Abstract
Objective:
This study explored the best treatment strategies for stable coronary artery disease (SCAD) patients with differing levels of ischemic severity.
Methods:
We conducted a comprehensive search of the PubMed, EMBASE, and Cochrane databases – searching for relevant articles through 4 February 2021. We selected studies comparing different treatments for patients with SCAD who had received ischemia assessments. The primary outcome was death. The secondary outcomes were major adverse cardiovascular events (MACEs) and myocardial infarction (MI).
Results:
A total of 11 studies, including 35,607 subjects, were selected for this meta-analysis. Results showed that, compared with medical therapy, revascularization could reduce MACE incidence (odds ratio (OR) 0.73, 95% confidence interval (CI): 0.57–0.94, p < 0.05) in SCAD patients with myocardial ischemia, but that it was not effective for patients without ischemia. For mild ischemia, the incidence of death (OR 0.78, 95% CI: 0.59–1.01, p = 0.063), MACE (OR 0.91, 95% CI: 0.48–1.70, p = 0.762), or MI (OR 1.44, 95% CI: 0.94–2.19, p = 0.093) was the same whether they were treated with revascularization or medical therapy. For moderate to severe ischemia, revascularization reduced the incidence of MACE (OR 0.59, 95% CI: 0.42–0.83, p < 0.05) and MI (OR 0.83, 95% CI: 0.71–0.98, p < 0.05), but the incidence of death (OR 0.73, 95% CI: 0.47–1.12, p = .145) was similar. For SCAD patients with severe ischemia, revascularization may confer survival benefits (OR 0.46, 95% CI: 0.21–1.00, p = 0.05).
Conclusion:
For SCAD patients with moderate to severe ischemia, revascularization reduces the MACE and MI incidences, but does not change the incidence of death. Evaluation for myocardial ischemia is vital when selecting a therapeutic strategy.
Keywords: ischemia, myocardial perfusion, revascularization, stable coronary disease, therapy
Introduction
In recent years, with the rapid development of coronary revascularization technology, revascularization therapy for stable coronary artery disease (SCAD) has become very common. 1 In China, 58% of SCAD patients received revascularization, but this treatment was not suitable for 20.9% of patients. 2 In the United States, more than 1 million revascularization operations are performed each year, and approximately 85% of percutaneous coronary intervention (PCI) operations are performed in patients with SCAD. 3
However, for SCAD patients with obvious coronary artery stenosis, the benefits of revascularization based on drug therapy are controversial. No convincing conclusions have been obtained from early (TIME, 4 MASS-II, 5 SWISSI-II, 6 J-ACCESS, 7 COURAGE, 8 and JSAP 9 ) or later (BARI 2D, 10 ORBITA, 11 and CLARIFY 12 ) studies. The 2019 ESC Chronic Coronary Syndrome (CCS) Guidelines 13 recommended revascularization therapy for CCS patients with high event risk, which included ischemic areas ⩾ 10% of the left ventricle myocardium in single photon emission computed tomography (SPECT); ⩾ 2/16 segments with stress perfusion defects in cardiac magnetic resonance (CMR); or ⩾ 3/16 segments with stress-induced hypokinesia or akinesia in stress echocardiograms. However, findings from the COURAGE study showed that long-term mortality following PCI was consistent with drug treatment in SCAD patients. 8 The 2020 ISCHEMIA study showed that revascularization did not reduce the risk of major adverse cardiovascular events (MACEs) in SCAD patients with moderate to severe ischemia after optimal drug treatment. 14 However, Marcos-Garces et al. 15 found that, compared with medical therapy (MT), revascularization could improve the prognosis of SCAD patients with extensive ischemic burden (⩾5/17 segments ischemia in CMR). Thus, whether SCAD patients with moderate to severe ischemia should receive revascularization therapy is still controversial.
An additional complication is that there are currently no uniform standards for ischemia grading. At present, ESC guidelines 13 and reviews 16 identify moderate to severe ischemia based on the incidence of cardiovascular death and myocardial infarction (MI) events. However, there are no clear guidelines for switching between different segment divisions (16, 17, or 20 segments) or how to perform equivalent conversions with summed difference scores (SDSs).
Here, we aimed to explore appropriate conditions for revascularization of SCAD patients and to determine whether SCAD patients with moderate to severe ischemia needed to receive revascularization at all. In addition, we summarized definitions for ischemic grading. We hope our findings will help improve treatment of SCAD patients.
Methods
Search strategy
We searched the PubMed, EMBASE, and Cochrane databases to find relevant studies from the inception of these databases through 4 February 2021. Search terms included ‘stable coronary disease’, ‘stable ischemic heart disease’, ‘chronic coronary syndrome’, ‘SCAD’, ‘SIHD’, ‘CCS’, ‘percutaneous coronary intervention’, ‘coronary revascularization PCI’, ‘coronary artery bypass grafting (CABG)’, ‘CABG’, ‘medical therapy (MT)’, ‘MT’, ‘drug treatment’, ‘optimal medication therapy (OMT)’, ‘OMT’ and ‘coronary magnetic resonance imaging’, ‘single photon emission computed tomography’, ‘cardiac magnetic resonance (CMR)’, ‘Single photon emission computed tomography (SPECT)’, ‘myocardial perfusion’, and ‘stress test’. We also screened the included reference list of manuscripts as a supplement. These studies were independently searched by two authors (J.W.Y. and J.F.T.). A third author (X.T.S.) resolved the inconsistencies. This meta-analysis was registered in PROSPERO (CRD42021250635). Our research also followed PRISMA guidelines.
