Abstract
The influences of inoculum size and glucose supplementation on the growth kinetics of 60 Candida spp. clinical isolates (Candida albicans, Candida tropicalis, Candida parapsilosis, Candida glabrata, Candida krusei, and Candida lusitaniae [10 isolates each]) are assessed. The combined influence of growth and reading method (visual or spectrophotometric) on the determination of the MICs of amphotericin B, flucytosine, fluconazole, itraconazole, ketoconazole, and voriconazole is also analyzed, and the MICs are compared with those determined by the National Committee for Clinical Laboratory Standards standard microdilution method (NCCLS document M27-A). Glucose supplementation and inoculum size had a significant influence on the growth cycles of these yeasts, and a statistically significant denser growth (optical density at 540 nm) was seen for both incubation periods, 24 and 48 h (P < 0.01). A longer exponential phase and shorter lag phase were also observed. The A540 values at 24 h of incubation with medium containing glucose and an inoculum of 105 CFU/ml were >0.4 U for all species, with the exception of that for C. parapsilosis (A540 = 0.26 ± 0.025). The MICs at 24 h determined by testing with 2% glucose and an inoculum of 105 CFU/ml showed the strongest agreement (96.83%) with MICs determined by the reference method. MICs were not falsely elevated, and good correlation indexes were obtained. The reproducibility of results with this medium-inoculum combination was high (intraclass correlation coefficient, 0.955). The best agreement and reproducibility of results for spectrophotometric readings were achieved with endpoints of 50% growth inhibition for flucytosine and azoles and 95% for amphotericin B. Supplementation of test media with glucose and an inoculum size of 105 CFU/ml yielded a reproducible technique that shows elevated agreement with the reference procedures and a shorter incubation period for obtaining reliable MIC determinations. The spectrophotometric method offers an advantage over the visual method by providing a more objective and automated MIC determination.
Susceptibility testing of fungi has recently been standardized by the National Committee for Clinical Laboratory Standards (NCCLS) Subcommittee on Antifungal Susceptibility Tests (10). Significant progress has been made in this field, and testing continues to develop into a clinically useful tool (1). However, the methodology still has some unresolved problems and limitations (15). At present, the main problem is the determination of endpoint MICs due to the partial inhibition of growth with azole drugs (trailing) (8, 11, 14). The NCCLS method recommends visual reading, but this aspect is an important source of variability and inaccuracy due to the trailing phenomenon (6, 8). For amphotericin B (AMB), a fungicidal drug, endpoints are easily defined and the MIC is the lowest drug concentration that prevents any discernible growth compared with the growth of the control (drug-free tube or well). However, fungistatic agents as azoles (ketoconazole [KTC], fluconazole [FLC], and itraconazole [ITC]) and flucytosine (5FC) show less defined endpoints and introduce significant subjectivity into the reading of results (13, 21). Document M27-A proposes the agitation of antifungal susceptibility testing (AST) solutions and quantification of endpoint determinations to improve the reliability of techniques, but these proposals do not settle the issue. In addition, the reference procedure requires 48 h of incubation to obtain the AST results (7, 10).
Several studies trying to overcome these limitations have been published. Some of these works include spectrophotometric determination of endpoints, higher-inoculum utilization, and glucose supplementation (2, 5, 12, 13, 17). The utility of these modifications remains to be established because of a lack of agreement between study findings. Recent reports have pointed out that a large inoculum size and glucose supplementation may falsely elevate the MICs (11). However, works previously published had indicated that supplemental glucose, large inoculum size, and spectrophotometric reading may serve as a less subjective method and one that requires only 24 h of incubation for AST of yeasts (8, 12, 18, 19).
Our study addresses first the influence of inoculum size and glucose supplementation on the growth (growth kinetics [GKs]) of six species of Candida (Candida albicans, Candida tropicalis, Candida parapsilosis, Candida glabrata, Candida krusei, and Candida lusitaniae). In a second set of experiments, we analyze the combined influence of growth and reading method (visual or spectrophotometric) on the determination of the MICs of AMB, 5FC, FLC, ITC, KTC, and voriconazole (VRC) and compare these MICs with those determined by the NCCLS standard microdilution method. The details of these analyses and their implications are the subject of this work. Isolates of Cryptococcus neoformans have not been included in this study. A second major limitation of the NCCLS reference procedure is the poor growth of Cryptococcus neoformans and other nonfermentative yeasts with medium recommended for AST (15). So, a recent report has pointed out that cultivation under constant agitation, higher inoculum size, and an assay medium different from RPMI is necessary to perform AST of Cryptococcus neoformans (20).
MATERIALS AND METHODS
Organisms.
A collection of 60 clinical isolates was tested. This collection included 10 isolates of each of six Candida spp. The majority of isolates (n = 44) were obtained from blood cultures, and the remainder were obtained from specimens of deep sites. Each strain represented a unique isolate from a patient and was sent to our laboratory for identification or AST. Isolates were identified by routine microbiological techniques and were maintained at −70°C.
C. parapsilosis ATCC 22019 and C. krusei ATCC 6258 were incorporated as quality control strains in each set of experiments (3).
Assay media.
RPMI is RPMI 1640 medium without sodium bicarbonate and with l-glutamine (Sigma Aldrich Química, Madrid, Spain). It was buffered to pH 7.0 with 0.165 M morpholinepropanesulfonic acid (MOPS; Sigma Aldrich Química). RPMI–2% glucose (RPMI–2%G) is RPMI 1640 supplemented with 18 g of glucose per liter to reach a final concentration of 2%. Media were prepared as double-strength solutions. Media were sterilized by filtration.
Inocula.
The yeast isolates were grown on Sabouraud dextrose agar (Oxoid, Madrid, Spain) for 24 h at 35°C. Suspensions were prepared from five colonies of each culture, and the spectrophotometric method for inoculum preparation was used (10). Three final inocula prepared in sterile distilled water containing (i) 0.5 × 103 to 2.5 × 103 CFU/ml, (ii) 0.5 × 104 to 2.5 × 104 CFU/ml, and (iii) 0.5 × 105 to 2.5 × 105 CFU/ml were obtained.
