Abstract
Introduction
Previous drug survival studies of dupilumab in atopic dermatitis (AD) show that many patients continue treatment through 1 year, suggesting that patients experience clinically relevant benefits with long-term treatment.
Methods
This post hoc analysis included data through week 100 from 391 adult patients from the dupilumab open-label extension (OLE) study who had not achieved the endpoints of at least 75% improvement from baseline in the Eczema Area and Severity Index (EASI-75) or an Investigator’s Global Assessment (IGA) score of 0 or 1 with short-term (16 weeks, 300 mg qw or q2w) dupilumab treatment in the parent SOLO 1 or 2 studies. All patients received dupilumab 300 mg qw in the OLE study, irrespective of whether they received qw or 2qw dosing in the parent study.
Results
Among those who had not achieved EASI-75 or IGA 0/1 during the 16-week parent study, the proportion of patients achieving EASI-75 by week 100 was 91%. The proportion achieving IGA 0 or 1 at week 100 was 45% for patients initially on q2w week dosing and 49% for those on initial qw dosing.
Conclusion
Long-term dupilumab treatment may be associated with improvement in AD in patients with suboptimal responses during the initial 16 weeks of treatment.
Clinical Trial Registration
LIBERTY AD SOLO 1: ClinicalTrials.gov identifier NCT02277743; EudraCT 2014-001198-15. LIBERTY AD SOLO 2: ClinicalTrials.gov identifier NCT02277769; EudraCT 2014-002619-40. LIBERTY AD OLE: ClinicalTrials.gov Identifier NCT01949311; EudraCT 2013-001449-15.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13555-021-00643-4.
Keywords: Atopic dermatitis, Dupilumab, Efficacy
Video Abstract (MP4 65362 kb)
Key Summary Points
| Why carry out this study? |
| Drug survival studies of dupilumab show that many patients continue treatment through 1 year, suggesting that patients that do not achieve an optimal response during short-term treatment experience clinically relevant benefits over time. This analysis was conducted specifically to assess long-term (100-week) response in patients who did not initially achieve the pre-specified clinical trial endpoints. |
| What was learned from the study? |
| The study showed that long-term (100-week) treatment with dupilumab is associated with improvement in AD in most patients with suboptimal responses at 16 weeks. Regardless of the dosing option (qw or q2w) in the parent study, most patients achieved clinically relevant benefits. |
Digital Features
This article is published with digital features, including a video abstract, to facilitate understanding of the article. To view digital features for this article go to 10.6084/m9.figshare.17071910.
Introduction
Atopic dermatitis (AD), a chronic, relapsing, type 2 inflammatory disease, is characterized by skin lesions and pruritus that can significantly impair quality of life [1]. The disease affects as many as 20% of children and 2–8% of adults worldwide [2, 3]. Dupilumab is a fully human monoclonal antibody that blocks the shared receptor component for interleukin (IL)-4 and IL-13, inhibiting signaling of both IL-4 and IL-13, key and central drivers of type 2-mediated inflammation in multiple diseases [4, 5]. In phase 3 randomized trials, dupilumab with or without topical corticosteroids (TCS) versus placebo showed significant improvement in AD signs, symptoms, and quality of life with an acceptable safety profile in adults with moderate-to-severe AD [6–8]. As clinical studies may differ from longer-term patient treatment, open-label extension studies have also been carried out with dupilumab, and a study in adults shows that dupilumab treatment benefits were sustained through 3 years [9]. Data from both the open-label and a 1-year long-term study suggests that continued treatment with dupilumab may benefit adults with moderate-to-severe AD who do not optimally respond to initial short-term treatment [7, 9]. In addition, drug survival studies report high long-term retention rates through 1 year, also suggesting that patients not achieving clear or almost clear skin at 16 weeks may experience clinically relevant benefits over time [10]. To further explore this hypothesis, we conducted a post hoc analysis to assess whether continued treatment with dupilumab in an open-label extension (OLE) study (LIBERTY AD OLE) improves efficacy in adults who did not achieve optimal responses with short-term (16-week) monotherapy.
