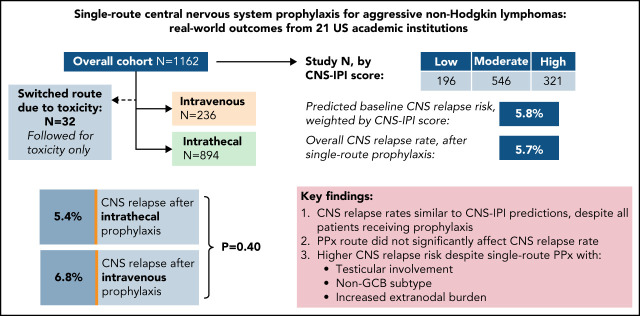
Prophylaxis to prevent central nervous system (CNS) relapse in diffuse large B-cell lymphoma (DLBCL) is widely used but has no defined standard of care. Orellana-Noia and colleagues assessed the efficacy of CNS prophylaxis in a retrospective multicenter analysis of 1162 patients. They demonstrate that the route of prophylaxis (intrathecal vs high-dose systemic therapy) does not affect relapse rate. CNS relapse remains very close to the rate predicted without prophylaxis, suggesting that the criteria and choice of therapy for CNS prophylaxis in DLBCL need to be reevaluated.
Key Points
In a large multicenter analysis of adult DLBCL patients, route of CNS prophylaxis did not affect CNS relapse rates
Testicular involvement, non-GCB subtype, and high extranodal burden predicted increased CNS risk despite MTX-based prophylaxis by either route.
Visual Abstract
Abstract
Prophylaxis is commonly used to prevent central nervous sy stem (CNS) relapse in diffuse large B-cell lymphoma (DLBCL), with no clear standard of care. We retrospectively evaluated 1162 adult patients across 21 US academic centers with DLBCL or similar histologies who received single-route CNS prophylaxis as part of frontline therapy between 2013 and 2019. Prophylaxis was administered intrathecally(IT) in 894 (77%) and using systemic high-dose methotrexate (HD-MTX) in 236 (20%); 32 patients (3%) switched route due to toxicity and were assessed separately. By CNS-International Prognostic Index (IPI), 18% were considered low-risk, 51% moderate, and 30% high. Double-hit lymphoma (DHL) was confirmed in 243 of 866 evaluable patients (21%). Sixty-four patients (5.7%) had CNS relapse after median 7.1 months from diagnosis, including 15 of 64 (23%) within the first 6 months. There was no significant difference in CNS relapse between IT and HD-MTX recipients (5.4% vs 6.8%, P = .4), including after propensity score matching to account for differences between respective recipient groups. Weighting by CNS-IPI, expected vs observed CNS relapse rates were nearly identical (5.8% vs 5.7%). Testicular involvement was associated with high risk of CNS relapse (11.3%) despite most having lower CNS-IPI scores. DHL did not significantly predict for CNS relapse after single-route prophylaxis, including with adjustment for treatment regimen and other factors. This large study of CNS prophylaxis recipients with DLBCL found no significant difference in CNS relapse rates between routes of administration. Relapse rates among high-risk subgroups remain elevated, and reconsideration of prophylaxis strategies in DLBCL is of critical need.
Introduction
Central nervous system (CNS) relapse in diffuse large B-cell lymphoma (DLBCL) occurs in ∼5% of patients overall and carries a median overall survival (OS) of only 3 to 7 months.1,2 This dreaded complication historically occurs within months of initial DLBCL diagnosis, prompting the use of CNS-directed prophylaxis with frontline therapy in select patients.3,4 Compared with Burkitt lymphoma, where CNS risk is higher and prophylaxis is considered standard practice,5,6 allocation of CNS prophylaxis in DLBCL is generally reserved for patients with high-risk features.7 These may include involvement of specific extranodal (EN) sites, such as kidneys, adrenal glands, or testes; molecular features, such as double-hit (DH) status; or a combination thereof. Which factor(s) may be used to justify CNS prophylaxis commonly varies across providers and institutions,8 and there is no clear standard of care in terms of which patients should or should not receive prophylaxis or by which route(s) this should be administered.
Consensus guidelines commonly recommend using the CNS-International Prognostic Index (CNS-IPI) to help guide prophylaxis use.4,9 This scoring system is validated for CNS relapse risk estimation and allocates 1 point each for conventional IPI risk factors as well as renal/adrenal EN involvement, where a score of ≥4 points is associated with a CNS relapse risk of 12% or greater.10 Prophylaxis was used in only a subset of included patients for both initial and subsequent validation studies of the CNS-IPI and was not standardized across the respective cohorts. Additionally, disease features not included in the CNS-IPI, such as double-hit status or testicular involvement, may affect the ability of this model to accurately discriminate “low-risk” patients. Thus, it remains unknown whether prophylaxis modifies CNS relapse risk across settings where it is commonly prescribed, and substantial differences exist between routes of administration which may further impact this.
Most modern regimens use MTX by either intrathecal (IT) or via HD systemic administration, the latter typically defined as doses of ≥3g/m2 for adequate CNS penetration.11 Differences between routes of administration include greater parenchymal penetration with HD-MTX at the cost of greater hematologic, renal, and other toxicities, vs more restricted distribution with IT administration but generally fewer toxicities or related delays in backbone therapy. While these distinctions may result in different patient subgroups receiving a given administration route, the relative efficacies of single-route IT vs HD-MTX have not been compared head-to-head in the context of modern regimens beyond single-center analyses.
Given these uncertainties, we performed a large, multicenter, real-world analysis of patients with aggressive B-cell non-Hodgkin lymphomas (NHL) across 21 US cancer centers who received single-route CNS prophylaxis. We collected detailed clinical and outcomes data with the principal aim of comparing CNS relapse rate across prophylaxis routes, hypothesizing that HD-MTX would result in fewer CNS relapses at the expense of added toxicity.
Methods
We conducted a multicenter retrospective study of adult (age ≥ 18) patients with DLBCL, high-grade B-cell lymphoma (HGBL), or transformation to either histology from an indolent B-NHL who received frontline chemoimmunotherapy plus single-route CNS prophylaxis between 2013 and 2019. Burkitt lymphoma and transformations from underlying chronic lymphocytic leukemia were excluded. This study was approved by the institutional review board at each of the 21 participating centers. A total of 1277 cases were originally identified; 115 were excluded due to: planned receipt of dual-route prophylaxis (n = 35), CNS involvement prior to frontline therapy (n = 15), ineligible NHL histology (n = 8), or incomplete clinical information (n = 59). Patients who underwent initial diagnostic lumbar puncture (LP) with IT prophylaxis followed exclusively by prophylactic HD-MTX (n = 15) were included and analyzed as HD-MTX recipients. Those who switched prophylaxis route due to intolerance (n = 32) were assessed for toxicity but excluded from the primary analysis.