Selection criteria
The selected studies had to meet the following requirements: (1) They included stable coronary heart disease or CCS patients; (2) they included patients receiving ischemia assessments (SPECT; CMR; Stress tests) before treatment; (3) the study compared at least two treatment methods (MT, PCI, or CABG); (4) the study included at least one of these outcomes: death (without a specific cause), cardiac death, MACEs, and MIs; and (5) the design was either a randomized controlled trial (RCT) or an observational study.
We removed duplicate studies and used titles and abstracts to filter them. Exclusionary criteria included (1) patients did not have SCAD; (2) the study did not include at least one valid outcome; (3) there was no comparison between treatment groups; and (4) the study included patients who did not undergo ischemic evaluations.
Data extraction and outcomes
Each study was independently screened by two authors (J.W.Y. and J.F.T.) and verified by a third author (X.T.S.). The extracted data included study type, SCAD diagnosis, ischemia diagnosis method, ischemia grading standard, follow-up time, patient characteristics, and endpoint events. The main outcome examined was death (both all-cause death and cardiac death). Secondary outcomes included MACEs and MIs. MACEs were defined as all-cause death/cardiac death, non-fatal MI, or repeat revascularization/re-hospitalization (Supplementary Table 1). We classified myocardial ischemia based on each study’s own definition, and divided it into minimal, mild, moderate, and severe.
Statistical analysis
Stata11.0 (StataCorp, College Station, Tx) was used for analysis. We conducted a meta-analysis to compare the difference between revascularization and conservative medication. Revascularization includes both CABG and PCI. We also performed subgroup analyses based on ischemic grades and endpoints. Odds ratios (ORs) with 95% confidence intervals (CI) were calculated. The I2 test was used to assess the heterogeneity between studies (low heterogeneity: I 2 0–25%; moderate heterogeneity: 25–50%; severe heterogeneity: > 50%). If I 2 < 50%, Mantel-Haenszel (M-H) fixed effects models were used; but if I 2 > 50%, MH random effects models were used. A p value < 0.05 was considered to be statistically significant.
Sensitivity analysis and quality assessment
When heterogeneity was high, sensitivity analyses were performed by excluding all study endpoints one by one. Random effects were used to recalculate set estimates. We used Egger’s linear regression test and funnel chart visual inspection to assess publication bias. We used the Cochrane risk bias rating scale to assess the quality of RCTs, 17 and the Newcastle–Ottawa Scale (NOS) for retrospective studies. 18
Results
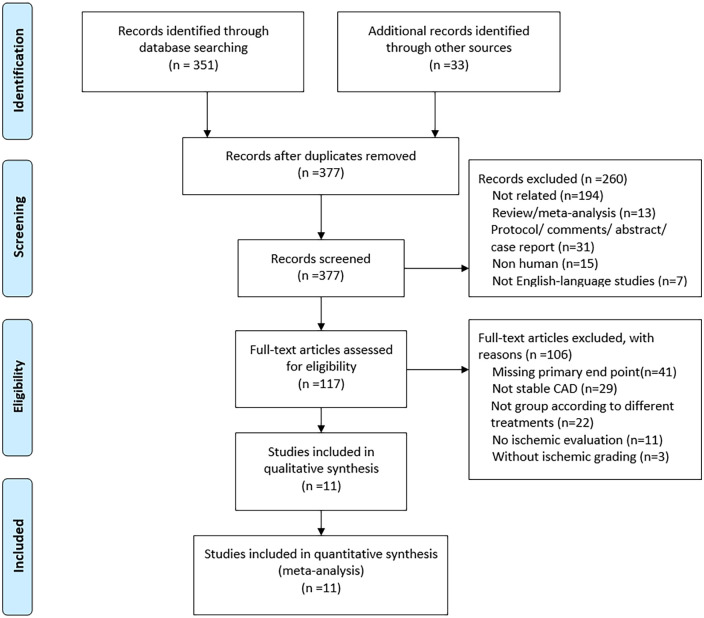
A total of 351 articles were identified from the database search analysis. Thirty-three articles were published between 1997 and 2020. Among these articles, 260 were excluded from the screening process at the title and summary levels. Figure 1 shows the search and screen protocol. Of the remaining 117 studies, 103 were excluded for the following reasons: missing the primary endpoint (n = 41), not including SCAD patients (n = 29), not grouping studies according to different treatment modalities (n = 22), not including ischemic evaluations (n = 11), and not including ischemic grading (n = 3). The remaining 11 studies reported death and did not meet any other exclusion criteria (3 reported cardiac death; 8 reported all-cause death).
Figure 1.
Flowchart of study selection.
Definition of ischemia
Table 2 details the definitions of 10 ischemic classifications for three different methods (SPECT, CMR, Echocardiographic). The images were divided into 6/16/17/20 segments. Although the standardized 17-segment model was recommended for quantification of wall motion and perfusion, the 17th segment (i.e. apical cap) was not evaluated separately on stress echocardiography or CMR myocardial perfusion imaging.
Table 2.
Definition of ischemia.
| Inspection method | Stress drugs | Evaluation means | Segment-model | Normal to minimal | Mild | Moderate | Severe |
|---|---|---|---|---|---|---|---|
| SPECT 24 | Exercise or dipyridamole stress | SDS | 17 | 0–4 | 5–9 | 10–19 | ⩾20 |
| SPECT 25 | Exercise or adenosine | SDS | 20 | 0–4 | 5–10 | 11–20 | >20 |
| SPECT 7 | NA | SDS | 20 | 0–4 | 5–8 | >8 | |
| SPECT19,23 | NA | % | 17 | 0–4% | 5–9% | 10–14% | >14% |
| SPECT 20 | Exercise or adenosine | % | 20 | 0–5% | 6–10% | 11–20% | >20% |
| SPECT 8 | NA | Segment | 6 | ⩾3 | |||
| CMR 15 | Exercise or adenosine | Segment with stress perfusion defects | 17 | 0 | 1 | 2–5 | ⩾6 |
| CMR 16 | Dobutamine | Segment with dysfunction | 16 | 0 | 1–2 | 3–4 | ⩾5 |
| CMR 16 | NA | Segment with stress perfusion defects | 16 | 0 | 1 | 2–4 | ⩾5 |
| Echocardiographic 16 | NA | Segment with stress-induced hypokinesis or akinesis | 16 | ⩾3 |
CMRI, coronary magnetic resonance imaging; SDS, summed difference score; SPECT, single photon emission computed tomography.