Antifungal agents.
The antifungal agents used in this study were as follows: AMB (Sigma Aldrich Química), 5FC (Sigma Aldrich Química), FLC (Pfizer, Madrid, Spain), ITC (Janssen Farmacéutica, Madrid, Spain), KTC (Janssen Farmacéutica), and VRC (Pfizer). Stock solutions were prepared in 100% dimethyl sulfoxide (Sigma Aldrich Química), except 5FC, which was dissolved in sterile distilled water. Stock solutions were prepared at concentrations 100 times the highest concentration to be tested and were frozen at −70°C until used.
Influence of inoculum size and glucose supplementation on growth.
This set of experiments was performed by means of GKs. GKs for the 60 isolates and the control strains were determined with the microdilution format. GKs were done with RPMI, RPMI–2%G, and each of the inocula. The wells of the trays were inoculated with 200 μl and sealed with a gas-permeable sealing membrane for microtiter plates (Breathe Easy membrane; Sigma Aldrich Química). The microplates were incubated for 48 h at 35°C inside a IEMS Reader MF (Labsystems, Madrid, Spain). The reader carried out an hourly spectrophotometric reading at a wavelength of 540 nm (A540). All procedures were repeated on two different days. Hourly spectrophotometric readings were saved and analyzed with the software package Ascent Research Edition, version 2.1 (Labsystems). Curves were constructed with help from the SigmaPlot, version 5.0, graph package (SPSS S. L., Madrid, Spain).
Susceptibility testing.
Each isolate was subcultured onto Sabouraud dextrose agar plates at 35°C for 24 h prior to testing. AST was performed simultaneously with RPMI and RPMI–2%G assay media.
(i) AST with RPMI.
The methodology used strictly followed the NCCLS recommendations for the microdilution procedure, which include a final yeast inoculum of 0.5 × 103 to 2.5 × 103 CFU/ml.
(ii) AST with RPMI–2%G.
The NCCLS methodology was again followed for testing with RPMI–2%G, but three final inocula were tested: (i) 0.5 × 103 to 2.5 × 103 CFU/ml, (ii) 0.5 × 104 to 2.5 × 104 CFU/ml, and (iii) 0.5 × 105 to 2.5 × 105 CFU/ml.
Sterile plastic microtitration plates containing flat-bottomed wells were used. The plates contained twofold serial dilutions of the antifungal drugs with a volume of assay medium of 100 μl per well. We used two drug-free-medium wells as sterility and growth controls. Each well of the trays was inoculated with a 100-μl final inoculum. The microtiter plates were incubated at 35°C for 48 h in a humid atmosphere. Stationary cultures were performed.
The MICs were determined at 24 and 48 h both visually and spectrophotometrically for each assay medium and final inoculum. After incubation, the microplates were mechanically agitated at 1,400 rpm for 30 s in a microplate shaker and then read. All procedures were repeated on two different days.
Endpoint determination.
By visual endpoint determination, the MICs of 5FC, FLC, ITC, KTC, and VRC were determined according to a 0-to-4 scale, with 0 indicating an optically clear culture, 1 indicating a slightly hazy culture, 2 indicating a prominent decrease in turbidity, 3 indicating a slight decrease in turbidity, and 4 indicating no reduction in turbidity. The MIC was defined as the lowest concentration of a drug with which the score was ≤2. The AMB MIC was defined as the lowest concentration of drug that completely inhibited the growth of the strain (10).
By spectrophotometric endpoint determination, the MICs were obtained by measuring the absorbance at 540 nm with a Labsystems IEMS Reader MF. Two endpoints were defined for each antifungal agent tested, with Spec-80% indicating the lowest drug concentration resulting in a reduction in growth of 80% or more (determined spectrophotometrically) compared with the growth of the control as recommended by NCCLS document M27-A for macrodilution testing (10) and with Spec-50% indicating the lowest drug concentration resulting in a reduction in growth of 50% or more (determined spectrophotometrically) compared with the growth of the control. Previous reports have demonstrated that the Spec-50% produced results both with a higher in vitro-in vivo correlation index and better agreement with results of the NCCLS document M27-A method (8); therefore, the Spec-50% was also defined as the spectrophotometric endpoint. For AMB we also calculated the Spec-95%, the lowest drug concentration exhibiting a reduction in growth of 95% or more compared with the growth of the drug-free well.
Statistical analysis.
The significance of the differences in the GKs and AST results between methodologies was determined by the Student t test (unpaired, unequal variance) or by the Mann-Whitney U test. When the effect of one variable was studied, the others were fixed as constants. Differences in proportions were determined by Fisher's exact test or by chi-square analysis. A P value of <0.01 was considered significant.
The lag phase of the growth cycle was defined as the time during which optical density (A540) does not increase. The beginning of the growth phase was defined as a change in A540 of ≥0.015 U (the lowest detectable optical density). Exponential-phase broth cultures were marked by a specific growth rate of >0.02 U per h. Stationary-phase broth cultures were marked by the lack of a continued exponential increase in A540.
The optical density in the drug-free well must be >0.2 to calculate the spectrophotometric MICs. The mean of the absorbance of eight sterility control wells was subtracted from the absorbance value obtained for each well, and then spectrophotometric MICs were calculated. The reproducibility of the results for each medium-inoculum combination employed in AST was evaluated by an intraclass correlation coefficient (ICC) which compared the results of 30 consecutive determinations of the MICs of antifungal agents with those of the two quality control strains included. Reproducibility was calculated by means of a scales analysis in which reliability was the extent to which endpoint determinations yielded the same MICs over time. The ICC assesses reliability as an internal consistency statistic by means of interitem correlations. A one-way random effect model was used to calculate the ICCs that were expressed over a maximum value of 1 and with a confidence interval of 95% (CI95%) (9). The agreement between results determined by different AST methodologies was defined as a difference in MICs equal to one twofold dilution. The correlation between AST methods was determined by Pearson's coefficient, which was expressed over a maximum value of 1. These analyses were performed for each species and for all isolates considered together. MICs were transformed on log2-unit data. All statistical analysis was done with the Statistical Package for the Social Sciences (version 10.0; SPSS S. L.).
