gerndt CH, Budde MD, et al. (2018): Effects of mild blast traumatic brain injury on cognitive- and addiction-related behaviors. Sci Rep 8:9941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nawarawong NN, Slaker M, Muelbl M, Shah AS, Chiariello R, Nelson LD, et al. (2019): Repeated blast model of mild traumatic brain injury alters oxycodone self-administration and drug seeking. Eur J Neurosci 50:2101–2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ferland JN, Winstanley CA (2017): Risk-preferring rats make worse decisions and show increased incubation of craving after cocaine self-administration. Addict Biol 22:991–1001. [DOI] [PubMed] [Google Scholar]
- 50.Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ (2008): High impulsivity predicts the switch to compulsive cocaine-taking. Science 320:1352–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cervantes MC, Laughlin RE, Jentsch JD (2013): Cocaine self-administration behavior in inbred mouse lines segregating different capacities for inhibitory control. Psychopharmacology (Berl) 229:515–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Levis SC, Bentzley BS, Molet J, Bolton JL, Perrone CR, Baram TZ, Mahler SV (2019): On the early life origins of vulnerability to opioid addiction [published online ahead of print Dec 10]. Mol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ordoñes Sanchez E, Bavley CC, Deutschmann AU, Carpenter R, Peterson DR, Karbalaei R, et al. (2021): Early life adversity promotes resilience to opioid addiction-related phenotypes in male rats and sex-specific transcriptional changes. Proc Natl Acad Sci U S A 118: e2020173118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Giza CC, Hovda DA (2014): The new neurometabolic cascade of concussion. Neurosurgery 75(suppl 4):S24–S33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kumar A, Loane DJ (2012): Neuroinflammation after traumatic brain injury: Opportunities for therapeutic intervention. Brain Behav Immun 26:1191–1201. [DOI] [PubMed] [Google Scholar]
- 56.Pearn ML, Niesman IR, Egawa J, Sawada A, Almenar-Queralt A, Shah SB, et al. (2017): Pathophysiology associated with traumatic brain injury: Current treatments and potential novel therapeutics. Cell Mol Neurobiol 37:571–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Simon DW, McGeachy MJ, Bayır H, Clark RS, Loane DJ, Kochanek PM (2017): The far-reaching scope of neuroinflammation after traumatic brain injury. Nat Rev Neurol 13:171–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kilinc D, Gallo G, Barbee KA (2008): Mechanically induced membrane poration causes axonal beading and localized cytoskeletal damage. Exp Neurol 212:422–430. [DOI] [PubMed] [Google Scholar]
- 59.Farkas O, Lifshitz J, Povlishock JT (2006): Mechanoporation induced by diffuse traumatic brain injury: An irreversible or reversible response to injury? J Neurosci 26:3130–3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wolf JA, Stys PK, Lusardi T, Meaney D, Smith DH (2001): Traumatic axonal injury induces calcium influx modulated by tetrodotoxin-sensitive sodium channels. J Neurosci 21:1923–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Corps KN, Roth TL, McGavern DB (2015): Inflammation and neuroprotection in traumatic brain injury. JAMA Neurol 72:355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Korgaonkar AA, Li Y, Sekhar D, Subramanian D, Guevarra J, Swietek B, et al. (2020): Toll-like receptor 4 signaling in neurons enhances calcium-permeable alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor currents and drives post-traumatic epileptogenesis. Ann Neurol 87:497–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Spaethling JM, Klein DM, Singh P, Meaney DF (2008): Calcium-permeable AMPA receptors appear in cortical neurons after traumatic mechanical injury and contribute to neuronal fate. J Neurotrauma 25:1207–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bell JD, Park E, Ai J, Baker AJ (2009): PICK1-mediated GluR2 endocytosis contributes to cellular injury after neuronal trauma. Cell Death Differ 16:1665–1680. [DOI] [PubMed] [Google Scholar]