SDS segments and percentages were widely used in ischemia grading. SDS was defined as the summed difference between each of the total segments on the stress and rest images (every segment was scored from 0 to 4). When the image was divided into 20 segments and SDS = 8, the area was equal to approximately 10% (8 / 4 × 20).
Normal or minimal ischemia was defined as no ischemia. Mild to severe ischemia was defined as ischemia. The definition of moderate to severe ischemia in the literature varied, including either >10% or 11%, >2/17 or > 2/16 or 3/16 segments, or SDS > 8, 10, or 11. The definition of moderate to severe ischemia in the ISCHEMIA study was ⩾10% ischemic myocardium on stress nuclear imaging; ⩾3/16 newly dysfunctional segments in stress echocardiography; ⩾4/32 stress perfusion defects or ⩾3/16 newly dysfunctional segments. 14 This was mainly based on mortality. Previous studies have shown that the risk of cardiac death and MI increases as the ischemic area increases. The incidence of cardiac death and MI is between 4.5% and 4.9%, 16 which correlates with moderate to severe ischemia.
Baseline characteristics
Characteristics of this study are listed in Table 1. The 11 selected studies included 38,970 patients. Two of the studies were RCTs, four were observational studies, and five were retrospective studies. Sharir et al. 24 and the COURAGE study 8 compared PCI with MT, while all the others compared CABG/PCI with MT. SPECT was used in nine studies; CMR was used in one study. The other studies used different forms of stress tests, including CMRI, Echocardiography, and Nuclear imaging. The follow-up period ranged from 2 to 5 years. The average age of patients ranged from 59 to 67 years old. The majority of patients were men who were older than 60 years. More than 66% of the patients had hypertension, more than 25% had diabetes, and more than half had high levels of cholesterol. A total of 12–74% of the patients smoked, 10–51% had histories of revascularization, and 6–44% had a history of MI. The mean LVEF was more than 50%.
Table 1.
Baseline characteristics of the patients.
| Author | Study design | Ischemic grade | Method for ischemia | Follow-up time | n | Age | Male (%) | HT (%) | DM (%) | HC (%) | Smoke (%) | PrRev (%) | PrMI (%) | Mean LVEF (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Marcos-Garces et al. 15 | Retrospective | 0–1; 2–5; 6–8; ⩾9 segments (17-segment) | CMR (vasodilator drugs) | 5.7 years | 6389 | 65 ± 12 | 62 | 64 | 28 | 56 | 18 | 23 | 18 | 61 |
| Li et al. 19 | Retrospective | <10%; ⩾10% (17-segment) | SPECT (bicycle exercise or pharmacologic stress) | 46 ± 21 months | 286 | 64 ± 10 | 69.23 | 69.93 | 26.57 | 30.42 | 74.48 | NA | NA | 63 |
| Yoda et al. 20 | Retrospective propensity score-match |
⩽5%, 6–10%, >10% (20-segment) | SPECT (exercise or adenosine) | 3 years | 900 | 67.5 ± 10 | 81.11 | 78.89 | 42.67 | 54.89 | 32.22 | 40.89 | 32.67 | 56.00 |
| Boiten et al. 21 | Observational | <3, ⩾3 segments (6-segment) |
SPECT (exercise bicycle or dobutamine) | 5 years | 538 | 59.8 ± 11 | 73 | 40 | 16 | 35 | 29 | 30 | 41 | NA |
| Carvajal-Juarez et al. 22 | Retrospective | >10% | SPECT | 44 months | 100 | 64/59 (median) | 84 | 63 | 37 | 48 | 40 | NA | NA | 54 |
| Simonsen et al. 23 | Observational | 5–9%; 10–14%; >14% | SPECT (exercise test or pharmacological stress by adenosine, dipyridamole or dobutamine.) | 6.1 years | 1327 | 59.5 ± 11.8 | 43 | NA | 15 | NA | 15.30 | 15.67 | 6.56 | NA |
| Sharir et al. 24 | Observational cohort | <5%, 5–9%, ⩾10% (17-segment) |
SPECT (Treadmill exercise or dipyridamole stress) | 4.04 ± 1.86 years | 7973 | 64.5 ± 11.1 | 61.60 | 63.10 | 30.90 | 69 | 12.70 | 32.70 | 15.90 | 60.30 |
| Hachamovitch et al. 25 | Observational | 0%; 1–5%; 5–10%; 11–20%; >20% (20-segemnt) |
SPECT (exercise or adenosine) | 1.9 ± 0.6 years | 10,627 | 65/69 (median) | 54.61 | 46.19 | 12.99 | 41.60 | 13.24 | 10.15 | NA | NA |
| COURAGE 20128 | RCT | 0–2, ⩾3 segments (6-segment) |
SPECT | 4.6 years | 1381 | 61/62 | 87 | 69.73 | 36.21 | 47.79 | 28.53 | 26.69 | 38.83 | NA |
| J-ACCESS 20127 | Retrospective | SDS ⩽4; 5–8; >8 (20-segment) | SPECT | 3 years | 632 | 65.7 ± 9.4 | 71.35 | 58.15 | 39.55 | 58.25 | 19.35 | 51.60 | 43.35 | 58.00 |
| ISCHEMIA 202014 | RCT | NA | Stress test (CMRI; Echocardiography; Nuclear imaging) | 3.2 years | 5176 | 64 | 77.40 | 73.40 | 41.80 | NA | 57.40 | 23.20 | 19.20 | 60 |
CMR, cardiac magnetic resonance; CMRI, coronary magnetic resonance imaging; DM, diabetes mellites; HC, hyper-cholesterol; HT, hypertension; LVEF, left ventricular ejection fraction; PrMI, Previous myocardial infarction; PrRev, Previous revascularization; RCT, randomized controlled trial; SDS, summed difference score; SPECT, single photon emission computed tomography.
