Abstract
Many of the commercial slide agglutination tests for Staphylococcus aureus incorporate antibodies against cell surface antigens associated with methicillin resistance, including capsular polysaccharides and an uncharacterized antigen, serotype 18. These tests are more sensitive than the first-generation agglutination procedures that detected only bound coagulase and protein A, but they suffer from false-positive reactions with some coagulase-negative staphylococci. The aim of this study was to elucidate the mechanism for false-positive agglutination by S. epidermidis in these tests. A group of methicillin-resistant S. aureus (MRSA) isolates, including a serotype 18 strain, that were not detectable in the first-generation tests were found to be of capsular polysaccharide type 8. All of these isolates were deficient in bound coagulase and/or protein A, and they possessed a heat-stable, proteinaceous antigen that was absent from a prototype capsule type 8 strain. Enzyme-linked immunosorbent assay and agarose gel immunodiffusion experiments demonstrated that this proteinaceous antigen was also present on both methicillin-sensitive and methicillin-resistant S. epidermidis clinical isolates. S. epidermidis strains that gave false-positive agglutination test results had a considerably higher level of this antigen than strains that gave the correct negative result. These findings reveal the importance of the careful selection of MRSA strains for raising anti-capsular type 8 antibodies for use in agglutination tests. Strains devoid of the antigen shared with S. epidermidis should be used to eliminate potential cross-reactions with this coagulase-negative coccus.
The rapid and accurate diagnosis of Staphylococcus aureus infection is of vital importance so that appropriate antimicrobial therapy can be initiated. The first-generation rapid identification tests for S. aureus were based on the agglutination of particles coated with plasma to detect bound coagulase (clumping factor) and protein A (8, 9).
Methicillin-resistant S. aureus (MRSA) strains may fail to produce agglutination in these tests since they commonly have undetectable levels of bound coagulase and protein A (10, 18, 32, 33). All of these strains possess capsular polysaccharide (3, 10), and this may physically mask other cell surface components (19, 31). Eight serologically distinct types of capsular polysaccharide were demonstrated by cell agglutination in a scheme originally described by Karakawa et al. (20). Capsule types 5 and 8 appear to predominate among clinical isolates of varied geographical locations (17, 30).
A separate serotyping scheme for S. aureus was established in France some years earlier (27, 28). In the 1960s a new serotype of MRSA emerged in French hospitals, and it was designated serotype 18 on the basis of an uncharacterized cell surface antigen (26). This serotype became the predominant cause of MRSA infections in France (4, 14) and more recently in other European countries (21).
The incorporation of antibodies against capsule types 5 and 8 or against serotype 18 into several commercial agglutination tests has improved the sensitivity for MRSA (6, 12, 34). Some of these tests, however, suffer from false-positive reactions with coagulase-negative staphylococci (7, 15, 34). Strains of S. haemolyticus and S. hominis have been reported to possess type 8 capsular polysaccharide (11, 12). A strain of S. epidermidis that gave a false-positive reaction in a commercial agglutination test, however, did not contain either type 5 or 8 capsules (12).
This study was undertaken to elucidate the mechanism for false-positive results by S. epidermidis in commercial agglutination tests for S. aureus that incorporate antibodies against methicillin resistance-associated antigens. MRSA isolates (including the original serotype 18 strain) that were undetectable in the first-generation agglutination tests were examined for the presence of type 5 or 8 capsules using specific antisera. The effect of encapsulation on the detectability of cell-bound coagulase and protein A was determined. The immunological relationship between these MRSA strains and clinical S. epidermidis isolates was investigated. Two groups of S. epidermidis strains were selected for this purpose: one that gave false-positive agglutination test results and one that gave the correct negative result.
MATERIALS AND METHODS
Bacteria.
The four MRSA strains selected for this study gave strong agglutination in three commercially available tests that use latex sensitized with fibrinogen and antibodies against methicillin resistance-associated antigens, but they failed to agglutinate latex coated with plasma only. One of these strains (Institute Pasteur, 64002) was the original serotype 18 strain described by Pillet et al. (26). The other isolates (MRSA1, MRSA2, and MRSA3) were collected between 1995 and 1999 from hospitals in France, Canada, and Belgium, respectively. S. aureus reference strains were the nonencapsulated strain Wood (NCIMB 11852), the capsule type 5 strain Reynolds (Nabi, Rockville, Md.), and the capsule type 8 strain Becker (NABI). Twelve S. epidermidis strains were obtained, 1 from a culture collection and 11 from hospitals in France and the United Kingdom between 1996 and 1999 (see Table 1). Six of these were “problematic” strains that gave false-positive reactions in commercial agglutination tests for S. aureus that incorporate antibodies against methicillin resistance-associated antigens. These isolates did not, however, agglutinate latex coated with plasma only. The other six were “nonproblematic” strains that gave the correct negative result in these commercial tests. The isolates were identified by conventional procedures, including API 32 STAPH (bioMerieux, Basingstoke, United Kingdom).
TABLE 1.
Initial characterization of S. epidermidis strains
| Strain | Source | Oxacillin susceptibility |
|---|---|---|
| Nonproblematic strains | ||
| 12228 | ATCC,a Rockville, Md. | Sensitive |
| 1632 | Colchester, United Kingdom | Sensitive |
| 2022 | Basildon, United Kingdom | Resistant |
| 2023 | Basildon, United Kingdom | Sensitive |
| 2026 | Basildon, United Kingdom | Sensitive |
| 2214 | Grenoble, France | Resistant |
| Problematic strains | ||
| 2034 | Basildon, United Kingdom | Sensitive |
| 2038 | Basildon, United Kingdom | Sensitive |
| 2213 | Grenoble, France | Resistant |
| 2216 | Grenoble, France | Resistant |
| 2222 | Carshalton, United Kingdom | Resistant |
| 2227 | Bridgend, United Kingdom | Resistant |
ATCC, American Type Culture Collection.
