Abstract
Protothecosis is a severe form of mastitis in cattle that is caused by colorless algae of the genus Prototheca. So far, no suitable serological test for the identification of infected animals is available for routine diagnosis. In this study an indirect enzyme-linked immunosorbent assay (ELISA) for the identification of infected cows and for discriminating among infected cows at various clinical stages was developed. Immunoglobulin G (IgG) in serum and IgA and IgG1 in whey were used as antibody isotypes. The ELISA was evaluated using serum and whey from animals at different clinical stages of infection. A total of 12 cows with acute clinical manifestation of protothecal mastitis, 22 cows with clinical signs of chronic mastitis, 40 Prototheca zopfii-negative cows, and 18 cows with chronic clinical signs and earlier cultures positive for P. zopfii but with presently negative culturing results were investigated. A sensitivity of 96% and a specificity of 94% were calculated for the ELISA based on IgA levels. Intra-assay and interassay variations were calculated to be 6.08 and 6.32%, respectively. Based on these data, this ELISA was found to be suitable for discrimination between infected and uninfected animals and might therefore be useful for screening affected herds.
Members of the species of the genus Prototheca are unicellular colorless algae. They are saprophytes which can be isolated from a variety of environmental sources, including plants, soil, drinking and marine water, sludge, the feces of domestic animals (e.g., cattle, dogs, salmon, and pigs) or wild animals (e.g., deer, rats, mice, or rabbits), and barn floors (2, 10, 23, 30).
Both, Prototheca zopfii and P. wickerhamii have been reported as the etiologic agents of protothecoses in humans and animals. The rare cases of human protothecoses are caused predominantly by P. wickerhamii and occur as local and systemic infections, mainly in immune suppressed patients, e.g., patients infected with human immunodeficiency virus, or treated with cortisone (7, 20, 32, 34; A. Kunova, T. Kollar, S. Spanik, and V. Kremery, Jr., Letter, J. Chemother. 8:166–167, 1996).
P. zopfii can cause severe local and systemic infections in domestic animals, especially in dogs and cows. The first case of bovine mammary infection was reported in 1952 (17). Whereas in the past only sporadic cases of Prototheca mastitis have been observed, cases of acute to chronic mastitis are recognized increasingly today to be endemic worldwide (1, 9, 13, 18, 24).
Due to the ubiquitous occurrence of P. zopfii, fecal samples of dairy cows in herds without a history of protothecal mastitis can be found to be culturally positive at rates of 20 to 70% (2, 11). Prototheca mastitis can be transmitted from cow to cow during milking (9, 28, 31). The incidence of infections depends on predisposing factors such as poor environmental conditions or insufficient milking hygiene (3, 9, 30). The existence of a particular mastitis-associated variant of P. zopfii (variant II) has been discussed elsewhere (4, 5, 29).
Since P. zopfii is highly resistant to all known chemotherapeutics, infected cows should be removed from the herd (3, 8). Additionally, chronically infected cows can become intermittent shedders (27). A reliable identification of those individuals would reduce the risk of infection of uninfected cows or contamination of the farm environment.
The diagnosis of Prototheca mastitis is still based upon the time-consuming cultivation on Sabouraud-dextrose-agar medium and on the additional investigation of lactophenol cotton blue-stained cells by light microscopy (3, 9, 24). However, due to the slow growth of most Prototheca strains and the intermittent excretion of the organisms, these methods cannot be used for stringent control measures (27).
In the few available previous immunological studies, detection of anti-Prototheca immunoglobulin G (IgG) in serum using counterimmunoelectrophoresis tests and an enzyme-linked immunosorbent assay (ELISA) showed poor sensitivity and specificity. Additionally, their use for routine diagnosis (5, 15) was limited. Although, the presence of specific IgA antibodies in whey from lactating cows could be demonstrated by immunodiffusion, this test system was unsuitable for herd screening because it is too labor-intensive (12).
Therefore, the aim of this study was to develop a highly specific and sensitive ELISA suitable for diagnostics at the herd level. Our results demonstrate the potential of the ELISA to discriminate cows showing different clinical stages of infection from uninfected animals.
MATERIALS AND METHODS
Alga strains.
