Germline mutations in the adenomatous polyposis coli (APC) gene are responsible for familial adenomatous polyposis (FAP). FAP patients develop multiple colonic adenomas early in life. The number of polyps can vary considerably because of different mutations1 but also for family members carrying the same mutation.2 Several genes have been considered as modifiers for the intrafamilial severity differences.3
We generated APC1311/+ mutant pigs, orthologous to the hotspot APC1309 mutation,4 which is associated with severe FAP in humans. These pigs recapitulate major hallmarks of the human disease5 and provide a translational model for preclinical studies.6 As observed in human patients, the polyposis varies significantly among APC1311/+ pigs, including between siblings (Figure 1A). The study aimed to identify genetic variants responsible for the phenotype.
Figure 1.
Identification of 3'UTR polymorphism that influences polyposis severity in APC1311/+pigs.(A) Endoscopic view showing different degrees of polyposis. (B) Genomic structure of the wild-type (wt) and mutated (mut) APC gene. The transcription termination cassette is shown as yellow box. SNPs are marked with arrows. The c.10046A/G SNP is indicated by red asterisks. The analyzed CpG sites are shown as open circles. (C) Association analysis between the c.10046A/G SNP and polyp number in APC1311/+ pigs (n = 201). The box plot shows the average number and standard deviation values. (D) Pedigree chart showing distribution of c.10046A/G genotypes and colon polyposis over 5 generations. Red indicates high and blue indicates low polyp number. Male, square symbol; female, circle. (E and F) mRNA laser microdissected normal colon crypts (n = 20), and protein (normal mucosa) expression from wild-type APC allele. Quantitative measurement of Western blot (n = 8 per genotype) (G) DNA methylation of APC promoter, exon 17, and 3'UTR in colon mucosa samples with A∗A (n = 26), A∗G (n = 25), and GG (n = 4) genotype. Average and standard deviations are shown. (H) In vitro 3'UTR luciferase assay (2 triplicate experiments per variant). The mimic-17-5P was cotransfected with both 3'UTR variants. ∗ P < 0.05, ∗∗ P < 0.01, ∗∗∗ P < 0.001.
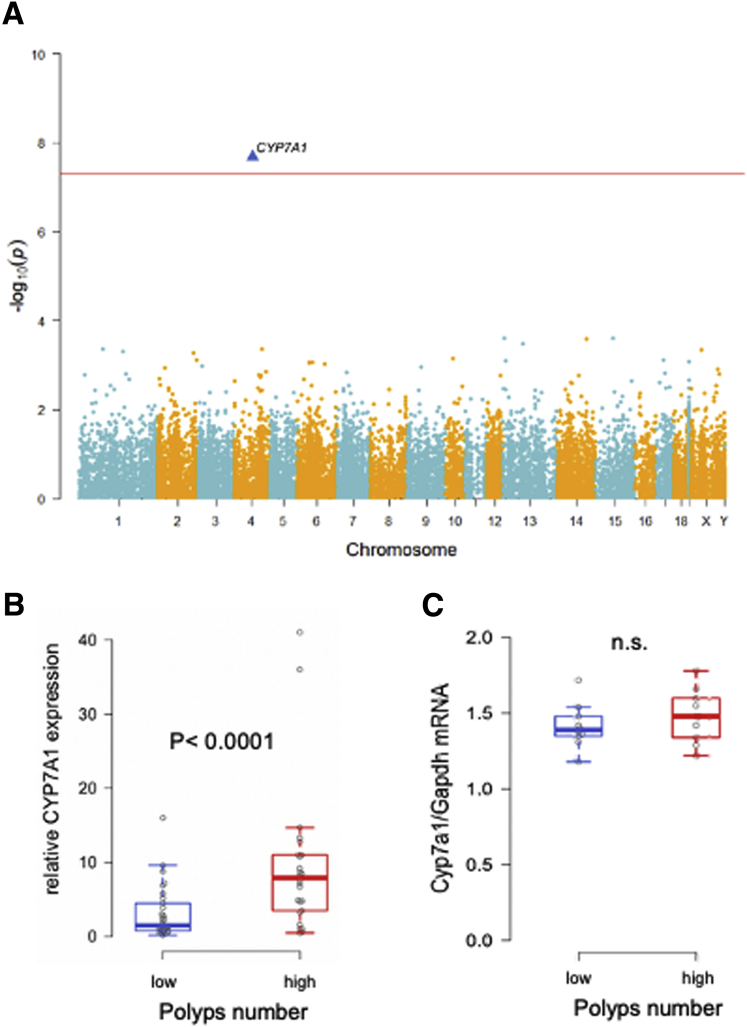
To identify differentially expressed genes, RNA sequencing of 35 normal mucosa samples from APC1311/+ pigs with low or high polyp number was carried out. After multi-comparison testing, the only significant difference was obtained for cholesterol 7 alpha-hydroxylase (Supplementary Figure 1A and B). However, the up-regulation of the gene is likely the result of colon inflammation,7 as confirmed by similar cholesterol 7 alpha-hydroxylase mRNA expression in non-inflamed colon mucosa of young APC1311/+ pigs (Supplementary Figure 1C).