RESULTS
GKs.
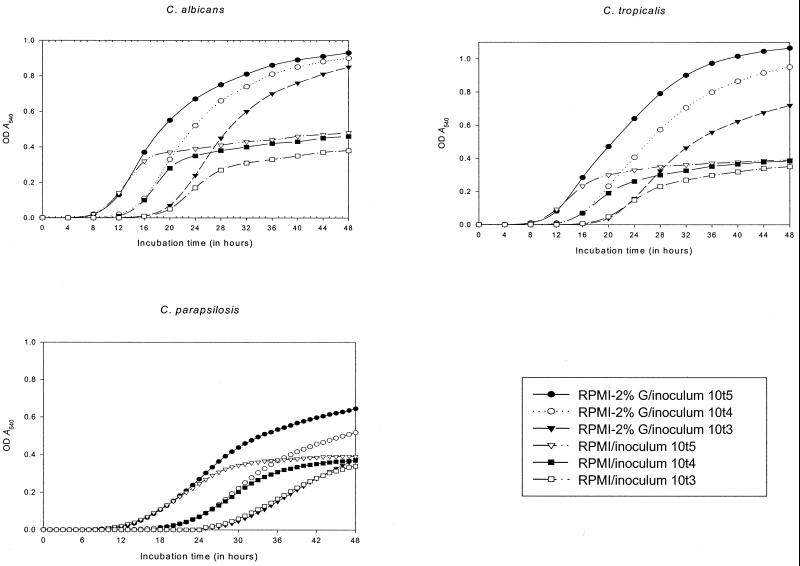
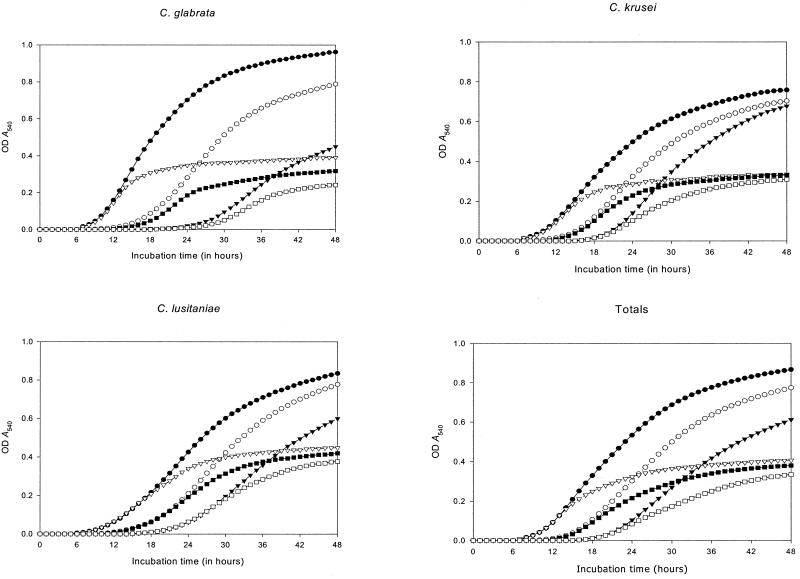
Figure 1 displays the results of GKs by species with each medium-inoculum combination. The figure also shows a curve generated for the 60 isolates analyzed. Curves are constructed with mean ± standard error values of the individual GK of each isolate.
FIG. 1.
GKs by species and totals for each medium-inoculum combination. OD, optical density.
Several significant pieces of evidence of the relationship between the inoculum-medium combination and the cycle of growth of the strains were encountered.
(i) Lag phase.
Higher inoculum sizes significantly shortened the length of the lag phase (P = 0.0012), but glucose supplementation did not have a significant influence on the length of the lag phase.
(ii) Growth phase.
Evidence of a relationship between the inoculum size and the duration of exponential growth was observed only for the inoculum size of 105 CFU/ml, which significantly extended the length of the growth phase (P = 0.0056). In addition, glucose supplementation was significantly related to longer growth phases (P = 0.0032).
(iii) Growth after a 24-h incubation time (A540).
Higher growth rates at 24 h of incubation were significantly associated with both higher inoculum sizes (P < 0.001) and glucose supplementation (P < 0.001).
(iv) Final growth at the 48-h incubation time.
The final growth rates reached after 48 h of incubation were statistically related to both greater inoculum sizes (P < 0.001) and glucose supplementation (P < 0.001). The A540 values reached with each inoculum-medium combination at 24 h of incubation and levels of final growth at 48 h are displayed in Table 1. Table 1 also shows times to reach growth-phase and stationary-phase broth cultures. Data are expressed as means ± standard errors.
TABLE 1.
Growth parameters of 60 Candida spp. isolates determined with medium-inoculum combinationsa
| Inoculum (CFU/ml) | Time to growth phaseb
|
Time to stationary phaseb
|
Growth at 24 hc
|
Final growth at 48 hc
|
||||
|---|---|---|---|---|---|---|---|---|
| RPMI–2%G | RPMI | RPMI–2%G | RPMI | RPMI–2%G | RPMI | RPMI–2%G | RPMI | |
| 105 | 6:54 ± 0:25 | 7:01 ± 0:33 | 26:49 ± 1:45 | 22:32 ± 2:01 | 0.53 ± 0.16 | 0.32 ± 0.05 | 0.86 ± 0.15 | 0.40 ± 0.05 |
| 104 | 10:25 ± 0:42 | 10:32 ± 0:31 | 34:07 ± 2:32 | 30:05 ± 2:47 | 0.30 ± 0.10 | 0.21 ± 0.09 | 0.77 ± 0.15 | 0.37 ± 0.05 |
| 103 | 17:32 ± 0:53 | 17:39 ± 0:57 | 41:01 ± 2:59 | 38:27 ± 3:07 | 0.10 ± 0.09 | 0.08 ± 0.06 | 0.61 ± 0.17 | 0.33 ± 0.05 |
Data are means ± standard errors.
