Abstract
Youth growing up in disadvantaged neighborhoods are more likely than their advantaged peers to face negative behavioral and mental health outcomes. Although studies have shown that adversity can undermine positive development via its impact on the developing brain, few studies have examined the association between neighborhood disadvantage and neural function, and no study has investigated potential social mechanisms within the neighborhood that might link neighborhood disadvantage to altered neural function. The current study evaluated the association between neighborhood disadvantage and amygdala reactivity during socioemotional face processing. We also assessed whether and which neighborhood-level social processes were related to amygdala reactivity, and whether these social processes mediated or moderated the association between neighborhood disadvantage and altered amygdala reactivity. We examined these aims in a registered report, using a sample of twins aged 7–19 years (N = 354 families, 708 twins) recruited from birth records with enrichment for neighborhood disadvantage. Twins completed a socioemotional face processing fMRI task and a sample of unrelated participants from the twins’ neighborhoods were also recruited to serve as informants on neighborhood social processes. We found that neighborhood disadvantage was associated with greater right amygdala reactivity to threat, but only when neighborhood informants perceived norms in the neighborhood to be more permissive regarding general safety and management. The findings from this research add to the growing literature highlighting the influence of neighborhood disadvantage on amygdala function and the ways that supportive social processes may buffer the impact of adversity on brain function.
Keywords: Neighborhood, Amygdala, Social cohesion, Ecological neuroscience
1. Introduction
Today, more than 8.5 million children in the U.S. live in high poverty neighborhoods, where at least 30% of residents live below the poverty line (The Annie E. Casey Foundation, 2019). Growing up in these high poverty neighborhood contexts negatively impacts developmental outcomes, including school readiness and academic achievement, as well as behavioral and emotional problems (Aneshensel and Sucoff, 1996, Kohen et al., 2008, Leventhal and Brooks-Gunn, 2000, Sastry, 2012, Xue et al., 2005). Despite the significant costs of growing up in neighborhood poverty for youth’s development, there is limited research to elucidate the underlying mechanisms by which exposures within the neighborhood are instantiated in the brains and bodies of young children to undermine positive development.
A growing body of literature suggests that exposure to early adversity may undermine child development via structural and functional changes in the brain, particularly within the amygdala – a key node of the stress response system – which is responsible for determining the emotional significance of stimuli, threat processing, and fear conditioning (Davis and Whalen, 2001, LeDoux, 2003, LeDoux, 2000, Tottenham and Sheridan, 2010). The amygdala is sensitive to emotional facial expressions, especially those that signal threat or uncertainty (Fusar-Poli et al., 2009, LeDoux, 2003, Ochsner et al., 2012, Shi et al., 2013, Whalen and Phelps, 2009). Many forms of adversity, including childhood poverty, maltreatment, and extreme social deprivation, are associated with heightened amygdala reactivity during socioemotional processing in late childhood, adolescence, and adulthood (Hein and Monk, 2017, Javanbakht et al., 2015, Tottenham et al., 2011). Moreover, individual variability in amygdala reactivity during socioemotional processing has been associated with major psychiatric disorders (Etkin et al., 2004, Hyde et al., 2016, Monk, 2008). Thus, examination of the amygdala as a key node within the limbic system as it responds to socially relevant threat can help to elucidate how early life stress is linked to adverse outcomes in youth.
Although studies are beginning to delineate the potential impacts of adversity on amygdala function, most have focused on family processes (e.g., parenting, maltreatment) or distal factors (e.g., income), often with small convenience samples and little attention paid to the neighborhood context. This omission is surprising given the robust literature demonstrating a strong association between concentrated neighborhood disadvantage and negative behavioral, cognitive, and socioemotional outcomes (Aneshensel and Sucoff, 1996, Burt et al., 2016, Kohen et al., 2008, Leventhal and Brooks-Gunn, 2000, Sastry, 2012). The few studies that have examined neighborhood effects on the brain have found that greater neighborhood disadvantage is associated with greater increases in left and right amygdala volume longitudinally during adolescence (Whittle et al., 2017) and greater amygdala reactivity to emotional faces in adolescence and adulthood (Gard et al., 2017, Gard et al., 2020).
Much of the research examining adversity and amygdala reactivity to emotional faces has purposefully focused on faces indicating threat and distress (i.e., anger and fear) (e.g., Gard et al., 2017; Hein and Monk, 2017). However, amygdala reactivity to neutral facial expressions may also be impacted by neighborhood-level adversities (Gard et al., 2017, Gard et al., 2020). Due to the ambiguity of neutral faces, they may be interpreted as hostile or threatening, especially for those exposed to adversity (Gard et al., 2017, Marusak et al., 2017, Pollak et al., 2000). Moreover, given the role of the amygdala in processing the emotional significance of stimuli and prompting physiological and behavioral responses to perceived threats, the unpredictability of ambiguous neutral faces may be especially salient for youth growing up in disadvantaged neighborhoods (Davis and Whalen, 2001, LeDoux, 2003). Thus, the first aim of the current study will be to examine the association between neighborhood disadvantage and amygdala reactivity to facial expressions of threat and distress (i.e., anger and fear), as well as ambiguity (i.e., neutral faces).
Although important, studies of neighborhood disadvantage alone cannot explain how neighborhood disadvantage predicts child outcomes at the neural level. In more disadvantaged neighborhoods, structural characteristics, such as poverty and residential instability, are associated with the breakdown of social ties and norms, which undermines residents’ abilities to maintain community-level mechanisms of control that curb violent crime and promote the safety and wellbeing of residents (Morenoff et al., 2001, Sampson et al., 1997, Sampson and Groves, 1989). In particular, a wealth of developmental and sociological literature points to three important neighborhood-level social processes that are associated with neighborhood disadvantage and mediate associations between neighborhood disadvantage and youth behavioral outcomes: (1) neighborhood norms, (2) social cohesion, and (3) informal social control (Aneshensel and Sucoff, 1996, Elliott et al., 1996; Henry et al., 2014; Leventhal and Brooks-Gunn, 2000; Sampson et al., 2002; Xue et al., 2005).
Neighborhood norms are the shared beliefs regarding appropriate or expected behaviors and attitudes of neighborhood residents, especially regarding youth management, protection, and behavior, as well as neighborhood safety and management (Henry et al., 2014). In general, youth in the neighborhood are more likely to engage in deviant behaviors (e.g., smoking, violent behavior) when neighbors approve of, or fail to disapprove of, such behavior (Musick et al., 2008, Reed et al., 2011, Wright and Fagan, 2013). Social cohesion refers to the degree of mutual support, help, and trust amongst neighbors, and informal social control refers to the shared expectation and willingness of neighbors to intervene for the common good in accordance with socially prescribed neighborhood norms (Henry et al., 2014; Sampson et al., 1997). The degree of social cohesion and informal social control within a neighborhood is often combined to form the construct of collective efficacy (Sampson et al., 1997). Neighborhood disadvantage is associated with lower levels of neighborhood collective efficacy, which directly and indirectly impacts youths’ mental health and subjective well-being, academic achievement, and antisocial behaviors (Aneshensel and Sucoff, 1996, Brody et al., 2001, Dawson et al., 2019, Jackson et al., 2016, Odgers et al., 2009, Wang and Fowler, 2019, Woolley et al., 2008). Thus, a wealth of studies demonstrate that these neighborhood social processes are important for youth behavioral outcomes. However, no study has addressed whether these social processes influence brain development broadly or amygdala reactivity to socioemotional faces specifically. Thus, our second aim is to investigate whether and which social processes within the neighborhood, including neighborhood norms and collective efficacy (i.e., the combination of social cohesion and informal social control), are related to amygdala reactivity to threat and ambiguity, and whether these social processes are a potential mechanism through which neighborhood disadvantage predicts amygdala reactivity during socioemotional processing.
Finally, in examining these questions there are several methodological gaps in the existing literature. First, much of the research focused on linking early adversity to brain function has focused on samples of convenience or on samples of extreme groups/clinical cases (e.g., families reported to child protective services). To best understand neighborhood effects, studies are needed that use representative sampling, but also are enriched for exposure to neighborhood poverty to increase representation of those in the most adverse contexts (Falk et al., 2013). Second, one potential weakness of some research on neighborhood effects is the sole reliance on parent-report of neighborhood conditions, which may include shared method variance with outcomes or bias that may reflect person-specific views of the neighborhood or gene-environment correlation between the reporting of neighborhood processes and youth brain function. Cutting-edge approaches can leverage objective, census-level data and the views of independent raters who live in the same context (i.e., multiple neighbors who are not related to the child) (Burt et al., 2019, Burt et al., 2020). The current study utilized both approaches.
1.1. Specific aims and hypotheses
In the current study, we examined the association between neighborhood disadvantage and amygdala reactivity during socioemotional face processing of threat (i.e., angry and fearful faces) and ambiguity (i.e., neutral faces) in a relatively large sample of youth (age 7–19 years, N = 708). Second, we assessed whether and which social processes within the neighborhood were associated with sensitized amygdala reactivity to threat and ambiguity, and whether these social processes mediated the effects of neighborhood disadvantage on amygdala reactivity during socioemotional processing. Given the wide age range of our sample (age 7 – 19) and the rapid development of the brain during this period, we also assessed age as a moderator in the associations between neighborhood disadvantage, neighborhood social processes, and amygdala reactivity. Participants were recruited via birth records to be representative of families with twins living in southcentral Michigan with a high oversampling for exposure to neighborhood disadvantage. Thus, the study boasts a representative sampling frame, but with substantial (over)representation of families living in high poverty neighborhoods, which increases representation of youth facing substantial adversity; often the exact youth missing from other neuroimaging studies. In addition, we supplemented this important sampling approach with census-reported data on neighborhood disadvantage and a novel assessment of neighborhood social processes by collecting reports of neighborhood social processes from sets of randomly selected individuals residing in the families’ neighborhoods (i.e., neighbors). The current project was thus well-positioned, both in its design and analytic approach, to explore whether and how neighborhood social processes mediate the association between neighborhood disadvantage and amygdala reactivity. Specifically, we hypothesized that 1) neighborhood disadvantage would be associated with greater amygdala reactivity to both threat and ambiguity; 2) low levels of collective efficacy and permissive neighborhood norms would be associated with greater amygdala reactivity to threat and ambiguity; and 3) these social processes would mediate the pathway between neighborhood disadvantage and amygdala reactivity to socioemotional faces. Additionally, beyond mediation, literature has shown that high levels of collective efficacy often moderate associations between neighborhood factors and youth outcomes (e.g., Browning et al., 2014; Dawson et al., 2019; Fagan et al., 2014; Kingsbury et al., 2019) – that is, these social processes can be protective in high poverty environments. Thus, 4) we conducted an exploratory analysis examining whether positive neighborhood social processes (e.g., high collective efficacy) buffered (i.e., moderated) the effects of neighborhood impoverishment on amygdala reactivity to threat and ambiguity.