- 65.Lüscher C (2016): The emergence of a circuit model for addiction. Annu Rev Neurosci 39:257–276. [DOI] [PubMed] [Google Scholar]
- 66.Wolf ME (2016): Synaptic mechanisms underlying persistent cocaine craving. Nat Rev Neurosci 17:351–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Loweth JA, Tseng KY, Wolf ME (2013): Using metabotropic glutamate receptors to modulate cocaine’s synaptic and behavioral effects: mGluR1 finds a niche. Curr Opin Neurobiol 23:500–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pascoli V, Terrier J, Espallergues J, Valjent E, O’Connor EC, Lüscher C (2014): Contrasting forms of cocaine-evoked plasticity control components of relapse. Nature 509:459–464. [DOI] [PubMed] [Google Scholar]
- 69.Vezzani A, Viviani B (2015): Neuromodulatory properties of inflammatory cytokines and their impact on neuronal excitability. Neuropharmacology 96:70–82. [DOI] [PubMed] [Google Scholar]
- 70.Zhu G, Okada M, Yoshida S, Mori F, Ueno S, Wakabayashi K, Kaneko S (2006): Effects of interleukin-1beta on hippocampal glutamate and GABA releases associated with Ca2+-induced Ca2+ releasing systems. Epilepsy Res 71:107–116. [DOI] [PubMed] [Google Scholar]
- 71.Vereyken EJ, Bajova H, Chow S, de Graan PN, Gruol DL (2007): Chronic interleukin-6 alters the level of synaptic proteins in hippocampus in culture and in vivo. Eur J Neurosci 25:3605–3616. [DOI] [PubMed] [Google Scholar]
- 72.Kasanetz F, Lafourcade M, Deroche-Gamonet V, Revest JM, Berson N, Balado E, et al. (2013): Prefrontal synaptic markers of cocaine addiction-like behavior in rats. Mol Psychiatry 18:729–737. [DOI] [PubMed] [Google Scholar]
- 73.Lu L, Uejima JL, Gray SM, Bossert JM, Shaham Y (2007): Systemic and central amygdala injections of the mGluR(2/3) agonist LY379268 attenuate the expression of incubation of cocaine craving. Biol Psychiatry 61:591–598. [DOI] [PubMed] [Google Scholar]
- 74.Hao Y, Martin-Fardon R, Weiss F (2010): Behavioral and functional evidence of metabotropic glutamate receptor 2/3 and metabotropic glutamate receptor 5 dysregulation in cocaine-escalated rats: Factor in the transition to dependence. Biol Psychiatry 68:240–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moussawi K, Kalivas PW (2010): Group II metabotropic glutamate receptors (mGlu2/3) in drug addiction. Eur J Pharmacol 639:115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Caprioli D, Justinova Z, Venniro M, Shaham Y (2018): Effect of novel allosteric modulators of metabotropic glutamate receptors on drug self-administration and relapse: A review of preclinical studies and their clinical implications. Biol Psychiatry 84:180–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stellwagen D, Beattie EC, Seo JY, Malenka RC (2005): Differential regulation of AMPA receptor and GABA receptor trafficking by tumor necrosis factor-alpha. J Neurosci 25:3219–3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vezzani A, French J, Bartfai T, Baram TZ (2011): The role of inflammation in epilepsy. Nat Rev Neurol 7:31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vezzani A, Maroso M, Balosso S, Sanchez MA, Bartfai T (2011): IL-1 receptor/toll-like receptor signaling in infection, inflammation, stress and neurodegeneration couples hyperexcitability and seizures. Brain Behav Immun 25:1281–1289. [DOI] [PubMed] [Google Scholar]
- 80.Galic MA, Riazi K, Pittman QJ (2012): Cytokines and brain excitability. Front Neuroendocrinol 33:116–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Frey LC (2003): Epidemiology of posttraumatic epilepsy: A critical review. Epilepsia 44:11–17. [DOI] [PubMed] [Google Scholar]
