Abstract
In the current study, the effects of 7-BIO as a specific GSK3β inhibitor was examined on cell survival and expression of miR-29a-3p and miR-34a-5p in neurotoxin MPP+ treated SH-SY5Y cells. Our findings revealed that while co-treatment of the cells with 7-BIO and MPP+ did not alter the toxicity induced by MPP+, pretreatment with 3.5 μM 7-BIO for 6 h increased the survival of the 2 mM MPP+ treated cells. Also, qRT-PCR analysis of gene expression showed that while miR-29a-3p was unchanged in cells treated with either 2 mM MPP+ or 3.5 μM 7-BIO alone, miR-34a-5p was increased by MPP+ but decreased by 7-BIO. Pretreatment with 3.5 μM 7-BIO prior to MPP+ however, increased miR-29a-3p but decreased miR-34a-5p induced by MPP+. We therefore suggest that 7-BIO inhibition of GSK3β alleviates the MPP+ induced neurotoxicity by regulating miR-29a-3p and miR-34a-5p expressions in Parkinson's disease model SH-SY5Y cells.
Keywords: Parkinson's disease, Neuroprotection, SH-SY5Y, 7-BIO, MPP+, miR-29a, miR-34a, GSK3β inhibitor, miR-29a-3p, miR-34a-5p
1. Introduction
Parkinson's disease (PD) is one of the most prevalent neurodegenerative diseases in elderlies. During years of the disease progression dopaminergic neuron death of substantia nigra located in midbrain results in PD. More than 10 million people worldwide are suffering from PD [1] with symptoms including difficulties in balance and coordination, muscle stiffness of the limbs, hands and head, tremor at rest and dementia at late phases of the disease [2]. Like most diseases, PD is modulated by various environmental and genetic factors. Genetic mutations including the mutation in A53T Synuclein [3] and environmental factors such as sedentary lifestyle, exposure to certain insecticides, neurotoxins, MPTP, 6-OHDA, rotenone and paraquat might induce PD [2,4]. MPTP is a lipid soluble substance, able to cross the blood-brain barrier, oxidized by the glial enzyme Monoamine oxidase B to a toxic agent called 1-methyl-4-phenylpyridinium-4-methyl-1 (MPP+) [[4], [5], [6]]. MPTP/MPP + are widely used experimentally both in vivo and in vitro not only for understanding the underlying mechanisms involved in dopaminergic neuron death but also for developing novel therapeutic strategies [7,8]. Survival and differentiation of dopaminergic neurons during pre- and postnatal development are regulated by many pathways including the Wnt pathway [[9], [10], [11], [12], [13], [14]]. A variety of ligands are known to activate the Wnt pathway, among them the indirubins known as natural alkaloids extracted from the indigo colored plants and molluscs [[15], [16], [17], [18]]. Indirubins are also found in human urine indicating that our body also synthesizes them [16]. Indirubins inhibit variety of kinases including CDK5 and the enzyme GSK3β and therefore recommended for the treatment of chronic bone marrow leukemia, cancer and neurodegenerative diseases [[19], [20], [21]]. 7-BIO is a derivative of indirubin-3-oxime improves the toxicity induced cognitive impairment by large amyloid-beta polymer fibrils in SH-SY5Y neuroblastoma [22]. The mechanism through which 7-BIO protects SH-SY5Y from toxicity could be mediated by microRNAs, a group of small non-coding RNAs that interfere with messenger RNAs (mRNAs). These RNAs are single-stranded and have lengths of 18–27 nucleotides [23]. So far, the expression of numerous microRNAs have been altered in patients' plasmas compared to the healthy individuals and introduced to be associated with PD [7]. Detecting the changes in microRNAs expression patterns if any, could be beneficial both for diagnosis and treatment of the PD. Two candidate microRNAs known to be altered in PD have been considered in our study, that is, hsa-miR-34a and hsa-miR-29a [24]. In the present study, we have examined if Wnt signaling activation by 7-BIO affects the survival of MPP+ treated SH-SY5Y cells as well as the expression of hsa-miR-34a and hsa-miR-29a. Understanding the mechanism of action of 7-BIO may have implication for PD diagnosis and therapy.
2. Materials and methods
2.1. Cell culture
Human neuroblastoma SH-SY5Y cell line (a kind gift by Dr. Dina Morshedi, Associate Professor in the National institute of Genetic Engineering and Biotechnology, Industry Research Institute, Tehran), was cultured in DMEM/F−12 (Sigma) media supplemented with 10% FBS (Biochrome) and 1% Penicillin-Streptomycin (Gibco), refreshed every two days and incubated at 37 °C with 5% CO2.
2.2. Cell viability assay
MTT assay was performed to determine cell viability. Briefly, SH-SY5Y cells were cultured in 96-well plates at a density of 12 × 103 cells/well. After 24 h, the media was removed from each well and the cells treated with either BIO, MPP+ or a combination. Following the treatments, cells were incubated with 0.5 mg/ml MTT solution for 4 h at 37 °C. MTT solution was then removed and 100 μl dimethyl sulfoxide (DMSO) added to each well to solubilize the formazan crystals. Using the ELISA reader, the absorbance of each well was read at 580 nm and tabulated as a ratio to that of the control group.