With and without ischemia
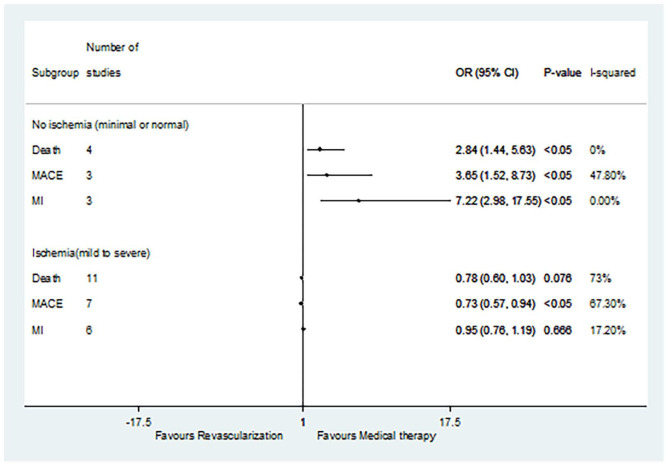
A total of 11 studies were selected, including 4 studies on non-ischemia (minimal or normal ischemia) and 11 studies on ischemia (mild to severe ischemia) (Figure 2). For patients without ischemia (minimal or normal ischemia), and compared with revascularization, MT not only reduced the incidence of death (OR 2.84, 95% CI: 1.44–5.63, p < 0.05) but also reduced the incidence of MACE (OR 3.65, 95% CI: 1.52–8.73, p < 0.05) and MI (OR 7.22, 95% CI: 2.98–17.55, p < 0.05). As for the ischemia patients, and compared with MT, revascularization was only associated with decreased incidence of MACEs (OR 0.73, 95% CI: 0.57–0.94, p < 0.05). For ischemia patients, there was no significant difference in death rates (OR 0.78, 95% CI: 0.60–1.03, p = 0.076) or MI incidence (OR 0.95, 95% CI: 0.76–1.19, p = 0.666) between revascularization and MT.
Figure 2.
Comparison of revascularization and medical therapy for SCAD patients with or without ischemia.
MACE, major adverse cardiovascular event; MI, myocardial infarction; SCAD, stable coronary artery disease.
Mild or moderate to severe ischemia
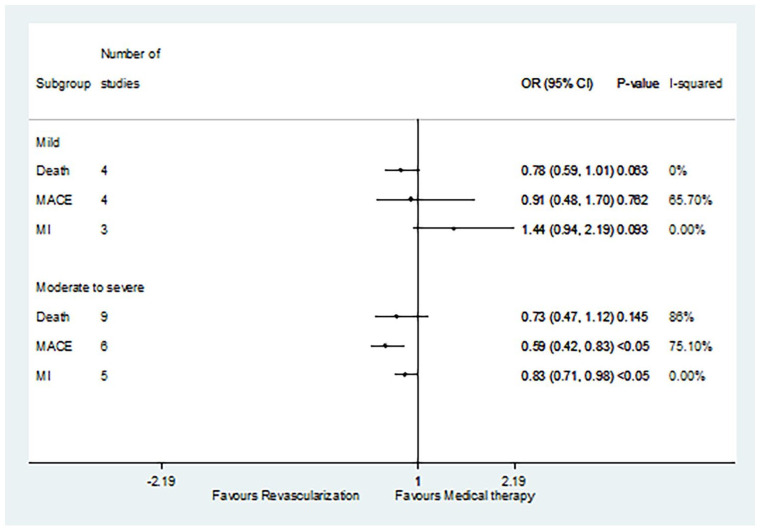
Four studies focused on mild ischemia, and nine focused on moderate to severe ischemia (Figure 3). For the mild ischemia patients, there was no difference in death rates (OR 0.78, 95% CI: 0.59–1.01, p = 0.063), MACE rates (OR 0.91, 95% CI: 0.48–1.70, p = 0.762), or MI rates (OR 1.44, 95% CI: 0.94–2.19, p = 0.093) between patients treated with revascularization and patients treated with MT. In contrast, for moderate to severe ischemia patients, revascularization did reduce the incidence of MACE (OR 0.59, 95% CI: 0.42–0.83, p < 0.05) and MI (OR 0.83, 95% CI: 0.71–0.98, p < 0.05) compared with MT. However, the incidence of death was similar between patients receiving revascularization and MT (OR 0.73, 95% CI: 0.47–1.12, p = 0.145).
Figure 3.
Comparison of revascularization and medical therapy for SCAD patients with different degrees of ischemia.
MACE, major adverse cardiovascular event; MI, myocardial infarction; SCAD, stable coronary artery disease.