Oxacillin resistance.
Susceptibility to oxacillin was determined by a standard disc diffusion procedure (23). Resistant strains were tested for the mecA gene by PCR (2). Control S. aureus strains for these tests were ATCC 25923 (sensitive) and NCTC 11939 (resistant).
Coagulase tests.
Bound coagulase was determined using 18- to 24-h brain heart infusion (BHI) broth cultures. Bacteria emulsified in distilled water were mixed with 10 μl of rabbit plasma (Difco, Surrey, United Kingdom) on a slide. The slide was rocked, and the strength of agglutination of the cells after 5 s was recorded. Overnight BHI broth cultures were tested for free coagulase using a tube test. One hundred microliters of culture was mixed with 500 μl of rabbit plasma in a test tube and examined for clot formation after 1, 3, 6, and 24 h at 37°C. The nonencapsulated S. aureus reference strain served as the positive control for these tests, and S. epidermidis ATCC 12228 served as the negative control.
Protein A determinations.
Bacteria were grown at 37°C for 24 h on Columbia blood agar (Oxoid Ltd., Basingstoke, United Kingdom) and harvested into phosphate-buffered saline (PBS; pH 7.3). These live cell suspensions were standardized by determinations of optical density at 550 nm. Extracts were also prepared by gently mixing bacteria at 0.5 g (wet weight)/ml with 25 μg of lysostaphin (Sigma, Poole, United Kingdom) per ml for 2 h at 37°C. Cell debris was removed by centrifugation at 25,000 × g for 20 min at 4°C, and supernatants were retained. The protein A contents of these bacterial preparations were determined by enzyme-linked immunosorbent assay (ELISA). Since the ELISA signal was proportional to the concentration of soluble protein A (Sigma) over a narrow range (30 to 70 ng/ml), it was possible to measure only relative amounts of protein A in the samples. The levels of protein A in the bacterial preparations were compared with those of a known high-level protein A producer, S. aureus Cowan NCTC 8530. The negative control for this assay was S. epidermidis ATCC 12228.
Immulon 2 HB plates (Dynex, Billingshurst, United Kingdom) were coated with 100 μl of 3.7-μg/ml ovalbumin (Sigma) per well in 0.05 M carbonate–bicarbonate buffer, pH 9.6. After incubation at 4°C overnight, plates were washed three times with PBS containing 0.05% Tween 20 (PBST). Plates were blocked with PBST plus 1% bovine serum albumin (PBST-BSA) for 1 h at 37°C. After being washed as described above, plates were incubated for 1 h at 37°C with 100 μl of 1.2-μg/ml rabbit anti-ovalbumin antibody (Sigma) per well in PBST-BSA. The ovalbumin coat orientated the antibody down onto the plate, leaving the Fc region available for binding to protein A. Plates were washed as described above and incubated for 1 h at 37°C with 100 μl of live bacteria or extract, diluted in PBST-BSA, per well. Plates were washed as described above and incubated at 37°C for 1 h with 100 μl of rabbit immunoglobulin-peroxidase conjugate (Sigma) per well at a dilution of 1:5,000 in PBST-BSA. Plates were washed and developed using tetramethylbenzidine (Sigma), and the resulting absorbance was measured at 450 nm.
Preparation of rabbit antisera for serotyping.
Antigens for immunization were prepared from bacteria grown overnight at 37°C on Columbia blood agar. Cells were harvested into PBS to give 2 g (wet weight)/ml, and they were inactivated by one of two methods. The S. aureus reference capsule type 5 strain and S. epidermidis strain 2213 were heat killed at 80°C for 2 h. The S. aureus nonencapsulated, capsule type 8, and serotype 18 reference strains were inactivated by incubation with 3% formaldehyde (Sigma) at room temperature for 18 h. Bacteria were collected by centrifugation (3,500 × g for 20 min at 4°C), washed twice, and resuspended in PBS. Antisera were prepared as previously described (20), and they were stored in aliquots at −20°C prior to use.
The sera were absorbed using the methicillin-sensitive, nonencapsulated S. aureus Wood strain to remove antibodies against common antigens. Heat-killed cells, prepared as described above, were diluted 1 in 10 in PBS. One mg of trypsin (Sigma) per ml and a few drops of chloroform were added, and the cells were mixed gently for 8 h at 37°C. After three washes in PBS, 0.5 g (wet weight) of cells was added per ml of antiserum and mixed gently at room temperature for 20 h. Cells were removed by centrifugation (10,000 × g for 10 min), and the sera were reabsorbed using fresh cells. Sodium azide was added at 0.1% to absorbed sera, and the serum solutions were stored at 4°C.
Serotyping ELISA.
Formaldehyde-killed bacteria, prepared as described earlier, were diluted in PBS containing 0.1% formaldehyde (Sigma) to an optical density of 0.6 at 550 nm and added to Immulon 2 HB plates at 100 μl/well. Plates were incubated at 4°C overnight, washed twice with PBST, and blocked as described for the protein A assay. Plates were washed twice with PBST, and 100 μl of rabbit antiserum per well (see the previous section), diluted in PBST-BSA, was added. Plates were incubated for 1 h at 37°C and washed three times in PBST. One hundred microliters of protein A-peroxidase (Sigma) per well at 0.25 μg/ml in PBST-BSA was added, and plates were reincubated for 1 h at 37°C. After three washes in PBST, plates were developed using tetramethylbenzidine and the absorbance was recorded at 450 nm against that of a blank well without serum.
