Abstract
Objective
Women with more adverse childhood experiences (ACEs) may face a triple threat of risk factors for cognitive concerns during the menopause transition: reduced estradiol, increased inflammation, and early life stress sequelae. Our objective was to determine the extent to which ACEs and peripheral basal inflammatory markers associate with verbal memory across the menopause transition.
Methods
Penn Ovarian Aging cohort participants (n = 167) were assessed for ACEs (low (0–1) or high (≥2)) and had remaining stored blood samples at study end assayed for interleukin (IL)-6, IL-1-beta (IL-1β), C-reactive protein (CRP), and tumor necrosis factor alpha (TNF-α). Annual assessment included a verbal memory test (the Buschke Selective Reminding Test) and menopause stage determination. To estimate the effects of menopause stage, ACEs, and cytokines on verbal memory, repeated cognitive outcome measures were modeled in generalized estimating equations. Covariates included body mass index, smoking, race, education, age at baseline, and baseline verbal memory performance. Cytokine levels were log-transformed.
Results
Advancing menopause stage was associated with worse performance on immediate verbal recall and delayed verbal recall (ps < 0.001). During perimenopause, higher ACE exposure was associated with worse immediate verbal recall at higher levels of TNF-α (slope difference p = 0.041).
Conclusions
Inflammation may mechanistically link ACEs and verbal memory for high ACE women during perimenopause. Reducing inflammation for these individuals may have positive impact on verbal memory across the menopause transition.
Keywords: Menopause, Menopausal status, Childhood adversity, Cognition, Inflammation
Highlights
-
•
Controlling for age, advancing menopause stage was associated with decrements in verbal memory.
-
•
In perimenopause, high TNF-α was associated with decreased verbal memory in high ACE women.
-
•
Inflammation may be a mechanism by which menopausal high ACE women are prone to cognitive deficits.
1. Introduction
Subjective cognitive complaints such as “memory difficulties” are common and distressing among peri- and early postmenopausal woman (Gold et al., 2000; Schaafsma et al., 2010; Weber et al., 2012). While once attributed to aging only, the majority of longitudinal studies including work from our lab demonstrate a decline in cognitive performance, particularly in the verbal memory domain, across the menopause transition (Epperson et al., 2013; Fuh et al., 2006; Greendale et al., 2009; Weber et al., 2014). Multiple biological and psychosocial factors are thought to contribute to this phenomenon. Biologically, fluctuations and decline in 17β-estradiol (E2) that characterize perimenopause affect multiple brain systems and physiology including—but not limited to—the hippocampus, which is critical to verbal memory performance (Beltz and Moser, 2020; Russell et al., 2019), and the immune system, which impacts “whole body” health (Kase et al., 2020; Pozzi et al., 2006). E2 loss has been shown across numerous studies to relate to suboptimal cognitive aging (Au et al., 2016). Likewise, psychosocial factors such as experiencing multiple adverse childhood experiences (ACEs) affect adulthood cognitive ability (Richards and Wadsworth, 2004; Ritchie et al., 2011) and inflammatory profiles (Baumeister et al., 2016), including during the menopause transition (Matthews et al., 2014; Metcalf et al., 2021; Nguyen and Thurston, 2020; Shanmugan et al., 2020).
An underexplored but growing area of research examines how distal life events, including ACEs, influence how physiology is affected by the transition to menopause (e.g., Epperson et al., 2017; Heys et al., 2011; Ryan et al., 2009). ACEs have demonstrated deleterious cognitive and inflammatory effects following surgical menopause and during natural menopause transition (Matthews et al., 2014; Metcalf et al., 2021; Nguyen and Thurston, 2020; Shanmugan et al., 2020). During the menopause transition, women with more ACEs may face a trifecta of risk factors for cognitive concerns: declines in E2, increased inflammation, and early life stress sequelae. These three factors independently influence cognition; however, potential interactive effects of menopausal status, early life stress, and inflammation have yet to be adequately examined. Thus, determining whether inflammation links ACEs and menopause transition-specific cognitive decrements (e.g., decreased verbal memory performance)—in addition to identifying relevant inflammatory markers and menopause transition stages—is a novel and unexplored area of aging.
The present study sought to determine whether exposure to childhood adversity contributes to the age-independent decrement in immediate and delayed verbal memory performance we previously reported across the menopause transition among women participating in the Penn Ovarian Aging Study (POAS) (Epperson et al., 2013). We predicted that advancing menopause stage would be associated with worse verbal memory performance to a greater extent for participants with high ACEs (≥2 ACEs). Our second aim was to investigate whether inflammation further modified the interaction between menopause stage and ACEs on verbal memory performance. We predicted that peripheral basal inflammation would have a differential relationship to verbal memory performance for participants with high ACEs (≥2 ACEs) in advancing menopause stages as compared to participants with low ACEs (0–1 ACEs).
2. Methods
2.1. Cohort description
Fourteen years after study enrollment, 167 POAS participants completed the 10-item ACE Questionnaire (ACE-Q) and had remaining, unused blood samples for assessment of the following cytokines: CRP (CRP), interleukin 6 (IL-6), interleukin-beta (IL-1β), and tumor necrosis factor-alpha (TNF-ɑ). The POAS Cohort sample selection and recruitment have been described in detail previously (Freeman and Sammel, 2016). Broadly, eligibility criteria included age between 35 and 47 years; premenopausal with regular, normal length cycles; and presence of a uterus and at least 1 ovary. Substance abuse or major psychiatric disorder within the past year, psychotropic drug use, hormone therapy or hormone contraception use, and history of health problems affecting hormone function were exclusionary. Recruitment was stratified to enroll equal numbers of African American and Caucasian participants. Written informed consent was obtained for all POAS participants at study enrollment and verbal assent was obtained prior to ACE-Q completion. Only blood samples that research records confirmed were not collected during a cold or infection or during use of antibiotic, corticosteroid, psychotropic, over-the-counter cold or allergy medication were used for assays in the present study. The University of Pennsylvania institutional review board approved all study procedures.
2.2. Assessment periods for the POAS cohort
Data were collected approximately annually. Research interviewers administered the verbal memory test, conducted a structured questionnaire, measured height and weight, and collected blood samples during each assessment period. Information about demographics, menstrual cycle dates, reproductive history, health status and behaviors, and menopausal symptoms was obtained at each in-home assessment. Participants completed the ACE-Q at the end of the study, 14 year post-enrollment.