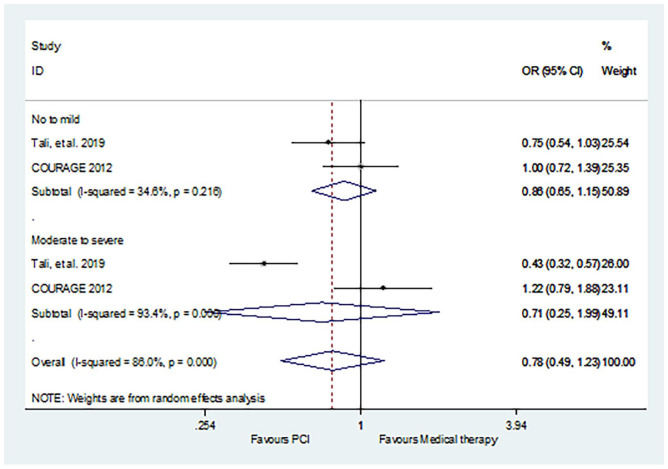
The result of death in PCI versus MT was consistent with revascularization (CABG/PCI) versus MT (Figure 4): no to mild (OR 0.86, 95% CI: 0.65–1.15, p = 0.311); moderate to severe (OR 0.71, 95% CI: 0.25–1.99, p = 0.515).
Figure 4.
Comparison of PCI and medical therapy for SCAD patients with different degrees of ischemia: death.
PCI, percutaneous coronary intervention; SCAD, stable coronary artery disease.
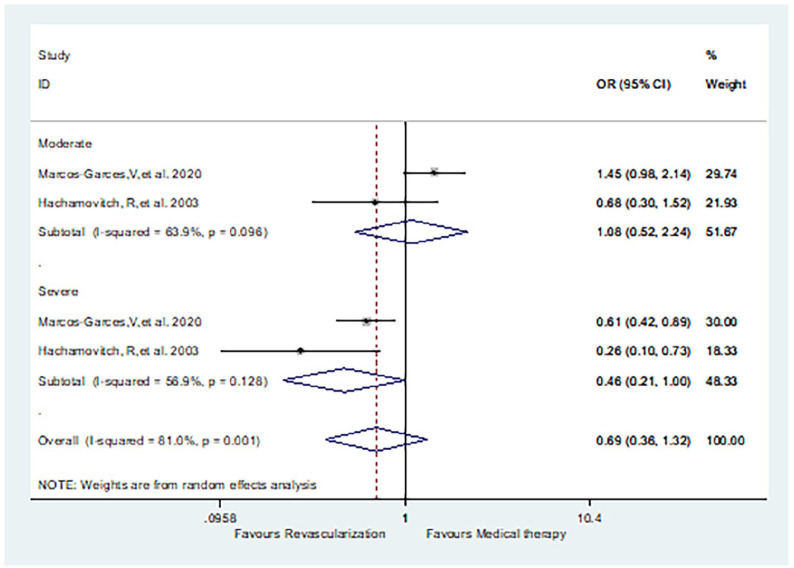
Moderate or severe ischemia
Marcos-Garces et al. 15 and Hachamovitch et al. 25 clearly distinguished results between moderate and severe ischemia. The incidence of death was similar between patients receiving revascularization and MT, for both moderate (OR 1.08, 95% CI: 0.52–2.24, p = 0.515) and severe (OR 0.46, 95% CI: 0.21–1.00, p = 0.05) ischemia patients (Figure 5).
Figure 5.
Comparison of revascularization and medical therapy for SCAD patients with moderate or severe ischemia: death.
SCAD, stable coronary artery disease.
Sensitivity analysis, quality of studies, and publication bias
In sensitivity analyses, when each trial was individually excluded, conclusions around outcomes related to death remained stable. The statistical heterogeneity of subgroups of minimal or normal, PCI versus MT, and moderate or severe ischemia (as measured by I 2 ) was 0–47.8% and 23.11–50%, indicating low to moderate heterogeneity (p = 0.145; 0.086). Because there appeared to be a high degree of heterogeneity between studies for both incidences of death and MACE (I 2 : 69–94.7%), we performed a subgroup analysis based on ischemia grading. When each study was excluded individually, conclusions were consistent with the original findings.
Our quality assessments of RCTs and observational studies were shown in Supplementary Table 2. A preliminary assessment of the quality of the two RCTs was conducted. These five studies were considered low-risk because they specified the randomization methods applied. However, none of the studies reported employing blindness. All RCTs reported data on each major outcome indicator (including lost to follow-up and dropped out). Therefore, follow-up deviations were considered low-risk. As for the observational studies, four studies scored seven points on the NOS scale, and the remaining five studies scored eight points.
We found evidence of publication bias based on funnel plots and the Begg test (t = 1.85; p = 0.593).
Discussion
A total of 11 studies were included in this meta-analysis, involving 38,970 patients. Our findings showed that, compared with MT, revascularization could reduce the risk of death and MACE in SCAD patients with ischemia (mild to severe ischemia). However, in subgroup analyses based on the ischemic grade, revascularization only reduced the risk of MACE and MI in SCAD patients with moderate to severe ischemia, and, compared with drug treatment, there was no difference in the risk of death.
The 2019 ESC Chronic Coronary Syndrome Guidelines 13 recommend assessment of event risk for all SCAD patients, especially non-invasive assessment of ischemia. Revascularization was recommended for patients with high event risk whose ischemic area was more than 10%. 26 However, many meta-analyses, including those completed by Laukkanen et al., 27 and Bangalore et al., 28 showed that there was no survival advantage of an initially invasive strategy over conservative MT in SCAD patients. Furthermore, using an initial invasive strategy only reduced the overall risk of the combined outcomes of death, MI, revascularization, readmission, and stroke. In addition, these studies did not grade ischemia. Many SCAD patients are faced with the choice to accept revascularization.2,29 Although current guidelines recommended revascularization therapy for patients with moderate to severe ischemia,13,26,30 evidence around conduct in revascularization based on ischemia grading is still lacking. Thus, resolving these issues is urgent. Our study performed a detailed analysis of SCAD patients according to different grades of ischemia, which has further guiding value for the selection of treatment options for SCAD patients.