- 82.Bruns J Jr, Hauser WA (2003): The epidemiology of traumatic brain injury: A review. Epilepsia 44:2–10. [DOI] [PubMed] [Google Scholar]
- 83.Marshall SA, McClain JA, Kelso ML, Hopkins DM, Pauly JR, Nixon K (2013): Microglial activation is not equivalent to neuroinflammation in alcohol-induced neurodegeneration: The importance of microglia phenotype. Neurobiol Dis 54:239–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Calipari ES, Godino A, Peck EG, Salery M, Mervosh NL, Landry JA, et al. (2018): Granulocyte-colony stimulating factor controls neural and behavioral plasticity in response to cocaine. Nat Commun 9:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kashima DT, Grueter BA (2017): Toll-like receptor 4 deficiency alters nucleus accumbens synaptic physiology and drug reward behavior. Proc Natl Acad Sci U S A 114:8865–8870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang X, Northcutt AL, Cochran TA, Zhang X, Fabisiak TJ, Haas ME, et al. (2019): Methamphetamine activates toll-like receptor 4 to induce central immune signaling within the ventral tegmental area and contributes to extracellular dopamine increase in the nucleus accumbens shell. ACS Chem Neurosci 10:3622–3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schwarz JM, Smith SH, Bilbo SD (2013): FACS analysis of neuronalglial interactions in the nucleus accumbens following morphine administration. Psychopharmacology (Berl) 230:525–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hutchinson MR, Bland ST, Johnson KW, Rice KC, Maier SF, Watkins LR (2007): Opioid-induced glial activation: Mechanisms of activation and implications for opioid analgesia, dependence, and reward. ScientificWorldJournal 7:98–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Crews FT, Nixon K (2009): Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol 44:115–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Krasnova IN, Cadet JL (2009): Methamphetamine toxicity and messengers of death. Brain Res Rev 60:379–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sekine Y, Ouchi Y, Sugihara G, Takei N, Yoshikawa E, Nakamura K, et al. (2008): Methamphetamine causes microglial activation in the brains of human abusers. J Neurosci 28:5756–5761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Erickson EK, Grantham EK, Warden AS, Harris RA (2019): Neuroimmune signaling in alcohol use disorder. Pharmacol Biochem Behav 177:34–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Crews FT, Lawrimore CJ, Walter TJ, Coleman LG Jr (2017): The role of neuroimmune signaling in alcoholism. Neuropharmacology 122:56–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang Y, Liang Y, Levran O, Randesi M, Yuferov V, Zhao C, Kreek MJ (2017): Alterations of expression of inflammation/immune-related genes in the dorsal and ventral striatum of adult C57BL/6J mice following chronic oxycodone self-administration: A RNA sequencing study. Psychopharmacology (Berl) 234:2259–2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Witcher KG, Eiferman DS, Godbout JP (2015): Priming the inflammatory pump of the CNS after traumatic brain injury. Trends Neurosci 38:609–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Block ML, Zecca L, Hong JS (2007): Microglia-mediated neurotoxicity: Uncovering the molecular mechanisms. Nat Rev Neurosci 8:57–69. [DOI] [PubMed] [Google Scholar]