2.3. Study variables
2.3.1. Menopausal status
The initial staging system for reproductive aging in women (Soules et al., 2001) informed the current menopause stage groupings. Three menopause stages were assessed in the present work:
-
1.
Premenopause: premenopause (i.e., regular menstrual cycles ranging between 22 and 35 days) to late premenopause (i.e., change in cycle length of ≥7 days in either direction from baseline and observed for at least 1 cycle);
-
2.
Perimenopause: early transition (i.e., a change in ≥7 days in either direction from participant personal baseline for at least 2 consecutive cycles or 60 days amenorrhea) to late transition (i.e., 3–11 months of amenorrhea);
-
3.
Postmenopause (i.e., amenorrhea for ≥12 months without hysterectomy).
2.3.2. Adverse childhood experiences
Exposure to early life stress was assessed using the ACE-Q (Felitti et al., 1998). The ACE-Q is a self-report scale assessing exposure to 10 types of early life adversity—including experiences of abuse, neglect, and household dysfunction—before age 18. Participants indicate whether they have experienced each category of early life adversity prior to the age 18 or not with a yes (1) or no (0) response. Total responses range from 0 to 10 with higher scores indicating exposure to more categories of early life adversity. ACEs negatively impact a range of health outcomes in a graded fashion (Chapman et al., 2004; Felitti et al., 1998). Informed by the established association between two or more ACEs and increased depression risk in the POAS (Epperson et al., 2017) and ACEs studies (Felitti et al., 1998), in addition to worse cognitive outcomes for this group following surgical menopause (Shanmugan et al., 2020), total ACE-Q scores were dichotomized into two groups: low ACE (0–1 ACEs) or high ACE (≥2 ACEs).
2.3.3. Cognitive assessments
Buschke Selective Reminding Test (Buschke and Fuld, 1974). Participants were read a list of 16 words over the course of 6 trials. On the first trial, participants were read the entire word list. Thereafter, participants are reminded of only the words that they forgot on the previous trial. After each trial, participants were asked to recall as many words as possible. The number of words recalled after each of these trials were averaged to create an immediate recall score ranging from 0 to 16. Twenty minutes after the sixth trial, participants were asked to recall as many words from the list as possible (delayed recall, ranging from 0 to 16). To prevent possible memorization of words, a different word set was used each year for 5 years, then repeated in another 5-year cycle. Interviewers performing the test had no access to previous information collected from participants and did not know how participants had performed in previous years.
2.3.4. Inflammatory marker assessment
At each POAS assessment, menstruating participants provided blood samples for serum analysis between days 2 and 6 of 2 consecutive cycles, whereas non-menstruating participants provided 2 blood samples 1 month apart. For menstruating women, samples were taken during the early follicular phase (days 2–6) as this is a time when ovarian hormones are low and relatively stable compared to other times in the cycle (Taylor et al., 2019). Because blood samples were initially intended for measurements of hormones, the early follicular phase was chosen as this has been the preferred time for hormonal assay in longitudinal studies of menopause (El Khoudary et al., 2016; Epperson et al., 2013). Following 10 min of centrifuge, blood samples were stored at −80 °C in aliquots using polyproylene containers. Blood samples were initially collected for hormone assays (reported in Freeman et al., 2006, Gracia et al., 2005). In order for remaining blood samples to be assayed for inflammatory markers, there had to be adequate volum left and confirmation via records that the participant did not have symptoms of infection or was taking medications that might influence inflammation at time of collection (See Cohort description above for details). Appropriate blood samples were then assayed for CRP, IL-6, IL-1β, and TNF-ɑ. Serum levels of CRP were assessed by Immunoephelometry (Siemens, BNII Malvern PA), measured in singlicate on BNII. Serum levels of IL-6, IL-1β, and TNF-α were measured using Human High Sensitivity TNF-ɑ, IL-1β and IL-6 Cytokine premixed magnetic Luminex performance assay (R&D Systems) and were run in duplicate. Multiple samples for individual participants were blocked appropriately and quantified together. Duplicate values differing by more than 20% were rerun. Intra- and inter-assay coefficients of variation were 5.2% and 9.6% (IL-6), 5.3% and 12.8% (IL-1β), and 5.2% and 9.6% (TNF-α), respectively, and are not available for CRP. Sensitivity thresholds were as follows: 0.16 mg/L for CRP, 0.14 pg/mL for IL-6, 0.08 pg/mL for IL-1β, and 0.29 pg/mL for TNF-α.
2.3.5. Statistical analysis
Demographic, inflammatory markers, and cognitive differences between low or high ACEs groups were compared using two-sample t tests and Fischer's exact tests. Cytokines exhibited extreme skew and as such were compared after natural log transformation. Generalized estimating equation models with an exchangeable correlation structure to account for repeated measures were used to estimate associations between our outcomes of interest (i.e., verbal memory performance and inflammatory marker levels) and exposures (i.e., menopause stage, ACEs status, their interaction) while controlling for relevant covariates, described below. Further GEE models explored potential three-way interactions among menopause stage, ACEs status, and inflammatory marker levels on repeated cognitive outcomes. Two-sided p-values of <0.05 were considered statistically significant. As secondary analyses of the POAS cohort, these models were exploratory and did not necessitate corrections for multiple comparisons.
Covariates were determined a priori and are the same used in our previous publication focusing on cognitive changes across the menopause transition for the entire POAS cohort (n = 403, Epperson et al., 2013). Covariates included baseline cognitive performance (continuous), BMI (continuous), and the categorical variables of race (African American or Caucasian), education (high school degree or less versus any college or training after high school), smoking status, and age at baseline (<40; 40–44.99; ≥45) and were included in all models. A categorical measure of age at baseline was selected as a covariate due to the repeated nature of the data; as menopause stage is an important risk factor strongly correlated with age, selecting age at baseline plus changing values for menopausal stage with additional repeated measures within a participant reduces the extent of this correlation and allows for better interpretation of the associations between menopause stage and outcomes of interest. Models with inflammatory marker levels further included categorical smoking status as a covariate. Menopause stage was categorized into three groups: premenopause, perimenopause, and postmenopause. R (version 3.6.3) was used for analyses as well as creation of all figures.
3. Results
3.1. Participants and assessments
One hundred sixty-seven POAS cohort participants provided ACE-Q data and had remaining blood specimens appropriate for inflammatory marker assays, totaling 640 blood specimens. Over the longitudinal study, participants contributed an average of 3.2 cognitive assessments each for a toal of 503 assessments. Table 1 includes baseline demographic information, inflammatory marker levels, performance on cognitive tests, and comparisons between low and high ACE groups. The high ACE group contained significantly more individuals who identified as African American (p = 0.044) and who smoked (p = 0.047).