Time expressed in hours:minutes.
Growth is optical density at 540 nm.
Similar significant differences were found when data were analyzed by Candida species. The highest specific growth rates were observed for RPMI–2%G with an inoculum of 105 CFU/ml for all species included. With this inoculum-medium combination, the A540 values at 24 h of incubation were >0.4 U, with the exception of that for C. parapsilosis (A540 = 0.26 ± 0.025).
AST. (i) Reproducibility and reliability.
Table 2 shows the degree of reproducibility and reliability of each AST method evaluated. This analysis was performed with 30 consecutive determinations of MICs of antifungal agents for C. parapsilosis ATCC 22019 and C. krusei ATCC 6258. Reproducibility was high for all antifungal agents in each AST analyzed. In general, worse reproducibility was observed with visual endpoint determinations than with spectrophotometric readings (P = 0.002). For 5FC and the azoles, the best ICCs were obtained with RPMI–2%G, an inoculum of 105 CFU/ml, and the Spec-50% endpoint determination after 24 h of incubation, and the ICC was 0.955 (range, 0.938 to 0.972) (mean and CI95% of the ICCs of 5FC and the azoles). For AMB, the more reliable AST conditions were RPMI–2%G, an inoculum of 105 of CFU/ml, and the Spec-95% endpoint (ICC = 0.965; range 0.939 to 0.981). Small differences were observed between the MICs determined with the Spec-50% or Spec-80% in each of the four AST evaluated. The best reproducibility for the visual endpoint determination was obtained with RPMI, an inoculum of 103 CFU/ml, and 48 h of incubation time. The ICC under those conditions was 0.848 (range, 0.805 to 0.892) (mean and CI95% of the ICCs of six antifungal agents tested). For AST performed with RPMI and an inoculum of 103 CFU/ml, reliability was higher at 48 h than at 24 h of incubation for all spectrophotometric and visual endpoint determinations (P = 0.007).
TABLE 2.
Degree of reproducibility of results of four AST methodologies with regard to 30 consecutive MICs for reference strains C. parapsilosis ATCC 22019 and C. krusei ATCC 6258
| Medium | Inoculum (CFU/ml) | Method of endpoint determination | Time of incubation (h) | ICCa with:
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| AMB | 5FC | FLC | ITC | KTC | VRC | ||||
| RPMI–2%G | 105 | Visual inspection | 24 | 0.843 | 0.956 | 0.791 | 0.789 | 0.825 | 0.777 |
| 48 | 0.856 | 0.942 | 0.722 | 0.783 | 0.812 | 0.799 | |||
| Spec-50% | 24 | 0.854 | 0.978 | 0.932 | 0.962 | 0.971 | 0.934 | ||
| 48 | 0.823 | 0.921 | 0.908 | 0.927 | 0.937 | 0.936 | |||
| Spec-80% | 24 | 0.935 | 0.943 | 0.912 | 0.897 | 0.907 | 0.886 | ||
| 48 | 0.921 | 0.956 | 0.899 | 0.904 | 0.911 | 0.901 | |||
| Spec-95% | 24 | 0.965 | |||||||
| 48 | 0.964 | ||||||||
| RPMI–2%G | 104 | Visual inspection | 24 | 0.813 | 0.914 | 0.786 | 0.756 | 0.863 | 0.797 |
| 48 | 0.824 | 0.931 | 0.798 | 0.765 | 0.821 | 0.804 | |||
| Spec-50% | 24 | 0.845 | 0.932 | 0.911 | 0.941 | 0.962 | 0.908 | ||
| 48 | 0.865 | 0.941 | 0.903 | 0.916 | 0.924 | 0.912 | |||
| Spec-80% | 24 | 0.921 | 0.916 | 0.895 | 0.868 | 0.894 | 0.870 | ||
| 48 | 0.919 | 0.932 | 0.891 | 0.857 | 0.904 | 0.868 | |||
| Spec-95% | 24 | 0.943 | |||||||
| 48 | 0.931 | ||||||||
| RPMI–2%G | 103 | Visual inspection | 24 | 0.813 | 0.907 | 0.782 | 0.743 | 0.829 | 0.799 |
| 48 | 0.819 | 0.906 | 0.784 | 0.725 | 0.822 | 0.812 | |||
| Spec-50% | 24 | 0.853 | 0.896 | 0.853 | 0.842 | 0.895 | 0.892 | ||
| 48 | 0.855 | 0.885 | 0.849 | 0.834 | 0.894 | 0.867 | |||
| Spec-80% | 24 | 0.901 | 0.893 | 0.842 | 0.840 | 0.881 | 0.867 | ||
| 48 | 0.888 | 0.871 | 0.856 | 0.829 | 0.874 | 0.866 | |||
| Spec-95% | 24 | 0.929 | |||||||
| 48 | 0.921 | ||||||||
| RPMI | 103 | Visual inspection | 24 | 0.845 | 0.723 | 0.654 | 0.765 | 0.798 | 0.723 |
| 48 | 0.898 | 0.912 | 0.867 | 0.781 | 0.825 | 0.804 | |||
| Spec-50% | 24 | 0.734 | 0.723 | 0.712 | 0.698 | 0.687 | 0.714 | ||
| 48 | 0.742 | 0.889 | 0.863 | 0.821 | 0.886 | 0.843 | |||
| Spec-80% | 24 | 0.765 | 0.678 | 0.703 | 0.843 | 0.812 | 0.823 | ||
| 48 | 0.845 | 0.829 | 0.851 | 0.820 | 0.839 | 0.818 | |||
| Spec-95% | 24 | 0.870 | |||||||
| 48 | 0.871 | ||||||||
Data are expressed over a maximum value of 1.