- 97.Loane DJ, Kumar A (2016): Microglia in the TBI brain: The good, the bad, and the dysregulated. Exp Neurol 275:316–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Green-Fulgham SM, Ball JB, Kwilasz AJ, Fabisiak T, Maier SF, Watkins LR, Grace PM (2019): Oxycodone, fentanyl, and morphine amplify established neuropathic pain in male rats. Pain 160:2634–2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ellis A, Grace PM, Wieseler J, Favret J, Springer K, Skarda B, et al. (2016): Morphine amplifies mechanical allodynia via TLR4 in a rat model of spinal cord injury. Brain Behav Immun 58:348–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Grace PM, Strand KA, Galer EL, Urban DJ, Wang X, Baratta MV, et al. (2016): Morphine paradoxically prolongs neuropathic pain in rats by amplifying spinal NLRP3 inflammasome activation. Proc Natl Acad Sci U S A 113:E3441–E3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Crews FT, Zou J, Qin L (2011): Induction of innate immune genes in brain create the neurobiology of addiction. Brain Behav Immun 25(suppl 1):S4–S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mantsch JR, Baker DA, Funk D, Lê AD, Shaham Y (2016): Stress-induced reinstatement of drug seeking: 20 years of progress. Neuropsychopharmacology 41:335–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Farrell MR, Schoch H, Mahler SV (2018): Modeling cocaine relapse in rodents: Behavioral considerations and circuit mechanisms. Prog Neuropsychopharmacol Biol Psychiatry 87:33–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Peck JA, Ranaldi R (2014): Drug abstinence: Exploring animal models and behavioral treatment strategies. Psychopharmacology (Berl) 231:2045–2058. [DOI] [PubMed] [Google Scholar]
- 105.Shaham Y, Shalev U, Lu L, de Wit H, Stewart J (2003): The reinstatement model of drug relapse: History, methodology and major findings. Psychopharmacology (Berl) 168:3–20. [DOI] [PubMed] [Google Scholar]
- 106.Richtand NM, Ahlbrand R, Horn PS, Chambers B, Davis J, Benoit S (2012): Effects of prenatal immune activation and peri-adolescent stress on amphetamine-induced conditioned place preference in the rat. Psychopharmacology (Berl) 222:313–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Borçoi AR, Patti CL, Zanin KA, Hollais AW, Santos-Baldaia R, Ceccon LM, et al. (2015): Effects of prenatal immune activation on amphetamine-induced addictive behaviors: Contributions from animal models. Prog Neuropsychopharmacol Biol Psychiatry 63: 63–69. [DOI] [PubMed] [Google Scholar]
- 108.Randall PA, Vetreno RP, Makhijani VH, Crews FT, Besheer J (2019): The toll-like receptor 3 agonist poly(I:C) induces rapid and lasting changes in gene expression related to glutamatergic function and increases ethanol self-administration in rats. Alcohol Clin Exp Res 43:48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Warden AS, Azzam M, DaCosta A, Mason S, Blednov YA, Messing RO, et al. (2019): Toll-like receptor 3 activation increases voluntary alcohol intake in C57BL/6J male mice. Brain Behav Immun 77:55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Brown KT, Levis SC, O’Neill CE, Northcutt AL, Fabisiak TJ, Watkins LR, Bachtell RK (2018): Innate immune signaling in the ventral tegmental area contributes to drug-primed reinstatement of cocaine seeking. Brain Behav Immun 67:130–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schwarz JM, Bilbo SD (2013): Adolescent morphine exposure affects long-term microglial function and later-life relapse liability in a model of addiction. J Neurosci 33:961–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sinha R (2001): How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berl) 158:343–359. [DOI] [PubMed] [Google Scholar]
- 113.Frank MG, Weber MD, Watkins LR, Maier SF (2015): Stress sounds the alarmin: The role of the danger-associated molecular pattern HMGB1 in stress-induced neuroinflammatory priming. Brain Behav Immun 48:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Niraula A, Sheridan JF, Godbout JP (2017): Microglia priming with aging and stress. Neuropsychopharmacology 42:318–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lo Iacono L, Catale C, Martini A, Valzania A, Viscomi MT, Chiurchiù V, et al. (2018): From traumatic childhood to cocaine abuse: The critical function of the immune system. Biol Psychiatry 84:905–916. [DOI] [PubMed] [Google Scholar]