2.3. SH-SY5Y treatments
To determine the non-toxic doses of 7-BIO and IC50 dose of MPP+, 12 × 103 cells were cultured in 96 well plates and treated with different concentrations of 7-BIO (1, 3, 5, 7, 10 μM) or MPP+ (0.3, 0.5, 1.5, 2 mM). Further analyses were performed by either co-treatment of 7-BIO (1, 3 μM) and MPP+ (2 mM) for 24 h or pretreatment with 7-BIO (2.0, 2.5, 3.0, 3.5, 4.0 μM) for 2, 4, 6 and 8 h followed by 2 mM MPP+ (dissolved in H2O) for 24 h. Since 7-BIO was dissolved in DMSO, the control group were treated with the same volumes of DMSO as those of 7-BIO. Following 7-BIO pretreatments, each well was gently washed with sterile PBS prior to the addition of MPP+. For the analysis of miRNAs expression, 3 × 106 cells were plated in T75 flasks followed by treatments with 7-BIO, MPP+ or the above mentioned pre-treatments with BIO followed by MPP+.
2.4. Western blotting
Cells treated with 7-BIO were washed with ice-cold PBS. Total cell lysate was prepared using protein lysis buffer (Tris 62.5 mM, pH 6.8; DTT 50 mM, NaF 10 mM, SDS 2% w/v), protease/phosphatase inhibitor cocktail and stored at 20 °C. Subsequently, 60 mg protein was subjected to 10% SDS-PAGE gel electrophoresis, followed by blotting onto the PVDF membrane. Molecular weight protein marker was laoded and run in parallel with the samples. The blots were then blocked with skimmed milk (5%) in TBS (Tris buffered saline) at room temperature for 1 h. The membrane was probed with Phospho GSK3β (1:1000; Santa Cruz) and reprobed with GSK3β (1:2000; Santa Cruz) primary antibodies. The membranes were subsequently probed with the corresponding HRP-conjugated secondary rabbit and mouse IgGs (Sigma) at 1:20,000 dilutions. Signals sized at 47 kDa corresponding to PGSK3β/GSK3β were visualized using ECL (chemilluminescence) on the Kodak film.
2.5. RNA extraction and cDNA synthesis
Easy-BLUE™ Total RNA extraction solution (iNtRON) was used to extract total RNA according to the manufacturer's instructions. The extracted RNAs were dissolved in DEPC treated water, their qualities and concentrations were measured using nanodrop (Thermo-fisher scientific). cDNA was synthesized by cDNA synthesis Kit (Yekta Tajhiz Azma, Iran). At this stage, specific stem-loop primers, dNTP's, RNase inhibitor, Reverse transcriptase M-MLV enzyme were added to the extracted RNAs and diluted in DEPC water to a total volume of 20 μl cDNAs were then synthesized using the Bio-Rad thermal cycler (Bio molecular systems; 10 min at 25 °C, 15 min at 37 °C, 60 min at 42 °C, and 10 min at 75 °C).
2.6. Real-time q RT-PCR
BIO FACT Real-Time PCR Master Mix was used to perform real time PCR using mic bms Real Time PCR machine (Australia). U6 was used as an internal control. The primer sequences are listed as Table 1. The real time PCR programs were set as follow:
Table 1.
Primer sequences.
| Gene | Primer sequences |
|---|---|
| miR-34a-5p | Forward primer for real time PCR: 5′ ATGCCTGCCGTGTGAAC3′ Stem loop primer for cDNA synthesis: 5′ GGTCGTATGCAGAGCAGGGTCCGAGGTATCCATCGCACGCATCGCACTGCATACGACCACAACC3′ |
| miR-29a-3p | Forward primer for real time PCR:5′ AGCCTAGCACCATCTGAA3′ Stem loop primer for cDNA synthesis: 5′GTCGTATGCAGAGCAGGGTCCGAGGTATTCGCACTGCATACGACTAACCG3′ |
| U6 | Forward: AAGGATGACACGCAAATTC Stem loop primer for cDNA synthesis: GTC GTATGCAGAGCAGGGTCCGAGGTATTCGCACTGCATACGACAAAAATATGG |
| Universal primer for real time PCR | Reverse: 5′ GAGCAGGGTCCGAGGT 3′ |
The program for U6 and miR-29a-3p: 15 min at 95 °C for 1 cycle, followed by 20 s at 95 °C, 30 s at 60 °C, and 20 s at 72 °C for 40 cycles as the followings.
The program for miR-34a-5p: 15 min at 95 °C for 1 cycle, followed by 15 s at 95 °C, 20 s at 62 °C, and 15 s at 72 °C for 40 cycles. ΔΔCt method was used for relative quantification of changes in the gene expression levels compared to those of the control group.
2.7. Statistical analyses
All statistical analyses were performed using Graph Pad Prism 8 software. Data of the experimental and control groups were compared using one-way ANOVA. A value of p < 0.05 was considered as a significant difference. All the data are expressed as Mean ± Standard Error.