Methods
This post hoc analysis includes data from 391 adult patients from the OLE study who had not achieved the endpoints of at least 75% improvement from baseline in the Eczema Area and Severity Index (EASI-75) or an Investigator’s Global Assessment (IGA) score of 0 or 1 with short-term (16-week) dupilumab treatment in SOLO 1 or 2 studies; of these, 178 patients had received dupilumab 300 mg weekly (qw), and 213 had received dupilumab 300 mg every 2 weeks (q2w). In total, 469 patients in SOLO 1 and 2 were potentially eligible for this study.
Detailed methodology, efficacy and safety results have been reported previously for the studies included in this analysis [8, 9]. In brief, SOLO 1 and SOLO 2 were two identically designed trials that evaluated the efficacy and safety of dupilumab monotherapy (300 mg qw or q2w) for 16 weeks in adults with moderate-to-severe AD [6]. The OLE study included patients from SOLO 1 and 2 and other studies in the phase 1–3 development stages (including patients in the placebo groups) who had adequately completed the required parent study assessments or were screened for phase 3 studies but were not randomized because of randomization closure [9]. During the OLE, all patients received a 300 mg qw dupilumab dose regimen, with an additional loading dose of dupilumab 600 mg if the gap between studies was longer than 4 weeks [9]. Use of topical therapies was permitted.
Outcomes assessed in this analysis include proportions of patients achieving the secondary endpoints of EASI-75 or IGA 0 or 1 through week 100 of the OLE among the initial non-achiever patients in the parent study. Data are presented on the basis of assigned treatment regimen in the original parent study, and patients were included in this analysis if they received at least dose of dupilumab in the OLE. All analyses presented here were performed using observed values at the indicated time point with no imputation for missing values. Data are reported on the basis of a cutoff date of December 15, 2018 (database lock February 2019).
The study was conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonization Good Clinical Practice guideline, and applicable regulatory requirements. An independent data and safety monitoring committee conducted blinded monitoring of patient safety data. The local institutional review board or ethics committee at each study center oversaw trial conduct and documentation. All patients provided written informed consent before participating in the trial.
Results
Of the 391 patients in this analysis, 229 (59%) completed 100 weeks in the study. Of those discontinuing, the most common reason was the commercial availability of the study drug (79 patients, 49% of patients discontinuing); 12 patients (7% of patients who discontinued) discontinued because of lack of efficacy. Other reasons for discontinuation included adverse events, subject’s own choice, and loss to follow-up (Table 1).
Table 1.
Baseline demographics and disease characteristics
| Non-achievers at week 16 of SOLO 1 and 2 | ||
|---|---|---|
| EASI-75 (n = 364) | IGA 0 or 1 (n = 383) | |
| Age, years, mean ± SD | 39.1 (13.9) | 39.3 (14.1) |
| Male gender, n (%) | 245 (67.3) | 256 (66.8) |
| Race, n (%) | ||
| White | 238 (65.4) | 252 (65.8) |
| Black/African American | 19 (5.2) | 21 (5.5) |
| Asian | 100 (27.5) | 103 (26.9) |
| Other/not reported | 7 (1.9) | 7 (1.8) |
| BMI, kg/m2, mean ± SD | 26.7 (5.7) | 26.7 (5.6) |
| Duration of AD, years, mean ± SD | 29.5 (14.8) | 29.5 (14.9) |
| EASI (range 0–72), mean ± SD | 20.3 (13.3) | 19.8 (13.3) |
| IGA (range 0–4), mean ± SD | 3.1 (0.6) | 3.0 (0.6) |
| Average treatment gap between parent study and OLE, n (%), weeks | ||
| < 6 | 39 (10.7) | 43 (11.2) |
| ≥ 6 and ≤ 13 | 283 (77.7) | 291 (76.0) |
| > 13 | 42 (11.5) | 49 (12.8) |
| Reasons for discontinuation, n (%) | ||
| Commercialization of study drug | 73 (20.1) | 79 (20.6) |
| Patients’ choice | 70 (19.2) | 72 (18.8) |
| Lack of efficacy | 12 (3.3) | 12 (3.1) |
| Adverse event | 18 (4.9) | 20 (5.2) |
| Lost to follow-up | 11 (3.0) | 11 (2.9) |
| Protocol deviation | 5 (1.4) | 5 (1.3) |
| Missing | 37 (10.2) | 39 (10.2) |
AD atopic dermatitis, BMI body mass, EASI Eczema Area and Severity Index, EASI-75 at least 75% improvement from baseline in EASI, IGA Investigator’s Global Assessment, OLE open-label extension, SD standard deviation
Baseline demographics (Table 1) were similar to those reported in the parent studies [8, 9]; however, the patients not achieving EASI-75 or IGA 0 or 1 in the initial 16 weeks of dupilumab treatment had a more severe disease profile than those who did achieve those endpoints (Table 2).