Table 1.
Baseline demographics, inflammatory marker levels, and cognitive test performance for low and high ACE groups.
| Characteristic | Low ACE (n = 78) |
High ACE (n = 89) |
p value |
|---|---|---|---|
| Race, n (%) | 0.044 | ||
| White | 47 (60.3%) | 39 (43.8%) | |
| African American | 31 (39.7%) | 50 (56.2%) | |
| Education, n (%) | 0.431 | ||
| High school or less | 34 (43.6%) | 33 (37.1%) | |
| Some college/training after HS | 44 (56.4%) | 56 (62.9%) | |
| BMI, mean (SD) | 29.3 (8.5) | 31.5 (8.8) | 0.111 |
| Age, mean (SD) | 44.6 (4.0) | 44.7 (3.9) | 0.977 |
| Smoker, n (%) | 19 (24.4%) | 35 (39.3%) | 0.047 |
| Log CRP, mean (SD) | 0.4 (1.6) | 0.5 (1.5) | 0.767 |
| Log IL-6, mean (SD) | 1.2 (1.8) | 1.5 (2.2) | 0.349 |
| Log IL-1β, mean (SD) | −0.6 (2.2) | −0.4 (2.5) | 0.448 |
| Log TNF-α, mean (SD) | 2.4 (1.4) | 2.4 (1.6) | 0.753 |
| Immediate word recall, mean (SD) | 11.3 (2.3) | 10.8 (1.9) | 0.149 |
| Delayed word recall, mean (SD) | 13.2 (2.8) | 12.7 (2.8) | 0.315 |
3.2. Menopause stage
Over ninety percent of the sample (94.6%, 158/167) were observed in the study from premenopause into perimenopause. Over half of the sample (56.3%, 94/167) were observed in the study from perimenopause into postmenopause. Participants spent an average (SD) of 4.14 (2.56) years in premenopause and 5.28 (2.21) years in perimenopause.
3.3. Early life adversity
Approximately 46.7% (78/167) endorsed experiencing 0 or 1 ACEs and 53.3% (89/167) endorsed experiencing 2 or more ACEs. From most frequently to least frequently endorsed, ACEs included emotional abuse (51/167; 30.5%), physical abuse (49/167; 29.3%), substance abuse in parent (49/167; 29.3%), divorce (48/167; 28.70%), sexual abuse (40/167; 24.0%), witnessing abuse of mother (40/167; 24.0%), emotional neglect (36/167; 21.6%), mental illness in parent (31/167; 18.6%), physical neglect (22/167; 13.2%), and imprisoned parent (15/167; 9.0%).
3.4. Relationship between menopause stage and cognitive performance
In models adjusted for BMI, race, education, age at baseline, and baseline cognitive performance, menopause stage was significantly associated with immediate verbal recall and delayed verbal recall. Specifically, for immediate verbal recall, the premenopause stage was associated with significantly better performance compared to the perimenopause stage (p < 0.001) and postmenopause stage. A similar finding was found for delayed verbal recall in which the premenopause stage was associated with significantly better performance compared to the perimenopause stage (p < 0.001) and postmenopause stage (p < 0.001). See Table 2 for details.
Table 2.
Interaction between menopause phase and verbal memory.
| Unadjusted estimate (95% CI) | Unadjusted p value | Adjusted∗ estimate (95% CI) | Adjusted∗ p value | |
|---|---|---|---|---|
| Immediate Recall | ||||
| Menopause phase (reference: premenopause) | <0.001 | <0.001 | ||
| Perimenopause | −0.55 (−0.72, −0.39) | <0.001 | −0.49 (−0.67, −0.32) | <0.001 |
| Postmenopause | −1.06 (−1.31, −0.80) | <0.001 | −1.01 (−1.27, −0.75) | <0.001 |
| Delayed Recall | ||||
| Menopause phase (reference: premenopause) | <0.001 | <0.001 | ||
| Perimenopause | −0.77 (−1.05, −0.50) | <0.001 | −0.70 (−0.98, −0.42) | <0.001 |
| Postmenopause | −1.17 (−1.57, −0.77) | <0.001 | −1.13 (−1.54, −0.72) | <0.001 |
Adjusted for baseline cognitive performance, BMI, race, education, and age at baseline.
3.5. Interactive effects of menopause stage and early life adversity on verbal memory performance
ACEs did not modify the relationship between menopause stage and verbal memory performance in models adjusted for BMI, race, education, age at baseline, and baseline cognitive performance for either immediate verbal recall (p = 0.377) or delayed verbal recall (p = 0.465) in these models.
3.6. Interactive effects of menopause stage, early life adversity, and inflammatory markers on verbal memory performance
3.6.1. Immediate verbal recall
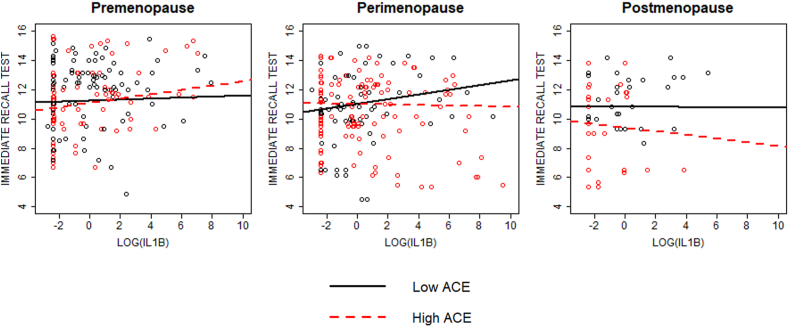
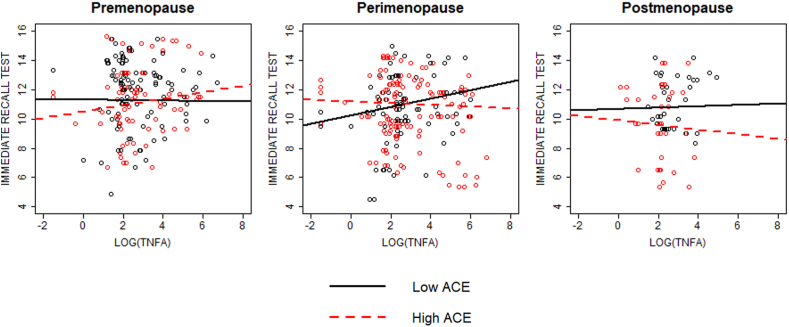
In models adjusted for covariates, menopause stage and ACEs interacted with log IL-1β and log TNF-α to impact immediate verbal recall performance (p = 0.009 and 0.026, respectively). During perimenopause, participants with more ACEs immediately recalled fewer words at higher levels of log IL-1β and log TNF-α at the marginal and statistically significant levels, respectively, relative to those with fewer ACEs (slope difference ps = 0.061 and 0.041, respectively; Fig. 1, Fig. 2). During premenopause, participants with more ACEs immediately recalled more words at higher levels of log IL-1β and log TNF-α at the trend level, relative to those with fewer ACEs (slope difference p = 0.089 and 0.062, respectively; Fig. 1, Fig. 2). Log CRP and log IL-6 did not interact with menopause stage and ACEs to impact immediate verbal recall (p = 0.971 and 0.335, respectively).