P. zopfii type strain SAG 263-4 and reference strain SAG 2021 were obtained from the Culture Collection of Algae at the University of Göttingen, Göttingen, Germany. Strain SAG 263-4 was originally isolated from human intestine. P. zopfii SAG 2021 is a virulent isolate associated with an outbreak of mastitis in a dairy herd in Saxony, Germany, and was isolated from a case of a severe acute mammary infection in a lactating cow.
Alga cultivation and biochemical analyses.
All strains were routinely grown on Sabouraud-dextrose-agar medium (Difco Laboratories, Detroit, Mich.) at 37°C under aerobic conditions. For diagnostic purposes, aliquots of 50 μl from quarter or composite milk samples were streaked onto plates. After 72 and 120 h, plates were examined for the growth of Prototheca. Any colonies resembling Prototheca spp. were subcultured once. Smears were made from colonies of interest and stained with lactophenol cotton blue. Specimens were investigated microscopically for characteristic morphology, i.e., the presence of sporangiospores in the sporangium. In order to distinguish P. zopfii strains from P. wickerhamii, each isolate was additionally tested for assimilation properties in OF-Broth Medium (Difco Laboratories). The assimilation of glucose but not of trehalose indicated the growth of P. zopfii. The assimilation of trehalose was taken as a discriminating feature for the presence of P. wickerhamii. To identify different variants of P. zopfii, each isolate was tested auxanographically for the assimilation of glycerol or galactose on Prototheca isolation medium (22). A strong assimilation activity of galactose and glycerol within 48 h indicated P. zopfii variant I. P. zopfii variant II did not show the assimilation of galactose, whereas the P. zopfii variant III was not able to utilize glycerol (4).
Preparation of genomic DNA.
Alga cultures were grown on Sabouraud-agar plates for 48 h at 37°C. Cells were harvested by centrifugation (ca. 5,000 × g, 10 min) and broken in a mortar with a pestle and sterile sea sand in liquid nitrogen. Then, 30 mg of the powder was transferred to 1.5-ml Eppendorf tubes and mixed with 500 μl of preheated CTAB buffer (2% cetyltrimethylammonium bromide [CTAB; wt/vol]; 20 mM EDTA; 1.4 M NaCl; 1% polyvinylpyrrolidone; 100 mM Tris, pH 8.0). The mixture was held at 65°C for 5 min. One volume (percent [vol/vol]) of chloroform-isoamyl alcohol (24:1) was added and mixed. After centrifugation (5,000 × g, 10 min), the supernatant was transferred to a new tube and mixed with a 1/5 volume (percent [vol/vol]) of a 5% CTAB solution (5% CTAB, 0.7 M NaCl). DNA was precipitated by adding 2 volumes of cold ethanol (96%) to the supernatant and pelleted by centrifugation (15,000 × g, 30 min, 4°C). The pellet was dried under vacuum and resuspended in 20 μl of double-deionized H2O for further use.
Species confirmation of the coating strain by rDNA-PCR analysis.
P. zopfii strains SAG 293-4 and SAG 2021 were compared by partial 18S ribosomal DNA (rDNA) sequencing. Total DNA of both strains was prepared as described above. For amplification of the 18S rDNA, the primer pair wicker-18f (5′-AACCTGGTTGATCCTGCCAGT-3′) and wicker-18r (5′-TGATCCTTCTGCAGGTTCACC-3′) was designed on the basis of the known sequence information of the 18S rDNA of P. wickerhamii (GenBank accession no. X56099). PCR amplification was carried out with 1 U of Taq DNA polymerase, 1 μg of DNA, 1 μmol of each primer, and a 200 μM concentration of each deoxynucleoside triphosphate in a Perkin-Elmer 2400 thermal cycler. The cycle conditions were as follows: 80 s of denaturation at 94°C and 90 s of extension at 72°C. The annealing conditions for amplification were chosen according to the guanine-cytosine content of the corresponding oligonucleotides. The amplification product was analyzed on a 1% (wt/vol) agarose gel and purified using PCR Purification Kit (Qiagen, Inc., Chatsworth, Calif.). The PCR fragment was sequenced directly using the internal primer wicker-18S-fseq1 (5′-TGCCAGTAGTCATATGCTTGT-3′) and further sequence-derived oligonucleotides. Nucleotide sequence determination was carried out with a LI-COR DNA sequencer model 4000 by the dideoxy chain termination method (26). The sequence was analyzed using the Wisconsin Package version 8.1 UNIX (GCG) software package.
Preparation of polyclonal antibodies.