Table 2.
Baseline disease characteristics of pooled dupilumab-treated “achiever” and “non-achiever” patients from SOLO 1 and 2
| IGA 0/1 or EASI-75 at 16 weeks | SOLO 1 and 2 dupilumab-treated patients | |
|---|---|---|
| Non-achievers (n = 391) |
Achievers (n = 529) |
|
| Duration of AD, years, mean ± SD | 29.1 (15.0) | 26.7 (15.4) |
| IGA score (range 0–4), mean ± SD | 3.6 (0.5) | 3.4 (0.5) |
| EASI total score (range 0–72), mean ± SD | 35.7 (14.1) | 29.9 (12.1) |
| Weekly peak pruritus NRS (range 1–10), mean ± SD | 6.5 (1.8) | 6.4 (2.0) |
| BSA affected (range 0–100), mean ± SD | 58.9 (22.6) | 50.0 (21.3) |
| POEM total score (range 0–28), means ± SD | 21.4 (5.8) | 19.8 (6.0) |
| Prior use of systemic immunosuppressants (including corticosteroids), n (%) | 234 (59.8) | 238 (45.0) |
AD atopic dermatitis, BSA Body Surface Area Index, EASI Eczema Area and Severity Index, IGA Investigator’s Global Assessment, NRS numerical rating scale, POEM Patient-Oriented Eczema Measure, SD standard deviation
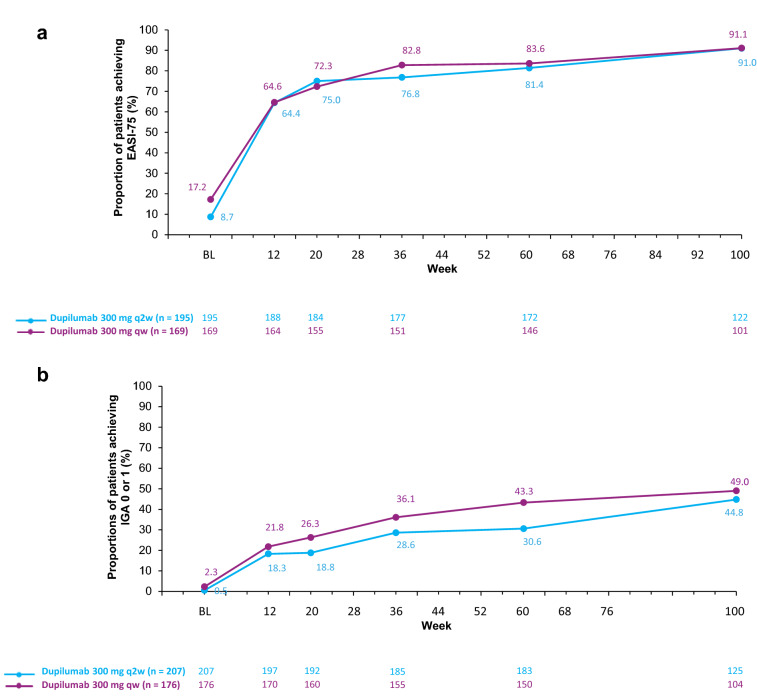
Among adults with moderate-to-severe AD treated with dupilumab 300 mg qw or q2w not achieving EASI-75 or IGA 0 or 1 at week 16 in SOLO 1 and 2, a large proportion achieved these respective endpoints after continued treatment with dupilumab 300 mg qw, regardless of the initial dupilumab treatment received in the 16-week parent studies (Fig. 1). By week 12 of the OLE study, almost 65% of patients with a suboptimal response at week 16 of the parent study achieved EASI-75, and 18–22% achieved IGA 0 or 1. For both endpoints, the number of responders increased progressively through 100 weeks, with 91% achieving EASI-75 by week 100. Among those who had not achieved EASI-75 or IGA 0/1 during the 16-week parent study, the proportion of patients achieving IGA 0 or 1 at week 100 was 45% for patients initially on q2w week dosing and 49% for the qw dosing. Results for patients with a gap in treatment between the parent study and the OLE (at least 4 weeks’ interruption in treatment) were similar to those with uninterrupted treatment, and by week 36, there were no discernible differences in response. The average treatment gaps per group are shown in Table 1, with most patients having a 6–13-week gap in treatment between the parent study and OLE. TCS use was allowed during the OLE study; 59.1% of patients that did not initially achieve EASI-75, and 58% of the IGA 0/1 non-achievers used TCS at least once by the end of the 100-week OLE study.
Fig. 1.