Fig. 1.
Interaction between menopausal stage, ACE group, and IL-1β on immediate verbal recall performance. Significant interaction between menopause stage, ACE group, and IL-1β on immediate recall performance (p = 0.026). During premenopause, there was a trend level difference in slopes between ACE groups (p = 0.089) such that higher levels of in the high ACE group were associated with increased cognitive performance. During perimenopause, there was a tend level difference in slopes between ACE groups (p = 0.061) such that higher levels of IL-1β in the high ACE group were associated with decreased performance. No significant difference in slopes between ACE groups during postmenopause (p = 0.591).
Fig. 2.
Interaction between menopausal stage, ACE group, and TNF-α on immediate verbal recall performance. Significant interaction between menopause stage, ACE group, and TNF-α on immediate recall performance (p = 0.009). During premenopause, there was a trend level difference in slopes between ACE groups (p = 0.062) such that higher levels of TNF-α in the high ACE group were associated with increased cognitive performance. During perimenopause, there was a significant difference in slopes between ACE groups (p = 0.041) such that higher levels of TNF-α in the high ACE group were associated with decreased performance. No significant difference in slopes between ACE groups during postmenopause (p = 0.666).
3.6.2. Delayed verbal recall
In models adjusted for the same covariates, menopause stage and ACEs did not interact with log CRP, IL-1β, IL-6, or TNF-α to impact delayed verbal recall performance (p = 0.138, 0.692, 0.424, and 0.268, respectively).
4. Discussion
Despite the smaller sample size (n = 167 vs. n = 403) and relatively fewer verbal memory test observations between this study using POAS cohort participants with ACE-Q data and remaining blood specimens versus our previously published report (Epperson et al., 2013), we observed a significant reduction in performance on an immediate and delayed word recall task from the pre-to postmenopause stages, replicating our previous finding.
Partially consistent with our hypothesis, participants in the high ACE group with higher levels of the pro-inflammatory cytokines IL-1β and TNF-α performed worse on the immediate but not delayed verbal recall task than participants in the low ACE group during perimenopause. Surprisingly, relative to their peers with in the low ACE group, participants in the high ACE group had a trend towards better immediate verbal recall at higher log IL-1β and log TNF-α during premenopause.
This longitudinal work underscores the importance of considering ACE history and staging within the menopause transition in studies of inflammation and cognitive performance. These results not only complement previous work by characterizing verbal memory performance changes over the menopause transition, but also provide novel contributions in evidencing interactive contributions of the menopause transition, early life stress history, and inflammatory marker levels to impact verbal memory performance. This study provides evidence from a longitudinal study of healthy participants who were premenopausal at study start that inflammation's relationship to verbal memory performance at different menopause stages depends on ACE status.
Our work builds on that of others demonstrating effects of the menopause transition on cognitive performance. Prior studies highlight the late transition and early postmenopause as periods of vulnerability to decrements in memory and attention (Epperson et al., 2013; Fuh et al., 2006; Greendale et al., 2009; Rentz et al., 2017; Weber et al., 2014). However, these studies did not consider ACEs, inflammation, or other potential mechanisms underlying these cognitive changes. The present study extends previous research by providing evidence that high inflammatory markers differentially impacted immediate verbal memory performance based on ACE status.
In perimenopause, participants in the high ACE group with high levels of cytokines performed worse on cognitive tasks relative to their peers in the low ACE group. For instance, the relationship between log TNF-α and immediate verbal recall was different for participants in the high versus low ACE groups; participants in the high ACE group with relatively high log TNF-α had relatively lower immediate verbal recall during perimenopause (Fig. 2. Similar patterns were observed for IL-1β, with participants in the high ACE group with high levels of this inflammatory marker performing comparatively poorly on immediate verbal recall (Fig. 1). For the participants in the high ACE group, the 5.28 (SD 2.21) years spent in perimenopause on average were associated with unique negative relationships between inflammation and verbal memory performance relative to participants with fewer ACEs.
Some of our findings highlighted the premenopause and postmenopause periods as windows where inflammation may continue to relate to cognitive performance differentially by ACE history. Unexpectedly, greater levels of inflammatory markers (IL-1β and TNF-α) were associated with relatively better immediate verbal recall among participants with more ACEs during premenopause at the trend level. There is some evidence from animal models that low levels of IL-1β improve performance in various learning paradigms (reviewed in Yirmiya and Goshen, 2011). However, the mechanism by which inflammatory cytokines might have a pro-cognitive effect specifically in the context of ACEs and premenopausal status remains unknown and will likely require additional translational research.
Reviews and meta-analytic findings support associations between increased inflammation and negative mental health and cognitive outcomes (Köhler et al., 2017; Osimo et al., 2019; Trollor and Agars, 2010; Valkanova et al., 2013), though in general this literature is mixed. Our findings provide evidence that higher levels of inflammatory markers do not associate uniformly with negative consequences and that ACEs history matters. Most studies assessing the relationship between inflammation and mental health and cognitive outcomes do not consider ACEs; our results demonstrate that they should.