2. Materials and methods
2.1. Participants
2.1.1. Twin families
Participants were part of an on-going longitudinal twin study, the Michigan Twins Neurogenetics Study (MTwiNS). Twins were recruited from the Twin Study of Behavioral and Emotional Development – Child (TBED-C), a project within the Michigan State University Twin Registry (MSUTR) (Burt and Klump, 2013). TBED-C identified twins via birth records, a strong epidemiologic sampling frame, and included both a population-based sample of 528 twin families (1056 twins) and “at-risk” sample of 502 twin families (1004 twins). In collaboration with the Department of Vital Records in the Michigan Department of Health and Human Services (MDHHS), primary recruitment was carried out via anonymous mailings to twin families within a 120-mile radius of Michigan State University, an area including Detroit, Flint, Lansing, and other urban areas, as well as substantial parts of suburban and rural Michigan. Recruitment procedures for the population-based and the at-risk samples were identical except that for the latter, mailings were restricted to families residing in modestly-to-severely impoverished neighborhoods that contained over 10.5% of families living below the poverty line (the mean for the state of Michigan at the time, e.g., Burt et al., 2016). This recruitment strategy yielded overall response rates of 62% for the population-based sample and 57% for the at-risk sample. To be eligible for participation in the TBED-C, neither twin could have a cognitive or physical condition that would preclude completion of the study protocol, such as a significant developmental delay or deficit (as assessed via parental screen). Families participating in the population-based sample reported racial group memberships at rates approximating those of area inhabitants (e.g., 86.4% White, 5.4% Black; Burt and Klump, 2013). Compared to the population-based sample, the at-risk sample was significantly more racially diverse (76.3% White, 14.2% Black) and less advantaged, reporting lower family incomes (means of $72,027 versus $57,281; Cohen’s d effect size = −0.38) (Burt et al., 2016, Burt et al., 2018). For more details on recruitment procedures, see (Burt and Klump, 2019).
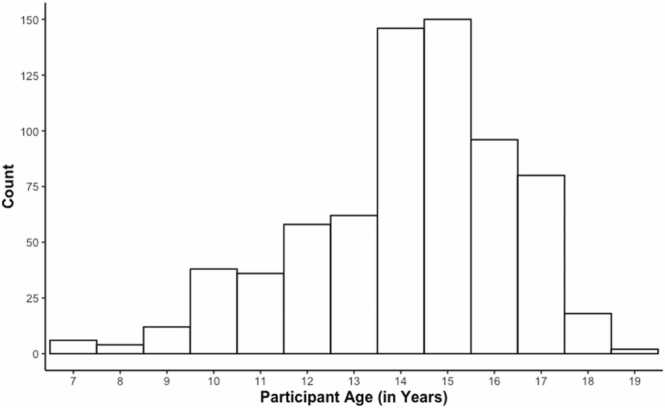
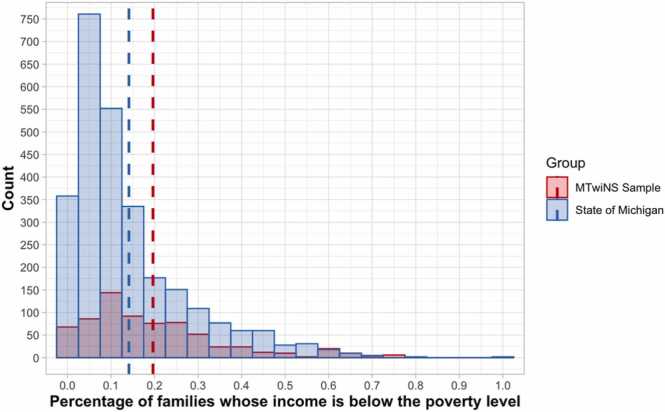
The current sample drew from the families originally eligible for the “at-risk” sample, including all at-risk sample families and those in the population-based sample that would have met criteria for the at-risk sample by living in a neighborhood with above average levels of neighborhood poverty. The sample included 708 twins (354 families) (54.5% boys; 78.5% White, 13.0% Black, 8.5% other racial/ethnic group membership) aged 7–19 years (Mage = 14.14, SD = 2.24; 94.2% of the sample was between 10 and 17 years old, with only 11 twin pairs < 10 years old and 10 twin pairs > 17 years old; see Fig. 1), resulting in a sample that represented families living in south-central Michigan with substantial oversampling for families living in impoverished neighborhoods. In the current MTwiNS sample, 64.1% of twin families lived in neighborhoods with > 10.5% of families living below the poverty line (mean percentage of families below the poverty line in the neighborhood is 19.6% and ranges as high as 77.0%) (see Fig. 2). Note that, though families were originally recruited based on living in neighborhoods with above average levels of poverty, this recruitment occurred when children were 6 – 10 years old and since then some families no longer live in neighborhoods with above average poverty because they moved or because neighborhoods shifted (e.g., gentrified). Participants included in the present analyses met basic fMRI eligibility criteria, such as the absence of metal in their body and willingness to participate in the scanning session (i.e., 557 of 708 twins were eligible for scanning and agreed to scan; see Table 1). This study is ongoing, and we will wait until we have accrued the full sample (>2 years in the future) to test core aims of the grant. However, to ask important, but non-central grant aim questions, we have used successive “freezes” of the data to make sure that papers use the same groups of families and there is no concern about “stopping rules” (e.g., only included enough data to find significant results). In this case, we used a freeze of 354 families (708 twins) for this report. We had not analyzed any data yet at this freeze and the stopping time of this freeze was based on the pause of in-person research due to the COVID-19 pandemic (and thus was random and could not reflect investigator-motivated stopping rules).
Fig. 1.
Histogram of participant age.
Fig. 2.
American community survey 5-year neighborhood poverty ratings for the MTwiNS sample and the State of Michigan. Note. Dashed lines mark the mean percentage of families below the poverty line for each group (State of Michigan:N = 2736, M = 0.14, SD = 0.13; MTwiNS Sample:N = 708, M = 0.20, SD = 0.16).
Table 1.
Summary of data included in MTwiNS analysis.
| Number Lost | Participants with Data | |
|---|---|---|
| Original Sample | 708 | |
| Declined MRI scan (including declining to remove jewelry/piercings) | 27 | |
| Uncomfortable with MRI scan | 14 | |
| Dental (e.g., braces, retainer) | 17 | |
| Metal in/on the body1 (including recent surgery) | 12 | |
| Exceeding scanner size restrictions (e.g., overweight, broad shoulders) | 5 | |
| Major medical/neurological disorder (e.g., Autism, TBI, tumor) | 17 | |
| Incomplete Scan | 17 | |
| Total Lost | 1091 | |
| Sample with imaging data | 557 | |
| Exceeding movement thresholds (ART outliers > 20.0%) | 5 | |
| Low task performance (< 70%) | 36 | |
| Low bilateral amygdala coverage (< 90%) | 3 | |
| Failed Visual Inspection (prefrontal artifacts) | 1 | |
| Total Lost | 37 | |
| Sample with usable imaging data | 512 |
Note. An additional 21 families (42 twins) received an earlier/pilot version of the task that was not comparable to the current version; these participants were excluded from all analyses.
Includes non-MRI safe implanted medical devices, having BBs/pellets or other non-removable metal inside of body, recent surgery, metallic tattoos, unremovable jewelry.
2.1.2. Neighborhood informants
A randomly selected sample of unrelated participants (i.e., neighbors of study families) from the same neighborhoods were recruited to serve as neighborhood informants on social processes. Mailing packets were sent to 10 randomly chosen addresses in each twin family’s Census tract, inviting one adult resident per household to complete a survey. When an address was no longer inhabited (i.e., the letter was undeliverable), one attempt was made to find a replacement address. All participants provided informed consent. There was at least one neighborhood informant report available for all but one family, with an average number of 4.39 (SD = 1.64) informant reports per neighborhood. For the current timepoint, we collected data from 983 neighbors (61% female, 34% male, 5% missing/prefer not to answer; 86.6% White; 6.7% Black; 4.6% other; 2.1% missing/prefer not to answer). The response rate for the current timepoint was 51%, of which 62% agreed to participate to date.
2.2. Procedure
Youth and their primary caregivers took part in a day-long visit to the University of Michigan (UM) which included a one-hour fMRI scan for each youth at the UM fMRI lab. Twins provided assent and parents provided informed consent for themselves and their children. Twins were then introduced to the scanning environment using a mock scanner and completed practice versions of several fMRI tasks. Youth were then scanned using blood-oxygen-level-dependent (BOLD) fMRI while completing several tasks, including the socioemotional face processing task described below. Families also completed a battery of questionnaires and were provided lunch. Additionally, primary caregivers completed a demographic interview with an examiner. Approval was obtained from the UM Institutional Review Board. Participants were compensated for their time.
2.3. Measures
2.3.1. Socioemotional face processing fMRI task
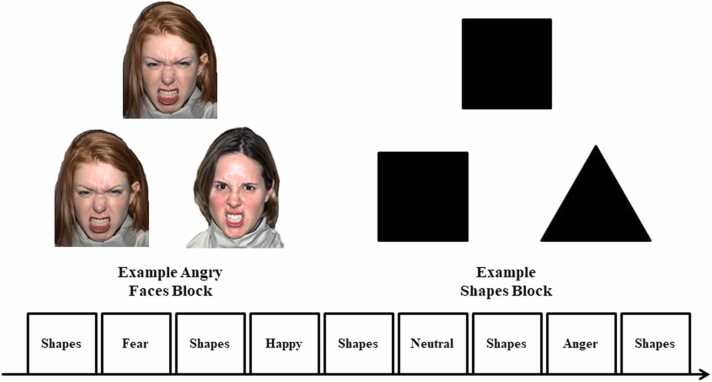
Participants performed an implicit emotional face processing task, which consisted of four blocks of perceptual face processing interleaved with five blocks of sensorimotor control (see also Gard et al., 2018; Hariri et al., 2002; Manuck et al., 2007). Participants viewed a trio of faces and selected one of two faces (bottom) identical to a target face (top; Fig. 3). Each face processing block consisted of 18 images, balanced for sex, all derived from the NimStim standard set of pictures of facial affect (Tottenham et al., 2009). Each of the four face processing blocks consisted of a different emotional facial expression (i.e., anger, fear, happy, neutral), and participants were randomly assigned to one of four different orders of block presentation. During the sensorimotor control blocks, participants viewed 12 trios of simple geometric shapes (circles, squares, triangles) and selected one of two shapes (bottom) identical to a target shape (top; Fig. 3). In the face processing blocks, each of the 18 face trios were presented for 2 s with a variable interstimulus interval (ISI) of 2 – 6 s for a total block length of 98 s. A variable ISI was used to minimize expectancy effects and resulting habituation, as well as to maximize amygdala reactivity throughout the paradigm. In the sensorimotor control blocks, each of the 12 shape trios was presented for 2 s followed by a fixation cross for 0.5 s, for a total block length of 30 s. An additional 4 s of crosshair presentation followed each block. Total task time was 578 s
Fig. 3.
Socioemotional face processing fMRI Task.