Variables and endpoints
Lactate dehydrogenase (LDH) was reported according to institutional reference ranges. Performance status (PS) was standardized according to the Eastern Cooperative Oncology Group (ECOG) scale. CNS-IPI and National Comprehensive Cancer Network (NCCN)-IPI12 scores were extrapolated for each patient based on available clinical data; those with missing data who still met criteria for high risk (eg, missing LDH value but with a CNS-IPI already scoring >4) were included in the respective analyses, whereas those with incomplete data that may affect their classification were excluded.
DH lymphoma was defined according to fluorescence in situ hybridization FISH as genetic rearrangements involving Myc as well as BCL2 and/or BCL6. DH evaluability included patients with confirmed DH or those with known testing of Myc, BCL2, and BCL6 irrespective of results (ie, not all patients underwent requisite fluorescence in situ hybridization testing to evaluate for DH). Cell-of-origin (COO) was assessed locally by immunohistochemistry (IHC) using the Hans criteria.13 Primary endpoint was CNS relapse, defined as new involvement of the brain, cranial nerves, leptomeninges, cerebrospinal fluid, and/or spinal cord after initiation of frontline therapy, confirmed either histologically or by irrefutable radiographic findings. Progression free survival (PFS) was defined as time from diagnosis to disease progression/recurrence, death, or last follow-up. OS was defined as time from diagnosis to death or last follow-up.
Statistical methods
Categorical data were analyzed using χ2 tests, Fisher exact tests, and logistic regression modeling. Continuous data were assessed using 2-sample Student t tests given approximately normal distribution. Variables found to be significant on univariate analyses were included in multivariate comparisons. Kaplan-Meier estimates and log-rank tests were used for time-to-event analyses. To minimize selection bias from baseline characteristics between prophylaxis routes (Table 1), traditional propensity score matching was performed using 1:1 matching without replacement via a greedy 5:1 digit match algorithm.14 After matching, the group balance was evaluated using standardized differences, with values <0.1 considered negligible.15 IV vs IT prophylaxis recipients were compared using McNemar’s test for paired proportions, with 179 patients for each respective route being successfully matched. Nine of the 11 baseline characteristics were well balanced, with standardized differences <0.1 (supplemental Table 2 available on the Blood Web site). Two variables, B symptoms and chemotherapy regimen, had slight imbalance (standardized difference between 0.1 and 0.2).
Table 1.
Demographics and baseline clinical features of single-route prophylaxis recipients
| Characteristic | Overall (n = 1130) | IT (n = 894) | IV (n = 236) | P |
|---|---|---|---|---|
| Male sex, n (%) | 666 (58.9) | 540 (60.4) | 126 (53.4) | .051 |
| Median age, y (range) | 62 (18-86) | 62 (18-86) | 60 (20-82) | .02 |
| <70, n (%) | 852 (75.4) | 658 (73.6) | 194 (82.2) | Ref |
| ≥70, n (%) | 278 (24.6) | 236 (26.4) | 42 (17.8) | .007 |
| ECOG PS 0-1, n (%) | 844 (80.8) | 669 (80.1) | 175 (83.7) | .24 |
| Baseline renal impairment, n (%) | 172 (16.8) | 144 (17.8) | 28 (13.1) | 0.1 |
| B symptoms, n (%) | 415 (38.3) | 314 (36.6) | 101 (44.7) | .03 |
| Serum LDH, n (%) | ||||
| Not elevated | 339 (31.3) | 262 (30.5) | 77 (34.4) | Ref |
| Elevated, <3× ULN | 598 (55.2) | 480 (55.8) | 118 (52.7) | .58 |
| Elevated, ≥3× ULN | 147 (13.6) | 118 (13.7) | 29 (13.0) | .69 |
| Missing/unknown | 46 (4.1) | 34 (3.8) | 12 (5.1) | .61 |
| Stage, n (%) | ||||
| Limited (I-II) | 227 (20.1) | 176 (19.7) | 51 (21.6) | Ref |
| Advanced (III-IV) | 903 (79.9) | 718 (80.3) | 185 (78.4) | 0.51 |
| No. EN sites, n (%) | ||||
| 0 | 190 (16.8) | 174 (19.5) | 16 (6.8) | Ref |
| 1 | 523 (46.3) | 415 (46.4) | 108 (45.8) | .051 |
| ≥2 | 417 (36.9) | 305 (34.1) | 112 (47.5) | <.0001 |
| EN site(s) involved, n (%) | ||||
| Renal/adrenal | 133 (11.8) | 107 (12.0) | 26 (11.0) | .69 |
| Testis | 69 (6.1) | 44 (4.9) | 25 (10.6) | .001 |
| Breast | 34 (3.0) | 21 (2.4) | 13 (5.5) | .01 |
| Sinus | 82 (7.3) | 58 (6.5) | 24 (10.2) | .053 |
| Bone marrow | 124 (11.0) | 95 (10.6) | 29 (12.3) | .47 |
| CNS-IPI score, n (%) | ||||
| 0-1 (low) | 196 (18.4) | 151 (19.2) | 45 (19.2) | Ref |
| 2-3 (moderate) | 546 (51.4) | 432 (51.4) | 114 (48.5) | .33 |
| ≥4 (high) | 321 (30.2) | 245 (29.6) | 76 (32.3) | .67 |
| Histology, n (%) | ||||
| DLBCL | 750 (67.0) | 575 (65.0) | 175 (74.5) | Ref |
| HGBL | 305 (27.2) | 270 (30.5) | 35 (14.9) | <.0001 |
| Transformed FL | 43 (3.8) | 28 (3.2) | 15 (6.4) | .15 |
| Other | 22 (1.9) | 10 (1.1) | 12 (5.1) | .01 |
| Cell of origin (DLBCL only), n (%) | ||||
| Germinal center (GCB) | 340 (45.3) | 258 (44.9) | 82 (46.9) | Ref |
| Non-GCB | 316 (42.1) | 248 (43.1) | 68 (38.9) | .43 |
| Double-hit status, n (%) | ||||
| DH evaluable | 875 (77.4) | 687 (78.5) | 188 (21.5) | Ref |
| Confirmed DH /TH* | 243 (27.7) | 223 (32.5) | 20 (10.6) | <.0001 |
| Not DH evaluable | 255 (22.6) | 207 (23.2) | 48 (20.3) | .36 |
| Frontline chemotherapy regimen, n (%) | ||||
| R-CHOP | 536 (47.5) | 377 (42.2) | 159 (67.4) | Ref |
| R-EPOCH (+/− dose adjustment) | 509 (45.1) | 441 (49.4) | 68 (28.8) | .04 |
| Other | 85 (7.4) | 75 (8.4) | 9 (3.8) | .09 |
All listed P values are 2-sided.
CNS, central nervous system; DH, double-hit; DLBCL, diffuse large B-cell lymphoma; EN, extranodal; FL, follicular lymphoma; HGBL, high grade B-cell lymphoma; iNHL, indolent non-Hodgkin lymphoma; IPI, international prognostic index; IT, intrathecal; LDH, lactate dehydrogenase; TH, triple hit; ULN, upper limit of normal.
