Abstract
Discussion of the hematologic complications of vaccination for severe acute respiratory syndrome coronavirus-2 (COVID-19) has primarily focused on the development of vaccine-associated immune thrombosis with thrombocytopenia (VITT). Other hematologic complications are uncommon. We report the case of a patient who developed immunoglobulin G (IgG)-mediated autoimmune hemolytic anemia (AIHA) after the Moderna COVID-19 messenger ribonucleic acid (mRNA) vaccine.
Keywords: autoimmune hemolytic anemia, COVID, COVID vaccine, hematology oncology, hemolysis, rheumatology
Introduction
Vaccination of a large proportion of the population is an effective approach for control of the COVID-19 pandemic. Messenger RNA (mRNA) vaccines encoding the spike protein of the coronavirus are a major tool in this vaccination campaign. These vaccines are generally safe and effective, with the most common side effects being local pain, fatigue, and myalgias. 1 Attention to hematologic complications has largely focused on rare but severe cases of vaccine-associated thrombosis with thrombocytopenia (VITT). 2 Reports of other hematologic complications are uncommon. We report the case of a patient who presented with moderate immunoglobulin G (IgG)-mediated autoimmune hemolytic anemia (AIHA) after receiving the Moderna mRNA-1273 COVID-19 vaccine.
Case Presentation
The patient is a 66-year-old woman with long-term psoriatic arthritis on adalimumab therapy for more than 5 years. At a routine clinic visit, she was found to have hemoglobin (Hgb) 9.9 g/dL/hematocrit (Hct) 29.4% and total bilirubin 2.5 mg/dL with direct bilirubin 0.7 mg/dL (Table 1). At her previous visit 4 months earlier, Hgb/Hct were normal at 14.0 g/dL/39.9% with normal total bilirubin 0.9 mg/dL. Leukocyte and platelet counts were normal. During the clinic visit, she was completely asymptomatic. Additional laboratory testing a few days later confirmed anemia and hyperbilirubinemia. Further testing showed an elevated absolute reticulocyte count at 152 000/µL (normal range 50 000-100 000/µL), an elevated serum lactate dehydrogenase (LDH) concentration at 282 U/L (normal < 246 U/L) and serum haptoglobin concentration below the measured range (<30 mg/dL). Physical examination at the time of further testing showed only slight uvular icterus. A review of the peripheral blood smear by a hematologist showed a moderate number of spherocytes. Direct antiglobulin testing (DAT) showed 4+ IgG without C3, confirming IgG-mediated (warm) AIHA diagnosis.
Table 1.
Table 1 Showing Her Laboratory Values Before and After Moderna Vaccine.
| Parameters | Laboratory values before the vaccine | Laboratory values after the vaccine | Laboratory values after the initiation of steroids | Reference ranges |
|---|---|---|---|---|
| Hemoglobin | 14.0 g/dL | 9.9 g/dL | 10.6 g/dL | 11.1-15.9 g/dL |
| Hematocrit | 41.7% | 29.5% | 32.8% | 34-46.6% |
| Total bilirubin | 0.9 mg/dL | 2.0 mg/dL | 1.5 mg/dL | 0.2-1.2 mg/dL |
| Direct bilirubin | N/A | 0.6 mg/dL | N/A | <0.3 mg/dL |
| LDH (lactate dehydrogenase) | N/A | 282 U/L | N/A | 120-246 U/L |
| Haptoglobin | N/A | <30 mg/dL | N/A | 30-200 mg/dL |
| Absolute reticulocyte count | N/A | 152 000/µL | 83 400/µL | 50 000-100 000/µL |
The patient had had no illnesses or medication changes in the preceding 6 months. She received her second dose of the Moderna mRNA COVID vaccine 3 months prior to presenting with anemia, and her hemoglobin at a routine follow-up visit 10 days after her second vaccine dose was 13.2 g/dL, and her total bilirubin concentration was normal (0.9 mg/dL).
She was started on prednisone 60 mg/d (approximately 1 mg/kg/d). Her Hgb/Hct began to improve within 2 weeks of treatment and continues to improve with gradual prednisone tapering (Figure 1).
Figure 1.