3. Results
3.1. 7-BIO inactivated GSK3β
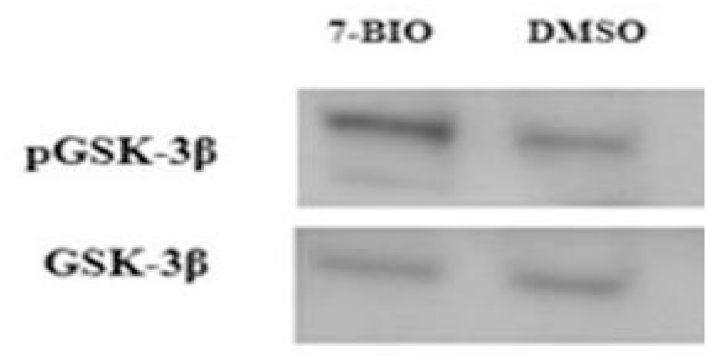
Following the treatment with 7-BIO, western blotting was performed and the result showed a 2-fold increase in the ratio of p-GSK3β/total GSK3β protein level compared to that following the DMSO treatment (Fig. 1).
Fig. 1.
Western blot analysis of the increased level of phosphorylated GSK3β (67 kDa) relative to that of the total GSK3β (as the loading control) following 7-BIO treatment. DMSO treatment was used as a control.
3.2. Co-treatment of the SH-SY5Y cells with 7-BIO and MPP+ did not reduce the MPP+ induced toxicity
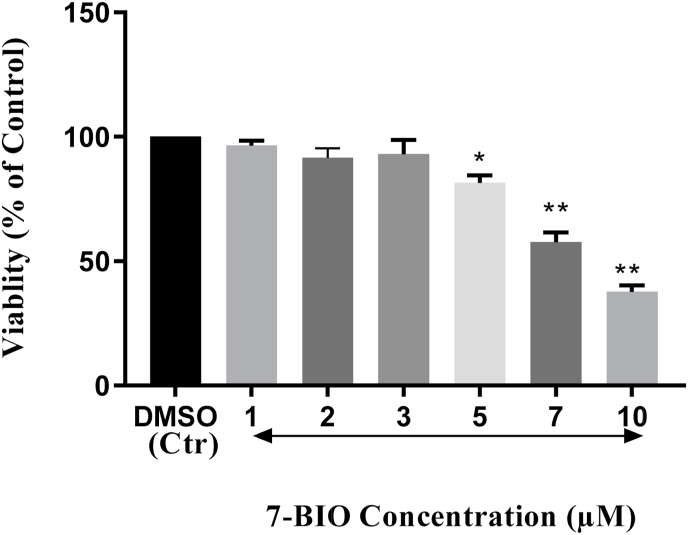
To determine the IC50 concentrations of 7-BIO and MPP+, we firstly treated the cells with either 7-BIO or MPP+ alone. 7-BIO treatments were performed at 1, 2, 3, 5, 7, and 10 μM. As indicated in Fig. 2, the results of the MTT assay results showed that 7-BIO did not decrease the viabilities at concentrations 1 μM (98%), 2 μM (91%) and 3 μM (97%) for 24 h, hence were considered for co-treatment with MPP+ (Fig. 2). Other concentrations however, decreased the viabilities significantly, e.g. 5 μM (81%), 7 μM (57%) and 10 μM (37%), thus were excluded from the rest of the experiments (Fig. 2).
Fig. 2.
Viability analysis of the SH-SY5Y cells treated with different concentrations of 7-BIO. Since 7-BIO did not decrease viabilities at concentrations 1 μM (98%), 2 μM (91%) and 3 μM (97%) for 24 h, these concentrations were used for all subsequent experiments with MPP. * indicates p < 0.05 and ** indicates p < 0.001.
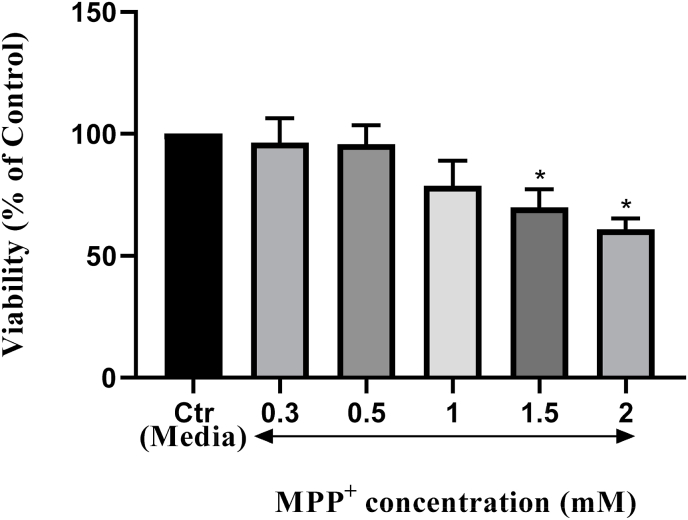
With regards to MPP+, significant reductions in viabilities were observed at concentrations the MTT analysis at different concentrations of 1, 1.5 and 2 mM; viabilities were 96 % at 0.3 mM, 95% at 0.5 mM, 78% at 1 mM, 69% at 1.5 mM, and 60% at 2 mM. The latest concentration (2 mM) was therefore considered for treatments with 7-BIO (Fig. 3).
Fig. 3.