Percentage of DH evaluable patients. Percentages are otherwise referenced within each column.
Competing risk analyses were performed according to the Fine-Gray method,16 assessing CNS relapse and death as competing events; as those experiencing non-CNS relapse were still at risk for subsequent CNS involvement, this was not considered a competing endpoint. Analyses were performed in SAS 9.4 (SAS institute, Cary NC), with associations considered to be significant at a 2-sided value of P < .05.
Results
Patient characteristics
Among 1162 eligible patients, median age was 62 (range 18-86), 60% were male, and 75% had ECOG PS 0-1; 79% had advanced stage (III/IV) at diagnosis and 37% had B symptoms. Serum LDH was elevated in 66% (n = 767), including 20% (n = 151) at ≥3 times upper limit of normal (ULN). Most patients (n = 782, 67%) had DLBCL; 305 (26%) had HGBL, of whom 243 (80%) had confirmed double-hit lymphoma (DHL), and 67 (5.8%) had aggressive transformation from either follicular lymphoma (n = 59) or other non–chronic lymphocytic leukemia indolent histologies (n = 8). At least 1 EN site was documented in 972 patients (83.6%); notable sites included renal/adrenal (12%), bone marrow (11%), sinus (7.4%), and testis (6.2%); 449 patients (39%) had ≥2 distinct EN sites.
In terms of frontline regimen, 536 (47.5%) received rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP); 509 (45.1%) received rituximab, etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin (R-EPOCH); and 85 (7.4%) received other regimens. In those with DHL, 209 of 243 (86%) received R-EPOCH, 15 (6.2%) R-CHOP, and 19 (7.8%) other regimens. As most patients had advanced stage disease, median number of chemoimmunotherapy cycles overall was 6; those with limited stage disease had a median of 4 cycles. There was no significant difference in number of cycles by chemotherapy regimen (median of 6 cycles each for R-CHOP and R-EPOCH, P = .24), including when adjusting for stage or DH status.
In total, 894 (77%) received IT prophylaxis and 236 (20%) received HD-MTX; 32 (2.8%) changed approaches due to toxicity and are assessed below for toxicity only. Table 1 lists baseline characteristics among single-route prophylaxis recipients.
Overall prognosis by NCCN-IPI was estimated as: low (0-1; n = 12, 1.0%), low-intermediate (2-3; n = 198, 18%), high-intermediate (4-5; n = 495, 43%), or high (≥6; n = 302, 30%); 155 (13%) had incomplete NCCN-IPI data. Baseline CNS relapse risk by CNS-IPI was estimated as: low (0-1; n = 196, 18%), moderate (2-3; n = 546, 51%), or high (≥4; n = 321, 30%); 67 (5.9%) had incomplete CNS-IPI data. Using published risk predictions of 0.8, 3.9, and 12% by respective CNS-IPI category,10 the weighted CNS relapse risk for the study population at large was estimated at 5.8%.
Prophylaxis allocation
All patients received methotrexate, with 121 of 894 IT recipients (13.5%) also receiving cytarabine. No other prophylactic agents were reported. HD-MTX was most commonly dosed at 3.5 g/m2 (n = 176, 75.5%), with 17 (7.3%) receiving less than 3 g/m2 (range 2.0−2.75 g/m2) due to baseline renal dysfunction and 4 (1.6%) receiving doses of 4 g/m2 or greater. There were no documented CNS relapses among patients who required dose reduction below 3 g/m2.
Median number of prophylaxis doses was 4 for IT administration (interquartile range[IQR] 3-5) and 3 for HD-MTX (IQR 2-4); number of IT prophylaxis doses did not vary by whether patients received MTX monotherapy or MTX plus cytarabine. Delayed initiation of prophylaxis by either route until after completion of all planned chemotherapy cycles occurred in 130 patients (12%) but more commonly among HD-MTX recipients (n = 86 vs 44, P < .0001). Of those receiving delayed IT prophylaxis, 41 of 44 (93%) received MTX monotherapy.
Compared with HD-MTX, IT recipients had a higher proportion with age ≥70 (26% vs 18%, P = .006), baseline renal impairment (17.8% vs 13.1%, P = .10), and/or R-EPOCH chemoimmunotherapy backbone (87% vs 13%, P < .0001). Patients with DHL were also more likely to receive IT prophylaxis (90% vs 10%, P < .0001), reflecting more frequent R-EPOCH use in this subgroup. In contrast, HD-MTX recipients had higher proportions with testicular involvement (11% vs 4.9%, P = .001) and increased (≥2 sites) total EN burden (48% vs 34%, P < .0001).
Patient outcomes
With a median follow-up of 2.4 years, median PFS and OS have not been reached; 2-year PFS was 71% and 2-year OS was 82%. Sixty-four (5.7%) of 1130 single-route prophylaxis recipients had CNS relapse after frontline therapy. Anatomic site was documented in 53 of 64 CNS relapses, of which 22 were leptomeningeal only, 29 were parenchymal (including 11 with concurrent leptomeningeal involvement), and 2 involved other CNS sites (ocular, spinal cord; n = 1 each). With low numbers in each respective subcategory, there was no significant difference in neuroanatomic site(s) of relapse by prophylaxis route (data not shown).