Hyperimmune sera directed against P. zopfii were developed in rabbits. A total number of 107 cells/ml in phosphate-buffered saline of either strain SAG 263-4 or strain SAG 2021 were emulsified with an equal volume of Freund incomplete adjuvant (Sigma), and 1 ml was used to inoculate New Zealand White rabbits with an approximate body mass of 2.5 to 3.5 kg intradermally. Starting at 3 weeks postinfection, the rabbits were boosted intravenously biweekly three times with 107 viable cells of the homologous strain. At 7 days after the last application (10 weeks after the original inoculations), the rabbits were bled and sera were obtained.
Antigen preparation procedures, SDS-PAGE, and immunoblotting.
Pools were made from whey and blood serum samples from cows with chronic mastitis due to naturally P. zopfii infection and from noninfected lactating cows. Specimens were analyzed for specific IgG, IgG1, and IgA concentrations. Different antigen preparations were tested in order to gain the most suitable antigen preparation for coating or immunoblotting. Prototheca cells were thus resuspended in distilled water without any further treatment, broken by repeated freezing-thawing cycles in liquid nitrogen, ultrasonicated, heated (100°C, 5 min), and digested with proteinase K. Protein fractions (pellets or supernatants) of each preparation were separated on 10% denaturing sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels (Protean II; Bio-Rad) (16). Proteins were transferred to a polyvinylidene difluoride membrane for immunoblotting. Immunodetection procedures were carried out by standard procedures (25). To minimize nonspecific background during immunoblotting, a synthetic blocking reagent (Roti-Block; Carl Roth, Ltd., Karlsruhe, Germany) was used. Detection was carried out colorimetrically using VIP-Kits (Vector Laboratories, Inc.) or by chemiluminescence using ECL-Kits (Amersham Pharmacia Biotech, Ltd., Buckinghamshire, United Kingdom) using peroxidase-conjugated secondary antibodies.
Classification of P. zopfii-infected cows.
Lactating cows with a history of either acute or chronic P. zopfii infection were included in the present study. A total of 92 cows were evaluate by anamnestic, cultural, and clinical criteria. The animals were then assigned to the following groups: group A, cows (n = 12) with acute Prototheca mastitis (swollen udder with hard quarters, quarters secreting watery milk with white flakes, no fever) and positive cultural Prototheca isolation; group B, cows (n = 22) with chronic clinical symptoms of Prototheca mastitis (atretic quarters reduced in size, significant decrease in milk production, no fever, duration of clinical signs for >2 weeks) and a current positive culture; and group C, cows (n = 18) showing chronic clinical signs of mastitis (atretic udder, significant decrease in milk production, no fever, duration of clinical signs for >2 weeks) combined with earlier positive cultural findings but with negative cultural results when investigated. The negative control group (group D) consisted of healthy, uninfected cows (n = 40) without a history of Prototheca mastitis. Each animal in this group was tested negatively for P. zopfii by culture. In addition, the milk of all cows was routinely investigated by culture on day 5 postpartum.
Sample collection and preparations.
Serum and milk samples were collected in parallel from cows included in the present study. To extract whey, milk samples were mixed with 0.1 mU of chymotrypsin (Rennin; Fluka, Ltd.) per 10 ml of milk. After incubation for 30 min at 37°C, the samples were centrifuged. Serum samples obtained from blood and whey preparations were allowed to clot overnight at 4°C before separation.
ELISA procedures.
In order to obtain antigen for whole-cell ELISA, cultures were incubated for 36 h at 37°C on Sabouraud-dextrose-agar medium plates. Algae were rinsed from the plates with 2 ml of carbonate buffer (pH 9.6) and subsequently pooled. The total cell count was adjusted to 107 cells/ml in carbonate buffer. For coating, 100 μl of this suspension was transferred to each well of 96-well microtiter plates (Maxisorp; lot no. 4-42404; Nunc, Ltd., Roskilde, Denmark). All incubations and wash steps were done as described previously (12). As a positive reference standard, a randomly selected serum pool obtained from 15 chronically infected cows was used. The negative control consisted of a pooled serum from 10 healthy lactating cows obtained from a herd without any history of Prototheca mastitis over a period of 5 years. The following affinity-purified polyclonal monospecific peroxidase-conjugated conjugates were used: anti-bovine IgG, anti-bovine IgG1, anti-bovine IgA, or anti-bovine IgM (Bethyl Laboratories, Inc., Montgomery, Tex.). The plates were washed again four times with phosphate-buffered saline–Tween. The enzymatic reaction was developed with an ABTS (Boehringer-Mannheim)-based chromogen and measured by a computer-controlled photometer (Multiscan MCC-340; Flow Lab, Inc., McLean, Va.) (12). Antibody concentrations were expressed as ELISA units (EU) using the positive reference standard method (6).