Proportions of patients who achieved a EASI-75 or b an IGA score of 0 or 1 beyond week 16, but who did not achieve either of these outcomes at week 16 of SOLO 1 and 2. Treatment groups are shown by treatment allocation in the parent study (16 weeks); all patients in this analysis subsequently received 300 mg qw in the OLE study. BL baseline, EASI Eczema Area and Severity Index, EASI-75 at least 75% improvement from baseline in EASI, IGA Investigator’s Global Assessment, OLE open-label extension, q2w, every 2 weeks, qw weekly
Discussion
Previously published controlled phase 3 trials show that the 1-year safety and efficacy of q2w and qw regimens are comparable. Treatment with dupilumab 300 mg qw for up to 3 years showed an overall acceptable risk–benefit profile with a sustained efficacy and safety profile, consistent with the known safety profile of dupilumab [8, 9, 11]. This analysis of the OLE data suggests that for patients with suboptimal responses to short-term (16-week) dupilumab treatment, continued treatment with dupilumab is often associated with improvement in AD signs and symptoms. Of note, this subgroup of patients also had a more severe disease profile at baseline, which likely also impacted the length of time needed to reach the trial endpoints of IGA 0 or 1 and EASI-75. In this study, the number of patients that achieved EASI-75 was approximately 64% following an additional 12 weeks of treatment, and this rose to approximately 76–82% of patients by 36 weeks (which corresponds to 1 year of dupilumab treatment in total). By the end of the study, when patients had remained on dupilumab for over 2 years, approximately 91% of patients achieved EASI-75.
Since the OLE study is ongoing and as a result of its study design, a limitation of this analysis is a diminishing patient population over time because of study withdrawals, partly due to the study termination on regulatory approval. Only a small number of patients (3%) withdrew because of lack of efficacy. In addition, differences in the use of adjunct and rescue medications may be a confounding factor in the analysis, as TCS and topical calcineurin inhibitors were allowed in the OLE study but only as rescue therapy in SOLO 1 and 2. However, only approximately 58–59% of patients used adjunct TCS at some point in the 100-week study period, whereas approximately 91% achieved EASI-75.
Conclusions
Patients with suboptimal responses to short-term (16-week) dupilumab treatment often benefit from continued long-term treatment, regardless of the dose frequency (qw or q2w) of the original treatment.