2.3.2. Neighborhood characteristics
Neighborhood disadvantage was measured using the Area Deprivation Index (ADI) at the Census block group level (Kind et al., 2014). ADI scores measure indices of concentrated disadvantage in the neighborhood via indicators of neighbors’ education, employment, income, and poverty (e.g., home ownership rates, percentage of single-parent households, percentage of families living below the poverty line, percentage of those 16 years or older unemployed). ADI scores were calculated using the Singh method, which entails summing Singh’s 17 census indicator weights by Singh’s factor score coefficients for each indicator (Singh, 2003). The ADI uses the American Community Survey Five Year Estimates in its construction (e.g., the 2015 ADI uses the ACS data from 2015, which is a 5-year average of data obtained from 2011 to 2015). Importantly, this five-year period overlapped with the timing of data collection for the twin sample. The ADI provides a national percentile ranking at the block group level from 1 to 100. These percentiles were constructed by ranking the ADI from low-to-high for the nation and grouping the block groups into bins corresponding to each 1% range of the ADI. Group 1 was the lowest ADI, indicating the lowest level of “disadvantage” within the nation; whereas the group 100 was the highest ADI, indicating the highest level of “disadvantage.”
Neighborhood Social Processes were assessed from neighborhood informants using the Neighborhood Matters questionnaire, which included three subscales (Henry et al., 2014). Within the Neighborhood Matters questionnaire, the 30-item Social Cohesion scale assesses perceptions of support, help, and trust within the neighborhood. Each item begins with the stem, “In general, people in this neighborhood…” (e.g., In general, people in this neighborhood…are willing to help their neighbors). Responses are on a 5-point Likert-type scale on which 1 represented “Strongly Agree” and 5 represented “Strongly Disagree.” The 29-item Informal Social Control scale assesses perceptions that community residents will undertake activities to maintain social order. Each item starts with the stem, “In general, what would someone in this neighborhood most likely do if…” (e.g., In general, what would someone in this neighborhood most likely do if…a child is left at home alone during the evening?). Participants selected 1 of 4 responses: (1) Do nothing; (2) Complain to or discuss with other neighbors; (3) Talk to someone who can do something about it, for example the police, a landlord, or a parent; (4) Do something directly, for example, step in and/or talk to the person or people involved. The 22-item Norms scale assesses perceptions of behavioral norms in the neighborhood, with a focus on norms regarding child welfare and neighborhood safety. Each item starts with the stem, “In general, people in this neighborhood think…” (e.g., In general, people in this neighborhood think…adults should do something if a child is doing something dangerous, even if it is not their child). Responses are on a 5-point Likert-type scale on which 1 represented “Strongly Agree” and 5 represented “Strongly Disagree.” The social cohesion and informal social control scales were standardized and combined into a single measure of collective efficacy.
2.3.3. Covariates
2.3.3.1. Demographics
Primary caregivers completed a demographic interview with an examiner. To control for racial group in analyses, race was coded as: 0 = White, 1 = Non-White. We controlled for race, a socially constructed category, to control for differences in exposure to systemic racism and the unequal exposures to poverty, stress, trauma, and opportunity for people of color and those not identifying as White in the United States (Pager and Shepherd, 2008; Roberts and Rizzo, 2020; Sellers et al., 2006). Twin’s chronological age and gender were also included as covariates. In addition, if we found significant associations between neighborhood disadvantage or neighborhood social processes and amygdala reactivity, we planned to control for other potential confounding variables related to socioeconomic context in sensitivity analyses, including family income, as defined via primary caregiver reported monthly household gross income and any outside additional sources of income (e.g., government assistance or child support), as well as maternal education, defined via the primary caregiver’s highest completed level of education.
2.3.3.2. Parenting
Twin’s perceptions of parenting were assessed using the Parental Environment Questionnaire (PEQ). The PEQ is a 42-item inventory that assesses five factorially derived aspects of the parent-child relationship: Conflict, Parental Involvement, Child Regard for Parent, Parent Regard for Child, and Structure (Elkins et al., 1997). For the present study, we used twin self-reports of the Conflict scale (12 items; = 0.75) to index harsh parenting. Each item was rated on a 4-point Likert scale ranging from “definitely false” to “definitely true.” Sample items from the Conflict scale include, “My parent often criticizes me” and “My parent sometimes hits me in anger.” Possible scores for the Conflict scale range from 12 to 48 with higher scores indicating greater levels of harsh, conflictual parenting.
2.4. fMRI acquisition and processing
Each participant was scanned with one of two research-dedicated GE Discovery MR750 3 T scanners located at the University of Michigan Functional MRI Laboratory. To take advantage of improvements in MRI data acquisition and harmonize our protocol with the Adolescent Brain Cognitive Development Study (Casey et al., 2018), we altered our acquisition protocol after the first 140 families (i.e., 280 twins). For the first 140 families (i.e., 280 twins), one run of 298 volumes was collected for each participant with blood oxygenation level–dependent (BOLD) functional images acquired via an 8-channel head coil and a reverse spiral sequence (TR/TE=2000/30 ms, flip angle = 90°, FOV = 22 cm), which covered 43 interleaved oblique slices of 3-mm thickness. High-resolution T1-weighted SPGR images (156, 1 mm-thick slices) were aligned with the AC-PC plane, and later used during normalization of the functional images. For the remaining 214 families (i.e., 428 twins), one run of 730 volumes was collected for each participant in which BOLD functional images were acquired with a 32-channel head coil and a gradient-echo sequence with multiband acquisition (TR/TE=800/30 ms, flip angle = 52°, FOV = 21.6 cm), which covered 742 interleaved axial slices of 2.4-mm thickness. High-resolution T1-weighted SPGR images (208, 1 mm-thick slices) were aligned with the AC-PC plane and used during normalization of the functional images. For both acquisition sequences, BOLD functional images encompassed the entire cerebrum and most of the cerebellum to maximize coverage of limbic structures.
Preprocessing for both acquisition sequences was identical, unless otherwise specified. Functional data were preprocessed and analyzed using Statistical Parametric Mapping version 12 (SPM12; Wellcome Trust Centre, London, United Kingdom). Raw k-space data from reverse-spiral sequence acquisition were de-spiked before reconstruction to image space. For multiband data, task-specific field maps were constructed from volumes of both anterior-to-posterior and posterior-to-anterior phase encoding; field maps were applied after image construction to reduce spatial distortions and minimize movement artifacts. Slice timing correction was performed using the 23rd slice as the reference slice (reverse-spiral data) or the 2nd slice of each 10-slice band (gradient-echo data with multiband acquisition). Data from both acquisition sequences were then spatially realigned to the 10th slice of the volume. These spatially realigned data were coregistered to the high-resolution T1-weighted image, and segmented and spatially normalized into standard stereotactic space (MNI template). Finally, functional data were smoothed to minimize noise and residual difference in gyral anatomy with a Gaussian filter set at 8 mm FWHM. After preprocessing, the Artifact detection Tools (ART) software package (http://www.nitrc.org/projects/artifact_detect/) was used to detect global mean intensity and translation or rotational motion outliers (> 4.5 SD from the mean global brain activation, >2 mm movement or 2° translation in any direction). For each participant with outliers, individual nuisance covariates were created for each outlier volume and included in the individual-level model (i.e., via spike regression). Any participant with > 20% motion outliers identified using ART were excluded from analyses.
Additionally, because of the relatively extensive signal loss typically observed in the amygdala, single-subject BOLD fMRI data were only included in subsequent analyses if there was a minimum of 90% signal coverage in the bilateral amygdala, defined using the Automated Anatomical Labeling (AAL) atlas in the WFU PickAtlas Tool, version 1.04 (Maldjian et al., 2003). Lastly, participants were excluded if accuracy performance on the task was less than 70%. Youth with valid imaging data were compared to youth without valid imaging data to ensure that they did not differ (all ps > .05) on youth characteristics (i.e., chronological age, gender, and self-reported race) or primary caregiver characteristics (i.e., education or annual income). If included versus missing participants differed on any of these variables, these variables were included as covariates in all models (Graham, 2009).
3. Experimental design and statistical analyses
3.1. Functional data analysis
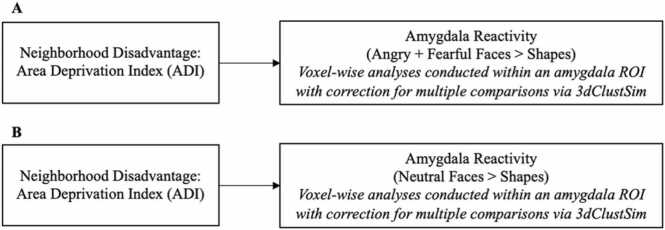
The general linear model (GLM) was used to conduct fMRI data analyses in SPM12. Linear contrasts employing canonical hemodynamic response functions were used to estimate condition specific BOLD activation for each individual scan. These individual contrast images (i.e., weighted sum of the beta images) were then used in second-level random effects models that account for both scan-to-scan and participant-to-participant variability to determine mean emotion-specific reactivity using one-sample t-tests. The main goal of this study was to examine amygdala reactivity to emotional faces relative to a non-faces condition (shapes), with a focus on threat and distress (i.e., angry and fearful faces) and ambiguity (i.e., neutral faces). Thus, we examined amygdala reactivity within two planned contrasts: (1) angry and fearful faces > shapes and (2) neutral faces > shapes. We provided supplementary, exploratory analyses to examine whether these results were more highly related to anger versus fear by examining angry faces > shapes and fearful faces > shapes, separately. Consistent with past publications from our lab (Gard et al., 2018), a bilateral amygdala region of interest (ROI) was defined structurally using the AAL Atlas definition in the WFU PickAtlas Tool, version 1.04 (Maldjian et al., 2003). We then examined activation within this anatomically defined ROI. For completeness, in supplementary materials, a whole-brain analysis was conducted and reported for the two planned contrasts: angry and fearful faces > shapes and neutral faces > shapes. We conducted all amygdala and whole brain analyses using a cluster correction method via the most updated version of the 3dClustSim program using Analysis of Functional NeuroImages (AFNI) software version 16.1.14 (Cox, 1996, Cox et al., 2017) (within the amygdala ROI for the main analyses and across the entire brain for whole-brain supplementary analyses). Consistent with recommendations by Cox and colleagues (2017), we implemented the spatial autocorrelation function (i.e., the -acf option) to model the spatial smoothness of noise volumes. Group-level smoothing values were estimated from participants’ individual-model residuals using the program 3dFWHMX, and then averaged across those subjects. 3dClustSim uses a Monte Carlo simulation to provide thresholds that achieve a family-wise error (FWE) correction for multiple comparisons of p < .05 within each ROI (k = 1 for amygdala analyses and k = 77 and 80 for whole brain analyses for the neutral faces > shapes and fearful + angry faces > shapes contrasts, respectively). We used a voxel-wise threshold of p < 0.001 for cluster sizes. Our cluster thresholds were based on 2-sided tests and used the nearest neighbor definition of “face and edge” (i.e., 3dClustSim command: NN=2). (Figs. Fig. 4, Fig. 5, Fig. 6).
Fig. 4.
Aim 1 models linking neighborhood disadvantage to amygdala reactivity. Note. Covariates: age, sex, race, and scan type.
Fig. 5.
Aim 2 models linking neighborhood social processes to amygdala reactivity. Note. Covariates: age, sex, race, and scan type.