Inhibition ELISAs.
Autoclave extracts of Columbia agar-grown bacteria were prepared by suspending bacteria at 0.5 g (wet weight)/ml in PBS and autoclaving at 121°C for 60 min. The cell suspensions were centrifuged at 25,000 × g for 20 min at 4°C, and the supernatants were stored in aliquots at −20°C. The ability of these extracts to inhibit the binding of antisera in the serotyping ELISA was examined. Dilutions of extract and of antiserum were prepared in PBST-BSA, and 70-μl aliquots of each were mixed for 10 min at room temperature. One hundred microliters of the serum-extract mixtures per well was added in triplicate to microtiter plates, and the assay was continued as described above. The mean absorbance of the test wells was deducted from that of reference wells without extract, and the difference was expressed as a percentage of the latter. This percent inhibition of the ELISA signal was plotted against the reciprocal of the extract dilution.
In some experiments extracts were pretreated prior to analysis by inhibition ELISA. Extracts were incubated with 0.05 M sodium m-periodate (Sigma) for 18 h at 4°C in the dark, and the reaction was stopped by addition of 1.4% ethylene glycol. In other experiments extracts were mixed at 37°C overnight with trypsin (Sigma) at 1 mg/ml. Trypsin inhibitor (Sigma) was added at 5 mg/ml, and incubation was continued for a further 2 h. Alternatively, 0.5 mg of proteinase K (Sigma) per ml was added to extracts, and these were mixed at 37°C overnight. Proteinase K was inhibited by incubation for a further hour with α1-antichymotrypsin (Sigma) at 0.1 mg/ml.
Agarose gel immunodiffusion.
Bacterial autoclave extracts were also analyzed by double immunodiffusion in 1.5% agarose (Sigma) in PBS. Extracts were diluted as appropriate in PBS. Antisera were used undiluted or concentrated in Centricon YM-10 devices (Amicon Ltd., Stonehouse, United Kingdom). Thirty-six microliters of extract and the same amount of antiserum were added to separate wells (5 mm in diameter), and gels were placed at 4°C overnight. Gels were washed in several changes of 1 M sodium chloride (1 liter in total) over 2 days and in several changes of distilled water (1 liter in total) for 4 h. Gels were immersed for 5 min in Amido Black stain (5 g of Amido Black [Sigma] per liter in 45% methanol–10% acetic acid–45% distilled water) and destained in a solution containing 45% methanol, 10% acetic acid, and 45% distilled water.
Detection of extracellular slime.
Slime production was examined qualitatively using the Congo red agar method (13). Slime producers formed black, dry, and crystalline colonies, whereas non-slime producers formed pink and shiny colonies.
Slime production was measured quantitatively using a modified version of the microplate adherence assay (5). Overnight cultures were diluted 1:50 in 200 μl of BHI broth (Oxoid) supplemented with 0.25% glucose in triplicate wells of a 96-well flat-bottomed tissue culture plate (Life Technologies, Paisley, United Kingdom). After static incubation overnight at 37°C, the amount of growth in each well was measured as an optical density at 540 nm. Wells were emptied, washed three times with PBS and air dried, and adherent growth was stained with 0.1% Safranin (Sigma) for 1 h. Excess stain was removed by rinsing the wells in distilled water, wells were tapped dry, and adherent material was solubilized by incubation with 200 μl of 0.2 M sodium hydroxide for 1 h at 85°C. The absorbance for each strain was remeasured at 540 nm, and these values were corrected for the total amount of growth measured before staining.
S. epidermidis strains ATCC 14990, a non-slime producer, ATCC 35983, a moderate-level slime producer, and ATCC 35984, a high-level slime producer, served as controls for both methods.
RESULTS
Bacterial characterization.
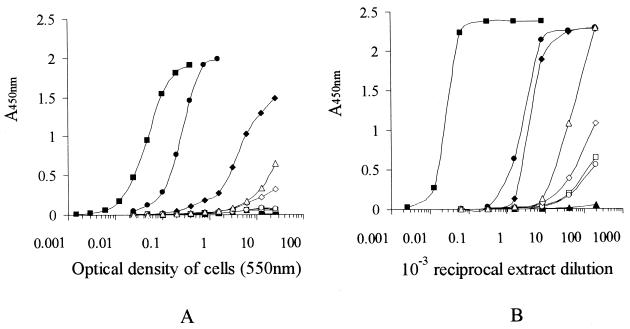
Coagulase tests on the four MRSA isolates that failed to agglutinate plasma-sensitized latex revealed that only one strain (MRSA2) had detectable levels of bound coagulase. The other strains appeared to produce only free coagulase. A highly sensitive ELISA for protein A was developed (detection limit, 20 ng/ml) in order to measure protein A in these isolates. Live bacterial suspensions of all four MRSA strains contained considerably less protein A than did S. aureus Wood, a well-documented low-level protein A producer, and a reference capsule 5 strain (Fig. 1A). The reference capsule 8 strain had no detectable cell surface protein A.
FIG. 1.
Assay of protein A in live cell suspensions (A) and extracts (B) of S. aureus strains Cowan (■), Wood (●), type 5 (⧫), type 8 (▴), MRSA1 (□), MRSA2 (○), MRSA3 (◊), serotype 18 (▵), and S. epidermidis ATCC 12228 ( ).
).
In order to reveal any masking of cell surface protein A by a capsule, protein A was also assayed in lysostaphin extracts of these bacteria (Fig. 1B). Since S. aureus Wood is a nonencapsulated strain, the position of the curve obtained for protein A in its extract should be unchanged relative to the positions of the protein A curves for extracts of other nonencapsulated strains. The protein A curves for all of the other S. aureus isolates had shifted upwards and to the left, indicating that there was relatively more protein A in extracts than on intact cells. These results provide evidence for the physical masking of protein A in these strains.