Acknowledgements
We thank patients and investigators who participated in the studies; El-Bdaoui Haddad, Tracy Chew, and Adriana Mello of Sanofi Genzyme; and Linda Williams of Regeneron Pharmaceuticals, Inc.
Funding
Research sponsored by Sanofi and Regeneron Pharmaceuticals, Inc. and was funded by Sanofi Genzyme and Regeneron Pharmaceuticals, Inc., according to the Good Publication Practice guideline. The Dermatology and Therapy rapid service fee was funded by Sanofi and Regeneron Pharmaceuticals Inc.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Andrew Blauvelt, Eric L Simpson, Catherine H Smith, Silvia M Ferrucci: study investigator, conceptualization, review and editing of all drafts, and approval of final manuscript; April Armstrong, Pedro Herranz, Yoko Kataoka, Seong Jun Seo, conceptualization, review and editing of all drafts, and approval of final manuscript; Jingdong Chao: review and editing of drafts, and approval of final manuscript; Ana B. Rossi, Brad Shumel, Paul Tomondy: conceptualization, data analysis definition and interpretation, review and editing of drafts, and approval of final manuscript; Zhen Chen: data analysis, methodology, review and editing of drafts and approval of final manuscript.
Medical Writing, Editorial, and Other Assistance
Medical writing and editorial support were provided by Carolyn Ellenberger, PhD of Excerpta Medica and was funded by Sanofi Genzyme and Regeneron Pharmaceuticals, Inc.
Disclosures
April Armstrong served as a research investigator and/or scientific advisor to AbbVie, Boehringer Ingelheim, Bristol Myers Squibb, Dermavant, Dermira, Eli Lilly, EPI, Incyte, Janssen, LEO Pharma, Modernizing Medicine, Novartis, Ortho Dermatologics, Pfizer, Sanofi, Sun Pharma, Regeneron Pharmaceuticals Inc., UCB. Andrew Blauvelt served as a consultant and investigator for AbbVie, Aligos, Almirall, Amgen, Arcutis Antiobix, Arena Pharmaceuticals, Athenex, Boehringer Ingelheim, Bristol Myers Squibb, Dermavant, Eli Lilly, Evommune, Forté, Galderma, Incyte, Janssen, LEO Pharma, Novartis, Pfizer, Rapt, Regeneron Pharmaceuticals Inc., Sanofi Genzyme, Sun Pharma, and UCB Pharma. Eric L. Simpson is an investigator for AbbVie, Eli Lilly, Galderma, Kyowa Hakko Kirin, LEO Pharma, Merck, Pfizer, Regeneron Pharmaceuticals, Inc.; has received consultant honorarium from AbbVie, Boehringer Ingelheim, Dermavant, Eli Lilly, Forté Bio, Incyte, LEO Pharma, Menlo Therapeutics, Pfizer, Pierre Fabre Dermo Cosmétique, Regeneron Pharmaceuticals, Inc., Sanofi Genzyme, Valeant. Catherine Smith received departmental funding for commercially sponsored clinical trials that involved dupilumab from Regeneron Pharmaceuticals, Inc., Pfizer, Roche, AbbVie and Novartis. Seong Jun Seo served as a consultant, speaker or investigator for AbbVie, Eli Lilly, LEO Pharma, Sanofi Genzyme. Silvia M. Ferrucci served as a consultant and investigator for Regeneron Pharmaceuticals, Inc., AbbVie, Eli Lilly, and Sanofi Genzyme. Jingdong Chao, Zhen Chen, and Brad Shumel are employees and shareholders of Regeneron Pharmaceuticals, Inc. Ana B. Rossi is an employees may hold stock and/or stock options in the company Sanofi Genzyme. Paul Tomondy is a former employee of Sanofi Genzyme; current affiliation is Pvalue Communications Inc.
Compliance with Ethics Guidelines
The study was conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonization Good Clinical Practice guideline, and applicable regulatory requirements. An independent data and safety monitoring committee conducted blinded monitoring of patient safety data. The local institutional review board or ethics committee at each study center oversaw trial conduct and documentation. All patients provided written informed consent before participating in the trial.