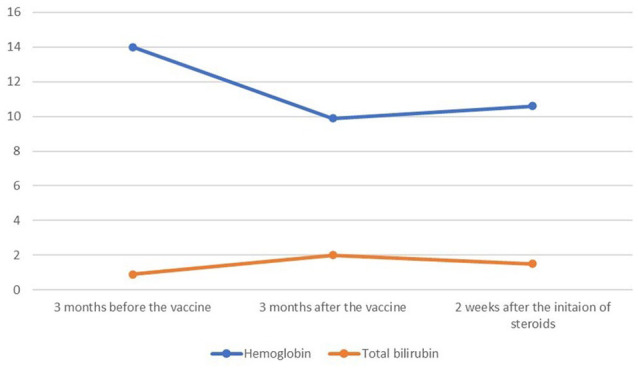
The relationship between the messenger ribonucleic acid vaccine and hemolytic parameters.
The patient gave written informed consent for her information to be submitted as a case report.
Discussion
A Medline search did not identify any cases of AIHA associated with psoriatic arthritis. A single case of adalimumab-induced AIHA has been reported, 3 but has been questioned because of the multi-year interval between drug initiation and AIHA onset. 4 Our patient has no clinical diagnosis or drug exposure that would predispose the AIHA other than COVID-19 vaccination.
Three cases of AIHA following COVID-19 mRNA vaccination have been reported, 2 with the Moderna vaccine,5,6 and 1 with the Pfizer vaccine. 7 All were associated with symptomatic anemia causing the patient to seek urgent medical care. The 2 episodes with the Moderna vaccine each presented a few days after the second dose, and the DAT in each case was positive for both IgG and C3. The case following the Pfizer vaccine presented 19 days after the first dose, and the DAT was positive for IgG only. The presentation of our patient was less acute and was identified later after vaccination than any of the previous reports. The direct antiglobulin test results differed from the 2 previous cases reported following the Moderna-1273 vaccine.
Conclusion
We present a case of moderate AIHA presenting 4 months after the second dose of the Moderna mRNA COVID-19 vaccine. Although 2 other cases of AIHA following the Moderna vaccine have been reported, we propose that the delayed timing of onset, lesser degree of severity, and different immunohematology suggests that the case reported here may be either a different immunologic process or a different stage in the evolution of the same immune process. In addition, the less-acute presentation raises the possibility that unrecognized mild autoimmune hemolysis may occur following COVID-19 vaccination. Continued monitoring of patients after vaccination may yield a greater understanding of the hematologic effects of these novel agents.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics Approval: Our institution does not require ethical approval for reporting individual cases or case series.
Informed Consent: Written informed consent was obtained from the patient(s) for their anonymized information to be published in this article.
ORCID iDs: Zainab Fatima  https://orcid.org/0000-0002-6284-1070
https://orcid.org/0000-0002-6284-1070
Blair R. A. Reece  https://orcid.org/0000-0002-4936-2681
https://orcid.org/0000-0002-4936-2681
Robert T. Means  https://orcid.org/0000-0002-0947-2624
https://orcid.org/0000-0002-0947-2624
References
- 1. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Franchini M, Liumbruno GM, Pezzo M. COVID-19 vaccine-associated immune thrombosis and thrombocytopenia (VITT): diagnostic and therapeutic recommendations for a new syndrome. Eur J Haematol. 2021;107(2):173-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Harada Y, Yamamoto H, Sato M, Kodaira M, Kono T. Autoimmune hemolytic anemia during adalimumab treatment for plaque psoriasis. Intern Med. 2015;54(9):1103-1104. [DOI] [PubMed] [Google Scholar]
- 4. Nagashima T, Minota S. Autoimmune hemolytic anemia induced by adalimumab. Intern Med. 2016;55(6):715. [DOI] [PubMed] [Google Scholar]
- 5. Gaignard ME, Lieberherr S, Schoenenberger A, Benz R. Autoimmune hematologic disorders in two patients after mRNA COVID-19 vaccine. Hemasphere. 2021;5(8):e618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brito S, Ferreira N, Mateus S, et al. A case of autoimmune hemolytic anemia following COVID-19 messenger ribonucleic acid vaccination. Cureus. 2021;13(5):e15035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Murdych TM. A case of severe autoimmune hemolytic anemia after a receipt of a first dose of SARS-CoV-2 vaccine [published online ahead of print July 14, 2021]. Int J Lab Hematol. doi: 10.1111/ijlh.13653. [DOI] [PMC free article] [PubMed] [Google Scholar]


