Abstract
Objectives:
To investigate a 6-month intervention with an olive leaf extract (OLE) on knee functionality and biomarkers of bone/cartilage metabolism and inflammation.
Design:
This randomized, double-blind, placebo-controlled, multi-centric trial included 124 subjects with knee pain or mobility issues. Subjects received twice a day one capsule of placebo or 125 mg OLE (Bonolive™, an OLE containing 50 mg of oleuropein) for 6 months. The co-primary endpoints were Knee injury and Osteoarthritis Outcome Score (KOOS) and serum Coll2-1NO2. The secondary endpoints were the subscales of the KOOS, knee pain VAS at rest and at walking, OARSI core set of performance-based tests and multiple inflammatory and bone or cartilage remodeling serum biomarkers and concentration of oleuropein’s metabolites in urine.
Results:
At 6 months, OLE group was not efficient on global KOOS score, changes of inflammatory and cartilage remodeling biomarkers compared to placebo. Post hoc analyses demonstrated a large and significant treatment effect of OLE in a sub-group of subjects with high walking pain at baseline (p = 0.03). This was observed at 6 months for the global KOOS score, and each different subscale and for pain at walking (p = 0.02). OLE treatment was well tolerated.
Conclusion:
OLE was not effective on joint discomfort excepted in a sub-group of subjects with high pain at treatment initiation. As oleuropein is well tolerated, OLE can be used to relieve knee joint pain and enhance mobility in subjects with articular pain.
Keywords: function, joint pain, nutritional supplement, oleuropein
Introduction
Mobility is important for quality of life and is a core indicator of health in aging. Most aged people experience mobility impairment slinked to at least one of the three components of the musculoskeletal system (joints, bones, and muscles), which may affect their ability to move. During aging, some people suffer of joint discomfort and pain leading to reduced mobility, affecting their ability to carry out regular activities. Joint discomfort may result from mechanical stress and repetitive movement during exercise and physical activity in aging populations. 1 Current medical treatments aim at decreasing discomfort and increasing mobility. They generally include nonsteroidal anti-inflammatory drugs (NSAIDs) or paracetamol to control discomfort and inflammation. Unfortunately, chronic use of these medications, particularly in elderly subjects with comorbidities, can lead to significant adverse effects, including gastrointestinal bleeding, loss of kidney function, and/or hepatotoxicity.2–4
Specific nutritional concepts have been developed to help maintain mobility in aged people by optimizing the structure and function of the musculoskeletal system, one of them is based on oral intake of olive leaf extract (OLE). Olive leaves are the richest source of olive phenolic compounds, and OLE is now a popular nutraceutical taken either as liquid or capsules. 5 OLE provides oleuropein, a secoiridoid, considered as the most prevalent phenolic component in olive leaves and has been shown to have anti-inflammatory and anti-oxidant effects potentially interesting for joint health. 6 In vitro, oleuropein significantly inhibits the interleukin (IL)-1β-induced production of nitric oxide (NO) and prostaglandin (PG)E2, expression of cyclooxygenase (COX)-2, inducible Nitric Oxide Synthase (iNOS), Matrix Metalloprotease (MMP)-1, -13, and A Disintegrin And Metalloproteinase with Thrombospondin Motifs (ADAMTS-5) and degradation of aggrecan and collagen-II by human osteoarthritis (OA) chondrocytes. 7 Oleuropein exerts anti-inflammatory and anti-oxidant effects via down-regulation of MAPK and NF-κB signaling pathways and induction of Nrf2-linked HO-1 controlling the production of inflammatory mediators.7,8 It has recently been demonstrated that oleuropein protects against collagen II–induced arthritis in mice 9 and slows down the natural cartilage degradation and synovial membrane changes in Dunkin–Hartley guinea pigs developing spontaneous aging-related joint degeneration. 10
Benefits of oleuropein have also been reported for bone health in several in-vivo studies. The consumption of olives, 11 olive oil, 12 and oleuropein 13 prevents the loss of bone mass in experimental models mimicking osteoporosis that occurs with aging. 14 Ex-vivo studies have demonstrated that oleuropein inhibits the differentiation of mesenchymal stem cells (MSCs) isolated from human bone marrow into adipocytes 15 and enhances differentiation into osteoblasts, suggesting it could prevent age-related bone loss and osteoporosis. 16
In this study, we report the effect of an OLE containing 40% oleuropein (OLE) on joint health. This OLE was previously tested on osteopenic postmenopausal women at the dose of 250 mg/day (providing 100 mg oleuropein) for 12 months. Women taking this OLE showed a reduce loss of bone mineral density at the lumbar spine and an increased gain of bone mineral density at the femur neck compared to those taking placebo. 17 We conducted a randomized, double-blinded, placebo-controlled trial to assess whether supplementation with OLE improves knee pain, discomfort, and loss of mobility in elderly subjects who reported having mild to moderate functional knee pain during/after physical activity. In addition, blood markers reflecting inflammation and cartilage, or bone metabolism were investigated. Considering that patients with higher pain intensity are the most responders to antalgic treatment, we have also conducted a post hoc analysis by dividing the experimental population into three groups: low pain, medium and high pain at baseline. This study is the first ever to have been conducted in full accordance with the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human use (ICHE6) that examine the impact of ingesting a food supplement composed by OLE on alleviating pain (discomfort), function and physical performance in elderly subjects who reported having mild to moderate functional knee pain during/after physical activity.
Population and design
Study design
This study was a randomized, double-blind, placebo-controlled with two parallel-groups and multicenter trial in free-living healthy male and female subjects with moderate knee pain and lost of mobility. This study included subjects from Belgium enrolled by 19 health professionals between June 2016 and April 2018 and was conducted by an independent contract research organization (CRO, Artialis SA, Liège, Belgium). The main inclusion criteria were age of 55 years or over with moderate knee pain evaluated on VAS (0–100) between 40 and 75 at walking over the last 24 hours. The most painful knee was considered. The main exclusion criteria were pregnancy or lactation, planned knee replacement surgery, allergy or contraindication to oleuropein, recent trauma of the knee or rheumatic diseases responsible of the symptomatic knee. Use of any intra-articular injection in the target knee in the last 3 months, symptomatic slow-acting drugs in OA (SYSADOA) in the last month, oral corticotherapy in the last 3 months, other dietary supplements used for joint health in the last 3 months were also exclusion criteria. Subjects enrolled could have taken paracetamol and/or oral NSAIDs to manage knee pain. Patients were then asked to use these rescue medications only when needed during the trial. Twenty-four hours before a visit, subjects were asked to stop rescue medication for the evaluation of clinical parameters by the investigator. The trial has been conducted in accordance with the European Directive 2001/20/EC and the Belgian law of May 7, 2004, relating to experiments on the human person, following Good Clinical Practices (GCP) guidelines and according to the ‘Declaration of Helsinki’ published by the World Medical Association. The study protocol was approved by the Central Ethics Committee of the University Hospital of Liège in Belgium (namely Comité d’Ethique Hospitalo-Facultaire Universitaire de Liège), agreement number: 707. This RCT was also registered on Clinical trial.gov on March 7, 2017 (https://clinicaltrials.gov/ct2/show/NCT03072108).
Treatment assignment
The subjects were randomly assigned to one of the study groups using minimization (dynamic allocation with p best = 15%): (1) investigational product one cap twice a day and (2) placebo one cap twice a day. Randomization was performed with Medidata Balance and included gender as a stratification factor. The administration scheme consisted of one 125-mg capsule of OLE (Bonolive, consisting of a mixture of polyphenols derived from olive leaf, standardized for its oleuropein content (40%), BioActor BV, Maastricht, The Netherlands) or placebo twice a day, in the morning and in the evening at the beginning of the meal during 6 months. The investigational product was per capsule 125 mg of OLE containing 50 mg ± 10 mg of oleuropein plus additives (microcrystalline cellulose, vegetable magnesium stearate, silicon dioxide). The placebo was Maltodextrin Glucidex (IT 19) plus additives (microcrystalline cellulose, vegetable magnesium stearate, and silicon dioxide). Compliance with the study treatments was established by counting unused study products. A good compliance has been defined as ⩾85% of product taken through the entire study. Participants were allowed to consume habitual foods. Participants were also allowed to take rescue treatment to manage knee pain, that is, authorized analgesics except 24 h before each visit.