Our study confirmed that revascularization was meaningful for SCAD patients with ischemia (mild to severe ischemia). Revascularization could reduce the risk of death and MACEs. This result is consistent with a prior ACIP study. 31 However, the TIME, MASS-II, BARI 2D, and COURAGE studies found different results. TIME 4 showed similar long-term survival rates for SCAD patients who received invasive care or MT. The MASS-II 5 study showed that 10-year survival rates were similar in CABG, PCI, and MT (74.9%, 75.1%, and 69%, p = 0.089) patients. The BARI 2D 10 and COURAGE 8 studies showed that PCI did not reduce the risk of death, MI, or MACEs in SCAD after OMT.
For SCAD patients with mild ischemia, there were no differences in the risk of death, MI, or MACE between groups of patients who received revascularization and groups of patients who received drug therapy. For SCAD patients with moderate to severe ischemia, revascularization did not reduce the risk of death. This result is consistent with the results of the COURAGE 8 and ISCHEMIA 14 studies. Subgroups of the COURAGE studies 8 showed that incidence of death was similar in OMT and PCI + OMT groups for patients with both no to mild (18% and 19%) and moderate to severe (19% and 22%) ischemia. The ISCHEMIA study 14 also revealed that, for SCAD patients with moderate to severe ischemia, revascularization did not reduce the risk of death compared with conservative treatment. However, Sharir et al. found a different result. Their study showed that the rate of death (1.42% versus 3.12%) was lower in the revascularization group compared with MT in patients with moderate to severe ischemia (⩾10%). 24
For SCAD patients with moderate to severe ischemia, although revascularization did not reduce the risk of death, it did reduce the risk of non-fatal events (MACE and MI). This result was consistent with those from the J-ACCESS and TIME studies. The J-ACCESS study showed that early revascularization could reduce the risk of cardiac events in >10% ischemic myocardium compared with MT (0% versus 12.3%). 7 The TIME study showed that early revascularization could reduce the risk of non-fatal events and could significantly improve symptoms and quality of life, particularly for patients with obvious symptoms. 4 Similarly, according to Sharir et al., 24 MI and MACE were lower in the revascularization group compared with MT in patients with moderate to severe ischemia (⩾10%). However, Yoda et al. 20 showed the opposite result (i.e. that early revascularization may lead to MACE related to treatment procedures, but could improve the prognosis of SCAD patients with moderate to severe ischemia). Thus, more RCTs are needed to provide more evidence for the effects of revascularization in these patients.
We also performed subgroup analysis for moderate and severe ischemia patients. We found that there was no difference between the risk of death for patients treated with revascularization or MT who had either moderate (or 1.08, 95% CI: 0.52–2.24) or severe ischemia (or 0.46, 95% CI: 0.21–1.00, p = 0.05). This indicates that revascularization may only improve the long-term prognosis of SCAD patients with severe ischemia. This result is consistent with previous observational studies, including those by Marcos-Garces et al. 15 and Hachamovitch et al., 25 but further RCT studies are needed to confirm these findings.
Limitations
Our study has several limitations. First, we included mostly observational studies (9/11), which may have decreased the validity of our findings. Second, we did not use Fractional flow reserve (FFR) assessments. FFR can be used as a decision-making basis for intervention in patients with SCAD. However, this article mainly focuses on myocardial perfusion and ischemic grading, which is why FFR was excluded. Third, there is no unified standard for ischemia grading. Fourth, due to limited data, lifestyle changes in the included articles were not evaluated. And the standards of drug treatment were not uniform. Fifth, due to limited data availability about CMR, we mostly included studies using SPECT to assess ischemia (9/11). Finally, definitions of ischemia differ in the literature (Table 2). The lack of unified standards for ischemia grading contributed to variable results from different detection methods. We need a clearer definition for ischemic grading, including different detection methods and criteria. Overall, we would recommend that the research community determine a unified segment standard, as well as a transformation formula to apply to different ischemic grades. In addition, more studies are needed to examine differences in prognosis of different ischemic grades.
Conclusion
For patients with ischemia, revascularization can reduce the risk of death and MACE compared with MT interventions. For SCAD patients with mild ischemia, risk of death, MACE, and MI outcomes were the same whether patients experienced revascularization or MT.
For SCAD patients with moderate to severe ischemia, revascularization reduced the risk of MACE and MI, but did not reduce the risk of death.
Myocardial ischemia evaluations are vital when choosing therapeutic strategies. More RCTs are needed to confirm the best treatments for SCAD patients with moderate to severe ischemia.
Supplemental Material
Supplemental material, sj-doc-2-taj-10.1177_20406223211056713 for Revascularization or medical therapy for stable coronary artery disease patients with different degrees of ischemia: a systematic review and meta-analysis of the role of myocardial perfusion by JingWen Yong, JinFan Tian, Xin Zhao, XueYao Yang, MingDuo Zhang, Yuan Zhou, Yi He and XianTao Song in Therapeutic Advances in Chronic Disease
Supplemental material, sj-docx-2-taj-10.1177_20406223211056713 for Revascularization or medical therapy for stable coronary artery disease patients with different degrees of ischemia: a systematic review and meta-analysis of the role of myocardial perfusion by JingWen Yong, JinFan Tian, Xin Zhao, XueYao Yang, MingDuo Zhang, Yuan Zhou, Yi He and XianTao Song in Therapeutic Advances in Chronic Disease
Acknowledgments
When writing this paper, we benefited from the participation of teachers and classmates. They generously helped us collect the required information and gave us many valuable suggestions. In particular, we are very grateful to a specific patient – Ms. Chen, a patient with SCAD who was struggling to decide whether to receive revascularization therapy. She was the inspiration for this analysis.