- 116.Beardsley PM, Shelton KL, Hendrick E, Johnson KW (2010): The glial cell modulator and phosphodiesterase inhibitor, AV411 (ibudilast), attenuates prime- and stress-induced methamphetamine relapse. Eur J Pharmacol 637:102–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shulman GD (1989): Experience with the cocaine trigger inventory. Adv Alcohol Subst Abuse 8:71–85. [DOI] [PubMed] [Google Scholar]
- 118.Kubera M, Filip M, Budziszewska B, Basta-Kaim A, Wydra K, Leskiewicz M, et al. (2008): Immunosuppression induced by a conditioned stimulus associated with cocaine self-administration. J Pharmacol Sci 107:361–369. [DOI] [PubMed] [Google Scholar]
- 119.Theberge FR, Li X, Kambhampati S, Pickens CL, St Laurent R, Bossert JM, et al. (2013): Effect of chronic delivery of the toll-like receptor 4 antagonist (+)-naltrexone on incubation of heroin craving. Biol Psychiatry 73:729–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hånell A, Greer JE, Jacobs KM (2015): Increased network excitability due to altered synaptic inputs to neocortical layer V intact and axotomized pyramidal neurons after mild traumatic brain injury. J Neurotrauma 32:1590–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Carron SF, Alwis DS, Rajan R (2016): Traumatic brain injury and neuronal functionality changes in sensory cortex. Front Syst Neurosci 10:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Smith CJ, Xiong G, Elkind JA, Putnam B, Cohen AS (2015): Brain injury impairs working memory and prefrontal circuit function. Front Neurol 6:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Muelbl MJ, Glaeser B, Shah AS, Chiariello R, Nawarawong NN, Stemper BD, et al. (2021): Repeated blast mild traumatic brain injury and oxycodone self-administration produce interactive effects on neuroimaging outcomes. bioRxiv. 10.1101/2020.11.18.388421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hayes JP, Bigler ED, Verfaellie M (2016): Traumatic brain injury as a disorder of brain connectivity. J Int Neuropsychol Soc 22:120–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sharp DJ, Scott G, Leech R (2014): Network dysfunction after traumatic brain injury. Nat Rev Neurol 10:156–166. [DOI] [PubMed] [Google Scholar]
- 126.Sun Y, Wang GB, Lin QX, Lu L, Shu N, Meng SQ, et al. (2017): Disrupted white matter structural connectivity in heroin abusers. Addict Biol 22:184–195. [DOI] [PubMed] [Google Scholar]
- 127.Eierud C, Craddock RC, Fletcher S, Aulakh M, King-Casas B, Kuehl D, LaConte SM (2014): Neuroimaging after mild traumatic brain injury: Review and meta-analysis. Neuroimage Clin 4:283–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Stielper ZF, Fucich EA, Middleton JW, Hillard CJ, Edwards S, Molina PE, Gilpin NW (2021): Traumatic brain injury and alcohol drinking alter basolateral amygdala endocannabinoids in female rats. J Neurotrauma 38:422–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fucich EA, Mayeux JP, McGinn MA, Gilpin NW, Edwards S, Molina PE (2019): A novel role for the endocannabinoid system in ameliorating motivation for alcohol drinking and negative behavioral affect after traumatic brain injury in rats. J Neurotrauma 36:1847–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mayeux JP, Teng SX, Katz PS, Gilpin NW, Molina PE (2015): Traumatic brain injury induces neuroinflammation and neuronal degeneration that is associated with escalated alcohol self-administration in rats. Behav Brain Res 279:22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bachtell RK, Jones JD, Heinzerling KG, Beardsley PM, Comer SD (2017): Glial and neuroinflammatory targets for treating substance use disorders. Drug Alcohol Depend 180:156–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bachtell R, Hutchinson MR, Wang X, Rice KC, Maier SF, Watkins LR (2015): Targeting the toll of drug abuse: The translational potential