Outcome measures
There were two co-primary endpoints, and both were assessed as the change from baseline to 6 months. They were analyzed by ANCOVA correcting for the measurement at baseline. One endpoint was the Knee injury and Osteoarthritis Outcome Score (KOOS) using a self-administered questionnaire. An average of the five subscales was used. The other endpoint was the serum levels of Coll2-1NO2, a specific amino acid sequence located in the triple helicoidal part of type-II collagen and considered as a biomarker of cartilage degradation. 18 sColl2-1NO2 was measured in diluted serum using an Enzyme Linked Immuno-Sensitive Assay ELISA method (Artialis SA, Liège, Belgium). These two endpoints have been considered hierarchically. A hierarchical testing strategy assuring a type-1 error rate equal to 2.5% was used. This consisted in first testing the KOOS score, and then, in case of statistically significant results, the biomarker Coll2-1NO2 was analyzed. The secondary endpoints included each of the five sub-scales of the KOOS questionnaire, knee pain on a 100 mm VAS at rest and at walking, the OARSI core set of performance-based tests (30 second Chair test, timed up and go, stair climb test), a set of biomarkers evaluating bone (osteocalcin, CTX-1) and cartilage (Coll2-1) metabolism and inflammation (IL-8, tumor necrosis factor (TNF)-α, and PGE2,) in serum and concentration of oleuropein’s metabolites in urine by liquid chromatography and identified/quantified by mass spectrometry coupled to the chromatography (LC-MS/MS). Serum analyses for biomarkers have been done by Artialis SA (Liège, Belgium) using validated immunoassays (ELISA) and according to written procedures. All adverse events (AEs) and abnormal laboratory test results were recorded. The compliance based on pill consumption was computed as the number of caps dispensed minus the number of caps returned. The percentage of compliance was then assessed by dividing the consumption by the number of caps assumed to be taken. For secondary end-points, a p value < 0.05 was considered as significant.
Statistical analysis
Determination of the sample size
The effects to be estimated were the treatment difference between OLE and placebo in KOOS at 6 months and the treatment difference between OLE and placebo in Coll2-1NO2 at 6 months. In order to show in a two-group parallel design a difference of 10 score point in KOOS pain subscale with a standard deviation of 16 score points as statistically significant at an alpha level of 5% and a power of 90%, 55 subjects should be analyzed. In order to show a difference of 5.1 nM in Coll2-1NO2 with a standard deviation of 8.73 nM as statistically significant at an alpha level of 5% and a power of 80%, 47 subjects needed to be analyzed. So, 55 subjects were needed by group, these were 110 in total. Considering a 15% drop-out rate, a maximal number of 126 subjects were required.
Statistical analyses for primary endpoints
For each endpoint, the descriptive statistics presented were number of subjects (n), mean, standard deviation (SD), standard error of the mean (SEM), min and max, lower quartile (Q1), median and upper quartile (Q3) as well as the p value of Shapiro–Wilk. A linear mixed model was used with baseline value, visit and treatment as covariates. Within-subject correlations were described through a covariance pattern model and specified with a covariance structure. Three structures were tested, that is, compound symmetry, unstructured, and time-series-type, and chosen according to the Akaike information criterion (AIC).
Statistical analyses for secondary endpoints
The same ANCOVA model correcting for baseline value as mentioned in the primary analysis was used to analyze secondary endpoints. If the statistical assumptions of the ANCOVA model were not fulfilled, a log transformation was used to meet them. In this case, n, geometric mean (geo. mean), the lower and upper bounds defined as [geom. Mean/geom. SD, geom. Mean * geom SD], min and max were presented. If a log transformation did not help, it was planned to use the non-parametric Wilcoxon rank-sum test which was not necessary in this study.
For biomarkers with a percentage of measurements below limit of detection (LOD) or lower limit of detection (LLOQ) which was superior to 30%, a comparison of the proportion of subjects with detectable versus non-detectable values between the two treatment groups was performed at each visit. Detectable values were defined as values measured as well as values below LLOQ, whereas non-detectable values were defined as values below LOD. For biomarkers with a percentage of measurements below LOD or LLOQ which is inferior to 30%, linear mixed models were performed. In order to do it, biomarkers have to be considered as continuous variables. Therefore, measurements below LOD were replaced by 0. Regarding measurements below LLOQ, if a measurement was still provided, this measurement was used. Otherwise, measurements below LLOQ were replaced by LLOQ/2.
Post hoc analysis
We also performed a post hoc analysis based on the tertiles of VAS score at walking measured at baseline. The group of subjects having a low VAS score at baseline included subjects with a VAS score inferior to the first tertile. The group of subjects having a medium VAS score at baseline included subjects with a VAS stablecore superior to the first tertile and inferior to the second tertile. Finally, the groups of subjects having a high VAS score at baseline included subjects with a VAS score superior to the second tertile. Only data on both extreme tertiles will be presented. Same linear mixed models than those used for main analyses were performed to assess treatment difference between sub-groups. These sub-group analyses were performed on primary endpoints as well as the five different subscales of the KOOS score.
Results
Population
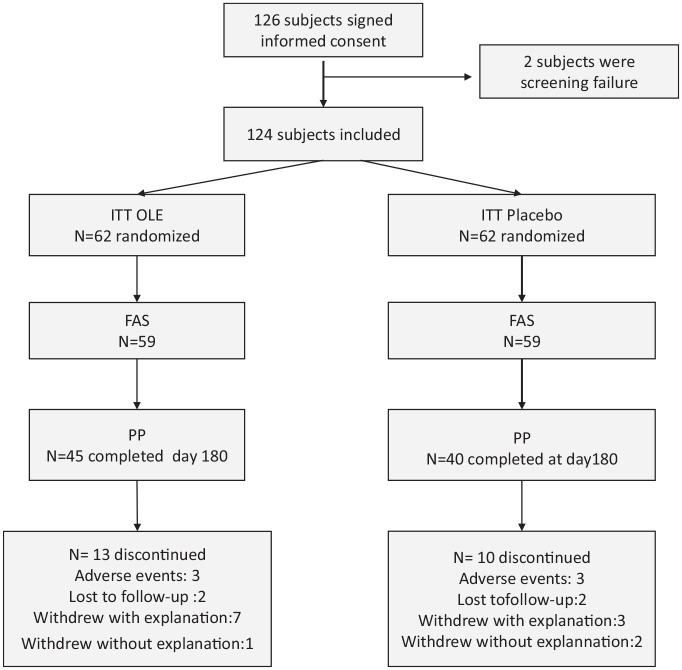
Out of the 138 subjects screened, 126 were randomized and considered eligible for the Intention-To-Treat (ITT) analysis and 118 for the full analysis set (FAS) analysis. The per-protocol set included 85 subjects of the FAS who also completed the study and did not have any major protocol deviation. Among the FAS population, 59 received Bonolive and 59 received placebo (Figure 1). The cumulative time distribution of withdrawals was similar in the two groups without significant differences in the reasons for withdrawals. At baseline, participants in each group were well-matched (Table 1). Female and male were equally distributed between the two groups, with 49.1% and 52.5% of male in the placebo and OLE groups, respectively. About 52% and 54% of the subjects were overweigth (25 < BMI< 29.9 kg/m2) among placebo and OLE groups, respectively. No significant differences were observed between the two treatment groups according to demographic characteristics and BMI. The global KOOS score was similar in both groups (mean ± SEM: placebo 54.1 (2.5) vs OLE 49.8 (2.0), p = 0.22). The KOOS pain subscore was higher in the placebo group as compared with the OLE group at baseline (p = 0.025; Table 1)) sColl2-1NO2 levels were also comparable (mean ± SEM: placebo 2694 (181) pg/mL vs OLE 2638 (249) pg/mL, p = 0.35).