Viability analysis of the SH-SY5Y cells treated with different concentrations of MPP+. Among different concentrations of MPP+, 1.5 mM and 2 mM reduced the viability significantly; at 0.3 mM (96%), 0.5 mM (95%), 1 mM (78%), 1.5 mM (69%), and 2 mM (60%). 2 mM MPP+ was therefore considered for co-treatment with 7-BIO. * indicates p <0.05.
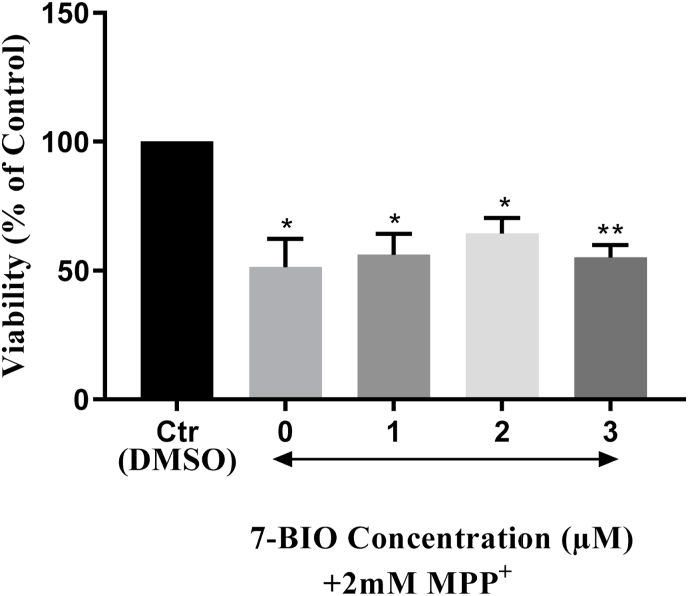
MTT assay results of the co-treatments with 7-BIO (1, 2 and 3 μM) and MPP+ (2 mM) showed that 7-BIO co-treatment did not reduce the toxicity induced by 2 mM MPP+; the viabilities were still down to 56.16%, 64.38% and 55.02%, after co-treatment with 1, 2 and 3 μM 7-BIO, respectively (Fig. 4).
Fig. 4.
Viability analysis of the SH-SY5Y co-treated with 1, 2, and 3 μM 7-BIO and 2 mM MPP+ for 24h. Co-treatments did not reduce the toxicity induced by 2 mM MPP+; the viabilities were down to 56.16%, 64.38% and 55.02%, respectively. * indicates p < 0.05 and ** indicates p < 0.001.
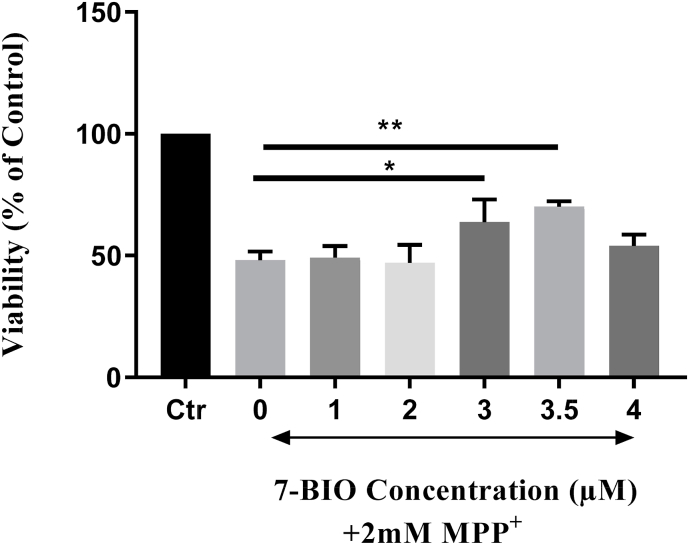
3.3. 7-BIO pretreatment alleviated the MPP + induced toxicity
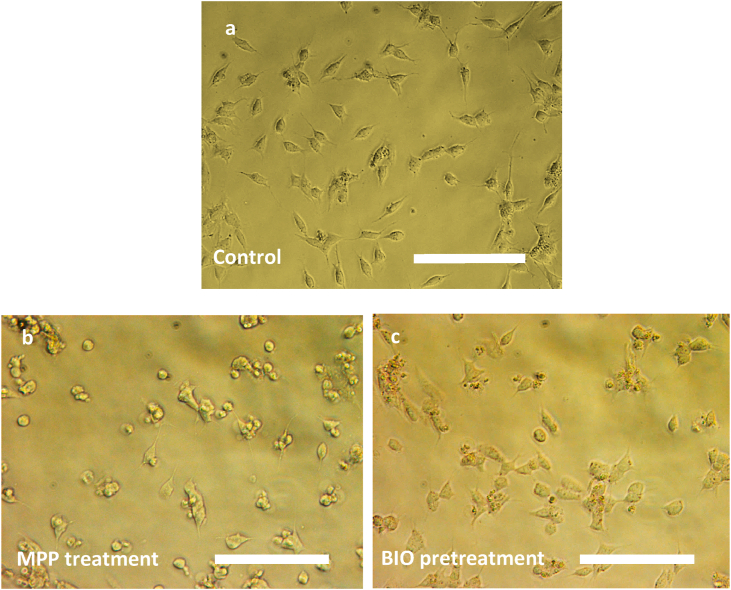
To further investigate if neuroprotection could be induced by pretreatment with 7-BIO in MPP+ treated cells, SH-SY5Y cells were pretreated with 1, 2, 3 and 4 μM concentrations of 7-BIO for 2, 4, 6, and 8 h prior to the treatment with 2 mM MPP+ for 24 h (data not shown). Results of the MTT assay showed that pretreatment with 7-BIO for 6 h at 3 μM and 3.5 μM enhanced survival against MPP+ significantly; up to 63% at 3 μM and to 70% at 3.5 μM (Fig. 5). Other concentrations e.g. 1 μM (49%), 2 μM (47%) and 4 μM (54%) however, did not improve the survival of the MPP+ treated cells. 3.5 μM 7-BIO was therefore selected as the effective pretreatment concentration to evaluate the changes in the expression levels of microRNAs following the treatment with MPP+. More viable cells are clearly visible in photomicrographs (Fig. 6c) of the 3.5 μM 7-BIO pretreated cells for 6 h compared to those of the neurotoxin treated where many apoptotic cells are present (Fig. 6b).