Supplementary Figure 1.
Differentially expressed genes in normal mucosa of APC1311/+ pigs with low alnd high polyp number. (A) Whole genome association analysis.Quantitative PCR analysis showing CYP7A1 expression in normal mucosa of 4- month old (B) and 1- month old (C) APC1311/+ pigs.
Because renewed wild-type APC expression increased apoptosis of colorectal cancer cells,8 we asked whether expression differences in APC itself could be responsible for the polyposis variance. Sequencing of DNA regions controlling expression, eg, promoter (-2000 base pairs) and 2500 base pairs of 3'UTR, from APC1311/+ paired siblings with low (<20, n = 10) or high polyp number (∼200, n = 10) detected no single nucleotide polymorphism (SNP) in the promoter region but 3 SNPs in the 3'UTR (c.8831A/C, c.9724G/T, c.10046A/G). These segregated as a common haplotype, and c.10046A/G SNP falls within the conserved recognition site for miR17-5P (Figure 1B), which has been shown to play a role in colorectal cancer progression in humans.9 At this SNP position the mutant APC1311 allele showed adenine (A∗), whereas the wild-type allele was either guanine (G) or adenine (A). Two hundred one APC1311/+ pigs, representing 5 generations, were genotyped, revealing a highly significant correlation (Kruskal-Wallis test; P = 2.04e-23); pigs with A∗A genotype averaged 12 and with A∗G 107 polyps (last 40 cm of the colon) (interquartile range = 28.5 versus interquartile range = 138) (Figure 1C and D). These results indicated that the genotype of the wild-type APC allele determines the severity of polyposis in APC1311/+ pigs.
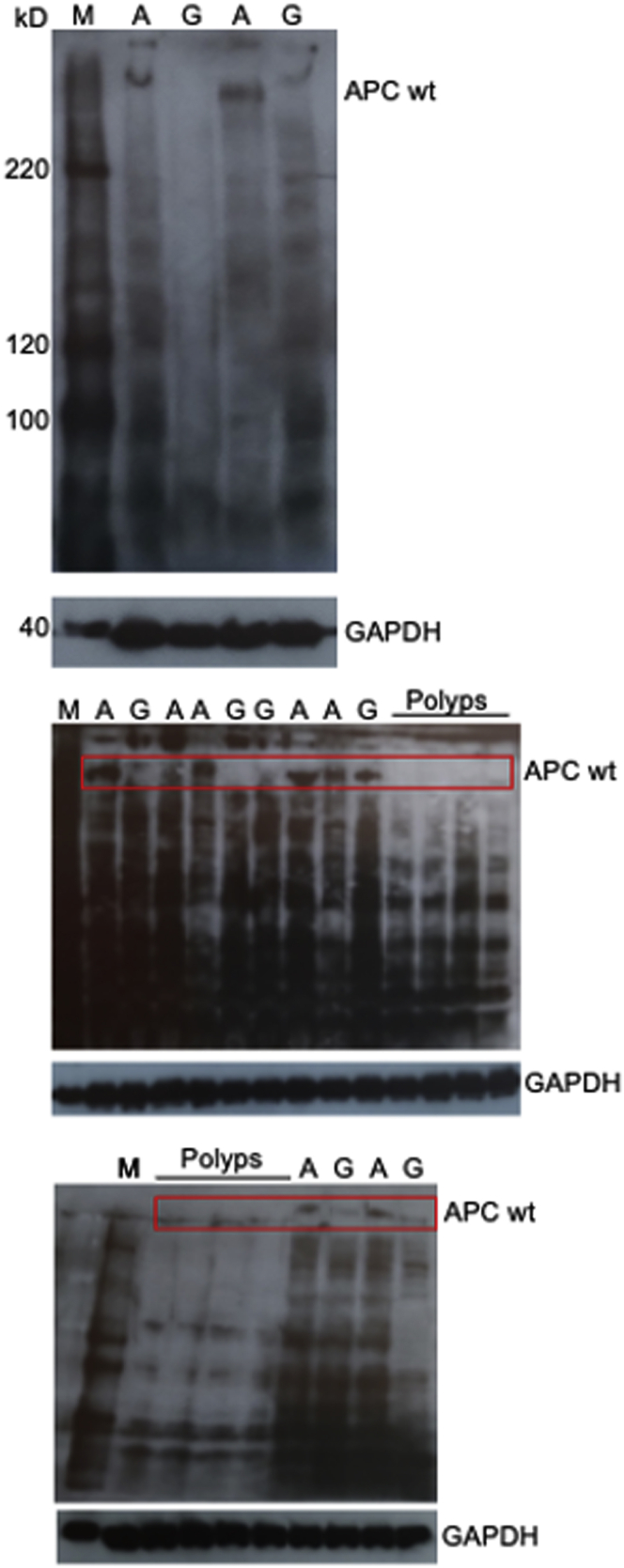
The mutant APC1311 allele generates a shortened mRNA and a truncated protein. By using reverse transcriptase polymerase chain reaction, primers specific for the 3' end or antibodies for the C-terminus mRNA and protein derived solely from the wild-type alleles could be quantified. In normal mucosa, the expression of the G allele was reduced by approximately 2-fold for both mRNA and protein (Figure 1E and F, Supplementary Figure 2). This was not due to altered CpG methylation of the promoter; here too changes were restricted to the 3'UTR (Figure 1G). A luciferase-based 3'UTR assay showed a significant activity reduction for the G compared with the A variant (Figure 1H). Addition of the Mimic-17-5P resulted in decreased luciferase values for both alleles. Together, these results indicated that the c.10046A/G SNP was responsible for the difference in APC expression and disease severity.