Figure 1.
Disposition of subjects.
FAS, full analysis set; ITT, intention to treat; N, number; PP, per-protocol.
Table 1.
Demographic and baseline characteristics.
| Placebo (N = 59) | OLE (N = 59) | Total (N = 118) | p values | ||
|---|---|---|---|---|---|
| Gender | n/n miss | 59/0 | 59/0 | 118/0 | 0.71 |
| Male, n (%) | 29 (49.1%) | 31 (52.5%) | 60 (50.8%) | ||
| Female, n (%) | 30 (50.9%) | 28 (47.5%) | 58 (49.1%) | ||
| Ethnicity | n/n miss | 59/0 | 59/0 | 118/0 | 0.19 |
| Asian, n (%) | 1 (1.7%) | 0 (0%) | 1 (0.8%) | ||
| African, n (%) | 2 (3.4%) | 1 (1.7%) | 3 (2.5%) | ||
| Caucasian, n (%) | 56 (94.9%) | 58 (98.3%) | 114 (96.6%) | ||
| Age (years) | n/n miss | 59/0 | 59/0 | 118/0 | 0.23 |
| Mean (SEM) | 64.3 (0.89) | 62.8 (0.85) | 63.6 (0.61) | ||
| Median | 64 | 62 | 63 | ||
| Q1, Q3 | 59, 69 | 57, 67 | 57, 68 | ||
| Min, Max | 55, 82 | 53, 79 | 53, 82 | ||
| BMI (kg/m2) | n/n miss | 59/0 | 59/0 | 118/0 | 0.55 |
| Mean (SEM) | 25.6 (0.46) | 25.3 (0.32) | 25.4 (0.28) | ||
| Median | 25.5 | 25.2 | 25.4 | ||
| Q1, Q3 | 23.5, 28.5 | 23.9, 27.1 | 23.7, 27.7 | ||
| Min, Max | 18.5, 35.2 | 19.2, 29.9 | 18.5, 35.2 | ||
| Overall KOOS | n/n miss | 56/3 | 59/0 | 115/3 | 0.22 |
| Mean (SEM) | 54.1 (2.5) | 49.8 (2.0) | 51.9 (1.6) | ||
| Median | 52.8 | 51.3 | 52.3 | ||
| Q1, Q3 | 41.9, 70.2 | 39.7, 60.6 | 40.6, 65.0 | ||
| Min, Max | 11.8, 87.8 | 15.2, 87.6 | 11.8, 87.8 | ||
| Coll2-1 NO2 (pg/mL) | n/n miss | 57/2 | 59/0 | 116/2 | 0.35 |
| Mean (SEM) | 2694 (181) | 2638 (249) | 2666 (154) | ||
| Median | 2309 | 2068 | 2136 | ||
| Q1, Q3 | 1686, 3421 | 1517, 2963 | 1614, 3345 | ||
| Min, Max | 932, 6466 | 253, 10304 | 253, 10304 | ||
| KOOS pain | n/n miss | 59/0 | 59/0 | 118/0 | 0.025 |
| Mean (SEM) | 61.0 (2.5) | 52.2 (2.4) | 56.6 (1.8) | ||
| Median | 58.3 | 52.8 | 58.3 | ||
| Q1, Q3 | 47.2, 75.0 | 38.9, 66.7 | 41.7, 69.4 | ||
| Min, Max | 16.7, 94.4 | 0.0, 86.1 | 0.0, 94.4 | ||
| KOOS Adl | n/n miss | 59/0 | 59/0 | 118/0 | 0.44 |
| Mean (SEM) | 63.0 (2.9) | 60.1 (2.5) | 61.6 (1.9) | ||
| Median | 63.2 | 63.2 | 63.2 | ||
| Q1, Q3 | 44.1, 82.4 | 44.1, 76.5 | 44.1, 76.5 | ||
| Min, Max | 7.4, 98.5 | 14.7, 97.1 | 7.4, 98.5 | ||
| KOOS symptom | n/n miss | 59/0 | 59/0 | 118/0 | 0.5 |
| Mean (SEM) | 65.9 (2.5) | 63.6 (2.2) | 64.7 (1.7) | ||
| Median | 64.3 | 67.9 | 67.9 | ||
| Q1, Q3 | 50.0, 82.1 | 50.0, 78.6 | 50.0, 78.6 | ||
| Min, Max | 28.6, 100.0 | 28.6, 89.3 | 28.6, 100.0 | ||
| KOOS sport | n/n miss | 56/3 | 59/0 | 115/3 | 0.27 |
| Mean (SEM) | 36.4 (3.4) | 30.5 (2.6) | 33.4 (2.1) | ||
| Median | 30 | 30 | 30 | ||
| Q1, Q3 | 12.5, 60.0 | 15.0, 40.0 | 15.0, 50.0 | ||
| Min, Max | 0.0, 90.0 | 0.0, 95.0 | 0.0, 95.0 | ||
| KOOS Qol | n/n miss | 59/0 | 59/0 | 118/0 | 0.88 |
| Mean (SEM) | 43.8 (2.9) | 42.6 (2.2) | 43.2 (1.8) | ||
| Median | 37.5 | 43.8 | 43.8 | ||
| Q1, Q3 | 31.3, 62.5 | 31.3, 50.0 | 31.3, 56.3 | ||
| Min, Max | 0.0, 87.5 | 6.3, 75.0 | 0.0, 87.5 | ||
| Knee pain VAS score at rest | n/n miss | 59/0 | 59/0 | 118/0 | 0.76 |
| Mean (SEM) | 45.2 (2) | 45.4 (2.4) | 45.3 (1.5) | ||
| Median | 45 | 47 | 45 | ||
| Q1, Q3 | 40, 58 | 40, 60 | 40, 59 | ||
| Min, Max | 10, 73 | 0, 74 | 0, 74 | ||
| Knee pain VAS score at walking | n/n miss | 59/0 | 59/0 | 118/0 | 0.82 |
| Mean (SEM) | 58.1 (1.4) | 57.3 (1.4) | 57.7 (1) | ||
| Median | 59 | 59 | 59 | ||
| Q1, Q3 | 50, 65 | 48, 70 | 49, 66 | ||
| Min, Max | 40, 100 | 32, 75 | 32, 100 |
BMI, body mass index; KOOS, Knee injury and Osteoarthritis Outcome Score; OLE, olive leaf extract; SEM, standard error of the mean; VAS, Visual analog scale.
Primary outcomes
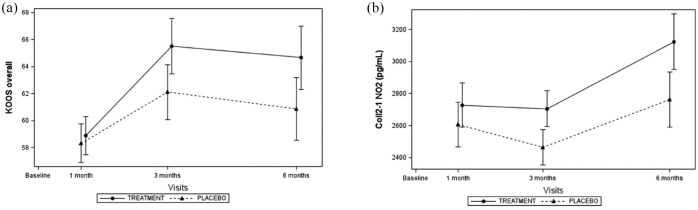
Both primary endpoints increased significantly across visits (Figure 2). Global KOOS score tended to be higher in the OLE group at each time point but the difference with the placebo group was not significant (Figure 2(a)). The difference of the least squares (LS) mean also tended to increase with time suggesting that the magnitude of OLE effect enhanced with time (Table 2). At each time point, Coll2-1NO2, a specific marker of oxidative-related cartilage degradation, tended to be higher in OLE group compared to placebo (Figure 2(b)), but no significant difference between groups was reached (Table 3). These observations were similar in the FAS and PP population.