Fig. 5.
Viability analysis of the SH-SY5Y cells pretreated with either the media alone or with 7-BIO for 6h at different concentrations of 1, 2, 3, 3.5 and 4 μM followed by 24 h treatment with MPP+. The results showed that 7-BIO pretreated cells at 3 μM and 3.5 μM for 6 h enhanced the survival against MPP+ significantly; other concentrations however, did not have significant effects.
Fig. 6.
Photomicrographs of the SH-SY5Y untreated control (a), 2 mM MPP+ treated (b) and 7-BIO pretreated for 6 h followed by 2 mM MPP+ treatment for 24 h (c). More viable cells are clearly visible after 7-BIO treatment (c) compared to the MPP+ treated cells (b). Scale bar 200 μm.
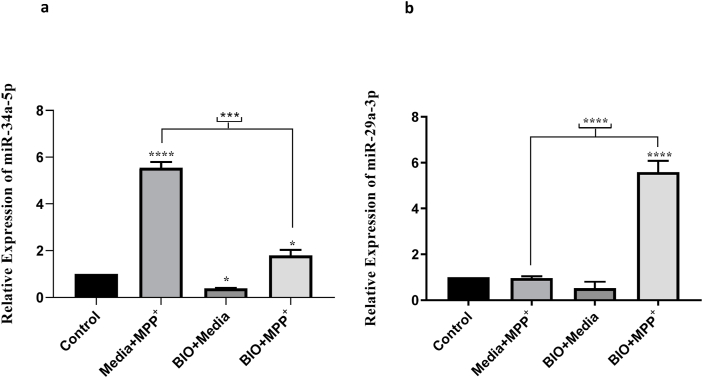
3.4. 7-BIO pretreatment altered the expression level of miR-29a-3p and miR-34a-5p following the MPP+ treatment
As shown in Fig. 7a, the expression level of miR-34a-5p was upregulated significantly after the MPP+ treatment by almost 6 folds. On the other hand, treatment with 7-BIO alone reduced the level down to almost zero or to 2 folds when was treated prior to MPP+. We therefore suggest that 7-BIO pretreatment relatively rectifies the induced expression of miR-34a-5p by MPP+. With regards to miR-29a-5p, the expression level was unchanged after MPP+ or 7-BIO treatment alone but induced following the pretreatment with 3.5 μM 7-BIO in 2 mM MPP+ treated cells (Fig. 7b). These results are taken to indicate that 7-BIO efficiently alters the expressions of miR-29a-3p and miR-34a-5p.
Fig. 7.
Pretreatment with 7-BIO in MPP+ exposed cells altered the expression levels of (a) miR-34a and (b) miR-29a. 7-BIO pretreatment drastically reduced the elevated expression of miR-34a due to the MPP+ treatment, but increased that of miR-29a. (* indicates p < 0.05, *** indicates p < 0.001 and **** indicates p < 0.0001).