(ii) Agreement and correlation among AST methodologies.
Percentages of agreement and correlation indexes are shown in Table 3. The table displays the agreement and correlation between AST performed with RPMI–2%G and an inoculum size of 105 CFU/ml and the reference AST (RPMI, an inoculum of 103 CFU/ml, and visual reading at 48 h of incubation). It exactly shows the degree of agreement between MICs obtained by reference AST and MICs obtained with RPMI–2%G and an inoculum of 105 CFU/ml by both visual and spectrophotometric endpoint determination. Table 3 includes data for the six antifungal agents and 60 isolates analyzed.
TABLE 3.
Percentages of agreement and correlation indexes between MICs obtained by the NCCLS reference AST method and MICs obtained both visually and spectrophotometricallya
| Method of endpoint determination | Time of incubation (h) | Antifungal agent
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AMB
|
5FC
|
FLC
|
ITC
|
KTC
|
VRC
|
||||||||
| % | r | % | r | % | r | % | r | % | r | % | r | ||
| Visual inspection | 24 | 92 | 0.608∗ | 93 | 0.895∗ | 92 | 0.874∗ | 94 | 0.849∗ | 99 | 0.824∗ | 96 | 0.897∗ |
| 48 | 97 | 0.673∗ | 98 | 0.848∗ | 93 | 0.847∗ | 95 | 0.876∗ | 97 | 0.790∗ | 95 | 0.886∗ | |
| Spec-50% | 24 | 72 | 0.286 | 98 | 0.913∗ | 97 | 0.899∗ | 96 | 0.886∗ | 95 | 0.902∗ | 97 | 0.900∗ |
| 48 | 87 | 0.512∗ | 91 | 0.783∗ | 95 | 0.885∗ | 94 | 0.888∗ | 95 | 0.886∗ | 94 | 0.904∗ | |
| Spec-80% | 24 | 91 | 0.553∗ | 96 | 0.805∗ | 92 | 0.873∗ | 92 | 0.815∗ | 88 | 0.711∗ | 89 | 0.807∗ |
| 48 | 92 | 0.421 | 94 | 0.799∗ | 91 | 0.806∗ | 89 | 0.774∗ | 91 | 0.734∗ | 90 | 0.846∗ | |
| Spec-95% | 24 | 98 | 0.654∗ | ||||||||||
| 48 | 94 | 0.623∗ | |||||||||||
The NCCLS reference method uses RPMI, an inoculum of 103 CFU/ml, and a visual reading of optical density at 48 h of incubation. The tested method uses RPMI–2%G, an inoculum of 105 CFU/ml, and spectrophotometric determinations of optical density after 24 and 48 h of incubation. Data are for 60 strains tested. %, percentage of agreement between AST methods; r, correlation index expressed over a maximum value of 1. An asterisk indicates a correlation index with a statistically significant P value of <0.01.
The agreement and correlation between MICs were high, with the exception of those obtained with the Spec-50% endpoint determination for AMB. When data were considered globally, visually determined MICs of AST performed with RPMI–2%G and an inoculum of 105 CFU/ml significantly correlated with MICs obtained using the NCCLS method. The percentage of agreement and correlation index were also elevated when analysis was performed with data obtained by spectrophotometric determination of the endpoint and by the reference procedure. For 5FC and the azoles, agreement and correlation were slightly better with MICs obtained using the Spec-50% endpoint determination. For AMB, the best results were observed with the Spec-95% determination. For all antifungal and endpoint determinations, differences between the percentages of agreement obtained for the two periods of incubation were minimal, with the exception of those obtained with the Spec-50% endpoint determination for AMB.
A less strong agreement was seen between the MICs determined by the standard microdilution method and spectrophotometric MICs obtained with AST employing RPMI–2%G and an inoculum size of 103 or 104 CFU/ml. Overall, percentages of agreement in tests with six species and six antifungal agents were 79% after 24 h of incubation and 85% after 48 h for an inoculum size of 103 CFU/ml and 90% at 24 h and 94% at 48 h for an inoculum size of 104 CFU/ml. The lowest percentages of agreement were observed for azole agents after 24 h of incubation.
(iii) AST and Candida spp.
The ranges of MICs by Candida spp. appear in Table 4. Table 4 includes data noted from AST performed with RPMI–2%G and an inoculum size of 105 CFU/ml after 24 and 48 h of incubation in comparison with those obtained by the AST reference procedure. For AST using RPMI–2%G as the incubation medium, Table 4 displays only spectrophotometric MICs, the Spec-50%s for 5FC and the azoles, and the Spec-95% for AMB. It can be appreciated that MICs determined by spectrophotometric endpoint determination and by the reference procedure were in similar ranges.
TABLE 4.