Indeed, our data are consistent with observations that ACEs modify or otherwise provide nuance to the relationship between inflammation and aspects of mental health like cognition and depression. For instance, experimental research indicates that inflammatory response to the influenza vaccine (i.e., change from pre-to-post vaccine) relates to later depression symptoms and cognitive difficulty only among participants with more exposure to early life stress (Kuhlman et al., 2020). Moreover, in prospective longitudinal research among infants followed through adulthood, current depression and history of childhood maltreatment was associated with higher inflammation levels compared to current depression without childhood maltreatment (Danese et al., 2008), suggesting that depression and elevated inflammation may be more closely related among those with a childhood maltreatment history. Prospective longitudinal research among adolescents further supports a link between inflammation and depression for individuals with a history of childhood adversity, but not for individuals without such history (Miller and Cole, 2012). The link between inflammation, depression, and ACEs does raise the question of whether decrements in cognition in perimenopausal high ACE individuals with higher inflammatory burden was a byproduct of depression. While possible, evidence suggests that inflammation is an intermediary for both depression (Kuhlman et al., 2020; Miller and Cole, 2012) and decreases in cognitive performance (Au et al., 2016; Krabbe et al., 2005; Reichenberg et al., 2001). As such, including levels of depression in our models would not have been appropriate.
While unable to draw causal conclusions, our findings raise the intriguing possibility of inflammation as a mechanistic link between menopause stage and cognitive performance among individuals with a history of childhood adversity. One pathway through which peripheral inflammation may affect brain systems underlying cognition is through decreased integrity of the blood brain barrier (BBB) in the context of lower estrogen states (reviewed in Au et al., 2016) Some evidence indicates that age-related estrogen changes (i.e., reproductive senescence) may increase BBB permeability, permitting entry of potentially harmful substances into the brain (Bake and Sohrabji, 2004; Sohrabji, 2018)—including peripheral inflammatory products (reviewed in (Au et al., 2016; Banks et al., 2002; Quan and Banks, 2007). Animal research supports increased BBB permeability in the context of ovariectomy, and further indicates that E2 treatment among ovariectomized rats restores BBB permeability to levels observed in non-ovariectomized rats (Burek et al., 2010). Elevated peripheral inflammation observed among individuals with more ACEs (Baumeister et al., 2016) combined with evidence of higher sensitivity to inflammation among individuals with more ACEs (e.g., Kuhlman et al., 2020) indicate that an ACE history combined with (1) decreased neuroprotective effects of E2 during the menopause transition and (2) deleterious effects of inflammation may put this group at higher risk for decrements in cognition.
The present results found that immediate verbal recall, but not delayed verbal recall, was specifically vulnerable to the combination of ACE, perimenopausal status, and increasing inflammatory markers. This may be due to the multiple brain functions required for immediate recall in the Buschke Selective Reminding Test, making it more sensitive to pertubation. The immediate recall measure of the Buschke Selective Reminding Test requires a mix of semantic memory, episodic long-term and short-term memory as well as working memory (Burkart and Heun, 2000). Thus, performance is likely dependent upon function of the prefrontal cortex, the hippocampus, as well as communication between the two structures (Duff et al., 2020; Emch et al., 2019; Sigurdsson and Duvarci, 2016). ACEs (Hanson et al., 2012; Irigaray et al., 2013; Philip et al., 2016; Richards and Wadsworth, 2004), perimenopause (Epperson et al., 2013; Greendale et al., 2009; Maki, 2015), and inflammation (Donzis and Tronson, 2014; Yirmiya and Goshen, 2011) have all been associated with deficits in both hippocampal-dependent memory as well as prefrontal cortex-dependent processes like working memory. Interestingly, some evidence in animals suggests that inflammation may only disrupt working memory in the context of pre-existing hippocampal synaptic loss (Skelly et al., 2019). ACE-induced decreases in hippocampal volume (Teicher et al., 2012) may increase vulnerability to the effects of inflammatory cytokines on cognitive tasks involving working memory, like immediate recall, that require coordination between the cortex and hippocampus. This is probably especially true in the context of estradiol withdrawal in perimenopause, during which the effects of inflammation on the brain are likely further magnified (Au et al., 2016).
Clinical implications of this work include the importance of identifying and counseling individuals with two or more ACEs, who may differentially experience greater increases in inflammation during the menopause transition (Metcalf et al., 2021). Clinicians ought to consider modifiable factors with respect to reducing inflammation for this high-risk group, particularly in light of findings demonstrating differentially elevated inflammation markers among participants with two or more ACEs during the late perimenopause transition relative to premenopause (Metcalf et al., 2021). Determining the cognitive benefits of interventions to reduce inflammation among high ACE women during the menopause is an area that is ripe for research. Indeed, efforts to improve sleep, diet, and exercise and reduce stress may be even more important among those whose early life experiences affect physical and cognitive responses to the menopause transition. Because behavior change can be difficult, providing research-based information to individuals with two or more ACEs about the potential negative consequences of unmitigated inflammation on cognition during the menopause transition may prove motivational. Impaired cognition is a common subjective complaint among midlife individuals (Mitchell and Woods, 2011). Unfortunately, it is impossible to change early life stress exposure after the fact, but inflammation represents a risk factor for these individuals that is modifiable with intervention.
4.1. Strengths & limitations
Strengths of this work include that all participants were generally healthy and premenopausal at study start. We statistically accounted for variables known to impact inflammation and cognitive outcomes, including age at baseline, BMI, education, and baseline cognitive performance. Data analyses in this work revealed one statistically significant and three trend-level 3-way interactions between variables of interest, representing a strength of the present work and indicating promise of further exploration between ACEs, inflammation, and cognition during the menopause transition.
Analyses in the present work were secondary and used a sample of convenience, and as such were subject to some limitations. Evaluation of childhood adversity was done by retrospective self-report, which can differ from prospective measurements due to memory limitations, recall bias, or individual preference for disclosure (Baldwin et al., 2019). The limitations of human memory specifically precluded participants in our study from reporting adversity that occurred either in utero or during the early postnatal period. This is relevant given that prenatal and early postnatal stress has effects on the brain and immune system (Creutzberg et al., 2021; Dutcher et al., 2020; Weinstock, 2016). An additional limitation includes focus on cross-sectional effects in three menopause stages due to limited availability of leftover blood samples for cytokine measurement. Examining associations between within-participant changes in inflammatory markers over the menopause transition and cognitive performance in future large longitudinal studies will be valuable, as will ensuring inflammatory marker measurement in all menopause stages for every participant. As our statistical approach featured cross-sectional analyses within menopause stage, causal interpretations are not possible.