of toll-like receptor 4. CNS Neurol Disord Drug Targets 14:692–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Koob GF, Volkow ND (2016): Neurobiology of addiction: A neuro-circuitry analysis. Lancet Psychiatry 3:760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Reiner DJ, Fredriksson I, Lofaro OM, Bossert JM, Shaham Y (2019): Relapse to opioid seeking in rat models: Behavior, pharmacology and circuits. Neuropsychopharmacology 44:465–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kalivas PW, O’Brien C (2008): Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology 33:166–180. [DOI] [PubMed] [Google Scholar]
- 136.Corrigan JD (2021): Traumatic brain injury and treatment of behavioral health conditions [published online ahead of print Feb 24]. Psychiatr Serv. [DOI] [PubMed] [Google Scholar]
- 137.Purewal Boparai SK, Au V, Koita K, Oh DL, Briner S, Burke Harris N, Bucci M (2018): Ameliorating the biological impacts of childhood adversity: A review of intervention programs. Child Abuse Negl 81:82–105. [DOI] [PubMed] [Google Scholar]
- 138.McKinlay A, Corrigan J, Horwood LJ, Fergusson DM (2014): Substance abuse and criminal activities following traumatic brain injury in childhood, adolescence, and early adulthood. J Head Trauma Rehabil 29:498–506. [DOI] [PubMed] [Google Scholar]
- 139.Hearing M (2019): Prefrontal-accumbens opioid plasticity: Implications for relapse and dependence. Pharmacol Res 139:158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Marinelli M, Rudick CN, Hu XT, White FJ (2006): Excitability of dopamine neurons: Modulation and physiological consequences. CNS Neurol Disord Drug Targets 5:79–97. [DOI] [PubMed] [Google Scholar]
- 141.Beattie MS, Ferguson AR, Bresnahan JC (2010): AMPA-receptor trafficking and injury-induced cell death. Eur J Neurosci 32:290–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kigerl KA, de Rivero Vaccari JP, Dietrich WD, Popovich PG, Keane RW (2014): Pattern recognition receptors and central nervous system repair. Exp Neurol 258:5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Burnstock G, Krügel U, Abbracchio MP, Illes P (2011): Purinergic signalling: From normal behaviour to pathological brain function. Prog Neurobiol 95:229–274. [DOI] [PubMed] [Google Scholar]
- 144.Mattson MP, Camandola S (2001): NF-kappaB in neuronal plasticity and neurodegenerative disorders. J Clin Invest 107:247–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Maroso M, Balosso S, Ravizza T, Liu J, Aronica E, Iyer AM, et al. (2010): Toll-like receptor 4 and high-mobility group box-1 are involved in ictogenesis and can be targeted to reduce seizures. Nat Med 16:413–419. [DOI] [PubMed] [Google Scholar]
- 146.Northcutt AL, Hutchinson MR, Wang X, Baratta MV, Hiranita T, Cochran TA, et al. (2015): DAT isn’t all that: Cocaine reward and reinforcement require toll-like receptor 4 signaling. Mol Psychiatry 20:1525–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Pando-Naude V, Toxto S, Fernandez-Lozano S, Parsons CE, Alcauter S, Garza-Villarreal EA (2021): Gray and white matter morphology in substance use disorders: A neuroimaging systematic review and meta-analysis. Transl Psychiatry 11:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Büki A, Povlishock JT (2006): All roads lead to disconnection?–Traumatic axonal injury revisited. Acta Neurochir (Wien) 148:181–193; discussion 193–194. [DOI] [PubMed] [Google Scholar]
- 149.McGinn MJ, Povlishock JT (2016): Pathophysiology of traumatic brain injury. Neurosurg Clin N Am 27:397–407. [DOI] [PubMed] [Google Scholar]