AST results by Candida spp. obtained with RPMI–2%G and an inoculum of 105 CFU/ml after 24 and 48 h of incubation and comparison with those obtained by the NCCLS reference procedure
| Species (no. of isolates) | Antifungal agent | Range of MIC (μg/ml)
|
||
|---|---|---|---|---|
| RPMI–2%G and inoculum of 105 CFU/ml after incubation fora:
|
NCCLS reference method | |||
| 24 h | 48 h | |||
| C. albicans (10)b | AMB | 0.50–1.00 | 0.50–2.00 | 0.50–2.00 |
| 5FC | 0.12–0.25 | 0.12–1.00 | 0.12–0.50 | |
| FLC | 0.12–0.50 | 0.12–0.50 | 0.25–0.50 | |
| ITC | 0.12–0.25 | 0.12–0.25 | 0.12–0.50 | |
| KTC | ≤0.01–0.03 | ≤0.01–0.03 | ≤0.01–0.03 | |
| VRC | ≤0.01–0.03 | ≤0.01–0.06 | ≤0.01–0.06 | |
| C. tropicalis (10) | AMB | 0.50–1.00 | 0.50–2.00 | 1.00–2.00 |
| 5FC | 0.12–1.00 | 0.12–2.00 | 0.12–2.00 | |
| FLC | 0.25–8.00 | 0.25–8.00 | 0.25–8.00 | |
| ITC | 0.06–0.50 | 0.12–0.50 | 0.12–1.00 | |
| KTC | ≤0.01–0.06 | ≤0.01–0.12 | ≤0.01–0.25 | |
| VRC | ≤0.01–0.03 | ≤0.01–0.03 | ≤0.01–0.03 | |
| C. parapsilosis (10) | AMB | 0.50–2.00 | 0.50–2.00 | 0.50–2.00 |
| 5FC | 0.12–0.50 | 0.12–0.50 | 0.12–0.25 | |
| FLC | 0.25–2.00 | 0.25–4.00 | 0.25–2.00 | |
| ITC | 0.12–0.25 | 0.12–0.25 | 0.12–0.50 | |
| KTC | ≤0.01–0.03 | ≤0.01–0.06 | ≤0.01–0.06 | |
| VRC | ≤0.01–0.06 | ≤0.01–0.12 | ≤0.01–0.12 | |
| C. glabrata (10) | AMB | 0.25–2.00 | 1.00–2.00 | 1.00–2.00 |
| 5FC | 0.12–0.50 | 0.12–0.50 | 0.12–0.50 | |
| FLC | 4.00–16.0 | 8.00–64.0 | 4.00–64.0 | |
| ITC | 1.00–4.00 | 1.00–16.0 | 1.00–16.0 | |
| KTC | 0.06–0.25 | 0.25–0.50 | 0.12–0.50 | |
| VRC | 0.12–4.00 | 0.25–8.00 | 0.12–8.00 | |
| C. krusei (10) | AMB | 0.12–2.00 | 0.25–2.00 | 0.06–1.00 |
| 5FC | 1.00–8.00 | 1.00–8.00 | 0.50–16.0 | |
| FLC | 16.0–64.0 | 32.0–128.0 | 16.0–128.0 | |
| ITC | 0.25–1.00 | 0.25–1.00 | 0.12–2.00 | |
| KTC | 0.06–0.50 | 0.12–1.00 | 0.12–0.50 | |
| VRC | 0.12–0.50 | 0.25–1.00 | 0.12–1.00 | |
| C. lusitaniae (10) | AMB | 0.12–2.00 | 0.12–2.00 | 0.12–1.00 |
| 5FC | 0.12–1.00 | 0.12–4.00 | 0.12–4.00 | |
| FLC | 0.25–0.50 | 0.25–0.50 | 0.25–1.00 | |
| ITC | ≤0.01–0.12 | ≤0.01–0.25 | ≤0.01–0.25 | |
| KTC | ≤0.01–0.03 | ≤0.01–0.03 | ≤0.01–0.03 | |
| VRC | ≤0.01–0.03 | ≤0.01–0.03 | ≤0.01–0.03 | |
| C. parapsilosis ATCC 22019c | AMB | 0.25–2.00 | 1.00–2.00 | 0.50–2.00 |
| 5FC | 0.12–0.50 | 0.12–0.25 | 0.12–0.5 | |
| FLC | 1.00–4.00 | 2.00–4.00 | 1.00–4.00 | |
| ITC | 0.12–0.25 | 0.12–0.50 | 0.25–0.50 | |
| KTC | ≤0.01–0.06 | 0.03–0.12 | 0.03–0.12 | |
| VRC | ≤0.01–0.12 | 0.03–0.25 | 0.03–0.12 | |
| C. krusei ATCC 6258c | AMB | 0.25–2.00 | 1.00–2.00 | 0.50–2.00 |
| 5FC | 4.00–8.00 | 4.00–8.00 | 8.00–16.0 | |
| FLC | 16.0–32.0 | 32.0–64.0 | 16.0–64.0 | |
| ITC | 0.12–0.5 | 0.12–0.50 | 0.25–1.00 | |
| KTC | 0.06–0.50 | 0.12–0.50 | 0.12–0.50 | |
| VRC | 0.12–0.50 | 0.12–0.50 | 0.25–0.50 | |
MICs represented were obtained with the Spec-95% for AMB and the Spec-50% for 5FC, FLC, ITC, KTC, and VRC.
All procedures were repeated on two different days.
Data from 30 consecutive determinations on different days.
A good agreement (ICC > 0.85) was also observed between data obtained with RPMI–2%G and an inoculum size of 104 or 103 CFU/ml and MICs obtained by the reference methodology. However, as it was stated above, the agreement was lower for MICs determined at 24 h of incubation. In addition, spectrophotometric MICs could not be determined for all organisms after 24 h of incubation when AST was performed with RPMI–2%G and 103 CFU/ml as the inoculum size. Briefly, 11 isolates of 60 tested (5 C. parapsilosis, 3 C. krusei, and 3 C. lusitaniae) grew less than 0.2 U (A540) (P < 0.001 by the chi-square test).
Finally, spectrophotometric MICs obtained with RPMI as the incubation medium and an inoculum size of 103 CFU/ml were also compared with those determined by the AST reference method (RPMI with an inoculum of 103 CFU/ml and visual reading). The agreement and correlation indexes among methods of reading were elevated after 48 h of incubation (89%) but weak at 24 h of incubation (68%). Nevertheless, spectrophotometric MICs for 17 of 60 isolates tested (P < 0.001) could not be determined at 24 h of incubation (6 C. parapsilosis isolates, 1 C. tropicalis isolate, 5 C. krusei isolates, and 5 C. lusitaniae isolates).
DISCUSSION
A great deal of effort has gone into the development of a standardized method for AST, and the reference techniques are now more reliable and reproducible. However, the use of this methodology still has some limitations as regards visual endpoint determination, which is a subjective operation, particularly with fungistatic agents (14, 15). Several studies have demonstrated the value of automation in all stages of yeast susceptibility testing in the utilization of spectrophotometric readings, which offer the advantage of permitting a less subjective automated MIC determination (12, 18).