Supplementary Figure 2.
Uncropped Western blots for wild-type APC protein.
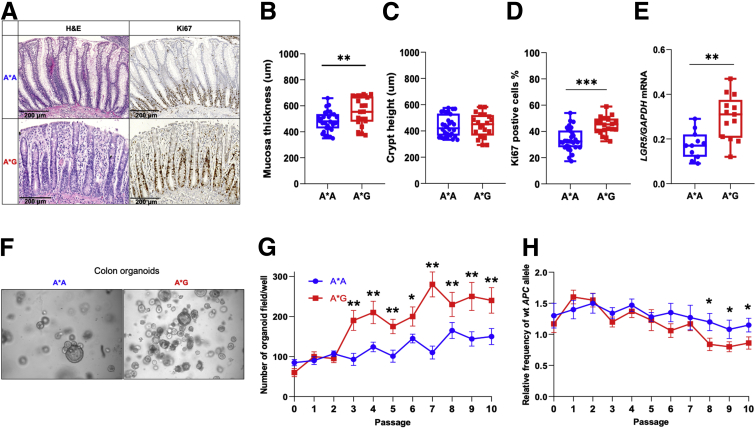
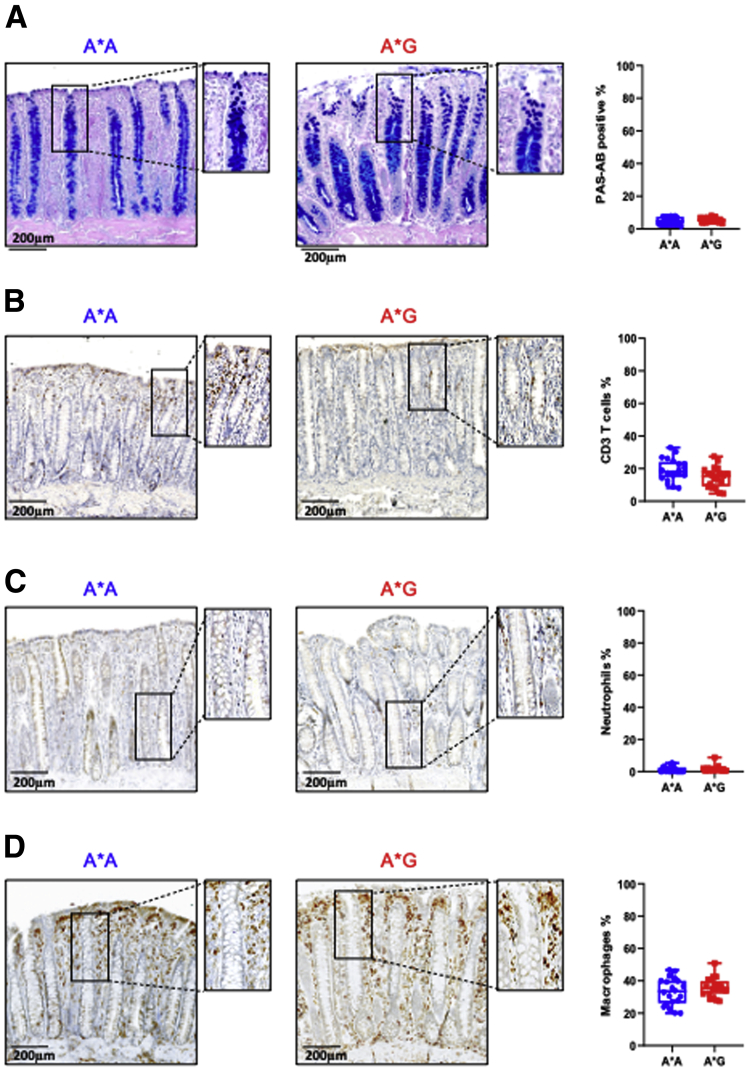
Functional analysis of normal mucosa showed increased mucosa thickness for the A∗G genotype, but similar crypt length, a higher number of Ki67 positive cells, and increased mRNA expression of the colon stem cells marker LGR5 (Figure 2A–E). This was reflected in the increased ability to form organoids for the colon epithelium of A∗G genotype (Figure 2F and G), which was associated with increased inactivation of the wild-type APC allele (Figure 2H). Immunostaining revealed no differences for goblet cells, CD3+, neutrophils, and macrophages between A∗A and A∗G genotypes (Supplementary Figure 3).
