Abstract
Meningiomas are the most common primary intracranial brain tumor and have been divided into 15 histologic subtypes, which are further classified into 3 grades according to biological behavior. Lymphoplasmacyte-rich meningioma is a rare histologic subtype of benign (grade 1) meningiomas characterized by prominent infiltration of plasma cells and lymphocytes, with a variable proportion of meningothelial elements. These benign meningioma variants usually cause significant peritumoral brain edema and mimic higher-grade lesions, which is believed to represent inflammatory cell infiltration rather than true neoplastic invasion. Bone invasion in these tumors is exceedingly rare and its clinical significance remains elusive. We describe the case of a lymphoplasmacyte-rich meningioma with skull invasion and peritumoral brain edema in a 57-year-old female patient presenting with left hemiparesis. Gross total resection of the lesion and adjacent skull were performed and histophatological examination disclosed a lymphoplasmacyte-rich meningioma. Gradual decrease of the parenchymal edema was seen on postoperative imaging studies and the patient showed progressive improvement of the motor deficit. This case report depicts rare bone invasion by lymphoplasmacyte-rich meningioma and highlights the other imaging features of this rare histologic subtype of benign meningioma. Due to the paucity of cases, gross total resection, and long-term follow-up are warranted as the prognosis of these tumors is still not fully understood.
Kewyords: Lymphoplasmacyte-rich meningioma, WHO grade 1 tumor, Vasogenic edema, Magnetic resonance imaging
Introduction
Meningiomas are the most common primary intracranial brain tumor, representing approximately 37% of all primary central nervous system (CNS) tumours in adults [1,2]. The 2021 World Health Organization (WHO) classification of CNS tumors divides meningiomas into 15 histologic subtypes, which are further classified into 3 grades that predict the prognosis of the tumor [3,4,5]. Lymphoplasmacyte-rich (LPR) meningioma is a rare histologic subtype of benign (WHO grade 1) meningiomas with approximately 60 cases reported in the literature [6,7]. This variant is known to be associated with peritumoral brain edema in roughly 60% of cases [6], which may explain the accompanying clinical features. In addition, bone invasion is extremely rare [8]. Therefore, we report the rare case of a LPR meningioma with bone invasion presenting with left hemiparesis, which resolved after tumor excision.
Case presentation
A 57-year-old female patient with unremarkable medical history presented to our hospital after several falls without traumatic head injury. Moderate left hemiparesis (grade 3 in the medical research council [MRS] scale) was noted on the neurologic examination, with no evidence of sensory disturbances or cranial nerve injury. A full laboratorial work-up, chest radiography and electrocardiogram were performed and showed no abnormalities.
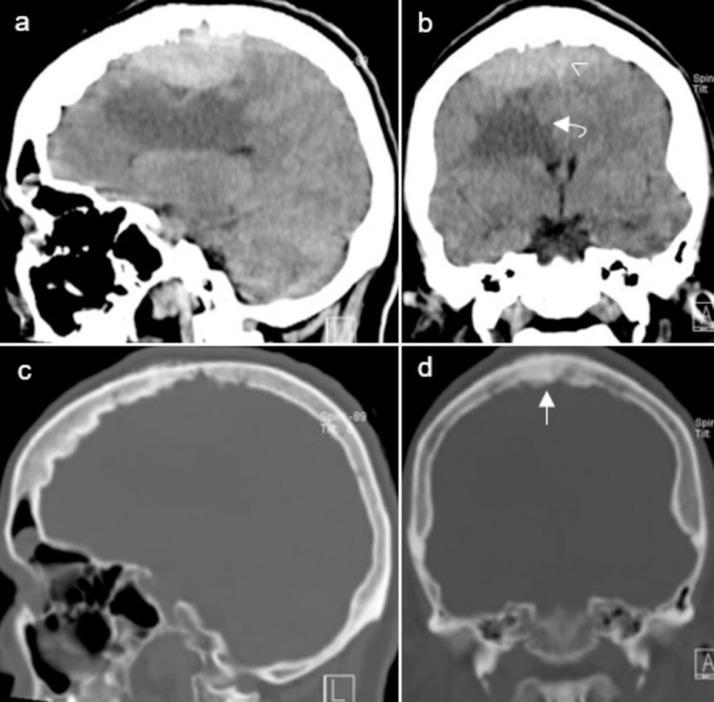
Due to the left-sided motor deficit, the patient underwent computed tomography (CT) scan as first-choice imaging technique. The CT scan revealed a bilateral extra-axial frontoparietal space-occupying lesion, measuring approximately 55 × 70 × 18 millimeters (anterior-posterior x transverse x craniocaudal). The lesion was slightly hyperdense relative to the cerebral cortex, caused hyperostotic reaction of the adjacent skull, and encased the middle third of the superior sagittal sinus (SSS). The underlying right frontal lobe and body of the corpus callosum showed prominent edema. Mass effect was seen with effacement of the adjacent sulci and buckling of the ventricular system (Fig. 1).