Figure 2.
Effects of OLE on global KOOS (a) score and on serum Coll2-1NO2 (b).
Table 2.
Estimates of treatment differences by visits between OLE and placebo group in FAS population.
| LS mean (SE) | 95% CI | p value | ||
|---|---|---|---|---|
| KOOS global | Overall | 2.58 (2.41) | [–2.2;7.36] | 0.29 |
| T1 | 0.56 (2.01) | [–3.43;4.54] | 0.78 | |
| T3 | 3.39 (2.9) | [–2.36;9.14] | 0.25 | |
| T6 | 3.79 (3.31) | [–2.77;10.35] | 0.25 | |
| kOOS pain | Overall | 2.88 (2.64) | [–2.34;8.1] | 0.28 |
| T1 | 2.07 (2.35) | [–2.59;6.73] | 0.38 | |
| T3 | 3.07 (3.16) | [–3.19;9.33] | 0.33 | |
| T6 | 3.5 (3.59) | [–3.61;10.61] | 0.33 | |
| KOOS Adl | Overall | 0.16 (2.52) | [–4.84;5.16] | 0.95 |
| T1 | –2.02 (2.22) | [–6.42;2.38] | 0.37 | |
| T3 | 0.15 (2.95) | [–5.71;6] | 0.96 | |
| T6 | 2.35 (3.51) | [–4.61;9.31] | 0.51 | |
| KOOS symptom | Overall | 1.74 (2.24) | [–2.71;6.18] | 0.44 |
| T1 | 1.08 (2.09) | [–3.06;5.21] | 0.61 | |
| T3 | 1.77 (2.76) | [–3.7;7.23] | 0.52 | |
| T6 | 2.37 (3.11) | [–3.8;8.54] | 0.45 | |
| KOOS sport | Overall | 3.06 (3.8) | [–4.47;10.59] | 0.42 |
| T1 | 0.03 (3.72) | [–7.34;7.41] | 0.99 | |
| T3 | 4.98 (4.5) | [–3.93;13.89] | 0.27 | |
| T6 | 4.16 (5.14) | [–6.03;14.35] | 0.42 | |
| KOOS Qol | Overall | 3.56 (2.69) | [–1.77;8.89] | 0.19 |
| T1 | 0.58 (2.53) | [–4.44;5.6] | 0.82 | |
| T3 | 6.44 (3.38) | [–0.25;13.13] | 0.06 | |
| T6 | 3.66 (3.6) | [–3.47;10.8] | 0.31 | |
| Knee pain VAS score at rest | Overall | 3.6 (3.1) | [–2.6;9.7] | 0.26 |
| T1 | 7.1 (3.5) | [0.2;14.1] | 0.04 | |
| T3 | 3.5 (4.1) | [–4.6;11.7] | 0.39 | |
| T6 | 0.01 (4) | [–7.9;7.9] | 1 | |
| Knee pain VAS score at walking | Overall | 0.9 (3.6) | [–6.2;8.1] | 0.79 |
| T1 | 6.3 (3.3) | [–0.3;12.8] | 0.06 | |
| T3 | –1.4 (4.6) | [–10.5;7.7] | 0.75 | |
| T6 | –2 (4.8) | [–11.6;7.6] | 0.68 | |
| 30 second chair test | Overall | 0.3 (0.5) | [–0.7;1.2] | 0.59 |
| T1 | 0.1 (0.5) | [–0.9;1.1] | 0.86 | |
| T3 | 0.3 (0.6) | [–0.8;1.4] | 0.61 | |
| T6 | 0.4 (0.6) | [–0.8;1.6] | 0.52 | |
| Stair test | Overall | –0.1 (1.5) | [–3;2.8] | 0.94 |
| T1 | –0.1 (1.4) | [–2.8;2.7] | 0.96 | |
| T3 | –0.1 (1.3) | [–2.7;2.5] | 0.96 | |
| T6 | 0.2 (1.5) | [–2.8;3.3] | 0.88 | |
| Timed up and go | Overall | 0.2 (0.38) | [–0.6;0.9] | 0.68 |
| T1 | 0.1 (0.39) | [–0.7;0.9] | 0.77 | |
| T3 | 0.4 (0.48) | [–0.5;1.4] | 0.39 | |
| T6 | 0 (0.46) | [–1;0.9] | 0.92 |
CI, confidence interval; KOOS, Knee injury and Osteoarthritis Outcome Score; LS, Least Squares; SE, standard error; VAS, Visual analog scale.
Table 3.
Estimates of treatment differences by visits between OLE and placebo group for different biomarkers in FAS population.
| LS mean (SE) | 95% CI | p value | ||
|---|---|---|---|---|
| Coll2-1NO2 (pg/mL) | Overall | 242 (155) | [–67;550] | 0.12 |
| T1 | 122 (197) | [–269;512] | 0.54 | |
| T3 | 241 (157) | [–71;554] | 0.13 | |
| T6 | 362 (245) | [–124;848] | 0.14 | |
| Coll2-1 (nM) | Overall | 21.31 (27.06) | [–32.33;74.96] | 0.43 |
| T1 | 19.95 (29.14) | [–37.8;77.7] | 0.5 | |
| T3 | 8.37 (30.28) | [–51.63;68.37] | 0.78 | |
| T6 | 35.62 (38.87) | [–41.53;112.77] | 0.36 | |
| PGE2 (pg/mL) | Overall | –22 (64.1) | [–149;105] | 0.73 |
| T1 | –48.2 (113.7) | [–274;177] | 0.67 | |
| T3 | 23.5 (79.2) | [–134;181] | 0.77 | |
| T6 | –41.2 (68.3) | [–177;94.3] | 0.55 | |
| CTX1 (ng/mL) | Overall | 0.03 (0.014) | [0;0.05] | 0.09 |
| T1 | 0.02 (0.018) | [–0.01;0.06] | 0.22 | |
| T3 | 0.04 (0.02) | [0;0.08] | 0.05 | |
| T6 | 0.02 (0.019) | [–0.02;0.05] | 0.45 | |
| Osteocalcin (ng/mL) | Overall | 0.19 (0.38) | [–0.56;0.94] | 0.62 |
| T1 | 0.33 (0.39) | [–0.45;1.11] | 0.41 | |
| T3 | –0.04 (0.45) | [–0.93;0.85] | 0.93 | |
| T6 | 0.28 (0.58) | [–0.87;1.43] | 0.63 | |
| IL-8 (pg/mL) | Overall | –1.53 (4.26) | [–9.98;6.92] | 0.72 |
| T1 | –8.61 (4.85) | [–18.21;1.00] | 0.08 | |
| T3 | 3.26 (10.74) | [–18.04;24.56] | 0.76 | |
| T6 | 0.76 (1.91) | [–3.03;4.56] | 0.69 | |
| TNF-alpha (pg/mL) | Overall | –0.01 (0.09) | [–0.19;0.17] | 0.95 |
| T1 | –0.09 (0.1) | [–0.29;0.11] | 0.36 | |
| T3 | –0.04 (0.11) | [–0.26;0.18] | 0.73 | |
| T6 | 0.11 (0.13) | [–0.15;0.38] | 0.4 |
CI, confidence interval; LS, least squares; SE, standard error.
Secondary outcomes
The KOOS subscores (pain, other symptoms, function in daily living (ADL), function in sport and recreation (Sport/Rec), and knee-related quality of life (QOL)) increased during the first 3 months and then remained stable until month 6 in both PP and FAS population (Supplemental Appendix 1). No difference between treatment groups was observed using the FAS and PP population. Compared to placebo, OLE did not significantly improve the KOOS subscores (Table 2) (Supplemental Appendix 1). The pain intensity at rest decreased with time in both FAS and PP population (Supplemental Appendix 2), but no difference between treatment groups was observed (Table 2). Similarly, pain during walking decreased with time in both treatment group (Supplemental Appendix 2) with no differences between treatment groups.