Footnotes
Author contributions: Jingwen Yong: Conceptualization; Data curation; Formal analysis; Methodology; Writing-original draft.
JinFan Tian: Conceptualization; Data curation; Funding acquisition; Methodology; Supervision; Writing-review & editing.
Xin Zhao: Methodology; Project administration; Resources.
XueYao Yang: Data curation; Project administration; Validation.
MingDuo Zhang: Data curation; Resources.
Yuan Zhou: Conceptualization; Data curation; Resources.
Yi He: Conceptualization; Funding acquisition; Methodology; Writing-review & editing.
Xiantao Song: Conceptualization; Funding acquisition; Project administration; Supervision; Visualization; Writing-review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Capital Health Development Research Project (No. 2018-2-2063), National Natural Science Foundation of China (No. 82100486 and No. 81971569), coronary artery microvascular disease innovation foundation (No. 2018-CCA-CMVD-01), Beijing Lab for Cardiovascular Precision Medicine (PXM2018_014226_000013), Beijing Municipal Science and Technology Project (Z161100000516139), and 2018 Beijing Excellent Talent Fund (No. 2018000021469G241).
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Consent statement and ethical approval: As this study consisted only of published articles, consent statements and ethical approvals were not required.
ORCID iD: XianTao Song  https://orcid.org/0000-0002-2114-2230
https://orcid.org/0000-0002-2114-2230
Data availability: All data included in this study are available from the corresponding author upon reasonable request.
Supplemental material: Supplemental material for this article is available online.
Contributor Information
JingWen Yong, Department of Cardiology, Beijing Anzhen Hospital, Capital Medical University, Beijing, China.
JinFan Tian, Department of Cardiology, Beijing Anzhen Hospital, Capital Medical University, Beijing, China.
Xin Zhao, Department of Cardiology, Beijing Anzhen Hospital, Capital Medical University, Beijing, China.
XueYao Yang, Department of Cardiology, Beijing Anzhen Hospital, Capital Medical University, Beijing, China.
MingDuo Zhang, Department of Cardiology, Beijing Anzhen Hospital, Capital Medical University, Beijing, China.
Yuan Zhou, Department of Cardiology, Beijing Anzhen Hospital, Capital Medical University, Beijing, China.
Yi He, Department of Radiology, Beijing Friendship Hospital, Capital Medical University, Yongan Road 95, Beijing 100050, China.
XianTao Song, Department of Cardiology, Beijing Anzhen Hospital, Capital Medical University, Anzhen Road No. 2, Chaoyang District, Beijing 100029, China.
References
- 1. Compilation group of China cardiovascular health and disease report. Report on cardiovascular health and disease in China 2019: an updated summary. Chin Circulation J 2020; 35: 833–854. [Google Scholar]
- 2. Shen L, Chunyu Y, Chenfei R, et al. Appropriateness of coronary revascularization in patients with stable coronary artery disease: a multicenter clinical trial. Chin Circulation J 2019; 34: 859–865. [Google Scholar]
- 3. Virani SS, Alonso A, Benjamin EJ, et al. Heart disease and stroke statistics – 2020 update: a report from the American Heart Association. Circulation 2020; 141: e139–e596. [DOI] [PubMed] [Google Scholar]
- 4. Pfisterer M, Buser P, Osswald S. Outcome of elderly patients with chronic symptomatic coronary artery disease with an invasive vs optimized medical treatment strategy: one-year results of the randomized TIME trial. Acc Current J Rev 2003; 12: 11–11. [DOI] [PubMed] [Google Scholar]
- 5. Hueb W, Lopes NH, Gersh BJ, et al. Five-year follow-up of the medicine, angioplasty, or surgery study (MASS II): a randomized controlled clinical trial of 3 therapeutic strategies for multivessel coronary artery disease. Circulation 2007; 115: 1082–1089. [DOI] [PubMed] [Google Scholar]
- 6. Kalay N, Ozdogru I. Effects of percutaneous coronary interventions in silent ischemia after myocardial infarction: the SWISSI II randomized controlled trial. JAMA 2007; 298: 1985–1991. [DOI] [PubMed] [Google Scholar]
- 7. Moroi M, Yamashina A, Tsukamoto K, et al. Coronary revascularization does not decrease cardiac events in patients with stable ischemic heart disease but might do in those who showed moderate to severe ischemia. Int J Cardiol 2012; 158: 246–252. [DOI] [PubMed] [Google Scholar]
- 8. Shawa LJ, Maron DJ, Hartigan PM, et al. Baseline stress myocardial perfusion imaging results and outcomes in patients with stable ischemic heart disease randomized to optimal medical therapy with or without percutaneous coronary intervention. Am Heart J 2012; 164: 243–250. [DOI] [PubMed] [Google Scholar]
- 9. Nishigaki K, Yamazaki T, Kitabatake A, et al. Percutaneous coronary intervention plus medical therapy reduces the incidence of acute coronary syndrome more effectively than initial medical therapy only among patients with low-risk coronary artery disease: a randomized, comparative, multicenter study. JACC Cardiovasc Interv 2008; 1: 469–479. [DOI] [PubMed] [Google Scholar]
- 10. BARI 2D Study Group, Frye RL, August P, et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med 2009; 360: 2503–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Al-Lamee R, Thompson D, Dehbi HM, et al. Percutaneous coronary intervention in stable angina (ORBITA): a double-blind, randomised controlled trial. Lancet 2018; 391: 31–40. [DOI] [PubMed] [Google Scholar]