Fig. 1.
Preoperative CT scan of the lesion. Sagittal (A) and coronal (B) brain window scans show a bilateral extra-axial frontoparietal space-occupying lesion. The lesion was slightly hyperdense relative to the cerebral cortex and encased the middle third of the SSS (arrowhead). The underlying right frontal lobe and body of the corpus callosum showed prominent edema (curved arrow). Sagittal (C) and coronal (D) bone window scans show hyperostosis (straight arrow) of the adjacent skull.
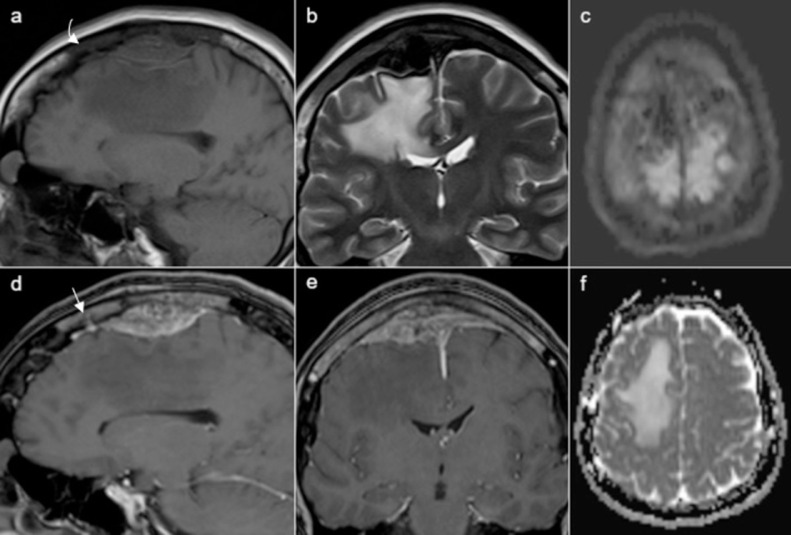
On magnetic resonance imaging (MRI) the lesion was isointense relative to the cerebral cortex on T1-weighted imaging (T1WI), hypointense on T2-weighted imaging (T2WI), did not show restricted diffusion, and exhibited relatively homogeneous enhancement after intravenous gadolinium injection. The middle third of the SSS was completely encased and occluded by the mass lesion. The underlying area of cerebral vasogenic edema displayed both high T2-signal intensity and facilitated diffusion on the apparent diffusion coefficient (ADC) map. The adjacent calvaria also showed abnormal signal intensity, displaying hypointensity on both T1WI and T2WI and enhancement on postcontrast fat-suppression T1WI (Fig. 2).
Fig. 2.
Preoperative MRI of the lesion. The lesion was isointense relative to the cerebral cortex on T1WI (A), hypointense on T2WI (B), did not show restricted diffusion on the diffusion-weighted imaging sequence (DWI, C) and exhibited relatively homogeneous enhancement on postcontrast fat-suppression T1WI (D,E). The underlying area of cerebral vasogenic edema displayed high signal intensity on T2WI (B) and facilitated diffusion on the ADC map (F). The adjacent skull also showed abnormal signal intensity, displaying hypointensity on both T1WI (a, curved arrow) and T2WI (B) and relatively homogeneous enhancement on sagittal (D, straight arrow) and coronal (E) postcontrast fat-suppression T1WI.