4. Discussion
PD as one of the incurable age related neurodegenerative disorder has demanded intensive research to unravel the underlying mechanism of disease progression for more effective diagnostic tools and therapeutic approaches. In view of the increasing evidence that microRNAs are involved in pathogenesis of PD, we have proposed if the changed expressions of miR-34a-5p and miR-29a-3p could be considered as possible biomarkers for PD. In the present study, neurotoxin (MPP+) treated dopaminergic cell line, SH-SY5Y, were treated with 7-BIO as a GSK3β inhibitor and examined for their survival and the expression of miR-29a-3p and miR-34a-5p. Our results showed that GSK3 inhibition by 7-BIO enhanced the survival of the MPP+ treated SH-SY5Y. Neuroprotective effects of GSK3β inhibition in differentiated neurons have also been shown by others. for example, Li and colleagues (2020) reported the positive effect of GSK3β knockout on MPTP treated mice model of Parkinson's disease as well as that of the injected specific GSK3β inhibitor (Tideglusib) in MPTP treated mice. They showed enhanced resistance of these mice models to Parkinson's disease following GSK3β inhibition. Quantification of TH immunoreactive striatal dopaminergic neurons showed that in GSK3β inhibited or knockout mice, the TH intensity was significantly increased in these differentiated neurons [25]. Also, Hu and colleagues (2015) showed that indirubin-3-oxime effectively prevented 6OHDA-induced neurotoxicity in PC12 cells through the inhibition of GSKβ [26]. With regards to the expressions of miR 29a and 34a, our data analysis showed that following the treatment with MPP+ alone, the expression of miR-34a-5p was increased significantly, whereas that of the miR-29a-3p was unaltered. Referring to previous studies who showed that in neurotoxin treated SH-SY5Y and PC12 cells, there was a raised expression of miR-34a-5p versus a dropped level of the anti-apoptotic gene, BCL-2 [27,28], one would suggest that the BCL-2 mRNA could be targeted by miR-34a-5p. Also considering that miR-34a promoter has binding sites for Nuclear factor-kappa B (NF-κB), it is conceivable that the inflammatory and apoptotic effects of miR-34a could be mediated via binding with NF-κB [29]. Regarding miR-29a-3p, to our knowledge we have provided the first in vitro evidence on its unaltered expression after the MPP + treatment. While some in vivo evidence were indicative of a declined expression of the level of miR-29a in PD patients [7,[30], [31], [32]], Bai and colleagues (2017) interestingly reported a differential expression of miR-29a-3p in peripheral blood monocytes (PBMC) versus that in sera and CSF. They found no altered levels in PBMCs but decreased levels in sera and CSF samples of the PD patients, declining with the disease severity. Thus, based on the source of the samples, the in vivo evidence might differ from or correspond with that from in vitro. Different pathways have been predicted to modulate/to be modulated by the expression of miRNAs, among them the MAPK and the Wnt pathways [31] sharing GSK3β as a common key element. We therefore sought to examine the effect of 7-BIO as a specific inhibitor of GSK3β on the expression of miR-34a-5p and miR-29a-3p as well as on cell survival in MPP+ treated SH-SY-5Y. Our results showed that 7-BIO alleviated the elevated expression of miR-34a-5p, either alone or prior to the MPP+ treatment, as well as the toxicity induced by MPP+. The neuroprotective and anti-inflammatory effects through the inhibition of GSK3β have also been shown elsewhere both in vitro and in vivo [33]. Also, decreased toxicity and miR-34a expression by GSK3β inhibition have been evidenced by Alural and colleagues (2015) who showed that in paraquat (PQ) intoxicated SH-SY5Y cells [34] the neuroprotective effects of lithium was reversed by miR-34a-5p mimic. The correlation between miR-34a-5p and induced apoptosis could also be found in a comprehensive review by Wan and colleagues (2017) who have highlighted the oxidative stress-responsive microRNAs [35]. Neuroprotective effect induced by GSK3β inhibition via miR-34a-5p regulation could also be explained by the suggested targets of GSK3β, one of which is the redox sensitive transcription factor Nuclear factor erythroid 2-related factor 2 (NRF2). Alural and colleagues (2015) showed that the effects of lithium against PQ-toxicity was reversed by NRF2 siRNA transfection. Also, Jain and colleagues (2007) showed that GSK3β siRNA-transfection in Human Hepatoma (HepG2) cells resulted in nuclear accumulation, stabilization of NRF2 and activation of its downstream genes [36]. Another target of GSK3β is P53, also known to regulate miR-34. Feng and colleagues (2011) showed that the ectopic expression of miR-34 promoted the p53 mediated apoptosis, cell cycle arrest and senescence, whereas its inactivation resulted in the inhibition of p53 mediated apoptosis [37]. Considering the above findings, it is conceivable that the decreased expression of miR-34a-5p that we have seen following 7-BIO pretreatment of the MPP+ treated SH-SY5Y might have been resulted by the activation of NRF2 and BCL-2 as well as suppression of P53, all of which require to be further analyzed in future experiments. While we have seen a reduced expression of miR-34a-5p following 7-BIO treatment either alone or prior to the MPP+ treatment, that of the miR-29a-3p was induced following 7-BIO pretreatment in MPP+ exposed cells. This upregulated level of miR-29a-3p has also been reported in a clinical study by Bai and colleagues (2017) in l-dopa-treated patients [32]. According to Bai and colleagues (2017), miR-29 family of microRNAs reduced the oxidative stress and enhanced neuronal survival and protection by targeting the candidate targets of the PD related genes such as PARK7 (DJ-1), Parkin substrate GPR37 and apoptosis related genes such as BCL-2. Also, miR-29 expression were downregulated in PD patients diagnosed with idiopathic rapid eye movement behavior disorder, dementia and Lewy bodies [38]. It appears therefore that miR-29 expression is conversely related to PD and its upregulation might have therapeutic beneficial. Collectively, we suggest that GSK3β inhibition either in general or specifically e.g. by 7-BIO rectifies the altered expression of key microRNAs in PD such as miR-29a-3p and miR-34a-5p, thus might alleviate the toxicity induced in dopaminergic neurons. We also suggest that the expression patterns of miR-29a-3p and miR-34a-5p in dopaminergic neurons can be considered as signatures for diagnosis, progression of the disease and therapy in PD patients.
Compliance with ethical standards
All studies were accomplished in accordance with the guidelines provided by “Tehran University of Medical Sciences Research Ethics Committee.”