The methicillin susceptibilities of the problematic and nonproblematic groups of S. epidermidis were determined in order to establish whether there was a correlation between the problematic characteristic and methicillin resistance. Both groups of S. epidermidis were found to include methicillin-sensitive and methicillin-resistant phenotypes (Table 1). All of the strains that were phenotypically resistant were shown by PCR to possess the mecA gene.
Serotyping of strains by ELISA.
High-titer rabbit sera (giving an ELISA signal at 450 nm of >1.0 for a 1-in-3,000 serum dilution) were obtained against the S. aureus reference strains (nonencapsulated, capsule type 5, capsule type 8, and serotype 18) and against a problematic S. epidermidis strain (2213). The specificities of the latter four sera were improved by absorption with the nonencapsulated S. aureus Wood strain (data not shown).
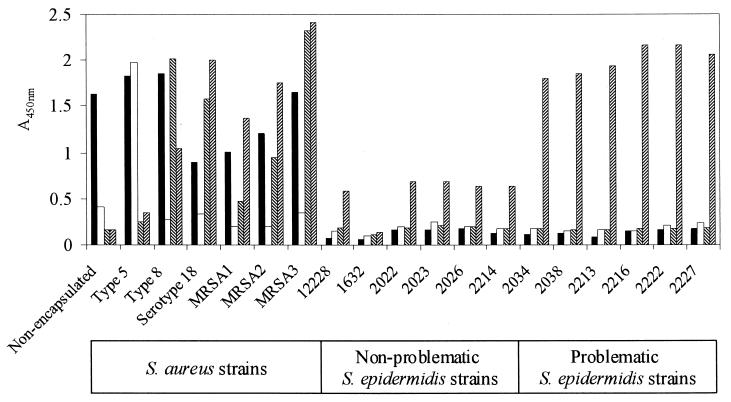
The anti-S. aureus Wood serum appeared to contain antibodies against species-specific antigens since it bound to all of the S. aureus isolates but failed to bind to the S. epidermidis isolates (Fig. 2). The anti-capsule type 5 serum was specific for the type 5 strain, whereas the anti-capsule type 8 and anti-serotype 18 sera contained antibodies that were cross-reactive for S. aureus type 8 and serotype 18 strains (Fig. 2). The pattern of binding of sera to the other MRSA isolates was similar to the pattern for serotype 18; all of these strains possessed an antigen recognized by anti-capsular type 8 antibodies, although MRSA1 had only a low level of this antigen (Fig. 2).
FIG. 2.
Serotyping of S. aureus and S. epidermidis isolates by ELISA. Rabbit sera were as follows: anti-nonencapsulated S. aureus Wood serum (■), diluted 1:1,000; anti-type 5 (□) and anti-type 8 (▧) sera, diluted 1:2,000; and anti-serotype 18 serum (▨), diluted 1:4,000. All determinations were done in duplicate.
The anti-serotype 18 serum bound to 11 out of the 12 S. epidermidis isolates. The level of serum binding to problematic strains was approximately three times higher than that to nonproblematic strains. Antiserum raised against the problematic S. epidermidis strain 2213 bound strongly to both this strain and to the serotype 18 MRSA and moderately to a nonproblematic S. epidermidis strain, 2214 (data not shown). This serum failed to bind, however, to the nonencapsulated, capsule type 5, and type 8 S. aureus reference strains.
Inhibition ELISAs.
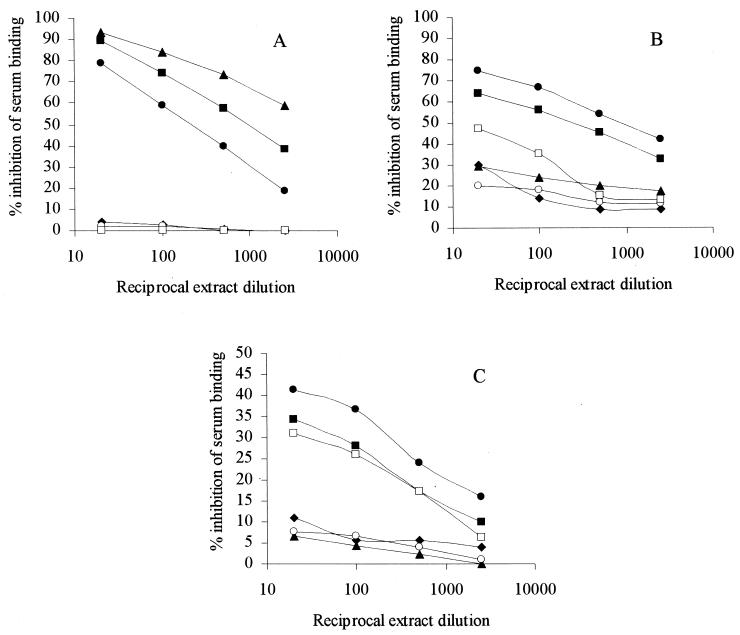
In order to investigate further the cross-reactions between strains, the ability of autoclave extracts of the bacteria to inhibit serum binding in the serotyping ELISA was examined. The binding of anti-type 8 serum to type 8 cells was almost completely inhibited by extract of this type 8 strain; extracts of serotype 18 and of the strain MRSA3 also exhibited high levels of inhibition (Fig. 3A). In contrast, extracts of the nonencapsulated S. aureus reference strain and of the 12 S. epidermidis isolates gave no inhibition (Fig. 3A shows the data for a representative problematic S. epidermidis isolate, 2213, and a representative nonproblematic S. epidermidis isolate, 2214). All of these levels of inhibition were essentially unchanged when anti-serotype 18 serum replaced anti-type 8 serum in the ELISA and also when the binding of anti-type 8 serum to serotype 18 was examined (data not shown). These results confirm that the MRSA isolates possess the type 8 capsular polysaccharide but that S. epidermidis lacks this capsule.