The patient underwent surgery through a bilateral parasagittal approach. Gross total resection of the lesion was performed with excision of the occluded segment of the SSS, followed by cranioplasty with a titanium mesh.
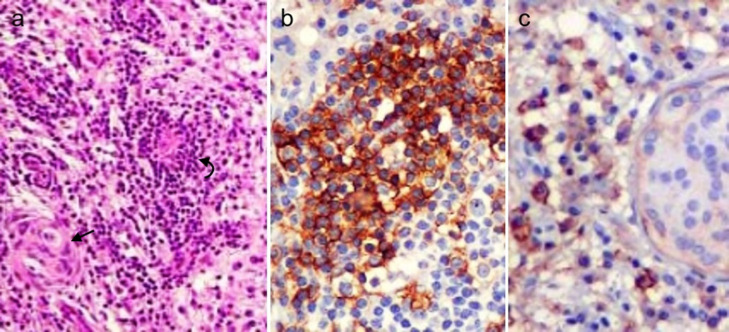
Pathologic examination of the surgical specimen showed dense dural infiltration by lymphocytes and plasma cells, admixed with multiple whorls of meningothelial cells. The inflammatory cells displayed immunoreactivity for CD3 and CD20 and the meningothelial cells were weakly reactive for epithelial membrane antigen (EMA) (Fig. 3). These findings were consistent with LPR meningioma (WHO grade 1).
Fig. 3.
Microphotographs of the histologic preparations of the surgical specimen. Low-magnification view of the hematoxylin and eosin stain (A) displays abundant lymphoplasmacytic infiltrates, mainly in a perivascular distribution (curved arrow), admixed with whorls of meningothelial cells (straight arrow). The lymphocytic infiltrates with big nuclei showed strong CD20 immunoreactivity (B) and the meningothelial cells were weakly reactive to EMA (C).
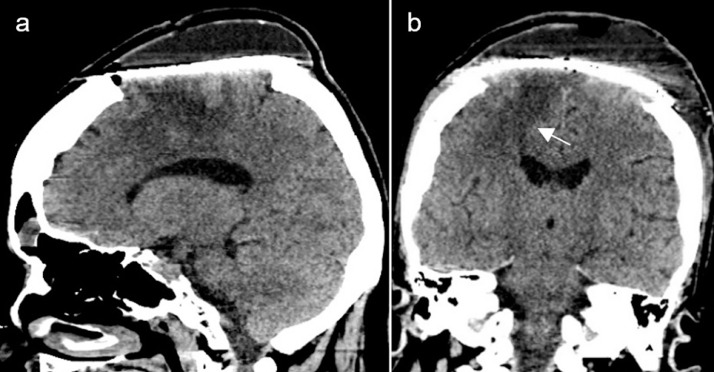
The postoperative course was uneventful and the patient showed progressive improvement of the left hemiparesis in the following days, presenting mild left-sided motor deficit at discharge (grade 4 in the MRS scale). A postoperative CT scan performed the day before discharge showed gross total resection of the lesion and middle third of the SSS, as well as partial resolution of the right frontal lobe vasogenic edema, and related mass effect (Fig. 4).
Fig. 4.
Postoperative CT scan before discharge. Sagittal (A) and coronal (B) brain window scans show gross total resection of the lesion and middle third of the SSS, as well as partial resolution of the right frontal lobe vasogenic edema (arrow) and related mass effect.
The patient remained under follow-up appointments after discharge and has fully recovered from the motor deficit, with no evidence of tumour recurrence during a 1-year follow-up period.
Discussion
Meningiomas are common brain tumors derived from arachnoid cap cells, which form the outer lining of the arachnoid membrane [9]. The 2021 WHO classification of CNS tumors divides meningiomas into 3 grades and 15 histologic subtypes, including the LPR meningioma.
Meningothelial, fibrous and transitional meningiomas are the most common subtypes, accounting for approximately 80% of all meningiomas [4,5]. For this reason, these variants have been so-called ‘typical’ meningiomas, while the remaining 12 subtypes correspond to the uncommon ‘atypical’ meningiomas [4].