ELISA performances and statistical analyses.
Data were calculated using a computer-based program developed for ELISA evaluation (21). The values of positive reference standards were set to 100 EU. The cutoff EU value was calculated to be three standard deviations above the mean of the negative controls. The activities of isotype-specific antibodies were calculated and plotted as notch boxes. The median (internal horizontal line), upper, and lower quartiles (the oblique margins of the boxes), the 95% confidence limits (the upper and lower horizontal margins of the boxes), and the extreme values are shown (19). Significant differences between EU data of infected cows at the three various clinical stages (groups A, B, and C) and the uninfected control group (group D) were tested by Student t tests for unpaired observations or by Welch's test. A P value of ≤0.05 was considered to be significant. ELISA results were evaluated by calculation of the sensitivity and the specificity (34).
To investigate the reproducibility of all ELISA systems, intra-assay and interassay variations were determined for IgG, IgG1, and IgA isotypes. For intra-assay variation, each well of two plates was coated with positive standard serum diluted 1:800 for IgG in serum and 1:400 for IgA or IgG1 in whey. For interassay variation, specimens obtained from 10 positively and 10 negatively tested cows were investigated at least five times each. In order to compare the whole-cell ELISA antigens of strains SAG 263-4 and SAG 2021, suspensions of both alga strains were weighed and adjusted. Microtiter plates were coated with both of these antigens and then tested by checkerboard titration. The evaluation of intra-assay variation and interassay variation was performed as described previously (14).
Due to similarities in culture morphology, pathogenesis, and the clinical signs of bovine mastitis, the pathogenic yeast Cryptococcus neoformans was chosen for the testing of cross-reactivity. A rabbit-derived hyperimmune sera developed against C. neoformans was tested on microtiter plates coated with P. zopfii SAG 2021 as the antigen.
RESULTS
Species confirmation.
Based on genetic, morphologic, and biochemical properties, all Prototheca strains isolated in this study could be assigned to the species P. zopfii, variant II.
Choice of strains, antigen preparation procedures, and immunoblotting.
In order to select a strain for coating, P. zopfii SAG 263-4 and SAG 2021 were compared. Noticeable differences in their immunogenic properties were obvious when Western blotting with rabbit hyperimmune serum was performed. Two immunogenic components at molecular masses of 90 kDa and 100 kDa were detected for SAG 263-4 and SAG 2021. An additional common signal occurred at a molecular mass of 45 kDa which was weak in case of SAG 263-4 (data not shown). In their partial 18S rDNA sequence they did not differ (data not shown).
When compared as whole-cell antigens in a checkerboard titration, SAG 2021 revealed consistently significant stronger signals than SAG 263-4. Based on these results and on the human origin of strain SAG 263-4, SAG 2021 was chosen as the strain coated for all of the ELISAs. Immunoblot analyses of the different antigen preparations revealed that the main immunogenic component was already released when the cells were diluted in distilled water. No additional immunogenic components were set free from the cells by further treatment, such as heating or sonication (data not shown)
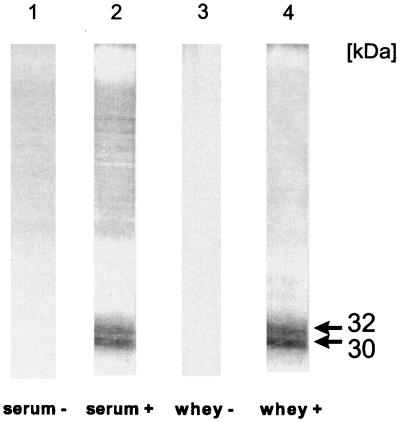
The presence of specific IgG antibodies in serum and IgA antibodies in whey samples of cows with protothecal mastitis is shown by an identical immunogenic double band, located at 30 and 32 kDa (Fig. 1, lanes 2 and 4). No signal was obtained with systemic and local antibodies obtained from Prototheca-negative cows (lanes 1 and 3).
FIG. 1.