FIG. 3.
Ability of bacterial extracts to inhibit the binding in an ELISA of anti-type 8 serum to type 8 cells (A), of anti-serotype 18 serum to serotype 18 cells (B), and of anti-serotype 18 serum to a problematic S. epidermidis strain, 2213 (C). Extracts of S. aureus strains were nonencapsulated (⧫), type 8 (▴), serotype 18 (●), and MRSA3 (■). Extracts of S. epidermidis strains were 2214 (○) and 2213 (□). Serum dilutions are given in the legend to Fig. 2. All determinations were done in triplicate.
An entirely different pattern of inhibition by the extracts was obtained for anti-serotype 18 serum binding to serotype 18 cells (Fig. 3B). In this case extracts of serotype 18 and of MRSA3 exhibited high levels of inhibition, whereas inhibition by the type 8 extract was approximately threefold lower than in the previous ELISA (Fig. 3A). Extracts of the six problematic S. epidermidis isolates (represented by strain 2213 in Fig. 3B) exhibited considerable inhibition in this system, whereas extracts of the nonproblematic S. epidermidis strains (represented by 2214 in Fig. 3B) gave only low levels of inhibition. These data indicate that the two MRSA strains have a second antigen that is absent from the reference capsule type 8 strain but present on problematic S. epidermidis isolates. Further evidence for this shared antigen was obtained when the inhibition of binding of the anti-serotype 18 serum to a problematic S. epidermidis strain, 2213, was examined. Extract of serotype 18 again gave the greatest inhibition in this system (Fig. 3C), and extracts of MRSA3 and of the six problematic S. epidermidis strains (represented by strain 2213 in Fig. 3C) also exhibited significant inhibition. Extracts of S. aureus type 8 and of the six nonproblematic S. epidermidis isolates (represented by strain 2214 in Fig. 3C) gave levels of inhibition similar to those for the nonencapsulated S. aureus reference strain. When a nonproblematic S. epidermidis strain, 2214, replaced strain 2213 on the ELISA plate, the same pattern of inhibition occurred, although the levels of inhibition were approximately twofold higher (data not shown). These findings suggest that nonproblematic S. epidermidis isolates may also possess low levels of the antigen that is shared between problematic S. epidermidis isolates and the MRSA strains.
Agarose gel immunodiffusion.
Anti-type 8 serum gave a single fused line of precipitation in an agarose gel with autoclave extracts of S. aureus type 8 and the four MRSA strains (Fig. 4A), providing further evidence that all of these strains possess the type 8 capsule. The serum gave no precipitation, however, with extract of a problematic S. epidermidis strain, 2213. Anti-serotype 18 serum gave a reaction of identity with extracts of type 8 and the MRSA strains, and it appeared to recognize an additional antigen in the MRSA extracts, giving a second line of precipitation that was not fully resolved from the first line (Fig. 4B). Anti-serotype 18 serum recognized a single identical antigen in extracts of the six problematic S. epidermidis isolates (Fig. 4C). This antigen gave a reaction of identity with the serotype 18 extract but not with the type 8 preparation (Fig. 4D). Although a low level of binding of the anti-serotype 18 serum to the nonproblematic S. epidermidis strains was demonstrated by ELISA, this serum failed to give a precipitin line with extract of a representative nonproblematic isolate, 2214 (Fig. 4D). This is probably due to the lower sensitivity of agarose gel immunodiffusion. Antiserum raised against a representative problematic S. epidermidis isolate, 2213, gave a fused precipitin line with extracts of this isolate and the four MRSA strains (Fig. 4E), confirming that these organisms share a common antigen. This serum gave no precipitation with extract of the nonproblematic S. epidermidis isolate, 2214, which presumably contained a lower level of the shared antigen (Fig. 4E).
FIG. 4.
Agarose gel immunodiffusion analysis of bacterial extracts (1 to 12) with the following absorbed sera: anti-type 8 serum, unconcentrated (A); anti-serotype 18 serum, concentrated threefold (B to D); and anti-S. epidermidis 2213 serum, concentrated threefold (E). S. aureus extracts were as follows: 1, type 8; 2, serotype 18; 3, MRSA1; 4, MRSA2; and 5, MRSA3. S. epidermidis extracts were as follows: 6, 2034; 7, 2038; 8, 2213; 9, 2216; 10, 2222; 11, 2227; and 12, 2214. All extracts were diluted 1:2 in PBS.
Detection of extracellular slime.
Since slime production has been demonstrated for clinical isolates of S. epidermidis (5, 22) and also more recently for S. aureus (1) we wanted to determine whether the common antigen on the MRSA isolates and S. epidermidis was slime associated. The colonial morphology of the 12 S. epidermidis isolates on Congo red agar (pink and shiny) indicated that none was a slime producer. The microtiter plate adherence assay for slime appeared to be more sensitive since it detected low levels of slime secretion in some of these strains (Table 2). Slime secretion was not, however, exclusive to the problematic isolates. Furthermore, the MRSA isolates did not produce detectable slime (Table 2), suggesting that the antigen they share with S. epidermidis is not a component of slime.
TABLE 2.