LPR is a rare pathologic subtype of WHO grade 1 meningiomas with an estimated incidence of less than 1% of all meningiomas [10]. This variant was originally reported by Banerjee and Blackwood in 1971 [11] and has been recognized by the WHO classification of CNS tumors since 1993 [6]. It is characterized by a prominent infiltration of plasma cells and lymphocytes, with a variable proportion of meningothelial elements [6,9].
LPR meningioma may occur at any age and most commonly affects young and middle-age adults without sex predominance [4,6], differing from the most common subtypes of meningiomas which usually affect women. Hematological abnormalities such as hypergammaglobulinemia and iron refractory anemia have also been reported with an estimated prevalence of about 21% [6], which tend to resolve after tumor excision, and reappear with tumor recurrence. It is believed that the inflammatory cell infiltrates are responsible for the blood abnormalities and that the inflammatory cell reaction in this meningioma subtype is secondary, possibly reflecting an uncommon immunologic response of the host individual [6].
Zhu et al [6] reviewed 62 cases of LPR meningiomas published in the literature, the majority of which were located along the convexity, skull base, falx and cervical canal. These patients presented with nonspecific clinical features including headache, hemiparesis, seizures, vomiting and visual disturbance, most likely as the result of intracranial hypertension, which tends to occur earlier and with greater severity comparing to other subtypes of WHO grade 1 meningiomas [12].
On imaging studies these tumors usually present as dural-based en-plaque lesions, with irregular margins and unclear tumor boundaries, which may resemble atypical or malignant meningiomas [9,12]. Nakayama et al [13] reported a LPR meningioma with multiple foci of linear enhancement spreading into the adjacent sulci and brain parenchyma. Histologic examination of the surgical specimen showed inflammatory cell infiltrates in the dura mater, as well as the subarachnoid, and Virchow-Robin spaces. In addition, there were no signs of cellular atypia or atypical lymphoid proliferation, and the Ki-67 index of the tumor was less than 1%. Taken together, these findings suggest that the irregular margins, and unclear boundaries with neighboring brain tissue seen in most LPR meningiomas may be explained by the presence of rich inflammatory infiltrates rather than neoplastic meningeal cells.
Another common imaging feature of this benign meningioma variant is the presence of peritumoral brain edema, which was seen in roughly 60% of all reported cases of LPR meningiomas [6], and may be multifactorial in origin. First, Lee et al [14] retrospectively reviewed 79 cases of meningiomas and showed peritumoral brain edema was mainly related with the type of blood supply (pial vs dural blood supply). This study found a positive correlation between pial-cortical blood supply and the occurrence of brain edema, as it reflects a close spatial relationship between the tumor surface, and the adjacent brain parenchyma. Whether LPR meningiomas have a dominant pial blood supply remains to be elucidated. Second, Park el al [15] found on immunohistochemical analysis a significantly higher expression of IL-6 in the cytoplasm of tumor cells in edematous benign meningiomas compared to non–edematous tumors, suggesting a contribution of inflammatory cells in the pathogenesis of peritumoral brain edema. Third, we also hypothesize that the inflammatory cells found by Nakayama et al [13] in the Virchow-Robin spaces may also infiltrate the brain parenchyma and account for the occurrence of peritumoral brain edema in LPR meningiomas.
LPR meningiomas are usually hyperdense on non–contrast CT scans and may show restricted diffusion on MRI, owing to the high cellularity derived from abundant lymphocyte infiltrates. In addition, these tumors are frequently isointense to hypointense on T1WI and show variable signal intensity on T2WI, depending on its cellularity. Intense homogeneous enhancement and dural tail sign may be seen after intravenous gadolinium injection [4,5], although heterogeneous enhancement has also been described [6]. Cyst formation in the adjacent brain parenchyma has been reported to occur in about 30% of LPR meningiomas [5,9].
Although the biological behavior of this rare meningioma variant is mostly benign, roughly a third of reported cases showed a proliferation index higher than 3%. Zhu at al [6] counted 7 cases of tumor recurrence and 2 deaths in 2 years after surgery in a total of 62 reported LPR meningiomas, although total resection was achieved in only about 60% of the cases. Therefore, the prognosis of LPR is not clear, even though recurrence after complete resection has not been reported till date [8].