- 12. Steg PG, Greenlaw N, Tendera M, et al. Prevalence of anginal symptoms and myocardial ischemia and their effect on clinical outcomes in outpatients with stable coronary artery disease. JAMA Intern Med 2014; 174: 1651–1659. [DOI] [PubMed] [Google Scholar]
- 13. Knuuti J, Wijns W, Saraste A, et al. 2019. ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J 2020; 41: 407–477. [DOI] [PubMed] [Google Scholar]
- 14. Maron DJ, Hochman JS, Reynolds HR, et al. Initial invasive or conservative strategy for stable coronary disease. N Engl J Med 2020; 382: 1395–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Marcos-Garces V, Gavara J, Monmeneu JV, et al. Vasodilator stress CMR and all-cause mortality in stable ischemic heart disease: a large retrospective registry. JACC Cardiovasc Imaging 2020; 13: 1674–1686. [DOI] [PubMed] [Google Scholar]
- 16. Shaw LJ, Berman DS, Picard MH, et al. Comparative definitions for moderate-severe ischemia in stress nuclear, echocardiography, and magnetic resonance imaging. JACC Cardiovasc Imaging 2014; 7: 593–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jørgensen L, Paludan-Müller AS, Laursen DR, et al. Evaluation of the Cochrane tool for assessing risk of bias in randomized clinical trials: overview of published comments and analysis of user practice in Cochrane and non-Cochrane reviews. Syst Rev 2016; 5: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wells G. The Newcastle-Ottawa Scale (NOS) for assessing the quality of non-randomised studies in meta-analyses. In: Symposium on systematic reviews: beyond the basics, Catherine’s college, Oxford, 2014. [Google Scholar]
- 19. Li J, Yang X, Tian Y, et al. Complete revascularization determined by myocardial perfusion imaging could improve the outcomes of patients with stable coronary artery disease, compared with incomplete revascularization and no revascularization. J Nucl Cardiol 2019; 26: 944–953. [DOI] [PubMed] [Google Scholar]
- 20. Yoda S, Hori Y, Hayase M, et al. Correlation between early revascularization and major cardiac events demonstrated by ischemic myocardium in Japanese patients with stable coronary artery disease. J Cardiol 2017; 71: 44–51. [DOI] [PubMed] [Google Scholar]
- 21. Boiten HJ, van den Berge JC, Valkema R, et al. Ischemia burden on stress SPECT MPI predicts long-term outcomes after revascularization in stable coronary artery disease. J Nucl Cardiol 2018; 25: 958–966. [DOI] [PubMed] [Google Scholar]
- 22. Carvajal-Juarez I, Espínola-Zavaleta N, Antonio-Villa NE, et al. Optimal medical treatment or invasive approach in patients with significantly obstructive coronary artery disease and ischemia. Arch Med Res 2020; 51: 413–418. [DOI] [PubMed] [Google Scholar]
- 23. Simonsen JA, Mickley H, Johansen A, et al. Outcome of revascularisation in stable coronary artery disease without ischaemia: a Danish registry-based follow-up study. BMJ Open 2017; 7: e016169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sharir T, Hollander I, Hemo B, et al. Survival benefit of coronary revascularization after myocardial perfusion SPECT: the role of ischemia. J Nucl Cardiol 2021; 28: 1676–1687. [DOI] [PubMed] [Google Scholar]
- 25. Hachamovitch R, Hayes SW, Friedman JD, et al. Comparison of the short-term survival benefit associated with revascularization compared with medical therapy in patients with no prior coronary artery disease undergoing stress myocardial perfusion single photon emission computed tomography. Circulation 2003; 107: 2900–2907. [DOI] [PubMed] [Google Scholar]
- 26. Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018. ESC/EACTS guidelines on myocardial revascularization. Eur Heart J 2019; 40: 87–165. [DOI] [PubMed] [Google Scholar]
- 27. Laukkanen JA, Kunutsor SK. Revascularization versus medical therapy for the treatment of stable coronary artery disease: A meta-analysis of contemporary randomized controlled trials. Int J Cardiol 2021; 324: 13–21. [DOI] [PubMed] [Google Scholar]
- 28. Bangalore S, Maron DJ, Stone GW, et al. Routine revascularization versus initial medical therapy for stable ischemic heart disease: a systematic review and meta-analysis of randomized trials. Circulation 2020; 142: 841–857. [DOI] [PubMed] [Google Scholar]
- 29. Katritsis DG, Mark DB, Gersh BJ. Revascularization in stable coronary disease: evidence and uncertainties. Nat Rev Cardiol 2018; 15: 408–419. [DOI] [PubMed] [Google Scholar]
- 30. Montalescot G, Sechtem U, Achenbach S, et al. 2013. ESC guidelines on the management of stable coronary artery disease. Russ J Cardiol 2014; 7: 7–79. [Google Scholar]
- 31. Davies RF, Goldberg A, Forman S, et al. Asymptomatic Cardiac Ischemia Pilot (ACIP) study two-year follow-up: outcomes of patients randomized to initial strategies of medical therapy versus revascularization. Circulation 1997; 95: 2037–2043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-doc-2-taj-10.1177_20406223211056713 for Revascularization or medical therapy for stable coronary artery disease patients with different degrees of ischemia: a systematic review and meta-analysis of the role of myocardial perfusion by JingWen Yong, JinFan Tian, Xin Zhao, XueYao Yang, MingDuo Zhang, Yuan Zhou, Yi He and XianTao Song in Therapeutic Advances in Chronic Disease
Supplemental material, sj-docx-2-taj-10.1177_20406223211056713 for Revascularization or medical therapy for stable coronary artery disease patients with different degrees of ischemia: a systematic review and meta-analysis of the role of myocardial perfusion by JingWen Yong, JinFan Tian, Xin Zhao, XueYao Yang, MingDuo Zhang, Yuan Zhou, Yi He and XianTao Song in Therapeutic Advances in Chronic Disease