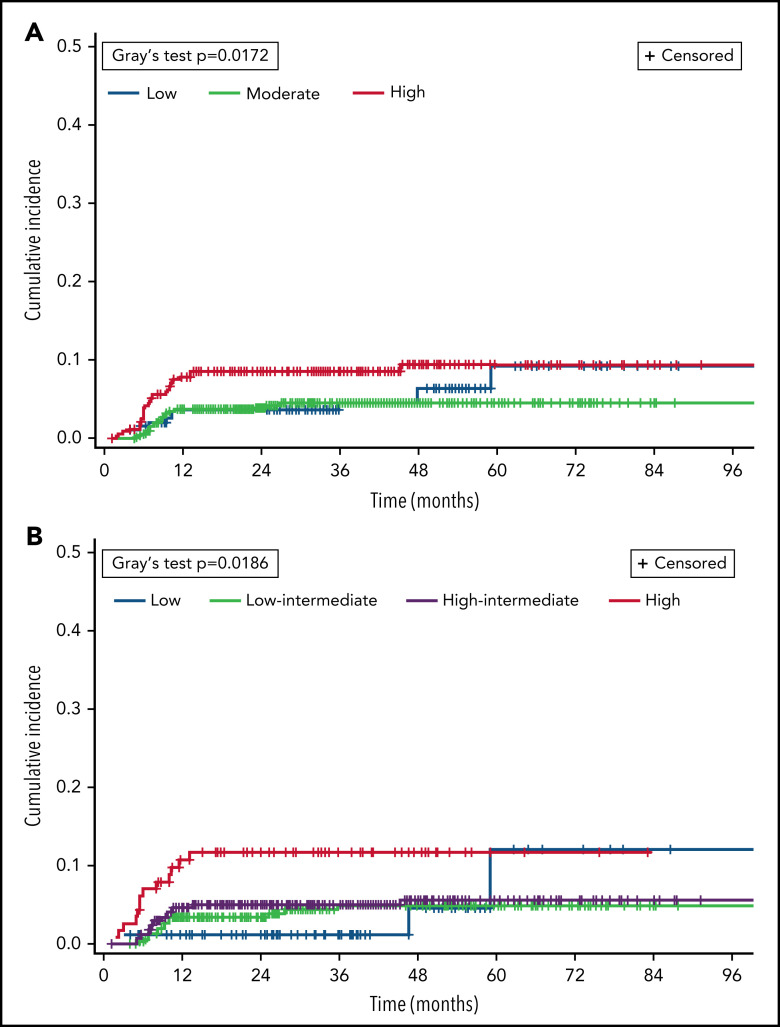
Differences in CNS relapse by baseline risk factors are listed in Table 2. By CNS-IPI category, incidence was 5.1% with low risk, 3.8% with moderate risk, and 8.4% with high risk (overall significance P = .02). Figure 1 shows cumulative incidence of CNS relapse according to CNS-IPI and NCCN-IPI scores. Testicular involvement (n = 69) occurred predominantly among patients with low (n = 29, 42%) or moderate CNS-IPI (n = 25, 36%), with 8 (12%) total and 6 (11%) otherwise low- to moderate-risk patients having CNS relapse after single-route prophylaxis. Excluding patients with testicular involvement from CNS-IPI risk groups, the adjusted CNS relapse rates for low (n = 167), moderate (n = 521), and high risk (n = 306) were 3.6%, 3.7%, and 8.2%, respectively and 5.0% overall. In terms of chemoimmunotherapy regimens, CNS relapse rates did not significantly differ between recipients of R-CHOP vs R-EPOCH (OR 1.25, 95% CI 0.51-3.10; P = .74), including when adjusting for DH status and CNS-IPI (data not shown).
Table 2.
Univariate analyses of clinical features with CNS relapse among single-route prophylaxis recipients
| Characteristic | Overall (n = 1130) |
CNS relapse (n = 64) |
No CNS relapse (n = 1066) |
OR | 95% CI | P |
|---|---|---|---|---|---|---|
| Male sex, n (%) | 666 (58.4) | 39 (5.9) | 627 (94.1) | 1.09 | 0.65-1.83 | .78 |
| Median age, y (range) | 62 (16-86) | 61 (21-83) | 62 (18-86) | — | — | 0.33 |
| < 70, n (%) | 852 (75.3) | 54 (6.3) | 798 (93.7) | — | — | Ref |
| ≥ 70, n (%) | 278 (24.6) | 10 (3.6) | 268 (96.4) | 0.55 | 0.28-1.10 | .1 |
| ECOG PS 0-1, n (%) | 844 (74.7) | 42 (5.0) | 802 (95.0) | 0.70 | 0.37-1.30 | .25 |
| Baseline renal impairment, n (%) | 172 (15.2) | 9 (5.2) | 163 (94.8) | 0.90 | 0.43-1.87 | .78 |
| B symptoms, n (%) | 415 (36.7) | 27 (6.5) | 388 (93.5) | 1.30 | 0.77-2.18 | .76 |
| Serum LDH, n (%) | ||||||
| Not elevated | 339 (30.0) | 8 (12.5) | 331 (31.1) | — | — | Ref |
| Elevated, <3× ULN | 598 (53.0) | 42 (65.6) | 556 (52.2) | 3.13 | 1.45-6.74 | .002 |
| Elevated, ≥3× ULN | 147 (13.0) | 12 (18.8) | 135 (12.7) | 3.68 | 1.47-9.20 | .003 |
| Missing/unknown | 46 (4.0) | 2 (5.7) | 44 (4.1) | 1.92 | 0.40-9.36 | .43 |
| Stage, n (%) | ||||||
| Limited (I-II) | 227 (20.1) | 13 (5.7) | 214 (94.3) | — | — | Ref |
| Advanced (III-IV) | 903 (79.9) | 51 (5.6) | 852 (94.4) | 0.99 | 0.53-1.85 | .96 |
| Number EN sites, n (%) | ||||||
| 0 | 190 (16.8) | 5 (2.6) | 185 (97.4) | — | — | Ref |
| 1 | 523 (46.3) | 27 (5.2) | 496 (94.8) | 2.01 | 0.76-5.31 | .15 |
| ≥2 | 417 (36.9) | 32 (7.8) | 385 (92.2) | 3.08 | 1.18-8.02 | .02 |
| EN site(s) involved, n (%) | ||||||
| Renal/adrenal | 133 (11.8) | 8 (6.0) | 125 (94.0) | 1.08 | 0.50-2.31 | .85 |
| Testis | 69 (6.1) | 8 (11.6) | 61 (88.4) | 2.36 | 1.08-5.16 | .03 |
| Breast | 34 (3.0) | 4 (11.8) | 30 (88.2) | 2.30 | 0.79-6.75 | .13 |
| Sinus | 82 (7.3) | 2 (2.4) | 80 (97.4) | 0.40 | 0.10-1.66 | .19 |
| Bone marrow | 124 (11.0) | 11 (8.9) | 113 (91.1) | 1.75 | 0.89-3.45 | .1 |
| CNS-IPI score, n (%) | ||||||
| 0-1 (low) | 196 (18.4) | 10 (5.1) | 186 (94.9) | — | — | Ref |
| 2-3 (moderate) | 546 (51.4) | 21 (3.8) | 525 (96.2) | 0.74 | 0.34-1.61 | .055 |
| ≥4 (high) | 321 (30.2) | 27 (8.4) | 294 (91.6) | 1.71 | 0.81-3.61 | .02 |
| Histology, n (%) | ||||||
| DLBCL | 750 (67.0) | 41 (5.5) | 709 (94.5) | — | — | Ref |
| HGBL | 305 (27.2) | 20 (6.6) | 285 (93.4) | 1.21 | 0.69-2.09 | .49 |
| Cell of origin (DLBCL only), n (%) | ||||||
| Germinal center (GCB) | 340 (45.3) | 11 (3.2) | 329 (96.8) | — | — | Ref |
| Non-GCB | 316 (42.1) | 21 (6.6) | 295 (93.4) | 2.13 | 1.01-4.49 | .047 |
| Double-hit status, n (%) | ||||||
| DH evaluable | 875 (77.4) | 52 (5.9) | 823 (94.1) | — | — | Ref |
| Confirmed DH/TH* | 243 (27.7) | 15 (6.2) | 228 (93.8) | 1.06 | 0.57-1.97 | .86 |
| Frontline chemotherapy regimen, n (%) | ||||||
| R-CHOP | 536 (47.5) | 31 (5.8) | 505 (94.2) | — | — | Ref |
| R-EPOCH (+/− dose adjustment) | 509 (45.1) | 27 (5.3) | 482 (94.7) | 1.25 | 0.51-3.10 | .74 |
| Other | 85 (7.4) | 6 (7.1) | 79 (92.9) | 0.91 | 0.54-1.55 | .64 |
| CNS prophylaxis route, n (%) | ||||||
| Intrathecal | 894 (79.1) | 48 (5.4) | 846 (94.6) | — | — | Ref |
| Intravenous (HD-MTX) | 236 (20.9) | 16 (6.8) | 220 (93.2) | 1.28 | 0.71-2.30 | .4 |
All listed P values are 2-sided.