- 150.Goldstein RZ, Volkow ND (2011): Dysfunction of the prefrontal cortex in addiction: Neuroimaging findings and clinical implications. Nat Rev Neurosci 12:652–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Jasinska AJ, Chen BT, Bonci A, Stein EA (2015): Dorsal medial prefrontal cortex (MPFC) circuitry in rodent models of cocaine use: Implications for drug addiction therapies. Addict Biol 20:215–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Aoki Y, Inokuchi R (2016): A voxel-based meta-analysis of diffusion tensor imaging in mild traumatic brain injury. Neurosci Biobehav Rev 66:119–126. [DOI] [PubMed] [Google Scholar]
- 153.Zhang R, Volkow ND (2019): Brain default-mode network dysfunction in addiction. Neuroimage 200:313–331. [DOI] [PubMed] [Google Scholar]
- 154.Büttner A, Rohrmoser K, Mall G, Penning R, Weis S (2006): Widespread axonal damage in the brain of drug abusers as evidenced by accumulation of beta-amyloid precursor protein (beta-APP): An immunohistochemical investigation. Addiction 101:1339–1346. [DOI] [PubMed] [Google Scholar]
- 155.Shlosberg D, Benifla M, Kaufer D, Friedman A (2010): Blood-brain barrier breakdown as a therapeutic target in traumatic brain injury. Nat Rev Neurol 6:393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chodobski A, Zink BJ, Szmydynger-Chodobska J (2011): Blood-brain barrier pathophysiology in traumatic brain injury. Transl Stroke Res 2:492–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hammad A, Westacott L, Zaben M (2018): The role of the complement system in traumatic brain injury: A review. J Neuroinflammation 15:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Presumey J, Bialas AR, Carroll MC (2017): Complement system in neural synapse elimination in development and disease. Adv Immunol 135:53–79. [DOI] [PubMed] [Google Scholar]
- 159.Lacagnina MJ, Rivera PD, Bilbo SD (2017): Glial and neuroimmune mechanisms as critical modulators of drug use and abuse. Neuropsychopharmacology 42:156–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Williamson LL, Bilbo SD (2013): Chemokines and the hippocampus: A new perspective on hippocampal plasticity and vulnerability. Brain Behav Immun 30:186–194. [DOI] [PubMed] [Google Scholar]
- 161.Trotti D, Danbolt NC, Volterra A (1998): Glutamate transporters are oxidant-vulnerable: A molecular link between oxidative and excitotoxic neurodegeneration? Trends Pharmacol Sci 19:328–334. [DOI] [PubMed] [Google Scholar]
- 162.Beckhauser TF, Francis-Oliveira J, De Pasquale R (2016): Reactive oxygen species: Physiological and physiopathological effects on synaptic plasticity. J Exp Neurosci 10:23–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Nennig SE, Schank JR (2017): The role of NFkB in drug addiction: Beyond inflammation. Alcohol 52:172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Chan YY, Yang SN, Lin JC, Chang JL, Lin JG, Lo WY (2015): Inflammatory response in heroin addicts undergoing methadone maintenance treatment. Psychiatry Res 226:230–234. [DOI] [PubMed] [Google Scholar]
- 165.Araos P, Pedraz M, Serrano A, Lucena M, Barrios V, García-Marchena N, et al. (2015): Plasma profile of pro-inflammatory cytokines and chemokines in cocaine users under outpatient treatment: Influence of cocaine symptom severity and psychiatric co-morbidity. Addict Biol 20:756–772. [DOI] [PubMed] [Google Scholar]
- 166.Bland ST, Beckley JT, Watkins LR, Maier SF, Bilbo SD (2010): Neonatal Escherichia coli infection alters glial, cytokine, and neuronal gene expression in response to acute amphetamine in adolescent rats. Neurosci Lett 474:52–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Linker KE, Cross SJ, Leslie FM (2019): Glial mechanisms underlying substance use disorders. Eur J Neurosci 50:2574–2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Miller AH, Haroon E, Raison CL, Felger JC (2013): Cytokine targets in the brain: Impact on neurotransmitters and neurocircuits. Depress Anxiety 30:297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Santello M, Volterra A (2012): TNFalpha in synaptic function: Switching gears. Trends Neurosci 35:638–647. [DOI] [PubMed] [Google Scholar]