Data Availability
The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. Qualified researchers may request access to patient level data and related study documents including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and data set specifications. Patient level data will be anonymized, and study documents will be redacted to protect the privacy of our trial participants. Further details on Sanofi’s data sharing criteria, eligible studies, and process for requesting access can be found at http://www.clinicalstudyrequest.com/.
References
- 1.Weidinger S, Beck LA, Bieber T, Kabashima K, Irvine AD. Atopic dermatitis. Nat Rev Dis Primers. 2018;4:1. doi: 10.1038/s41572-018-0001-z. [DOI] [PubMed] [Google Scholar]
- 2.Silverberg JI, Barbarot S, Gadkari A, et al. Atopic dermatitis in the pediatric population: a cross-sectional, international, epidemiologic study. Ann Allergy Asthma Immunol. 2021;126:417–28.e2. doi: 10.1016/j.anai.2020.12.020. [DOI] [PubMed] [Google Scholar]
- 3.Barbarot S, Auziere S, Gadkari A, et al. Epidemiology of atopic dermatitis in adults: results from an international survey. Allergy. 2018;73:1284–1293. doi: 10.1111/all.13401. [DOI] [PubMed] [Google Scholar]
- 4.Gandhi NA, Pirozzi G, Graham NMH. Commonality of the IL-4/IL-13 pathway in atopic diseases. Expert Rev Clin Immunol. 2017;13:425–437. doi: 10.1080/1744666X.2017.1298443. [DOI] [PubMed] [Google Scholar]
- 5.Le Floc'h A, Allinne J, Nagashima K, et al. Dual blockade of IL-4 and IL-13 with dupilumab, an IL-4Rα antibody, is required to broadly inhibit type 2 inflammation. Allergy. 2020;75:1188–1204. doi: 10.1111/all.14151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Bruin-Weller M, Thaci D, Smith CH, et al. Dupilumab with concomitant topical corticosteroid treatment in adults with atopic dermatitis with an inadequate response or intolerance to ciclosporin A or when this treatment is medically inadvisable: a placebo-controlled, randomized phase III clinical trial (LIBERTY AD CAFE) Br J Dermatol. 2018;178:1083–1101. doi: 10.1111/bjd.16156. [DOI] [PubMed] [Google Scholar]
- 7.Blauvelt A, de Bruin-Weller M, Gooderham M, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017;389:2287–2303. doi: 10.1016/S0140-6736(17)31191-1. [DOI] [PubMed] [Google Scholar]
- 8.Simpson EL, Bieber T, Guttman-Yassky E, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375:2335–2348. doi: 10.1056/NEJMoa1610020. [DOI] [PubMed] [Google Scholar]
- 9.Beck LA, Thaçi D, Deleuran M, et al. Dupilumab provides favorable safety and sustained efficacy for up to 3 years in an open-label study of adults with moderate-to-severe atopic dermatitis. Am J Clin Dermatol. 2020;21:567–577. doi: 10.1007/s40257-020-00527-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spekhorst LS, Ariëns LFM, van der Schaft J, et al. Two-year drug survival of dupilumab in a large cohort of difficult-to-treat adult atopic dermatitis patients compared to cyclosporine A and methotrexate: results from the BioDay registry. Allergy. 2020;75:2376–2379. doi: 10.1111/all.14324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Worm M, Simpson EL, Thaçi D, et al. Efficacy and safety of multiple dupilumab dose regimens after initial successful treatment in patients with atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156:131–143. doi: 10.1001/jamadermatol.2019.3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. Qualified researchers may request access to patient level data and related study documents including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and data set specifications. Patient level data will be anonymized, and study documents will be redacted to protect the privacy of our trial participants. Further details on Sanofi’s data sharing criteria, eligible studies, and process for requesting access can be found at http://www.clinicalstudyrequest.com/.