CI, confidence interval; CNS, central nervous system; DH, double-hit; DLBCL, diffuse large B-cell lymphoma; EN, extranodal; FL, follicular lymphoma; GCB, germinal center subtype (Hans criteria); HGBL, high grade B-cell lymphoma; iNHL, indolent non-Hodgkin lymphoma; IPI, international prognostic index; LDH, lactate dehydrogenase; OR, odds ratio; PS, performance status; TH, triple hit; ULN, upper limit of normal.
Percentage of DH evaluable patients. Percentages are otherwise referenced within each row.
Figure 1.
Cumulative incidence of CNS relapse, by risk category in validated prognostic indices. CNS relapse and death were analyzed as competing events. (A) CNS-IPI. (B) NCCN-IPI.
COO by IHC was reported in 656 of 782 patients with DLBCL. Of these, 32 (4.9%) experienced CNS relapse, more frequently among non-GCB patients (6.7% vs 3.2%, P = .04). Incorporating IHC-based COO estimations into the CNS-IPI,17 217 of 656 (33%) were identified as low risk, 303 (46%) moderate risk, and 101 (15%) high risk; 35 (5.3%) had incomplete CNS-IPI data. Ten of 101 high-risk patients (9.9%) experienced CNS relapse vs a rate of 15% previously reported for high-risk patients based on gene expression profiling.
CNS relapse by prophylaxis route
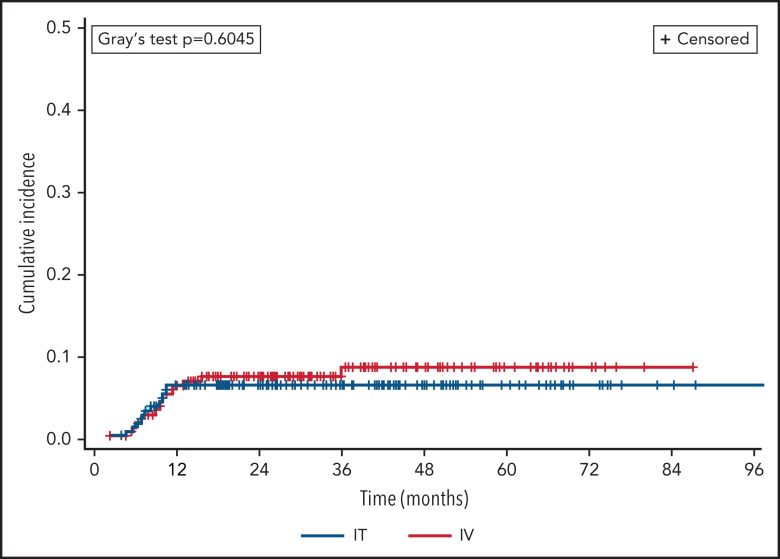
There was no significant difference in CNS relapse rates by prophylaxis route: 48 (5.4%) IT vs 16 (6.8%) HD-MTX (OR 1.28, 95% CI 0.71-2.30; P = .4). This finding persisted when adjusting for differences in number of prophylaxis doses received and backbone chemotherapy regimen (adjusted OR 1.38, 95% CI 0.74-2.57; P = .31). In terms of prophylactic agent, there was no significant difference between recipients of IT MTX monotherapy vs those receiving IT MTX plus cytarabine (OR 0.91, 95% CI 0.38-2.18; P = .61), and there remained no difference between routes when comparing HD-MTX vs either respective IT approach (data not shown).
Two hundred twenty-five (20%) patients died during the study period (n = 194 IT vs 32 HD-MTX), including 45 following CNS relapse (n = 36 IT vs 9 HD-MTX). A competing risk analysis was performed with death as a competing event to CNS relapse (Figure 2), which continued to find no difference between prophylaxis routes (Fine-Gray P = .60). In terms of dose timing, there was no significant difference in CNS relapse following intercalated vs delayed administration overall (OR 0.79, 95% CI 0.38-1.63; P = .52) or when adjusted for route of administration (adjusted OR 0.87, 95% CI 0.39-1.95, P = .74).
Figure 2.
Cumulative incidence of CNS relapse, by prophylaxis route. CNS relapse and death were analyzed as competing events.
Among 358 PS-matched patients (n = 179 per arm; Table 3), there were 19 CNS relapses (5.3%), with no significant difference between prophylaxis routes (5.0% IT vs 5.6% HD-MTX, P = .81). There remained no significant difference in CNS relapse among matched patients by prophylaxis route when stratified across CNS-IPI and NCCN-IPI categories, nor did DH status appear to be predictive of CNS relapse in this setting.
Table 3.
Univariate analyses of CNS relapse among propensity score matched patients
| Characteristic | Overall n = (358) |
CNS relapse (n = 19) |
No CNS relapse (n = 339) |
P |
|---|---|---|---|---|
| Prophylaxis route, n (%) | ||||
| Intrathecal | 179 (50) | 9 (5.0) | 170 (95.0) | .81 |
| HD-MTX | 179 (50) | 10 (5.6) | 169 (94.4) | |
| CNS-IPI, n (%) | ||||
| 0-1 (low) | 79 (22.1) | 5 (6.5) | 72 (93.5) | .10 |
| 2-3 (moderate) | 177 (49.4) | 5 (2.8) | 172 (97.2) | |
| ≥4 (high) | 100 (27.9) | 9 (8.7) | 95 (91.3) | |
| NCCN-IPI, n (%) | ||||
| Low | 27 (7.5) | 0 (0) | 27 (100) | .37 |
| Low-Int | 154 (43.0) | 8 (5.2) | 146 (94.8) | |
| High-Int | 149 (41.6) | 8 (5.4) | 141 (94.6) | |
| High | 28 (7.8) | 3 (10.7) | 25 (89.3) | |
| Double-hit positive | 30 (8.4) | 2 (6.7) | 28 (93.3) | .86 |
All listed P values are 2-sided.
CNS, central nervous system; HD, high dose; IPI, international prognostic index; MTX, methotrexate; NCCN, National Comprehensive Cancer Network.
Timing of CNS relapse
Median time to CNS relapse overall was 7.8 months (IQR 6.1-10.4 months) and was inversely proportional to risk according to CNS-IPI: 7.0 months (high) vs 8.8 months (moderate) vs 9.8 months (low). Timing did not significantly differ across routes: 7.5 months after IT vs 9.5 months after HD-MTX (P = .86). Fifteen of 64 CNS relapses (23%) occurred 6 months or less from diagnosis, with nonsignificant trend toward more events among IT recipients (n = 13 vs 2, P = .23); all early relapses occurred among recipients of intercalated prophylaxis. Excluding these early events, CNS relapses remained similar across prophylaxis routes: n = 35 (IT) vs 14 (HD-MTX) (P = .21). Further subgroup analyses of CNS relapse timing were not performed due to low numbers in each respective category.