- 170.Merkel SF, Cannella LA, Razmpour R, Lutton E, Raghupathi R, Rawls SM, Ramirez SH (2017): Factors affecting increased risk for substance use disorders following traumatic brain injury: What we can learn from animal models. Neurosci Biobehav Rev 77:209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Volkow ND, Fowler JS, Wang GJ, Swanson JM, Telang F (2007): Dopamine in drug abuse and addiction: Results of imaging studies and treatment implications. Arch Neurol 64:1575–1579. [DOI] [PubMed] [Google Scholar]
- 172.Liddelow SA, Barres BA (2017): Reactive astrocytes: Production, function, and therapeutic potential. Immunity 46:957–967. [DOI] [PubMed] [Google Scholar]
- 173.Takahashi DK, Jin S, Prince DA (2018): Gabapentin prevents progressive increases in excitatory connectivity and epileptogenesis following neocortical trauma. Cereb Cortex 28:2725–2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kutlu MG, Brady LJ, Peck EG, Hofford RS, Yorgason JT, Siciliano CA, et al. (2018): Granulocyte colony stimulating factor enhances reward learning through potentiation of mesolimbic dopamine system function. J Neurosci 38:8845–8859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ferrini F, De Koninck Y (2013): Microglia control neuronal network excitability via BDNF signalling. Neural Plast 2013:429815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wise RA, Robble MA (2020): Dopamine and addiction. Annu Rev Psychol 71:79–106. [DOI] [PubMed] [Google Scholar]
- 177.Willuhn I, Burgeno LM, Groblewski PA, Phillips PE (2014): Excessive cocaine use results from decreased phasic dopamine signaling in the striatum. Nat Neurosci 17:704–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ko D, Wanat MJ (2016): Phasic dopamine transmission reflects initiation vigor and exerted effort in an action- and region-specific manner. J Neurosci 36:2202–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Donnemiller E, Brenneis C, Wissel J, Scherfler C, Poewe W, Riccabona G, Wenning GK (2000): Impaired dopaminergic neurotransmission in patients with traumatic brain injury: A SPECT study using 123I-beta-CIT and 123I-IBZM. Eur J Nucl Med 27:1410–1414. [DOI] [PubMed] [Google Scholar]
- 180.Wagner AK, Scanlon JM, Becker CR, Ritter AC, Niyonkuru C, Dixon CE, et al. (2014): The influence of genetic variants on striatal dopamine transporter and D2 receptor binding after TBI. J Cereb Blood Flow Metab 34:1328–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Fox HC, D’Sa C, Kimmerling A, Siedlarz KM, Tuit KL, Stowe R, Sinha R (2012): Immune system inflammation in cocaine dependent individuals: Implications for medications development. Hum Psychopharmacol 27:156–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Heneka MT, McManus RM, Latz E (2018): Inflammasome signalling in brain function and neurodegenerative disease. Nat Rev Neurosci 19:610–621. [DOI] [PubMed] [Google Scholar]
- 183.Cadet JL, Bisagno V, Milroy CM (2014): Neuropathology of substance use disorders. Acta Neuropathol 127:91–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Egervari G, Akpoyibo D, Rahman T, Fullard JF, Callens JE, Landry JA, et al. (2020): Chromatin accessibility mapping of the striatum identifies tyrosine kinase FYN as a therapeutic target for heroin use disorder. Nat Commun 11:4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kovacs GG, Horvath MC, Majtenyi K, Lutz MI, Hurd YL, Keller E (2015): Heroin abuse exaggerates age-related deposition of hyperphosphorylated tau and p62-positive inclusions. Neurobiol Aging 36:3100–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Flanagan ME, Larson EB, Walker RL, Keene CD, Postupna N, Cholerton B, et al. (2018): Associations between use of specific analgesics and concentrations of amyloid-beta 42 or phospho-tau in regions of human cerebral cortex. J Alzheimers Dis 61:653–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.