5. Conclusions
When considering contributors to poor cognitive function during midlife for women, our data supported menopause stage, early life adversity, and inflammation as relevant risk factors. Broadly, our findings suggest the importance of perimenopause as a window of risk for worsened verbal memory performance. Inflammation was associated with worsened immediate verbal recall for participants with a history of early life adversity during the perimenopause, a stage that can last for multiple years. To our knowledge, this study represents the first longitudinal research to examine how inflammation markers interact with ACEs and menopause stage to impact verbal memory outcomes. Evidence indicated that ACEs history is an important modifier. Historically, ACEs have not been considered in studies of inflammation, cognition, and menopause transition; examining ACEs may help to explain previous mixed findings and uncover novel relationships in these domains.
Funding/support
Grants from the National Institute of Mental Health (T32 MH015442, CAM), the National Institute on Aging (U54 AG062319, AMN; R01 AG048839, CNE, MDS; and R01 AG012745-15, EWF, MDS), the Center for Women's Health Research at the University of Colorado (AMN), the Office of Research on Women's Health (P50 MH099910, CNE, MDS), and the National Institute on Drug Abuse (K24 DA030301, CNE and R01 DA37289, CNE).
Declaration of interest/financial disclosure
CAM, RLJ, AMN, EWF, MDS, and LGA have nothing to disclose. CNE serves on the Advisory Board for Sage Therapeutics, Asarina Pharma, BabyScripts and the Parthenon Management Group. She receives research grant support from Sage Therapeutics. Portions of this manuscript were presented as a poster abstract at the 2020 Annual Meeting of the North American Menopause Society.
Contributor Information
Christina A. Metcalf, Email: christina.metcalf@cuanschutz.edu.
Andrew M. Novick, Email: andrew.m.novick@cuanschutz.edu.
References
- Au A., Feher A., McPhee L., Jessa A., Oh S., Einstein G. Estrogens, inflammation and cognition. Front. Neuroendocrinol. 2016;40:87–100. doi: 10.1016/j.yfrne.2016.01.002. [DOI] [PubMed] [Google Scholar]
- Bake S., Sohrabji F. 17β-Estradiol differentially regulates blood-brain barrier permeability in young and aging female rats. Endocrinology. 2004;145:5471–5475. doi: 10.1210/en.2004-0984. [DOI] [PubMed] [Google Scholar]
- Baldwin J.R., Reuben A., Newbury J.B., Danese A. Agreement between prospective and retrospective measures of childhood maltreatment: a systematic review and meta-analysis. JAMA Psychiatr. 2019;76:584–593. doi: 10.1001/jamapsychiatry.2019.0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks W.A., Farr S.A., Morley J.E. Entry of blood-borne cytokines into the central nervous system: effects on cognitive processes. Neuroimmunomodulation. 2002;10:319–327. doi: 10.1159/000071472. [DOI] [PubMed] [Google Scholar]
- Baumeister D., Akhtar R., Ciufolini S., Pariante C.M., Mondelli V. Childhood trauma and adulthood inflammation: a meta-analysis of peripheral C-reactive protein, interleukin-6 and tumour necrosis factor-α. Mol. Psychiatr. 2016;21:642–649. doi: 10.1038/mp.2015.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltz A.M., Moser J.S. Ovarian hormones: a long overlooked but critical contributor to cognitive brain structures and function. Ann. N. Y. Acad. Sci. 2020;1464:156–180. doi: 10.1111/nyas.14255. [DOI] [PubMed] [Google Scholar]
- Burek M., Arias-Loza P.A., Roewer N., Förster C.Y. Claudin-5 as a novel estrogen target in vascular endothelium. ATVB. 2010;30:298–304. doi: 10.1161/ATVBAHA.109.197582. [DOI] [PubMed] [Google Scholar]
- Burkart M., Heun R. Psychometric analysis of the selective reminding procedure in a sample from the general elderly population. Dement. Geriatr. Cognit. Disord. 2000;11:74–80. doi: 10.1159/000017218. [DOI] [PubMed] [Google Scholar]
- Buschke H., Fuld P.A. Evaluating storage, retention, and retrieval in disordered memory and learning. Neurology. 1974;24:1019–1025. doi: 10.1212/wnl.24.11.1019. [DOI] [PubMed] [Google Scholar]
- Chapman D.P., Whitfield C.L., Felitti V.J., Dube S.R., Edwards V.J., Anda R.F. Adverse childhood experiences and the risk of depressive disorders in adulthood. J. Affect. Disord. 2004;82:217–225. doi: 10.1016/j.jad.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Creutzberg K.C., Sanson A., Viola T.W., Marchisella F., Begni V., Grassi-Oliveira R., Riva M.A. Long-lasting effects of prenatal stress on HPA axis and inflammation: a systematic review and multilevel meta-analysis in rodent studies. Neurosci. Biobehav. Rev. 2021;127:270–283. doi: 10.1016/j.neubiorev.2021.04.032. [DOI] [PubMed] [Google Scholar]
- Danese A., Moffitt T.E., Pariante C.M., Ambler A., Poulton R., Caspi A. Elevated inflammation levels in depressed adults with a history of childhood maltreatment. Arch. Gen. Psychiatr. 2008;65:409–415. doi: 10.1001/archpsyc.65.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donzis E.J., Tronson N.C. Modulation of learning and memory by cytokines: signaling mechanisms and long term consequences. Neurobiol. Learn. Mem. 2014;115:68–77. doi: 10.1016/j.nlm.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff M.C., Covington N.V., Hilverman C., Cohen N.J. Semantic memory and the Hippocampus: revisiting, reaffirming, and extending the reach of their critical relationship. Front. Hum. Neurosci. 2020;13:471. doi: 10.3389/fnhum.2019.00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutcher E.G., Pama E.A.C., Lynall M.-E., Khan S., Clatworthy M.R., Robbins T.W., Bullmore E.T., Dalley J.W. Early-life stress and inflammation: a systematic review of a key experimental approach in rodents. Brain Neurosci Adv. 2020;4 doi: 10.1177/2398212820978049. 2398212820978049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Khoudary S.R., Santoro N., Chen H.-Y., Tepper P.G., Brooks M.M., Thurston R.C., Janssen I., Harlow S.D., Barinas-Mitchell E., Selzer F., Derby C., Jackson E.A., McConnell D., Matthews K.A. Trajectories of estradiol and follicle stimulating hormone over the menopause transition and early markers of atherosclerosis after menopause. Eur. J. Prev. Cardiol. 