Fig. 6.
Aim 3 path models linking neighborhood disadvantage to amygdala reactivity via neighborhood social processes.
3.2. Aim 1: Is neighborhood disadvantage related to amygdala reactivity?
To determine the association between neighborhood disadvantage and amygdala reactivity, we estimated multiple regression models using Neuropointillist (Madhyastha et al., 2018; https://github.com/IBIC/neuropointillist) in conjunction with the Mplus Automation package in R (Hallquist and Wiley, 2018) (at the group level, across all participants), with census-derived ADI scores predicting amygdala activation for the contrasts angry and fearful faces > shapes and neutral faces > shapes. Group level activation was analyzed within an anatomically defined bilateral amygdala ROI from WFU PickAtlas. Our main models controlled for twin’s age, sex, and race, as well as the scan type (i.e., multiband or spiral acquisition). Given the wide age range of our sample, we also examined age as an exploratory moderator in the association between neighborhood disadvantage and amygdala reactivity. If we found significant associations between neighborhood disadvantage and amygdala reactivity controlling for twin’s age, sex, and race, and scan type, we ran additional sensitivity models controlling for family income, maternal education, and harsh parenting to determine if neighborhood disadvantage uniquely predicts amygdala reactivity over and above these family-level adversities. Although these stringent models have rarely been used in other adversity-brain studies, controlling for the effects of harsh parenting and family SES (income and education), allows us to better attribute effects directly to neighborhood disadvantage, rather than potentially confounded family-level adversities (e.g., Gard et al., 2017). Lastly, all analyses used full information Maximum Likelihood estimation with cluster corrected bootstrapping (5000 draws) to account for missing data and twin nesting within family (Falk, 2018). This approach via Neuropointillist and Mplus Automation provides efficient and robust estimates even in the face of substantial missingness and is robust to skewed data.
3.3. Aim 2: are neighborhood social processes related to amygdala reactivity?
To determine whether or not neighborhood social processes (i.e., neighborhood norms and collective efficacy) were associated with amygdala reactivity, we estimated multiple regression models using Neuropointillist (https://github.com/IBIC/neuropointillist) in conjunction with the Mplus Automation package in R (at the group level, across all participants) to test whether each neighborhood social process predicts amygdala activation for the contrasts angry and fearful faces > shapes and neutral faces > shapes. Group level activation was analyzed within an anatomically defined bilateral amygdala ROI from WFU PickAtlas. Again, in our main models we controlled for twin’s age, sex, and race, and we used full information Maximum Likelihood estimation with cluster corrected bootstrapping (5000 draws) to account for missing data and twin nesting within family. Given the wide age range of our sample, we also examined age as an exploratory moderator in the association between neighborhood social processes and amygdala reactivity.
Furthermore, if we found significant associations between neighborhood social processes and amygdala reactivity while controlling for twin’s age, sex, and race, and scan type, we estimated additional sensitivity models controlling for the other neighborhood social process, in addition to other potential confounders: family income, maternal education, and parenting. Though these stringent models have rarely been used in other adversity-brain studies, we believe it is important to examine the extent to which associations are due specifically to neighborhood processes, rather than other confounding variables associated with neighborhood disadvantage (i.e., family poverty, lower maternal education, harsh parenting). Finally, given that neighborhood crime, in particular, has been found to be associated with child mental health and individual differences in adolescents’ emotional regulatory processing (McCoy et al., 2016, Ramey and Harrington, 2019), if we found significant associations between neighborhood norms and amygdala reactivity, we also proposed to conduct specificity models including items on the Norms scale that are and are not related to neighborhood crime in order to determine if norms related to crime are associated with amygdala reactivity over and above other neighborhood norms.
3.4. Aim 3: do neighborhood social processes mediate the relation between neighborhood disadvantage and amygdala reactivity?
Path analyses were conducted voxel-wise (with correction for multiple comparisons using 3DClustSim) using Neuropointillist in conjunction with the Mplus Automation R package. We only proceeded with testing our path models if two conditions were met: First, amygdala activation from either the angry + fearful faces > shapes or the neutral faces > shapes contrast must be correlated with either neighborhood social process, neighborhood norms and/or neighborhood collective efficacy (i.e., 1 or more of these 4 correlations must be statistically significant). Second, neighborhood disadvantage must also be significantly correlated with the same neighborhood social process that was associated with amygdala reactivity. That is, if we had significant a and b paths for the same neighborhood social process, we proceeded with testing our overall path model. To limit analytic flexibility and control for the overlap of neighborhood social processes, we included all neighborhood-level social processes (i.e., collective efficacy and neighborhood norms) in the same path model on the full sample (N = 708). We used full information Maximum Likelihood estimation with cluster corrected bootstrapping (5000 draws) to accommodate missing data, to protect against distortion of effects from violations of distributional assumptions, and to account for nesting within families. Our conservative model controlled for covariates, including twin age, sex, and race, and scan type. If any paths or indirect effects were significant, we added in potential confounders (i.e., family income, maternal education, parenting) to the model to examine the specificity of effects to neighborhood processes.
Lastly, given that previous research has also found high levels of collective efficacy to be protective in high poverty environments (e.g., Browning et al., 2014; Dawson et al., 2019; Fagan et al., 2014; Kingsbury et al., 2019), we examined neighborhood social processes as a potential moderator in the association between neighborhood disadvantage and amygdala reactivity to threat and ambiguity. In the moderation models, predictors were mean-centered, and the interaction term was created as the product of the centered predictors and the models were estimated in Neuropointillist with Mplus Automation as described above.
4. Results
4.1. Preliminary analyses
Descriptive statistics and correlations for all demographic and study variables are displayed in Table 2. Of note, all neighborhood social processes variables were positively associated with each other. Social cohesion was significantly positively associated with informal social control (r = .40) and neighborhood norms (r = .56), and informal social control and norms were positively correlated (r = .44). Moreover, as expected, neighborhood disadvantage was significantly negatively correlated with social cohesion (r = −.27), informal social control (r = −.17), norms (r = −.12), and collective efficacy (r = −.26).
Table 2.
Means, standard deviations, and correlations among study constructs.
| Variable | M | SD | N | 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Twin Gender | 708 | |||||||||||
| 2. Twin Race/Ethnicity | 708 | |||||||||||
| 3. Twin Age | 14.1 | 2.2 | 708 | .15** | .19** | |||||||
| 4. Parent Education | 6.3 | 1.3 | 706 | -.02 | .16** | .18** | ||||||
| 5. Parent Income | 9.4 | 3.0 | 702 | .04 | .37** | .10* | .45** | |||||
| 6. Neighborhood Disadvantage | 60.7 | 21.1 | 689 | -.04 | -.31** | -.01 | -.25** | -.41** | ||||
| 7. Social Cohesion | 107.3 | 12.7 | 572 | .01 | .15** | .06 | .09* | -.01 | -.27** | |||
| 8. Informal Social Control | 25.1 | 3.4 | 570 | .10* | .10* | .14* | .12* | .11* | -.17** | .40** | ||
| 9. Neighborhood Norms | 89.9 | 6.4 | 572 | .05 | .01 | .01 | .06 | -.03 | -.12* | .56** | .44** | |
| 10. Collective Efficacy | 0.0 | 0.8 | 572 | .07 | .15** | .11* | .12* | .06 | -.26** | .84** | .84** | .60** |
Note. M and SD are used to represent mean and standard deviation, respectively. Twin gender: 1 =Females (45.5%) and 0 = Males; Twin race/ethnicity: 1 = Non-Hispanic White (76.0%) and 0 = Minoritized other. *= p < .05. **= p < .001.
Pearson correlation was used for all estimates except for those including twin gender and twin race/ethnicity, which relied on point biserial correlations.
4.2. Neighborhood disadvantage and amygdala reactivity to threat and ambiguity
Accounting for twin demographic characteristics (i.e., age, gender, race/ethnicity) and scan type (i.e., multiband vs spiral) and nesting within twin families, we did not find any associations between neighborhood disadvantage and amygdala reactivity to threat (i.e., angry + fearful faces > shapes) or ambiguity (neutral faces > shapes) that survived correction for multiple comparisons using our registered voxel-wise threshold of p < .001. Of note, the cluster correction method applied to control for multiple comparisons results in a trade-off between the voxelwise significance level and the cluster extent threshold. On the one hand, a more lenient threshold (p < .01) demands a larger cluster size and ultimately results in a loss of spatial specificity, in which case it becomes difficult to detect effects that are highly concentrated within a region. On the other hand, a more stringent threshold (p < .001) may fail to detect weaker effects that are more diffuse across a particular region. Thus, researchers have argued that the currently accepted cluster correction approach tends to discriminate against intrinsically small anatomical regions, such as the amygdala, and clustering often fails to reveal an effect unless the statistical evidence is unusually strong (Chen et al., 2020). Due to these statistical challenges, some researchers advocate for the reporting of the continuous spectrum of statistical evidence. Therefore, as unregistered, exploratory analyses, we examined the associations using a voxel-wise threshold of p < .01 (cluster threshold = 16 voxels for the angry + fearful faces > shapes contrast and 14 voxels for the neutral faces > shapes contrast). Note that this change still resulted in an overall region of interest correction for multiple comparisons of p < .05, it just used a slightly less extreme p value (p < .01 rather than p < .001 at the voxel level), which resulted in a larger cluster threshold (16 and 14 voxel threshold instead of 1 voxel) to achieve correction for multiple corrections. Using this threshold, we found that neighborhood disadvantage was significantly associated with greater right amygdala reactivity to threat – i.e., angry + fearful faces > shapes (peak centered within right amygdala: [x, y, z] = [24, 4, −20]; T extent threshold = 3.13, k cluster size = 20; Table 3). These results do meet our registered goal of an ROI threshold of p < .05 corrected for multiple comparisons, but do not conform to our registered use of voxel-wise threshold of p < .001. Exploratory analyses revealed that these results were more related to amygdala reactivity to the contrast fearful faces > shapes (peak centered within right amygdala: [x, y, z] = [26, 4, −20]; T extent threshold = 2.82; k cluster size = 4; Table 3) than angry faces > shapes (no suprathreshold clusters); however, this cluster did not pass the cluster extent threshold at a voxelwise threshold of p < .01 and thus does not survive multiple correction thresholding of p < .05 in the region of interest (we present this only as evidence of which type of face may be most important in this association). When examining age as a potential moderator, we did not find any significant interaction between neighborhood disadvantage and age when predicting amygdala reactivity to threat or ambiguity.
Table 3.