Other clinical and performance parameters were not significantly modified by OLE (Table 2). The 30s chair score increased with time in both group in the FAS and PP population, while the time-up and go scores remained stable overtime and stair climb time decreased (Supplemental Appendix 3).
The biomarker Coll2-1NO2 and Coll2-1 increased significantly between baseline and month 6, while IL-8 and PGE2 levels decreased and CTX-1, osteocalcin, and TNFα remained stable (Supplemental Appendix 4). However, no significant treatment difference was observed for both the overall treatment effect over the 6-month period as well as at each visit (Table 3).
Post hoc analysis
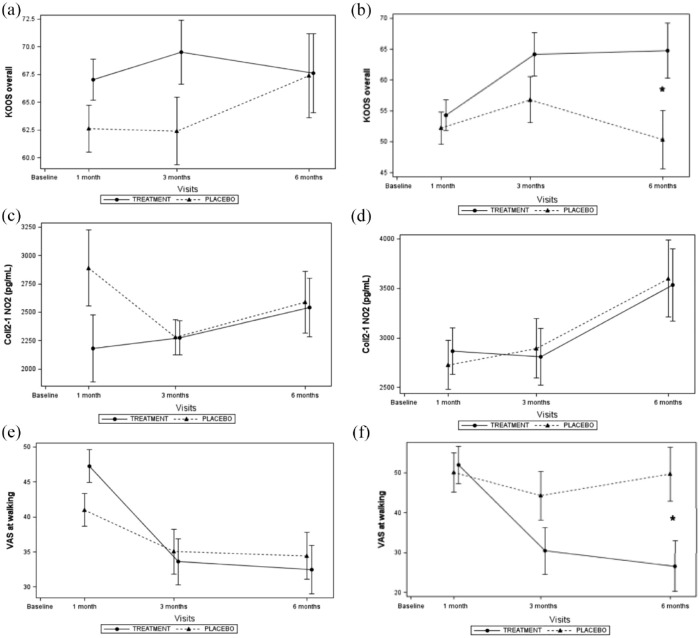
The effect of OLE was also assessed according to the level of walking pain (VAS score at walking) at baseline. Stratification was performed in three groups based on walking pain: subjects with low walking pain with a VAS score between 30 and 50 (n = 40 subjects), subjects with medium walking pain with a VAS score above 50 and inferior to 62 (n = 40), and subjects with high walking pain with a VAS score above 62 (n = 38). Sub-group analyses showed significant treatment difference in favor of the OLE. No effect was observed in subjects with low pain intensity at walking at baseline (Figure 3(a), (c) and (e)). In contrast, 6 months of OLE treatment significantly increased the KOOS global score and decreased pain at walking at month 6 in subjects with high walking pain at baseline (Figure 3(b) and (f)). At 6 month, the effect sizes (ESs) were 0.36 and 0.40 for KOOS global score and the walking pain respectively. Serum Coll2-1NO2 levels were not affected by treatments, whether the pain when walking is high or low (Figure 3(c) and (d)).
Figure 3.
Effects of OLE on global KOOS (a, b), serum Coll2-1NO2 (c, d) and walking pain (e, f) according the level of walking pain at baseline. (a, c, e); subject included in the lowest tertile of pain; (b, d, f): subjects included in the highest tertile of pain.
The intensity of walking pain at baseline had no significant impact on the effect of OLE on serum biomarker levels and VAS at rest (Tables 2 and 3).
Compliance and bioavailability
The percentage of compliance was high at each visit, with median compliance always superior to 93% and the first quartile above 87%.
The four metabolites of oleuropein (oleuropein aglycone, homovanillyl alcohol, hydroxytyrosol, and an isomer of homovanillyl alcohol were significantly increased in urine of OLE-treated subjects compared to placebo. The metabolites levels rapidly increased and reached a steady state after one month (Supplemental Appendix 5).
Adverse effects
Among 114 AEs, 67 occurred in the placebo group and 47 in the OLE group. The most frequent AE in both groups according the MedDRA System Organ Class (SOC) were gastrointestinal disorders (abdominal pain, nausea, dyspepsia; 6 (8.96%) in placebo vs 7 in OLE group (14.89%)) and musculoskeletal and connective tissue disorders (arthralgia and back pain; 22 (32.84%) in placebo vs 12 (25.53%) in OLE group) (Supplemental Appendix 6). No significant difference in terms of number of subjects with at least one AE or severity of AE was observed between the two treatment groups.
Discussion
In this study, we report the results of a placebo-controlled randomized clinical trial investigating the clinical efficacy of OLE administered orally during 6 months in subjects with knee pain, discomfort, and loss of mobility. This is the first study investigating the effects of OLE in knee pain/discomfort in a fully controlled prospective multicenter trial. This study indicates that OLE at the posology tested had no significant effect on knee pain and function in an aged population without diagnosed OA. However, OLE improved KOOS score and reduced walking pain in subjects with high pain at baseline. This effect corresponds to an ES of 0.05 for pain at walking at 1 month, increasing to 0.27 at 3 months to reach 0.40 at 6 months, corresponding to a higher ES than that observed with paracetamol. 19 Similarly, in this sub-group, KOOS global score was also significantly increased after 6 months of treatment with a corresponding ES of 0.36 at 6 months.
The beneficial effects of OLE on pain and locomotion were not associated with any adverse effect after 6 months of treatment. This is in line with toxicological studies in Wistar rat in which no adverse effect was observed after 90-day ingestion of the highest dose tested (1000 mg/kg bw/d). 20 Considering the adverse effects of NSAIDs and analgesic, mainly in patients with comorbidities, OLE could be of value as an alternative to these drugs in pain management of active subjects. 21 One strength of this study was that oleuropein levels have been measured in the urine of subjects to evaluate the compliance and the bioavailability of the product. Oleuropein metabolites levels raised rapidly in the urine of all treated subjects but remained undetectable or very low in the placebo group. This clearly demonstrates a good bioavaibility of the main metabolites of OLE, oleuropein aglycone, hydrotyrosol and homovanillyl alcool and its isomer, and suggests that the typical diet of the participants of the study do not provide significant levels of dietary oleuropein. The OLE supplement was well-tolerated, and compliance with the study protocol was excellent.
Another key observation from this study was the absence of OLE effect on inflammatory blood parameters compared to placebo. Except the level of PGE2 which decreased over time, the other markers of inflammation remained stable until the sixth month. This partially corroborated the data of de Bock et al. 5 demonstrating that OLE increased plasma IL-6 but did not modulated C-reactive protein (CRP), IL-8, and TNF-α levels. In contrast to these authors, we have not observed IL-6 levels modification after OLE treatment. This difference can be explained by differences in the population recruited. We have recruited healthy elderly people while de Bock et al. 5 have enrolled younger overweigth volunteers. Another explanation could be the origin and/or the genotype of the olive used to make the extract. Oleuropein was the primary component of olive leaves extract and exhibiting a content of 21.0 to 98.0 mg/g extract according the genotype. 22 In addition, no effect of OLE was observed on the cartilage degradation biomarkers Coll2-1 and Coll2-1NO2. This was not anticipated as previous animal studies suggested a preventive effect of OLE on cartilage degradation in OA model and a decrease of Coll2-1 and PGE2. In STR/ort mice, OLE supplementation starting at the initial stages of OA, can suppress OA progression evaluated by histological scores. 23 Similarly, oleuropein treatment for 31 weeks prevented joint degeneration and osteoarthritis in Dunkin–Hartley guinea pigs that spontaneously develop OA, 10 and was superior to other polyphenols such as rutin and curcumin. Interestingly, all polyphenols significantly reduced the cartilage degradation score and Coll2-1NO2, but only oleuropein significantly decreased the synovial histological score and serum PGE2 levels compared to the control group. The preclinical benefits of oleuropein were established in the context of advanced OA where joint degradation was severe. It is therefore difficult to anticipate the effect of OLE on metabolic and structural changes occurring in our experimental population with early symptoms of loss of cartilage integrity.