2016;23:694–703. doi: 10.1177/2047487315607044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emch M., von Bastian C.C., Koch K. Neural correlates of verbal working memory: an fMRI meta-analysis. Front. Hum. Neurosci. 2019;13:180. doi: 10.3389/fnhum.2019.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epperson C.N., Sammel M.D., Bale T.L., Kim D.R., Conlin S., Scalice S., Freeman K., Freeman E.W. Adverse childhood experiences and risk for first-episode major depression during the menopause transition. J. Clin. Psychiatr. 2017;78:298–307. doi: 10.4088/JCP.16m10662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epperson C.N., Sammel M.D., Freeman E.W. Menopause effects on verbal memory: findings from a longitudinal community cohort. J. Clin. Endocrinol. Metabol. 2013;98:3829–3838. doi: 10.1210/jc.2013–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felitti V.J., Anda R.F., Nordenberg D., Williamson D.F., Spitz A.M., Edwards V., Koss M.P., Marks J.S. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: the adverse childhood experiences (ACE) study. Am. J. Prev. Med. 1998;14:245–258. doi: 10.1016/S0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Freeman E.W., Sammel M.D. Anxiety as a risk factor for menopausal hot flashes: evidence from the Penn Ovarian Aging cohort. Menopause. 2016;23:942–949. doi: 10.1097/GME.0000000000000662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman E.W., Sammel M.D., Lin H., Nelson D.B. Associations of hormones and menopausal status with depressed mood in women with no history of depression. Arch. Gen. Psychiatr. 2006;63:375–382. doi: 10.1001/archpsyc.63.4.375. [DOI] [PubMed] [Google Scholar]
- Fuh J.-L., Wang S.-J., Lee S.-J., Lu S.-R., Juang K.-D. A longitudinal study of cognition change during early menopausal transition in a rural community. Maturitas. 2006;53:447–453. doi: 10.1016/j.maturitas.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Gold E.B., Sternfeld B., Kelsey J.L., Brown C., Mouton C., Reame N., Salamone L., Stellato R. Relation of demographic and lifestyle factors to symptoms in a multi-racial/ethnic population of women 40–55 Years of age. Am. J. Epidemiol. 2000;152:463–473. doi: 10.1093/aje/152.5.463. [DOI] [PubMed] [Google Scholar]
- Gracia C.R., Sammel M.D., Freeman E.W., Lin H., Langan E., Kapoor S., Nelson D.B. Defining menopause status: creation of a new definition to identify the early changes of the menopausal transition. Menopause. 2005;12:128–135. doi: 10.1097/00042192-200512020-00005. [DOI] [PubMed] [Google Scholar]
- Greendale G.A., Huang M.-H., Wight R.G., Seeman T., Luetters C., Avis N.E., Johnston J., Karlamangla A.S. Effects of the menopause transition and hormone use on cognitive performance in midlife women. Neurology. 2009;72:1850–1857. doi: 10.1212/WNL.0b013e3181a71193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson J.L., Chung M.K., Avants B.B., Rudolph K.D., Shirtcliff E.A., Gee J.C., Davidson R.J., Pollak S.D. Structural variations in prefrontal cortex mediate the relationship between early childhood stress and spatial working memory. J. Neurosci. 2012;32:7917–7925. doi: 10.1523/JNEUROSCI.0307-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heys M., Jiang C., Cheng K.K., Zhang W., Yeung S.L.A., Lam T.H., Leung G.M., Schooling C.M. Life long endogenous estrogen exposure and later adulthood cognitive function in a population of naturally postmenopausal women from Southern China: the Guangzhou Biobank Cohort Study. Psychoneuroendocrinology. 2011;36:864–873. doi: 10.1016/j.psyneuen.2010.11.009. [DOI] [PubMed] [Google Scholar]
- Irigaray T.Q., Pacheco J.B., Grassi-Oliveira R., Fonseca R.P., Leite J.C. de C., Kristensen C.H. Child maltreatment and later cognitive functioning: a systematic review. Psicol. Reflexão Crítica. 2013;26:376–387. doi: 10.1590/S0102-79722013000200018. [DOI] [Google Scholar]
- Kase N.G., Gretz Friedman E., Brodman M., Kang C., Gallagher E.J., LeRoith D. The midlife transition and the risk of cardiovascular disease and cancer Part I: magnitude and mechanisms. Am. J. Obstet. Gynecol. 2020;223:820–833. doi: 10.1016/j.ajog.2020.05.051. [DOI] [PubMed] [Google Scholar]
- Köhler C.A., Freitas T.H., Maes M., Andrade N.Q. de, Liu C.S., Fernandes B.S., Stubbs B., Solmi M., Veronese N., Herrmann N., Raison C.L., Miller B.J., Lanctôt K.L., Carvalho A.F. Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies. Acta Psychiatr. Scand. 2017;135:373–387. doi: 10.1111/acps.12698. [DOI] [PubMed] [Google Scholar]
- Krabbe K.S., Reichenberg A., Yirmiya R., Smed A., Pedersen B.K., Bruunsgaard H. Low-dose endotoxemia and human neuropsychological functions. brain, behavior, and immunity, physical activity, behavior, immunity and health. 2005. 19, 453-460. [DOI] [PubMed]
- Kuhlman K.R., Robles T.F., Haydon M.D., Dooley L., Boyle C.C., Bower J.E. Early life stress sensitizes individuals to the psychological correlates of mild fluctuations in inflammation. Dev. Psychobiol. 2020;62:400–408. doi: 10.1002/dev.21908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki P.M. Verbal memory and menopause. Maturitas. 2015;82:288–290. doi: 10.1016/j.maturitas.2015.07.023. [DOI] [PubMed] [Google Scholar]
- Matthews K.A., Chang Y.-F., Thurston R.C., Bromberger J.T. Child abuse is related to inflammation in mid-life women: role of obesity. Brain Behav. Immun. 2014;36:29–34. doi: 10.1016/j.bbi.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf C.A., Johnson R.L., Freeman E.W., Sammel M.D., Epperson C.N. Influences of the menopause transition and adverse childhood experiences on peripheral basal inflammatory markers. Brain Behav. Immunity Health. 2021;15:100280. doi: 10.1016/j.bbih.2021.100280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G.E., Cole S.W. Clustering of depression and inflammation in adolescents previously exposed to childhood adversity. biological psychiatry, endocrinology, epigenetics, extinction, and early life traumatization. 2012. 72, 34-40. [DOI] [PMC free article] [PubMed]
- Mitchell E.S., Woods N.F. Cognitive symptoms during the menopausal transition and early postmenopause. Climacteric. 2011;14:252–261. doi: 10.3109/13697137.2010.516848. [DOI] [PubMed] [Google Scholar]