Immunoblot of a preparative SDS-PAGE analysis of whole-cell antigen of P. zopfii to detect specific IgG antibodies in serum and IgA antibodies in whey. Serum and whey were obtained from culture-positive (+) and culture-negative (−) dairy cows. Immunogenic components of 30 and 32 kDa are indicated by arrows.
ELISAs.
The statistical evaluation of the ELISA is summarized in Table 1. Animals with a clinical history of Prototheca mastitis but with negative culturing results were not considered. As a positive cutoff value, the total of the three standard deviations and the mean EU value of the culture negative animals was defined. The highest sensitivity (96%) and the lowest cutoff value (1 EU) was calculated for the ELISA when whey-IgA was used. Whereas the sensitivity of the serum IgG ELISA was significantly lower. No significant levels of IgM and IgA were detected in serum. A cross-reactivity with a hyperimmune serum of a rabbit immunized with C. neoformans was not observed.
TABLE 1.
Statistical characteristics of ELISAs suitable for identification of P. zopfii infection in dairy cows
| Antibody isotype testeda | % Sensitivity | % Specificity | Positive cutoff value (EU) | % Intra-assay variation | % Interassay variation |
|---|---|---|---|---|---|
| Serum-IgG | 81 | 100 | 38 | 8.49 | 22.50 |
| Whey-IgA | 96 | 94 | 1 | 6.08 | 6.32 |
| Whey-IgG1 | 92 | 94 | 1 | 7.20 | 9.74 |
IgA in serum was not detectable.
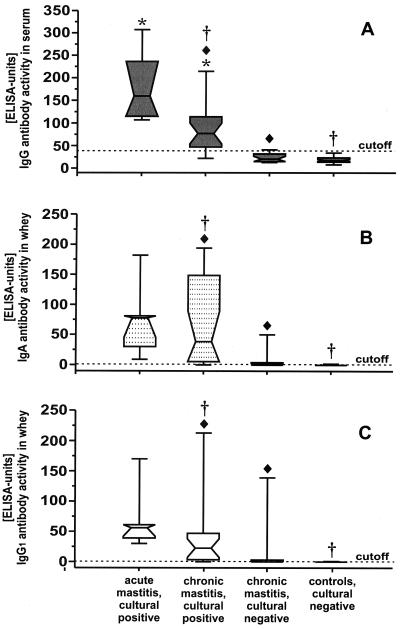
The antibody concentrations against P. zopfii measured with different ELISAs are depicted in Fig. 2. In comparison to all other clinical stages of infection, acutely infected cows revealed the highest antibody quantity in serum and in whey. A significant discrimination between acute and chronically infected cows (P < 0.05) was observed when serum IgG was measured (Fig. 2A). A similar result was found when the sera of chronically infected cows with positive culture results were compared to chronically infected cows that had earlier positive culture results but a negative culture result at the time of investigation. A significant discrimination for IgG could also be demonstrated when the sera of chronically infected cows with positive culture results and the sera of uninfected animals (P < 0.05) were analyzed.
FIG. 2.
ELISA activity of IgG (dark gray boxes) in serum (A) and of IgA (dotted boxes) (B) and IgG1 (white boxes) (C) in whey of cows with various clinical stages of P. zopfii infection. A significant discrimination (P < 0.05) of acutely infected culture-positive versus chronically infected culture-positive animals is indicated by asterisks. ⧫, Significant discrimination (P < 0.05) of chronically infected culture-positive versus chronically infected culture-negative animals. A significant discrimination (P < 0.05) of chronically infected culture-positive versus noninfected cows is indicated by a dagger symbol. The cutoff value for positive test results is pointed by the dotted horizontal line.
Analyzing whey antibodies reacting with P. zopfii antigens, chronically infected culture positive cows could be clearly distinguished from previously culture positive animals with an actual negative finding (Fig. 2B and C). A significant discrimination between chronically infected culture positive cows and uninfected animals (P < 0.05) was found in whey samples. A significant number of chronically infected culture positive animals showed a higher IgA antibody concentration in whey compared to cows with acute diseased.
On the basis of the low cutoff level chosen for the whey samples, 50 and 39% of the animals with previous cultural findings of Prototheca, which were currently culture negative, could be identified by investigating the IgA level and the level of IgG1 in whey. Specific antibodies were not detectable in uninfected animals when milk serum was used.