Figure 2.
Altered wild-type APC expression affects the function of the normal colon epithelium in APC1311/+pigs.(A) Representative hematoxylin-eosin and Ki67 immunohistochemistry staining. Scale bar, 200 μm. (B) Measurements of mucosa thickness. (C) Measurements of crypts heights.(D) Percentage of Ki67 positive cells (n = 30 per genotype). (E) LGR5 mRNA expression in laser microdissected epithelial crypts from normal mucosa samples (n = 12). (F) Representative pictures showing formation of organoids derived from normal colon mucosa. (G) Number of organoids counted on bright field images (n = 6 per passage). (H) Relative frequency of wild-type (wt) APC allele measured by quantitative polymerase chain reaction in colon organoids (n = 2 per genotype). ∗P < .05, ∗∗P < .01, ∗∗∗P < .001.
Supplementary Figure 3.
Immunohistochemistry staining and quantitative measurement of normal colon mucosa sections from APC1311/+ pigs with A∗A (n = 20) and A∗G (n = 20) genotype. Immunostaing for Goblet cells (A), CD3 T cells (B), neutrophils (C), and macrophages (D).
In summary, normal tissue hemostasis in the APC1311 model is only possible if the effect of the mutant allele is counteracted by strong expression of the wild-type APC allele. Expression of truncated APC in combination with reduced expression of wild-type allele enhances Lgr5 and Wnt signaling, cell proliferation, and the risk of second mutations, eg, loss of heterozygosity resulting in polyposis. The results provide evidence supporting previous observations showing that reduced APC mRNA expression can be associated with polyp formation in human FAP patients,10 thus arguing that APC itself can function as an FAP modifier gene and that expression imbalance is a disease risk factor.
A detailed description of methods is included in the Supplementary Material (www.cmghjournal.org or http://doi.org/10.1016/j.jcmgh.2021.11.002).
Acknowledgments
The authors thank Alexander Carrapeiro and Johanna Tebbing for their technical support and Steffen and Viola Löbnitz for animal husbandry. The authors also thank Prof. Riccardo Fodde, Erasmus UMC Rotterdam for the critical discussion of the work.
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding Supported by the Mildred Scheel Foundation for cancer research in Germany, grant number 111902, SFB1321 (Project-ID 329628492) and National Science Centre in Poland, grant number 2013/10/M/NZ2/00284 and the Studienstiftung des deutschen Volkes - German Academic Scholarship Foundation.
Supplementary Material
References
- 1.Nugent K.P., et al. Gut. 1994;35:1622–1623. doi: 10.1136/gut.35.11.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crabtree M.D., et al. Gut. 2002;51:420–423. doi: 10.1136/gut.51.3.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kwong L.N., et al. Adv Exp Med Biol. 2009;656:85–106. doi: 10.1007/978-1-4419-1145-2_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flisikowska T., et al. Gastroenterology. 2012;143:1173–1175. doi: 10.1053/j.gastro.2012.07.110. [DOI] [PubMed] [Google Scholar]
- 5.Flisikowska T., et al. Sci Rep. 2017;7 doi: 10.1038/s41598-017-06741-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yim J.J., et al. Proc Natl Acad Sci U S A. 2021;118 [Google Scholar]
- 7.Jia W., et al. Nat Rev Gastroenterol Hepatol. 2018;15:111–128. doi: 10.1038/nrgastro.2017.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morin P.J., et al. Proc Natl Acad Sci U S A. 1996;93:7950–7954. doi: 10.1073/pnas.93.15.7950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma Y., et al. Nat Commun. 2012;3 [Google Scholar]
- 10.Yan H., et al. Nat Genetics. 2002;30:25–26. doi: 10.1038/ng799. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.