Various investigators have employed spectrophotometric endpoint determination, but they have not used either the same growth conditions or the same endpoint (2, 19). Some of these studies have pointed out that the combination of spectrophotometric reading and the NCCLS reference inoculum size and incubation medium yielded poor agreement with results of both the standard broth macrodilution procedure and the reference micromethod (5, 11, 13). The lowest agreement was seen in azole susceptibility testing after 24 h of incubation (11). Better agreement was obtained for MICs of AMB after an incubation period of 48 h. Other findings have indicated that the addition of 2% glucose and a larger inoculum size improve the agreement between the results of the spectrophotometric method and those of the reference procedures (4, 12, 19). Others have shown, however, that glucose supplementation or a larger inoculum size may falsely elevate the MICs, mainly at 48 h of incubation (11).
In this study, a low agreement was seen between spectrophotometric and visual readings in AST performed without glucose and with an inoculum size of 103 CFU/ml at 24 h (68%). It should be noted that 17 isolates (28.3%) grew less than 0.2 U (A540) and that the spectrophotometric MICs were not determined. After 48 h of incubation, the percentage of agreement was higher (89%). As in previous reports, the lowest agreement was achieved in azole AST (13). In general, both the addition of glucose and larger inocula improve percentages of agreement between spectrophotometric readings and readings by the reference method. An important finding of this work is that the best percentages of agreement were achieved with AST with 2% glucose and 105 CFU/ml as the inoculum after 24 h of incubation. The automated readings that yielded the largest percentages under these conditions were the Spec-50%s for all azoles and 5FC and the Spec-95% for AMB (96.83%). Globally, results of the other ASTs using glucose with lower inocula, another percentage of growth inhibition as the endpoint, and the two periods of incubation showed good agreement with those of the NCCLS reference micromethod with the exception of the Spec-50% for AMB (Table 3).
Given the strong agreement observed for ASTs employing glucose and an inoculum size of 105 CFU/ml, we analyzed the reproducibility of this medium-inoculum combination and its correlation with MICs obtained by the reference methodology. The ICCs were elevated (Table 2), indicating that AST with glucose and larger inoculum sizes is a reliable technique. Correlation was assessed by Pearson's index (r) (Table 3), and MICs of antifungal agents obtained with RPMI–2%G and an inoculum of 105 CFU/ml correlated well with the MICs obtained by the standard method. MICs from AST with glucose and the larger inoculum size were not falsely elevated. A lower correlation index was seen for AMB, perhaps because RPMI yields a range of MICs that spans only three or four twofold dilutions, which can have an influence on the correlation index determination.
The supplementation of the test medium with glucose at a final concentration of 20 g/liter has been reported to simplify endpoint determination, mainly with fungistatic agents (8, 12). However, this modification is not considered part of the formal document M27-A procedures (10). Moreover, a recent report has indicated that glucose supplementation does not stimulate heavier growth after 24 h of incubation (11). The GKs observed in this study demonstrate that glucose supplementation has a significant effect on the growth of 60 Candida isolates included. The addition of glucose increased both the growth observed at 24 h and final growth after 48 h of incubation. The length of the exponential phase of growth was also prolonged. Larger inocula had an additive effect on the growth cycle, so the best cycle was observed with RPMI–2%G and an inoculum of 105 CFU/ml. This medium-inoculum combination shortened the lag phase and yielded elevated optical densities after 24 h, permitting an easy calculation of percentages of growth inhibition. By species, similar data were obtained, although for C. parapsilosis, isolate growth at 24 h of incubation was not so elevated (A540, 0.26 U). As we stated above, results of AST including supplementation with glucose, a larger inoculum size, and spectrophotometric endpoint determination showed strong agreement with those of the NCCLS reference micromethod at 24 h of incubation. Heavier growth and good agreement indicate that techniques performed with 2% glucose and an inoculum of 105 CFU/ml may be an optimal procedure for testing the susceptibility of Candida species to AMB, 5FC, and azoles. Regarding the choice of the percentage of the inhibition of growth to determine the spectrophotometric MIC, our data demonstrate that the Spec-95% for AMB and the Spec-50%s for 5FC and azoles at 24 h result in the best agreement and reliability. The importance of these results is further reinforced by a recent demonstration that the Spec-50% MIC of azoles at 24 h is associated with an increased correlation with the outcome in vivo (16).
In conclusion, these data confirm that the modified M27-A technique with 2% glucose has the advantage of reducing the incubation time to obtain AST results. Glucose supplementation and larger inoculum size have an additive effect on the growth cycle of Candida spp. in that they simplify endpoint determination and lack significant inoculum effects in the determination of MICs. The spectrophotometric method offers an advantage over the visual method by providing a more objective and automated MIC determination. Supplementation of test media with glucose and an inoculum size of 105 CFU/ml yield a reproducible and reliable technique that produces results that show elevated agreement with the results of the reference procedures. This technique may become a standard micromethod for testing antifungal agents against Candida spp. since it shows enhanced reproducibility and allows for an easier determination of definitive endpoints.
ACKNOWLEDGMENTS
This work was supported in part by research project 99/1199 from the Instituto de Salud Carlos III. T. M. Díaz-Guerra is a fellow of the Instituto de Salud Carlos III (grant 99/4149).
We thank Pfizer and Janssen Farmaceútica for supplying the antifungal powders.