Associations between neighborhood disadvantage and social processes and amygdala reactivity to threat and ambiguity.
| Variable/ Contrast | Voxelwise significance level (t-val/pthr) | # of voxels needed for cluster significance (.05) |
Left |
Right |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Coordinates |
Coordinates |
|||||||||||
| x | y | z | t-val | k | x | y | z | t-val | k | |||
| Area Deprivation Index | ||||||||||||
| Fearful + Angry Faces > Shapes | 3.29/.001 | 1 | no suprathreshold clusters | no suprathreshold clusters | ||||||||
| 2.58/.01 | 16 | no suprathreshold clusters | 24 | 4 | -20 | 3.13 | 20 | |||||
| Neutral Faces > Shapes | 3.29/.001 | 1 | no suprathreshold clusters | no suprathreshold clusters | ||||||||
| 2.58/.01 | 14 | -24 | 0 | -12 | -2.97 | 6 | no suprathreshold clusters | |||||
| Fearful Faces > Shapes | 3.29/.001 | 1 | no suprathreshold clusters | no suprathreshold clusters | ||||||||
| 2.58/.01 | 15 | no suprathreshold clusters | 26 | 4 | -20 | 2.81 | 4 | |||||
| Angry Faces > Shapes | 3.29/.001 | 1 | no suprathreshold clusters | no suprathreshold clusters | ||||||||
| 2.58/.01 | 18 | no suprathreshold clusters | no suprathreshold clusters | |||||||||
| Collective Efficacy | ||||||||||||
| Fearful + Angry Faces > Shapes | 3.29/.001 | 1 | no suprathreshold clusters | no suprathreshold clusters | ||||||||
| 2.58/.01 | 16 | no suprathreshold clusters | no suprathreshold clusters | |||||||||
| Neutral Faces > Shapes | 3.29/.001 | 1 | no suprathreshold clusters | no suprathreshold clusters | ||||||||
| 2.58/.01 | 14 | -24 | 2 | -16 | -3.20 | 9 | 28 | 0 | -12 | -3.03 | 10 | |
| Neighborhood Norms | ||||||||||||
| Fearful + Angry Faces > Shapes | 3.29/.001 | 1 | no suprathreshold clusters | no suprathreshold clusters | ||||||||
| 2.58/.01 | 16 | no suprathreshold clusters | no suprathreshold clusters | |||||||||
| Neutral Faces > Shapes | 3.29/.001 | 1 | no suprathreshold clusters | no suprathreshold clusters | ||||||||
| 2.58/.01 | 14 | no suprathreshold clusters | no suprathreshold clusters | |||||||||
Note. N = 512. pthr = p-value threshold. t-val = t extent threshold. k = cluster size. The number of voxels needed for significance refers to the cluster thresholds determined to pass significance at ROI p-value of .05, based on 2-sided tests using the nearest neighbor definition of “face and edge” (i.e., 3dClustSim command: NN=2).
4.3. Neighborhood social processes and amygdala reactivity to threat and ambiguity
When examining the associations between neighborhood social processes (i.e., collective efficacy and neighborhood norms) and amygdala reactivity to threat and ambiguity, no voxels survived correction for multiple comparisons using the registered voxel-wise threshold of p < .001. Consistent with Aim 1, we also ran exploratory analyses with a voxel threshold of p < .01. In these unregistered exploratory analyses, neither neighborhood norms nor collective efficacy were significantly associated with amygdala reactivity to threat or ambiguity. Age did not significantly moderate the associations between neighborhood social processes and amygdala reactivity to threat or ambiguity.
4.4. Supplementary whole brain analysis
Accounting for twin demographic characteristics (i.e., age, gender, race/ethnicity) and scan type (i.e., multiband vs spiral), we did not find any associations between neighborhood disadvantage and whole brain reactivity to threat (i.e., angry + fearful faces > shapes) or ambiguity (neutral faces > shapes). Also, we did not find any associations between neighborhood collective efficacy and whole brain reactivity to threat; however, collective efficacy was significantly related to decreased reactivity in the left and right fusiform gyrus in response to ambiguity (i.e., neutral faces > shapes) (peak centered in right fusiform gyrus: [x, y, z] = [46, −40, −24]; T extent threshold = −4.45; k cluster size = 226; peak centered in left fusiform gyrus: [x, y, z] = [−44, −34 −18]; T extent threshold = −5.09; k cluster size = 166, Table S1). Lastly, neighborhood norms were not associated with whole brain reactivity to ambiguity but were associated with increased reactivity in the right precentral gyrus in response to threat (peak centered: [x, y, z] = [62, −12, 34]; T extent threshold = 5.39; k cluster size = 903).
4.5. Examining neighborhood social processes as moderators of the association between neighborhood disadvantage and amygdala reactivity to threat and ambiguity
Finally, we examined neighborhood norms and collective efficacy as moderators of the association between neighborhood disadvantage and amygdala reactivity to threat and ambiguity. We first assessed whether the interaction between each neighborhood social process and neighborhood disadvantage was significantly associated with amygdala reactivity to threat or ambiguity. To probe any significant interactions, we used the model constraint command in Mplus to calculate the simple slopes for each value of the moderator (i.e., the mean and +/- 1 SD from the mean) by computing the direct effect of X on Y, conditional on each value of the moderator (i.e., b1 + b3W). Controlling for twin demographic characteristics (i.e., age, gender, race/ethnicity) and scan type (i.e., multiband vs spiral), we did not find a significant interaction between neighborhood social processes and neighborhood disadvantage in predicting amygdala reactivity to ambiguity. However, our results did reveal a significant interaction effect between neighborhood norms and neighborhood disadvantage predicting amygdala reactivity to threat (peak centered in the right amygdala: [x, y, z] = [22, 6, −16]; T extent threshold = −3.47; k cluster size = 1). Specifically, we found that neighborhood disadvantage was only significantly associated with greater right amygdala reactivity to threat at low (peak centered in right amygdala: [x, y, z] = [26, −2, −14]; T extent threshold = 3.56; k cluster size = 24), but not average or high levels of neighborhood norms. That is, neighborhood disadvantage was only associated with greater right amygdala reactivity to threat when neighborhood informants perceived norms in the neighborhood to be more permissive regarding general safety and management. Lastly, we also found a trend-level interaction between neighborhood disadvantage and collective efficacy predicting amygdala reactivity to threat (peak centered in the right amygdala: [x, y, z] = [24, 2, −22]; T extent threshold = −3.26; k cluster size = 1). Similar to the results for neighborhood norms, we found that neighborhood disadvantage was only associated with greater right amygdala reactivity to threat at low (peak centered in right amygdala: [x, y, z] = [24, 2, −22]; T extent threshold = 3.95; k cluster size = 33), but not average or high levels of collective efficacy. Thus, neighborhood norms appeared to buffer the association between neighborhood impoverishment and amygdala reactivity to threat in that neighborhood impoverishment was only associated with amygdala reactivity to threat when positive neighborhood norms were low. A very similar pattern (though only at a trend level) was seen for collective efficacy.
5. Discussion
In the current study, we examined the impact of neighborhood disadvantage and neighborhood social processes on amygdala reactivity to threat and ambiguity in a sample of adolescent twins with enrichment for families living in impoverished neighborhoods. Within this sample, we did not find significant associations between neighborhood disadvantage or neighborhood social processes and amygdala reactivity to threat or ambiguity when correcting for multiple comparisons at our originally registered stringent voxel-wise threshold. When examining the models at an unregistered voxel-wise threshold that still corrected for multiple comparisons, we found that neighborhood disadvantage was associated with increased amygdala reactivity to threat (i.e., angry and fearful faces). Our supplementary whole brain analyses revealed that neighborhood collective efficacy was related to less reactivity in the left and right fusiform gyrus in response to neutral faces, and neighborhood norms were associated with greater reactivity in the right precentral gyrus in response to angry and fearful faces. Lastly, neighborhood norms proved to be an important moderator in the association between neighborhood disadvantage and amygdala reactivity to threat, such that youth living in disadvantaged neighborhoods only exhibited greater amygdala reactivity to threat when neighborhood informants reported low endorsement of positive norms regarding neighborhood management and safety. Taken together, our results provide evidence that the neighborhood context impacts brain function during socioemotional processing, though several hypothesized direct associations between neighborhood social processes and amygdala reactivity were not supported and the association between neighborhood disadvantage and amygdala reactivity to threat may be contingent on neighborhood social processes.
Although we did not find a significant association between neighborhood disadvantage and amygdala reactivity at the more stringent voxel-wise significance level (p < .001) that we proposed, significant positive associations were identified using a lower voxel-wise (p < .01) threshold with cluster correction for multiple comparisons. In previous investigations, we have used a p < .001 voxel-wise threshold to identify activation that may be larger in effect size but smaller in cluster size. However, in practice, the cluster correction method employed may be less than ideal in spatially small regions, like the amygdala (Chen et al., 2020). Thus, although we tested our primary hypothesis using a different voxel-wise threshold than we had registered, we are careful in interpreting results that do not precisely conform to our pre-registered analysis and, thus, could be adding “degrees of freedom” to our analytic pipeline.
Our unregistered analysis at a different voxelwise threshold revealed that greater neighborhood disadvantage was related to greater right amygdala reactivity to threat. This finding is consistent with a growing literature, which finds that neighborhood disadvantage is linked to structural and functional changes within the amygdala and the broader corticolimbic circuit (Bell et al., 2021, Gard et al., 2020, Ramphal et al., 2020, Whittle et al., 2017). Neighborhood disadvantage has been longitudinally linked to larger left and right amygdala volumes in adolescence (Whittle et al., 2017) and increased amygdala reactivity to emotional faces in adolescence and adulthood (Gard et al., 2017, Gard et al., 2020). Importantly, these studies linking neighborhood disadvantage to amygdala function also report findings at similar lower p value voxelwise thresholds, perhaps suggesting that effects of neighborhood disadvantage on amygdala structure and function are weaker and more diffuse than originally hypothesized. Moreover, recent studies have found that neighborhood disadvantage is associated with differences in structural and functional connectivity between the amygdala and regions of the prefrontal cortex (PFC). For example, a recent cross-sectional study of participants aged 5–25 years, found weaker amygdala-ventromedial PFC functional connectivity in younger participants from more disadvantaged neighborhoods, and a recent longitudinal investigation found that greater neighborhood disadvantage in adolescence was associated with less white matter connectivity between brain regions supporting emotional processing in adulthood, including the prefrontal cortex, amygdala, hippocampus, and hypothalamus (Bell et al., 2021, Ramphal et al., 2020). Thus, our unregistered findings align with the existing evidence that the broader neighborhood context is critical to amygdala function and functional connectivity within the broader corticolimbic circuit. These findings have potential policy implications as they suggest that broader, non-familial contexts, exert important impacts on the brain, which may undermine positive behavioral development.
Key for policymakers is the identification of specific ‘active ingredients’ within disadvantaged neighborhoods that may be driving this association between neighborhood disadvantage and amygdala function. Thus, we investigated whether neighborhood social processes (i.e., collective efficacy and norms) were associated with altered amygdala reactivity to threat and ambiguity. Our results did not reveal any significant direct associations between neighborhood norms or collective efficacy and amygdala reactivity. It is not clear why these analyses did not support our hypotheses. It could be that our novel use of neighborhood informants impacted results. Though the use of neighborhood informants is a strength in assessing the collective assessment of neighborhood social processes, it may not reflect participant’s own perceptions of these social processes and, perhaps, own perceptions are most important. Alternatively, it could be that positive social dynamics in the neighborhood have a smaller impact than more extreme negative exposures (e.g., exposure to community violence), particularly when focused on socioemotional functioning within affective-related brain regions. Another possibility is that positive social connections do not directly impact basic activation in areas like the amygdala, but rather help to support connectivity between affective and control regions. Indeed, a recent study of urban adolescents reported that youth’s perception of neighborhood collective efficacy was associated with stronger negative functional connectivity between the amygdala and medial PFC during the processing of fearful faces (Gard et al., 2021). Thus, in line with perspectives that circuit-level neural function more closely reflects observable behavior and cognition (Menon, 2011), it may be that neighborhood social processes are more likely to sculpt functional connections within the brain, such as corticolimbic connectivity, as opposed to activation within single regions.