Slime production by S. aureus and S. epidermidis isolates
| Strain | Adherencea |
|---|---|
| S. epidermidis controls | |
| High-level slime producer | 0.518 ± 0.047 |
| Moderate-level slime producer | 0.246 ± 0.065 |
| Non-slime producer | 0.070 ± 0.020 |
| Nonproblematic S. epidermidis strains | |
| 12228 | 0.096 ± 0.017 |
| 1632 | 0.021 ± 0.019 |
| 2022 | 0.027 ± 0.010 |
| 2023 | 0.177 ± 0.047 |
| 2026 | 0.060 ± 0.031 |
| 2214 | 0.170 ± 0.081 |
| Problematic S. epidermidis strains | |
| 2034 | 0.008 ± 0.004 |
| 2038 | 0.024 ± 0.016 |
| 2213 | 0.105 ± 0.045 |
| 2216 | 0.047 ± 0.006 |
| 2222 | 0.124 ± 0.058 |
| 2227 | 0.070 ± 0.012 |
| S. aureus strains | |
| Serotype 18 | 0.014 ± 0.003 |
| MRSA1 | 0.038 ± 0.021 |
| MRSA2 | 0.008 ± 0.007 |
| MRSA3 | 0.000 ± 0.003 |
Mean absorbance at 540 nm, calculated from triplicate determinations, ± the standard deviation.
Further analysis of the nature of the antigen common to MRSA and S. epidermidis.
Autoclave extracts of the bacteria were treated with sodium periodate to destroy noncapsular polysaccharides and examined by inhibition ELISA. Periodate-treated extract of the nonencapsulated S. aureus strain Wood inhibited the binding of anti-Wood serum to Wood cells at only half the level of inhibition exhibited by untreated extract, serving as a positive control for periodate oxidation. In contrast, periodate oxidation of extracts of S. aureus serotype 18, MRSA3, and the problematic S. epidermidis isolates 2213 and 2216 did not alter their ability to inhibit the binding of anti-serotype 18 serum to serotype 18. Following incubation with trypsin, autoclave extracts of serotype 18, MRSA3, 2213, and 2216 exhibited reduced levels of inhibition of the binding of anti-serotype 18 serum to serotype 18 cells (70, 68, 50, and 51% reductions, respectively). The ability of these extracts to inhibit the binding of anti-type 8 serum to type 8 cells was unaffected by trypsin treatment. Similarly, incubation of serotype 18 and MRSA3 extracts with proteinase K decreased their ability to inhibit the binding of anti-serotype 18 serum to serotype 18 cells by 69 and 60%, respectively, but did not alter their ability to inhibit the binding of anti-type 8 serum to type 8 cells. It appears from these results that the antigen shared between S. aureus serotype 18 and S. epidermidis is a protein.
DISCUSSION
The detection of methicillin-resistant S. aureus by rapid agglutination procedures necessitates the incorporation into the test reagents of antibodies against resistance-associated antigens. These include capsular polysaccharide types 5 and 8 and a poorly characterized antigen, serotype 18 (6, 12, 33). Some of these tests suffer from false-positive reactions by coagulase-negative staphylococci (7, 15). This may be due to the production of type 5 and 8 capsular polysaccharides in some species, notably S. haemolyticus and S. hominis (11, 12). The frequency of isolation of coagulase-negative cocci that express these capsular types is 2% in humans (11) and 16% in livestock (29). There are no reports, however, of S. epidermidis possessing type 5 or 8 capsules, and Nelles et al. (24) observed that monoclonal antibodies raised against S. aureus capsule types 5 and 8 failed to react with clinical isolates of S. epidermidis. It follows, therefore, that some other mechanism is responsible for false-positive agglutination by S. epidermidis in tests for MRSA, and this mechanism is described here.
In the present study, four MRSA isolates that failed to agglutinate plasma-coated latex were all of capsular polysaccharide type 8 of the scheme of Karakawa et al. (20). The lack of bound coagulase on all but one of these strains may account for their inability to agglutinate this latex reagent. Furthermore, all of the isolates in common with a prototype capsule 8 strain were deficient in cell surface protein A. In contrast, a prototype capsule 5 strain had significantly higher levels of cell surface protein A. A study by Sutra et al. (31) suggested that type 8 strains produce greater amounts of capsule than type 5 strains. This may account for the more extensive masking of cell surface components such as protein A on type 8 isolates. Lysostaphin digests of capsule 5 and 8 reference strains, serotype 18, and the other MRSA isolates contained relatively higher levels of protein A than were present on intact bacteria, providing evidence for the physical masking of protein A in all of these encapsulated strains.
The MRSA isolates differed from the prototype capsule type 8 strain in that they possessed a second common antigen that was heat stable and protease sensitive. It is unclear whether this proteinaceous antigen is the same antigen that led to the designation of one of the strains, 64002, as the first serotype 18 isolate (26). This serotype 18 antigen is described as a heat-stable cell surface antigen, but the literature does not contain any further clues as to its nature.
None of the 12 S. epidermidis strains that we examined had either type 5 or 8 capsules. Eleven of these isolates, however, possessed a heat-stable protein that was immunologically identical to the proteinaceous material detected in the MRSA strains that failed to agglutinate plasma-sensitized latex. The level of this antigen in S. epidermidis isolates that give false-positive reactions in agglutination tests for MRSA was approximately three times higher than that in strains that give the correct negative result. Since the problematic S. epidermidis isolates included both methicillin-sensitive and methicillin-resistant phenotypes, the antigen that they share with certain MRSA strains may not be truly resistance associated.