- Nguyen J.K., Thurston R.C. Association of childhood trauma exposure with inflammatory biomarkers among midlife women. J. Wom. Health. 2020;29:1540–1546. doi: 10.1089/jwh.2019.7779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osimo E.F., Baxter L.J., Lewis G., Jones P.B., Khandaker G.M. Prevalence of low-grade inflammation in depression: a systematic review and meta-analysis of CRP levels. Psychol. Med. 2019;49:1958–1970. doi: 10.1017/S0033291719001454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip N.S., Sweet L.H., Tyrka A.R., Carpenter S.L., Albright S.E., Price L.H., Carpenter L.L. Exposure to childhood trauma is associated with altered n-back activation and performance in healthy adults: implications for a commonly used working memory task. Brain Imag. Behav. 2016;10:124–135. doi: 10.1007/s11682-015-9373-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzi S., Benedusi V., Maggi A., Vegeto E. Estrogen action in neuroprotection and brain inflammation. Ann. N. Y. Acad. Sci. 2006;1089:302–323. doi: 10.1196/annals.1386.035. [DOI] [PubMed] [Google Scholar]
- Quan N., Banks W.A. Brain-immune communication pathways. Brain Behav. Immun. 2007;21:727–735. doi: 10.1016/j.bbi.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Reichenberg A., Yirmiya R., Schuld A., Kraus T., Haack M., Morag A., Pollmächer T. Cytokine-associated emotional and cognitive disturbances in humans. Arch. Gen. Psychiatr. 2001;58:445–452. doi: 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- Rentz D.M., Weiss B.K., Jacobs E.G., Cherkerzian S., Klibanski A., Remington A., Aizley H., Goldstein J.M. Sex differences in episodic memory in early midlife: impact of reproductive aging. Menopause. 2017;24:400–408. doi: 10.1097/GME.0000000000000771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards M., Wadsworth M.E.J. Long term effects of early adversity on cognitive function. Arch. Dis. Child. 2004;89:922–927. doi: 10.1136/adc.2003.032490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie K., Jaussent I., Stewart R., Dupuy A.-M., Courtet P., Malafosse A., Ancelin M.-L. Adverse childhood environment and late-life cognitive functioning. Int. J. Geriatr. Psychiatr. 2011;26:503–510. doi: 10.1002/gps.2553. [DOI] [PubMed] [Google Scholar]
- Russell J.K., Jones C.K., Newhouse P.A. The role of estrogen in brain and cognitive aging. Neurotherapeutics. 2019;16:649–665. doi: 10.1007/s13311-019-00766-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan J., Carrière I., Scali J., Ritchie K., Ancelin M.-L. Life-time estrogen exposure and cognitive functioning in later life. Psychoneuroendocrinology. 2009;34:287–298. doi: 10.1016/j.psyneuen.2008.09.008. [DOI] [PubMed] [Google Scholar]
- Schaafsma M., Homewood J., Taylor A. Subjective cognitive complaints at menopause associated with declines in performance of verbal memory and attentional processes. Climacteric. 2010;13:84–98. doi: 10.3109/13697130903009187. [DOI] [PubMed] [Google Scholar]
- Shanmugan S., Sammel M.D., Loughead J., Ruparel K., Gur R.C., Brown T.E., Faust J., Domchek S., Epperson C.N. Executive function after risk-reducing salpingo-oophorectomy in BRCA1 and BRCA2 mutation carriers: does current mood and early life adversity matter? Menopause. 2020;27:746–755. doi: 10.1097/GME.0000000000001535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdsson T., Duvarci S. Hippocampal-prefrontal interactions in cognition, behavior and psychiatric disease. Front. Syst. Neurosci. 2016;9:190. doi: 10.3389/fnsys.2015.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelly D.T., Griffin É.W., Murray C.L., Harney S., O'Boyle C., Hennessy E., Dansereau M.-A., Nazmi A., Tortorelli L., Rawlins J.N., Bannerman D.M., Cunningham C. Acute transient cognitive dysfunction and acute brain injury induced by systemic inflammation occur by dissociable IL-1-dependent mechanisms. Mol. Psychiatr. 2019;24:1533–1548. doi: 10.1038/s41380-018-0075-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohrabji F. Guarding the blood–brain barrier: a role for estrogen in the etiology of neurodegenerative disease. Gene Expr. 2018;13:311–319. doi: 10.3727/000000006781510723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soules M.R., Sherman S., Parrott E., Rebar R., Santoro N., Utian W., Woods N. Executive summary: stages of reproductive aging workshop (STRAW) Climacteric. 2001;4:267–272. doi: 10.1080/cmt.4.4.267.272. [DOI] [PubMed] [Google Scholar]
- Taylor H.S., Pal L., Sell E. Lippincott Williams & Wilkins; 2019. Speroff's Clinical Gynecologic Endocrinology and Infertility. [Google Scholar]
- Teicher M.H., Anderson C.M., Polcari A. Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus, and subiculum. Proc. Natl. Acad. Sci. Unit. States Am. 2012;109:E563–E572. doi: 10.1073/pnas.1115396109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trollor J., Agars E. Neuropsychiatric Disorders. Springer; Tokyo: 2010. Systemic inflammation and cognition in the elderly; pp. 177–197. [Google Scholar]
- Valkanova V., Ebmeier K.P., Allan C.L. CRP, IL-6 and depression: a systematic review and meta-analysis of longitudinal studies. J. Affect. Disord. 2013;150:736–744. doi: 10.1016/j.jad.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Weber M.T., Maki P.M., McDermott M.P. Cognition and mood in perimenopause: a systematic review and meta-analysis. J. Steroid Biochem. Mol. Biol. Current Views Hormone Therapy Manag. Treatment Postmenopausal Women. 2014;142:90–98. doi: 10.1016/j.jsbmb.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M.T., Mapstone M., Staskiewicz J., Maki P.M. Reconciling subjective memory complaints with objective memory performance in the menopausal transition. Menopause. 2012;19:735–741. doi: 10.1097/gme.0b013e318241fd22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock M. Prenatal stressors in rodents: effects on behavior. Neurobiol Stress. 2016;6:3–13. doi: 10.1016/j.ynstr.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yirmiya R., Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav. Immun. 2011;25:181–213. doi: 10.1016/j.bbi.2010.10.015. [DOI] [PubMed] [Google Scholar]