CRediT authorship contribution statement
Morteza Ahmadzadeh-Darinsoo: Investigation. Claude Bernard: Supervision. Azita Parvaneh Tafreshi: Conceptualization, Supervision. Dr. Arefian: supervision and troublshooting the miRNA expression detection. Dr. Shahsanam Abbasi assisted with the SH-SY cells maintenance, culture establishment and troubleshooting the in vitro experiments.
Declaration of competing interest
There is no conflict of interest between the authors.
Acknowledgement
This study was supported by a grant from the National Research Institute of Genetic Engineering and Biotechnology (code#679). We thank Professor Bahram Soltani and Dr. Dokaneifard from Tarbiat Modarres University for their assistance, guidance and problem solving during initial steps of the experiments. SH-SY5Y cells were generously provided by Dr. Dina Morshedi from NIGEB.
References
- 1.Dorsey E.R., et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology. 2007;68(5):384–386. doi: 10.1212/01.wnl.0000247740.47667.03. [DOI] [PubMed] [Google Scholar]
- 2.Bove J., Perier C. Neurotoxin-based models of Parkinson's disease. Neuroscience. 2012;211:51–76. doi: 10.1016/j.neuroscience.2011.10.057. [DOI] [PubMed] [Google Scholar]
- 3.Chen L., et al. Indirubin derivative 7-Bromoindirubin-3-oxime (7Bio) attenuates Aβ Oligomer-induced cognitive impairments in mice. Front. Mol. Neurosci. 2017;10 doi: 10.3389/fnmol.2017.00393. 393-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mapa M.S.T., Le V.Q., Wimalasena K. Characteristics of the mitochondrial and cellular uptake of MPP+, as probed by the fluorescent mimic, 4'I-MPP. PLoS One. 2018;13(8) doi: 10.1371/journal.pone.0197946. e0197946-e0197946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schinelli S., et al. 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine metabolism and 1-methyl-4-phenylpyridinium uptake in dissociated cell cultures from the embryonic mesencephalon. J. Neurochem. 1988;50(6):1900–1907. doi: 10.1111/j.1471-4159.1988.tb02495.x. [DOI] [PubMed] [Google Scholar]
- 6.Langston J.W., et al. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983;219(4587):979–980. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- 7.Singh A., Sen D. MicroRNAs in Parkinson's disease. Exp. Brain Res. 2017;235(8):2359–2374. doi: 10.1007/s00221-017-4989-1. [DOI] [PubMed] [Google Scholar]
- 8.MacDonald B.T., Tamai K., He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev. Cell. 2009;17(1):9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akhlaghpour A., et al. TGFbeta and Wnt signaling pathways cooperatively enhance early dopaminergic differentiation of the unrestricted somatic stem cells. J. Mol. Neurosci. 2020;70(5):769–777. doi: 10.1007/s12031-020-01487-x. [DOI] [PubMed] [Google Scholar]
- 10.Kim T.W., et al. Biphasic activation of WNT signaling facilitates the derivation of midbrain dopamine neurons from hESCs for translational use. Cell Stem Cell. 2021;28(2):343–355 e5. doi: 10.1016/j.stem.2021.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arenas E., Salto C., Villaescusa C. WNT signaling in midbrain dopaminergic neuron development and cell replacement therapies for Parkinson's disease. SpringerPlus. 2015;4(Suppl 1):L49. doi: 10.1186/2193-1801-4-S1-L49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kele J., et al. SFRP1 and SFRP2 dose-dependently regulate midbrain dopamine neuron development in vivo and in embryonic stem cells. Stem Cell. 2012;30(5):865–875. doi: 10.1002/stem.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castelo-Branco G., et al. Differential regulation of midbrain dopaminergic neuron development by Wnt-1, Wnt-3a, and Wnt-5a. Proc. Natl. Acad. Sci. U. S. A. 2003;100(22):12747–12752. doi: 10.1073/pnas.1534900100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castelo-Branco G., Arenas E. Function of Wnts in dopaminergic neuron development. Neurodegener. Dis. 2006;3(1–2):5–11. doi: 10.1159/000092086. [DOI] [PubMed] [Google Scholar]
- 15.Fritz R.R., et al. Metabolism of the neurotoxin in MPTP by human liver monoamine oxidase B. FEBS Lett. 1985;186(2):224–228. doi: 10.1016/0014-5793(85)80713-4. [DOI] [PubMed] [Google Scholar]
- 16.Nicklas W.J., Vyas I., Heikkila R.E. Inhibition of NADH-linked oxidation in brain mitochondria by 1-methyl-4-phenyl-pyridine, a metabolite of the neurotoxin, 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine. Life Sci. 1985;36(26):2503–2508. doi: 10.1016/0024-3205(85)90146-8. [DOI] [PubMed] [Google Scholar]
- 17.Castelo-Branco G., Rawal N., Arenas E. GSK-3beta inhibition/beta-catenin stabilization in ventral midbrain precursors increases differentiation into dopamine neurons. J. Cell Sci. 2004;117(Pt 24):5731–5737. doi: 10.1242/jcs.01505. [DOI] [PubMed] [Google Scholar]