Prophylaxis-related toxicities
Significant prophylaxis-related toxicity was reported in 134 patients (12%), including 32 (2.8% of total) who switched prophylaxis route due to toxicity. Toxicities overall were more commonly reported among recipients of HD-MTX vs IT prophylaxis (25.4% vs 6%, P < .0001); individual toxicities by prophylaxis route are listed in supplemental Table 1. Common events included renal impairment (n = 47) primarily after HD-MTX, delayed MTX clearance (n = 27), and post-LP headache (n = 18). Low-grade mucositis was not captured, though severe mucosal toxicity was reported in 9 HD-MTX recipients and 6 IT recipients. Hematologic toxicities related to prophylaxis were uncommon with either route and resulted in only 1 patient changing from HD-MTX to IT prophylaxis. Delays in subsequent chemotherapy due to prophylaxis-related toxicity were noted in 37 patients, of whom 34 received HD-MTX and 5 ultimately switched to IT administration due to toxicity. Renal impairment due to HD-MTX was the most commonly cited reason for switching prophylaxis routes (n = 8), followed by difficulty with methotrexate clearance (n = 6). No patients received glucarpidase during the study period.
Among the 32 patients who switched route due to toxicity, 30 initially started prophylaxis with HD-MTX. Seventeen of 32 (53%) received R-CHOP and 15 (47%) received R-EPOCH; among the latter, 7 had confirmed DHL. One of 32 (3.1%) experienced CNS relapse after receiving 1 dose of HD-MTX at 3.5g/m2 and the remainder of prophylaxis via IT MTX monotherapy.
Discussion
To the best of our knowledge, this study represents 1 of the largest analyses of CNS prophylaxis recipients with DLBCL in the rituximab era. We identified no significant difference in rate of CNS relapse between routes of prophylaxis administration, using multiple techniques to account for variation in patient eligibility for a given route. Incidence of CNS relapse following single-route prophylaxis varied across numerous clinical and pathologic factors, suggesting heterogeneous benefit and/or baseline predisposing risk.
Features correlating with increased CNS relapse risk despite prophylaxis included testicular involvement, non-GCB subtype DLBCL, and high total burden of EN disease. Conversely, DHL did not appear a major risk factor for increased CNS relapse after single-route prophylaxis, nor did single-site involvement of other conventional high-risk EN sites, such as kidneys/adrenals, sinus, or bone marrow. Whether this represents a true preventive benefit following prophylaxis is unclear without a nonprophylaxis comparator. However, despite much higher prophylaxis use in our study population vs cohorts used in the development and validation of the CNS-IPI, CNS relapse rates overall were very similar to these historical benchmarks, raising the question of whether CNS prophylaxis provided meaningful protection against CNS relapse across settings.
While it remains common practice, no study to date has clearly demonstrated the utility of CNS prophylaxis for DLBCL patients in the rituximab era, nor has a definitive comparison of prophylaxis route been performed in this context. While recent data are limited regarding IT administration, Eyre and colleagues found that patients over age 70 had similar rates of CNS relapse with or without administration and that IT recipients experienced higher rates of infections during therapy.18 Similarly, a recent meta-analysis by this same group did not identify a significant decrease in CNS relapse following IT prophylaxis for any of the individual clinical trials included in the study, though analyses of pooled individual patient-level data were not reported.19 Prior reporting of SWOG-8516, which established CHOP as the standard chemotherapy backbone in DLBCL, likewise found no significant difference in CNS relapse with or without IT prophylaxis, though its use was restricted to patients with bone marrow involvement and results predated the use of rituximab.3
For HD-MTX, a recent Canadian analysis evaluated the Alberta health system’s recommendation for HD-MTX as the preferred prophylactic agent in high-risk DLBCL, defined as CNS-IPI ≥4, DHL, or testicular lymphoma.20 Incompletely adopted, 35.3% of the 906 patients identified as high risk between 2012-2019 received prophylaxis, with no significant difference after HD-MTX in CNS relapses in general or by CNS-IPI category. Timing of HD-MTX has likewise come into question, where one study found similar CNS relapse rates following intercalated administration with R-CHOP vs delaying prophylaxis until after completion of chemotherapy.21 Single-center experience at Memorial Sloan-Kettering has suggested that prophylaxis delays but does not prevent CNS relapse22; though ultimately underpowered to assess the impact of prophylaxis route and with limited numbers of HD-MTX recipients, their findings and ours are similar.
Our data provide a key contribution to this literature, noting that route of prophylaxis does not appear to meaningfully impact the ability to prevent CNS relapse in DLBCL. This is especially important in contexts such as DHL, where escalation beyond R-CHOP is standard of care and our data show no added benefit to using HD-MTX over IT prophylaxis. Whether either route provides protection against CNS relapse in DLBCL more broadly remains to be seen, and the absence of prospective clinical trials to address this question is increasingly apparent. We do not consider the currently available data, including those presented here, as sufficient to forgo the use of CNS prophylaxis in DLBCL altogether; however, given the scale and complexity needed to investigate a rare and heterogeneous outcome such as CNS relapse, further study to compare routes of methotrexate administration is likely of diminishing benefit compared with that of other key advances in this space.
Notable among these, emerging CNS-penetrant agents in lymphoma such as lenalidomide and ibrutinib may represent future alternatives to methotrexate as prophylactic agents. These agents have established single-agent activity in primary CNS lymphomas, where recurrent mutations in MyD88 and CD79b have been previously described and appear to be present in at least a subset of patients with CNS relapse after initial systemic presentation.23,24 These mutations are notably more frequent in testicular lymphomas as well and thus may represent a particular phenotype of aggressive NHL with propensity for sanctuary site involvement. Additional study is needed to determine whether these mutations are ultimately predictive of CNS relapse and/or response to targeted agents in larger DLBCL cohorts, both upfront and at the time of relapse. Furthermore, how these molecular features and the role of CNS prophylaxis in general interplay with the evolving classifications of DLBCL remain to be seen, especially as more tailored treatment paradigms emerge that reflect the biologic heterogeneity of this disease.