Finally, our results suggest that oleuropein may relieve nociceptive pain triggered by mechanical strain that could be explained by its calcium channel blocker property. 24 Indeed, N-type calcium channels are important for neuronal excitability and play a role in pain genesis. These channels are known to be the major route for Ca2+ entry into the nerve terminals of nociceptors and therefore, blockers of these channels would be expected to produce antinociceptive effects by reducing transmitter release. 25
This randomized, double-blinded, placebo-controlled trial, using well validated scientific methods (i.e. KOOS, ELISA, and OARSI core set) complements previous findings. Indeed, there have been four reports on the efficacy of olive derivatives on joint diseases, of which three were randomized controlled trials26–28 and one was a small-scale uncontrolled trial. 29 Subjects included in these studies were patients with knee OA. An intervention, in the form of topical (olive extract and virgin olive oil)27,29 or oral supplementation (olive extract and hydroxytyrosol)26,28 was given. Comparison with a placebo26,28 or an analgesic (piroxicam) 27 was performed in three of the clinical trials. Globally, it was reported that OLE supplement can decrease pain and thus improve daily activities in adults with OA.
This study showed promising effects of OLE on joint dysfunction in older subjects with pain at baseline but should also be interpretated with some limitations. The main limitation relies on the characteristic of the study population as we recruited older subjects without OA diagnosis which had early signs of joint discomfort but a continuum of joint pain, knee function, and physical performance at baseline. This heterogeneous population was chosen to reflect the general aging population looking for nutritional solutions to manage early symptoms of joint discomfort but reduced statistical power and limited the chances of observing treatment effects in subject with very limited joint dysfunction. The observation that OLE is efficacious in the subset of the population with moderate to high knee pain but without OA suggests that this intervention allows to manage the symptoms of joint dysfunction when they are prevalent but not delay the apparition of these symptoms in a preventive manner. Finally, recruited patients had in mean a moderate pain at inclusion while we observed an effect of OLE in only a part of patients with high pain intensity. It is possible that this choice to select patients with mild pain may have led to a floor effect leading to the inability to actually measure changes from baseline.
Conclusion
In conclusion, daily intake of OLE in subjects with low to moderate knee pain and loss of function did not improve knee functionality. Post hoc analysis indicates that in the most painful subjects OLE may reduce joint discomfort with a good safety profile and a good compliance. Moreover, this trial provides useful information for the design of a larger phase-III clinical trial including the sample size estimate, the choice of the dose, and the selection of primary outcomes.
Supplemental Material
Supplemental material, sj-docx-1-tab-10.1177_1759720X211070205 for An oleuropein-based dietary supplement may improve joint functional capacity in older people with high knee joint pain: findings from a multicentre-RCT and post hoc analysis by Marie-Noëlle Horcajada, Maurice Beaumont, Nicolas Sauvageot, Laure Poquet, Madleen Saboundjian, Berenice Costes, Peter Verdonk, Geoffrey Brands, Jean Brasseur, Didier Urbin-Choffray, Marc Vandenberghe, Karl Brabants, Kurt De Vlam, Werner Fache, Bernard Jandrain, Vincent Grek, Michel Malaise and Yves Henrotin in Therapeutic Advances in Musculoskeletal Disease
Supplemental material, sj-docx-2-tab-10.1177_1759720X211070205 for An oleuropein-based dietary supplement may improve joint functional capacity in older people with high knee joint pain: findings from a multicentre-RCT and post hoc analysis by Marie-Noëlle Horcajada, Maurice Beaumont, Nicolas Sauvageot, Laure Poquet, Madleen Saboundjian, Berenice Costes, Peter Verdonk, Geoffrey Brands, Jean Brasseur, Didier Urbin-Choffray, Marc Vandenberghe, Karl Brabants, Kurt De Vlam, Werner Fache, Bernard Jandrain, Vincent Grek, Michel Malaise and Yves Henrotin in Therapeutic Advances in Musculoskeletal Disease
Supplemental material, sj-docx-3-tab-10.1177_1759720X211070205 for An oleuropein-based dietary supplement may improve joint functional capacity in older people with high knee joint pain: findings from a multicentre-RCT and post hoc analysis by Marie-Noëlle Horcajada, Maurice Beaumont, Nicolas Sauvageot, Laure Poquet, Madleen Saboundjian, Berenice Costes, Peter Verdonk, Geoffrey Brands, Jean Brasseur, Didier Urbin-Choffray, Marc Vandenberghe, Karl Brabants, Kurt De Vlam, Werner Fache, Bernard Jandrain, Vincent Grek, Michel Malaise and Yves Henrotin in Therapeutic Advances in Musculoskeletal Disease
Supplemental material, sj-docx-4-tab-10.1177_1759720X211070205 for An oleuropein-based dietary supplement may improve joint functional capacity in older people with high knee joint pain: findings from a multicentre-RCT and post hoc analysis by Marie-Noëlle Horcajada, Maurice Beaumont, Nicolas Sauvageot, Laure Poquet, Madleen Saboundjian, Berenice Costes, Peter Verdonk, Geoffrey Brands, Jean Brasseur, Didier Urbin-Choffray, Marc Vandenberghe, Karl Brabants, Kurt De Vlam, Werner Fache, Bernard Jandrain, Vincent Grek, Michel Malaise and Yves Henrotin in Therapeutic Advances in Musculoskeletal Disease
Supplemental material, sj-docx-5-tab-10.1177_1759720X211070205 for An oleuropein-based dietary supplement may improve joint functional capacity in older people with high knee joint pain: findings from a multicentre-RCT and post hoc analysis by Marie-Noëlle Horcajada, Maurice Beaumont, Nicolas Sauvageot, Laure Poquet, Madleen Saboundjian, Berenice Costes, Peter Verdonk, Geoffrey Brands, Jean Brasseur, Didier Urbin-Choffray, Marc Vandenberghe, Karl Brabants, Kurt De Vlam, Werner Fache, Bernard Jandrain, Vincent Grek, Michel Malaise and Yves Henrotin in Therapeutic Advances in Musculoskeletal Disease
Supplemental material, sj-pptx-1-tab-10.1177_1759720X211070205 for An oleuropein-based dietary supplement may improve joint functional capacity in older people with high knee joint pain: findings from a multicentre-RCT and post hoc analysis by Marie-Noëlle Horcajada, Maurice Beaumont, Nicolas Sauvageot, Laure Poquet, Madleen Saboundjian, Berenice Costes, Peter Verdonk, Geoffrey Brands, Jean Brasseur, Didier Urbin-Choffray, Marc Vandenberghe, Karl Brabants, Kurt De Vlam, Werner Fache, Bernard Jandrain, Vincent Grek, Michel Malaise and Yves Henrotin in Therapeutic Advances in Musculoskeletal Disease
Acknowledgments
The authors would like to thank posthumously Dr Chapelle for its important contribution at project as well as C. Gimenez and S. Benet for their technical support for oleuropein metabolites analysis.
Footnotes
Author contributions: Marie-Noëlle Horcajada: Conceptualization; Writing – original draft; Writing – review & editing.
Meaumont: Methodology; Writing – review & editing.
Nicolas Sauvageot: Formal analysis; Methodology; Writing – review & editing.
Laure Poquet: Formal analysis; Methodology; Writing – review & editing.
Madleen Saboundjian: Conceptualization; Funding acquisition; Writing – review & editing.
Berenice Costes: Data curation; Investigation; Project administration; Writing – review & editing.
Peter Verdonk: Investigation; Writing – review & editing.