To our knowledge this study is the first in which a proteinaceous antigen has been implicated in cross-reactions with antibodies against MRSA-associated antigens. A fibrinogen-binding protein that is related to the bound coagulase (clumping factor) of S. aureus has been reported for several clinical isolates of S. epidermidis (25). Whereas the fibrinogen binding of bound coagulase causes clumping of S. aureus cells, the fibrinogen binding of the S. epidermidis protein does not result in cell clumping. Furthermore, since the problematic S. epidermidis isolates in our study failed to agglutinate latex coated only with plasma, this eliminates the involvement of a fibrinogen-binding protein in the false-positive agglutination results. Hilden et al. (16) described a 230-kDa carbohydrate-containing surface protein of S. aureus that is associated with a negative result in commercial tests designed to detect fibrinogen-binding proteins and/or protein A. This protein does not appear to be the antigen that we have detected in both MRSA and S. epidermidis since it is absent from coagulase-negative cocci (16).
Our findings have revealed the importance of the careful selection of strains for raising anti-capsular type 8 antibodies for use in agglutination tests. Strains devoid of the antigen shared with S. epidermidis should be used in order to eliminate potential cross-reactions with this coagulase-negative coccus.
ACKNOWLEDGMENTS
We are grateful to P. A. Lambert (Microbiology and Molecular Biology Research Group, Aston University, Birmingham, United Kingdom) for supplying the control strains for slime determination. We thank N. Woodford (PHLS Central Public Health Laboratory, London, United Kingdom) for mecA testing of the bacteria by PCR.
REFERENCES
- 1.Ammendolia M G, Di Rosa R, Montanaro L, Arciola C R, Baldassarri L. Slime production and expression of the slime-associated antigen by staphylococcal clinical isolates. J Clin Microbiol. 1999;37:3235–3238. doi: 10.1128/jcm.37.10.3235-3238.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bignardi G E, Woodford N, Chapman A, Johnson A P, Speller D C E. Detection of the mec-A gene and phenotypic detection of resistance in Staphylococcus aureus isolates with borderline or low-level methicillin resistance. J Antimicrob Chemother. 1996;37:53–63. doi: 10.1093/jac/37.1.53. [DOI] [PubMed] [Google Scholar]
- 3.Branger C, Goullet P, Boutonnier A, Fournier J M. Correlation between esterase electrophoretic types and capsular polysaccharide types 5 and 8 among methicillin-susceptible and methicillin-resistant strains of Staphylococcus aureus. J Clin Microbiol. 1990;28:150–151. doi: 10.1128/jcm.28.1.150-151.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chabbert Y A, Pillet J. Correlation between methicillin resistance and serotype in Staphylococcus. Nature. 1967;213:1137. doi: 10.1038/2131137a0. [DOI] [PubMed] [Google Scholar]
- 5.Christensen G D, Simpson W A, Younger J J, Baddour L M, Barrett F F, Melton D M, Beachey E H. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. J Clin Microbiol. 1985;22:996–1006. doi: 10.1128/jcm.22.6.996-1006.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Croize J, Gialanella P, Monnet D, Okada J, Orsi A, Voss A, Merlin S. Improved identification of Staphylococcus aureus using a new agglutination test. Results of an international study. APMIS. 1993;101:487–491. [PubMed] [Google Scholar]
- 7.Cuny C, Pasemann B, Witte W. The ability of the Dry Spot Staphytect Plus test, in comparison with other tests, to identify Staphylococcus species, in particular S. aureus. Clin Microbiol Infect. 1999;5:114–116. doi: 10.1111/j.1469-0691.1999.tb00117.x. [DOI] [PubMed] [Google Scholar]
- 8.Essers L, Radebold K. Rapid and reliable identification of Staphylococcus aureus by a latex agglutination test. J Clin Microbiol. 1980;12:641–643. doi: 10.1128/jcm.12.5.641-643.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flandrois J P, Carret G. Study of the staphylococcal affinity to fibrinogen by passive hemagglutination: a tool for the Staphylococcus aureus identification. Zentbl Bakteriol Hyg Abt 1 Orig A. 1981;251:171–176. [PubMed] [Google Scholar]
- 10.Fournier J M, Boutonnier A, Bouvet A. Staphylococcus aureus strains which are not identified by rapid agglutination methods are of capsular serotype 5. J Clin Microbiol. 1989;27:1372–1374. doi: 10.1128/jcm.27.6.1372-1374.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fournier, J. M., A. Bouvet, and A. Boutonnier. February 1990. Process for the preparation of capsular polysaccharides of staphylococci, the polysaccharides obtained, uses of these polysaccharides and strains for carrying out of the process. U.S. patent 4,902,616.