In fact, studies are beginning to demonstrate that neighborhood disadvantage impacts how the brain integrates information within and across multiple neural systems. For example, a recent study found that compared to youth in more disadvantaged neighborhoods, youth from more advantaged neighborhoods displayed a stronger positive relationship between age and increased local segregation within the limbic, somatomotor, and ventral attention systems (Tooley et al., 2020). Also, another study found that neighborhood disadvantage was associated with reduced resting state functional connectivity within and between multiple neural networks, including connectivity between the default mode network and both higher-order (e.g., ventral attention network) and sensory networks (e.g., auditory network) and connectivity within the higher-order networks (Rakesh et al., 2021). Collectively, these studies suggest that whole brain network approaches may yield stronger effects of neighborhood disadvantage and neighborhood social processes on brain development compared to studies examining activation within singular brain regions.
Furthermore, as noted above, other ‘active ingredients’ within disadvantaged neighborhoods may exert stronger effects on the brain than neighborhood social processes, and these should be examined as potential mechanisms through which neighborhood disadvantage impacts youth brain development and associated cognitive and socioemotional outcomes. This may include increased exposure to physical hazards (e.g., pollution, toxicants, street traffic), higher rates of violent crime, decreased access to healthy food options and lower school quality (Evans, 2004, Leventhal and Brooks-Gunn, 2000). Neuroimaging studies are starting to examine these more proximal experiences as mechanisms driving the associations between neighborhood disadvantage and brain development. For example, exposure to pollution and toxicants, which is more common in disadvantaged neighborhoods due to structural inequalities, has been linked to decreased functional integration and segregation across neural circuits, indicating slower brain maturation within a sample of children exposed to greater traffic-related air pollution (Pujol et al., 2016). Also, exposure to community violence is more prevalent within disadvantaged neighborhoods and has been linked to smaller hippocampal volumes (Saxbe et al., 2018) and heightened amygdala reactivity to emotional faces (White et al., 2019). Importantly, several of these neighborhood stressors may impact the brain at different points throughout development; thus, longitudinal studies will be needed to elucidate which proximal neighborhood factors are most impactful at various stages of development and the current study may have had more modest findings by only focusing on a more distal measure of neighborhood disadvantage.
In supplementary whole brain analyses, we found that neighborhood collective efficacy was related to decreased reactivity in the left and right fusiform gyrus in response to neutral faces, and neighborhood norms were associated with increased activation of the right precentral gyrus in response to angry and fearful faces. Given that these results were part of a supplementary analysis, we are cautious in drawing any specific conclusions regarding the links between neighborhood social processes and activation within the specific brain regions discussed; however, examining the roles of these brain regions provides some preliminary insights. The precentral gyrus, classically described as a “higher order motor area,” is important for both motor and nonmotor cognitive functions (e.g., voluntary movement, mental operation tasks, spatial attention) (Tanaka et al., 2005). Moreover, the precentral gyrus has been implicated in socioemotional processes, including emotion regulation (Fitzgerald et al., 2017, Hallam et al., 2014) and face specificity and recognition (Watson et al., 2016). The fusiform gyrus has been implicated as a region specialized for visual expertize, especially face recognition (Haxby et al., 2002). Studies have found decreased activation of the fusiform gyrus when viewing threatening faces for individuals scoring high on negative affectivity (Kret et al., 2011), and increased activation when subjects employ emotion regulation strategies (Vrtička et al., 2011). Studies also report conflicting results regarding the association between childhood SES and fusiform gyrus activation, with some studies finding increased activation among individuals from lower SES families (Kim et al., 2015, White et al., 2019), while other studies report decreased activation (Rosen et al., 2018). Thus, the precentral gyrus and fusiform gyrus have been implicated in various cognitive and socioemotional processes. Future studies should attempt to clarify the precise role of these brain regions in socioemotional face processing and how these regions may relate to socioemotional experiences within the neighborhood (e.g., social cohesion and informal social control).
Finally, one of the most notable findings in this study is the significant interaction between neighborhood social processes and neighborhood disadvantage predicting amygdala reactivity to threat. Although we did not find direct effects of neighborhood social processes on amygdala reactivity, the results of our moderation analyses revealed that neighborhood social processes serve an important moderating role in the association between neighborhood disadvantage and amygdala reactivity to threat. In this sample, greater levels of social cohesion and informal social control in the neighborhood and more protective norms regarding general neighborhood safety and management decreased the link between neighborhood disadvantage and heightened amygdala reactivity to threat. These results suggest that positive neighborhood social processes may help buffer the impacts of neighborhood disadvantage on the brain, or alternatively, that neighborhood disadvantage is most risky when neighbors show low positive norms (or low social cohesion). These findings are consistent with a host of developmental research showing that positive neighborhood social processes can protect youth exposed to neighborhood disadvantage from negative behavioral outcomes (Browning et al., 2014, Dawson et al., 2019, Fagan et al., 2014, Gard et al., 2021, Kingsbury et al., 2019). Moreover, these findings are consistent with a very recent study (in a separate sample) showing that neighborhood social ties buffered the behavioral and neural impacts of deadly gun violence (Gard et al., 2021). Although these results were exploratory, our findings contribute to an emerging literature highlighting the potential protective effects of neighborhood social processes in decreasing the impact of neighborhood structural disadvantage on amygdala reactivity. These results are especially encouraging because they suggest that social processes may offset the impact of larger structural issues within the neighborhood and help us better understand why so many youth are resilient, even in the face of adversity.
The present study included several methodological strengths, including a large population-based sample with a specific sampling frame that included families from rural, urban, and suburban communities, with oversampling for families living in impoverished neighborhoods. In addition, we included census-reported data on neighborhood disadvantage and a novel assessment of neighborhood social processes via reports from sets of randomly selected individuals residing in the families’ neighborhoods (i.e., neighbors). Still, the current study is not without limitations. First, a major limitation is that the analyses were cross-sectional. As MTwiNS is an ongoing longitudinal study, we plan to investigate associations between neighborhood resources and brain function longitudinally in future studies. Second, we only reported associations between neighborhood disadvantage and amygdala reactivity at a different voxel-wise threshold than we had registered. As previously mentioned, this may be due to the statistical challenges that researchers face when employing cluster-based methods to control for multiple comparisons (Chen et al., 2020). A more lenient voxel threshold requires a larger cluster size making it difficult to detect activation that may be larger in effect size but smaller in cluster size; whereas a more stringent threshold may fail to detect weaker effects that are more diffuse within a region. Additionally, researchers have recently drawn attention to the limited reliability of task-based fMRI, in which case, the neighborhood effects examined here would need be quite large to be detected and may not be detected reliably within a sample of this size (Elliott et al., 2020). Although our study boasts a relatively large sample size (N = 708) compared to other fMRI studies, researchers recently demonstrated that neuroimaging studies linking the brain to behavioral phenotypes stabilize and become more reproducible with sample sizes of N 2000 (Marek et al., 2020). Thus, future replication of these findings is highly encouraged, particularly in even larger samples.
Despite these limitations, we provide important evidence that neighborhood disadvantage may impact amygdala reactivity during socioemotional processing, but also that neighborhood social processes may buffer these impacts. Our findings add to a growing literature, which provides a model by which a seemingly distant experience, neighborhood disadvantage, affects the developing brain, moving us a step closer to understanding the pathways through which neighborhood disadvantage undermines positive developmental outcomes. Moreover, although our analyses did not provide evidence that neighborhood social processes exert a strong direct effect on amygdala function, we do provide evidence of the protective role that neighborhood social processes may play in decreasing the link between neighborhood disadvantaged and heightened amygdala reactivity. These findings help to elucidate why some youth are resilient even in the face of adversity and point to the power of positive social processes.
Data Statement
The protocol is registered via OSF, data shared (via NIMH requirements) through NDA, and unthresholded t-maps are available via neurovault:
Open Science Framework Registered Protocol: 10.17605/OSF.IO/KXPFT.
Publicly available study data: https://nda.nih.gov/edit_collection.html?id=2818.
Unthresholded Whole-Brain T Maps (Neurosynth): https://neurovault.org/collections/11604/.
Funding
Research reported in this publication related to MTwiNS was supported by the National Institute of Mental Health of the National Institutes of Health (NIMH) and the Office of the Director National Institute of Health (OD), under Award Number UG3MH114249 and the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number R01HD093334 to SAB and LWH. The content is solely the responsibility of the authors and does not necessarily represent the views of the National Institutes of Health. Funding was provided by a NARSAD young Investigator Grant from the Brain and Behavior Foundation (to LWH). GLS was supported by a National Science Foundation Graduate Research Fellowship and R01HD093334-S1. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to thank the staff of the TBED-C and MTwiNS studies fortheir hard work, and we thank the families who participated in TBED-C and MTwiNS for sharing their lives with us.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.dcn.2022.101061.
Appendix A. Supplementary material
Supplementary material
.