DISCUSSION
Thus far, no immunological test for the screening of dairy herds infected with P. zopfii exists. A rapid identification of these cows would reduce the risk of having the infection spread within the herd. In this study we developed an ELISA based on the detection of P. zopfii-specific IgA and IgG1 antibodies in whey and IgG antibodies in serum. This system allows the identification of infected animals and the discrimination of cows that are infected but are at various clinical stages. The ELISA might now be used as a control measure at the herd level.
The results of our ELISAs clearly demonstrated that anti-Prototheca IgA and IgG1 antibodies in whey are most suitable for the immunological detection of Prototheca-infected cows, which is underlined by the high sensitivities of 96 and 94%, and also by the low cutoff of 1.0 EU. In cows, the (natural) way of infection is believed to be the contact of the algae with the udder epithelium. This is reflected by the presence of IgA antibodies in the udder, the site of clinical manifestation (12). A sensitivity not sufficient for diagnostic purposes was obtained when (blood) serum IgG antibodies were investigated (Table 1). This finding might be explained by a frequent enteric contact with the algae (13). However, the ELISA for the detection of IgG in serum can be used for the identification of nonlactating cows or of animals with atretic udder quarters. Different methods of antigen preparation and the comparison of different strains demonstrated that the whole-cell antigen of the mastitis strain SAG 2021 is most suitable for coating.
Since the actual infection status of the cows with previous cultural findings of Prototheca but with presently culture negative results was uncertain, the sensitivity and specificity of the different ELISAs were calculated without using the data for these animals. On the one hand, these animals might represent frequently occurring intermittent shedders of the pathogen (16, 31). On the other hand, the cows might also have overcome the infection. The chronic clinical signs of mastitis such as the permanent decline of milk production and atretic udder quarters would persist in these cases (27). By using the ELISA for the detection of anti-Prototheca IgA antibodies in whey, 50% of these cows were identified as infected animals. When IgG1 antibodies against Prototheca were tested, the number of positively identified animals dropped to a rate of only 39%. A diagnostic interpretation of this finding is difficult, since controlled experimental infection, including the monitoring of specific immunoglobulin isotypes in serum and secretions was not carried out yet. One possible explanation for why 50% of the cows tested positive by ELISA in the group with chronic signs of infection but did not have positive cultural findings is that the test correctly identified persistently infected cows. This is underlined by the general finding that specific IgA antibodies are present in the sera of persistently infected animals and humans (14, 15, 19). Hence, a negative result obtained for this group could indicate convalescence. A third explanation is the occurrence of false-positives. However, this explanation is unlikely because the specificity was 94%. Negative findings may indicate falsely negative but nevertheless infected animals. In future investigations, the kinetics of antibody responses of chronically infected animals and intermittent shedders need to be clarified in greater detail.
The advantage of our ELISA compared to plate culturing is the simple and reliable identification of infected cows. In comparison to the classical culture method and the subsequent microscopic investigation, the use of our ELISA has reduced the time needed to make a diagnosis of an infected animal to 12 h.
A question yet to be addressed in literature, is whether antibodies can be protective or algicidal for P. zopfii organisms. Our findings demonstrate the presence of antibodies against immunogenic components at a molecular masses of 30 and 32 kDa in serum and in whey (Fig. 1). The role of these two structures in the pathogenesis of protothecal bovine mastitis has to be elucidated in further experiments in order to investigate the horizontal and vertical routes of infection.
In summary, serologic diagnostic measures for correct identification of protothecal mastitis in dairy cows by ELISA provide a rapid, reliable, and inexpensive screening test for infection with P. zopfii compared to microbiological examination by plate culturing. Early identification of subclinically infected animals will reduce the risk of new infections and contamination of the environment. The test might also be a promising tool for the remediation of infected dairy herds.
ACKNOWLEDGMENTS
We thank A. Meyer for collecting specimens. The technical assistance of E. Brumme and D. Rüster is also gratefully acknowledged. We thank F. Wagner for providing the rabbit anti-Cryptococcus sera. Finally, we thank H. Neubauer and L. D. Sprague for useful discussions, critically reading of the manuscript, and for correcting the English.