Bone invasion in these tumors seems to be exceedingly rare. Kurmi et al [8] reported a case of a LPR meningioma with parietal bone invasion in a 32-year-old male patient. Gross total removal of the lesion was performed and the overlying parietal bone was found to be thickened and soft with tumoral invasion. The Ki-67 antigen and/or MIB-1 monoclonal antibody index was low (0%-1%) and the patient remained clinically well during a 3-month follow-up period with no evidence of tumor recurrence on a follow-up MRI. To the best of our knowledge, our case is probably the second case report to document calvarial invasion from LPR meningioma. Indeed, we observed abnormal signal intensity in the adjacent skull, showing both T1 and T2 low-signal intensity and enhancement after contrast injection. Histopathologic examination of the surgical specimen confirmed skull infiltration by both inflammatory and neoplastic meningeal cells.
The differential diagnosis of LPR meningiomas is extensive, as the en-plaque morphology of these tumors may resemble different entities including hypertrophic pachymeningitis, granulomatous disease, histiocytic tumors, inflammatory pseudotumors, neurosarcoidosis, and lymphoma [4,7,16,17]. In some cases, imaging features of these entities may be indistinguishable, requiring histopathological, and immunohistochemical analysis to obtain a definitive diagnosis.
Treatment options for LPR meningiomas are similar to other histologic subtypes of WHO grade 1 meningiomas. Gross total resection is the treatment of choice and Simpson grade I excision is ideal [6,8,17]. The usefulness of radiation therapy in LPR meningiomas has not yet been established [18]. Hormonal therapy and immunotherapy are promising therapeutic strategies due to the immunohistochemical features of this meningioma variant, although these therapeutic options still need stronger evidence to confirm their clinical application [6]. Due to the paucity of cases, long term clinical, and radiological follow-up are warranted as the prognosis of these tumors is still not clear.
Conclusion
We described the rare case of a LPR meningioma with bone invasion presenting with hemiparesis. Due to the abundant inflammatory cell infiltrates, these benign meningioma variants are usually associated with peritumoral brain edema, which may explain accompanying clinical features not usually seen in other WHO grade 1 meningiomas. Skull invasion by these tumors is extremely rare and its clinical significance remains elusive. Gross total resection and long term clinical and radiological follow-up are warranted, as the biological behavior of LPR meningiomas is not still fully understood.
Authors’ contributions
All authors contributed to the first draft of the manuscript and made changes to its subsequent versions. All authors read and approved the final manuscript.
Patient consent
The patient has consented to the submission of the case report to the journal.
Declaration of Competing Interest
None.
Footnotes
Data availability: The data related to this article will remain confidential in order to respect the patient's right to privacy. The data will be shared on reasonable request to the corresponding author. This is an original work which has not been published and is not under consideration for publication elsewhere.