References
- Aneshensel C.S., Sucoff C.A. The neighborhood context of adolescent mental health. J. Health Soc. Behav. 1996;37(4):293. doi: 10.2307/2137258. [DOI] [PubMed] [Google Scholar]
- Bell K.L., Purcell J.B., Harnett N.G., Goodman A.M., Mrug S., Schuster M.A., Elliott M.N., Emery S.T., Knight D.C. White matter microstructure in the young adult brain varies with neighborhood disadvantage in adolescence. Neuroscience. 2021;466:162–172. doi: 10.1016/j.neuroscience.2021.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody G.H., Conger R., Gibbons F.X., Ge X., McBride Murry V., Gerrard M., Simons R.L. The influence of neighborhood disadvantage, collective socialization, and parenting on African American children’s affiliation with deviant peers. Child Dev. 2001;72(4):1231–1246. doi: 10.1111/1467-8624.00344. [DOI] [PubMed] [Google Scholar]
- Browning C.R., Gardner M., Maimon D., Brooks-Gunn J. Collective efficacy and the contingent consequences of exposure to life-threatening violence. Dev. Psychol. 2014;50(7):1878–1890. doi: 10.1037/a0036767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt S.A., Clark D.A., Pearson A.L., Klump K.L., Neiderhiser J.M. Do neighborhood social processes moderate the etiology of youth conduct problems? Psychol. Med. 2019:1–11. doi: 10.1017/S0033291719001521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt S.A., Klump K.L. The Michigan State University Twin Registry (MSUTR): an update. Twin Res. Hum. Genet. 2013;16(1):344–350. doi: 10.1017/thg.2012.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt S.A., Klump K.L. The Michigan State University Twin Registry (MSUTR): 15 years of twin and family research. Twin Res. Hum. Genet. 2019;22(6):741–745. doi: 10.1017/thg.2019.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt S.A., Klump K.L., Gorman-Smith D., Neiderhiser J.M. Neighborhood disadvantage alters the origins of children’s nonaggressive conduct problems. Clin. Psychol. Sci. 2016;4(3):511–526. doi: 10.1177/2167702615618164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt S.A., Pearson A.L., Carroll S., Klump K.L., Neiderhiser J.M. Child antisocial behavior is more environmental in origin in disadvantaged neighborhoods: evidence across residents’ perceptions and geographic scales in two samples. J. Abnorm. Child Psychol. 2020;48(2):265–276. doi: 10.1007/s10802-019-00587-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt S.A., Slawinski B.L., Klump K.L. Are there sex differences in the etiology of youth antisocial behavior? J. Abnorm. Psychol. 2018;127(1):66–78. doi: 10.1037/abn0000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B.J., Cannonier T., Conley M.I., Cohen A.O., Barch D.M., Heitzeg M.M., Soules M.E., Teslovich T., Dellarco D.V., Garavan H., Orr C.A., Wager T.D., Banich M.T., Speer N.K., Sutherland M.T., Riedel M.C., Dick A.S., Bjork J.M., Thomas K.M., Dale A.M. The Adolescent Brain Cognitive Development (ABCD) study: Imaging acquisition across 21 sites. Dev. Cogn. Neurosci. 2018;32:43–54. doi: 10.1016/j.dcn.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Taylor P.A., Cox R.W., Pessoa L. Fighting or embracing multiplicity in neuroimaging? Neighborhood leverage versus global calibration. NeuroImage. 2020;206 doi: 10.1016/j.neuroimage.2019.116320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R.W. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cox R.W., Chen G., Glen D.R., Reynolds R.C., Taylor P.A. FMRI clustering and false-positive rates. Proc. Natl. Acad. Sci. USA. 2017;114(17) doi: 10.1073/pnas.1614961114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M., Whalen P.J. The amygdala: vigilance and emotion. Mol. Psychiatry. 2001;6(1):13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Dawson C.T., Wu W., Fennie K.P., Ibañez G., Cano M.Á., Pettit J.W., Trepka M.J. Perceived neighborhood social cohesion moderates the relationship between neighborhood structural disadvantage and adolescent depressive symptoms. Health Place. 2019;56:88–98. doi: 10.1016/j.healthplace.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkins I.J., McGue M., Iacono W.G. Genetic and environmental influences on parent–son relationships: evidence for increasing genetic influence during adolescence. Dev. Psychol. 1997;33(2):351–363. doi: 10.1037/0012-1649.33.2.351. [DOI] [PubMed] [Google Scholar]
- Elliott D.S., Wilson W.J., Huizinga D., Sampson R.J., Elliott A., Rankin B. The effects of neighborhood disadvantage on adolescent development. J. Res. Crime. Delinquency. 1996;33(4):389–426. doi: 10.1177/0022427896033004002. [DOI] [Google Scholar]
- Elliott M.L., Knodt A.R., Ireland D., Morris M.L., Poulton R., Ramrakha S., Sison M.L., Moffitt T.E., Caspi A., Hariri A.R. What Is the test-retest reliability of common task-functional MRI measures? New empirical evidence and a meta-analysis. Psychol. Sci. 2020;31(7):792–806. doi: 10.1177/0956797620916786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A., Klemenhagen K.C., Dudman J.T., Rogan M.T., Hen R., Kandel E.R., Hirsch J. Individual differences in trait anxiety predict the response of the basolateral amygdala to unconsciously processed fearful faces. Neuron. 2004;44(6):1043–1055. doi: 10.1016/j.neuron.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Evans G.W. The environment of childhood poverty. Am. Psychol. 2004;59(2):77–92. doi: 10.1037/0003-066X.59.2.77. [DOI] [PubMed] [Google Scholar]
- Fagan A.A., Wright E.M., Pinchevsky G.M. The protective effects of neighborhood collective efficacy on adolescent substance use and violence following exposure to violence. J. Youth Adolesc. 2014;43(9):1498–1512. doi: 10.1007/s10964-013-0049-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk E.B., Hyde L.W., Mitchell C., Faul J., Gonzalez R., Heitzeg M.M., Keating D.P., Langa K.M., Martz M.E., Maslowsky J., Morrison F.J., Noll D.C., Patrick M.E., Pfeffer F.T., Reuter-Lorenz P.A., Thomason M.E., Davis-Kean P., Monk C.S., Schulenberg J. What is a representative brain? Neuroscience meets population science. Proc. Natl. Acad. Sci. USA. 2013;110(44):17615–17622. doi: 10.1073/pnas.1310134110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk C.F. Are robust standard errors the best approach for interval estimation with nonnormal data in structural equation modeling? Struct. Equ. Model. A Multidiscip. J. 2018;25(2):244–266. doi: 10.1080/10705511.2017.1367254. [DOI] [Google Scholar]
- Fitzgerald J.M., Phan K.L., Kennedy A.E., Shankman S.A., Langenecker S.A., Klumpp H. Prefrontal and amygdala engagement during emotional reactivity and regulation in generalized anxiety disorder. J. Affect. Disord. 2017;218:398–406. doi: 10.1016/j.jad.2017.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P., Placentino A., Carletti F., Landi P., Allen P., Surguladze S., Benedetti F., Abbamonte M., Gasparotti R., Barale F., Perez J., McGuire P., Politi P. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J. Psychiatry Neurosci. 2009;34(6):418–432. [PMC free article] [PubMed] [Google Scholar]
- Gard, A.M., Brooks-Gunn, J., McLanahan, S., Mitchell, C., Monk, C.S., Hyde, L.W., 2021. October 13). Deadly gun violence, neighborhood collective efficacy. and adolescent neurobehavioral outcomes. doi: 10.31234/osf.io/k37rf. [DOI] [PMC free article] [PubMed]
- Gard A.M., Maxwell A.M., Shaw D.S., Mitchell C., Brooks-Gunn J., McLanahan S.S., Forbes E.E., Monk C.S., Hyde L.W. Beyond family-level adversities: exploring the developmental timing of neighborhood disadvantage effects on the brain. Dev. Sci. 2020 doi: 10.1111/desc.12985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard A.M., Shaw D.S., Forbes E.E., Hyde L.W. Amygdala reactivity as a marker of differential susceptibility to socioeconomic resources during early adulthood. Dev. Psychol. 2018;54(12):2341–2355. doi: 10.1037/dev0000600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard A.M., Waller R., Shaw D.S., Forbes E.E., Hariri A.R., Hyde L.W. The long reach of early adversity: parenting, stress, and neural pathways to antisocial behavior in adulthood. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2017;2(7):582–590. doi: 10.1016/j.bpsc.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham J.W. Missing data analysis: making it work in the real world. Annu. Rev. Psychol. 2009;60(1):549–576. doi: 10.1146/annurev.psych.58.110405.085530. [DOI] [PubMed] [Google Scholar]
- Hallam G.P., Webb T.L., Sheeran P., Miles E., Niven K., Wilkinson I.D., Hunter M.D., Woodruff P.W.R., Totterdell P., Farrow T.F.D. The neural correlates of regulating another person’s emotions: an exploratory fMRI study. Front. Hum. Neurosci. 2014;8:376. doi: 10.3389/fnhum.2014.00376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallquist M.N., Wiley J.F. Mplusautomation: an R package for facilitating large-scale latent variable analyses in mplus. Struct. Equ. Model. A Multidiscip. J. 2018;25(4):621–638. doi: 10.1080/10705511.2017.1402334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri A.R., Mattay V.S., Tessitore A., Kolachana B., Fera F., Goldman D., Egan M.F., Weinberger D.R. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297(5580):400. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Haxby J.V., Hoffman E.A., Gobbini M.I. Human neural systems for face recognition and social communication. Soc. Anxiety.: Lab. Stud. Clin. Pract. 2002;51(1):59–67. doi: 10.1016/S0006-3223(01)01330-0. [DOI] [PubMed] [Google Scholar]
- Hein T.C., Monk C.S. Research Review: neural response to threat in children, adolescents, and adults after child maltreatment – a quantitative meta-analysis. J. Child Psychol. Psychiatry. 2017;58(3):222–230. doi: 10.1111/jcpp.12651. [DOI] [PubMed] [Google Scholar]
- Henry D., Gorman-Smith D., Schoeny M., Tolan P. “Neighborhood matters”: assessment of neighborhood social processes. Am. J. Community Psychol. 2014;54(3–4):187–204. doi: 10.1007/s10464-014-9681-z. [DOI] [PubMed] [Google Scholar]
- Hyde L.W., Shaw D.S., Murray L., Gard A., Hariri A.R., Forbes E.E. Dissecting the role of amygdala reactivity in antisocial behavior in a sample of young, low-income, urban men. Clin. Psychol. Sci. 2016;4(3):527–544. doi: 10.1177/2167702615614511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson N., Denny S., Sheridan J., Zhao J., Ameratunga S. The role of neighborhood disadvantage, physical disorder, and collective efficacy in adolescent alcohol use: a multilevel path analysis. Health Place. 2016;41:24–33. doi: 10.1016/j.healthplace.2016.07.005. [DOI] [PubMed] [Google Scholar]
- Javanbakht A., King A.P., Evans G.W., Swain J.E., Angstadt M., Phan K.L., Liberzon I. Childhood poverty predicts adult amygdala and frontal activity and connectivity in response to emotional faces. Front. Behav. Neurosci. 2015;9:154. doi: 10.3389/fnbeh.2015.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P., Ho S.S., Evans G.W., Liberzon I., Swain J.E. Childhood social inequalities influences neural processes in young adult caregiving. Dev. Psychobiol. 2015;57(8):948–960. doi: 10.1002/dev.21325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kind A.J.H., Jencks S., Brock J., Yu M., Bartels C., Ehlenbach W., Greenberg C., Smith M. Neighborhood socioeconomic disadvantage and 30-day rehospitalization. Ann. Intern. Med. 2014;161(11):765–774. doi: 10.7326/M13-2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury M., Clayborne Z., Colman I., Kirkbride J.B. The protective effect of neighbourhood social cohesion on adolescent mental health following stressful life events. Psychol. Med. 2019:1–8. doi: 10.1017/S0033291719001235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohen D.E., Leventhal T., Dahinten V.S., McIntosh C.N. Neighborhood disadvantage: pathways of effects for young children. Child Dev. 2008;79(1):156–169. doi: 10.1111/j.1467-8624.2007.01117.x. [DOI] [PubMed] [Google Scholar]