REFERENCES
- 1.Aalbaek B, Jensen H E, Huda A. Identification of Prototheca from bovine mastitis in Denmark. APMIS. 1998;106:483–488. [PubMed] [Google Scholar]
- 2.Anderson K L, Walker R L. Sources of Prototheca spp. in a dairy herd environment. J Am Vet Med Assoc. 1988;193:553–556. [PubMed] [Google Scholar]
- 3.Baumgärtner B. Vorkommen und Bekämpfung der Protothekenmastitis des Rindes im Einzugsgebiet des Staatlichen Veterinär- und Lebensmitteluntersuchungsamtes Potsdam. Prakt Tierarzt. 1997;78:406–414. [Google Scholar]
- 4.Blaschke-Hellmessen R, Schuster H, Bergmann V. Differenzierung von Varianten bei Prototheca zopfii (Krüger 1894) Arch Exp Veterinarmed. 1985;39:387–397. [PubMed] [Google Scholar]
- 5.Blaschke-Hellmessen R, Wilhelm A, Teichmann G, Schuster H, Boeltzig K. Orientierende Untersuchungen zum Nachweis von Antikörpern gegen Prototheca zopfii bei Rindern. Mh Vet Med. 1987;42:48–50. [Google Scholar]
- 6.Butler J E, Feldbush T L, McGivern P L, Stewart N. The enzyme-linked immunosorbent assay (ELISA): a measure of antibody concentration or affinity. Immunochemistry. 1978;15:131–136. doi: 10.1016/0161-5890(78)90053-6. [DOI] [PubMed] [Google Scholar]
- 7.Carey W P, Kaykova Y, Bandres J C, Sidhu G S, Brau N. Cutaneous protothecosis in a patient with AIDS and a severe functional neutrophil defect: successful therapy with amphotericin B. Clin Infect Dis. 1997;25:1265–1266. doi: 10.1086/516974. [DOI] [PubMed] [Google Scholar]
- 8.Casal M, Gutierrez A J. In vitro activity of ribostamycin against Prototheca sp. Mycopathologia. 1983;83:21–23. doi: 10.1007/BF00437408. [DOI] [PubMed] [Google Scholar]
- 9.Costa E O, Carciofi A C, Melville P A, Prada M S, Schalch U. Prototheca sp. outbreak of bovine mastitis. Zentbl Veterinarmed [B] 1996;43:321–324. doi: 10.1111/j.1439-0450.1996.tb00321.x. [DOI] [PubMed] [Google Scholar]
- 10.Da Costa E O, Ribeiro A R, Watanabe E T, Pardo R B, Silva J B, Sanches R B. An increased incidence of mastitis caused by Prototheca species and Nocardia species on a farm in Sao Paulo, Brazil. Vet Res Commun. 1996;20:237–241. doi: 10.1007/BF00366921. [DOI] [PubMed] [Google Scholar]
- 11.Enders F, Weber A. Untersuchungen zum Vorkommen von Prototheken in Kotproben von Rindern. Berl Munch Tierarztl Wochenschr. 1993;106:165–169. [PubMed] [Google Scholar]
- 12.Hensel A, Pabst R, Bunka S, Petzoldt K. Oral and aerosol immunization with viable or inactivated Actinobacillus pleuropneumoniae bacteria: antibody response to capsular polysaccharides in bronchoalveolar lavage fluids (BALF) and sera of pigs. Clin Exp Immunol. 1994;96:91–97. doi: 10.1111/j.1365-2249.1994.tb06236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hodges R T, Holland J T S, Neilson F J A, Wallace N M. Prototheca zopfii mastitis in a herd of dairy cows. NZ Vet J. 1985;33:108–111. doi: 10.1080/00480169.1985.35187. [DOI] [PubMed] [Google Scholar]
- 14.Jark U, Ringena I, Franz B, Gerlach G F, Beyerbach M, Franz B. Development of an ELISA technique for serodiagnosis of bovine paratuberculosis. Vet Microbiol. 1997;57:189–198. doi: 10.1016/s0378-1135(97)00125-9. [DOI] [PubMed] [Google Scholar]
- 15.Jensen H E, Aalbaek B, Bloch B, Huda A. Bovine mammary protothecosis due to Prototheca zopfii. Med Mycol. 1998;36:89–95. [PubMed] [Google Scholar]
- 16.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 17.Lerche M. Eine durch Algen (Prototheca) hervorgerufene Mastitis der Kuh. Berl Munch Tierarztl Wochenschr. 1952;4:64–69. [Google Scholar]