References
- 1.Huntoon K, Toland AMS, Dahiya S. Meningioma: a review of clinicopathological and molecular aspects. Front Oncol. 2020;10 doi: 10.3389/fonc.2020.579599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buerki RA, Horbinski CM, Kruser T, Horowitz PM, James CD, Lukas RV. An overview of meningiomas. Future Oncol. 2018;14(21):2161–2177. doi: 10.2217/fon-2018-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021;23(8):1231–1251. doi: 10.1093/neuonc/noab106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krishnan V, Mittal MK, Sinha M, Thukral BB. Imaging spectrum of meningiomas: a review of uncommon imagingappearances and their histopathological and prognostic significance. Pol J Radiol. 2019;84:e630–e653. doi: 10.5114/pjr.2019.92421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kunimatsu A, Kunimatsu N, Kamiya K, Katsura M, Mori H, Ohtomo K. Variants of meningiomas: a review of imaging findings and clinical features. Jpn J Radiol. 2016;34(7):459–469. doi: 10.1007/s11604-016-0550-6. [DOI] [PubMed] [Google Scholar]
- 6.Zhu HD, Xie Q, Gong Y, Mao Y, Zhong P, Hang FP, et al. Lymphoplasmacyte-rich meningioma: our experience with 19 cases and a systematic literature review. Int J Clin Exp Med. 2013;6(7):504–515. [PMC free article] [PubMed] [Google Scholar]
- 7.Yang X, Le J, Hu X, Zhang Y, Liu J. Lymphoplasmacyte-rich meningioma involving the whole intracranial dura mater. Neurology. 2018;90(20):934–935. doi: 10.1212/WNL.0000000000005532. [DOI] [PubMed] [Google Scholar]
- 8.Kurmi DJ, Sharma A, Mittal RS, Singhvi S. Lymphoplasmacyte-rich meningioma with invasion of bone: a case report and review of the literature. Asian J Neurosurg. 2016;11(4):360–363. doi: 10.4103/1793-5482.145084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yongjun L, Xin L, Qiu S, Jun-Lin Z. Imaging findings and clinical features of intracal of lymphoplasmacyte-rich meningioma. J Craniofac Surg. 2015;26(2):e132–e137. doi: 10.1097/SCS.0000000000001193. [DOI] [PubMed] [Google Scholar]
- 10.Moradi A, Semnani V, Djam H, Tajodini A, Zali AR, Ghaemi K, et al. Pathodiagnostic parameters for meningioma grading. J Clin Neurosci. 2008;15(12):1370–1375. doi: 10.1016/j.jocn.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 11.Banerjee AK, Blackwood W. A subfrontal tumour with the features of plasmacytoma and meningioma. Acta Neuropathol. 1971;18(1):84–88. doi: 10.1007/BF00684477. [DOI] [PubMed] [Google Scholar]
- 12.Liu JL, Zhou JL, Ma YH, Dong C. An analysis of magnetic resonance imaging and pathology of intracal lymphoplasmacyte-rich meningioma. Eur J Radiol. 2012;81(5):968–973. doi: 10.1016/j.ejrad.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 13.Nakayama Y, Watanabe M, Suzuki K, Usuda H, Emura I, Toyoshima Y, et al. Lymphoplasmacyte-rich meningioma: a convexity mass with regional enhancement in the adjacent brain parenchyma. Neuropathology. 2012;32(2):174–179. doi: 10.1111/j.1440-1789.2011.01236.x. [DOI] [PubMed] [Google Scholar]
- 14.Lee KJ, Joo WI, Rha HK, Park HK, Chough JK, Hong YK, et al. Peritumoral brain edema in meningiomas: correlations between magnetic resonance imaging, angiography and pathology. Surg Neurol. 2008;69(4):350–355. doi: 10.1016/j.surneu.2007.03.027. [DOI] [PubMed] [Google Scholar]
- 15.Park KJ, Kang SH, Chae YS, Yu MO, Cho TH, Suh JK, et al. Influence of interleukin-6 on the development of peritumoral brain edema in meningiomas. J Neurosurg. 2010;112(1):73–80. doi: 10.3171/2009.4.JNS09158. [DOI] [PubMed] [Google Scholar]
- 16.Hirunwiwatkul P, Trobe JD, Blaivas M. Lymphoplasmacyte-rich meningioma mimicking idiopathic hypertrophic pachymeningitis. J Neuroophthalmol. 2007;27(2):91–94. doi: 10.1097/WNO.0b013e31806773a5. [DOI] [PubMed] [Google Scholar]
- 17.Shah DB, Karki P, Paudel P, Joshi S, Upadhhya P, Sharma GR. Lymphoplasmacyte rich meningioma: a rare variant of meningioma mimicking diffuse granulomatous disease with leptomeningeal spread. Nepal J Neurosci. 2020;17(3):36–40. doi: 10.3126/njn.v17i3.33123. [DOI] [Google Scholar]
- 18.Nohara H, Furuya K, Kawahara N, Iijima A, Yako K, Shibahara J, et al. Lymphoplasmacyte-rich meningioma with atypical invasive nature. Neurol Med Chir. 2007;47(1):32–35. doi: 10.2176/nmc.47.32. [DOI] [PubMed] [Google Scholar]