- Kret M.E., Denollet J., Grèzes J., de Gelder B. The role of negative affectivity and social inhibition in perceiving social threat: an fMRI study. Neuropsychologia. 2011;49(5):1187–1193. doi: 10.1016/j.neuropsychologia.2011.02.007. [DOI] [PubMed] [Google Scholar]
- LeDoux J. The emotional brain, fear, and the amygdala. Cell. Mol. Neurobiol. 2003;23(4):727–738. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J.E. Emotion circuits in the brain. Annu. Rev. Neurosci. 2000;23(1):155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Leventhal T., Brooks-Gunn J. The neighborhoods they live in: the effects of neighborhood residence on child and adolescent outcomes. Psychol. Bull. 2000;126(2):309–337. doi: 10.1037/0033-2909.126.2.309. [DOI] [PubMed] [Google Scholar]
- Madhyastha T., Peverill M., Koh N., McCabe C., Flournoy J., Mills K., King K., Pfeifer J., McLaughlin K.A. Current methods and limitations for longitudinal fMRI analysis across development. Dev. Cogn. Neurosci. 2018;33:118–128. doi: 10.1016/j.dcn.2017.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian J.A., Laurienti P.J., Kraft R.A., Burdette J.H. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19(3):1233–1239. doi: 10.1016/S1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Manuck S.B., Brown S.M., Forbes E.E., Hariri A.R. Temporal stability of individual differences in amygdala reactivity. Am. J. Psychiatry. 2007;164(10):1613–1614. doi: 10.1176/appi.ajp.2007.07040609. [DOI] [PubMed] [Google Scholar]
- Marek S., Tervo-Clemmens B., Calabro F.J., Montez D.F., Kay B.P., Hatoum A.S., Donohue M.R., Foran W., Miller R.L., Feczko E., Miranda-Dominguez O., Graham A.M., Earl E.A., Perrone A.J., Cordova M., Doyle O., Moore L.A., Conan G., Uriarte J., Dosenbach N.U.F. Towards reproducible brain-wide association studies. BioRxiv. 2020 doi: 10.1101/2020.08.21.257758. [DOI] [Google Scholar]
- Marusak H.A., Zundel C.G., Brown S., Rabinak C.A., Thomason M.E. Convergent behavioral and corticolimbic connectivity evidence of a negativity bias in children and adolescents. Soc. Cogn. Affect. Neurosci. 2017;12(4):517–525. doi: 10.1093/scan/nsw182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy D.C., Roy A.L., Raver C.C. Neighborhood crime as a predictor of individual differences in emotional processing and regulation. Dev. Sci. 2016;19(1):164–174. doi: 10.1111/desc.12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn. Sci. 2011;15(10):483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Monk C.S. Vol. 20. Cambridge Core; 2008. The development of emotion-related neural circuitry in health and psychopathology; pp. 1231–1250. (Development and Psychopathology). [DOI] [PubMed] [Google Scholar]
- Morenoff J.D., Sampson R.J., Raudenbush S.W. Neighborhood inequality, collective efficacy, and the spatial dynamics of urban violence. Criminology. 2001;39(3):517–558. doi: 10.1111/j.1745-9125.2001.tb00932.x. [DOI] [Google Scholar]
- Musick K., Seltzer J.A., Schwartz C.R. Neighborhood norms and substance use among teens. Soc. Sci. Res. 2008;37(1):138–155. doi: 10.1016/j.ssresearch.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner K.N., Silvers J.A., Buhle J.T. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Ann. N. Y. Acad. Sci. 2012;1251(1):E1–E24. doi: 10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odgers C.L., Moffitt T.E., Tach L.M., Sampson R.J., Taylor A., Matthews C.L., Caspi A. The protective effects of neighborhood collective efficacy on british children growing up in deprivation: a developmental analysis. Dev. Psychol. 2009;45(4):942–957. doi: 10.1037/a0016162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pager D., Shepherd H. The sociology of discrimination: racial discrimination in employment, housing, credit, and consumer markets. Annu. Rev. Sociol. 2008;34(1):181–209. doi: 10.1146/annurev.soc.33.040406.131740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak S.D., Cicchetti D., Hornung K., Reed A. Recognizing emotion in faces: developmental effects of child abuse and neglect. Dev. Psychol. 2000;36(5):679–688. doi: 10.1037/0012-1649.36.5.679. [DOI] [PubMed] [Google Scholar]
- Pujol J., Martínez-Vilavella G., Macià D., Fenoll R., Alvarez-Pedrerol M., Rivas I., Forns J., Blanco-Hinojo L., Capellades J., Querol X., Deus J., Sunyer J. Traffic pollution exposure is associated with altered brain connectivity in school children. NeuroImage. 2016;129:175–184. doi: 10.1016/j.neuroimage.2016.01.036. [DOI] [PubMed] [Google Scholar]
- Rakesh D., Seguin C., Zalesky A., Cropley V., Whittle S. Associations between neighborhood disadvantage, resting-state functional connectivity, and behavior in the adolescent brain cognitive development study: the moderating role of positive family and school environments. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2021;6(9):877–886. doi: 10.1016/j.bpsc.2021.03.008. [DOI] [PubMed] [Google Scholar]
- Ramey D.M., Harrington N. Early exposure to neighborhood crime and child internalizing and externalizing behaviors. Health Place. 2019;57:228–237. doi: 10.1016/j.healthplace.2019.04.010. [DOI] [PubMed] [Google Scholar]
- Ramphal B., DeSerisy M., Pagliaccio D., Raffanello E., Rauh V., Tau G., Posner J., Marsh R., Margolis A.E. Associations between amygdala-prefrontal functional connectivity and age depend on neighborhood socioeconomic status. Cereb. Cortex Commun. 2020;1(1) doi: 10.1093/texcom/tgaa033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed E., Silverman J.G., Raj A., Decker M.R., Miller E. Male perpetration of teen dating violence: associations with neighborhood violence involvement, gender attitudes, and perceived peer and neighborhood norms. J. Urban Health. 2011;88(2):226–239. doi: 10.1007/s11524-011-9545-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen M.L., Sheridan M.A., Sambrook K.A., Meltzoff A.N., McLaughlin K.A. Socioeconomic disparities in academic achievement: a multi-modal investigation of neural mechanisms in children and adolescents. NeuroImage. 2018;173:298–310. doi: 10.1016/j.neuroimage.2018.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson R.J., Groves W.B. Community structure and crime: testing social-disorganization theory. Am. J. Sociol. 1989;94(4):774–802. doi: 10.1086/229068. [DOI] [Google Scholar]
- Sampson R.J., Morenoff J.D., Gannon-Rowley T. Assessing “neighborhood effects”: social processes and new directions in research. Annu. Rev. Sociol. 2002;28(1):443–478. doi: 10.1146/annurev.soc.28.110601.141114. [DOI] [Google Scholar]
- Sampson R.J., Raudenbush S.W., Earls F. Neighborhoods and violent crime: a multilevel study of collective efficacy. Science. 1997;277(5328):918. doi: 10.1126/science.277.5328.918. [DOI] [PubMed] [Google Scholar]
- Sastry N. Oxford University Press; 2012. Neighborhood Effects on Children’s Achievement: A Review of Recent Research. [DOI] [Google Scholar]
- Saxbe D., Khoddam H., Piero L.D., Stoycos S.A., Gimbel S.I., Margolin G., Kaplan J.T. Community violence exposure in early adolescence: longitudinal associations with hippocampal and amygdala volume and resting state connectivity. Dev. Sci. 2018;21(6) doi: 10.1111/desc.12686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellers R.M., Copeland-Linder N., Martin P.P., Lewis R.L. Racial identity matters: the relationship between racial discrimination and psychological functioning in African American adolescents. J. Res. Adolesc. 2006;16(2):187–216. doi: 10.1111/j.1532-7795.2006.00128.x. [DOI] [Google Scholar]
- Shi H., Wang X., Yao S. Comparison of activation patterns between masking and inattention tasks: a coordinate-based meta-analysis of implicit emotional face processing. Front. Hum. Neurosci. 2013;7:459. doi: 10.3389/fnhum.2013.00459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh G.K. Area Deprivation and Widening Inequalities in US Mortality, 1969–1998. Am. J. Public Health. 2003;93(7):1137–1143. doi: 10.2105/AJPH.93.7.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S., Honda M., Sadato N. Modality-specific cognitive function of medial and lateral human brodmann area 6. J. Neurosci. 2005;25(2):496. doi: 10.1523/JNEUROSCI.4324-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Annie E. Casey Foundation. (2019). 2019 Kids count data book state trends in child well-being. Baltimore, MD. Retrieved from www.aecf.org.
- Tooley U.A., Mackey A.P., Ciric R., Ruparel K., Moore T.M., Gur R.C., Gur R.E., Satterthwaite T.D., Bassett D.S. Associations between neighborhood SES and functional brain network development. Cereb. Cortex. 2020;30(1):1–19. doi: 10.1093/cercor/bhz066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N., Hare T.A., Millner A., Gilhooly T., Zevin J.D., Casey B.J. Elevated amygdala response to faces following early deprivation. Dev. Sci. 2011;14(2):190–204. doi: 10.1111/j.1467-7687.2010.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N., Sheridan M. A review of adversity, the amygdala and the hippocampus: a consideration of developmental timing. Front. Hum. Neurosci. 2010;3:68. doi: 10.3389/neuro.09.068.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N., Tanaka J.W., Leon A.C., McCarry T., Nurse M., Hare T.A., Marcus D.J., Westerlund A., Casey B., Nelson C. The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Res. 2009;168(3):242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.-C., Fowler P.J. Social cohesion, neighborhood collective efficacy, and adolescent subjective well-being in urban and rural Taiwan. Am. J. Community Psychol. 2019;63(3–4):499–510. doi: 10.1002/ajcp.12324. [DOI] [PubMed] [Google Scholar]
- Watson R., Huis in ’t Veld E.M.J., de Gelder B. The neural basis of individual face and object perception. Front. Hum. Neurosci. 2016;10:66. doi: 10.3389/fnhum.2016.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen P.J., Phelps E.A. Guilford Press; 2009. The Human Amygdala. [Google Scholar]
- White S.F., Voss J.L., Chiang J.J., Wang L., McLaughlin K.A., Miller G.E. Exposure to violence and low family income are associated with heightened amygdala responsiveness to threat among adolescents. Dev. Cogn. Neurosci. 2019;40 doi: 10.1016/j.dcn.2019.100709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle S., Vijayakumar N., Simmons J.G., Dennison M., Schwartz O., Pantelis C., Sheeber L., Byrne M.L., Allen N.B. Role of positive parenting in the association between neighborhood social disadvantage and brain development across adolescence. JAMA Psychiatry. 2017;74(8):824–832. doi: 10.1001/jamapsychiatry.2017.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley M.E., Grogan-Kaylor A., Gilster M.E., Karb R.A., Gant L.M., Reischl T.M., Alaimo K. Neighborhood social capital, poor physical conditions, and school achievement. Child. Sch. 2008;30(3):133–145. doi: 10.1093/cs/30.3.133. [DOI] [Google Scholar]
- Wright E.M., Fagan A.A. The cycle of violence in context: exploring the moderating roles of neighborhood disadvantage and cultural norms. Criminology. 2013;51(2):217–249. doi: 10.1111/1745-9125.12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y., Leventhal T., Brooks-Gunn J., Earls F.J. Neighborhood residence and mental health problems of 5- to 11-year-olds. Arch. Gen. Psychiatry. 2005;62(5):554–563. doi: 10.1001/archpsyc.62.5.554. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material