- 18.Meijer L., et al. GSK-3-selective inhibitors derived from Tyrian purple indirubins. Chem. Biol. 2003;10(12):1255–1266. doi: 10.1016/j.chembiol.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 19.Bove J., et al. Toxin-induced models of Parkinson's disease. NeuroRx. 2005;2(3):484–494. doi: 10.1602/neurorx.2.3.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Javitch J.A., et al. Parkinsonism-inducing neurotoxin, N-methyl-4-phenyl-1,2,3,6 -tetrahydropyridine: uptake of the metabolite N-methyl-4-phenylpyridine by dopamine neurons explains selective toxicity. Proc. Natl. Acad. Sci. U.S.A. 1985;82(7):2173–2177. doi: 10.1073/pnas.82.7.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sonsalla P.K., Zeevalk G.D., German D.C. Chronic intraventricular administration of 1-methyl-4-phenylpyridinium as a progressive model of Parkinson's disease. Park. Relat. Disord. 2008;14(Suppl 2):S116–S118. doi: 10.1016/j.parkreldis.2008.04.008. Suppl 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaboriaud-Kolar N., Vougogiannopoulou K., Skaltsounis A.L. Indirubin derivatives: a patent review (2010 - present) Expert Opin. Ther. Pat. 2015;25(5):583–593. doi: 10.1517/13543776.2015.1019865. [DOI] [PubMed] [Google Scholar]
- 23.Lee R.C., Feinbaum R.L., Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75(5):843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 24.Cho H.J., et al. MicroRNA-205 regulates the expression of Parkinson's disease-related leucine-rich repeat kinase 2 protein. Hum. Mol. Genet. 2013;22(3):608–620. doi: 10.1093/hmg/dds470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li J., et al. GSK-3β contributes to Parkinsonian dopaminergic neuron death: evidence from conditional knockout mice and tideglusib. Front. Mol. Neurosci. 2020;13(81) doi: 10.3389/fnmol.2020.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu S., et al. Indirubin-3-Oxime effectively Prevents 6OHDA-induced neurotoxicity in PC12 cells via activating MEF2D through the inhibition of GSK3β. J. Mol. Neurosci. 2015;57(4):561–570. doi: 10.1007/s12031-015-0638-y. [DOI] [PubMed] [Google Scholar]
- 27.Shanesazzade Z., et al. miR-34a/BCL-2 signaling axis contributes to apoptosis in MPP(+) -induced SH-SY5Y cells. Mol. Genet. Genom. Med. 2018;6(6):975–981. doi: 10.1002/mgg3.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rostamian Delavar M., et al. Differential expression of miR-34a, miR-141, and miR-9 in MPP+-treated differentiated PC12 cells as a model of Parkinson's disease. Gene. 2018;662:54–65. doi: 10.1016/j.gene.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 29.Li J., et al. Transcriptional activation of microRNA-34a by NF-kappa B in human esophageal cancer cells. BMC Mol. Biol. 2012;13:4. doi: 10.1186/1471-2199-13-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Margis R., Margis R., Rieder C.R. Identification of blood microRNAs associated to Parkinsonis disease. J. Biotechnol. 2011;152(3):96–101. doi: 10.1016/j.jbiotec.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 31.Botta-Orfila T., et al. Identification of blood serum micro-RNAs associated with idiopathic and LRRK2 Parkinson's disease. J. Neurosci. Res. 2014;92(8):1071–1077. doi: 10.1002/jnr.23377. [DOI] [PubMed] [Google Scholar]
- 32.Bai X., et al. Downregulation of blood serum microRNA 29 family in patients with Parkinson's disease. Sci. Rep. 2017;7(1) doi: 10.1038/s41598-017-03887-3. 5411-5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu J., et al. Indirubin-3-Oxime Prevents H2O2-induced neuronal apoptosis via concurrently inhibiting GSK3β and the ERK pathway. Cell. Mol. Neurobiol. 2016;37(4):655–664. doi: 10.1007/s10571-016-0402-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alural B., et al. Lithium protects against paraquat neurotoxicity by NRF2 activation and miR-34a inhibition in SH-SY5Y cells. Front. Cell. Neurosci. 2015;9 doi: 10.3389/fncel.2015.00209. 209-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wan Y., et al. Identification of four oxidative stress-responsive MicroRNAs, miR-34a-5p, miR-1915-3p, miR-638, and miR-150-3p, in Hepatocellular Carcinoma. Oxid. Med. Cell. Longev. 2017:5189138. doi: 10.1155/2017/5189138. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jain A.K., Jaiswal A.K. GSK-3beta acts upstream of Fyn kinase in regulation of nuclear export and degradation of NF-E2 related factor 2. J. Biol. Chem. 2007;282(22):16502–16510. doi: 10.1074/jbc.M611336200. [DOI] [PubMed] [Google Scholar]
- 37.Feng Z., et al. Tumor suppressor p53 meets microRNAs. J. Mol. Cell Biol. 2011;3(1):44–50. doi: 10.1093/jmcb/mjq040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fernández-Santiago R., et al. MicroRNA association with synucleinopathy conversion in rapid eye movement behavior disorder. Ann. Neurol. 2015;77(5):895–901. doi: 10.1002/ana.24384. [DOI] [PubMed] [Google Scholar]