REFERENCES
- 1.Alexander B D, Perfect J R. Antifungal resistance trends towards the year 2000: implications for therapy and new approaches. Drugs. 1997;54:657–678. doi: 10.2165/00003495-199754050-00002. [DOI] [PubMed] [Google Scholar]
- 2.Barchiesi F, Colombo A L, McGough D A, Rinaldi M G. Comparative study of broth macrodilution and microdilution techniques for in vitro antifungal susceptibility testing of yeasts by using the National Committee for Clinical Laboratory Standards' proposed standard. J Clin Microbiol. 1994;32:2494–2500. doi: 10.1128/jcm.32.10.2494-2500.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barry A L, Pfaller M A, Brown S D, Espinel-Ingroff A, Ghannoum M A, Knapp C, Rennie R P, Rex J H, Rinaldi M G. Quality control limits for broth microdilution susceptibility tests of ten antifungal agents. J Clin Microbiol. 2000;34:3457–3459. doi: 10.1128/jcm.38.9.3457-3459.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cantón E, Pemán J, Carrillo-Muñoz A, Orero A, Ubeda P, Viudes A, Gobernado M. Fluconazole susceptibilities of bloodstream Candida sp. isolates as determined by National Committee for Clinical Laboratory Standards method M27-A and two other methods. J Clin Microbiol. 1999;37:2197–2200. doi: 10.1128/jcm.37.7.2197-2200.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Espinel-Ingroff A, Kish C W, Kerkering T M, Fromtling R A, Bartizal K, Galgiani J N, Villareal K, Pfaller M A, Gerarden T, Rinaldi M G, Fothergill A. Collaborative comparison of broth macro- and microdilution antifungal tests. J Clin Microbiol. 1992;30:3138–3145. doi: 10.1128/jcm.30.12.3138-3145.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Espinel-Ingroff A, Rodriguez-Tudela J L, Martinez-Suarez J V. Comparison of two alternative microdilution procedures with the National Committee for Clinical Laboratory Standards reference macrodilution method M27-P for in vitro testing of fluconazole-resistant and -susceptible isolates of Candida albicans. J Clin Microbiol. 1995;33:3154–3158. doi: 10.1128/jcm.33.12.3154-3158.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Espinel-Ingroff A, White T, Pfaller M A. Antifungal agents and susceptibility tests. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 7th ed. Washington, D.C.: ASM Press; 1999. pp. 1640–1652. [Google Scholar]
- 8.Lozano-Chiu M, Arikan S, Paetznick V L, Anaissie E J, Rex J H. Optimizing voriconazole susceptibility testing of Candida: effects of incubation time, endpoint rule, species of Candida, and level of fluconazole susceptibility. J Clin Microbiol. 1999;37:2755–2759. doi: 10.1128/jcm.37.9.2755-2759.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Llop C, Pujol I, Aguilar C, Sala J, Riba D, Guarro J. Comparison of three methods of determining MICs for filamentous fungi using different end point criteria and incubation periods. Antimicrob Agents Chemother. 2000;44:239–242. doi: 10.1128/aac.44.2.239-242.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 11.Nguyen M H, Yu C Y. Influence of incubation time, inoculum size, and glucose concentration on spectrophotometric endpoint determinations for amphotericin B, fluconazole, and itraconazole. J Clin Microbiol. 1999;37:141–145. doi: 10.1128/jcm.37.1.141-145.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Odds F C, Messer S A, Woestenborghs F. Antifungal susceptibility testing of yeasts: evaluation of technical variables for tests automation. Antimicrob Agents Chemother. 1995;39:2051–2060. doi: 10.1128/aac.39.9.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pfaller M A, Messer S A, Coffmann S. Comparison of visual and spectrophotometric methods of MIC endpoint determinations by using broth microdilution methods to tests five antifungal agents, including the new triazole D0870. J Clin Microbiol. 1995;33:1094–1097. doi: 10.1128/jcm.33.5.1094-1097.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Revankar S G, Kirkpatrick W R, Mcatee R K, Fothergill A W, Redding S W, Rinaldi M G, Patterson T F. Interpretation of trailing endpoints in antifungal susceptibility testing by the National Committee for Clinical Laboratory Standards method. J Clin Microbiol. 1998;36:153–156. doi: 10.1128/jcm.36.1.153-156.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rex J H, Pfaller M A, Rinaldi M G, Polak A, Galgiani J N. Antifungal susceptibility testing. Clin Microbiol Rev. 1993;6:367–381. doi: 10.1128/cmr.6.4.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rex J H, Nelson P W, Paetznick V L, Lozano-Chiu M, Espinel-Ingroff A, Anaissie E J. Optimizing the correlation between results of testing in vitro and therapeutic outcome in vivo for fluconazole by testing critical isolates in a murine model of invasive candidiasis. Antimicrob Agents Chemother. 1998;42:129–134. doi: 10.1128/aac.42.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodriguez-Tudela J L, Martinez-Suarez J V. Improved medium for fluconazole susceptibility testing of Candida albicans. Antimicrob Agents Chemother. 1994;38:45–48. doi: 10.1128/aac.38.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodriguez-Tudela J L, Martinez-Suarez J V. Defining conditions for microbroth antifungal susceptibility testing: influence of RPMI and RPMI–2% glucose on the selection of endpoint criteria. J Antimicrob Chemother. 1995;35:739–749. doi: 10.1093/jac/35.6.739. [DOI] [PubMed] [Google Scholar]
- 19.Rodrigue-Tudela J L, Berenguer J, Martinez-Suarez J V, Sanchez R. Comparison of a spectrophotometric microdilution method with RPMI–2% glucose with the National Committee for Clinical Laboratory Standards reference macrodilution method M27-P for in vitro susceptibility testing of amphotericin B, flucytosine, and fluconazole against Candida albicans. Antimicrob Agents Chemother. 1996;40:1998–2003. doi: 10.1128/aac.40.9.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodriguez-Tudela J, Martín-Díez L F, Cuenca-Estrella M, Rodero L, Carpintero Y, Gorgojo B. Influence of shaking on antifungal susceptibility testing of Cryptococcus neoformans: a comparison of the NCCLS standard M27 A medium, buffered yeasts nitrogen base, and RPMI–2% glucose. Antimicrob Agents Chemother. 2000;44:400–404. doi: 10.1128/aac.44.2.400-404.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tornatore M A, Noskin G A, Hacek D M, Obias A A, Peterson L R. Effects of incubation time and buffer concentration on in vitro activities of antifungal agents against Candida albicans. J Clin Microbiol. 1997;35:1473–1476. doi: 10.1128/jcm.35.6.1473-1476.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]