- 12.Fournier J M, Bouvet A, Mathieu D, Nato F, Boutonnier A, Gerbal R, Brunengo P, Saulnier C, Sagot N, Slizewicz B, Mazie J-C. New latex reagent using monoclonal antibodies to capsular polysaccharide for reliable identification of both oxacillin-susceptible and oxacillin-resistant Staphylococcus aureus. J Clin Microbiol. 1993;31:1342–1344. doi: 10.1128/jcm.31.5.1342-1344.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freeman D J, Falkiner F R, Keane C T. New method for detecting slime production by coagulase negative staphylococci. J Clin Pathol. 1989;42:872–874. doi: 10.1136/jcp.42.8.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goullet P, Hieng H S. Increase in gentamicin- and tobramycin-resistant Staphylococcus aureus isolates from hospital patients. Nouv Presse Med. 1981;10:2645–2652. [PubMed] [Google Scholar]
- 15.Gupta H, McKinnon N, Louie L, Louie M, Simor A E. Comparison of six rapid agglutination tests for the identification of Staphylococcus aureus, including methicillin-resistant strains. Diagn Microbiol Infect Dis. 1998;31:333–336. doi: 10.1016/s0732-8893(98)00012-1. [DOI] [PubMed] [Google Scholar]
- 16.Hilden P, Savolainen K, Tyynela J, Vuento M, Kuusela P. Purification and characterisation of a plasmin-sensitive surface protein of Staphylococcus aureus. Eur J Biochem. 1996;236:904–910. doi: 10.1111/j.1432-1033.1996.00904.x. [DOI] [PubMed] [Google Scholar]
- 17.Hochkeppel H K, Braun D G, Vischer W, Imm A, Sutter S, Staeubli U, Guggenheim R, Kaplan E L, Boutonnier A, Fournier J M. Serotyping and electron microscopy studies of Staphylococcus aureus clinical isolates with monoclonal antibodies to capsular polysaccharide types 5 and 8. J Clin Microbiol. 1987;25:526–530. doi: 10.1128/jcm.25.3.526-530.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsueh P-R, Teng L-J, Yang P-C, Pan H-J, Chen Y-C, Wang L-H, Ho S-W, Luh K-T. Dissemination of two methicillin-resistant Staphylococcus aureus clones exhibiting negative Staphylase reactions in intensive care units. J Clin Microbiol. 1999;37:504–509. doi: 10.1128/jcm.37.3.504-509.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johne B, Jarp J, Haaheim L R. Staphylococcus aureus exopolysaccharide in vivo demonstrated by immunomagnetic separation and electron microscopy. J Clin Microbiol. 1989;27:1631–1635. doi: 10.1128/jcm.27.7.1631-1635.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karakawa W W, Fournier J M, Vann W F, Arbeit R, Schneerson R S, Robbins J B. Method for the serological typing of the capsular polysaccharides of Staphylococcus aureus. J Clin Microbiol. 1985;22:445–447. doi: 10.1128/jcm.22.3.445-447.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monzon-Moreno C, Aubert S, Morvan A, El Solh N. Usefulness of three probes in typing isolates of methicillin-resistant Staphylococcus aureus (MRSA) J Med Microbiol. 1991;35:80–88. doi: 10.1099/00222615-35-2-80. [DOI] [PubMed] [Google Scholar]
- 22.Mulder J G, Degener J E. Slime-producing properties of coagulase-negative staphylococci isolated from blood cultures. Clin Microbiol Infect. 1998;4:689–694. doi: 10.1111/j.1469-0691.1998.tb00653.x. [DOI] [PubMed] [Google Scholar]
- 23.National Committee for Clinical Laboratory Standards. Approved standard M2–A6. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 24.Nelles M J, Niswander C A, Karakawa W W, Vann W F, Arbeit R D. Reactivity of type specific monoclonal antibodies with Staphylococcus aureus clinical isolates and purified capsular polysaccharide. Infect Immun. 1985;49:14–18. doi: 10.1128/iai.49.1.14-18.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nilsson M, Frykberg L, Flock J-I, Pei L, Lindberg M, Guss B. A fibrinogen-binding protein of Staphylococcus epidermidis. Infect Immun. 1998;66:2666–2673. doi: 10.1128/iai.66.6.2666-2673.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pillet J, Orta B, Corrieras F, Perrier M. Characterisation of three new staphylococcal antigens. Differentiation of predominant and secondary agglutinating systems. Ann Inst Pasteur (Paris) 1966;110:422–435. [Google Scholar]
- 27.Pillet J, Orta B, Foucaud M, Perrier M. Serological studies on 559 strains of pathogenic staphylococci isolated in France. Ann Inst Pasteur (Paris) 1961;100:713–724. [PubMed] [Google Scholar]
- 28.Pillet J, Orta B, Perrier M, Corrieras F. A propos of the reference strains used as type-strains I, II and III in serological studies on staphylococci. Ann Inst Pasteur (Paris) 1964;106:267–278. [PubMed] [Google Scholar]
- 29.Poutrel B, Mendolia C, Sutra L, Fournier J M. Reactivity of coagulase-negative staphylococci isolated from cow and goat milk with monoclonal antibodies to Staphylococcus aureus capsular types 5 and 8. J Clin Microbiol. 1990;28:358–360. doi: 10.1128/jcm.28.2.358-360.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sompolinsky D, Samra Z, Karakawa W W, Vann W F, Schneerson R, Malik Z. Encapsulation and capsular types in isolates of Staphylococcus aureus from different sources and relationship to phage types. J Clin Microbiol. 1985;22:828–834. doi: 10.1128/jcm.22.5.828-834.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sutra L, Mendolia C, Rainard P, Poutrel B. Encapsulation of Staphylococcus aureus isolates from mastitic milk: relationship between capsular polysaccharide types 5 and 8 and colony morphology in serum-soft agar, clumping factor, teichoic acid, and protein A. J Clin Microbiol. 1990;28:447–451. doi: 10.1128/jcm.28.3.447-451.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wanger A R, Morris S L, Ericsson C, Singh K V, Larocco M T. Latex agglutination-negative methicillin resistant Staphylococcus aureus recovered from neonates: epidemiological features and comparison of typing methods. J Clin Microbiol. 1992;30:2583–2588. doi: 10.1128/jcm.30.10.2583-2588.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wichelhaus T A, Kern S, Schafer V, Brade V. Rapid detection of epidemic strains of methicillin resistant Staphylococcus aureus. J Clin Microbiol. 1999;37:690–693. doi: 10.1128/jcm.37.3.690-693.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wichelhaus T A, Kern S, Schafer V, Brade V, Hunfeld K-P. Evaluation of modern agglutination tests for identification of methicillin-susceptible and methicillin-resistant Staphylococcus aureus. Eur J Clin Microbiol Infect Dis. 1999;18:756–758. doi: 10.1007/s100960050396. [DOI] [PubMed] [Google Scholar]