Our study has several important limitations, including those intrinsic to its retrospective design. Eligibility was restricted to recipients of single-route prophylaxis, which was heavily skewed toward IT administration and showed imbalances across several key clinical features reflective of clinical practice which affected certain subgroup analyses. Propensity score matching was performed to address those potential confounders which were measured and collected. Despite the large sample size, our comparison of IT and HD-MTX was underpowered for the small number of CNS relapse events observed and the relative imbalances in their respective utilization. Intensified regimens were not assessed, including use of dual-route prophylaxis, nor was a prophylaxis-free comparator arm. COO was estimated by IHC, which incompletely captures activated B-cell subtype DLBCL by gene expression profiling,13 as was used in the COO integration to CNS-IPI scores reported by Klanova and colleagues.17 Given the large number of patients per site, we were unable to collect data points requiring more extensive review of pathology reports, such as double expressor25 and Epstein-Barr viral status, or additional treatment details, such as chemotherapy dose levels. While upfront CNS involvement was noted in 15 patients who were excluded on this basis, the incidence of pretreatment cerebrospinal fluid screening more broadly was not routinely captured. Few prophylaxis-related toxicities were reported, which may be underestimated due to incomplete documentation and/or collection. Community affiliates of each respective site were included, though our findings are likely under-representative of practice outside academic centers.
This real-world analysis found no difference between IT and HD-MTX in preventing CNS relapse in DLBCL. Relapse rates among high-risk subgroups remain elevated and reconsideration of prophylaxis strategies in DLBCL is of critical need. Future studies should focus less on route of methotrexate administration in favor of how to further leverage molecular features in risk stratification as well as the role of more biologically directed therapies in DLBCL.
Supplementary Material
The online version of this article contains a data supplement.
Acknowledgments
The authors acknowledge the input of Michael E. Williams in the early design of this study and the work of the UVA Office of Grants and Contracts for coordinating this effort.
This study originated at the University of Virginia with support from the University of Virginia Cancer Center (P30CA044579) (C.A.P.). Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under award number K12CA237806 from the Winship K12 Clinical Oncology Training Program (V.M.O.) and by the Biostatistics Shared Resource of Winship Cancer Institute of Emory University and NIH/NCI under award number P30CA138292 (A.M. and J.S.).
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
These data were virtually presented in part at the 62nd Annual Meeting of the American Society of Hematology, 5-8 December 2020.
Qualified researchers may request nonconfidential data from the corresponding author.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: V.M.O., D.R.R., and C.A.P. designed the study; and all authors participated in the collection and analysis of data and in writing/reviewing the manuscript.
Conflict-of-interest disclosure: B.S.K.: Acerta: consultancy and research funding; Janssen: consultancy and membership on an entity's board of directors or advisory committees; ADC Therapeutics: consultancy, membership on an entity's board of directors or advisory committees, and research funding; BeiGene: consultancy, membership on an entity's board of directors or advisory committees, and research funding; Roche Laboratories Inc: consultancy; Pharmacyclics LLC: consultancy; Genentech: consultancy; Celgene Corporation: consultancy; AstraZeneca Pharmaceuticals LP: consultancy and membership on an entity's board of directors or advisory committees; and AbbVie: consultancy. M.A.S.: Notable Labs: honoraria. R.A.: Celgene, Forty Seven, Inc., Genentech/Roche, Janssen Pharmaceutical, Kura, Merck, Millenium, Pharmacyclics, Regeneron, and Seattle Genetics: research funding; Astra Zeneca, Bayer Healthcare Pharmaceuticals, Cell Medica, Celgene, Genentech/Roche, Gilead, KitePharma, Kyowa, Portola Pharmaceuticals, Sanofi, Seattle Genetics, and Takeda: consultancy. T.J.V.: AstraZeneca: research funding. N.S.G.: Genentech: research funding; Tessa: consultancy; and Kite: consultancy. S.F.H.: Genentech: consultancy; Novartis: consultancy; Celgene: consultancy and research funding; TG Therapeutics: research funding; Pharmacyclics: honoraria; Bayer: consultancy and Honoraria; DTRM: research funding; Astrazeneca: honoraria; AbbVie: consultancy; COVIA Health: consultancy; and Flatiron Health: consultancy. A.L.: EUSA Pharma LCC, Avrobio, and BMS (limited past equities): consultancy. H.S.: Sanofi, Seagen, Bayer, Epizyme, and MorphoSys: consultancy. S.E.S.: Beigene: research funding; Gilead: research funding; Genentech: research funding; Bristol Myers Squibb: research funding; Pharmacyclics: consultancy; Janssen: consultancy and research funding; VelosBio: consultancy and research funding; Cardinal Health: honoraria; Verastem: research funding; Genmab: research funding; AstraZeneca: research funding; and Acerta: research funding. A.J.O.: Spectrum Pharmaceuticals: research funding; Genentech, Inc.: research funding; Adaptive Biotechnologies: research funding; and TG Therapeutics: research funding. D.J.L.: Seattle Genetics: speakers bureau; Morphosys: membership on an entity's board of directors or advisory committees; Karyopharm: membership on an entity's board of directors or advisory committees; Curis: Consultancy, membership on an entity's board of directors or advisory committees, research funding; Celgene: membership on an entity's board of directors or advisory committees; Takeda: research funding; and Triphase: research funding. M.K.: Roche: research funding. P.F.C.: Kite Pharma: other, advisory board; ADC Therapeutics: other, advisory board and research funding; Verastem: other: advisory board; Amgen, other: advisory board; Bayer: other, advisory board; and Celgene: speakers bureau. R.K.: Celgene Corporation, Gilead Sciences, Juno Therapeutics, Kite Pharma, Janssen, Karyopharm, Pharmacyclics, and Morphosys: consulting fees; Celgene Corporation/Juno Therapeutics/BMS, Takeda, BeiGene, and Gilead Sciences/Kite: grants/research support; and BeiGene and Gilead Sciences: speakers bureau. D.M.S.: Pharmacyclics: consultancy; Innate: consultancy; Verastem: research funding; Karyopharm: consultancy and research funding; Janssen: consultancy; Gilead: research funding; Arqule: research funding; Juno: research funding; MingSight: research funding; Acerta: research funding; and Beigene: consultancy. S.M.S.: Genentech/Roche: consultancy, other: support of parent study and funding of editorial support and research funding; TG Therapeutics: consultancy and research funding; Celgene: consultancy, research funding; Janssen: consultancy; BMS: consultancy; Karyopharm: consultancy and research funding; FortySeven: research funding; Pharmacyclics: research funding; and Acerta: research funding. N.K.: Celgene: research funding; Pharmacyclics: honoraria; Seattle Genetics: research funding; Bristol Myers Squibb: research funding; and Janssen: honoraria. B.T.H.: Kite: consultancy and honoraria; Gilead: consultancy, honoraria, and membership on an entity's board of directors or advisory committees; Pharmacyclics: consultancy, honoraria, membership on an entity's board of directors or advisory committees, and research funding; AstraZeneca: consultancy and honoraria; Celegene: consultancy, honoraria, and research funding; Seattle Genetics: consultancy and honoraria; Takeda: research funding; Amgen: research funding; TG therapeutics: research funding; Abbvie: consultancy, honoraria, membership on an entity's board of directors or advisory committees, and research funding; and Genentech: consultancy and research funding. J.C.: Genentech, BMS, Novartis, LAM, BioInvent, LRF, ASH, Astra Zeneca, and Seattle Genetics: research funding; and Janssen, Adicet, Astra Zeneca, Genentech, Aptitude Health, Cellectar, Kite/Gilead, and Loxo: consultancy. C.A.P.: Bayer: consultancy; Xencor: research funding; BeiGene: consultancy and research funding; Infinity: research funding; Roche/Genentech: consultancy and research funding; Amgen: consultancy; Janssen: consultancy; Pharmacyclics: consultancy; AbbVie: research funding; TG Therapeutics: research funding; Kite: consultancy and research funding; and Acerta/AstraZeneca: research funding. The remaining authors declare no competing financial interests.