Geoffrey brands: Investigation; Writing – review & editing.
Jean Brasseur: Investigation; Writing – review & editing.
Didier Urbin-Choffray: Investigation; Writing – review & editing.
Marc Vandenberghe: Investigation; Writing – review & editing.
Karl Brabant: Investigation; Writing – review & editing.
Kurt De Vlam: Investigation; Writing – review & editing.
Werner Faché: Investigation; Writing – review & editing.
Bernard Jandrain: Investigation; Writing – review & editing.
Vincent Grek: Investigation; Writing – review & editing.
Michel Malaise: Investigation; Writing – review & editing.
Yves Henrotin: Conceptualization; Methodology; Resources; Supervision; Writing – original draft; Writing – review & editing.
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: YH is the founder and chairman of Artialis SA, a spin-off company of the University of Liège developing biomarkers.
MNH, MB, NS, LP, and MS are employees of Société des produits Nestlé SA.
ACH, BC, and LG are employees of the Artialis’company.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: All the operational phase of this study (patient recruitment, data collection, and statistical analysis) was funded by Société des produits Nestlé SA.
Ethics Approval and Consent to Participate: The study protocol was approved by the Central Ethics Committee of the University Hospital of Liège in Belgium (namely Comité d’Ethique Hospitalo-Facultaire Universitaire de Liège), agreement number: 707. This RCT was also registered on Clinical trial.gov on March 7th, 2017 (https://clinicaltrials.gov/ct2/show/NCT03072108).
Consent for Publication: All authors have read the paper and agreed for its publication
ORCID iD: Yves Henrotin  https://orcid.org/0000-0003-1073-449X
https://orcid.org/0000-0003-1073-449X
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Marie-Noëlle Horcajada, Musculoskeletal Health Department, Nestle Research, EPFL Innovation Park, 1015 Lausanne, Switzerland.
Maurice Beaumont, Nestle Research, Vers-chez-les-Blanc, Lausanne, Switzerland.
Nicolas Sauvageot, Nestle Research, Vers-chez-les-Blanc, Lausanne, Switzerland.
Laure Poquet, Nestle Research, EPFL Innovation Park, Lausanne, Switzerland.
Madleen Saboundjian, Nestle Research, EPFL Innovation Park, Lausanne, Switzerland.
Berenice Costes, Artialis SA, CHU Sart-Tilman, Liège, Belgium.
Peter Verdonk, Antwerpen Orthopedic Center, Antwerpen, Belgium.
Geoffrey Brands, CHC, Clinique Saint-Joseph, Liège, Belgium.
Jean Brasseur, CHU UCL Namur, Site Mont-Godinne, Yvoir, Belgium.
Didier Urbin-Choffray, Centre Hospitalier Régional de la Citadelle, Liège, Belgium.
Marc Vandenberghe, Grand Hôpital de Charleroi, Charleroi, Belgium.
Karl Brabants, ZNA Middelheim, Antwerpen, Belgium.
Kurt De Vlam, ZNA Jan Palfijn, Merksem, Belgium.
Werner Fache, Linus Pauling Preventie Centrum, Gent, Belgium.
Bernard Jandrain, Clinical Pharmacology Unit, ATC S.A., Liège, Belgium.
Vincent Grek, 2B Clinic, Wavre, Belgium.
Michel Malaise, CHU Sart-Tilman, Liège, Belgium.
Yves Henrotin, musculoSKeletal Innovative research Lab (mSKIL), The Center for Interdisciplinary Research on Medicines (CIRM), Department of Motricity Center, Institute of Pathology, University of Liège, CHU Sart-Tilman, 4000 Liège, Belgium; Artialis SA, CHU Sart-Tilman, Liège, Belgium; Department of Physical Therapy and Rehabilitation, Princess Paola Hospital, Vivalia, Marche-en-Fammenne, Belgium.
References
- 1. Calmbach WL, Hutchens M. Evaluation of patients presenting with knee pain: part II. Differential diagnosis. Am Fam Physician 2003; 68: 917–922. [PubMed] [Google Scholar]
- 2. Atiquzzaman M, Karim ME, Kopec J, et al. Role of nonsteroidal antiinflammatory drugs in the association between osteoarthritis and cardiovascular diseases: a longitudinal study. Arthritis Rheumatol 2019; 71: 1835–1843. [DOI] [PubMed] [Google Scholar]
- 3. Bush TM, Shlotzhauer TL, Imai K. Nonsteroidal anti-inflammatory drugs. Proposed guidelines for monitoring toxicity. West J Med 1991; 155: 39–42. [PMC free article] [PubMed] [Google Scholar]
- 4. Conaghan PG, Arden N, Avouac B, et al. Safety of paracetamol in osteoarthritis: what does the literature say. Drugs Aging 2019; 36: 7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. de Bock M, Thorstensen EB, Derraik JG, et al. Human absorption and metabolism of oleuropein and hydroxytyrosol ingested as olive (Olea europaea L.) leaf extract. Mol Nutr Food Res 2013; 57: 2079–2085. [DOI] [PubMed] [Google Scholar]
- 6. Omar SH. Oleuropein in olive and its pharmacological effects. Sci Pharm 2010; 78: 133–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Feng Z, Li X, Lin J, et al. Oleuropein inhibits the IL-1β-induced expression of inflammatory mediators by suppressing the activation of NF-κB and MAPKs in human osteoarthritis chondrocytes. Food Funct 2017; 8: 3737–3744. [DOI] [PubMed] [Google Scholar]
- 8. Castejón ML, Ángeles Rosillo M, Montoya T, et al. Oleuropein down-regulated IL-1β-induced inflammation and oxidative stress in human synovial fibroblast cell line SW982. Food Funct 2017; 8: 1890–1898. [DOI] [PubMed] [Google Scholar]
- 9. Impellizzeri D, Esposito E, Mazzon E, et al. Oleuropein aglycone, an olive oil compound, ameliorates development of arthritis caused by injection of collagen type II in mice. J Pharmacol Exp Ther 2011; 339: 859–869. [DOI] [PubMed] [Google Scholar]
- 10. Horcajada MN, Sanchez C, Scalfo FM, et al. Oleuropein or rutin consumption decreases the spontaneous development of osteoarthritis in the Hartley guinea pig. Osteoarthritis Cartilage 2015; 23: 94–102. [DOI] [PubMed] [Google Scholar]
- 11. Puel C, Mardon J, Kati-Coulibaly S, et al. Black Lucques olives prevented bone loss caused by ovariectomy and talc granulomatosis in rats. Br J Nutr 2007; 97: 1012–1020. [DOI] [PubMed] [Google Scholar]
- 12. Puel C, Quintin A, Agalias A, et al. Olive oil and its main phenolic micronutrient (oleuropein) prevent inflammation-induced bone loss in the ovariectomised rat. Br J Nutr 2004; 92: 119–127. [DOI] [PubMed] [Google Scholar]
- 13. Puel C, Mathey J, Agalias A, et al. Dose-response study of effect of oleuropein, an olive oil polyphenol, in an ovariectomy/inflammation experimental model of bone loss in the rat. Clin Nutr 2006; 25: 859–868. [DOI] [PubMed] [Google Scholar]
- 14. Visioli F, Galli C. Biological properties of olive oil phytochemicals. Crit Rev Food Sci Nutr 2002; 42: 209–221. [DOI] [PubMed] [Google Scholar]
- 15. Casado-Díaz A, Anter J, Müller S, et al. Transcriptomic analyses of the anti-adipogenic effects of oleuropein in human mesenchymal stem cells. Food Funct 2017; 8: 1254–1270. [DOI] [PubMed] [Google Scholar]
- 16. Santiago-Mora R, Casado-Díaz A, De Castro MD, et al. Oleuropein enhances osteoblastogenesis and inhibits adipogenesis: the effect on differentiation in stem cells derived from bone marrow. Osteoporos Int 2011; 22: 675–684. [DOI] [PubMed] [Google Scholar]