- 18.Matsuda T, Matsumoto T. Protothecosis: a report of two cases in Japan and a review of the literature. Eur J Epidemiol. 1992;8:397–406. doi: 10.1007/BF00158575. [DOI] [PubMed] [Google Scholar]
- 19.McGill R, Tukey J W, Larson W A. Variations of box plots. Am Statist. 1978;32:12–16. [Google Scholar]
- 20.Mohabeer A J, Kaplan P J, Southern P M, Jr, Gander R M. Algaemia due to Prototheca wickerhamii in a patient with myasthenia gravis. J Clin Microbiol. 1997;35:3305–3307. doi: 10.1128/jcm.35.12.3305-3307.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paar F, Franz B, Nowotny N, Petzold K. Entwicklung eines Enzyme-linked-immunosorbent-assay (ELISA) zum Nachweis von Antikörpern in Perdeseren gegen das Equine Herpesvirus 3. Wien Tierarztl Mschr. 1989;76:401–404. [Google Scholar]
- 22.Pore R S. Selektive medium for the isolation of Prototheca. Appl Microbiol. 1973;26:648–649. doi: 10.1128/am.26.4.648-649.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pore R S, Barnett E A, Barnes W C, Jr, Walker J D. Prototheca ecology. Mycopathologia. 1983;81:49–62. doi: 10.1007/BF00443909. [DOI] [PubMed] [Google Scholar]
- 24.Pore R S, Shahan T A, Pore M D, Blauwiekel R. Occurrence of Prototheca zopfii, a mastitis pathogen, in milk. Vet Microbiol. 1987;15:315–323. doi: 10.1016/0378-1135(87)90019-8. [DOI] [PubMed] [Google Scholar]
- 25.Sambrook J, Fritsch E F, Maniatis T. Detection and analysis of proteins expressed from cloned genes. In: Sambrook J, Fritsch E F, Maniatis T, editors. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. pp. 18.3–18.74. [Google Scholar]
- 26.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schick W, Kutzer H. Zum Auftreten, zur Diagnostik und zur Bekämpfung der durch Prototheca trispora bedingten Mastitis des Rindes. Mh Vet Med. 1982;37:295–298. [Google Scholar]
- 28.Schlenstedt R, Zschock M, Kloppert B, Wolter W. Vorkommen von Protothekenmastitiden in hessischen Milcherzeugerbetrieben. Tierarztl Prax Ausg G Grosstiere. 1997;25:407–412. [PubMed] [Google Scholar]
- 29.Schmalreck A F, Trankle P, Vanca E, Blaschke-Hellmessen R. Differenzierung und Charakterisierung von humanpathogenen Hefen (Candida albicans, Exophiala dermatidis) und tierpathogenen Algen (Prototheca spp.) mittels Fourier-Transform-Infrarot-Spektroskopie (FT-IR) im Vergleich zu konventionellen Methoden. Mycoses. 1998;41(Suppl. 1):71–77. doi: 10.1111/j.1439-0507.1998.tb00587.x. [DOI] [PubMed] [Google Scholar]
- 30.Schuster H, Blaschke-Hellmessen R. Zur Epidemiologie der Protothekenmastitis des Rindes-Anzüchtung von Algen der Gattung Prototheca aus der Umgebung landwirtschaftlicher Nutztiere. Mh Vet Med. 1983;38:24–29. [Google Scholar]
- 31.Tenhagen B A, Kalbe P, Klunder G, Heuwieser W, Baumgartner B. Tierindividuelle Risikofaktoren für die Protothekenmastitis des Rindes. DTW Dtsch Tierarztl Wochenschr. 1999;106:376–380. [PubMed] [Google Scholar]
- 32.Thiele D. Protothekosen bei Mensch und Tier sowie In-vitro-Untersuchungen zur Wirksamkeit und lokalen Euterverträglichkeit von Polyvinylpyrrolidon-Iodlösung und Lugolscher Lösung. Ph.D. thesis. Leipzig, Germany: University of Leipzig; 1997. [Google Scholar]
- 33.Tyler J W, Cullor J S. Titers, tests, and truisms: rational interpretation of diagnostic serologic testing. J Am Vet Med Assoc. 1989;194:1550–1558. [PubMed] [Google Scholar]
- 34.Wirth F A, Passalacqua J A, Kao G. Disseminated cutaneous protothecosis in an immunocompromised host: a case report and literature review. Cutis. 1999;63:185–188. [PubMed] [Google Scholar]