Correspondence: Victor M. Orellana-Noia, Winship Cancer Institute, Department of Hematology and Medical Oncology, Emory University, 1365B Clifton Rd NE, Number 4011, Atlanta, GA 30322; e-mail: orellana-noia@emory.edu.
REFERENCES
- 1.Ghose A, Elias HK, Guha G, Yellu M, Kundu R, Latif T. Influence of rituximab on central nervous system relapse in diffuse large B-cell lymphoma and role of prophylaxis – a systematic review of prospective studies. Clin Lymphoma Myeloma Leuk. 2015;15(8):451-457. [DOI] [PubMed] [Google Scholar]
- 2.El-Galaly TC, Cheah CY, Bendtsen MD, et al. Treatment strategies, outcomes and prognostic factors in 291 patients with secondary CNS involvement by diffuse large B-cell lymphoma. Eur J Cancer. 2018;93:57-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernstein SH, Unger JM, Leblanc M, Friedberg J, Miller TP, Fisher RI. Natural history of CNS relapse in patients with aggressive non-Hodgkin’s lymphoma: a 20-year follow-up analysis of SWOG 8516 – the Southwest Oncology Group. J Clin Oncol. 2009;27(1):114-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peñalver F-J, Sancho J-M, de la Fuente A, et al. ; Spanish Lymphoma Group (GELTAMO) . Guidelines for diagnosis, prevention and management of central nervous system involvement in diffuse large B-cell lymphoma patients by the Spanish Lymphoma Group (GELTAMO). Haematologica. 2017;102(2):235-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evens AM, Danilov A, Jagadeesh D, et al. Burkitt lymphoma in the modern era: real-world outcomes and prognostication across 30 US cancer centers. Blood. 2021;137(3):374-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zayac AS, Evens AM, Danilov A, et al. Outcomes of Burkitt lymphoma with central nervous system involvement: evidence from a large multicenter cohort study. Haematologica. 2021;106(7):1932-1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qualls D, Abramson JS. Advances in risk assessment and prophylaxis for central nervous system relapse in diffuse large B-cell lymphoma. Haematologica. 2019; 104(1):25-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheah CY, Herbert KE, O’Rourke K, et al. A multicentre retrospective comparison of central nervous system prophylaxis strategies among patients with high-risk diffuse large B-cell lymphoma. Br J Cancer. 2014;111(6):1072-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zelenetz AD, Gordon LI, Abramson JS, et al. B-cell lymphomas, version 3.2019: featured updates to the NCCN Guidelines. J Natl Compr Cancer Netw. 2019;17(6):650-661. [DOI] [PubMed] [Google Scholar]
- 10.Schmitz N, Zeynalova S, Nickelsen M, et al. CNS International Prognostic Index: a risk model for CNS relapse in patients with diffuse large B-cell lymphoma treated with R-CHOP. J Clin Oncol. 2016;34(26):3150-3156. [DOI] [PubMed] [Google Scholar]
- 11.Abramson JS, Hellmann M, Barnes JA, et al. Intravenous methotrexate as central nervous system (CNS) prophylaxis is associated with a low risk of CNS recurrence in high-risk patients with diffuse large B-cell lymphoma. Cancer. 2010;116(18):4283-4290. [DOI] [PubMed] [Google Scholar]
- 12.Zhou Z, Sehn LH, Rademaker AW, et al. An enhanced International Prognostic Index (NCCN-IPI) for patients with diffuse large B-cell lymphoma treated in the rituximab era. Blood. 2014;123(6):837-842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103(1):275-282. [DOI] [PubMed] [Google Scholar]
- 14.Parsons LS. Reducing Bias in a Propensity Score Matched-Pair Sample Using Greedy Matching Techniques. Paper presented at SAS Users Group International 26 April 2001. Long Beach, CA.
- 15.Austin PC, Grootendorst P, Anderson GM. A comparison of the ability of different propensity score models to balance measured variables between treated and untreated subjects: a Monte Carlo study. Stat Med. 2007;26(4):734-753. [DOI] [PubMed] [Google Scholar]
- 16.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496-509. [Google Scholar]
- 17.Klanova M, Sehn LH, Bence-Bruckler I, et al. Integration of cell of origin into the clinical CNS International Prognostic Index improves CNS relapse prediction in DLBCL. Blood. 2019;133 (9):919-926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eyre TA, Kirkwood AA, Wolf J, et al. Stand-alone intrathecal central nervous system (CNS) prophylaxis provide unclear benefit in reducing CNS relapse risk in elderly DLBCL patients treated with R-CHOP and is associated increased infection-related toxicity. Br J Haematol. 2019;187(2):185-194. [DOI] [PubMed] [Google Scholar]
- 19.Eyre TA, Djebbari F, Kirkwood AA, Collins GP. Efficacy of central nervous system prophylaxis with stand-alone intrathecal chemotherapy in diffuse large B-cell lymphoma patients treated with anthracycline-based chemotherapy in the rituximab era: a systematic review. Haematologica. 2020;105(7):1914–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Puckrin R, El Darsa H, Ghosh S, Peters A, Owen C, Stewart D. Ineffectiveness of high‐dose methotrexate for prevention of CNS relapse in diffuse large B-cell lymphoma. Am J Hematol. 2021;96(7):764-771. [DOI] [PubMed] [Google Scholar]
- 21.Wilson MR, Eyre TA, Martinez-Calle N, et al. Timing of high-dose methotrexate CNS prophylaxis in DLBCL: an analysis of toxicity and impact on R-CHOP delivery. Blood Adv. 2020;4(15):3586-3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bobillo S, Joffe E, Venkatraman S, et al. Central nervous system prophylaxis with high-dose intravenous methotrexate or intrathecal chemotherapy in patients with diffuse large B-cell lymphoma and high-risk of CNS relapse treated in the rituximab era [abstract]. Blood. 2019;134(Suppl 1):1619.31409672 [Google Scholar]
- 23.Chapuy B, Roemer MGM, Stewart C, et al. Targetable genetic features of primary testicular and primary central nervous system lymphomas. Blood. 2016;127(7):869-881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ollila TA, Kurt H, Waroich J, et al. Genomic subtypes may predict the risk of central nervous system recurrence in diffuse large B-cell lymphoma. Blood. 2021;137(8):1120-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Savage KJ, Slack GW, Mottok A, et al. Impact of dual expression of MYC and BCL2 by immunohistochemistry on the risk of CNS relapse in DLBCL. Blood. 2016;127(18):2182-2188. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.