- 17. Filip R, Possemiers S, Heyerick A, et al. Twelve-month consumption of a polyphenol extract from olive (Olea europaea) in a double blind, randomized trial increases serum total osteocalcin levels and improves serum lipid profiles in postmenopausal women with osteopenia. J Nutr Health Aging 2015; 19: 77–86. [DOI] [PubMed] [Google Scholar]
- 18. Mobasheri AL, Lambert C, Henrotin Y. Coll2–1 and Coll2–1NO2 as exemplars of collagen extracellular matrix turnover – biomarkers to facilitate the treatment of osteoarthritis? Expert Rev Mol Diagn 2019; 19: 803–812. [DOI] [PubMed] [Google Scholar]
- 19. Bannuru RR, Schmid CH, Ken DM, et al. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med 2015; 162: 46–54. [DOI] [PubMed] [Google Scholar]
- 20. Clewell AE, Béres E, Vértesi A, et al. A comprehensive toxicological safety assessment of an extract of Olea Europaea L. Leaves (Bonolive™). Int J Toxicol 2016; 35: 208–221. [DOI] [PubMed] [Google Scholar]
- 21. Hayes CJ, Krebs EE, Hudson T, et al. Impact of opioid dose escalation on the development of substance use disorders, accidents, self-inflicted injuries, opioid overdoses and alcohol and non-opioid drug-related overdoses: a retrospective cohort study. Addiction 2020; 115: 1098–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Orak HH, Karamac M, Amarowicz R, et al. Genotype-related differences in the phenolic compound profile and antioxidant activity of extracts from olive (Olea europaea L.) leaves. Molecules 2019; 24: 1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Takuma M, Haruka K, Mutsuto W, et al. Olive leaf extract prevents cartilage degeneration in osteoarthritis of STR/ort mice. Biosci Biotechnol Biochem 2018; 82: 1101–1106. [DOI] [PubMed] [Google Scholar]
- 24. Zare L, Esmaeili-Mahani S, Abbasnejad M, et al. Oleuropein, chief constituent of olive leaf extract, prevents the development of morphine antinociceptive tolerance through inhibition of morphine-induced L-type calcium channel overexpression. Phytother Res 2012; 26: 1731–1737. [DOI] [PubMed] [Google Scholar]
- 25. Malfait AM, Miller RJ. Emerging targets for the management of osteoarthritis pain. Curr Osteoporos Rep 2016; 14: 260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bitler CM, Matt K, Hook G, et al. Olive extract supplement decreases pain and improves daily activities in adults with osteoarthritis and decreases plasma homocysteine in those with rheumatoid arthritis. Nutr Res 2007; 27: 470–477. [Google Scholar]
- 27. Bohlooli S, Jastan M, Nakhostin-Roohi B, et al. A pilot double-blinded, randomized, clinical trial of topical virgin olive oil versus piroxicam gel in osteoarthritis of the knee. J Clin Rheumatol 2012; 18: 99–101. [DOI] [PubMed] [Google Scholar]
- 28. Takeda R, Koike T, Tanaka K, et al. Double-blind placebo-controlled trial of hydroxytyrosol of Olea europaea on pain in gonarthrosis. Phytomedicine 2013; 20: 861–864. [DOI] [PubMed] [Google Scholar]
- 29. Gelmini F, Ruscica M, Macchi C, et al. Unsaponifiable fraction of unripe fruits of olea europaea: an interesting source of anti-inflammatory constituents. Planta Med 2016; 82: 273–278. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tab-10.1177_1759720X211070205 for An oleuropein-based dietary supplement may improve joint functional capacity in older people with high knee joint pain: findings from a multicentre-RCT and post hoc analysis by Marie-Noëlle Horcajada, Maurice Beaumont, Nicolas Sauvageot, Laure Poquet, Madleen Saboundjian, Berenice Costes, Peter Verdonk, Geoffrey Brands, Jean Brasseur, Didier Urbin-Choffray, Marc Vandenberghe, Karl Brabants, Kurt De Vlam, Werner Fache, Bernard Jandrain, Vincent Grek, Michel Malaise and Yves Henrotin in Therapeutic Advances in Musculoskeletal Disease
Supplemental material, sj-docx-2-tab-10.1177_1759720X211070205 for An oleuropein-based dietary supplement may improve joint functional capacity in older people with high knee joint pain: findings from a multicentre-RCT and post hoc analysis by Marie-Noëlle Horcajada, Maurice Beaumont, Nicolas Sauvageot, Laure Poquet, Madleen Saboundjian, Berenice Costes, Peter Verdonk, Geoffrey Brands, Jean Brasseur, Didier Urbin-Choffray, Marc Vandenberghe, Karl Brabants, Kurt De Vlam, Werner Fache, Bernard Jandrain, Vincent Grek, Michel Malaise and Yves Henrotin in Therapeutic Advances in Musculoskeletal Disease
Supplemental material, sj-docx-3-tab-10.1177_1759720X211070205 for An oleuropein-based dietary supplement may improve joint functional capacity in older people with high knee joint pain: findings from a multicentre-RCT and post hoc analysis by Marie-Noëlle Horcajada, Maurice Beaumont, Nicolas Sauvageot, Laure Poquet, Madleen Saboundjian, Berenice Costes, Peter Verdonk, Geoffrey Brands, Jean Brasseur, Didier Urbin-Choffray, Marc Vandenberghe, Karl Brabants, Kurt De Vlam, Werner Fache, Bernard Jandrain, Vincent Grek, Michel Malaise and Yves Henrotin in Therapeutic Advances in Musculoskeletal Disease
Supplemental material, sj-docx-4-tab-10.1177_1759720X211070205 for An oleuropein-based dietary supplement may improve joint functional capacity in older people with high knee joint pain: findings from a multicentre-RCT and post hoc analysis by Marie-Noëlle Horcajada, Maurice Beaumont, Nicolas Sauvageot, Laure Poquet, Madleen Saboundjian, Berenice Costes, Peter Verdonk, Geoffrey Brands, Jean Brasseur, Didier Urbin-Choffray, Marc Vandenberghe, Karl Brabants, Kurt De Vlam, Werner Fache, Bernard Jandrain, Vincent Grek, Michel Malaise and Yves Henrotin in Therapeutic Advances in Musculoskeletal Disease
Supplemental material, sj-docx-5-tab-10.1177_1759720X211070205 for An oleuropein-based dietary supplement may improve joint functional capacity in older people with high knee joint pain: findings from a multicentre-RCT and post hoc analysis by Marie-Noëlle Horcajada, Maurice Beaumont, Nicolas Sauvageot, Laure Poquet, Madleen Saboundjian, Berenice Costes, Peter Verdonk, Geoffrey Brands, Jean Brasseur, Didier Urbin-Choffray, Marc Vandenberghe, Karl Brabants, Kurt De Vlam, Werner Fache, Bernard Jandrain, Vincent Grek, Michel Malaise and Yves Henrotin in Therapeutic Advances in Musculoskeletal Disease
Supplemental material, sj-pptx-1-tab-10.1177_1759720X211070205 for An oleuropein-based dietary supplement may improve joint functional capacity in older people with high knee joint pain: findings from a multicentre-RCT and post hoc analysis by Marie-Noëlle Horcajada, Maurice Beaumont, Nicolas Sauvageot, Laure Poquet, Madleen Saboundjian, Berenice Costes, Peter Verdonk, Geoffrey Brands, Jean Brasseur, Didier Urbin-Choffray, Marc Vandenberghe, Karl Brabants, Kurt De Vlam, Werner Fache, Bernard Jandrain, Vincent Grek, Michel Malaise and Yves Henrotin in Therapeutic Advances in Musculoskeletal Disease